Enhanced Thermal Shock Resistance of High-Temperature Organic Adhesive by CF-SiCNWs Binary Phase Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. Experimental Procedures
2.2.1. Synthesis Modified Carbon Fibers (CFs)
2.2.2. Synthesis Modified HTOA
2.2.3. Preparation of Bonding Samples
2.3. Test Methods and Procedure
3. Results and Discussion
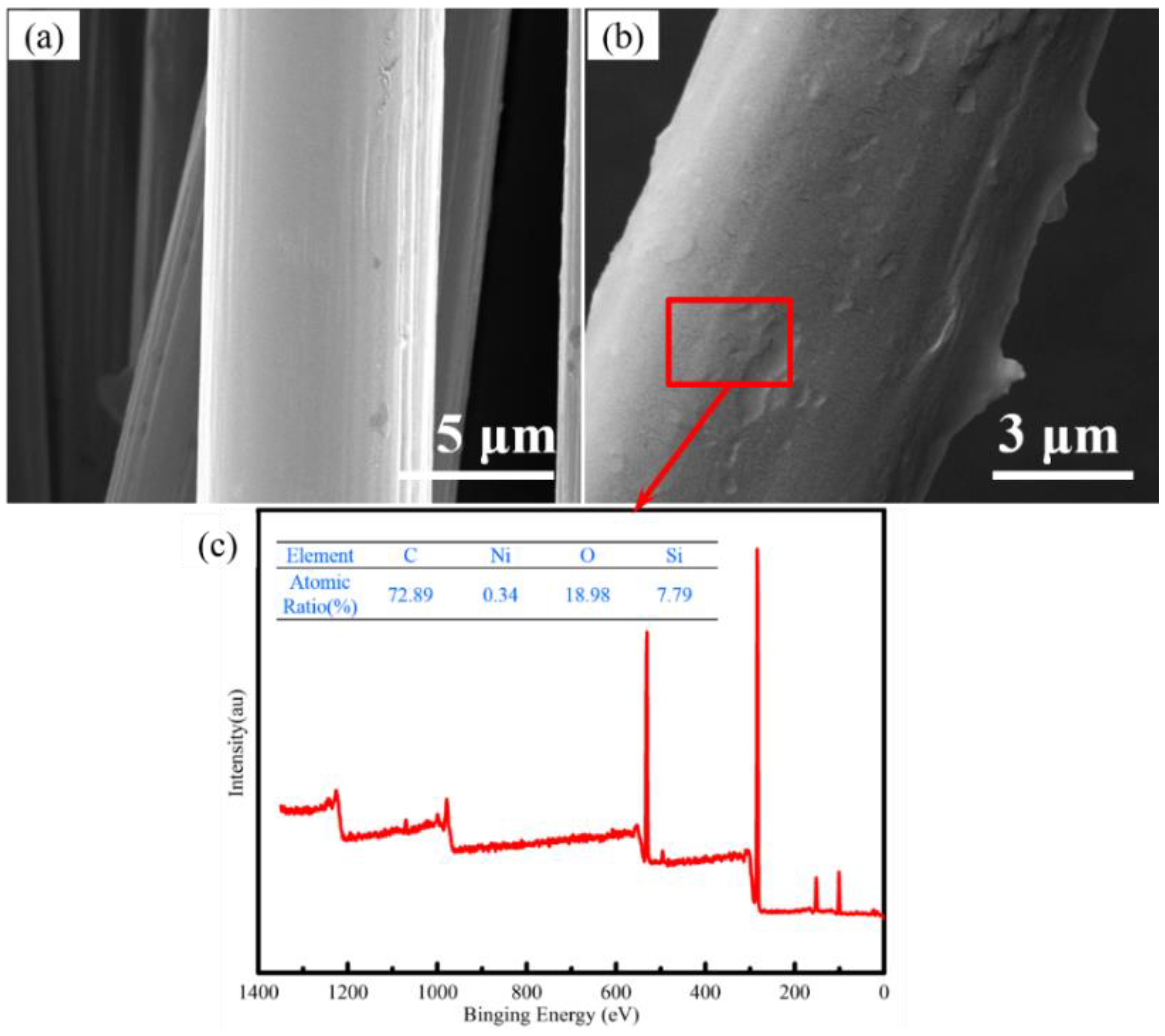
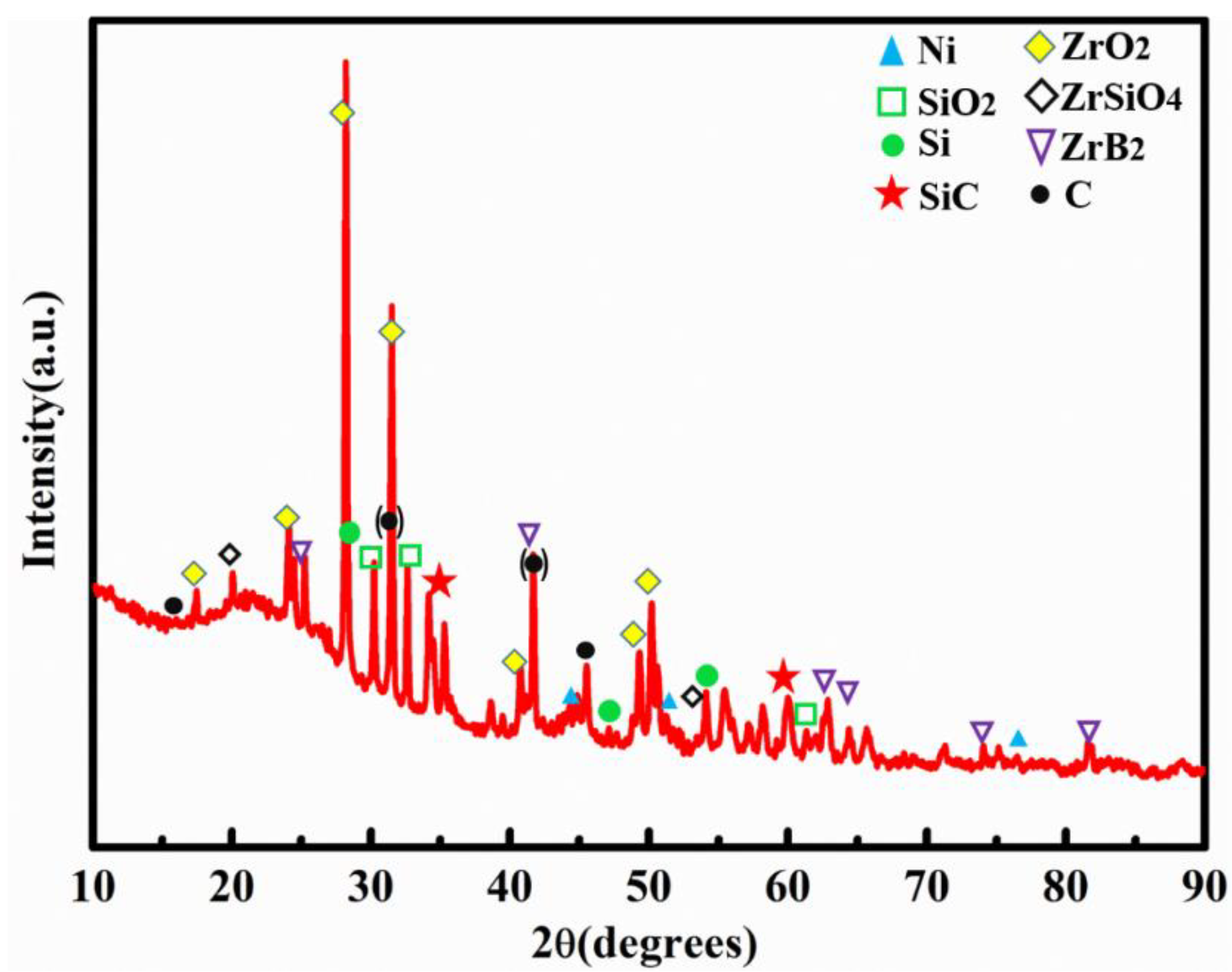
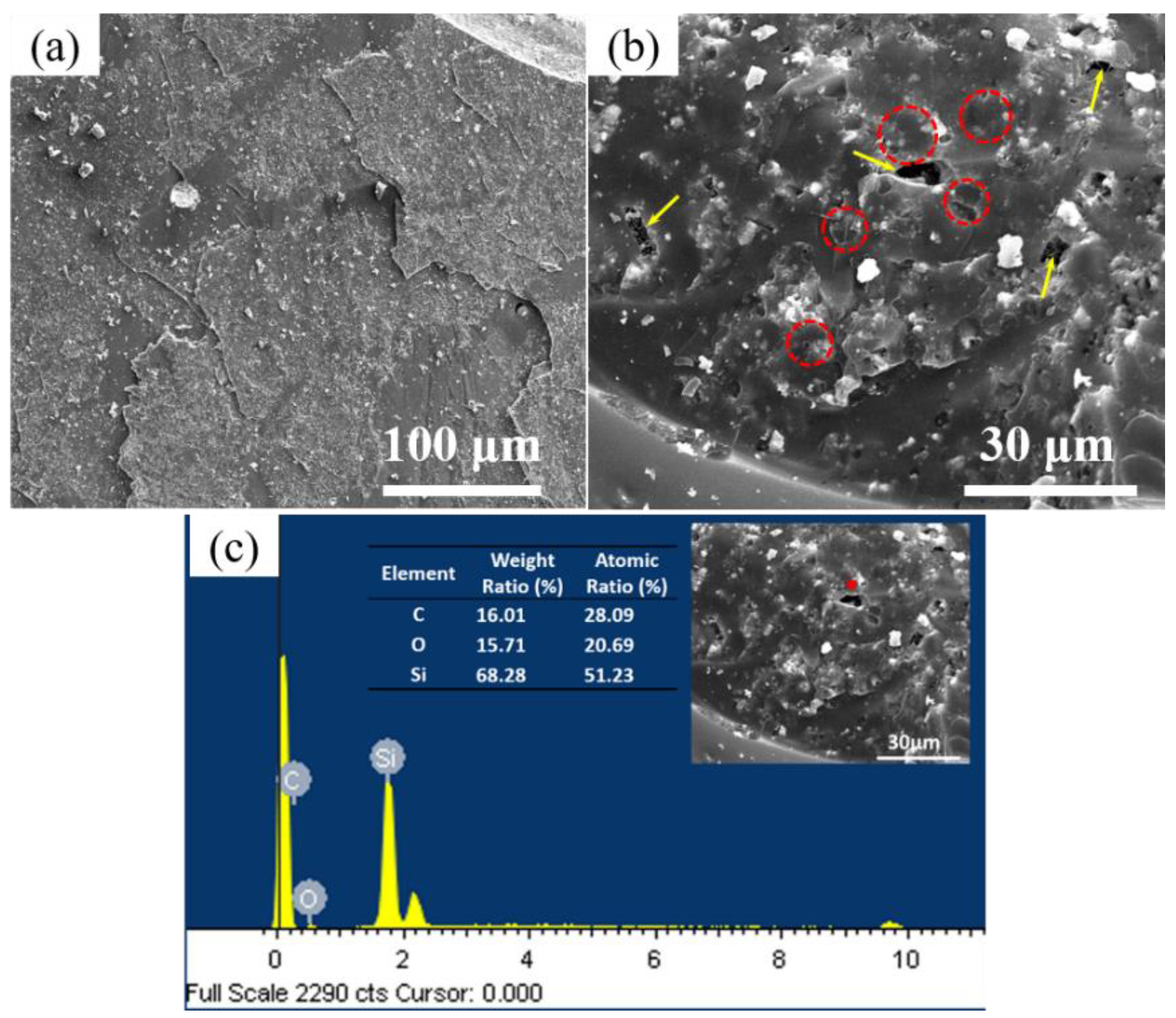
3.1. Construction and Analysis of Binary Phase Structure
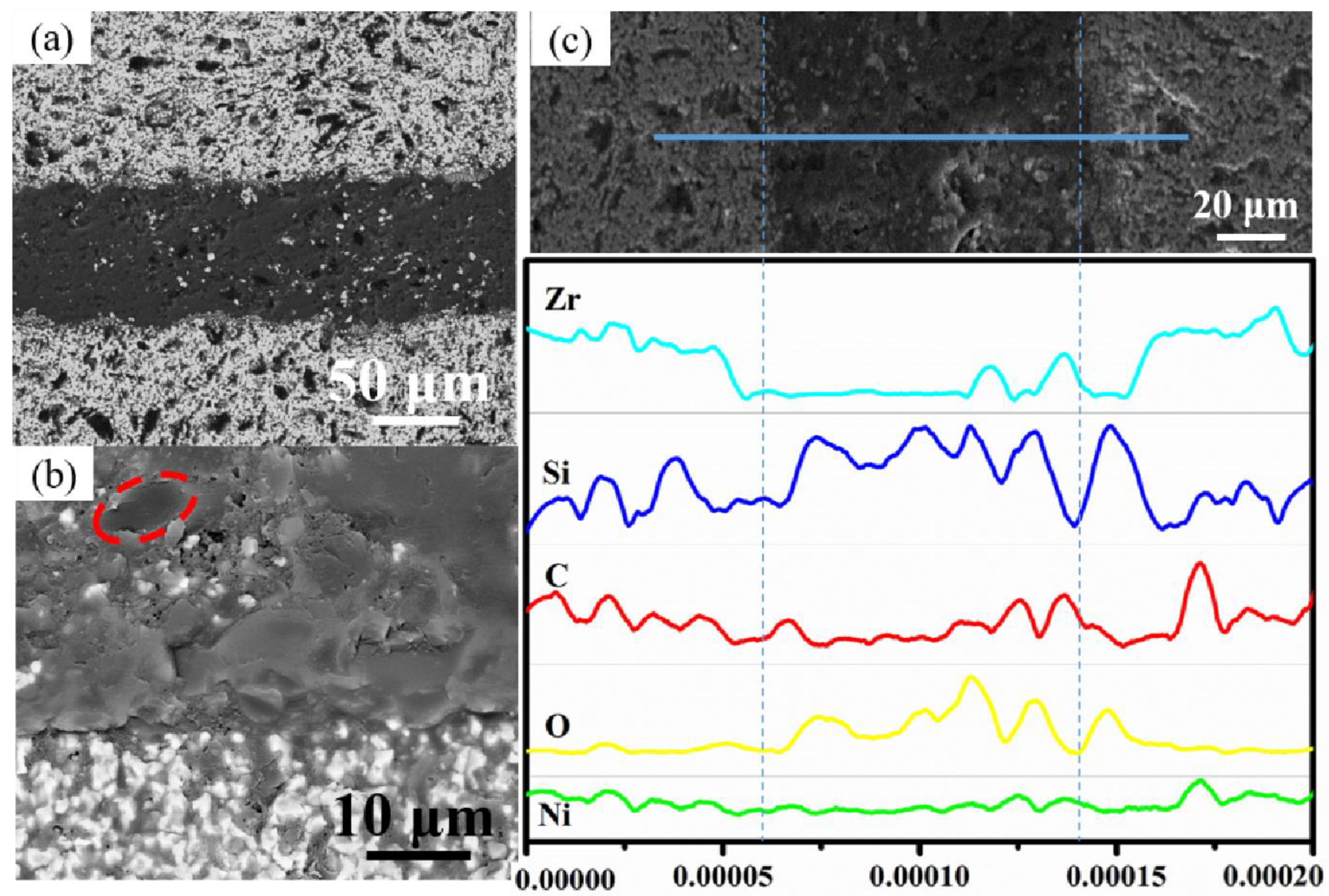
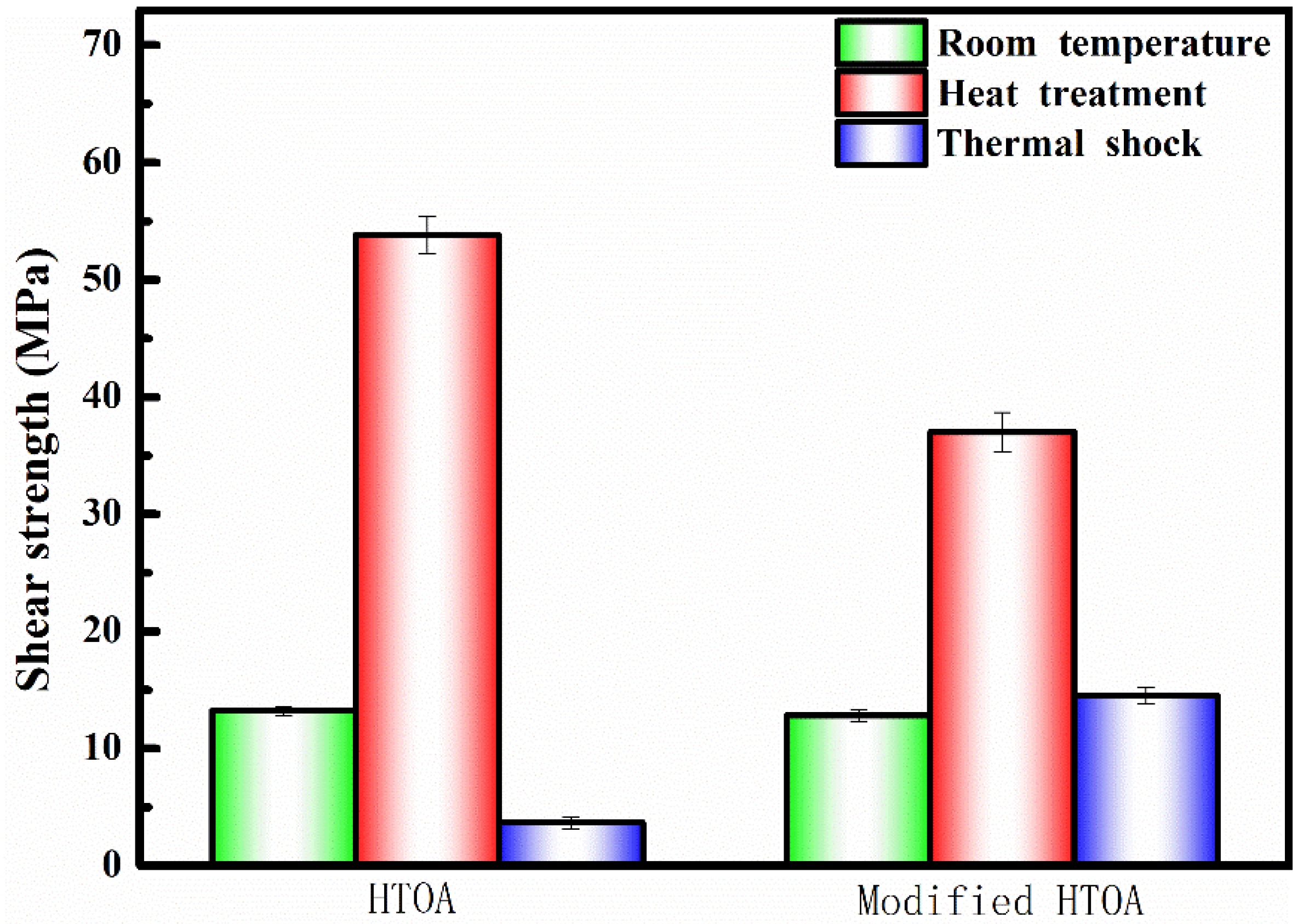
3.2. Structure Evolution and Shear Strength of ZSG Joints
3.3. Fracture Analysis and Toughening Mechanism of ZSG Joints
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayaseelan, D.D.; Sa, R.G.; Brown, P.; Lee, W.E. Reactive infiltration processing (RIP) of ultra high temperature ceramics (UHTC) into porous C/C composite tubes. J. Eur. Ceram. Soc. 2011, 31, 361–368. [Google Scholar] [CrossRef]
- Zhang, R.B.; He, R.J.; Zhang, X.H.; Fang, D.N. Microstructure and mechanical properties of ZrB2-SiC composites prepared by gelcasting and pressureless sintering. Int. J. Refract. Met. Hard Mater. 2014, 43, 83–88. [Google Scholar] [CrossRef]
- Asl, M.S.; Namini, A.S.; Kakroudi, M.G. Influence of silicon carbide addition on the microstructural development of hot pressed zirconium and titanium diborides. Ceram. Int. 2016, 42, 5375–5381. [Google Scholar]
- Muolo, M.L.; Ferrera, E.; Passerone, A. Wetting and spreading of liquid metals on ZrB2-based ceramics. Mater. Sci. 2005, 40, 2295–2300. [Google Scholar] [CrossRef]
- Zhong, Z.; Xu, H.; Zhang, X.; Xu, B.; Liu, L.; Huang, Y. Bonding ZrB2-SiC-G ceramics using modified organic adhesive for engineering applications at ultra high temperatures in air. Ceram. Int. 2018, 44, 3810–3815. [Google Scholar] [CrossRef]
- Singh, M.; Asthana, R. Joining and integration of ZrB2-based ultra-high temperature ceramic composites using advanced brazing technology. Mater. Sci. 2010, 45, 4308–4320. [Google Scholar] [CrossRef]
- Yang, W.Q.; Lin, T.S.; He, P.; Wei, H.M.; Xing, L.L.; Jia, D.C. Microstructure and mechanical properties of ZrB2–SiC joints fabricated by a contact-reactive brazing technique with Ti and Ni interlayers. Ceram. Int. 2014, 40, 7253–7260. [Google Scholar] [CrossRef]
- Lin, J.H.; Luo, D.L.; Chen, S.L.; Mao, D.S.; Wang, Z.Y.; Ma, Q.; Qi, J.L.; Feng, J.C. Control interfacial microstructure and improve mechanical properties of TC4-SiO2f/SiO2 joint by AgCuTi with Cu foam as interlayer. Ceram. Int. 2016, 42, 16619–16625. [Google Scholar] [CrossRef]
- Singh, M.; Asthana, R. Joining of zirconium diboride-based ultra high-temperature ceramic composites using metallic glass interlayers. Mater. Sci. Eng. A 2007, 460, 153–162. [Google Scholar] [CrossRef]
- Yang, W.Q.; Lin, T.S.; He, P.; Zhu, M.; Song, C.B.; Jia, D.C.; Feng, J.C. Microstructural evolution and growth behavior of in situ TiB whisker array in ZrB2–SiC/Ti6Al4V brazing joints. J. Am. Ceram. Soc. 2013, 96, 3712–3719. [Google Scholar] [CrossRef]
- Colombo, P.; Mera, G.; Riedel, R.; Sorarù, G.D. Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Liu, Y.R.; Huang, Y.D.; Liu, L. Effects of trisilanolisobutyl-POSS on thermal stability of methylsilicone resin. Polym. Degrad. Stab. 2006, 91, 2731–2738. [Google Scholar] [CrossRef]
- Zhang, J.S.; Luo, R.Y.; Jiang, M.; Xiang, Q.; Li, J.S. The preparation and performance of a novel room-temperature-cured heat-resistant adhesive for ceramic bonding. Mater. Sci. Eng. A 2011, 528, 2952–2959. [Google Scholar] [CrossRef]
- Wang, M.C.; Dong, X.; Tao, X.; Liu, M.M.; Liu, J.C.; Du, H.Y.; Guo, A.R. Joining of various engineering ceramics and composites by a modified preceramic polymer for high-temperature application. J. Eur. Ceram. Soc. 2015, 35, 4083–4097. [Google Scholar] [CrossRef]
- Jin, H.; Miller, G.M.; Pety, S.J.; Griffin, A.S.; Stradley, D.S.; Roach, D.; Sottos, N.R.; White, S.R. Fracture behavior of a self-healing, toughened epoxy adhesive. Int. J. Adhes. Adhes. 2013, 44, 157–165. [Google Scholar] [CrossRef]
- Jin, H.; Miller, G.M.; Sottos, N.R.; White, S.R. Fracture and fatigue response of a selfhealing epoxy adhesive. Polymer 2011, 52, 1628–1634. [Google Scholar] [CrossRef]
- Xu, B.S.; Zhou, S.B.; Hong, C.Q.; Han, J.C.; Zhang, X.H. Mechanical enhancement of lightweight ZrB2-modified carbon-bonded carbon fiber composites with self-grown carbon nanotubes. Carbon 2016, 102, 487–493. [Google Scholar] [CrossRef]
- Xu, B.S.; Hong, C.Q.; Zhang, X.H.; Han, J.C.; Han, W.B.; Qin, F.X. Nanostructured hybrid carbon nanotube/ultra high-temperature ceramic heterostructures: Microstructure microstructure evolution and forming mechanism. J. Am. Ceram. Soc. 2015, 98, 3699–3705. [Google Scholar] [CrossRef]
- Zhong, Z.X.; Yan, L.W.; Liu, L.; Xu, B.S. Fabrication of modified ultra high-temperature ceramic hybrid powders using in situ grown SiC nanowires. Ceram. Int. 2017, 43, 3462–3464. [Google Scholar] [CrossRef]
- Fan, J.; Chu, P.K. Silicon Carbide Nanostructures: Fabrication, Structure, and Properties; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Li, J.; Luo, R.; Bi, Y.; Xiang, Q.; Lin, C.; Zhang, Y.F.; An, N. The preparation and performance of short carbon fiber reinforced adhesive for bonding carbon/carbon composites. Carbon 2008, 46, 1957–1965. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Luo, R.Y.; Zhang, J.S.; Xiang, Q. The reinforcing mechanism of carbon fiber in composite adhesive for bonding carbon/carbon composites. J. Mater. Process. Technol. 2011, 211, 167–173. [Google Scholar] [CrossRef]
- Wong, E.W.; Sheehan, P.E.; Lieber, C.M. Nanobeam mechanics: Elasticity, strength, and toughness of nanorods and nanotubes. Science 1997, 277, 1971–1975. [Google Scholar] [CrossRef]
- Dai, H.; Wong, E.W.; Lu, Y.Z.; Fan, S.; Lieber, C.M. Synthesis and characterization of carbide nanorods. Nature 1995, 375, 769. [Google Scholar] [CrossRef]
- Huczko, A.; Dąbrowska, A.; Savchyn, V.; Popov, A.I.; Karbovnyk, I. Silicon carbide nanowires: Synthesis and cathodoluminescence. Phys. Status Solidi 2009, 246, 2806–2808. [Google Scholar] [CrossRef]
- Lebedev, A.S.; Suzdal’tsev, A.V.; Anfilogov, V.N.; Farlenkov, A.S.; Porotnikova, N.M.; Vovkotrub, E.G.; Akashev, L.A. Carbothermal Synthesis, Properties, and Structure of Ultrafine SiC Fibers. Inorg. Mater. 2020, 56, 20–27. [Google Scholar] [CrossRef]
- Tynyshbayeva, K.M.; Kadyrzhanov, K.K.; Kozlovskiy, A.L.; Kuldeyev, Y.I.; Uglov, V.; Zdorovets, M.V. Study of Helium Swelling and Embrittlement Mechanisms in SiC Ceramics. Crystals 2022, 12, 239. [Google Scholar] [CrossRef]
- Zhou, X.T.; Wang, N.; Lai, H.L.; Peng, H.Y.; Bello, I.; Wang, N.B.; Lee, C.S.; Lee, S.T. β-SiC nanorods synthesized by hot filament chemical vapor deposition. Appl. Phys. Lett. 1999, 74, 3942–3944. [Google Scholar] [CrossRef]
- Wei, J.; Li, K.Z.; Li, H.J.; Fu, Q.G.; Zhang, L. Growth and morphology of one-dimensional SiC nanostructures without catalyst assistant. Mater. Chem. Phys. 2006, 95, 140–144. [Google Scholar] [CrossRef]
- Sha, J.; Lv, Z.; Li, J.; Zhang, Z.; Dai, J. Effect of in-situ grown SiC nanowires on mechanical properties of short carbon fiber-reinforced polymer composites. Mater. Lett. 2017, 199, 17–20. [Google Scholar] [CrossRef]
- GB/T 3357-1982; Test Method for Interlaminar Shear Strength of Unidirectional Fiber Reinforced Plastics. Standardization Administration of China: Beijing, China, 1982.
- Qin, Y.; Rao, Z.L.; Huang, Z.X.; Zhang, H.; Jia, J.R.; Wang, F.Z. Preparation and performance of ceramizable heat-resistant organic adhesive for joining Al2O3 ceramics. Int. J. Adhes. Adhes. 2014, 55, 132–138. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, T.; Zhong, Z.; Zhang, X.; Liu, J.; Wang, W.; Wang, B.; Liu, L. Enhanced Thermal Shock Resistance of High-Temperature Organic Adhesive by CF-SiCNWs Binary Phase Structure. Materials 2023, 16, 5983. https://doi.org/10.3390/ma16175983
Zhao T, Zhong Z, Zhang X, Liu J, Wang W, Wang B, Liu L. Enhanced Thermal Shock Resistance of High-Temperature Organic Adhesive by CF-SiCNWs Binary Phase Structure. Materials. 2023; 16(17):5983. https://doi.org/10.3390/ma16175983
Chicago/Turabian StyleZhao, Tingyu, Zhengxiang Zhong, Xuanfeng Zhang, Jiangfeng Liu, Wenfang Wang, Bing Wang, and Li Liu. 2023. "Enhanced Thermal Shock Resistance of High-Temperature Organic Adhesive by CF-SiCNWs Binary Phase Structure" Materials 16, no. 17: 5983. https://doi.org/10.3390/ma16175983
APA StyleZhao, T., Zhong, Z., Zhang, X., Liu, J., Wang, W., Wang, B., & Liu, L. (2023). Enhanced Thermal Shock Resistance of High-Temperature Organic Adhesive by CF-SiCNWs Binary Phase Structure. Materials, 16(17), 5983. https://doi.org/10.3390/ma16175983




