Reduction-Induced Magnetic Behavior in LaFeO3−δ Thin Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Synthesis and Annealing
2.2. Sample Characterization Methods
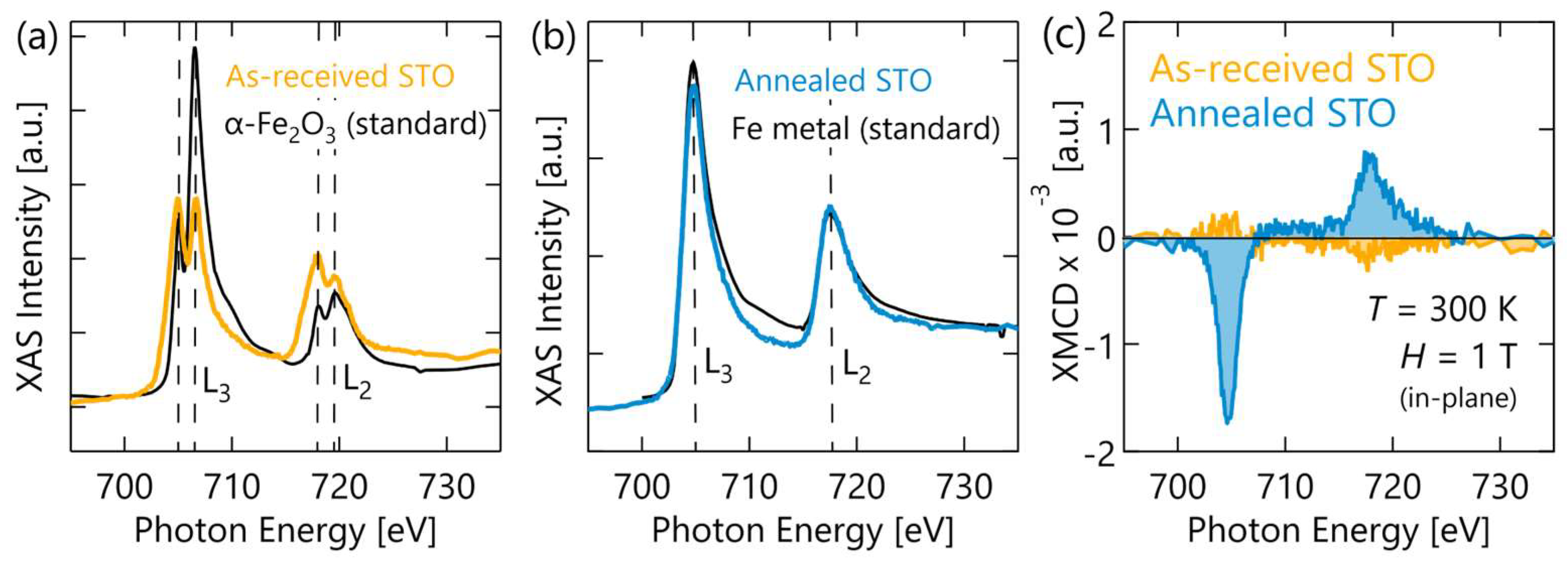
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bisri, S.Z.; Shimizu, S.; Nakano, M.; Iwasa, Y. Endeavor of Iontronics: From Fundamentals to Applications of Ion-Controlled Electronics. Adv. Mater. 2017, 29, 1607054. [Google Scholar] [CrossRef]
- Leighton, C. Electrolyte-Based Ionic Control of Functional Oxides. Nature Mater. 2019, 18, 13–18. [Google Scholar] [CrossRef]
- Cui, B.; Song, C.; Wang, G.; Yan, Y.; Peng, J.; Miao, J.; Mao, H.; Li, F.; Chen, C.; Zeng, F.; et al. Reversible Ferromagnetic Phase Transition in Electrode-Gated Manganites. Adv. Funct. Mater. 2014, 24, 7233–7240. [Google Scholar] [CrossRef]
- Bauer, U.; Yao, L.; Tan, A.J.; Agrawal, P.; Emori, S.; Tuller, H.L.; van Dijken, S.; Beach, G.S.D. Magneto-Ionic Control of Interfacial Magnetism. Nature Mater. 2015, 14, 174–181. [Google Scholar] [CrossRef]
- Li, H.-B.; Lu, N.; Zhang, Q.; Wang, Y.; Feng, D.; Chen, T.; Yang, S.; Duan, Z.; Li, Z.; Shi, Y.; et al. Electric-Field Control of Ferromagnetism through Oxygen Ion Gating. Nat. Commun. 2017, 8, 2156. [Google Scholar] [CrossRef]
- Walter, J.; Charlton, T.; Ambaye, H.; Fitzsimmons, M.R.; Orth, P.P.; Fernandes, R.M.; Leighton, C. Giant Electrostatic Modification of Magnetism via Electrolyte-Gate-Induced Cluster Percolation in La1−xSrxCoO3−δ. Phys. Rev. Mater. 2018, 2, 111406. [Google Scholar] [CrossRef]
- Eerenstein, W.; Mathur, N.D.; Scott, J.F. Multiferroic and Magnetoelectric Materials. Nature 2006, 442, 759–765. [Google Scholar] [CrossRef]
- Srinivasan, G. Magnetoelectric Composites. Annu. Rev. Mater. Res. 2010, 40, 153–178. [Google Scholar] [CrossRef]
- Song, C.; Cui, B.; Li, F.; Zhou, X.; Pan, F. Recent Progress in Voltage Control of Magnetism: Materials, Mechanisms, and Performance. Prog. Mater. Sci. 2017, 87, 33–82. [Google Scholar] [CrossRef]
- Spaldin, N.A.; Ramesh, R. Advances in Magnetoelectric Multiferroics. Nature Mater. 2019, 18, 203–212. [Google Scholar] [CrossRef]
- Gilbert, D.A.; Grutter, A.J.; Arenholz, E.; Liu, K.; Kirby, B.J.; Borchers, J.A.; Maranville, B.B. Structural and Magnetic Depth Profiles of Magneto-Ionic Heterostructures beyond the Interface Limit. Nat. Commun. 2016, 7, 12264. [Google Scholar] [CrossRef]
- Grutter, A.J.; Gilbert, D.A.; Alaan, U.S.; Arenholz, E.; Maranville, B.B.; Borchers, J.A.; Suzuki, Y.; Liu, K.; Kirby, B.J. Reversible Control of Magnetism in La0.67Sr0.33MnO3 through Chemically-Induced Oxygen Migration. Appl. Phys. Lett. 2016, 108, 082405. [Google Scholar] [CrossRef]
- Ahn, C.H.; Triscone, J.-M.; Mannhart, J. Electric Field Effect in Correlated Oxide Systems. Nature 2003, 424, 1015–1018. [Google Scholar] [CrossRef]
- Perez-Muñoz, A.M.; Schio, P.; Poloni, R.; Fernandez-Martinez, A.; Rivera-Calzada, A.; Cezar, J.C.; Salas-Colera, E.; Castro, G.R.; Kinney, J.; Leon, C.; et al. In Operando Evidence of Deoxygenation in Ionic Liquid Gating of YBa2Cu3O7−X. Proc. Natl. Acad. Sci. USA 2017, 114, 215–220. [Google Scholar] [CrossRef]
- Niu, W.; Chen, Y.; Gan, Y.; Zhang, Y.; Zhang, X.; Yuan, X.; Cao, Z.; Liu, W.; Xu, Y.; Zhang, R.; et al. Electrolyte Gate Controlled Metal-Insulator Transitions of the CaZrO3/SrTiO3 Heterointerface. Appl. Phys. Lett. 2019, 115, 061601. [Google Scholar] [CrossRef]
- Tan, A.J.; Huang, M.; Avci, C.O.; Büttner, F.; Mann, M.; Hu, W.; Mazzoli, C.; Wilkins, S.; Tuller, H.L.; Beach, G.S.D. Magneto-Ionic Control of Magnetism Using a Solid-State Proton Pump. Nature Mater. 2019, 18, 35–41. [Google Scholar] [CrossRef]
- Skinner, S.J. Recent Advances in Perovskite-Type Materials for Solid Oxide Fuel Cell Cathodes. Int. J. Inorg. Mater. 2001, 3, 113–121. [Google Scholar] [CrossRef]
- White, R.L. Review of Recent Work on the Magnetic and Spectroscopic Properties of the Rare-Earth Orthoferrites. J. Appl. Phys. 1969, 40, 1061–1069. [Google Scholar] [CrossRef]
- Zhang, K.; Sunarso, J.; Shao, Z.; Zhou, W.; Sun, C.; Wang, S.; Liu, S. Research Progress and Materials Selection Guidelines on Mixed Conducting Perovskite-Type Ceramic Membranes for Oxygen Production. RSC Adv. 2011, 1, 1661. [Google Scholar] [CrossRef]
- Sunarso, J.; Hashim, S.S.; Zhu, N.; Zhou, W. Perovskite Oxides Applications in High Temperature Oxygen Separation, Solid Oxide Fuel Cell and Membrane Reactor: A Review. Prog. Energy Combust. Sci. 2017, 61, 57–77. [Google Scholar] [CrossRef]
- Zamudio-García, J.; Caizán-Juanarena, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. A Review on Recent Advances and Trends in Symmetrical Electrodes for Solid Oxide Cells. J. Power Sources 2022, 520, 230852. [Google Scholar] [CrossRef]
- Tu, H.Y.; Takeda, Y.; Imanishi, N.; Yamamoto, O. Ln0.4Sr0.6Co0.8Fe0.2O3−δ (Ln = La, Pr, Nd, Sm, Gd) for the Electrode in Solid Oxide Fuel Cells. Solid State Ion. 1999, 117, 277–281. [Google Scholar] [CrossRef]
- Shao, Z.; Haile, S.M. A High-Performance Cathode for the next Generation of Solid-Oxide Fuel Cells. Nature 2004, 431, 170–173. [Google Scholar] [CrossRef]
- Jiang, S.P. Development of Lanthanum Strontium Cobalt Ferrite Perovskite Electrodes of Solid Oxide Fuel Cells—A Review. Int. J. Hydrogen Energy 2019, 44, 7448–7493. [Google Scholar] [CrossRef]
- Koehler, W.C.; Wollan, E.O. Neutron-Diffraction Study of the Magnetic Properties of Perovskite-like Compounds LaBO3. J. Phys. Chem. Solids 1957, 2, 100–106. [Google Scholar] [CrossRef]
- Scholl, A. Observation of Antiferromagnetic Domains in Epitaxial Thin Films. Science 2000, 287, 1014–1016. [Google Scholar] [CrossRef]
- Lüning, J.; Nolting, F.; Scholl, A.; Ohldag, H.; Seo, J.W.; Fompeyrine, J.; Locquet, J.-P.; Stöhr, J. Determination of the Antiferromagnetic Spin Axis in Epitaxial LaFeO3 Films by X-ray Magnetic Linear Dichroism Spectroscopy. Phys. Rev. B 2003, 67, 214433. [Google Scholar] [CrossRef]
- Folven, E.; Scholl, A.; Young, A.; Retterer, S.T.; Boschker, J.E.; Tybell, T.; Takamura, Y.; Grepstad, J.K. Effects of Nanostructuring and Substrate Symmetry on Antiferromagnetic Domain Structure in LaFeO3 Thin Films. Phys. Rev. B 2011, 84, 220410. [Google Scholar] [CrossRef]
- Hallsteinsen, I.; Moreau, M.; Chopdekar, R.V.; Christiansen, E.; Nord, M.; Vullum, P.-E.; Grepstad, J.K.; Holmestad, R.; Selbach, S.M.; Scholl, A.; et al. Magnetic Domain Configuration of (111)-Oriented LaFeO3 Epitaxial Thin Films. APL Mater. 2017, 5, 086107. [Google Scholar] [CrossRef]
- Kjærnes, K.; Hallsteinsen, I.; Chopdekar, R.V.; Moreau, M.; Bolstad, T.; Svenum, I.-H.; Selbach, S.M.; Tybell, T. Uniaxial Néel Vector Control in Perovskite Oxide Thin Films by Anisotropic Strain Engineering. Phys. Rev. B 2021, 103, 224435. [Google Scholar] [CrossRef]
- Qiu, Y.; Luo, Y.S.; Zou, Z.J.; Tian, Z.M.; Yuan, S.L.; Xi, Y.; Huang, L.Z. Size Effect on Magnetic and Dielectric Properties in Nanocrystalline LaFeO3. J. Mater. Sci Mater. Electron. 2014, 25, 760–764. [Google Scholar] [CrossRef]
- Rai, A.; Thakur, A.K. Tunability of Dielectric, Optical and Magnetic Property by Simultaneous Co-Substitution in LaFeO3. Mater. Sci. Eng. B 2017, 224, 139–149. [Google Scholar] [CrossRef]
- Gabal, M.A.; Al-Solami, F.; Al Angari, Y.M.; Awad, A.; Al-Juaid, A.A.; Saeed, A. Structural, Magnetic, and Electrical Characterization of Sr-Substituted LaFeO3 Perovskite Synthesized via Sucrose Auto-Combustion Route. J. Mater. Sci Mater. Electron. 2020, 31, 3146–3158. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, W.; Yang, S.; Yu, Z.; Gu, B.; Du, Y. Influences of La3+ Substitution on the Structure and Magnetic Properties of M-Type Strontium Ferrites. J. Magn. Magn. Mater. 2002, 238, 207–214. [Google Scholar] [CrossRef]
- Ni, W.; Ye, J.; Guo, Y.; Cheng, C.; Lin, Z.; Li, Y.; Wang, H.; Yu, Y.; Li, Q.; Huang, S.; et al. Decisive Role of Mixed-Valence Structure in Colossal Dielectric Constant of LaFeO3. J. Am. Ceram. Soc. 2017, 100, 3042–3049. [Google Scholar] [CrossRef]
- Li, J.; Miao, J.; Duan, X.; Dai, J.; Liu, Q.; Wang, S.; Zhou, W.; Shao, Z. Fine-Tuning Surface Properties of Perovskites via Nanocompositing with Inert Oxide toward Developing Superior Catalysts for Advanced Oxidation. Adv. Funct. Mater. 2018, 28, 1804654. [Google Scholar] [CrossRef]
- Salamon, M.B.; Jaime, M. The Physics of Manganites: Structure and Transport. Rev. Mod. Phys. 2001, 73, 583–628. [Google Scholar] [CrossRef]
- Serrate, D.; Teresa, J.M.D.; Ibarra, M.R. Double Perovskites with Ferromagnetism above Room Temperature. J. Phys. Condens. Matter 2007, 19, 023201. [Google Scholar] [CrossRef]
- Xu, P.; Han, W.; Rice, P.M.; Jeong, J.; Samant, M.G.; Mohseni, K.; Meyerheim, H.L.; Ostanin, S.; Maznichenko, I.V.; Mertig, I.; et al. Reversible Formation of 2D Electron Gas at the LaFeO3/SrTiO3 Interface via Control of Oxygen Vacancies. Adv. Mater. 2017, 29, 1604447. [Google Scholar] [CrossRef]
- Kang, K.T.; Zhang, B.; Sharma, Y.; Paudel, B.; Wang, H.; Dowden, P.; Chen, A. Substrate Oxygen Sponge Effect: A Parameter for Epitaxial Manganite Thin Film Growth. Appl. Phys. Lett. 2020, 117, 151601. [Google Scholar] [CrossRef]
- Cao, Q.; Lü, W.; Wang, X.R.; Guan, X.; Wang, L.; Yan, S.; Wu, T.; Wang, X. Nonvolatile Multistates Memories for High-Density Data Storage. ACS Appl. Mater. Interfaces 2020, 12, 42449–42471. [Google Scholar] [CrossRef]
- Maranville, B.; Ratcliff, W., II; Kienzle, P. Reductus: A Stateless Python Data Reduction Service with a Browser Front End. J. Appl. Crystallogr. 2018, 51, 1500–1506. [Google Scholar] [CrossRef]
- Kirby, B.J.; Kienzle, P.A.; Maranville, B.B.; Berk, N.F.; Krycka, J.; Heinrich, F.; Majkrzak, C.F. Phase-Sensitive Specular Neutron Reflectometry for Imaging the Nanometer Scale Composition Depth Profile of Thin-Film Materials. Curr. Opin. Colloid Interface Sci. 2012, 17, 44–53. [Google Scholar] [CrossRef]
- Maranville, B.B.; Green, A.; Kienzle, P.A. Distributed Error-Function Roughness in Refl1d Reflectometry Fitting Program. arXiv 2017, arXiv:1801.04975. [Google Scholar]
- Seo, J.W.; Fullerton, E.E.; Nolting, F.; Scholl, A.; Fompeyrine, J.; Locquet, J.-P. Antiferromagnetic LaFeO3 Thin Films and Their Effect on Exchange Bias. J. Phys. Condens. Matter 2008, 20, 264014. [Google Scholar] [CrossRef]
- Li, L.; Zhang, G.; Li, J.; Chen, D.; Cheng, Z.; Wang, Y. Electric Field Induced Two-Dimensional Electron Gas and Magnetism in LaFeO3/SrTiO3 (0 0 1) Heterostructures. Appl. Surf. Sci. 2019, 471, 185–195. [Google Scholar] [CrossRef]
- Chen, B.; Gauquelin, N.; Green, R.J.; Verbeeck, J.; Rijnders, G.; Koster, G. Asymmetric Interfacial Intermixing Associated Magnetic Coupling in LaMnO3/LaFeO3 Heterostructures. Front. Phys. 2021, 9, 698154. [Google Scholar] [CrossRef]
- De Souza, R.A. The Formation of Equilibrium Space-Charge Zones at Grain Boundaries in the Perovskite Oxide SrTiO3. Phys. Chem. Chem. Phys. 2009, 11, 9939. [Google Scholar] [CrossRef]
- Tang, A.S.; Pelliciari, J.; Song, Q.; Song, Q.; Ning, S.; Freeland, J.W.; Comin, R.; Ross, C.A. XMCD Study of Magnetism and Valence State in Iron-Substituted Strontium Titanate. Phys. Rev. Mater. 2019, 3, 054408. [Google Scholar] [CrossRef]
- Chen, C.T.; Idzerda, Y.U.; Lin, H.-J.; Smith, N.V.; Meigs, G.; Chaban, E.; Ho, G.H.; Pellegrin, E.; Sette, F. Experimental Confirmation of the X-ray Magnetic Circular Dichroism Sum Rules for Iron and Cobalt. Phys. Rev. Lett. 1995, 75, 152–155. [Google Scholar] [CrossRef]
- Sassi, M.; Pearce, C.I.; Bagus, P.S.; Arenholz, E.; Rosso, K.M. First-Principles Fe L2,3-Edge and O K-Edge XANES and XMCD Spectra for Iron Oxides. J. Phys. Chem. A 2017, 121, 7613–7618. [Google Scholar] [CrossRef]
- Anderson, H.U. Review of the Structural and Electrical Properties of the (La,Sr)(Co,Fe)O3 System. Proc. Vol. 1995, 1995-1, 375–384. [Google Scholar] [CrossRef]
- Navrotsky, A.; Lee, W.; Mielewczyk-Gryn, A.; Ushakov, S.V.; Anderko, A.; Wu, H.; Riman, R.E. Thermodynamics of Solid Phases Containing Rare Earth Oxides. J. Chem. Thermodyn. 2015, 88, 126–141. [Google Scholar] [CrossRef]
- Fallarino, L.; Kirby, B.J.; Pancaldi, M.; Riego, P.; Balk, A.L.; Miller, C.W.; Vavassori, P.; Berger, A. Magnetic Properties of Epitaxial CoCr Films with Depth-Dependent Exchange-Coupling Profiles. Phys. Rev. B 2017, 95, 134445. [Google Scholar] [CrossRef]
- De Souza, R.A. Oxygen Diffusion in SrTiO3 and Related Perovskite Oxides. Adv. Funct. Mater. 2015, 25, 6326–6342. [Google Scholar] [CrossRef]
- De Souza, R.A.; Martin, M. Using 18O/16O Exchange to Probe an Equilibrium Space-Charge Layer at the Surface of a Crystalline Oxide: Method and Application. Phys. Chem. Chem. Phys. 2008, 10, 2356. [Google Scholar] [CrossRef]
- De Souza, R.A.; Metlenko, V.; Park, D.; Weirich, T.E. Behavior of Oxygen Vacancies in Single-Crystal SrTiO3: Equilibrium Distribution and Diffusion Kinetics. Phys. Rev. B 2012, 85, 174109. [Google Scholar] [CrossRef]
- Connell, J.G.; Isaac, B.J.; Ekanayake, G.B.; Strachan, D.R.; Seo, S.S.A. Preparation of Atomically Flat SrTiO3 Surfaces Using a Deionized-Water Leaching and Thermal Annealing Procedure. Appl. Phys. Lett. 2012, 101, 251607. [Google Scholar] [CrossRef]
- Raisch, C.; Chassé, T.; Langheinrich, C.; Chassé, A. Preparation and Investigation of the A-Site and B-Site Terminated SrTiO3(001) Surface: A Combined Experimental and Theoretical X-ray Photoelectron Diffraction Study. J. Appl. Phys. 2012, 112, 073505. [Google Scholar] [CrossRef]
- Mugiraneza, S.; Hallas, A.M. Tutorial: A Beginner’s Guide to Interpreting Magnetic Susceptibility Data with the Curie-Weiss Law. Commun. Phys. 2022, 5, 95. [Google Scholar] [CrossRef]
- Papaefthymiou, G.C. Nanoparticle Magnetism. Nano Today 2009, 4, 438–447. [Google Scholar] [CrossRef]
- Carvell, J.; Ayieta, E.; Gavrin, A.; Cheng, R.; Shah, V.R.; Sokol, P. Magnetic Properties of Iron Nanoparticle. J. Appl. Phys. 2010, 107, 103913. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arndt, N.D.; Hershkovitz, E.; Shah, L.; Kjærnes, K.; Yang, C.-Y.; Balakrishnan, P.P.; Shariff, M.S.; Tauro, S.; Gopman, D.B.; Kirby, B.J.; et al. Reduction-Induced Magnetic Behavior in LaFeO3−δ Thin Films. Materials 2024, 17, 1188. https://doi.org/10.3390/ma17051188
Arndt ND, Hershkovitz E, Shah L, Kjærnes K, Yang C-Y, Balakrishnan PP, Shariff MS, Tauro S, Gopman DB, Kirby BJ, et al. Reduction-Induced Magnetic Behavior in LaFeO3−δ Thin Films. Materials. 2024; 17(5):1188. https://doi.org/10.3390/ma17051188
Chicago/Turabian StyleArndt, Nathan D., Eitan Hershkovitz, Labdhi Shah, Kristoffer Kjærnes, Chao-Yao Yang, Purnima P. Balakrishnan, Mohammed S. Shariff, Shaun Tauro, Daniel B. Gopman, Brian J. Kirby, and et al. 2024. "Reduction-Induced Magnetic Behavior in LaFeO3−δ Thin Films" Materials 17, no. 5: 1188. https://doi.org/10.3390/ma17051188
APA StyleArndt, N. D., Hershkovitz, E., Shah, L., Kjærnes, K., Yang, C.-Y., Balakrishnan, P. P., Shariff, M. S., Tauro, S., Gopman, D. B., Kirby, B. J., Grutter, A. J., Tybell, T., Kim, H., & Need, R. F. (2024). Reduction-Induced Magnetic Behavior in LaFeO3−δ Thin Films. Materials, 17(5), 1188. https://doi.org/10.3390/ma17051188





