Green Chemistry for Crosslinking Biopolymers: Recent Advances in Riboflavin-Mediated Photochemistry
Abstract
1. Introduction
2. Visible Light-Induced Photochemistry
2.1. Visible Light-Induced Photoinitiators
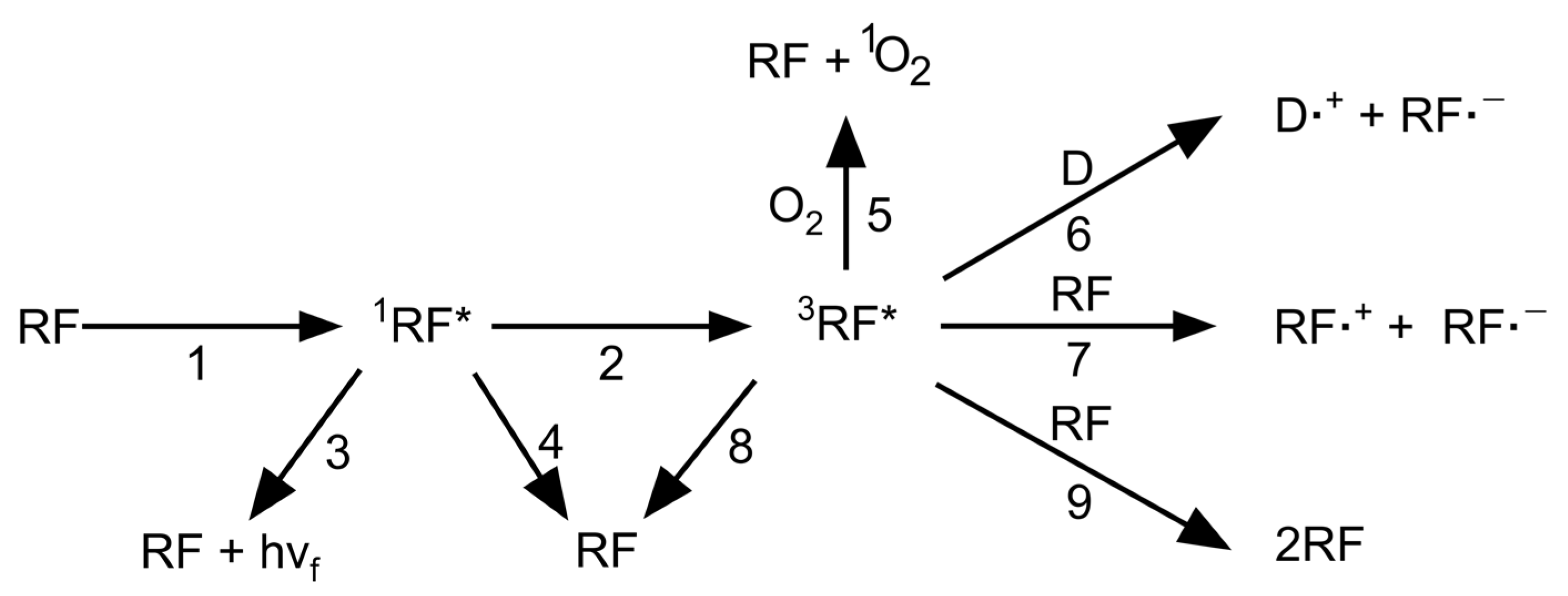
2.2. Mechanism of Riboflavin-Mediated Photochemistry
3. Riboflavin-Induced Photochemistry
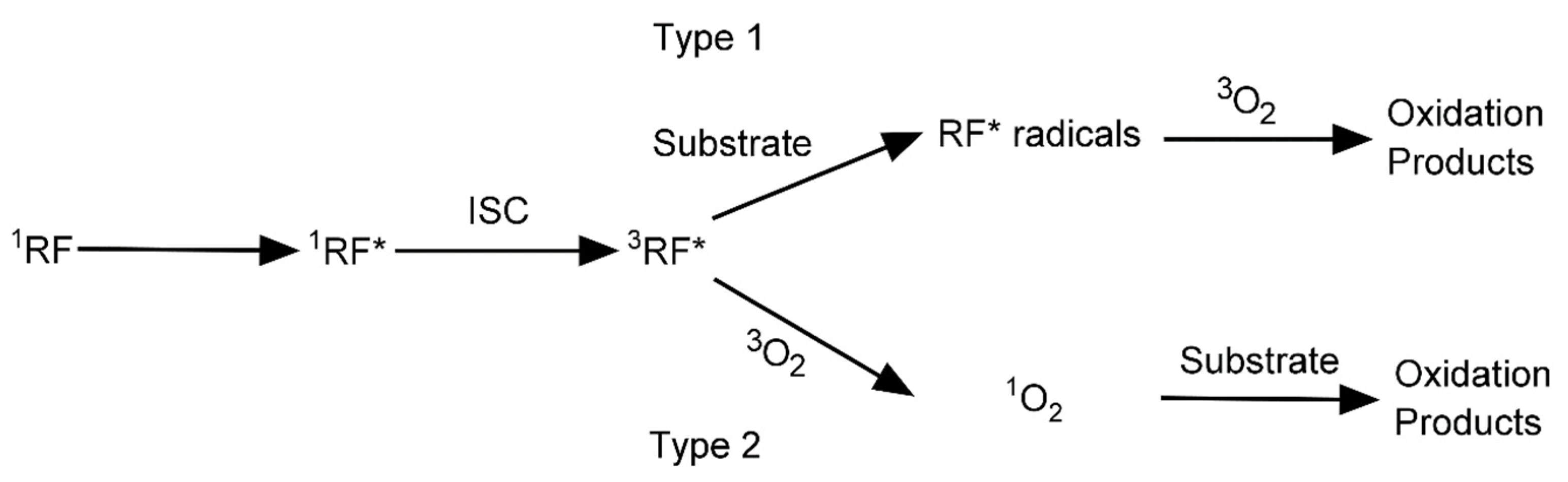
3.1. Oxidation
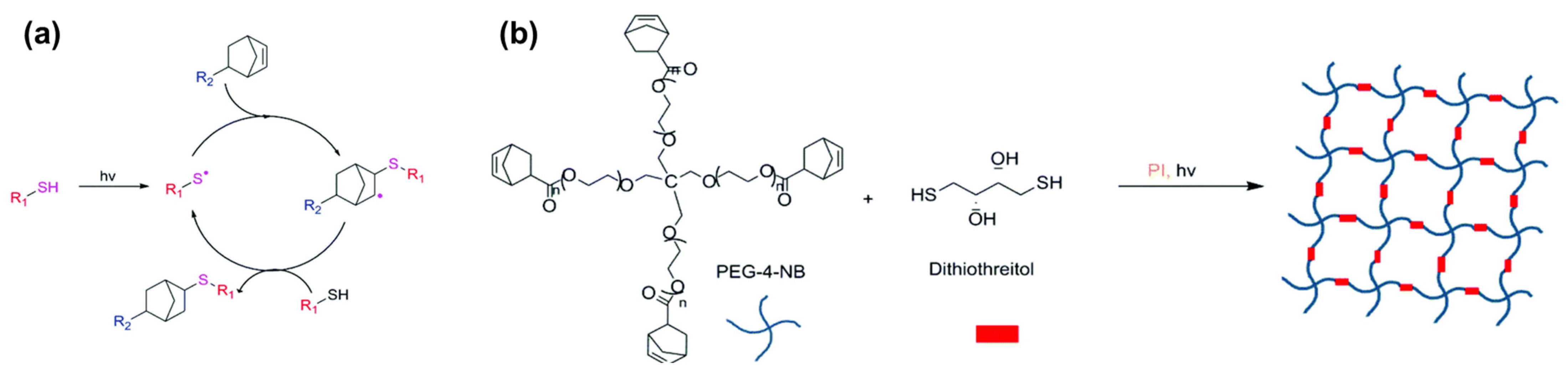
3.2. Thiol-Ene Reaction
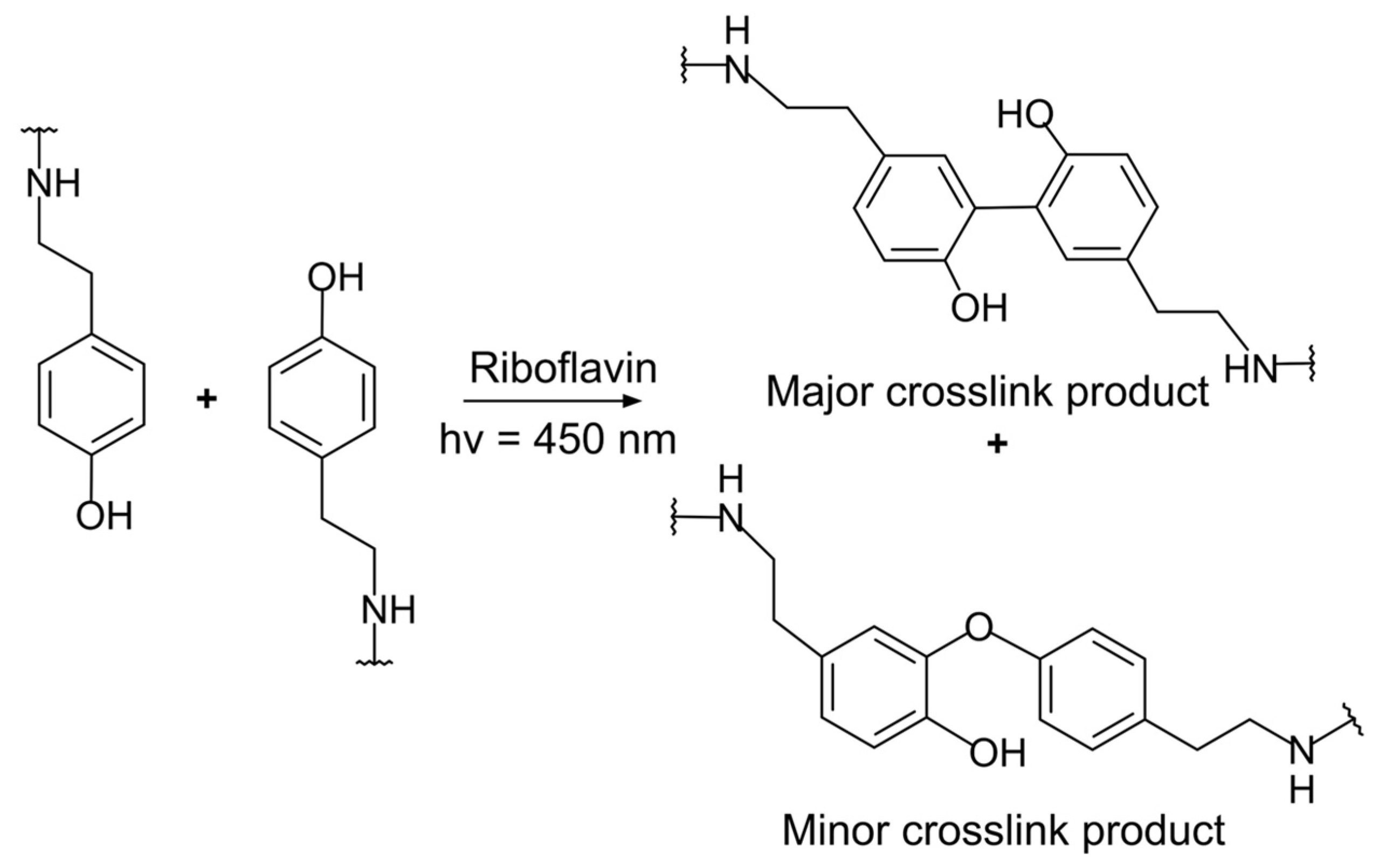
3.3. Tyramine and Tyrosine Groups
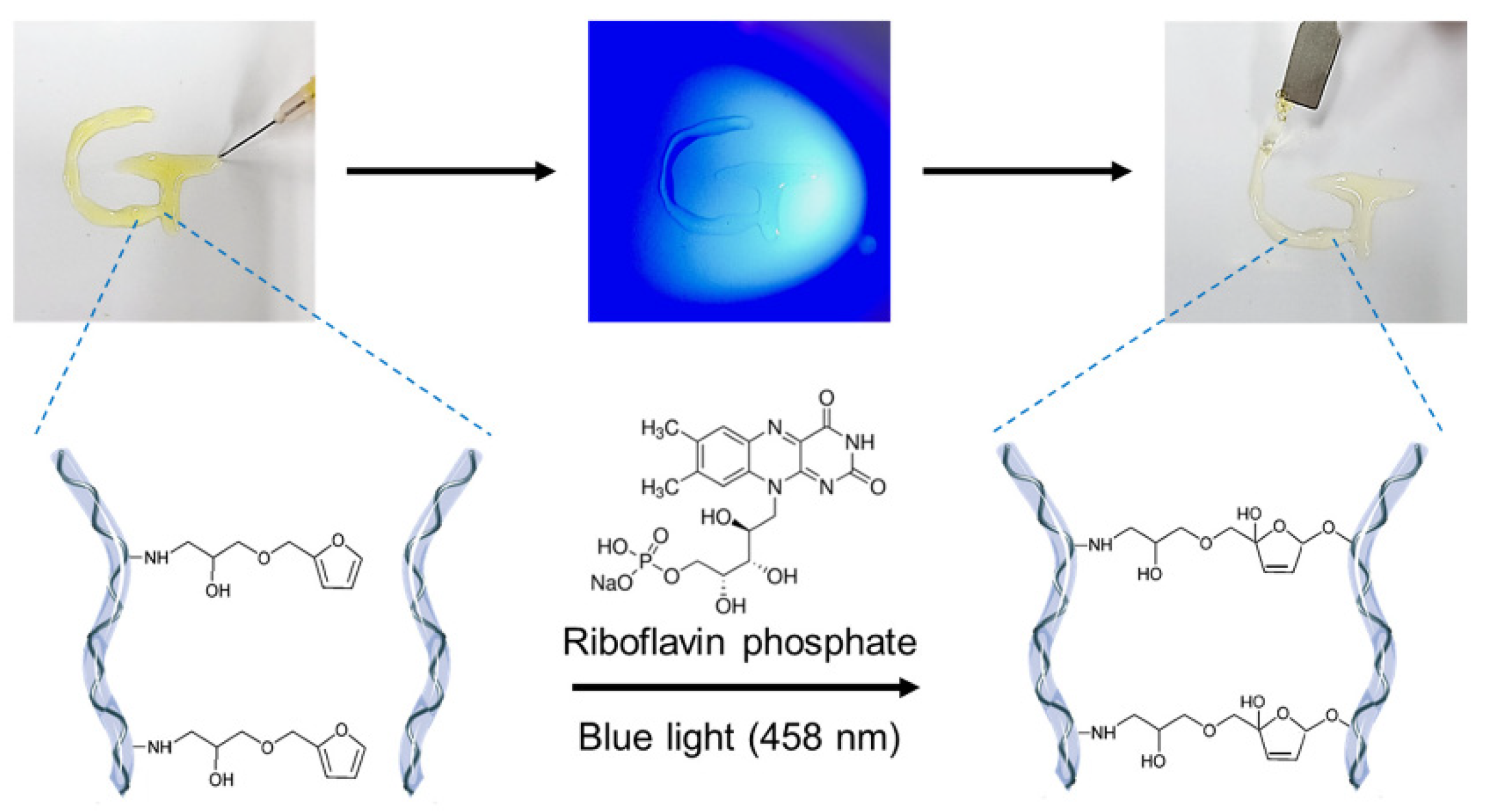
3.4. Furfuryl Group
4. Biomedical Applications
4.1. Tissue Crosslinking
4.2. Drug Delivery
4.3. Dermal Filler
4.4. Cellular Scaffolds for Tissue Engineering
5. Perspectives
5.1. Challenges in Riboflavin-Mediated Photochemistry for Clinical Applications
5.2. Future of Riboflavin-Mediated Photochemistry
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arif, Z.U.; Khalid, M.Y.; Sheikh, M.F.; Zolfagharian, A.; Bodaghi, M. Biopolymeric sustainable materials and their emerging applications. J. Environ. Chem. Eng. 2022, 10, 108159. [Google Scholar] [CrossRef]
- Qi, X.; Tong, X.; Pan, W.; Zeng, Q.; You, S.; Shen, J. Recent advances in polysaccharide-based adsorbents for wastewater treatment. J. Clean. Prod. 2021, 315, 128221. [Google Scholar] [CrossRef]
- Sun, J.; Shen, J.; Chen, S.; Cooper, M.A.; Fu, H.; Wu, D.; Yang, Z. Nanofiller Reinforced Biodegradable PLA/PHA Composites: Current Status and Future Trends. Polymers 2018, 10, 505. [Google Scholar] [CrossRef]
- Alaswad, S.O.; Mahmoud, A.S.; Arunachalam, P. Recent Advances in Biodegradable Polymers and Their Biological Applications: A Brief Review. Polymers 2022, 14, 4924. [Google Scholar] [CrossRef]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 50. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef]
- Badeau, B.A.; Comerford, M.P.; Arakawa, C.K.; Shadish, J.A.; DeForest, C.A. Engineered modular biomaterial logic gates for environmentally triggered therapeutic delivery. Nat. Chem. 2018, 10, 251–258. [Google Scholar] [CrossRef]
- Rapp, T.L.; DeForest, C.A. Visible Light-Responsive Dynamic Biomaterials: Going Deeper and Triggering More. Adv. Healthc. Mater. 2020, 9, 1901553. [Google Scholar] [CrossRef]
- Burdick, J.A.; Murphy, W.L. Moving from static to dynamic complexity in hydrogel design. Nat. Commun. 2012, 3, 1269. [Google Scholar] [CrossRef]
- Khoshakhlagh, P.; Bowser, D.A.; Brown, J.Q.; Moore, M.J. Comparison of visible and UVA phototoxicity in neural culture systems micropatterned with digital projection photolithography. J. Biomed. Mater. Res. Part A 2019, 107, 134–144. [Google Scholar] [CrossRef]
- Zheng, Z.; Eglin, D.; Alini, M.; Richards, G.R.; Qin, L.; Lai, Y. Visible Light-Induced 3D Bioprinting Technologies and Corresponding Bioink Materials for Tissue Engineering: A Review. Engineering 2021, 7, 966–978. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Schwartz, M.P.; Bowman, C.N.; Anseth, K.S. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: Polymerization rate and cytocompatibility. Biomaterials 2009, 30, 6702–6707. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, X.; Dai, R.; Holzman, J.F.; Kim, K. An ultrafast hydrogel photocrosslinking method for direct laser bioprinting. RSC Adv. 2016, 6, 21099–21104. [Google Scholar] [CrossRef]
- Hu, J.; Hou, Y.; Park, H.; Choi, B.; Hou, S.; Chung, A.; Lee, M. Visible light crosslinkable chitosan hydrogels for tissue engineering. Acta Biomater. 2012, 8, 1730–1738. [Google Scholar] [CrossRef] [PubMed]
- Ibusuki, S.; Halbesma, G.J.; Randolph, M.A.; Redmond, R.W.; Kochevar, I.E.; Gill, T.J. Photochemically cross-linked collagen gels as three-dimensional scaffolds for tissue engineering. Tissue Eng. 2007, 13, 1995–2001. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; El-Betany, A.; Menzel, H.; Chen, X. Influence of photoinitiator concentration and irradiation time on the crosslinking performance of visible-light activated pullulan-HEMA hydrogels. Int. J. Biol. Macromol. 2018, 120, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Al-Abboodi, A.; Zhang, S.; Al-Saady, M.; Ong, J.W.; Chan, P.P.Y.; Fu, J. Printing in situ tissue sealant with visible-light-crosslinked porous hydrogel. Biomed. Mater. 2019, 14, 045010. [Google Scholar] [CrossRef]
- Bertlein, S.; Brown, G.; Lim, K.S.; Jungst, T.; Boeck, T.; Blunk, T.; Tessmar, J.; Hooper, G.J.; Woodfield, T.B.F.; Groll, J. Thiol–Ene Clickable Gelatin: A Platform Bioink for Multiple 3D Biofabrication Technologies. Adv. Mater. 2017, 29, 1703404. [Google Scholar] [CrossRef]
- Fu, A.; Gwon, K.; Kim, M.; Tae, G.; Kornfield, J.A. Visible-Light-Initiated Thiol–Acrylate Photopolymerization of Heparin-Based Hydrogels. Biomacromolecules 2015, 16, 497–506. [Google Scholar] [CrossRef]
- Petta, D.; Grijpma, D.W.; Alini, M.; Eglin, D.; D’Este, M. Three-Dimensional Printing of a Tyramine Hyaluronan Derivative with Double Gelation Mechanism for Independent Tuning of Shear Thinning and Postprinting Curing. ACS Biomater. Sci. Eng. 2018, 4, 3088–3098. [Google Scholar] [CrossRef]
- Majima, T.; Schnabel, W.; Weber, W. Phenyl-2,4,6-trimethylbenzoylphosphinates as water-soluble photoinitiators. Generation and reactivity of O=P(C6H5)(O−) radical anions. Die Makromol. Chem. 1991, 192, 2307–2315. [Google Scholar] [CrossRef]
- Ki, C.S.; Shih, H.; Lin, C.-C. Facile preparation of photodegradable hydrogels by photopolymerization. Polymer 2013, 54, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.K.; Goering, P.L.; Elespuru, R.K.; Sarkar Das, S.; Narayan, R.J. The Photoinitiator Lithium Phenyl (2,4,6-Trimethylbenzoyl) Phosphinate with Exposure to 405 nm Light Is Cytotoxic to Mammalian Cells but Not Mutagenic in Bacterial Reverse Mutation Assays. Polymers 2020, 12, 1489. [Google Scholar] [CrossRef]
- Efrati, S.; Averbukh, M.; Berman, S.; Feldman, L.; Dishy, V.; Kachko, L.; Weissgarten, J.; Golik, A.; Averbukh, Z. N-Acetylcysteine ameliorates lithium-induced renal failure in rats. Nephrol. Dial. Transplant. 2004, 20, 65–70. [Google Scholar] [CrossRef]
- Jakubiak, J.; Allonas, X.; Fouassier, J.P.; Sionkowska, A.; Andrzejewska, E.; Linden, L.Å.; Rabek, J.F. Camphorquinone–amines photoinitating systems for the initiation of free radical polymerization. Polymer 2003, 44, 5219–5226. [Google Scholar] [CrossRef]
- Nicewicz, D.A.; MacMillan, D.W.C. Merging Photoredox Catalysis with Organocatalysis: The Direct Asymmetric Alkylation of Aldehydes. Science 2008, 322, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Carnizello, A.P.; Alves, J.M.; Pereira, D.E.; Campos, J.C.L.; Barbosa, M.I.F.; Batista, A.A.; Tavares, D.C. Study of the cytotoxic and genotoxic potential of the carbonyl ruthenium(II) compound, ct-[RuCl(CO)(dppb)(bipy)]PF6 [dppb = 1,4-bis(diphenylphosphino)butane and bipy = 2,2′-bipyridine], by in vitro and in vivo assays. J. Appl. Toxicol. 2019, 39, 630–638. [Google Scholar] [CrossRef]
- Shih, H.; Lin, C.-C. Visible-Light-Mediated Thiol-Ene Hydrogelation Using Eosin-Y as the Only Photoinitiator. Macromol. Rapid Commun. 2013, 34, 269–273. [Google Scholar] [CrossRef]
- Cheung, I.M.Y.; McGhee, C.N.J.; Sherwin, T. Beneficial effect of the antioxidant riboflavin on gene expression of extracellular matrix elements, antioxidants and oxidases in keratoconic stromal cells. Clin. Exp. Optom. 2014, 97, 349–355. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Dusemund, B.; Kouba, M.; Kos Durjava, M.; López-Alonso, M.; Puente, S.L.; et al. Safety and efficacy of vitamin B2 (riboflavin 5′-phosphate ester monosodium salt) for all animal species when used in water for drinking. EFSA J. 2018, 16, e05531. [Google Scholar]
- Heelis, P.F. The photophysical and photochemical properties of flavins (isoalloxazines). Chem. Soc. Rev. 1982, 11, 15–39. [Google Scholar] [CrossRef]
- Kotaki, A.; Yagi, K. Fluorescence Properties of Flavins in Various Solvents. J. Biochem. 1970, 68, 509–516. [Google Scholar] [CrossRef]
- Aleksandrov, A. A Molecular Mechanics Model for Flavins. J. Comput. Chem. 2019, 40, 2834–2842. [Google Scholar] [CrossRef]
- Wollensak, G.; Spoerl, E.; Seiler, T. Riboflavin/ultraviolet-a–Induced collagen crosslinking for the treatment of keratoconus. Am. J. Ophthalmol. 2003, 135, 620–627. [Google Scholar] [CrossRef]
- Raiskup, F.; Spoerl, E. Corneal Crosslinking with Riboflavin and Ultraviolet A. I. Principles. Ocul. Surf. 2013, 11, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Sheraz, M.A.; Kazi, S.H.; Ahmed, S.; Anwar, Z.; Ahmad, I. Photo, thermal and chemical degradation of riboflavin. Beilstein J. Org. Chem. 2014, 10, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Beztsinna, N.; Solé, M.; Taib, N.; Bestel, I. Bioengineered riboflavin in nanotechnology. Biomaterials 2016, 80, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Drössler, P.; Holzer, W.; Penzkofer, A.; Hegemann, P. pH dependence of the absorption and emission behaviour of riboflavin in aqueous solution. Chem. Phys. 2002, 282, 429–439. [Google Scholar] [CrossRef]
- Redmond, R.W.; Kochevar, I.E. Medical Applications of Rose Bengal- and Riboflavin-Photosensitized Protein Crosslinking. Photochem. Photobiol. 2019, 95, 1097–1115. [Google Scholar] [CrossRef] [PubMed]
- Gabriela, I.; Iulia, M. Application of Riboflavin Photochemical Properties in Hydrogel Synthesis. In Biophysical Chemistry; Mohammed, A.A.K., Ed.; IntechOpen: Rijeka, Primorje-Gorski Kotar, 2019; p. 2. [Google Scholar]
- Choe, E.; Huang, R.; Min, D.B. Chemical Reactions and Stability of Riboflavin in Foods. J. Food Sci. 2005, 70, R28–R36. [Google Scholar] [CrossRef]
- McCall, A.S.; Kraft, S.; Edelhauser, H.F.; Kidder, G.W.; Lundquist, R.R.; Bradshaw, H.E.; Dedeic, Z.; Dionne, M.J.C.; Clement, E.M.; Conrad, G.W. Mechanisms of Corneal Tissue Cross-linking in Response to Treatment with Topical Riboflavin and Long-Wavelength Ultraviolet Radiation (UVA). Investig. Ophthalmol. Vis. Sci. 2010, 51, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-R.; Spikes, J.D.; Kopečeková, P.; Kopeček, J. Photodynamic crosslinking of proteins. I. Model studies using histidine- and lysine-containing N-(2-hydroxypropyl) methacrylamide copolymers. J. Photochem. Photobiol. B Biol. 1996, 34, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, M.; Leinisch, F.; Leeming, D.J.; Svensson, B.; Davies, M.J.; Hägglund, P. Mass-Spectrometry-Based Identification of Cross-Links in Proteins Exposed to Photo-Oxidation and Peroxyl Radicals Using 18O Labeling and Optimized Tandem Mass Spectrometry Fragmentation. J. Proteome Res. 2018, 17, 2017–2027. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.; Kamma-Lorger, C.S.; Boote, C.; Young, R.D.; Quantock, A.J.; Rost, A.; Khatib, Y.; Harris, J.; Yagi, N.; Terrill, N.; et al. The Effect of Riboflavin/UVA Collagen Cross-linking Therapy on the Structure and Hydrodynamic Behaviour of the Ungulate and Rabbit Corneal Stroma. PLoS ONE 2013, 8, e52860. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, J.H.; Kim, S.Y.; Koh, W.-G.; Lee, H.J. Blue Light-Activated Riboflavin Phosphate Promotes Collagen Crosslinking to Modify the Properties of Connective Tissues. Materials 2021, 14, 5788. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, J.H.; Kang, Y.; Yoo, J.W.; Choi, J.; Lee, H.J. Green chemistry method for hair strengthening and setting using visible light-mediated protein crosslinking. J. Clean. Prod. 2022, 363, 132535. [Google Scholar] [CrossRef]
- Northrop, B.H.; Coffey, R.N. Thiol–Ene Click Chemistry: Computational and Kinetic Analysis of the Influence of Alkene Functionality. J. Am. Chem. Soc. 2012, 134, 13804–13817. [Google Scholar] [CrossRef]
- Batchelor, R.R.; Kwandou, G.; Spicer, P.T.; Stenzel, M.H. (−)-Riboflavin (vitamin B2) and flavin mononucleotide as visible light photo initiators in the thiol–ene polymerisation of PEG-based hydrogels. Polym. Chem. 2017, 8, 980–984. [Google Scholar] [CrossRef]
- Monfared, M.; Nothling, M.D.; Mawad, D.; Stenzel, M.H. Effect of cell culture media on photopolymerizations. Biomacromolecules 2021, 22, 4295–4305. [Google Scholar] [CrossRef]
- Lee, H.J.; Fernandes-Cunha, G.M.; Myung, D. In situ-forming hyaluronic acid hydrogel through visible light-induced thiol-ene reaction. React. Funct. Polym. 2018, 131, 29–35. [Google Scholar] [CrossRef]
- Goto, R.; Nishida, E.; Kobayashi, S.; Aino, M.; Ohno, T.; Iwamura, Y.; Kikuchi, T.; Hayashi, J.-i.; Yamamoto, G.; Asakura, M.; et al. Gelatin Methacryloyl–Riboflavin (GelMA–RF) Hydrogels for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 1635. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Koh, W.-G.; Lee, H.J. Effects of basic fibroblast growth factor combined with an injectable in situ crosslinked hyaluronic acid hydrogel for a dermal filler. React. Funct. Polym. 2021, 164, 104933. [Google Scholar] [CrossRef]
- Puré, E.; Cuff, C.A. A crucial role for CD44 in inflammation. Trends Mol. Med. 2001, 7, 213–221. [Google Scholar] [CrossRef]
- Abdul-Monem, M.M.; Kamoun, E.A.; Ahmed, D.M.; El-Fakharany, E.M.; Al-Abbassy, F.H.; Aly, H.M. Light-cured hyaluronic acid composite hydrogels using riboflavin as a photoinitiator for bone regeneration applications. J. Taibah Univ. Med. Sci. 2021, 16, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kim, M.H.; Song, J.H.; Kang, C.; Park, W.H. Dual crosslinked alginate hydrogels by riboflavin as photoinitiator. Int. J. Biol. Macromol. 2020, 154, 989–998. [Google Scholar] [CrossRef]
- Hong, B.M.; Park, S.A.; Park, W.H. Effect of photoinitiator on chain degradation of hyaluronic acid. Biomater. Res. 2019, 23, 21. [Google Scholar] [CrossRef]
- Hong, B.M.; Kim, H.C.; Jeong, J.E.; Park, S.A.; Park, W.H. Visible-light-induced hyaluronate hydrogel for soft tissue fillers. Int. J. Biol. Macromol. 2020, 165, 2834–2844. [Google Scholar] [CrossRef]
- Parker, S.T.; Domachuk, P.; Amsden, J.; Bressner, J.; Lewis, J.A.; Kaplan, D.L.; Omenetto, F.G. Biocompatible Silk Printed Optical Waveguides. Adv. Mater. 2009, 21, 2411–2415. [Google Scholar] [CrossRef]
- Applegate, M.B.; Partlow, B.P.; Coburn, J.; Marelli, B.; Pirie, C.; Pineda, R.; Kaplan, D.L.; Omenetto, F.G. Photocrosslinking of Silk Fibroin Using Riboflavin for Ocular Prostheses. Adv. Mater. 2016, 28, 2417–2420. [Google Scholar] [CrossRef]
- Piluso, S.; Flores Gomez, D.; Dokter, I.; Moreira Texeira, L.; Li, Y.; Leijten, J.; van Weeren, R.; Vermonden, T.; Karperien, M.; Malda, J. Rapid and cytocompatible cell-laden silk hydrogel formation via riboflavin-mediated crosslinking. J. Mater. Chem. B 2020, 8, 9566–9575. [Google Scholar] [CrossRef]
- Monnier, V.M.; Sell, D.R.; Saxena, A.; Saxena, P.; Subramaniam, R.; Tessier, F.; Weiss, M.F. Glycoxidative and Carbonyl Stress in Aging and Age-Related Diseases. In Critical Reviews of Oxidative Stress and Aging; World Scientific: Singapore, 2002; pp. 413–433. [Google Scholar]
- Park, S.-H.; Seo, S.-Y.; Lee, H.-J.; Na, H.-N.; Lee, J.-W.; Woo, H.-D.; Son, T.-I. Preparation of Furfuryl-fish gelatin (F-f.gel) cured using visible-light and its application as an anti-adhesion agent. Macromol. Res. 2012, 20, 842–846. [Google Scholar] [CrossRef]
- Kong, M.S.; Koh, W.-G.; Lee, H.J. Controlled Release of Epidermal Growth Factor from Furfuryl-Gelatin Hydrogel Using in Situ Visible Light-Induced Crosslinking and Its Effects on Fibroblasts Proliferation and Migration. Gels 2022, 8, 214. [Google Scholar] [CrossRef] [PubMed]
- AnilKumar, S.; Allen, S.C.; Tasnim, N.; Akter, T.; Park, S.; Kumar, A.; Chattopadhyay, M.; Ito, Y.; Suggs, L.J.; Joddar, B. The applicability of furfuryl-gelatin as a novel bioink for tissue engineering applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Nagiah, N.; El Khoury, R.; Othman, M.H.; Akimoto, J.; Ito, Y.; Roberson, D.A.; Joddar, B. Development and Characterization of Furfuryl-Gelatin Electrospun Scaffolds for Cardiac Tissue Engineering. ACS Omega 2022, 7, 13894–13905. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.; Akimoto, J.; Kobatake, E.; Ito, Y. Gelation and release behavior of visible light-curable alginate. Polym. J. 2020, 52, 323–332. [Google Scholar] [CrossRef]
- Noh, S.-H.; Kim, S.-W.; Kim, J.-W.; Lee, T.-H.; Nah, J.-W.; Lee, Y.-G.; Kim, M.-K.; Ito, Y.; Son, T.-I. Preparation of drug-immobilized anti-adhesion agent using visible light-curable alginate derivative containing furfuryl group. Int. J. Biol. Macromol. 2019, 121, 301–308. [Google Scholar] [CrossRef]
- Donnelly, P.E.; Chen, T.; Finch, A.; Brial, C.; Maher, S.A.; Torzilli, P.A. Photocrosslinked tyramine-substituted hyaluronate hydrogels with tunable mechanical properties improve immediate tissue-hydrogel interfacial strength in articular cartilage. J. Biomater. Sci. Polym. Ed. 2017, 28, 582–600. [Google Scholar] [CrossRef]
- Han, G.-D.; Kim, J.-W.; Noh, S.-H.; Kim, S.-W.; Jang, E.-C.; Nah, J.-W.; Lee, Y.-G.; Kim, M.-K.; Ito, Y.; Son, T.-I. Potent anti-adhesion agent using a drug-eluting visible-light curable hyaluronic acid derivative. J. Ind. Eng. Chem. 2019, 70, 204–210. [Google Scholar] [CrossRef]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- Nguyen, A.K.; Gittard, S.D.; Koroleva, A.; Schlie, S.; Gaidukeviciute, A.; Chichkov, B.N.; Narayan, R.J. Two-photon polymerization of polyethylene glycol diacrylate scaffolds with riboflavin and triethanolamine used as a water-soluble photoinitiator. Regen. Med. 2013, 8, 725–738. [Google Scholar] [CrossRef]
- Kim, S.-H.; Chu, C.-C. Fabrication of a biodegradable polysaccharide hydrogel with riboflavin, vitamin B2, as a photo-initiator and L-arginine as coinitiator upon UV irradiation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 390–400. [Google Scholar] [CrossRef] [PubMed]
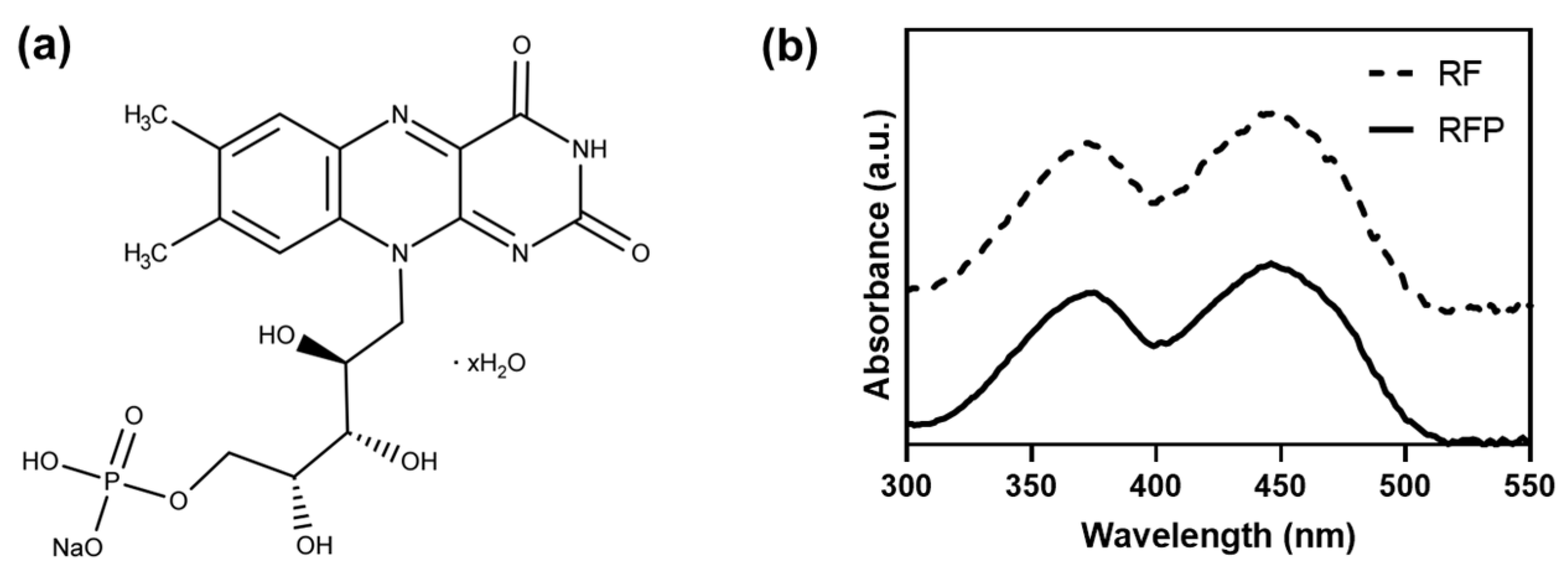
| Photoinitiator | Chemical Structure | Solubility in Water | Absorption Wavelength (nm) | Cell Viability (1 d) | Refs. |
|---|---|---|---|---|---|
| LAP |  | <30 mg mL−1 | 405 | >90% | [12,13] |
| RF |  | <0.5 mg mL−1 | 444 | >90% | [14,15] |
| Camphorquinone | 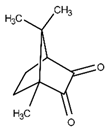 | Slightly soluble | 450 | 50–90% | [14,16] |
| [Ru(II)(bpy)3]2+ * | 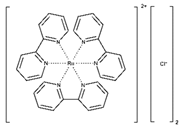 | Slightly soluble | 452 | 85–90% | [17,18] |
| Eosin Y | 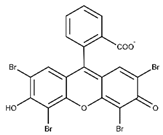 | <50 mg mL−1 | 515 | >96% | [19,20] |
| Photochemistry | Biopolymer | Photoinitiator | Light Source Wavelength (nm) | Light Intensity (mW cm−2) | Irradiation Time | Refs. |
|---|---|---|---|---|---|---|
| Oxidation | Collagen (Cornea) | RF | 405 | 3 | 30 min | [42,45] |
| Collagen (Skin) | RFP | 450 | 100 | 5 min | [46] | |
| Keratin (Hair) | RFP | 450 | 3 | 5 min | [47] | |
| Thiol-ene reaction | HA | RFP | 450 | 100 | 40 s | [51,53] |
| Tyramine group | Alginate | RF | 440 | 2500 | 3 min | [56] |
| HA | RFP | 440 | 2500 | 30 s | [57,58] | |
| HA | RF | 365 | 10 | 20 min | [69] | |
| Silk fibroin | RF | 450 | 18.7 | 20 min | [60] | |
| Silk fibroin | RF | 400–700 | - | 10 min | [61] | |
| Furfuryl group | Gelatin | RFP | 458 | 100 | 10 min | [64] |
| Gelatin | RF | 400 | - | 2.5 min | [65,66] | |
| Alginate | RF | 445 | 200 | 10 min | [67,68] | |
| HA | RF | - | - | 10 min | [70] |
| Application | Biopolymer | Photoinitiator | Photochemistry | Refs. |
|---|---|---|---|---|
| Tissue crosslinking | Collagen (Cornea) | RF and RFP | Oxidation | [34,35] |
| Collagen (Skin) | RFP | Oxidation | [46] | |
| Keratin (Hair) | RFP | Oxidation | [47] | |
| Drug delivery | Alginate | RF | Furfuryl group | [67,68] |
| HA | RF | Furfuryl group | [70] | |
| Gelatin | RFP | Furfuryl group | [64] | |
| Dermal filler | HA | RFP | Thiol-ene reaction | [53] |
| HA | RFP | Tyramine group | [58] | |
| Cellular scaffolds for tissue engineering | Silk fibroin | RF | Tyramine group | [61] |
| Gelatin | RF | Furfuryl group | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.B.; Lim, S.; Lee, Y.; Park, C.H.; Lee, H.J. Green Chemistry for Crosslinking Biopolymers: Recent Advances in Riboflavin-Mediated Photochemistry. Materials 2023, 16, 1218. https://doi.org/10.3390/ma16031218
Lee YB, Lim S, Lee Y, Park CH, Lee HJ. Green Chemistry for Crosslinking Biopolymers: Recent Advances in Riboflavin-Mediated Photochemistry. Materials. 2023; 16(3):1218. https://doi.org/10.3390/ma16031218
Chicago/Turabian StyleLee, Yoon Bok, Saebin Lim, Yerin Lee, Chan Ho Park, and Hyun Jong Lee. 2023. "Green Chemistry for Crosslinking Biopolymers: Recent Advances in Riboflavin-Mediated Photochemistry" Materials 16, no. 3: 1218. https://doi.org/10.3390/ma16031218
APA StyleLee, Y. B., Lim, S., Lee, Y., Park, C. H., & Lee, H. J. (2023). Green Chemistry for Crosslinking Biopolymers: Recent Advances in Riboflavin-Mediated Photochemistry. Materials, 16(3), 1218. https://doi.org/10.3390/ma16031218









