Development and Characterizations of Pullulan and Maltodextrin-Based Oral Fast-Dissolving Films Employing a Box–Behnken Experimental Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ZMT-OFDFs
2.3. Optimization of ZMT-OFDFs
2.4. Thickness, Weight Uniformity, and Folding Endurance
2.5. Water Content, Moisture Absorption, and Surface pH
2.6. Disintegration Time and Drug Content
2.7. Drug Dissolution
2.8. Mechanical Characterization
2.9. Compatibility Study of Optimized Formulation
2.10. Surface Morphology
2.11. Pharmacokinetics Study
2.12. Statistical Data Analysis
3. Results and Discussion
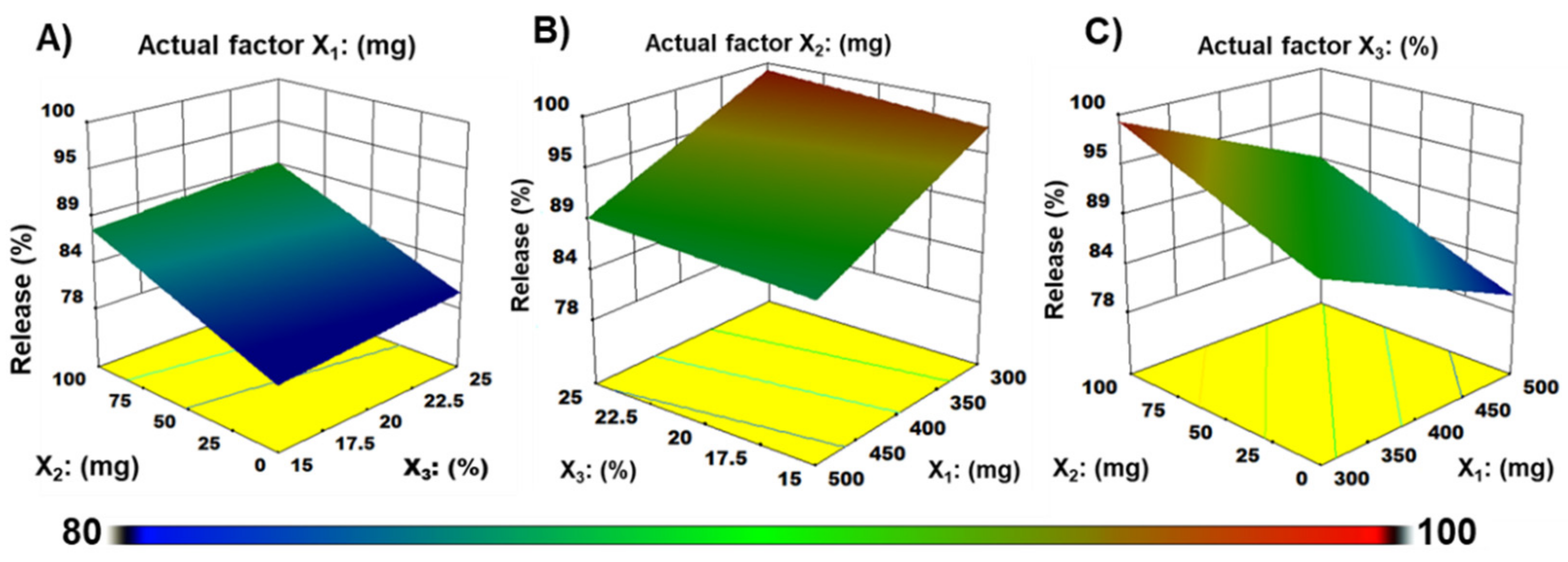
3.1. Formulation Optimization
3.2. Feasibility and Appearance of OFDFs
3.3. Thickness and Weight Uniformity
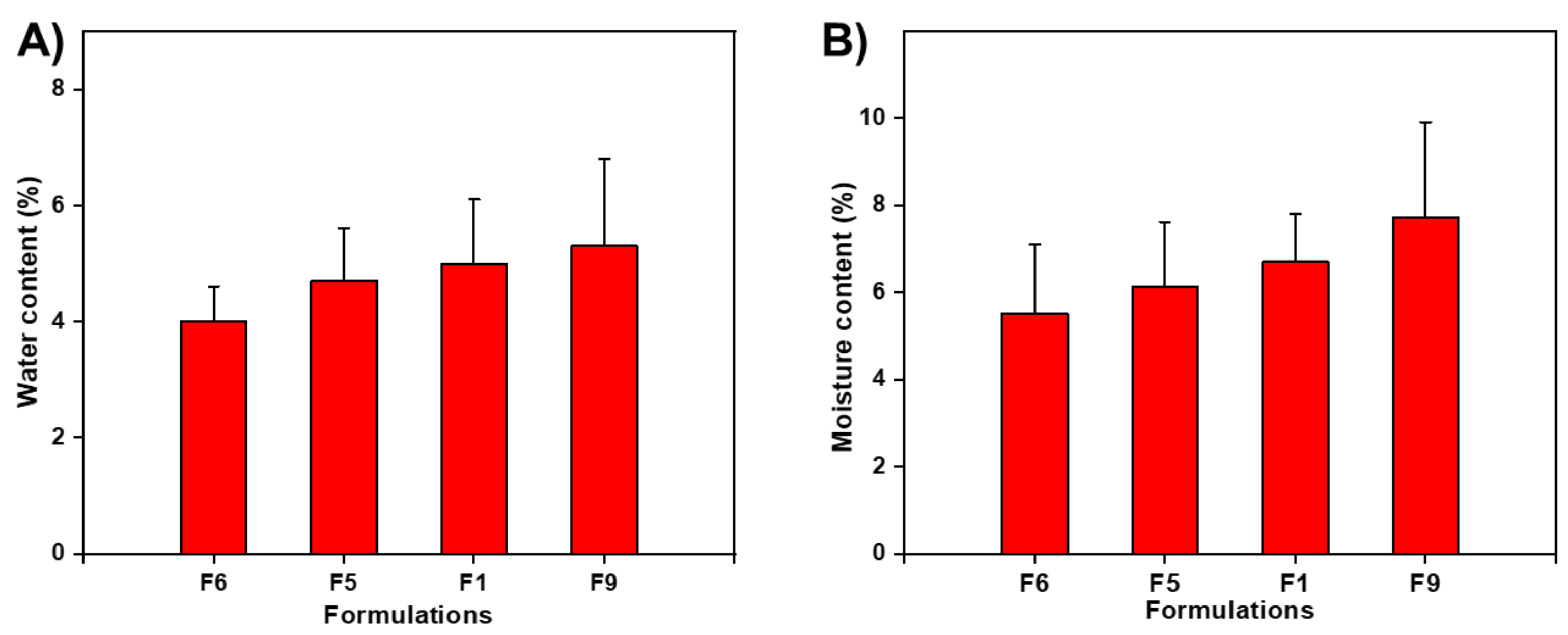
3.4. Water Content (%) and Moisture Uptake (%)
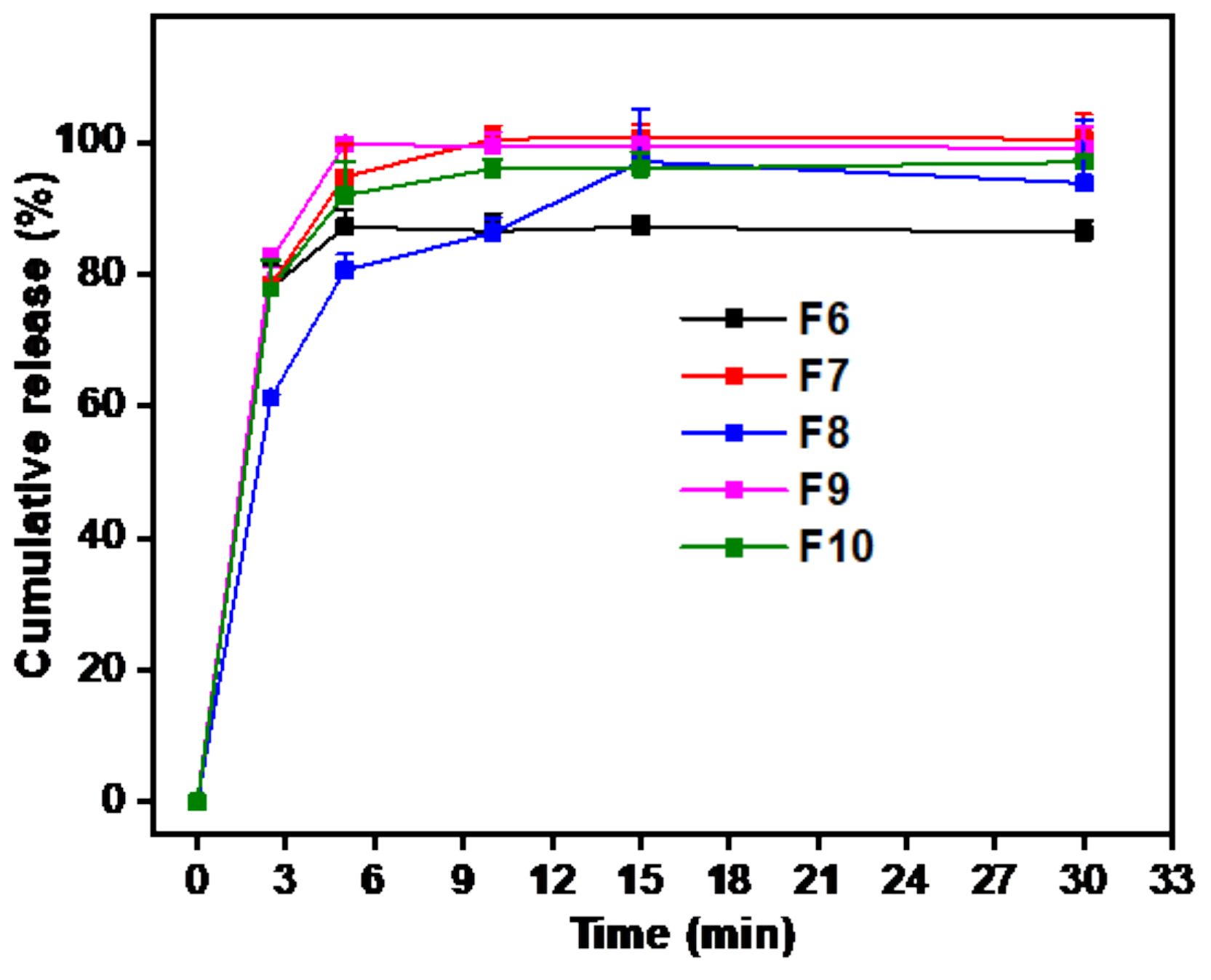
3.5. In Vitro Drug Release Study
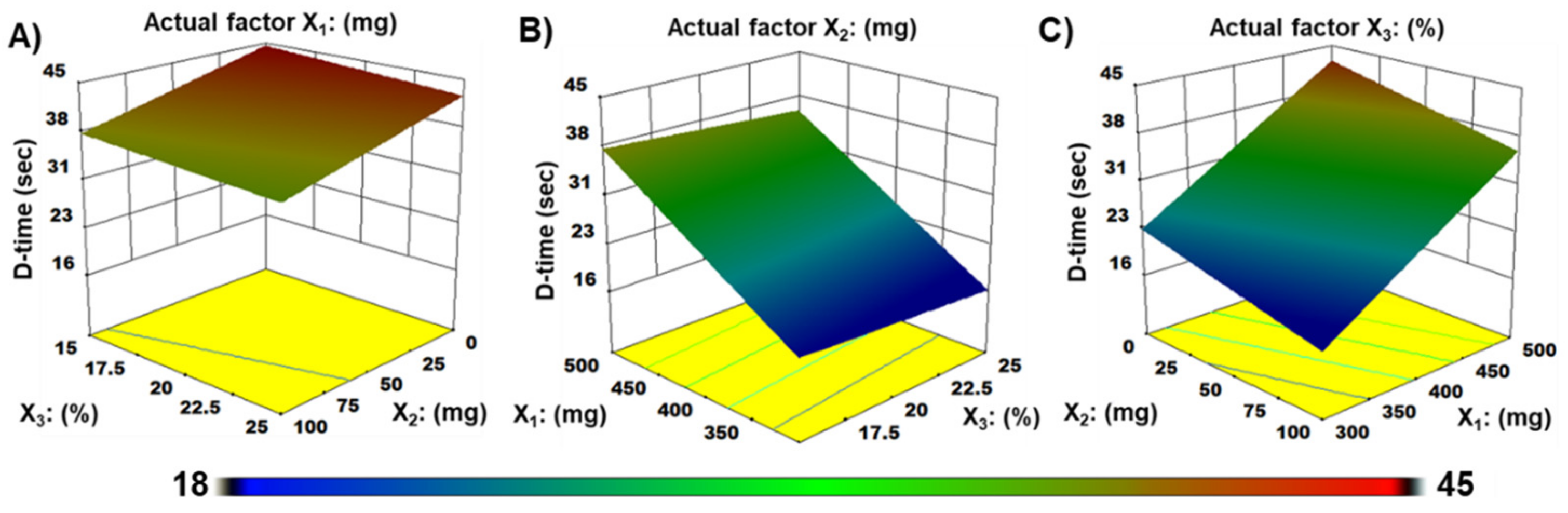
3.6. Disintegration Time
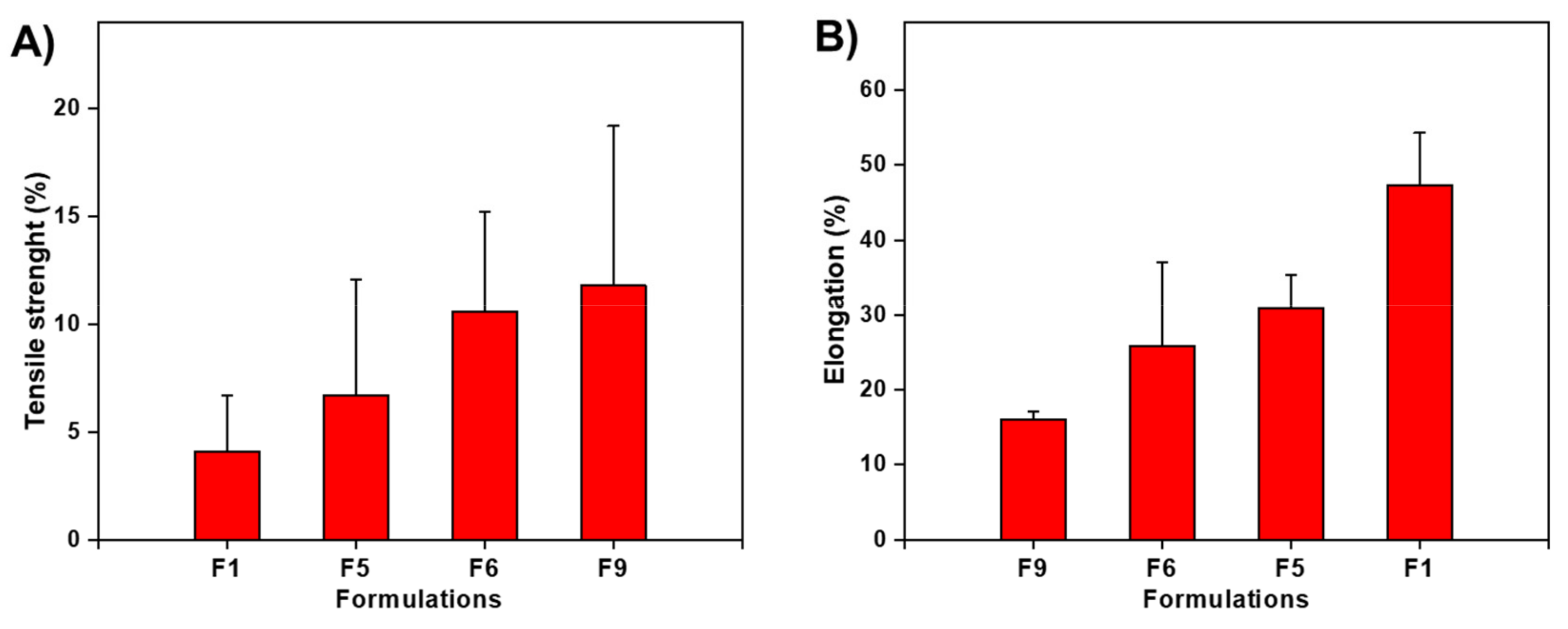
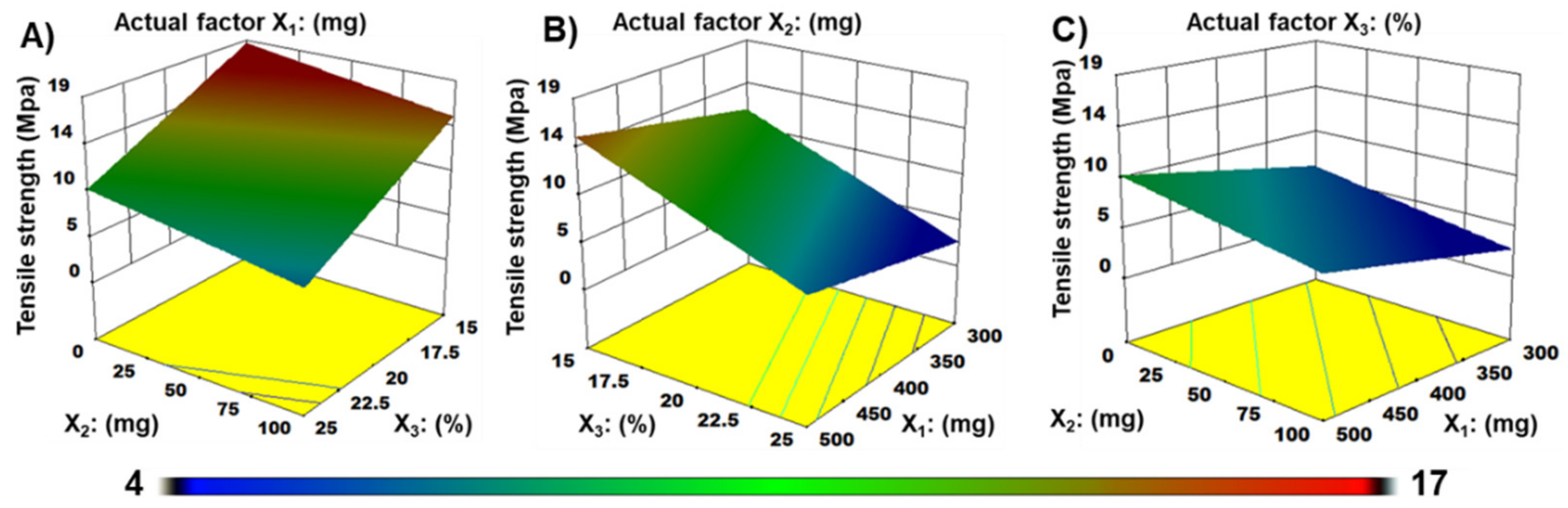
3.7. Mechanical Properties Analysis
3.8. Validation of Optimum Formulation
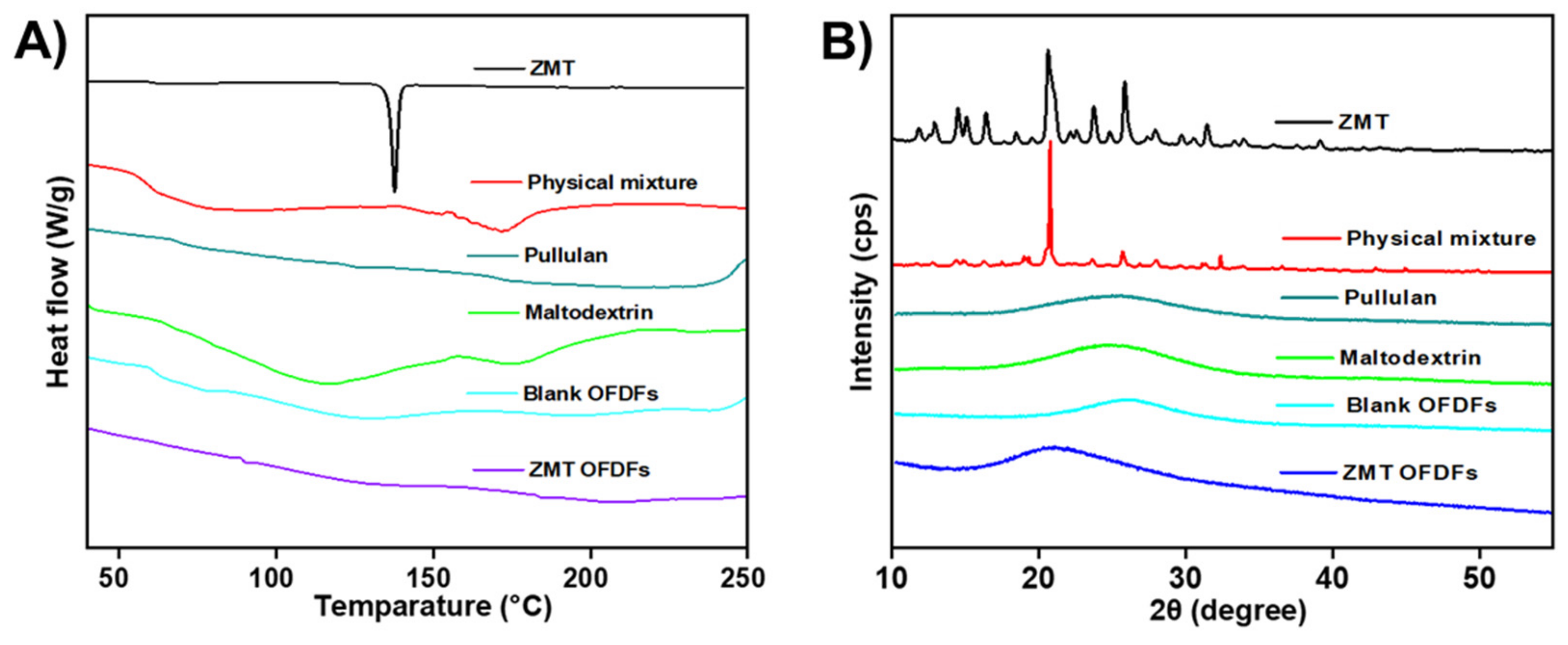
3.9. Compatibility Analysis
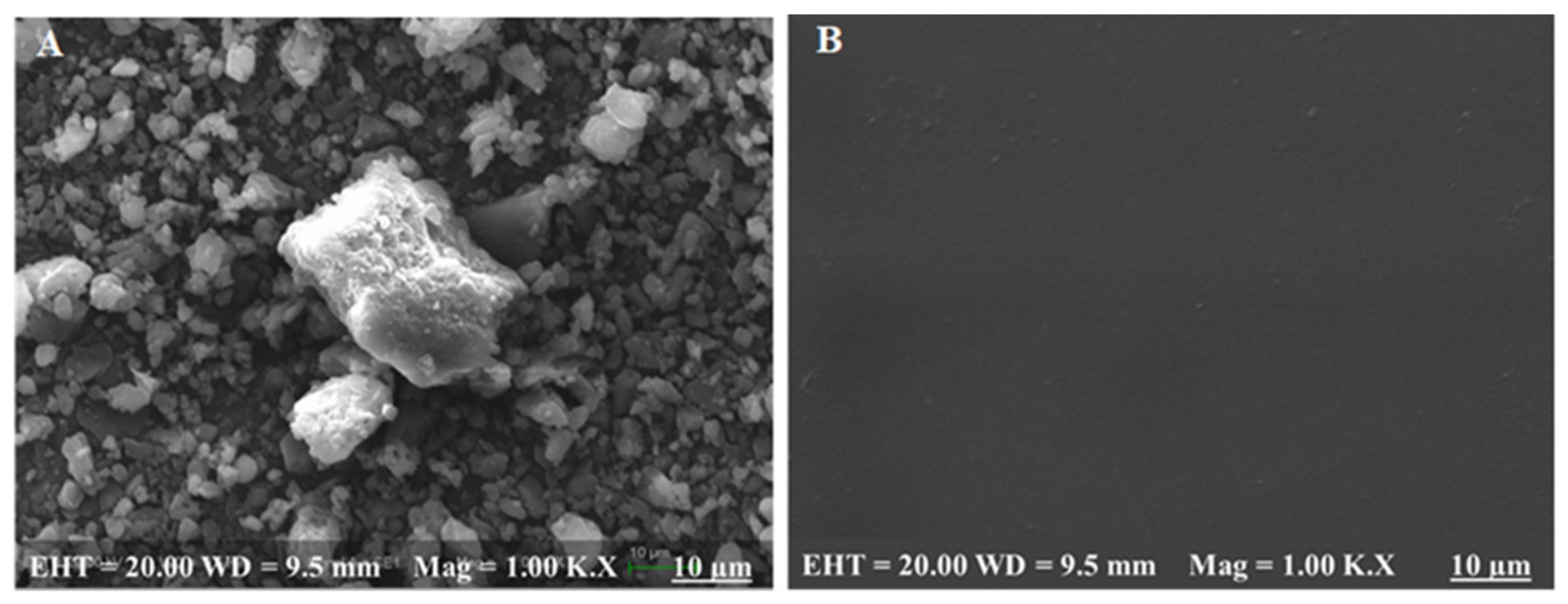
3.10. Surface Morphology Analysis
3.11. Pharmacokinetics Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stovner, L.; Hagen, K.; Jensen, R.; Katsarava, Z.; Lipton, R.; Scher, A.; Steiner, T.; Zwart, J. The global burden of headache: A documentation of headache prevalence and disability worldwide. Cephalalgia 2007, 27, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Shelke, S.; Shahi, S.; Jalalpure, S.; Dhamecha, D.; Shengule, S. Formulation and evaluation of thermoreversible mucoadhesive in-situ gel for intranasal delivery of naratriptan hydrochloride. J. Drug Deliv. Sci. Technol. 2015, 29, 238–244. [Google Scholar] [CrossRef]
- Galletti, F.; Cupini, L.M.; Corbelli, I.; Calabresi, P.; Sarchielli, P. Pathophysiological basis of migraine prophylaxis. Prog. Neurobiol. 2009, 89, 176–192. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, F.; Tian, Y.; Li, W.; Mao, G. Quantification of zolmitriptan in plasma by high-performance liquid chromatography–electrospray ionization mass spectrometry. J. Chromatogr. B 2004, 813, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Khezri, F.A.N.Z.; Lakshmi, C.; Bukka, R.; Nidhi, M.; Nargund, S.L. Pharmacokinetic study and brain tissue analysis of Zolmitriptan loaded chitosan nanoparticles in rats by LC-MS method. Int. J. Biol. Macromol. 2020, 142, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Alhalaweh, A.; Andersson, S.; Velaga, S.P. Preparation of zolmitriptan–chitosan microparticles by spray drying for nasal delivery. Eur. J. Pharm. Sci. 2009, 38, 206–214. [Google Scholar] [PubMed]
- Wu, D.; Tanaka, Y.; Jin, Y.; Yoneto, K.; Alama, T.; Quan, Y.; Kamiyama, F.; Kusamori, K.; Katsumi, H.; Sakane, T. Development of a novel transdermal patch containing sumatriptan succinate for the treatment of migraine: In vitro and in vivo characterization. J. Drug Deliv. Sci. Technol. 2014, 24, 695–701. [Google Scholar]
- Bharti, K.; Mittal, P.; Mishra, B. Formulation and characterization of fast dissolving oral films containing buspirone hydrochloride nanoparticles using design of experiment. J. Drug Deliv. Sci. Technol. 2019, 49, 420–432. [Google Scholar] [CrossRef]
- Song, Q.; Shen, C.; Shen, B.; Lian, W.; Liu, X.; Dai, B.; Yuan, H. Development of a fast dissolving sublingual film containing meloxicam nanocrystals for enhanced dissolution and earlier absorption. J. Drug Deliv. Sci. Technol. 2018, 43, 243–252. [Google Scholar] [CrossRef]
- Satyanarayana, D.A.; Keshavarao, K.P. Fast disintegrating films containing anastrozole as a dosage form for dysphagia patients. Arch. Pharmacal Res. 2012, 35, 2171–2182. [Google Scholar]
- Xu, L.-L.; Shi, L.-L.; Cao, Q.-R.; Xu, W.-J.; Cao, Y.; Zhu, X.-Y.; Cui, J.-H. Formulation and in vitro characterization of novel sildenafil citrate-loaded polyvinyl alcohol-polyethylene glycol graft copolymer-based orally dissolving films. Int. J. Pharm. 2014, 473, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Selmin, F.; Baldassari, S.; Gennari, C.; Caviglioli, G.; Cilurzo, F.; Minghetti, P.; Parodi, B. A focus on mucoadhesive polymers and their application in buccal dosage forms. J. Drug Deliv. Sci. Technol. 2016, 32, 113–125. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, V.; Pathak, K. Pharmaceutical evaluation and dynamic vapor sorption studies of fast dissolving intraoral films of Loratadine. Pharm. Dev. Technol. 2013, 18, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, V.D.; Chaudhari, A.M.; Gandhi, A.K.; Maheriya, P. Pullulan based oral thin film formulation of zolmitriptan: Development and optimization using factorial design. Int. J. Biol. Macromol. 2018, 107, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Cilurzo, F.; Cupone, I.E.; Minghetti, P.; Selmin, F.; Montanari, L. Fast dissolving films made of maltodextrins. Eur. J. Pharm. Biopharm. 2008, 70, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.L.; Fang, Y.; Han, H.; Li, Q.; Zhang, S.; Li, H.Y.; Chow, S.F.; Lam, T.N.; Lee, W.Y.T. Orally-dissolving film for sublingual and buccal delivery of ropinirole. Colloids Surf. B Biointerfaces 2018, 163, 9–18. [Google Scholar] [CrossRef]
- Milcovich, G.; Antunes, F.E.; Grassi, M.; Asaro, F. Stabilization of unilamellar catanionic vesicles induced by β-cyclodextrins: A strategy for a tunable drug delivery depot. Int. J. Pharm. 2018, 548, 474–479. [Google Scholar] [CrossRef]
- Reina, G.; González-Domínguez, J.M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416. [Google Scholar] [CrossRef] [Green Version]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules 2018, 23, 47. [Google Scholar] [CrossRef] [Green Version]
- Tong, Q.; Xiao, Q.; Lim, L.-T. Preparation and properties of pullulan–alginate–carboxymethylcellulose blend films. Food Res. Int. 2008, 41, 1007–1014. [Google Scholar] [CrossRef]
- Shibata, M.; Nozawa, R.; Teramoto, N.; Yosomiya, R. Synthesis and properties of etherified pullulans. Eur. Polym. J. 2002, 38, 497–501. [Google Scholar] [CrossRef]
- Rezaee, F.; Ganji, F. Formulation, characterization, and optimization of captopril fast-dissolving oral films. AAPS PharmSciTech 2018, 19, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Choi, Y.-G.; Kim, S.R.B.; Lim, S.-T. Humidity stability of tapioca starch–pullulan composite films. Food Hydrocoll. 2014, 41, 140–145. [Google Scholar] [CrossRef]
- Iqbal, H.; Khan, B.A.; Khan, Z.U.; Razzaq, A.; Khan, N.U.; Menaa, B.; Menaa, F. Fabrication, physical characterizations and in vitro antibacterial activity of cefadroxil-loaded chitosan/poly(vinyl alcohol) nanofibers against Staphylococcus aureus clinical isolates. Int. J. Biol. Macromol. 2020, 144, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.-Y.; Jia, X.-W.; Liu, Q.; Kong, B.-H.; Wang, H. Fast dissolving oral films for drug delivery prepared from chitosan/pullulan electrospinning nanofibers. Int. J. Biol. Macromol. 2019, 137, 224–231. [Google Scholar] [CrossRef]
- Cilurzo, F.; Cupone, I.E.; Minghetti, P.; Buratti, S.; Selmin, F.; Gennari, C.G.; Montanari, L. Nicotine fast dissolving films made of maltodextrins: A feasibility study. Aaps Pharmscitech 2010, 11, 1511–1517. [Google Scholar] [CrossRef] [Green Version]
- Elshafeey, A.H.; El-Dahmy, R.M. Formulation and Development of Oral Fast-Dissolving Films Loaded with Nanosuspension to Augment Paroxetine Bioavailability: In Vitro Characterization, Ex Vivo Permeation, and Pharmacokinetic Evaluation in Healthy Human Volunteers. Pharmaceutics 2021, 13, 1869. [Google Scholar] [CrossRef]
- Mashru, R.; Sutariya, V.; Sankalia, M.; Parikh, P. Development and evaluation of fast-dissolving film of salbutamol sulphate. Drug Dev. Ind. Pharm. 2005, 31, 25–34. [Google Scholar] [CrossRef]
- Göbel, A.; Breitkreutz, J. Concept of Orodispersible or Mucoadhesive “Tandem Films” and Their Pharmaceutical Realization. Pharmaceutics 2022, 14, 264. [Google Scholar] [CrossRef]
- Sha, H.; Yuan, C.; Cui, B.; Zhao, M.; Wang, J. Pre-gelatinized cassava starch orally disintegrating films: Influence of β-Cyclodextrin. Food Hydrocoll. 2022, 123, 107196. [Google Scholar] [CrossRef]
- Khan, Z.U.; Razzaq, A.; Khan, A.; Rehman, N.U.; Khan, H.; Khan, T.; Khan, A.U.; Althobaiti, N.A.; Menaa, F.; Iqbal, H.; et al. Physicochemical Characterizations and Pharmacokinetic Evaluation of Pentazocine Solid Lipid Nanoparticles against Inflammatory Pain Model. Pharmaceutics 2022, 14, 409. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, W.-W.; Shen, P.; Cao, Q.-R.; Lee, B.-J.; Cui, J.-H. pH-dependent release of platycodin mitigates its gastrointestinal mucosa irritation after oral administration in rats. Arch. Pharmacal Res. 2016, 39, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, H.; Gauri, S.; Rathee, P.; Kumar, V. Development and optimization of fast dissolving oro-dispersible films of granisetron HCl using Box–Behnken statistical design. Bull. Fac. Pharm. Cairo Univ. 2013, 51, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Esim, O.; Ozkan, C.K.; Kurbanoglu, S.; Arslan, A.; Tas, C.; Savaser, A.; Ozkan, S.A.; Ozkan, Y. Development and in vitro/in vivo evaluation of dihydroergotamine mesylate loaded maltodextrin-pullulan sublingual films. Drug Dev. Ind. Pharm. 2019, 45, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kamboj, S.; Singh, G.; Rana, V. Development of aprepitant loaded orally disintegrating films for enhanced pharmacokinetic performance. Eur. J. Pharm. Sci. 2016, 84, 55–69. [Google Scholar] [CrossRef] [PubMed]
- ElMeshad, A.N.; El Hagrasy, A.S. Characterization and optimization of orodispersible mosapride film formulations. Aaps Pharmscitech 2011, 12, 1384–1392. [Google Scholar] [CrossRef] [Green Version]
- Jantrawut, P.; Chaiwarit, T.; Jantanasakulwong, K.; Brachais, C.H.; Chambin, O. Effect of plasticizer type on tensile property and in vitro indomethacin release of thin films based on low-methoxyl pectin. Polymers 2017, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- Galus, S.; Mathieu, H.; Lenart, A.; Debeaufort, F. Effect of modified starch or maltodextrin incorporation on the barrier and mechanical properties, moisture sensitivity and appearance of soy protein isolate-based edible films. Innov. Food Sci. Emerg. Technol. 2012, 16, 148–154. [Google Scholar] [CrossRef]
- Yan, J.J.; Li, Z.; Zhang, J.F.; Qiao, C.S. Preparation and Properties of Pullulan Composite Films. In Advanced Materials Research; Trans Tech Publications Ltd.: Zurich, Switzerland, 2012; pp. 2100–2104. [Google Scholar]
- Palem, C.R.; Gannu, R.; Doodipala, N.; Yamsani, V.V.; Yamsani, M.R. Transmucosal delivery of domperidone from bilayered buccal patches: In vitro, ex vivo and in vivo characterization. Arch. Pharmacal Res. 2011, 34, 1701–1710. [Google Scholar] [CrossRef]
- Zhang, L.; Aloia, M.; Pielecha-Safira, B.; Lin, H.; Rajai, P.M.; Kunnath, K.; Davé, R.N. Impact of superdisintegrants and film thickness on disintegration time of strip films loaded with poorly water-soluble drug microparticles. J. Pharm. Sci. 2018, 107, 2107–2118. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, K.; Kim, M.; Choi, D.H.; Jeong, S.H. Orally disintegrating films focusing on formulation, manufacturing process, and characterization. J. Pharm. Investig. 2017, 47, 183–201. [Google Scholar] [CrossRef]
- Beigomi, M.; Mohsenzadeh, M.; Salari, A. Characterization of a novel biodegradable edible film obtained from Dracocephalum moldavica seed mucilage. Int. J. Biol. Macromol. 2018, 108, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Bhagawati, S.T.; Chonkar, A.D.; Dengale, S.J.; Reddy, S.M.; Bhat, K. Bioavailability enhancement of rizatriptan benzoate by oral disintegrating strip: In vitro and In vivo evaluation. Curr. Drug Deliv. 2016, 13, 462–470. [Google Scholar] [CrossRef]
- Singh, H.; Singla, Y.P.; Narang, R.S.; Pandita, D.; Singh, S.; Narang, J.K. Frovatriptan loaded hydroxy propyl methyl cellulose/treated chitosan based composite fast dissolving sublingual films for management of migraine. J. Drug Deliv. Sci. Technol. 2018, 47, 230–239. [Google Scholar] [CrossRef]

| Film Code | Pullulan (mg, X1) | Maltodextrin (mg, X2) | Propylene Glycol (%, X3) | Release at 5 min (Y1, %) | D-Time (Y2, s) | Tensile Strength (Y3, MPa) |
|---|---|---|---|---|---|---|
| F1 | 300 | 50 | 25 | 94.3 ± 1.7 | 20.5 ± 1.9 | 4.1 ± 2.6 |
| F2 | 400 | 50 | 20 | 90.3 ± 1.5 | 28.2 ± 3.7 | 10.4 ± 2.0 |
| F3 | 400 | 0 | 25 | 85.2 ± 4.2 | 31.7 ± 2.7 | 8.6 ± 8.1 |
| F4 | 500 | 50 | 25 | 82.9 ± 1.3 | 40.2 ± 3.2 | 7.6 ± 3.5 |
| F5 | 300 | 50 | 15 | 92.0 ± 5.2 | 22.2 ± 2.3 | 11.8 ± 7.4 |
| F6 | 300 | 0 | 20 | 87.2 ± 2.5 | 24.7 ± 1.4 | 10.6 ± 4.6 |
| F7 | 400 | 100 | 25 | 94.8 ± 4.8 | 25.2 ± 3.5 | 5.0 ± 4.0 |
| F8 | 500 | 0 | 20 | 80.6 ± 2.4 | 44.5 ± 3.5 | 14.6 ± 2.1 |
| F9 | 300 | 100 | 20 | 99.8 ± 0.5 | 18.3 ± 1.9 | 6.7 ± 5.4 |
| F10 | 400 | 100 | 15 | 92.0 ± 5.2 | 27.2 ± 3.1 | 15.7 ± 0.4 |
| F11 | 500 | 100 | 20 | 87.9 ± 3.0 | 36.2 ± 3.7 | 9.9 ± 6.5 |
| F12 | 500 | 50 | 15 | 82.2 ± 1.0 | 42.2 ± 5.9 | 16.4 ± 1.1 |
| F13 | 400 | 0 | 15 | 84.0 ± 1.2 | 33.8 ± 3.1 | 17.1 ± 2.0 |
| Film Code | Thickness (μm) | Elongation (%) | Folding Endurance (Folds) | Water Content (%) | Moisture Uptake (%) |
|---|---|---|---|---|---|
| F1 | 33.9 ± 5.1 | 47.3 ± 7.0 | 129.0 ± 11 | 5.0 ± 1.1 | 6.7 ± 1.6 |
| F2 | 56.8 ± 6.8 | 26.2 ± 0.8 | 100.3 ± 9.5 | 5.5 ± 1.9 | 6.0 ± 1.5 |
| F3 | 40.1 ± 5.2 | 27.7 ± 1.5 | 142.3 ± 16.5 | 5.0 ± 1.4 | 5.6 ± 1.3 |
| F4 | 75.1 ± 6.3 | 39.2 ± 8.4 | 193.7 ± 12.6 | 6.3 ± 2.8 | 5.1 ± 2.1 |
| F5 | 36.7 ± 4.9 | 16.1 ± 1.0 | 76.0 ± 12.5 | 4.7 ± 1.5 | 6.1 ± 1.5 |
| F6 | 25.5 ± 4.3 | 25.8 ± 11.3 | 121.7 ± 8.5 | 4.0 ± 0.6 | 5.5 ± 0.8 |
| F7 | 64.6 ± 6.3 | 53.2 ± 12.4 | 108.3 ± 8.0 | 6.0 ± 2.4 | 6.8 ± 2.6 |
| F8 | 66.4 ± 5.5 | 18.0 ± 0.3 | 178.7 ± 11 | 5.5 ± 2.4 | 6.5 ± 3.0 |
| F9 | 44.1 ± 5.8 | 30.9 ± 4.4 | 109.3 ± 9.5 | 5.3 ± 1.5 | 7.7 ± 2.2 |
| F10 | 62.0 ± 7.4 | 13.9 ± 0.2 | 88.7 ± 12.9 | 6.4 ± 2.6 | 6.4 ± 2.6 |
| F11 | 85.3 ± 6.5 | 24.0 ± 1.1 | 146.3 ± 7.4 | 6.9 ± 3.6 | 5.5 ± 3.0 |
| F12 | 73.2 ± 8.7 | 10.5 ± 3.9 | 103.3 ± 7.8 | 6.0 ± 2.9 | 4.8 ± 2.4 |
| F13 | 44.9 ± 6.1 | 11.8 ± 0.9 | 96.3 ± 9.6 | 4.9 ± 1.4 | 4.6 ± 1.4 |
| Responses | Model | Std. Dev. | R2 | Adjusted R2 | Predicted R2 | Press | Statistical Analysis |
|---|---|---|---|---|---|---|---|
| Drug release at 5 min (Y1) | Linear | 1.33 | 0.960 | 0.946 | 0.918 | 32.55 | * |
| 2FI | 1.13 | 0.981 | 0.961 | 0.920 | 31.43 | ||
| Quadratic | 0.75 | 0.996 | 0.983 | + | |||
| Cubic | + | # |
| Responses | Source | Sum of Squares | d.f. | Mean Square | F Value | p-Value Prob > F | Statistical Analysis |
|---|---|---|---|---|---|---|---|
| Drug release at 5 min (Y1) | Model | 378.9 | 3 | 126.3 | 71.4 | <0.0001 | ** |
| X1 | 197.0 | 1 | 197.0 | 111.3 | <0.0001 | ** | |
| X2 | 175.8 | 1 | 175.8 | 99.3 | <0.0001 | ** | |
| X3 | 6.1 | 1 | 6.1 | 3.5 | 0.0958 | # |
| Responses | Model | Std. Dev. | R2 | Adjusted R2 | Predicted R2 | Press | Statistical Analysis |
|---|---|---|---|---|---|---|---|
| Disintegration (Y2) | Linear | 1.26 | 0.984 | 0.9782 | 0.9695 | 26.42 | * |
| 2FI | 1.49 | 0.985 | 0.9694 | 0.9387 | 53.17 | ||
| Quadratic | 0.53 | 0.999 | 0.9961 | + | * | ||
| Cubic | + | # |
| Responses | Source | Sum of Squares | d.f. | Mean Square | F Value | p-Value Prob > F | Significant/ Non-Significant |
|---|---|---|---|---|---|---|---|
| Disintegration (Y2) | Model | 853.1 | 3 | 284.4 | 180.4 | <0.0001 | ** |
| X1 | 748.8 | 1 | 748.8 | 475.0 | <0.0001 | ||
| X2 | 96.6 | 1 | 96.6 | 61.3 | <0.0001 | ||
| X3 | 7.6 | 1 | 7.6 | 4.8 | <0.0557 |
| Responses | Model | Std. Dev. | R2 | Adjusted R2 | Predicted R2 | Press | Statistical Analysis |
|---|---|---|---|---|---|---|---|
| Tensile strength (Y3) | Linear | 1.05 | 0.955 | 0.9401 | 0.90 | 21.92 | ** |
| 2FI | 1.18 | 0.963 | 0.9252 | 0.79 | 46.01 | ||
| Quadratic | 0.94 | 0.988 | 0.9525 | + | |||
| Cubic | + | # |
| Responses | Source | Sum of Squares | d.f. | Mean Square | F Value | p-Value Prob > F | Significant/ Non-Significant |
|---|---|---|---|---|---|---|---|
| Tensile strength (Y3) | Model | 211.7 | 3 | 70.6 | 63.8 | <0.0001 | ** |
| X1 | 29.3 | 1 | 29.3 | 26.4 | <0.0006 | ||
| X2 | 23.1 | 1 | 23.1 | 20.9 | <0.0013 | ||
| X3 | 159.3 | 1 | 159.3 | 144.0 | <0.0001 |
| PU:MDX:PG = X1:X2 (mg):X3 (%) | Response Variables | Actual Values | Predicted Values | Relative Error (%) |
|---|---|---|---|---|
| Y1 (Release) | 99.8 | 98.4 | −1.5 | |
| PU:MDX:PG 300 mg:100 mg:20% | Y2 (D-time) | 18.3 | 17.2 | −6.2 |
| Y3 (Tensile strength) | 6.7 | 7.0 | 4.8 |
| No. | PK-Parameters | ZMT-OFDFs | ZMT-ig Suspension |
|---|---|---|---|
| 1 | Cmax (μg·mL−1) | 2.44 ± 0.34 | 1.56 ± 0.37 |
| 2 | Tmax (h) | 0.5 | 0.5 |
| 3 | AUC (0–t) (ng·h·mL−1) | 5.89 ± 0.94 | 2.82 ± 1.02 |
| 4 | MRT (h) | 2.9 ± 0.7 | 2.3 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, K.A.; Gao, B.; Kamal, R.; Razzaq, A.; Qi, S.; Zhu, Q.-N.; Lina, S.; Huang, L.; Cremin, G.; Iqbal, H.; et al. Development and Characterizations of Pullulan and Maltodextrin-Based Oral Fast-Dissolving Films Employing a Box–Behnken Experimental Design. Materials 2022, 15, 3591. https://doi.org/10.3390/ma15103591
Shah KA, Gao B, Kamal R, Razzaq A, Qi S, Zhu Q-N, Lina S, Huang L, Cremin G, Iqbal H, et al. Development and Characterizations of Pullulan and Maltodextrin-Based Oral Fast-Dissolving Films Employing a Box–Behnken Experimental Design. Materials. 2022; 15(10):3591. https://doi.org/10.3390/ma15103591
Chicago/Turabian StyleShah, Kiramat Ali, Binbin Gao, Robia Kamal, Anam Razzaq, Sun Qi, Qiu-Ning Zhu, Song Lina, Linyu Huang, Grainne Cremin, Haroon Iqbal, and et al. 2022. "Development and Characterizations of Pullulan and Maltodextrin-Based Oral Fast-Dissolving Films Employing a Box–Behnken Experimental Design" Materials 15, no. 10: 3591. https://doi.org/10.3390/ma15103591
APA StyleShah, K. A., Gao, B., Kamal, R., Razzaq, A., Qi, S., Zhu, Q.-N., Lina, S., Huang, L., Cremin, G., Iqbal, H., Menaa, F., & Cui, J.-H. (2022). Development and Characterizations of Pullulan and Maltodextrin-Based Oral Fast-Dissolving Films Employing a Box–Behnken Experimental Design. Materials, 15(10), 3591. https://doi.org/10.3390/ma15103591







