Insights into Nanomedicine for Head and Neck Cancer Diagnosis and Treatment
Abstract
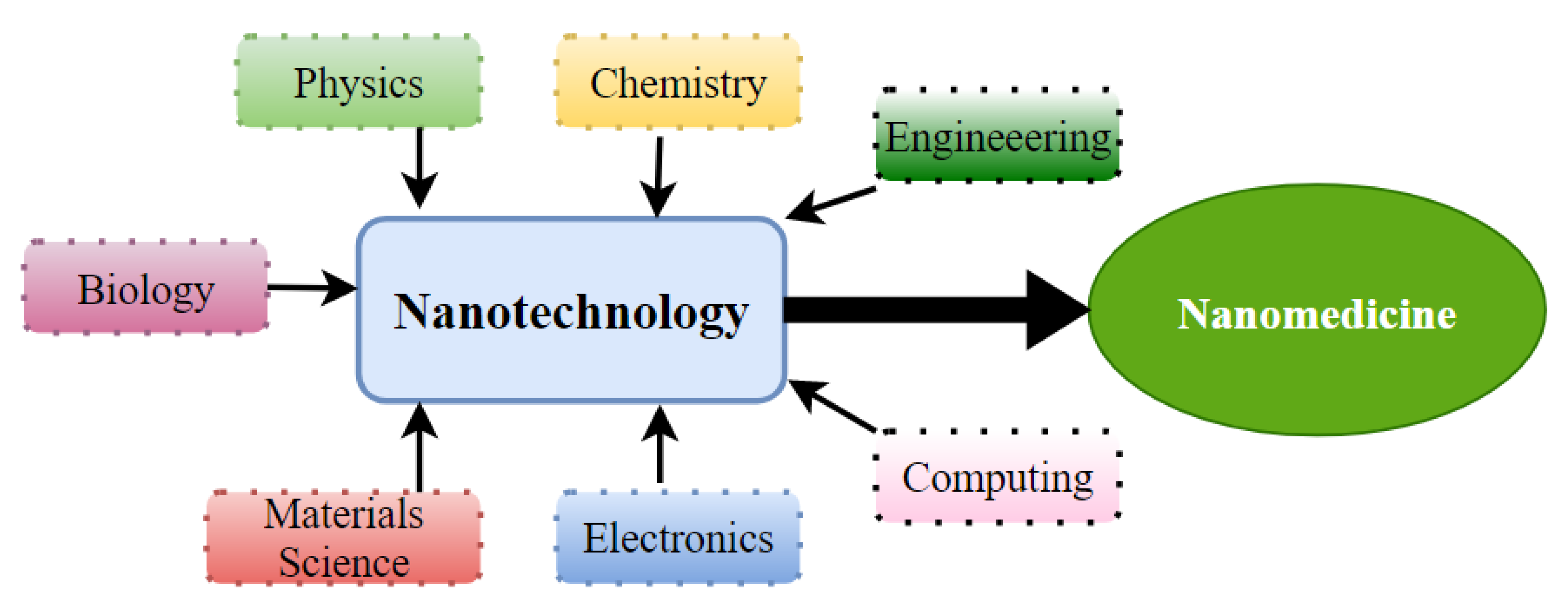
:1. Introduction
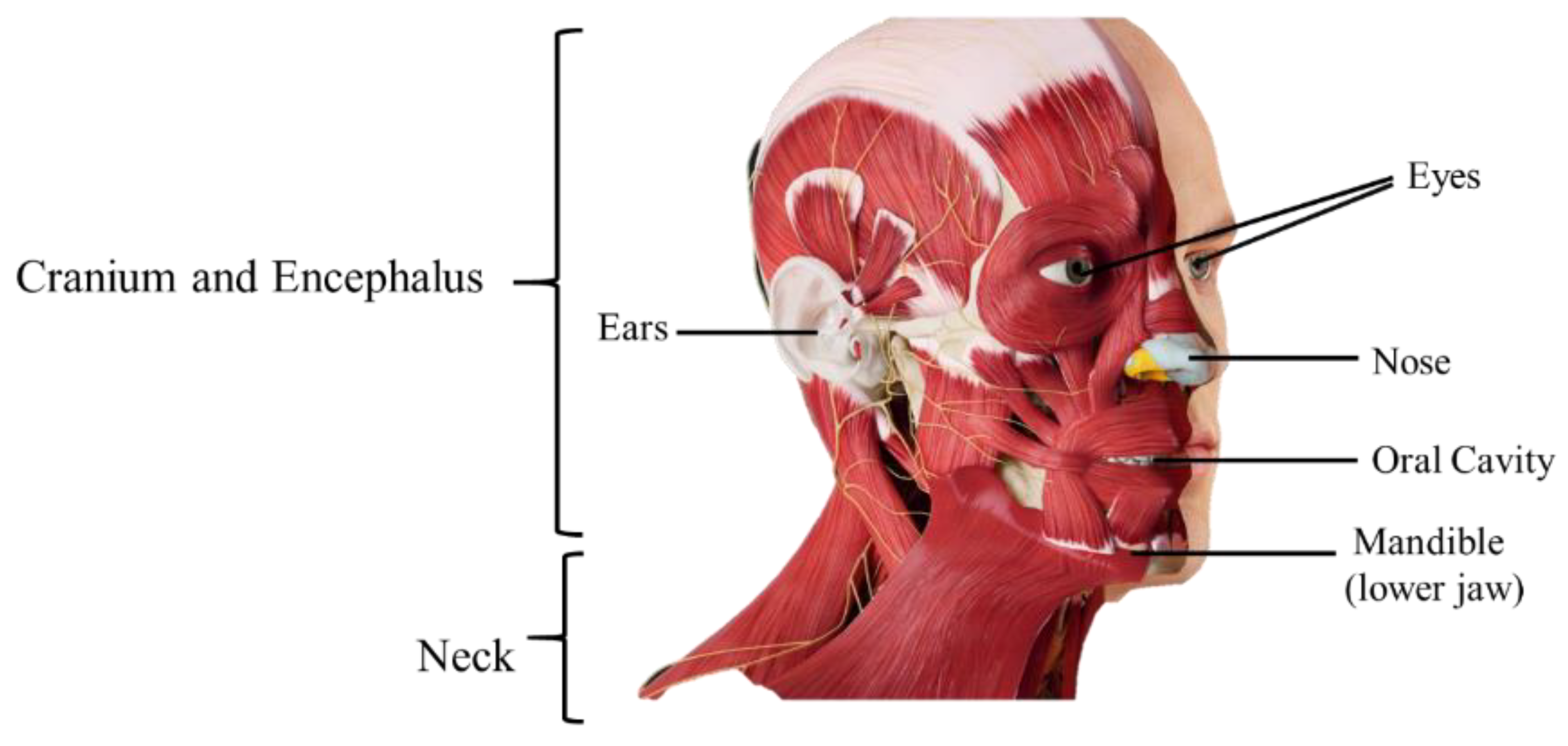
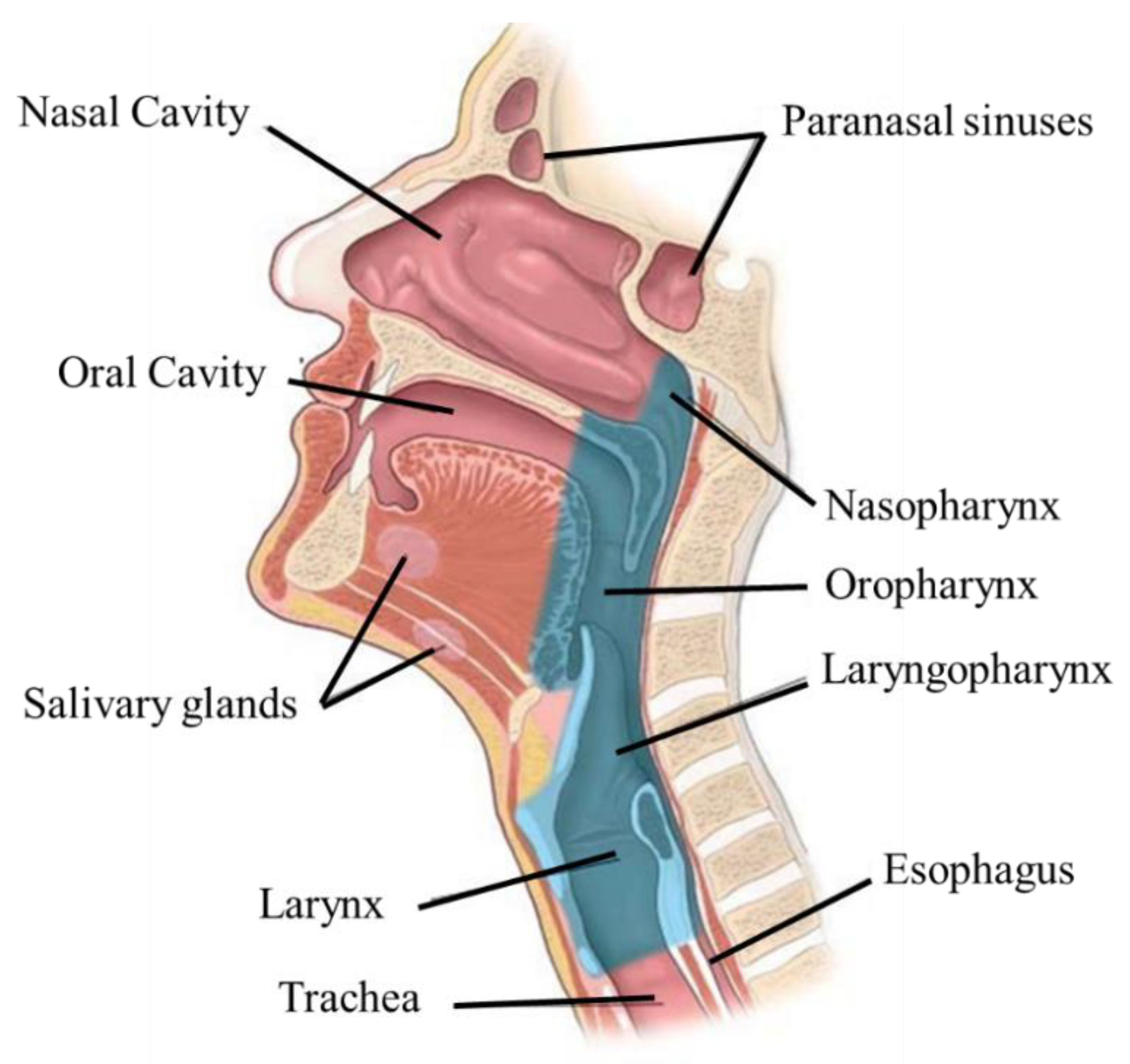
2. Anatomophysiology of the Head and Neck
3. Head and Neck Cancer
3.1. Epidemiology, Etiology, and Risk Factors
3.2. Pathophysiology
3.3. Signs and Symptoms
3.4. Diagnosis and Treatment
- Radiation enhancement/synchronous chemotherapy, used in conjunction with radiation therapy—reduces the risk of lymph node metastasis;
- Neo-adjuvant/induction chemotherapy—to reduce the tumor’s size before the main treatment;
- Adjuvant—acts on the small lesions that cannot be removed by surgery, reduces the recurrence rate, and improves the survival rate;
- Palliation—for distant metastases.
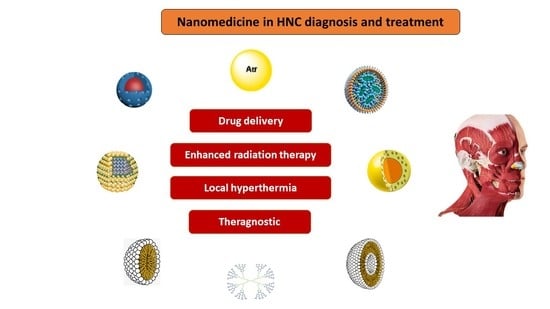
4. Nanomedicine as a Therapeutic Approach for HNC
- The improvement of diagnosis and the development of novel strategies for early detection of tumors, circulating tumor cells, and metastases;
- The improvement of treatment of solid tumors and chemo-resistant tumors;
- The improved precision and efficacy of radiotherapy, immunotherapy, photodynamic, individualized, and hyperthermia therapies
- Lower side effects through more targeted chemotherapy.
4.1. Passive Targeting
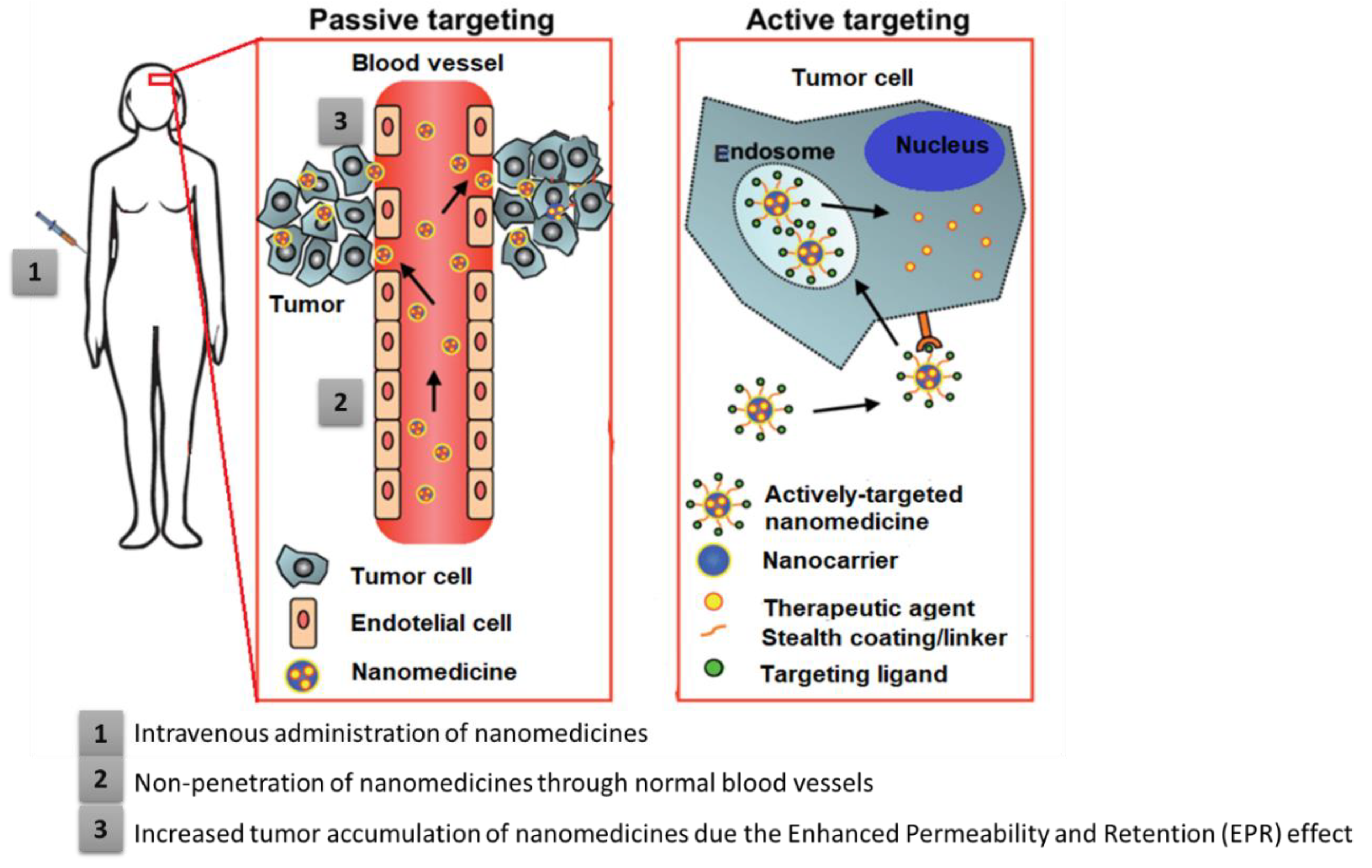
4.2. Active Targeting
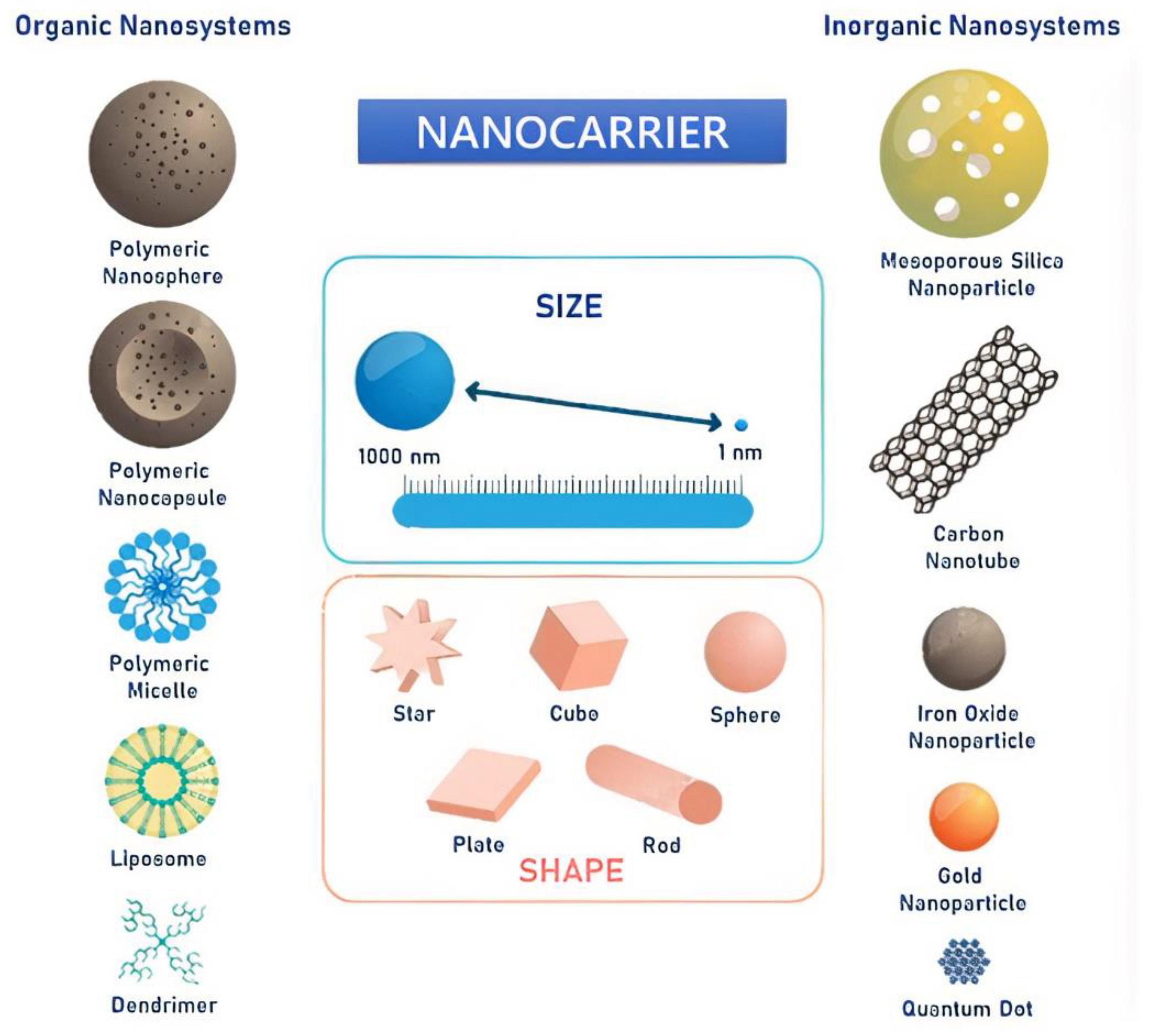
5. Nanocarriers for HNC Diagnosis and Treatment
| Nanocarrier | Load | Targeting Moiety | Targeted Cell/Tissue | Application | Ref. |
|---|---|---|---|---|---|
| Cationic lipid Nps | pre-microRNA-107 | MicroRNA-107 | Xenograft model of HNSCC cell lines (SCC15, SCC25, and CAL27 cells) | Targeted pre-miR-107 delivery | [86] |
| Liposomes | Curcumin-difluorinated | Cancer stem cell marker (CD44) | Cisplatin resistant HNSCC cell lines (CCL-23 and UM-SCC-1, laryngeal and oral cavity carcinomas respectively) | Targeted curcumin-difluorinated delivery | [87] |
| PEGylated liposomes | 188Rhenium | let-7 miRNA | Hypopharyngeal cancer (FaDu) cells and Human tongue squamous cancer (SAS) cells | Targeted 188Rhenium delivery | [88,89] |
| PEGylated NLC | Cisplatin and paclitaxel | Folate receptor (FR) | FaDu cells | Targeted co-delivery of DDP and PTX | [90] |
| Liposomes and SLNs modified with a pH-sensitive PEG shell and DSPE-PEG-peptide | Irinotecan and miR-200 microRNA | EMT-associated genes and topoisomerase-I | HNC SAS cells | Targeted Irinotecan and miR-200 delivery | [91] |
| Phospholipid complex (soybean lecithin, with phosphatidylcholine content of 70–97%) Nps | Salvianolic acid B | - | HNSCC cells lines (HN13, HN30) and Leuk1 precancerous cells | Drug delivery of SalB | [92] |
| Polymer delivery Nps Atrigel® (leuprolide acetate) | Cisplatin | - | C.B-17 severe combined immunodeficiency spontaneous mutant mouse | Cisplatin delivery | [93] |
| Polymer micelles -Poly(ethylene glycol)-poly(glutamic acid) block copolymers (PEG-P[Gu]) | Cisplatin | - | Oral HNSCC cell lines (OSC-19, OSC-20, HSC-3 and HSC-4) | Cisplatin delivery | [94] |
| pH-responsive diblock polymers cationic micelles (dimethylaminoethyl methacrylate blocks) | siRNA | Proapoptotic gene (Pkcδ) | Submandibular rat glands | Targeted siRNA delivery | [95] |
| Biocompatible polymer (poly(e-caprolactone):poly(lactide-cocaprolactone) (PLCL:PCL)) | CCL21 and cisplatin | - | HNSCC tumors | Delivery of CCL21 and Cisplatin | [96] |
| Dendrimers of polyamidoamine (PAMAM) | MTX | FR | HNSCC cell lines (xenografts of no folic acid receptor expression, intermediate folic acid receptor expression and high folic acid receptor expression) | Targeted MTX delivery | [97] |
| Gadolinium Nps | - | - | Radioresistant HNSCC (SQ20B) cell lines of the larynx after radiation therapy | Improvement of RT | [98] |
| Gadolinium Nps (AGuIX®) | DOTAGA | - | Radioresistant HNSCC cell lines (SQ20B, FaDu, and Cal33—tongue squamous cell carcinoma) | Improvement of irradiation of carbon ions (13C+6) | [99] |
| AGuIX® | DOTAGA | - | HNSCC cells lines (SQ20B) | Preparation for radiation application | [100] |
| Silica AuNanoshells | - | - | HNSCC cells lines (Human FaDu cancer cell line—ATCC, HTB-43 and rat alveolar macrophages (NR8383; ATCC# CRL-2192) | Plasmonic photothermal therapy (PPTT) and photodynamic therapy (PDT) | [101] |
| Gold nanorods (AuNRs) | - | - | HNSCC tumor | Photothermal tumor therapy (PTT) | [102] |
| Au Nps | anti-EGRF monoclonal antibody (host mouse) | EGFR | Oral squamous carcinoma cells (OSCC) | Targeted drug delivery of anti-EGFR antibody and photothermal agent (laser irradiation) | [103] |
| Au Nanorods | Rose Bengal | - | OSCC | Photodynamic and photothermal oral cancer therapy | [104] |
| Dextran-coated superparamagnetic iron oxide nanoparticles functionalized with hyaluronic acid (HA) | - | CD44 | Tongue squamous cell carcinoma cells | Magnetic fluid hyperthermia (MFH) | [105] |
| Superparamagnetic iron oxide nanoparticles | Mouse Anti Human CD44 antibody (sc-7297) | CD44 | HNSCC cancer stem cells | MFH | [106] |
| Magnetic iron oxide Nps | - | - | HNSCC cell line (Tu212) of mouse xenograft model | MFH | [107] |
| PAA-attached mesoporous Fe3O4 Nps | Bleomycin | - | Human tongue carcinoma cells (Cal-27) line | Magnetic targeting delivery | [108] |
| SLN (Compritol ATO888, lecithin, and glycerylmonostearate) | Andrographolide | - | Human immortalized oral epithelial (HIOEC), precancerous leucoplakia (Leuk1), HN6, and HN30 cells | Drug delivery of ADG | [109] |
| Multifunctional polymer (Linear-dendritic mPEG-BMA4) Nps | Saracatinib | Proto-oncogene tyrosine-protein kinase Src | HNSCC cell lines (HN6—tongue squamous cell carcinoma; HN8 -metastatic lymph node site from oral cavity; HN12—tongue squamous cell carcinoma) | Targeted Saracatinib delivery | [110] |
5.1. Nanocarriers for Drug Delivery
5.1.1. Lipid-Based Nanocarriers
5.1.2. Polymer-Based Nanocarriers
5.1.3. Metallic-Based Nanoparticles
5.2. Enhanced Radiation Therapy
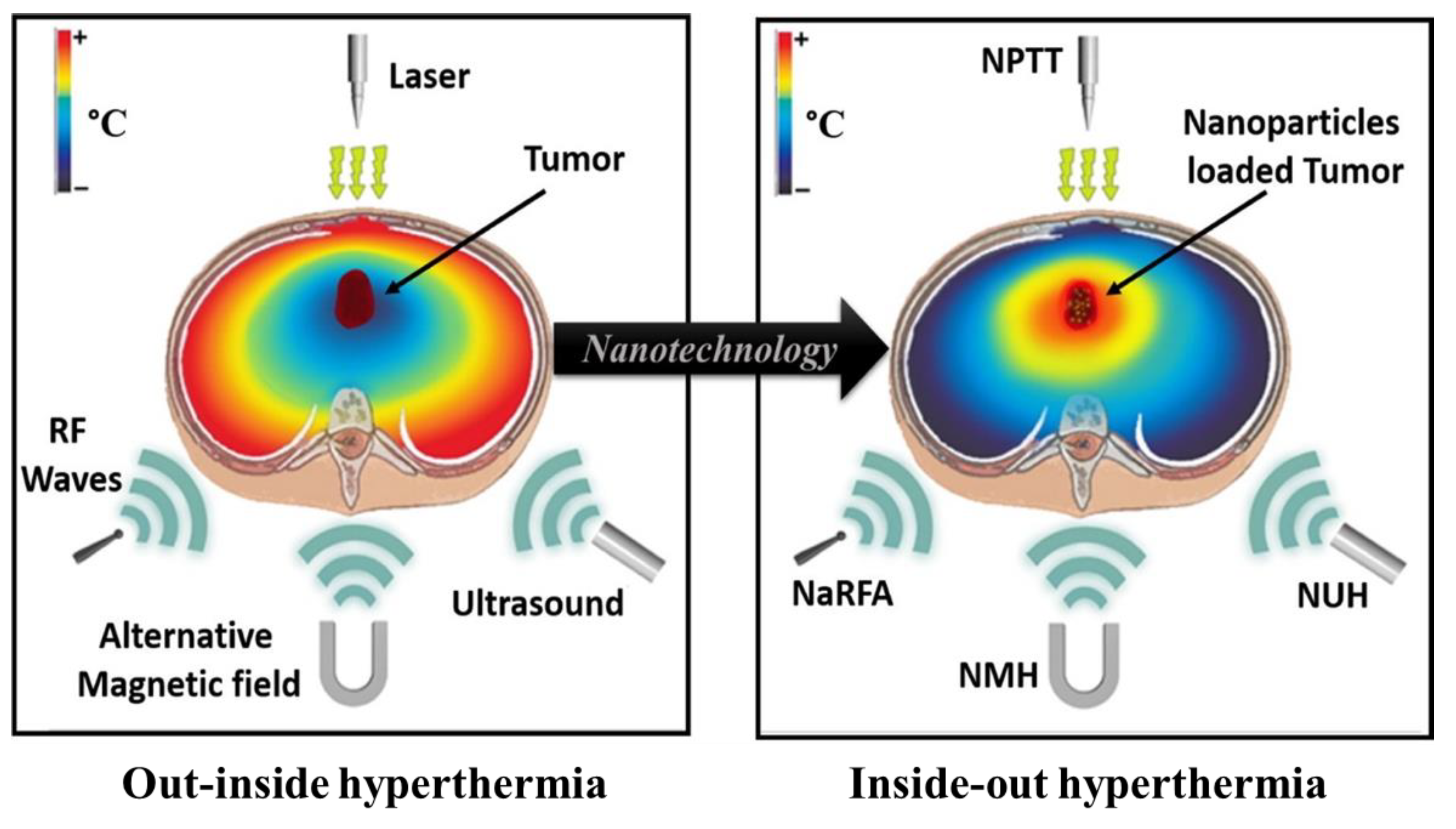
5.3. Local Hyperthermia
5.3.1. Plasmonic Photothermal Therapy
5.3.2. Magnetic Fluid Hyperthermia
5.4. Theragnostics
6. Challenges and Opportunities in the Application of Nanocarriers in HNC
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Acronyms
References
- World Health Organization (WHO)—Cancer Control Programme. Available online: http://www.who.int/cancer/en/ (accessed on 20 December 2021).
- Nayak, M.G.; George, A.; Vidyasagar, M.S.; Mathew, S.; Nayak, S.; Nayak, B.S.; Shashidhara, Y.N.; Kamath, A. Quality of life among cancer patients. Indian J. Palliat. Care 2017, 23, 445. [Google Scholar] [CrossRef] [PubMed]
- Weizman, B.; Golan, N.; Ronen, O. Effect of socioeconomic status on survival in patients with head and neck cancer. Head Neck 2021, 43, 3001–3009. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Fidler, M.M.; Bray, F.; Soerjomataram, I. The global cancer burden and human development: A review. Scand. J. Public Health 2018, 46, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Rezende, T.M.; de Souza Freire, M.; Franco, O.L. Head and neck cancer: Proteomic advances and biomarker achievements. Cancer 2010, 116, 4914–4925. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures. 2021. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html (accessed on 11 January 2022).
- Birkeland, A.C.; Swiecicki, P.L.; Brenner, J.C.; Shuman, A.G. A review of drugs in development for the personalized treatment of head and neck squamous cell carcinoma. Expert Rev. Precis. Med. Drug Dev. 2016, 1, 379–385. [Google Scholar] [CrossRef] [Green Version]
- de Lázaro, I.; Mooney, D.J. Obstacles and opportunities in a forward vision for cancer nanomedicine. Nat. Mater. 2021, 20, 1469–1479. [Google Scholar] [CrossRef]
- Kurmi, B.D.; Patel, P.; Paliwal, R.; Paliwal, S.R. Molecular approaches for targeted drug delivery towards cancer: A concise review with respect to nanotechnology. J. Drug Deliv. Sci. Technol. 2020, 57, 101682. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, H.; Chen, X.; Hollett, G.; Gu, Z.; Wu, J.; Liu, X. Targeted nanoparticles for head and neck cancers: Overview and perspectives. WIREs Nanomed. Nanobiotechnology 2017, 9, e1469. [Google Scholar] [CrossRef]
- Gharat, S.A.; Momin, M.M.; Bhavsar, C. Oral squamous cell carcinoma: Current treatment strategies and nanotechnology-based approaches for prevention and therapy. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 363–400. [Google Scholar] [CrossRef]
- Bharadwaj, R.; Medhi, S. Oral squamous cell carcinoma and the cutting edge of nanotechnology. Multidiscip. Cancer Investig. 2020, 4, 36–45. [Google Scholar] [CrossRef]
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Dinda, S.C.; Pattnaik, G. Nanobiotechnology-based drug delivery in brain targeting. Curr. Pharm. Biotechnol. 2013, 14, 1264–1274. [Google Scholar] [CrossRef]
- Chen, X.-J.; Zhang, X.-Q.; Liu, Q.; Zhang, J.; Zhou, G. Nanotechnology: A promising method for oral cancer detection and diagnosis. J. Nanobiotechnology 2018, 16, 52. [Google Scholar] [CrossRef]
- Wojtynek, N.E.; Mohs, A.M. Image-guided tumor surgery: The emerging role of nanotechnology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1624. [Google Scholar] [CrossRef] [PubMed]
- Mooore, K.L.; Dalley, A.F.; Agur, A.M.R. Moore Clinically Oriented Anatomy; Wolters Kluwer India Pvt Ltd.: Gurugram, India, 2017. [Google Scholar]
- Betts, J.G.; Y, K.A.; Wise, J.A.; Johnson, E.; Poe, B.; Kruse, D.H.; Korol, O.; Johnson, J.E.; Womble, M.; DeSaix, P. 7.2 The skull. In Anatomy and Physiology; OpenStax: Houston, TX, USA, 2013. [Google Scholar]
- Roesch, Z.K.; Tadi, P. Anatomy of head and neck. In StatPearls; StatPearls Publishing Copyright © 2020; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Lester, S.; Yang, W.-Y. Principles and management of head and neck cancer. Surgery 2012, 30, 617–623. [Google Scholar] [CrossRef]
- Crozier, E.; Sumer, B.D. Head and neck cancer. Med. Clin. N. Am. 2010, 94, 1031–1046. [Google Scholar] [CrossRef]
- Bose, P.; Brockton, N.T.; Dort, J.C. Head and neck cancer: From anatomy to biology. Int. J. Cancer 2013, 133, 2013–2023. [Google Scholar] [CrossRef]
- Johnson, N.W.; Amarasinghe, H.K. Epidemiology and aetiology of head and neck cancers. In Head and Neck Cancer; Bernier, J., Ed.; Springer: Geneva, Switzerland, 2011; p. 730. [Google Scholar] [CrossRef]
- Kawashita, Y.; Soutome, S.; Umeda, M.; Saito, T. Oral management strategies for radiotherapy of head and neck cancer. Jpn. Dent. Sci. Rev. 2020, 56, 62–67. [Google Scholar] [CrossRef]
- Wu, T.T.; Zhou, S.H. Nanoparticle-based targeted therapeutics in head-and-neck cancer. Int. J. Med. Sci. 2015, 12, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Zheng, M.; Ren, X.; Tang, Y.; Liang, X. Local hyperthermia in head and neck cancer: Mechanism, application and advance. Oncotarget 2016, 7, 57367–57378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef]
- Pezzuto, F.; Buonaguro, L.; Caponigro, F.; Ionna, F.; Starita, N.; Annunziata, C.; Buonaguro, F.M.; Tornesello, M.L. Update on head and neck cancer: Current knowledge on epidemiology, risk factors, molecular features and novel therapies. Oncology 2015, 89, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Aupérin, A. Epidemiology of head and neck cancers: An update. Curr. Opin. Oncol. 2020, 32, 178–186. [Google Scholar] [CrossRef]
- Hashibe, M.; Brennan, P.; Chuang, S.C.; Boccia, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Maso, L.D.; Daudt, A.W.; Fabianova, E.; et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: Pooled analysis in the international head and neck cancer epidemiology consortium. Cancer Epidemiol. Biomark. Prev. 2009, 18, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.Y.K.; Zhen, G.; Agrawal, N. The role of tumor DNA as a diagnostic tool for head and neck squamous cell carcinoma. Semin. Cancer Biol. 2019, 55, 1–7. [Google Scholar] [CrossRef]
- Zolkind, P.; Lee, J.J.; Jackson, R.S.; Pipkorn, P.; Massa, S.T. Untreated head and neck cancer: Natural history and associated factors. Head Neck 2021, 43, 89–97. [Google Scholar] [CrossRef]
- Chua, M.L.K.; Wee, J.T.S.; Hui, E.P.; Chan, A.T.C. Nasopharyngeal carcinoma. Lancet 2016, 387, 1012–1024. [Google Scholar] [CrossRef]
- Smatti, M.K.; Al-Sadeq, D.W.; Ali, N.H.; Pintus, G.; Abou-Saleh, H.; Nasrallah, G.K. Epstein–Barr virus epidemiology, serology, and genetic variability of LMP-1 oncogene among healthy population: An update. Front. Oncol. 2018, 8, 211. [Google Scholar] [CrossRef] [Green Version]
- Al-Thawadi, H.; Gupta, I.; Jabeen, A.; Skenderi, F.; Aboulkassim, T.; Yasmeen, A.; Malki, M.I.; Batist, G.; Vranic, S.; Al Moustafa, A.-E. Co-presence of human papillomaviruses and Epstein–Barr virus is linked with advanced tumor stage: A tissue microarray study in head and neck cancer patients. Cancer Cell Int. 2020, 20, 361. [Google Scholar] [CrossRef]
- Sroussi, H.Y.; Jessri, M.; Epstein, J. Oral assessment and management of the patient with head and neck cancer. Oral Maxillofac. Surg. Clin. 2018, 30, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Sahovaler, A.; Yeh, D.H.; Fung, K. General principles of head and neck cancer treatment. In Clinical Care and Rehabilitation in Head and Neck Cancer; Doyle, P.C., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 3–14. [Google Scholar]
- Vokes, E.E.; Weichselbaum, R.R.; Lippman, S.M.; Hong, W.K. Head and neck cancer. N. Engl. J. Med. 1993, 328, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Heroiu Cataloiu, A.-D.; Danciu, C.E.; Popescu, C.R. Multiple cancers of the head and neck. Maedica 2013, 8, 80–85. [Google Scholar] [PubMed]
- Chen, S.-W.; Li, S.-H.; Shi, D.-B.; Jiang, W.-M.; Song, M.; Yang, A.-K.; Li, Y.-D.; Bei, J.-X.; Chen, W.-K.; Zhang, Q. Expression of PD-1/PD-L1 in head and neck squamous cell carcinoma and its clinical significance. Int. J. Biol. Markers 2019, 34, 398–405. [Google Scholar] [CrossRef] [Green Version]
- Lenouvel, D.; González-Moles, M.; Talbaoui, A.; Ramos-García, P.; González-Ruiz, L.; Ruiz-Ávila, I.; Gil-Montoya, J.A. An update of knowledge on PD-L1 in head and neck cancers: Physiologic, prognostic and therapeutic perspectives. Oral Dis. 2020, 26, 511–526. [Google Scholar] [CrossRef]
- Stepnick, D.; Gilpin, D. Head and Neck Cancer—An Overview. Semin. Plast. Surg. 2009, 24, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Panikkanvalappil, S.R.; El-Sayed, M.A.; El-sayed, I.H. Advances in nanomedicine for head and neck cancer. In Head and Neck Cancer; Bernier, J., Ed.; Springer: Cham, Switzerland, 2016; pp. 827–843. [Google Scholar] [CrossRef]
- Ordoñez, R.; Otero, A.; Jerez, I.; Medina, J.A.; Lupiañez-Pérez, Y.; Gomez-Millan, J. Role of radiotherapy in the treatment of metastatic head and neck cancer. OncoTargets Ther. 2019, 12, 677–683. [Google Scholar] [CrossRef] [Green Version]
- Guidi, A.; Codecà, C.; Ferrari, D. Chemotherapy and immunotherapy for recurrent and metastatic head and neck cancer: A systematic review. Med. Oncol. 2018, 35, 37. [Google Scholar] [CrossRef]
- Saloura, V.; Langerman, A.; Rudra, S.; Chin, R.; Cohen, E.E.W. Multidisciplinary care of the patient with head and neck cancer. Surg. Oncol. Clin. N. Am. 2013, 22, 179–215. [Google Scholar] [CrossRef]
- Machiels, J.P.; René Leemans, C.; Golusinski, W.; Grau, C.; Licitra, L.; Gregoire, V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: Clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1462–1475. [Google Scholar] [CrossRef]
- Ferrari, D.; Ghi, M.G.; Franzese, C.; Codecà, C.; Gau, M.; Fayette, J. The slippery role of induction chemotherapy in head and neck cancer: Myth and reality. Front. Oncol. 2020, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Al Qaraghuli, M.M. Biotherapeutic antibodies for the treatment of head and neck cancer: Current approaches and future considerations of photothermal therapies. Front. Oncol. 2020, 10, 2710. [Google Scholar] [CrossRef] [PubMed]
- Herskovic, A.; Martz, K.; Al-Sarraf, M.; Leichman, L.; Brindle, J.; Vaitkevicius, V.; Cooper, J.; Byhardt, R.; Davis, L.; Emami, B. Combined Chemotherapy and Radiotherapy Compared with Radiotherapy Alone in Patients with Cancer of the Esophagus. N. Engl. J. Med. 1992, 326, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Pillai, G. Nanotechnology Toward Treating Cancer. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 221–256. [Google Scholar] [CrossRef]
- Xu, Q.; Fang, M.; Zhu, J.; Dong, H.; Cao, J.; Yan, L.; Leonard, F.; Oppel, F.; Sudhoff, H.; Kaufmann, A.M. Insights into nanomedicine for immunotherapeutics in squamous cell carcinoma of the head and neck. Int. J. Biol. Sci. 2020, 16, 2506. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Schipilliti, F.M.; Trudu, L.; Bertolini, F. Immunotherapy in head and neck cancer: The great challenge of patient selection. Crit. Rev. Oncol./Hematol. 2019, 144, 102829. [Google Scholar] [CrossRef] [PubMed]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Chang, E.H.; Harford, J.B.; Eaton, M.A.W.; Boisseau, P.M.; Dube, A.; Hayeshi, R.; Swai, H.; Lee, D.S. Nanomedicine: Past, present and future—A global perspective. Biochem. Biophys. Res. Commun. 2015, 468, 511–517. [Google Scholar] [CrossRef]
- Caruthers, S.D.; Wickline, S.A.; Lanza, G.M. Nanotechnological applications in medicine. Curr. Opin. Biotechnol. 2007, 18, 26–30. [Google Scholar] [CrossRef]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, properties, and regulatory issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Muthu, M.S.; Mei, L.; Feng, S.-S. Nanotheranostics: Advanced nanomedicine for the integration of diagnosis and therapy. Nanomedicine 2014, 9, 1277–1280. [Google Scholar] [CrossRef]
- Astruc, D. Introduction to Nanomedicine. Molecules 2016, 21, 4. [Google Scholar] [CrossRef] [PubMed]
- Cormode, D.P.; Skajaa, T.; Fayad, Z.A.; Mulder, W.J.M. Nanotechnology in medical imaging: Probe design and applications. Arterioscler. Thromb. Vasc. Biol. 2010, 29, 992–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, A.R.O.; Almeida, B.G.; Rodrigues, J.M.; Queiroz, M.J.R.P.; Calhelha, R.C.; Ferreira, I.C.F.R.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Coutinho, P.J.G.; et al. Magnetoliposomes as carriers for promising antitumor thieno[3,2-b]pyridin-7-arylamines: Photophysical and biological studies. R. Soc. Chem. Adv. 2017, 7, 15352–15361. [Google Scholar] [CrossRef] [Green Version]
- Clarke, J.; Wu, H.C.; Jayasinghe, L.; Patel, A.; Reid, S.; Bayley, H. Continuous base identification for single-molecule nanopore DNA sequencing. Nat. Nanotechnol. 2009, 4, 265–270. [Google Scholar] [CrossRef]
- Danie Kingsley, J.; Ranjan, S.; Dasgupta, N.; Saha, P. Nanotechnology for tissue engineering: Need, techniques and applications. J. Pharm. Res. 2013, 7, 200–204. [Google Scholar] [CrossRef]
- Nanomedicine, E.T.P. Nanomedicine Strategic Research & Innovation Agenda 2016–2030: Creating Junctions for Healthcare; European Technology Platform for Nanomedicine: Paris, France, 2016; pp. 1–31. [Google Scholar]
- Caban, S.; Aytekin, E.; Sahin, A.; Capan, Y. Nanosystems for drug delivery. OA Drug Des. Deliv. 2014, 2, 1–7. [Google Scholar]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Nikalje, A.P. Nanotechnology and its applications in medicine. Med. Chem. 2015, 5, 81–89. [Google Scholar] [CrossRef]
- Pradhan, P.; Giri, J.; Rieken, F.; Koch, C.; Mykhaylyk, O.; Döblinger, M.; Banerjee, R.; Bahadur, D.; Plank, C. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J. Control. Release 2010, 142, 108–121. [Google Scholar] [CrossRef]
- Aghebati-Maleki, A.; Dolati, S.; Ahmadi, M.; Baghbanzhadeh, A.; Asadi, M.; Fotouhi, A.; Yousefi, M.; Aghebati-Maleki, L. Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers. J. Cell. Physiol. 2020, 235, 1962–1972. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.R. The use of nanocarriers for drug delivery in cancer therapy. J. Cancer Sci. Ther. 2010, 2, 58–62. [Google Scholar] [CrossRef]
- Inagaki, F.F.; Furusawa, A.; Choyke, P.L.; Kobayashi, H. Enhanced nanodrug delivery in tumors after near-infrared photoimmunotherapy. Nanophotonics 2019, 8, 1673–1688. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- van der Meel, R. Targeted Inhibition of Tumor Growth and Angiogenesis. Ph.D. Thesis, Universiteit Utrecht, Utrecht, The Netherlands, 2013. [Google Scholar]
- Torchilin, V.P. Passive and active drug targeting: Drug delivery to tumors as an example. In Handbook of Experimental Pharmacology, 197th ed.; Springer: Berlin, Germany, 2010; Volume 197, pp. 3–53. [Google Scholar]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic nanoparticles: From design and synthesis to real world applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef]
- Pawar, P.V.; Domb, A.J.; Kumar, N. Systemic targeting systems. In Advances in Delivery Science and Technology; Springer: Boston, MA, USA, 2014; pp. 61–91. [Google Scholar] [CrossRef]
- Alexis, F.; Rhee, J.-W.; Richie, J.P.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. New frontiers in nanotechnology for cancer treatment. Urol. Oncol. Semin. Orig. Investig. 2008, 26, 74–85. [Google Scholar] [CrossRef]
- Couvreur, P. Nanoparticles in drug delivery: Past, present and future. Adv. Drug Deliv. Rev. 2013, 65, 21–23. [Google Scholar] [CrossRef]
- Caparica, R.; Júlio, A.; Araújo, M.E.; Baby, A.R.; Fonte, P.; Costa, J.G.; Santos de Almeida, T. Anticancer activity of rutin and its combination with ionic liquids on renal cells. Biomolecules 2020, 10, 233. [Google Scholar] [CrossRef] [Green Version]
- Andrade, F.; Fonte, P.; Costa, A.; Reis, C.C.; Nunes, R.; Almeida, A.; Ferreira, D.; Oliva, M.; Sarmento, B. Pharmacological and toxicological assessment of innovative self-assembled polymeric micelles as powders for insulin pulmonary delivery. Nanomedicine 2016, 11, 2305–2317. [Google Scholar] [CrossRef]
- Irimie, A.I.; Sonea, L.; Jurj, A.; Mehterov, N.; Zimta, A.A.; Budisan, L.; Braicu, C.; Berindan-Neagoe, I. Future trends and emerging issues for nanodelivery systems in oral and oropharyngeal cancer. Int. J. Nanomed. 2017, 12, 4593–4606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonte, P.; Andrade, F.; Azevedo, C.; Pinto, J.; Seabra, V.; van de Weert, M.; Reis, S.; Sarmento, B. Effect of the freezing step in the stability and bioactivity of protein-loaded PLGA nanoparticles upon lyophilization. Pharm. Res. 2016, 33, 2777–2793. [Google Scholar] [CrossRef] [PubMed]
- Piao, L.; Zhang, M.; Datta, J.; Xie, X.; Su, T.; Li, H.; Teknos, T.N.; Pan, Q. Lipid-based nanoparticle delivery of pre-miR-107 inhibits the tumorigenicity of head and neck squamous cell carcinoma. Mol. Ther. 2012, 20, 1261–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basak, S.K.; Zinabadi, A.; Wu, A.W.; Venkatesan, N.; Duarte, V.M.; Kang, J.J.; Dalgard, C.L.; Srivastava, M.; Sarkar, F.H.; Wang, M.B.; et al. Liposome encapsulated curcumin-difluorinated (CDF) inhibits the growth of cisplatin resistant head and neck cancer stem cells. Oncotarget 2015, 6, 18504. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.-T.; Chang, C.-Y.; Chang, C.-H.; Wang, H.-E.; Chiou, S.-H.; Liu, R.-S.; Lee, T.-W.; Lee, Y.-J. Involvement of let-7 microRNA for the therapeutic effects of Rhenium-188-embedded liposomal nanoparticles on orthotopic human head and neck cancer model. Oncotarget 2016, 7, 65782. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-Y.; Chen, C.-C.; Lin, L.-T.; Chang, C.-H.; Chen, L.-C.; Wang, H.-E.; Lee, T.-W.; Lee, Y.-J. PEGylated liposome-encapsulated rhenium-188 radiopharmaceutical inhibits proliferation and epithelial–mesenchymal transition of human head and neck cancer cells in vivo with repeated therapy. Cell Death Discov. 2018, 4, 100. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Ju, Z.; Dong, S. Cisplatin and paclitaxel co-delivered by folate-decorated lipid carriers for the treatment of head and neck cancer. Drug Deliv. 2017, 24, 792–799. [Google Scholar] [CrossRef] [Green Version]
- Lo, Y.-L.; Chang, C.-H.; Wang, C.-S.; Yang, M.-H.; Lin, A.M.-Y.; Hong, C.-J.; Tseng, W.-H. PEG-coated nanoparticles detachable in acidic microenvironments for the tumor-directed delivery of chemo-and gene therapies for head and neck cancer. Theranostics 2020, 10, 6695. [Google Scholar] [CrossRef]
- Li, H.; Shi, L.; Wei, J.; Zhang, C.; Zhou, Z.; Wu, L.; Liu, W. Cellular uptake and anticancer activity of salvianolic acid B phospholipid complex loaded nanoparticles in head and neck cancer and precancer cells. Colloids Surf. B Biointerfaces 2016, 147, 65–72. [Google Scholar] [CrossRef]
- Chen, F.A.; Kuriakose, M.A.; Zhou, M.X.; DeLacure, M.D.; Dunn, R.L. Biodegradable polymer-mediated intratumoral delivery of cisplatin for treatment of human head and neck squamous cell carcinoma in a chimeric mouse model. Head Neck 2003, 25, 554–560. [Google Scholar] [CrossRef]
- Endo, K.; Ueno, T.; Kondo, S.; Wakisaka, N.; Murono, S.; Ito, M.; Kataoka, K.; Kato, Y.; Yoshizaki, T. Tumor-targeted chemotherapy with the nanopolymer-based drug NC-6004 for oral squamous cell carcinoma. Cancer Sci. 2013, 104, 369–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arany, S.; Benoit, D.S.W.; Dewhurst, S.; Ovitt, C.E. Nanoparticle-mediated gene silencing confers radioprotection to salivary glands in vivo. Mol. Ther. 2013, 21, 1182–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellionisz, P.A.; Lin, Y.; Mallen-St Clair, J.; Luo, J.; Suwarnasarn, A.; Schaue, D.; Elashoff, D.A.; Palma-Diaz, F.; Dubinett, S.M.; Sharma, S.; et al. Use of a novel polymer in an animal model of head and neck squamous cell carcinoma. Otolaryngol.—Head Neck Surg. 2018, 158, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.B.; Dunham, T.; Majoros, I.J.; Baker, J.R. Targeted dendrimer chemotherapy in an animal model for head and neck squamous cell carcinoma. J. Oral Maxillofac. Surg. 2011, 69, 2452–2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miladi, I.; Aloy, M.T.; Armandy, E.; Mowat, P.; Kryza, D.; Magné, N.; Tillement, O.; Lux, F.; Billotey, C.; Janier, M.; et al. Combining ultrasmall gadolinium-based nanoparticles with photon irradiation overcomes radioresistance of head and neck squamous cell carcinoma. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 247–257. [Google Scholar] [CrossRef]
- Wozny, A.S.; Aloy, M.T.; Alphonse, G.; Magné, N.; Janier, M.; Tillement, O.; Lux, F.; Beuve, M.; Rodriguez-Lafrasse, C. Gadolinium-based nanoparticles as sensitizing agents to carbon ions in head and neck tumor cells. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2655–2660. [Google Scholar] [CrossRef]
- Simonet, S.; Rodriguez-Lafrasse, C.; Beal, D.; Gerbaud, S.; Malesys, C.; Tillement, O.; Lux, F.; Fayyad-Kazan, H.; Rachidi, W.; Ardail, D. Gadolinium-based nanoparticles can overcome the radioresistance of head and neck squamous cell carcinoma through the induction of autophagy. J. Biomed. Nanotechnol. 2020, 16, 111–124. [Google Scholar] [CrossRef]
- Trinidad, A.J.; Hong, S.J.; Peng, Q.; Madsen, S.J.; Hirschberg, H. Combined concurrent photodynamic and gold nanoshell loaded macrophage-mediated photothermal therapies: An in vitro study on squamous cell head and neck carcinoma. Lasers Surg. Med. 2014, 46, 310–318. [Google Scholar] [CrossRef] [Green Version]
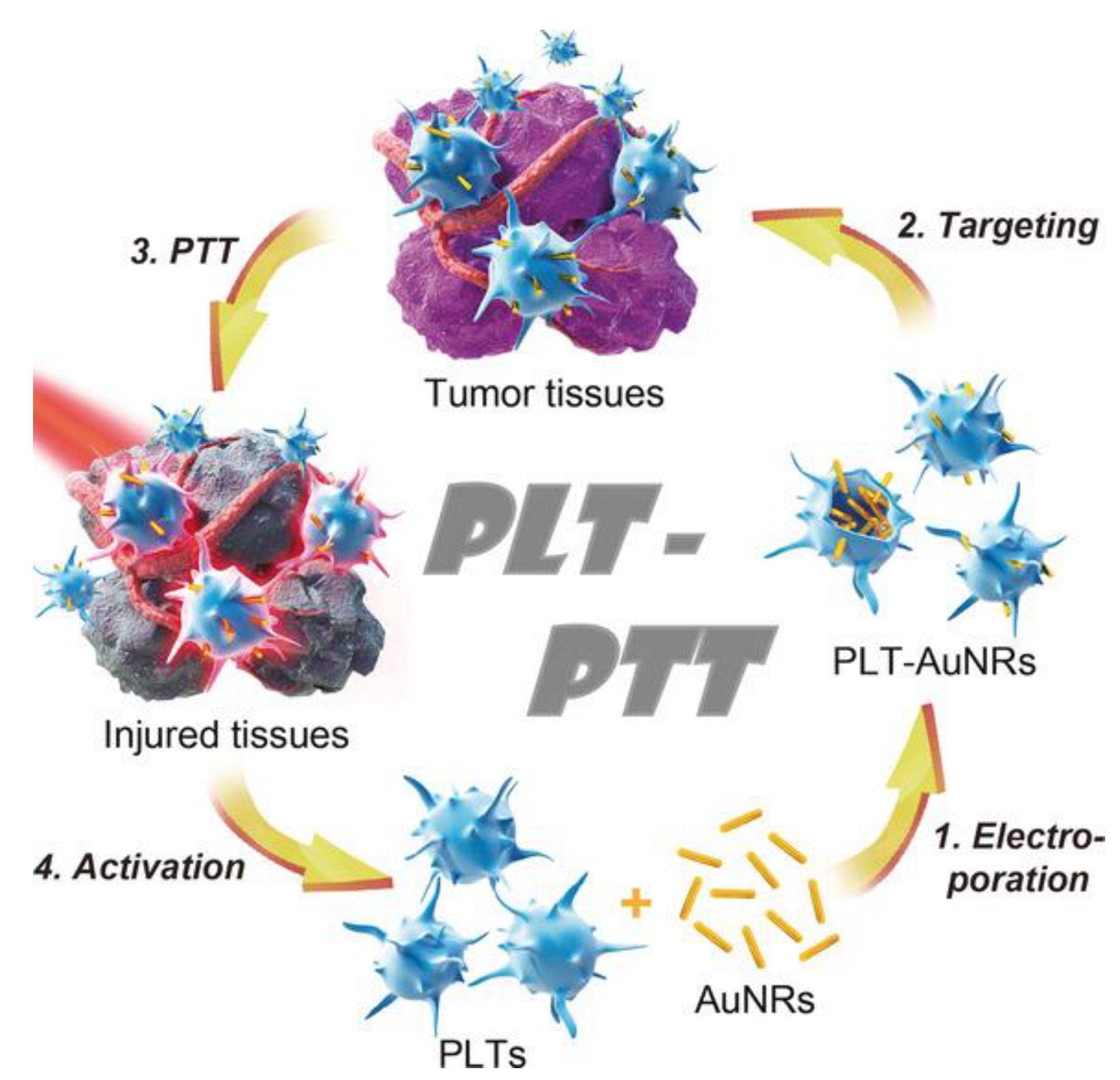
- Rao, L.; Bu, L.L.; Ma, L.; Wang, W.; Liu, H.; Wan, D.; Liu, J.F.; Li, A.; Guo, S.S.; Zhang, L.; et al. Platelet-Facilitated Photothermal Therapy of Head and Neck Squamous Cell Carcinoma. Angew. Chem. 2018, 57, 986–991. [Google Scholar] [CrossRef]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006, 239, 129–135. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.H.; Liu, Q.; Huang, H.; Chen, M.; Li, K.; Li, C.; Yu, X.F.; Chu, P.K. Rose-bengal-conjugated gold nanorods for invivo photodynamic and photothermal oral cancer therapies. Biomaterials 2014, 35, 1954–1966. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Thapa, R.; Galoforo, S.; Hang, X.; Buelow, K.; Wilson, G. Targeting Stem Cells in Head and Neck Cancer Using Superparamagnetic Iron Oxide Nanoparticles. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, E621. [Google Scholar] [CrossRef]
- Su, Z.; Liu, D.; Chen, L.; Zhang, J.; Ru, L.; Chen, Z.; Gao, Z.; Wang, X. CD44-targeted magnetic nanoparticles kill head and neck squamous cell carcinoma stem cells in an alternating magnetic field. Int. J. Nanomed. 2019, 14, 7549. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Wang, L.; Cheng, R.; Mao, L.; Arnold, R.D.; Howerth, E.W.; Chen, Z.G.; Platt, S. Magnetic nanoparticle-based hyperthermia for head & neck cancer in mouse models. Theranostics 2012, 2, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Zhuang, L.; Lin, Y.; Yan, M.; Lv, J.; Li, X.; Lin, H.; Zhu, P.; Lin, Q.; Xu, Y. Novel drug delivery system based on hollow mesoporous magnetic nanoparticles for head and neck cancers--targeted therapy in vitro and in vivo. Am. J. Cancer Res. 2020, 10, 350. [Google Scholar]
- Li, H.; Qu, X.; Qian, W.; Song, Y.; Wang, C.; Liu, W. Andrographolide-loaded solid lipid nanoparticles enhance anti-cancer activity against head and neck cancer and precancerous cells. Oral Dis. 2020, 28, 142–149. [Google Scholar] [CrossRef]
- Lang, L.; Shay, C.; Xiong, Y.; Thakkar, P.; Chemmalakuzhy, R.; Wang, X.; Teng, Y. Combating head and neck cancer metastases by targeting Src using multifunctional nanoparticle-based saracatinib. J. Hematol. Oncol. 2018, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Yeagle, P.L. The lipids of biological membranes. In The Membranes of Cells, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2016; p. 452. [Google Scholar]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.-H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef] [Green Version]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Borges, G.Á.; Rêgo, D.F.; Assad, D.X.; Coletta, R.D.; De Luca Canto, G.; Guerra, E.N.S. In vivo and in vitro effects of curcumin on head and neck carcinoma: A systematic review. J. Oral Pathol. Med. 2017, 46, 3–20. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Car, H. Nanocarriers in modern drug delivery systems. Chemik 2012, 66, 868–881. [Google Scholar]
- Rb, I.; Tj, Y. Role of Src expression and activation in human cancer. Oncogene 2000, 19, 5636–5642. [Google Scholar] [CrossRef] [Green Version]
- Dunn, L.A.; Fury, M.G.; Xiao, H.; Baxi, S.S.; Sherman, E.J.; Korte, S.; Pfister, C.; Haque, S.; Katabi, N.; Ho, A.L.; et al. A phase II study of temsirolimus added to low-dose weekly carboplatin and paclitaxel for patients with recurrent and/or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC). Ann. Oncol. 2017, 28, 2533–2538. [Google Scholar] [CrossRef] [PubMed]
- Fury, M.G.; Sherman, E.; Ho, A.; Katabi, N.; Sima, C.; Kelly, K.W.; Nwankwo, O.; Haque, S.; Pfister, D.G. A phase I study of temsirolimus plus carboplatin plus paclitaxel for patients with recurrent or metastatic (R/M) head and neck squamous cell cancer (HNSCC). Cancer Chemother. Pharmacol. 2012, 70, 121–128. [Google Scholar] [CrossRef]
- Damascelli, B.; Patelli, G.L.; Lanocita, R.; Tolla, G.D.; Frigerio, L.F.; Marchianò, A.; Garbagnati, F.; Spreafico, C.; Tichà, V.; Gladin, C.R.; et al. A novel intraarterial chemotherapy using paclitaxel in albumin nanoparticles to treat advanced squamous cell carcinoma of the tongue: Preliminary findings. Am. J. Roentgenol. 2003, 181, 253–260. [Google Scholar] [CrossRef]
- Haider, M.; Elsherbeny, A.; Jagal, J.; Hubatová-Vacková, A.; Saad Ahmed, I. Optimization and evaluation of poly(lactide-co-glycolide) nanoparticles for enhanced cellular uptake and efficacy of paclitaxel in the treatment of head and neck cancer. Pharmaceutics 2020, 12, 828. [Google Scholar] [CrossRef]
- Riestra Ayora, J.I.; Sánchez Rodríguez, C.; Palao Suay, R.; Yanes Díaz, J.; Martín Hita, A.; Aguilar de Armas, M.R.; Sanz Fernández, R. Paclitaxel-loaded polymeric nanoparticles based on a-tocopheryl succinate for the treatment of head and neck squamous cell carcinoma: In vivo murine model. Drug Deliv. 2021, 28, 1376–1388. [Google Scholar] [CrossRef]
- Baumann, M.; Krause, M.; Overgaard, J.; Debus, J.; Bentzen, S.M.; Daartz, J.; Richter, C.; Zips, D.; Bortfeld, T. Radiation oncology in the era of precision medicine. Nat. Rev. Cancer 2016, 16, 234–249. [Google Scholar] [CrossRef]
- Chen, H.H.W.; Kuo, M.T. Improving radiotherapy in cancer treatment: Promises and challenges. Oncotarget 2017, 8, 62742–62758. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Li, H.; Huo, B.; Meng, Z.; Xue, M.; Qiu, L.; Ma, S.; Yan, Z.; Piao, C.; Ma, X. Full-color mechanical sensor based on elastic nanocomposite hydrogels encapsulated three-dimensional colloidal arrays. Sens. Actuators B Chem. 2016, 234, 527–533. [Google Scholar] [CrossRef]
- Popovtzer, A.; Mizrachi, A.; Motiei, M.; Bragilovski, D.; Lubimov, L.; Levi, M.; Hilly, O.; Ben-Aharon, I.; Popovtzer, R. Actively targeted gold nanoparticles as novel radiosensitizer agents: An in vivo head and neck cancer model. Nanoscale 2016, 8, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Revannasiddaiah, S.; Susheela, S.P. Chemically enhanced radiotherapy: Visions for the future. Ann. Transl. Med. 2016, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Bergs, J.W.J.; Wacker, M.G.; Hehlgans, S.; Piiper, A.; Multhoff, G.; Rödel, C.; Rödel, F. The role of recent nanotechnology in enhancing the efficacy of radiation therapy. Biochim. Biophys. Acta Rev. Cancer 2015, 1856, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Ruba, T.; Tamilselvi, R. Radiosensitizers and radioprotectors for effective radiation therapy—A review. Asian J. Appl. Sci. Technol. 2018, 2, 77–86. [Google Scholar]
- Hainfeld, J.F.; Lin, L.; Slatkin, D.N.; Dilmanian, F.A.; Vadas, T.M.; Smilowitz, H.M. Gold nanoparticle hyperthermia reduces radiotherapy dose. Nanomedicine 2014, 10, 1609–1617. [Google Scholar] [CrossRef] [Green Version]
- Hainfeld, J.F.; Dilmanian, F.A.; Zhong, Z.; Slatkin, D.N.; Kalef-Ezra, J.A.; Smilowitz, H.M. Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys. Med. Biol. 2010, 55, 3045–3059. [Google Scholar] [CrossRef]
- Butterworth, K.T.; Coulter, J.A.; Jain, S.; Forker, J.; McMahon, S.J.; Schettino, G.; Currell, F.J.; Hirst, D.G. UKPMC Funders Group nm gold particles: Potential application for cancer therapy. Evaluation 2010, 21, 295101. [Google Scholar] [CrossRef]
- Nörenberg, D.; Schmidt, F.; Schinke, K.; Frenzel, T.; Pietsch, H.; Giese, A.; Ertl-Wagner, B.; Levin, J. Investigation of potential adverse central nervous system effects after long term oral administration of gadolinium in mice. PLoS ONE 2020, 15, e0231495. [Google Scholar] [CrossRef] [Green Version]
- Vergauwen, E.; Vanbinst, A.-M.; Brussaard, C.; Janssens, P.; De Clerck, D.; Van Lint, M.; Houtman, A.C.; Michel, O.; Keymolen, K.; Lefevere, B.; et al. Central nervous system gadolinium accumulation in patients undergoing periodical contrast MRI screening for hereditary tumor syndromes. Hered. Cancer Clin. Pract. 2018, 16, 2. [Google Scholar] [CrossRef] [Green Version]
- McDonald, J.S.; McDonald, R.J. MR imaging safety considerations of gadolinium-based contrast agents: Gadolinium retention and nephrogenic systemic fibrosis. Magn. Reson. Imaging Clin. 2020, 28, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Inácio, P. Nanobiotix Plans Nine NBTXR3 Clinical Trials for Six Cancer Types; Nanobiotix: Paris, France, 2019. [Google Scholar]
- Bonvalot, S.; Rutkowski, P.L.; Thariat, J.; Carrère, S.; Ducassou, A.; Sunyach, M.-P.; Agoston, P.; Hong, A.; Mervoyer, A.; Rastrelli, M. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act. In. Sarc): A multicentre, phase 2–3, randomised, controlled trial. Lancet Oncol. 2019, 20, 1148–1159. [Google Scholar] [CrossRef]
- Baronzio, G.F.; Hager, E.D. Hyperthermia in Cancer Treatment: A Primer; Springer Science & Business Media: Nova Iorque, NY, USA, 2006; p. 366. [Google Scholar] [CrossRef]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Horsman, M.R.; Overgaard, J. Hyperthermia: A potent enhancer of radiotherapy. Clin. Oncol. 2007, 19, 418–426. [Google Scholar] [CrossRef]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef]
- Horsman, M.R.; Siemann, D.W. Pathophysiologic effects of vascular-targeting agents and the implications for combination with conventional therapies. Cancer Res. 2006, 66, 11520–11539. [Google Scholar] [CrossRef] [Green Version]
- Hervault, A.; Thanh, N.T.K. Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nanoscale 2014, 6, 11553–11573. [Google Scholar] [CrossRef] [Green Version]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef]
- El-Sayed, I.H. Nanotechnology in head and neck cancer: The race is on. Curr. Oncol. Rep. 2010, 12, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Landon, C.D.; Park, J.Y.; Needham, D.; Dewhirst, M.W. Nanoscale drug delivery and hyperthermia: The materials design and preclinical and clinical testing of low temperature-sensitive liposomes used in combination with mild hyperthermia in the treatment of local cancer. Open Nanomed. J. 2011, 3, 38–64. [Google Scholar] [CrossRef]
- Bubnovskaya, L.; Belous, A.; Solopan, S.; Kovelskaya, A.; Bovkun, L.; Podoltsev, A.; Kondtratenko, I.; Osinsky, S. Magnetic fluid hyperthermia of rodent tumors using manganese perovskite nanoparticles. J. Nanopart. 2014, 2014, 278761. [Google Scholar] [CrossRef]
- Armour, M.; Hedayati, M.; Zhang, Y.; Theodore, L. Magnetic nanoparticle hyperthermia enhances radiation therapy: A study in mouse models of human prostate cancer. Int. J. Hyperth. 2015, 31, 359–374. [Google Scholar] [CrossRef]
- Venugopal, I.; Pernal, S.; Duproz, A.; Bentley, J.; Engelhard, H.; Linninger, A. Magnetic field-enhanced cellular uptake of doxorubicin loaded magnetic nanoparticles for tumor treatment. Mater. Res. Express 2016, 3, 095010. [Google Scholar] [CrossRef]
- Brusentsov, N.A. Magnetic fluid hyperthermia of the mouse experimental tumor. J. Magn. Magn. Mater. 2002, 252, 378–380. [Google Scholar] [CrossRef]
- Singh, A.; Sahoo, S.K. Magnetic nanoparticles: A novel platform for cancer theranostics. Drug Discov. Today 2014, 19, 474–481. [Google Scholar] [CrossRef]
- Kozissnik, B.; Bohorquez, A.C.; Dobson, J.; Rinaldi, C. Magnetic fluid hyperthermia: Advances, challenges, and opportunity. Int. J. Hyperth. 2013, 29, 706–714. [Google Scholar] [CrossRef]
- LeBrun, A.; Zhu, L. Magnetic nanoparticle hyperthermia in cancer treatment: History, mechanism, imaging-assisted protocol design, and challenges. Theory Appl. Heat Transf. Hum. 2018, 2, 631–667. [Google Scholar] [CrossRef]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characerization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Indira, T. Magnetic nanoparticles: A review. Int. J. Pharm. Sci. Nanotechnol. 2010, 3, 1035–1042. [Google Scholar]
- Saldívar-Ramírez, M.M.G.; Sánchez-Torres, C.G.; Cortés-Hernández, D.A.; Escobedo-Bocardo, J.C.; Almanza-Robles, J.M.; Larson, A.; Reséndiz-Hernández, P.J.; Acuña-Gutiérrez, I.O. Study on the efficiency of nanosized magnetite and mixed ferrites in magnetic hyperthermia. J. Mater. Sci. Mater. Med. 2014, 25, 2229–2236. [Google Scholar] [CrossRef]
- Legge, C.J.; Colley, H.E.; Lawson, M.A.; Rawlings, A.E. Targeted magnetic nanoparticle hyperthermia for the treatment of oral cancer. J. Oral Pathol. Med. 2019, 48, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Magforce—The Nanomedicine Company. Fighting Cancer with Nanotherm Therapy. 2019. Available online: https://www.magforce.com/fileadmin/user_upload/210812_E_AGM_Presentation_final.pdf (accessed on 11 January 2022).
- Lammers, T.; Aime, S.; Hennink, W.E.; Storm, G.; Kiessling, F. Theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lovell, J.F. Advanced functional nanomaterials for theranostics. Adv. Funct. Mater. 2017, 27, 1603524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
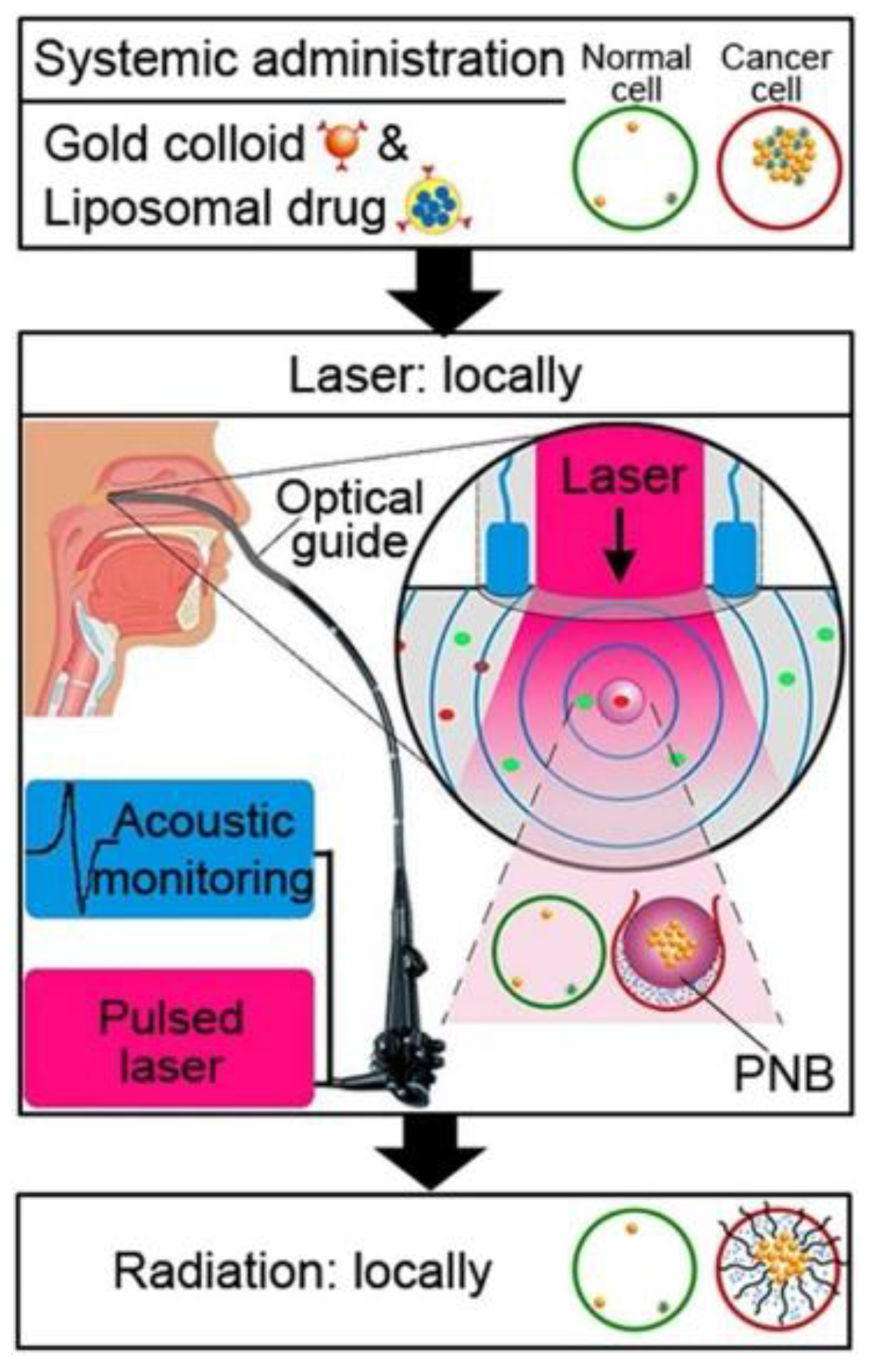
- Lukianova–Hleb, E.Y.; Lapotko, D.O. Rapid detection and destruction of squamous cell carcinoma of the head and neck by nano-quadrapeutics. Head Neck 2015, 37, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Muhanna, N.; Jin, C.S.; Huynh, E.; Chan, H.; Qiu, Y.; Jiang, W.; Cui, L.; Burgess, L.; Akens, M.K.; Chen, J.; et al. Phototheranostic porphyrin nanoparticles enable visualization and targeted treatment of head and neck cancer in clinically relevant models. Theranostics 2015, 5, 1428–1443. [Google Scholar] [CrossRef] [Green Version]
- Davidi, E.S.; Dreifuss, T.; Motiei, M.; Shai, E.; Bragilovski, D.; Lubimov, L.; Kindler, M.J.J.; Popovtzer, A.; Don, J.; Popovtzer, R. Cisplatin-conjugated gold nanoparticles as a theranostic agent for head and neck cancer. Head Neck 2018, 40, 70–78. [Google Scholar] [CrossRef]
- Li, R.; Gao, R.; Wang, Y.; Liu, Z.; Xu, H.; Duan, A.; Zhang, F.; Ma, L. Gastrin releasing peptide receptor targeted nano-graphene oxide for near-infrared fluorescence imaging of oral squamous cell carcinoma. Sci. Rep. 2020, 10, 11434. [Google Scholar] [CrossRef]
- Hensley, D.; Tay, Z.W.; Dhavalikar, R.; Goodwill, P.; Zheng, B.; Rinaldi, C.; Conolly, S. A theranostic platform for localized magnetic fluid hyperthermia and magnetic particle imaging. Energy-Based Treat. Tissue Assess. IX 2017, 10066, 10–17. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Wei, A.; Mehtala, J.G.; Patri, A.K. Challenges and opportunities in the advancement of nanomedicines. J. Control. Release 2012, 164, 236–246. [Google Scholar] [CrossRef] [Green Version]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- de Lima, J.M.; Bonan, P.R.; da Cruz Perez, D.E.; Hier, M.; Alaoui-Jamali, M.A.; da Silva, S.D. Nanoparticle-based chemotherapy formulations for head and neck cancer: A systematic review and perspectives. Nanomaterials 2020, 10, 1938. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhou, S.; Gao, W. What Went Wrong with Anticancer Nanomedicine Design and How to Make It Right. ACS Nano 2020, 14, 12281–12290. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H.; Brechbiel, M.W. Dendrimer-based macromolecular MRI contrast agents: Characteristics and application. Mol. Imaging 2003, 2, 15353500200303100. [Google Scholar] [CrossRef]
- Cho, W.-S.; Cho, M.; Jeong, J.; Choi, M.; Han, B.S.; Shin, H.-S.; Hong, J.; Chung, B.H.; Jeong, J.; Cho, M.-H. Size-dependent tissue kinetics of PEG-coated gold nanoparticles. Toxicol. Appl. Pharmacol. 2010, 245, 116–123. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Chan, W.C.W. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007, 7, 1542–1550. [Google Scholar] [CrossRef]
- Staal, A.H.J.; Becker, K.; Tagit, O.; Koen van Riessen, N.; Koshkina, O.; Veltien, A.; Bouvain, P.; Cortenbach, K.R.G.; Scheenen, T.; Flögel, U.; et al. In vivo clearance of (19)F MRI imaging nanocarriers is strongly influenced by nanoparticle ultrastructure. Biomaterials 2020, 261, 120307. [Google Scholar] [CrossRef]
- Dong, L.; Sun, L.; Li, W.; Jiang, Y.; Zhan, Y.; Yu, L.; Chen, Y.; Hong, G. Degradable and excretable ultrasmall transition metal selenide nanodots for high-performance computed tomography bioimaging-guided photonic tumor nanomedicine in NIR-II biowindow. Adv. Funct. Mater. 2021, 31, 2008591. [Google Scholar] [CrossRef]
- Torresan, V.; Forrer, D.; Guadagnini, A.; Badocco, D.; Pastore, P.; Casarin, M.; Selloni, A.; Coral, D.; Ceolin, M.; Fernández van Raap, M.B.; et al. 4D multimodal nanomedicines made of nonequilibrium Au-Fe alloy nanoparticles. ACS Nano 2020, 14, 12840–12853. [Google Scholar] [CrossRef]
| Stage | T | N | M |
|---|---|---|---|
| 0 | Defined shape | No invasion | No distant metastasis |
| I | Defined shape, less than 2 cm Does not invade the submucosa | No invasion | No distant metastasis |
| II | Between 2 and 4 cm Initial invasion of the submucosa | No invasion | No distant metastasis |
| III | Cancer cells rapidly divide Tumors with more than 4 cm | Invasion | No distant metastasis |
| IV | Cancer cells enter the bloodstream | Invasion | Distant metastasis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viegas, C.; Pereira, D.S.M.; Fonte, P. Insights into Nanomedicine for Head and Neck Cancer Diagnosis and Treatment. Materials 2022, 15, 2086. https://doi.org/10.3390/ma15062086
Viegas C, Pereira DSM, Fonte P. Insights into Nanomedicine for Head and Neck Cancer Diagnosis and Treatment. Materials. 2022; 15(6):2086. https://doi.org/10.3390/ma15062086
Chicago/Turabian StyleViegas, Cláudia, Daniela S. M. Pereira, and Pedro Fonte. 2022. "Insights into Nanomedicine for Head and Neck Cancer Diagnosis and Treatment" Materials 15, no. 6: 2086. https://doi.org/10.3390/ma15062086
APA StyleViegas, C., Pereira, D. S. M., & Fonte, P. (2022). Insights into Nanomedicine for Head and Neck Cancer Diagnosis and Treatment. Materials, 15(6), 2086. https://doi.org/10.3390/ma15062086