Corrosion Resistance of Novel Fly Ash-Based Forsterite-Spinel Refractory Ceramics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparation
2.3. Characterization
2.4. Testing of Corrosion Resistance of Forsterite–Spinel Ceramic Samples
3. Results and Discussion
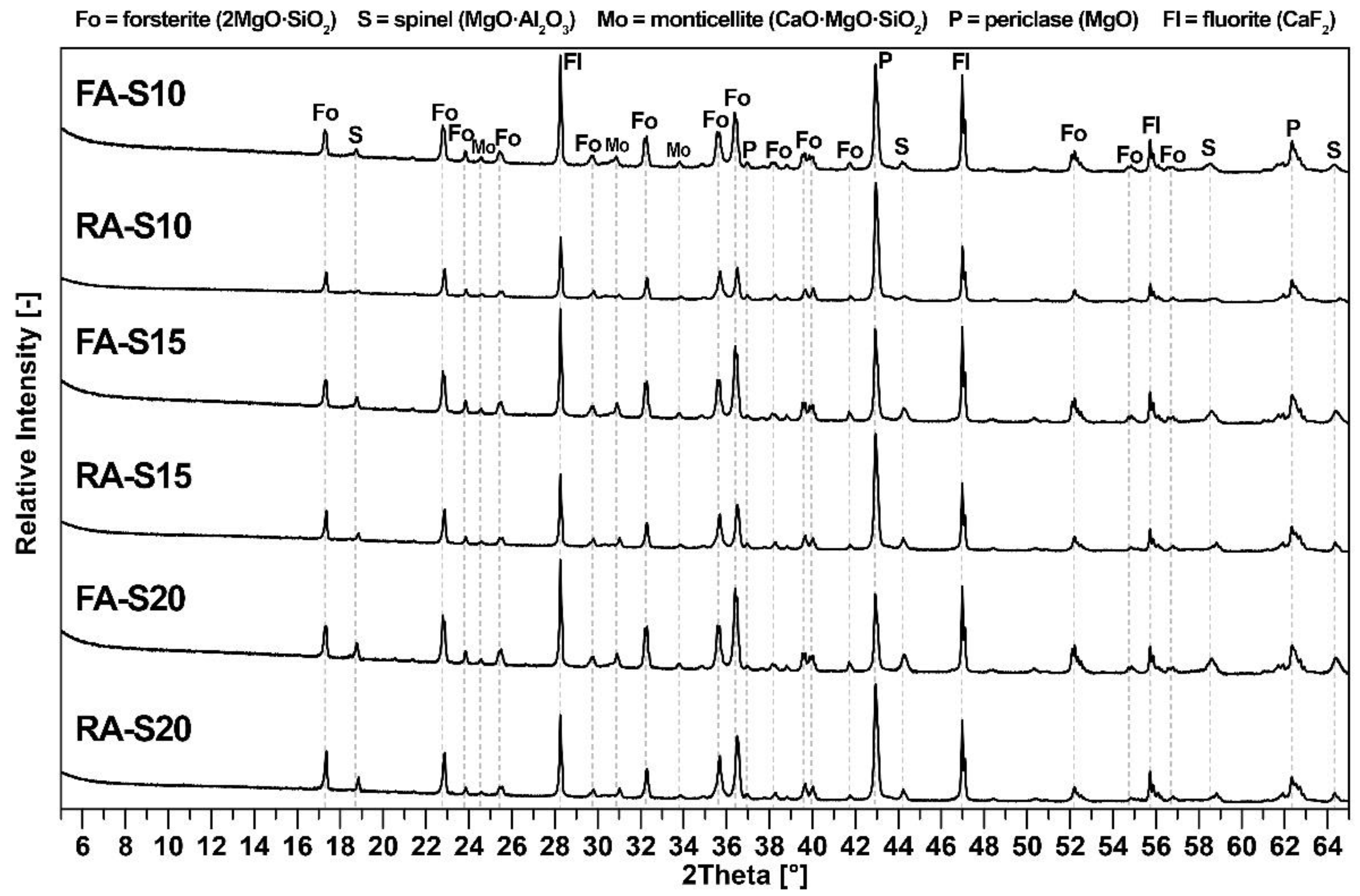
3.1. Mineralogical Composition and Microstructure
3.2. Physico-Mechanical Properties
3.3. Refractory and Thermo-Mechanical Parameters
3.4. Corrosion Resistance of Forsterite–Spinel Ceramics
3.4.1. Corrosion Resistance of Ceramics to Iron
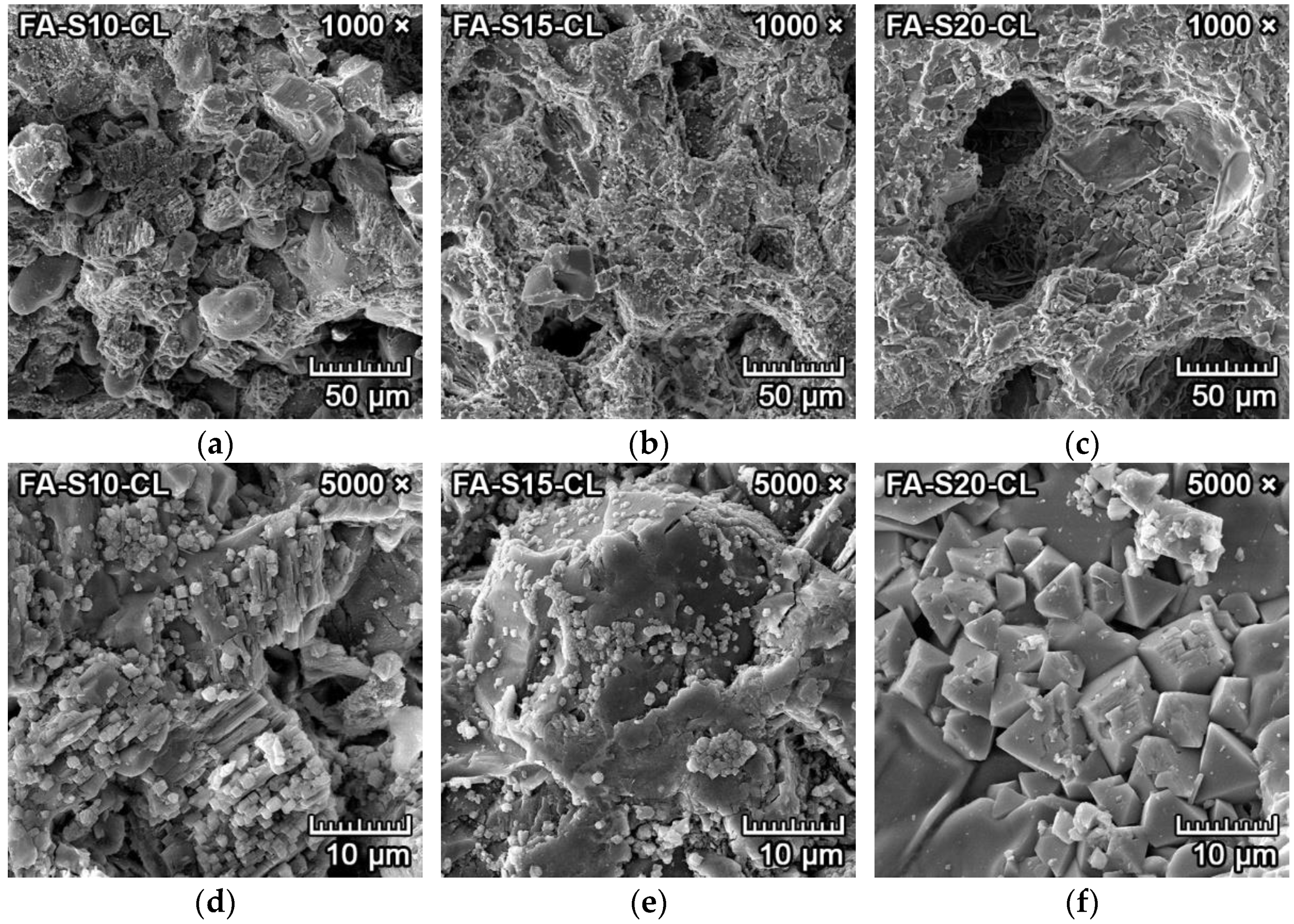
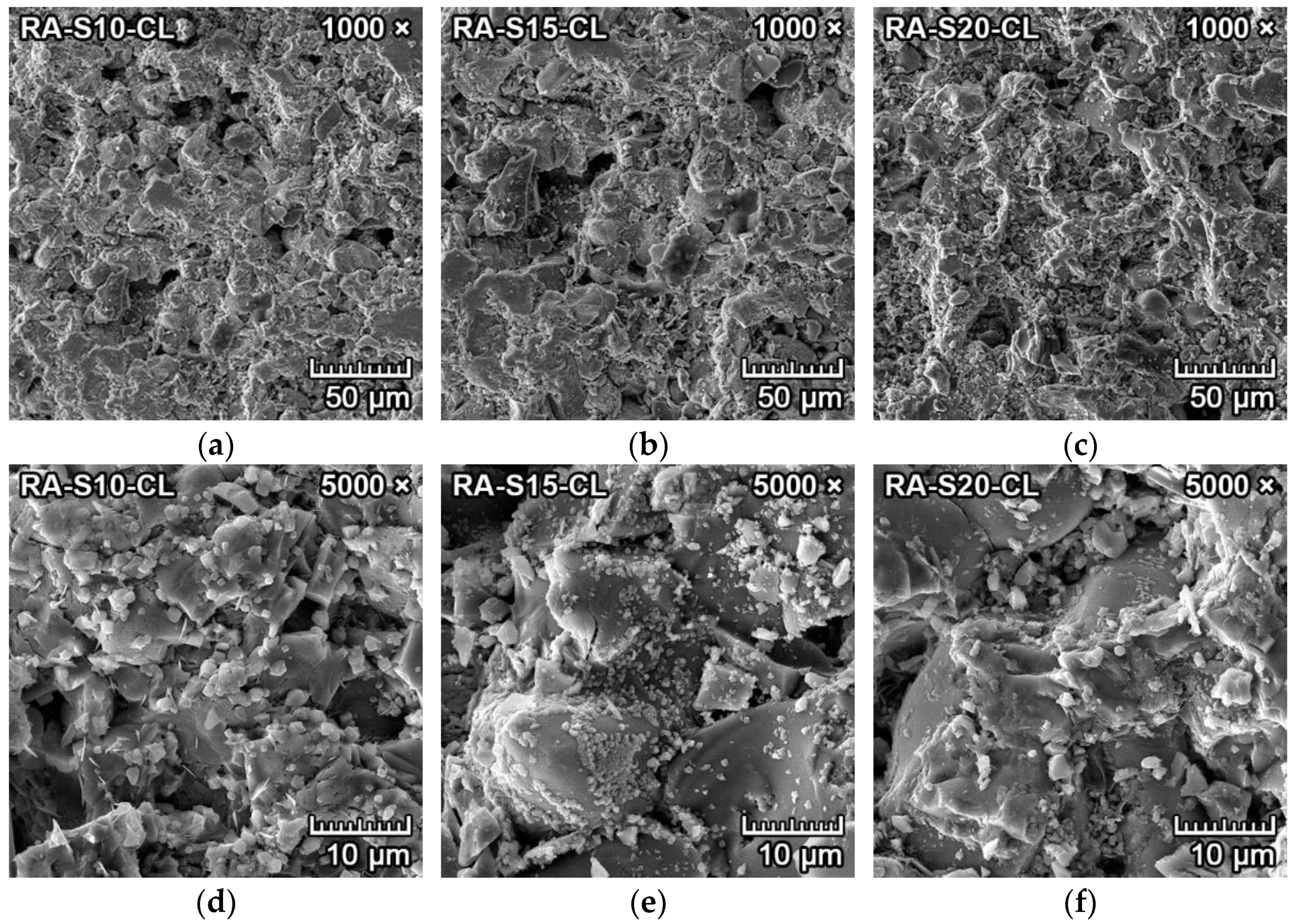
3.4.2. Corrosion Resistance of Ceramics to Clinker
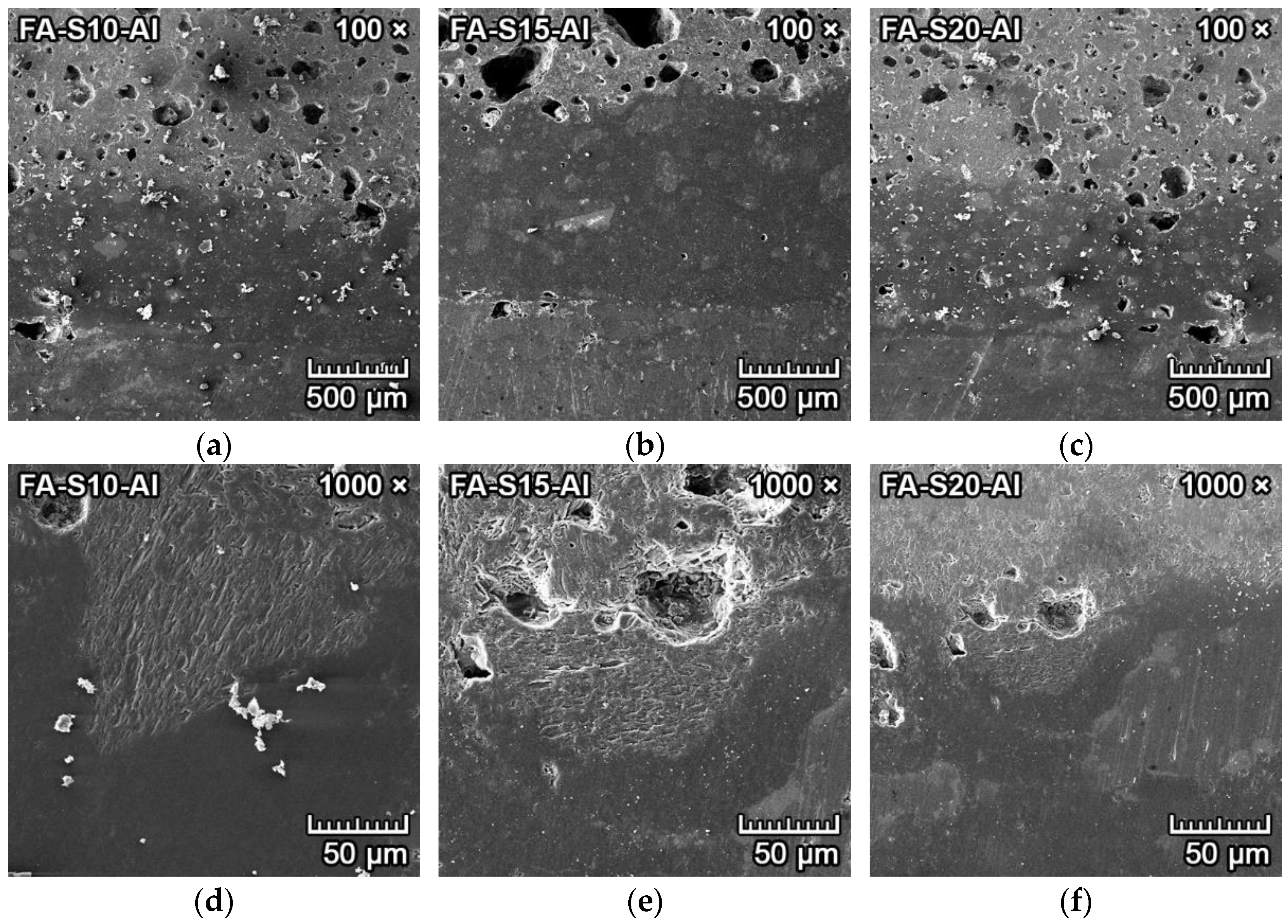
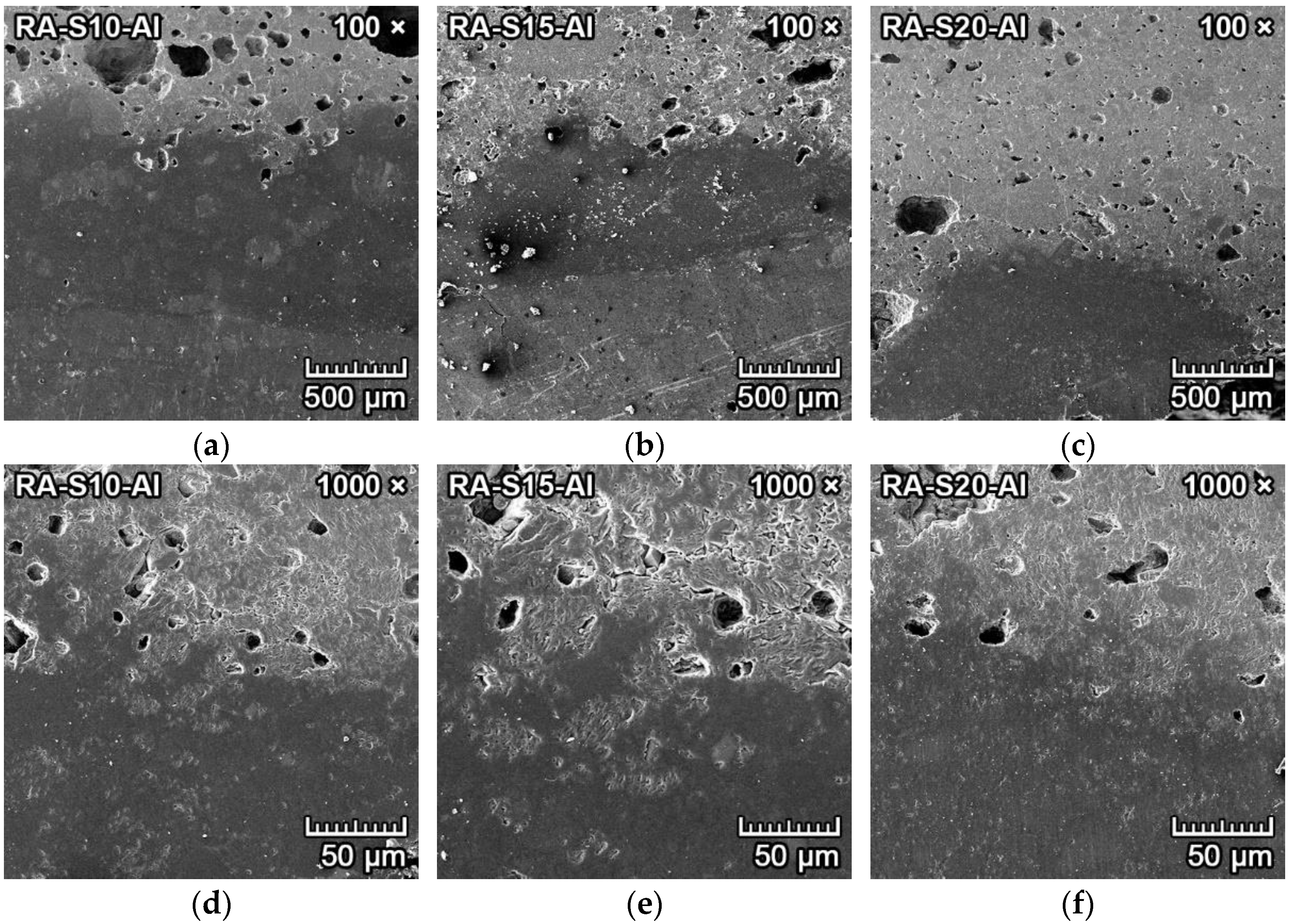
3.4.3. Corrosion Resistance of Ceramics to Aluminum
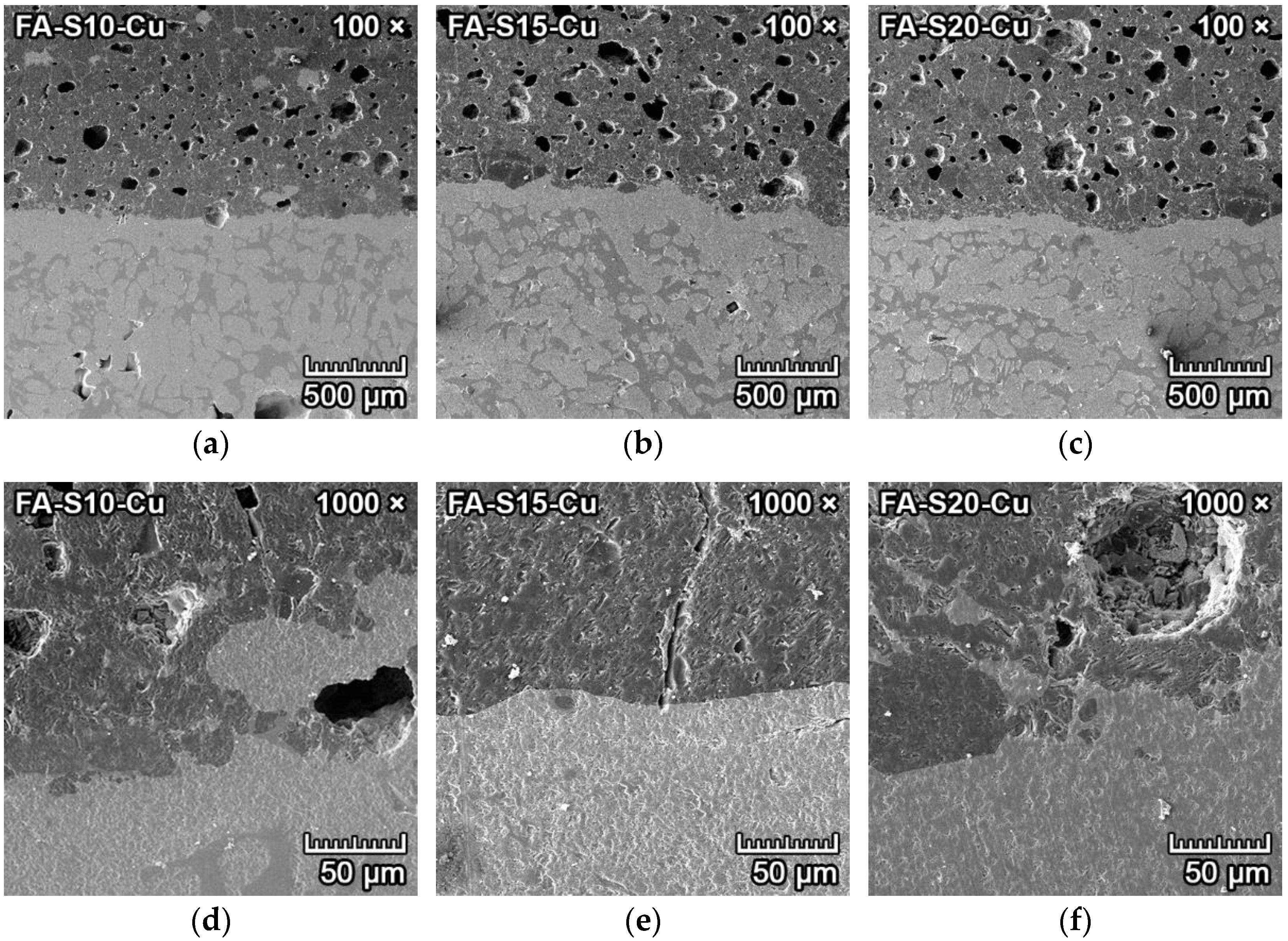
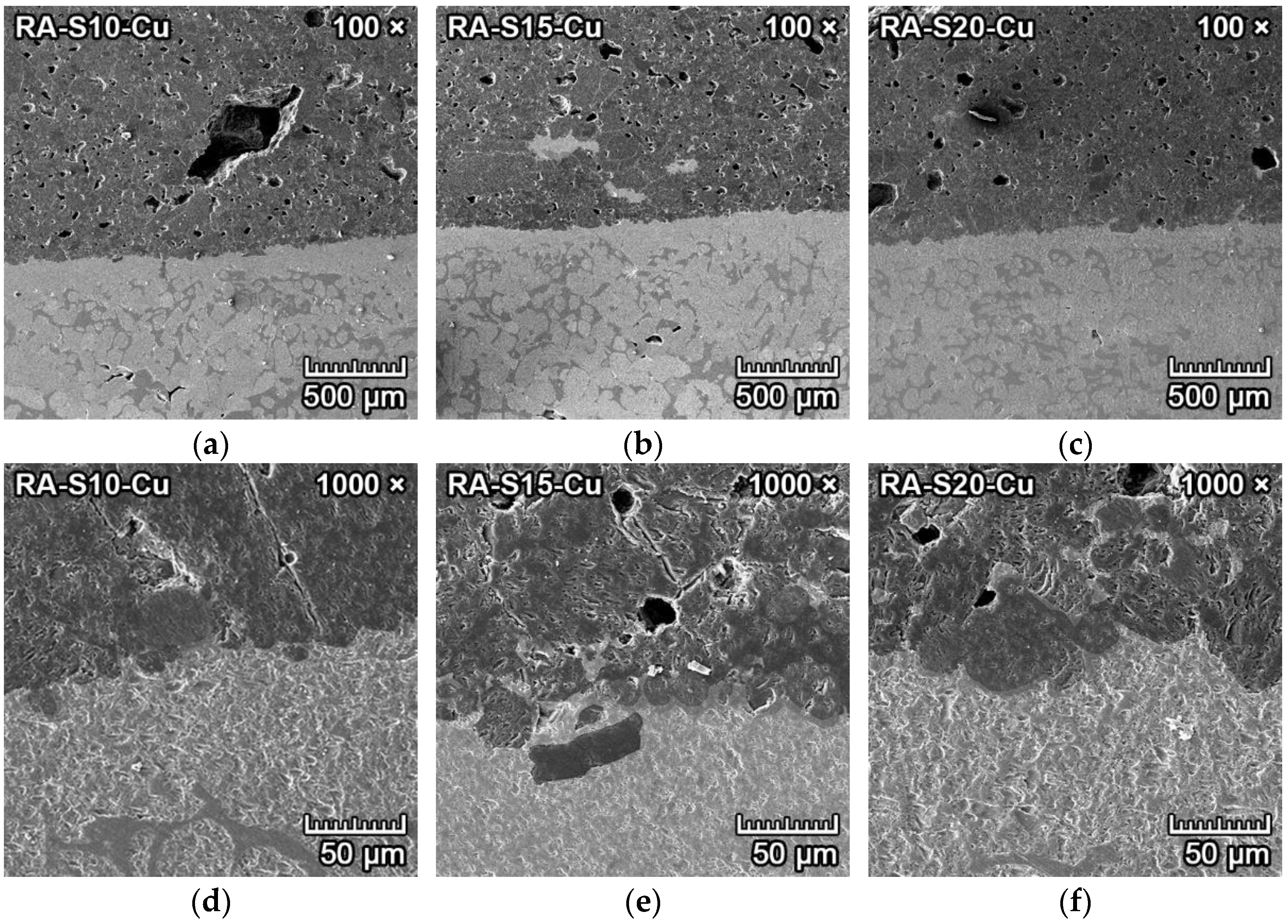
3.4.4. Corrosion Resistance of Ceramics to Copper
3.4.5. Discussion of Corrosion Resistance Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kingery, W. Introduction to Ceramics, 2nd ed.; Wiley: New York, NY, USA, 1960; pp. 261–284. ISBN 9780471478607. [Google Scholar]
- Budnikov, P.P. Technology of Ceramics and Refractories; MIT Press Ltd.: Cambridge, MA, USA, 1964; pp. 270–283. ISBN 9780262523776. [Google Scholar]
- Bouhifd, M.; Andrault, D.; Fiquet, G.; Richet, P. Thermal expansion of forsterite up to the melting point. Geoph. Res. Lett. 1996, 23, 1143–1146. [Google Scholar] [CrossRef]
- Ji, W.; Wei, H.; Cui, Y. Facile synthesis of porous forsterite nanofibres by direct electrospinning method based on the Kirkendall effect. Mater. Lett. 2018, 211, 319–322. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, L.; Ren, Z.; Gao, J.; Chen, X.; Liu, X.; Ge, T. A novel and green preparation of porous forsterite ceramics with excellent thermal isolation properties. Ceram. Int. 2019, 45, 2953–2961. [Google Scholar] [CrossRef]
- Tsai, M.T. Synthesis of nanocrystalline forsterite fiber via a chemical route. Mater. Res. Bull. 2002, 37, 2213–2226. [Google Scholar] [CrossRef]
- Chen, D.; Gu, H.; Huang, A.; Zhang, M.; Zhou, F.; Wang, C. Mechanical Strength and Thermal Conductivity of Modified Expanded Vermiculite/Forsterite Composite Materials. J. Mater. Eng. Perform. 2016, 25, 15–19. [Google Scholar] [CrossRef]
- Kharaziha, M.; Fathi, M.H. Synthesis and characterization of bioactive forsterite nanopowder. Ceram. Int. 2009, 35, 2449–2454. [Google Scholar] [CrossRef]
- Ni, S.; Chou, L.; Chang, J. Preparation and characterization of forsterite (Mg2SiO4) bioceramics. Ceram. Int. 2007, 33, 83–88. [Google Scholar] [CrossRef]
- Ramesh, S.; Yaghoubi, A.; Sara Lee, K.Y.; Christopher Chin, K.M.; Purbolaksono, J.; Hamdi, M.; Hassan, M.A. Nanocrystalline forsterite for biomedical applications: Synthesis, microstructure and mechanical properties. J. Mech. Behav. Biomed. 2013, 25, 63–69. [Google Scholar] [CrossRef]
- Saqaei, M.; Fathi, M.; Edris, H.; Mortazavi, V.; Hosseini, N. Effects of adding forsterite bioceramic on in vitro activity and antibacterial properties of bioactive glass-forsterite nanocomposite powders. Adv. Powder. Technol. 2016, 27, 1922–1932. [Google Scholar] [CrossRef]
- Zampiva, R.; Acauan, L.; Dos Santos, L.; De Castro, R.; Alves, A.; Bergmann, C. Nanoscale synthesis of single-phase forsterite by reverse strike co-precipitation and its high optical and mechanical properties. Ceram. Int. 2017, 43, 16225–16231. [Google Scholar] [CrossRef]
- Sun, H.T.; Fujii, M.; Nitta, N.; Mizuhata, M.; Yasuda, H.; Deki, S.; Hayashi, S. Molten-Salt Synthesis and Characterization of Nickel-Doped Forsterite Nanocrystals. J. Amer. Ceram. Soc. 2009, 92, 962–966. [Google Scholar] [CrossRef]
- Nguyen, M.; Sokolář, R. Formation and Influence of Magnesium-Alumina Spinel on Properties of Refractory Forsterite-Spinel Ceramics. Mater. Tehnol. 2020, 54, 135–141. [Google Scholar] [CrossRef]
- Nguyen, M.; Sokolář, R. Impact of Fly Ash as a Raw Material on the Properties of Refractory Forsterite–Spinel Ceramics. Minerals 2020, 10, 835. [Google Scholar] [CrossRef]
- Ewais, E.M.M.; El-Amir, A.A.M.; Besisa, D.H.A.; Esmat, M.; El-Anadouli, B.E.H. Synthesis of nanocrystalline MgO/MgAl2O4 spinel powders from industrial wastes. J. All. Com. 2017, 691, 822–833. [Google Scholar] [CrossRef]
- Mustafa, E.; Khalil, N.; Gamal, A. Sintering and microstructure of spinel–forsterite bodies. Ceram. Int. 2002, 28, 663–667. [Google Scholar] [CrossRef]
- Braulio, M.A.L.; Rigaud, M.; Buhr, A.; Parr, C.; Pandolfelli, V.C. Spinel-containing alumina-based refractory castables. Ceram. Int. 2011, 37, 1705–1724. [Google Scholar] [CrossRef]
- Ganesh, I. A Review on Magnesium Aluminate (MgAl2O4) Spinel: Synthesis, Processing and Applications. Int. Mater. Rev. 2013, 58, 63–112. [Google Scholar] [CrossRef]
- Graf, R.B.; Wahl, F.M.; Grim, R.E. Phase transformations in silica-alumina-magnesia mixtures as examined by continuous X-ray diffraction: II. Spinel-silica compositions. Am. Mineral. 1963, 48, 150–158. [Google Scholar]
- Michel, R.; Ammar, M.R.; Simon, P.; de Bilbao, E.; Poirier, J. Behaviour of olivine refractories at high temperature: Agglomeration in a fluidized bed reactor. Refract. Worldforum 2014, 6, 95–98. [Google Scholar]
- Orosco, P.; Barbosa, L.; Carmen Ruiz, M. Synthesis of magnesium aluminate spinel by periclase and alumina chlorination. Mater. Res. Bull. 2014, 59, 337–340. [Google Scholar] [CrossRef]
- Cao, J.; Dong, X.; Li, L.; Dong, Y.; Hampshire, S. Recycling of waste fly ash for production of porous mullite ceramic membrane supports with increased porosity. J. Eur. Ceram. Soc. 2014, 34, 3181–3194. [Google Scholar] [CrossRef]
- Dong, Y.; Hampshire, S.; Zhou, J.; Ji, Z.; Wang, J.; Meng, G. Sintering and characterization of fly ash-based mullite with MgO addition. J. Eur. Ceram. Soc. 2011, 31, 687–695. [Google Scholar] [CrossRef]
- Gengfu, L.; Yawei, L.; Tianbin, Z.; Yibiao, X.; Jun, L.; Shaobai, S.; Quanyou, L.; Yuanjin, L. Influence of the atmosphere on the mechanical properties and slag resistance of magnesia-chrome bricks. Ceram. Int. 2020, 46, 11225–11231. [Google Scholar] [CrossRef]
- Sako, E.Y.; Braulio, M.A.L.; Pandolfelli, V.C. The corrosion resistance of microsilica-containing Al2O3–MgO and Al2O3–spinel castables. Ceram. Int. 2012, 38, 4783–4789. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Pal, T.K.; DasPoddar, P.K. Improvement of corrosion resistance of spinel-bonded castables to converter slag. Ceram. Int. 2009, 35, 373–380. [Google Scholar] [CrossRef]
- Jeon, J.; Kang, Y.; Park, J.H.; Chung, Y. Corrosion-erosion behavior of MgAl2O4 spinel refractory in contact with high MnO slag. Ceram. Int. 2017, 43, 15074–15079. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, Y.; Kim, S.; Yoon, T.; Kim, Y.; Chung, Y. A study on the wetting behavior of liquid iron on forsterite, mullite, spinel and quasi-corundum substrates. Ceram. Int. 2018, 44, 17585–17591. [Google Scholar] [CrossRef]
- Guo, Z.; Ding, Q.; Liu, L.; Zhang, X.; Luo, X.; Duan, F. Microstructural characteristics of refractory magnesia produced from macrocrystalline magnesite in China. Ceram. Int. 2021, 47, 22701–22708. [Google Scholar] [CrossRef]
- Rutman, D.S.; Shchetnikova, I.L.; Kelareva, E.I.; Zhobolova, L.S.; Perepelitsyn, V.A. Effect of metallurgical slag on ceramics processed from pure oxides, spinels, and forsterite. Refractories 1973, 14, 701–705. [Google Scholar] [CrossRef]
- Braulio, M.A.L.; Tomba Martinez, A.G.; Luz, A.P.; Liebske, C.; Pandolfelli, V.C. Basic slag attack of spinel-containing refractory castables. Ceram. Int. 2011, 37, 1935–1945. [Google Scholar] [CrossRef]
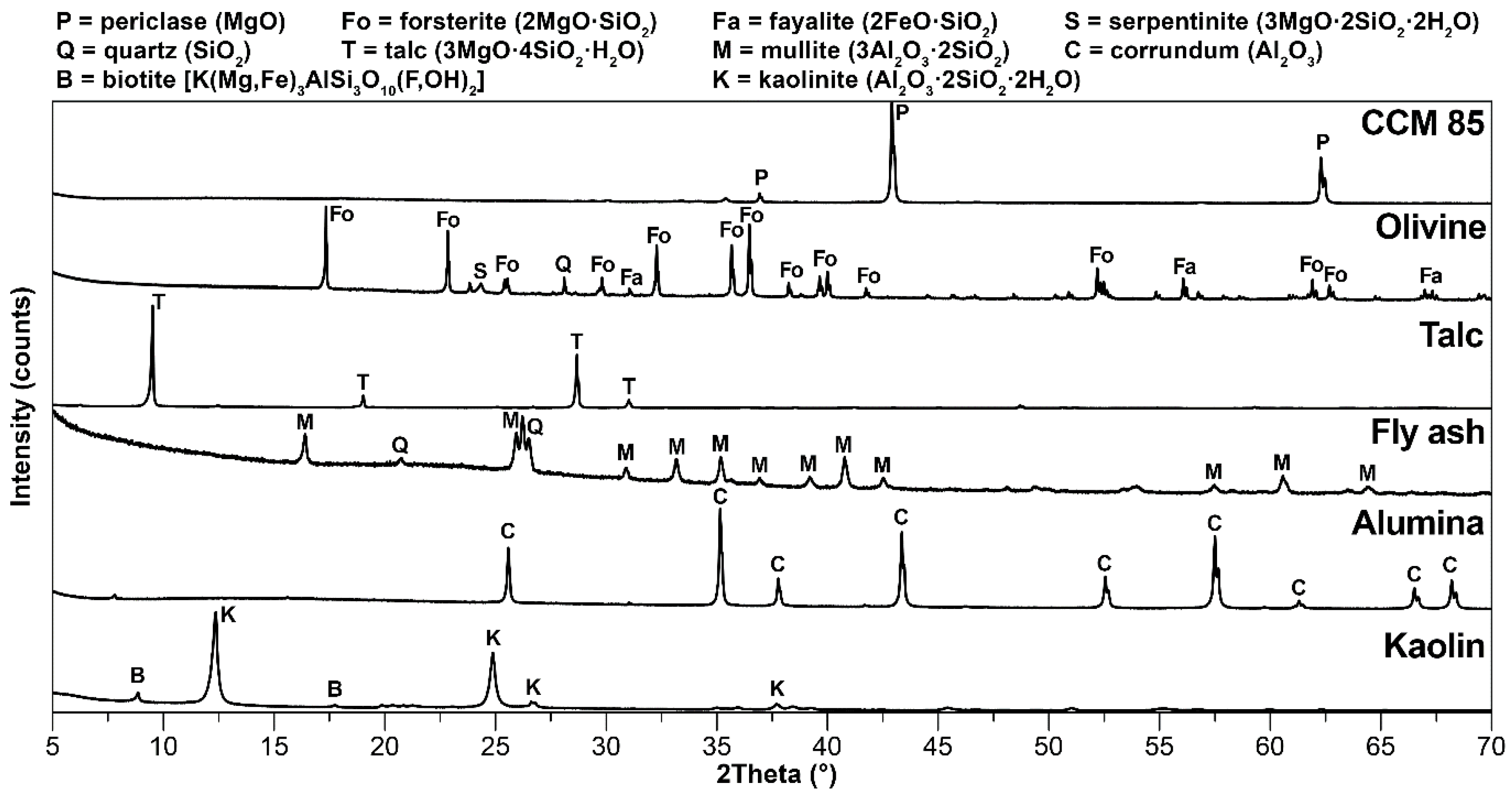
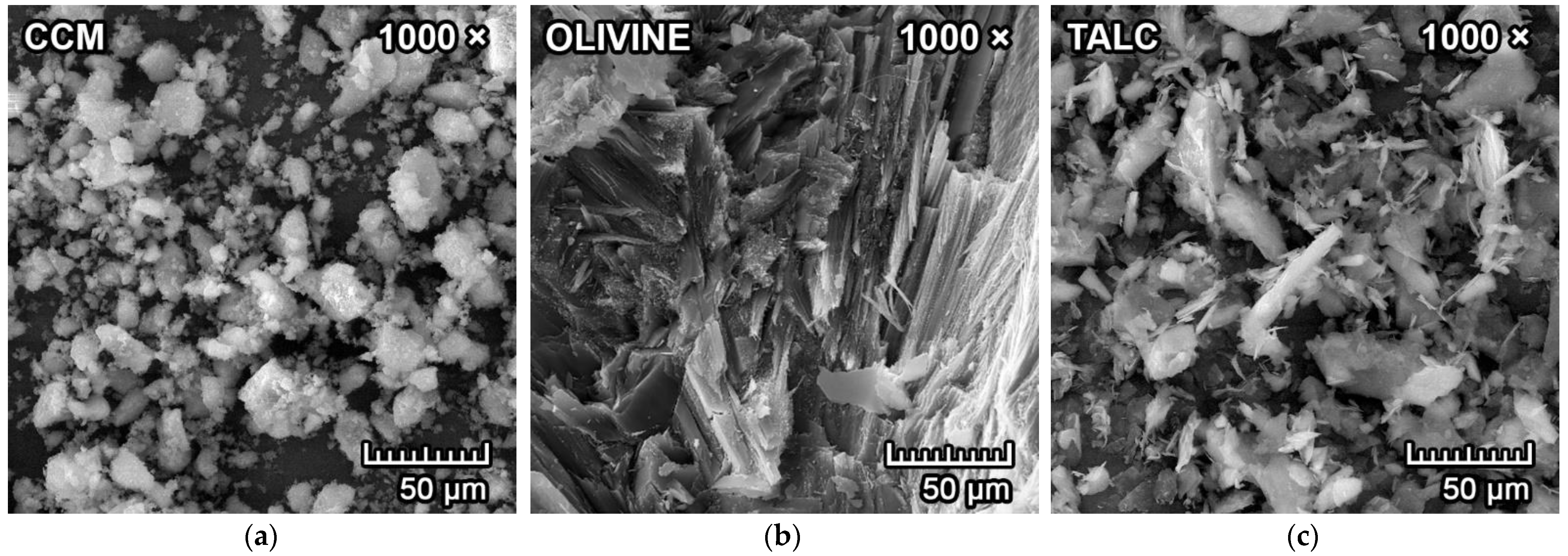
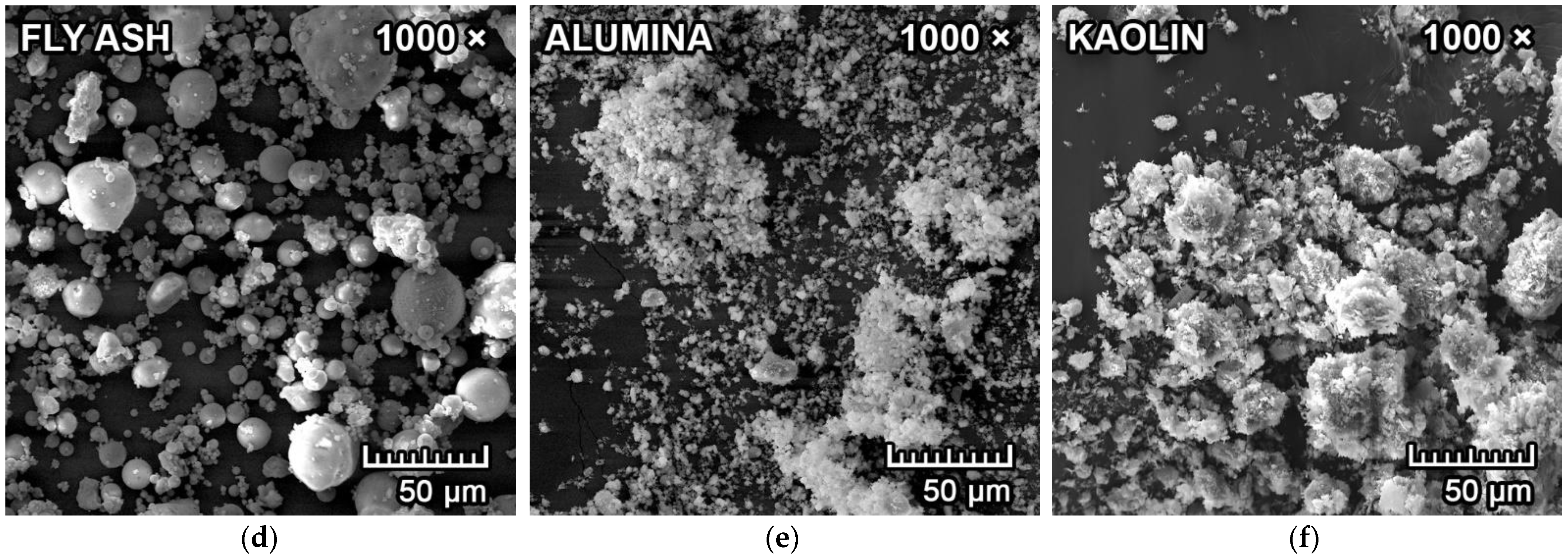

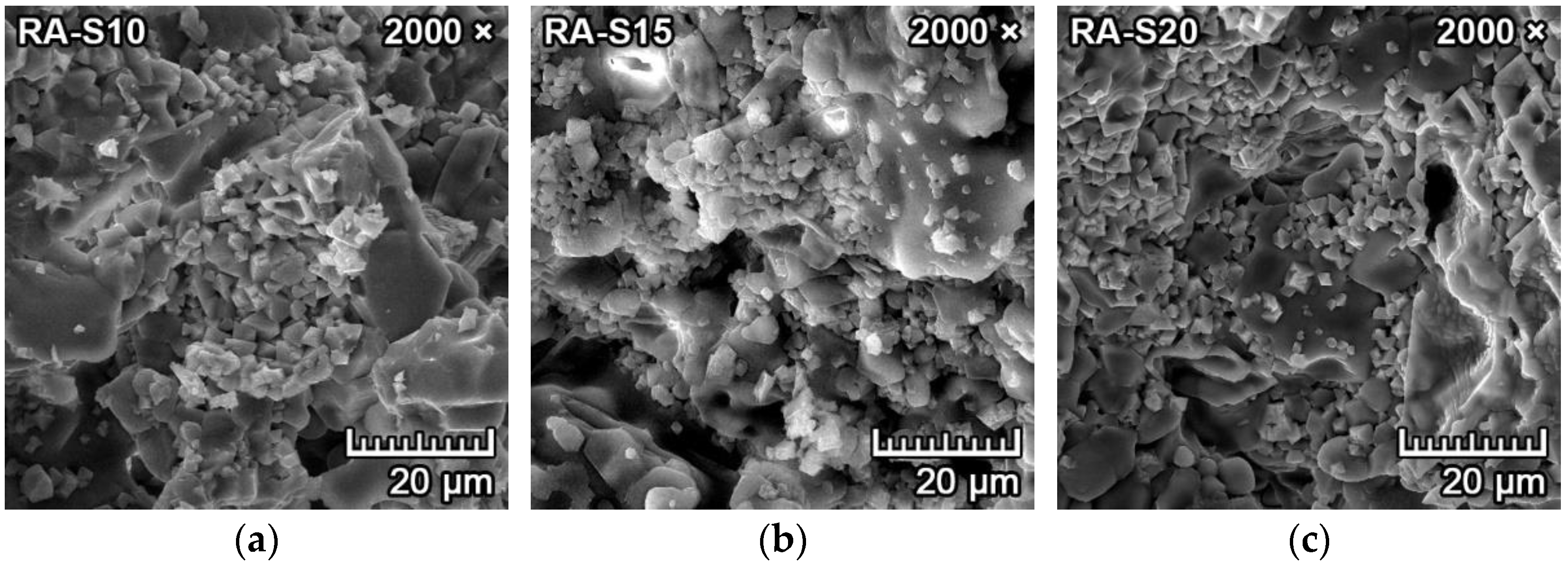
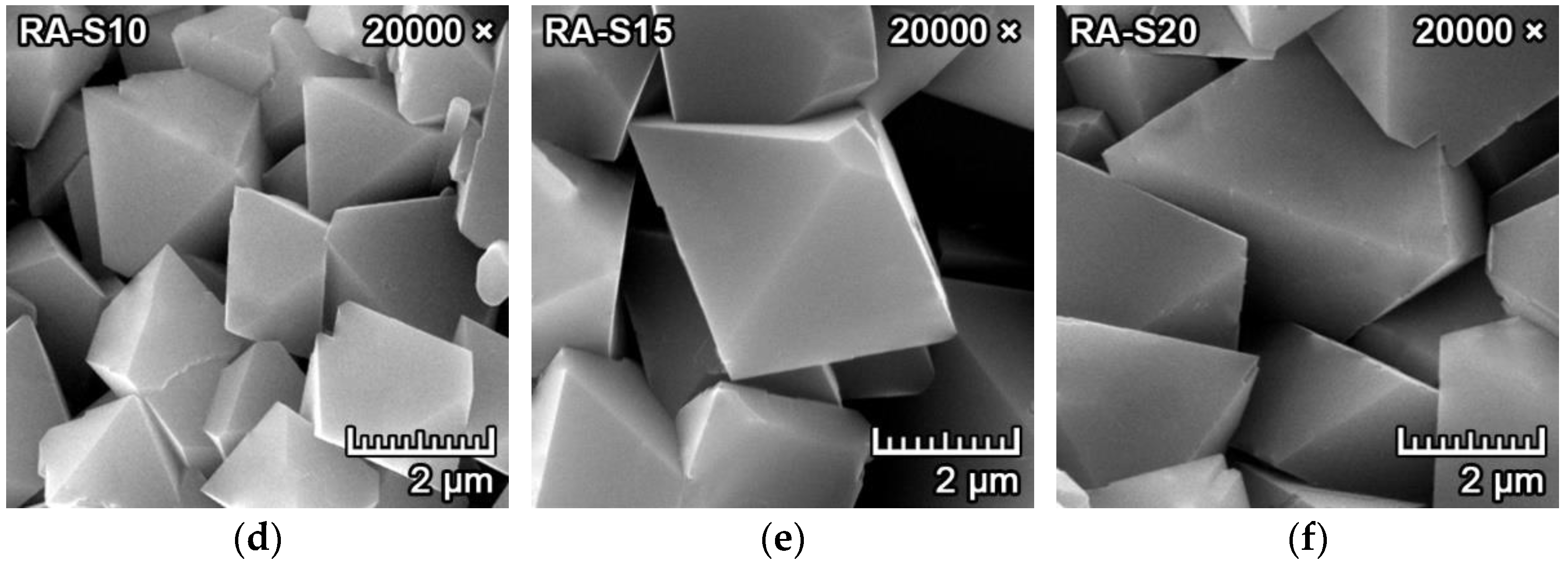


| Raw Materials | MgO | SiO2 | Al2O3 | CaO | Fe2O3 | K2O+Na2O | TiO2 | LOI 1 |
|---|---|---|---|---|---|---|---|---|
| CCM | 85.6 | 0.5 | 0.1 | 5.2 | 7.4 | 0.1 | 0.1 | 1.0 |
| Olivine | 24.1 | 64.7 | 1.0 | 0.7 | 8.8 | 0.5 | 0.1 | 0.1 |
| Talc | 31.5 | 59.1 | 1.0 | 1.0 | 0.7 | 0.2 | 0.0 | 6.5 |
| Fly ash | 1.4 | 57.4 | 29.3 | 2.2 | 5.1 | 1.7 | 1.7 | 1.2 |
| Alumina | 0.0 | 0.0 | 99.3 | 0.0 | 0.1 | 0.3 | 0.0 | 0.3 |
| Kaolin | 0.5 | 46.8 | 36.6 | 0.7 | 0.9 | 1.2 | 0.1 | 13.2 |
| Raw Materials | FA-S10 | FA-S15 | FA-S20 | RA-S10 | RA-S15 | RA-S20 |
|---|---|---|---|---|---|---|
| CCM [wt.%] | 43.5 | 45.9 | 48.2 | 40.0 | 39.6 | 39.2 |
| Olivine [wt.%] | 24.8 | 15.9 | 7.1 | 34.0 | 32.1 | 30.2 |
| Talc [wt.%] | 12.4 | 8.0 | 3.5 | 17.0 | 16.0 | 15.1 |
| Fly ash [wt.%] | 14.3 | 25.2 | 36.2 | - | - | - |
| Alumina [wt.%] | - | - | - | 4.1 | 7.3 | 10.5 |
| Kaolin [wt.%] | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Phase | FA-S10 | FA-S15 | FA-S20 | RA-S10 | RA-S15 | RA-S20 |
|---|---|---|---|---|---|---|
| Forsterite | 57.8 | 48.5 | 42.4 | 72.2 | 66.5 | 58.3 |
| Spinel | 11.1 | 14.2 | 19.7 | 9.8 | 14.7 | 19.8 |
| Periclase | 13.4 | 14.8 | 11.9 | 8.3 | 10.1 | 10.6 |
| Monticellite | 2.7 | 2.2 | 2.4 | 1.6 | 2.0 | 1.9 |
| Amorphous phase | 14.9 | 20.4 | 23.6 | 8.1 | 6.7 | 9.4 |
| Mixture | Apparent Porosity (%) | Water Absorption (%) | Bulk Density (kg·m−3) | Modulus of Rupture (MPa) |
|---|---|---|---|---|
| FA-S10 | 24.2 | 14.7 | 2365 | 15.5 |
| RA-S10 | 17.5 | 4.5 | 2745 | 16.0 |
| FA-S15 | 21.8 | 11.3 | 2460 | 18.4 |
| RA-S15 | 15.6 | 4.2 | 2735 | 22.6 |
| FA-S20 | 16.3 | 8.6 | 2510 | 17.3 |
| RA-S20 | 14.4 | 2.9 | 2750 | 19.1 |
| Mixture | Firing Shrinkage (%) | Refractoriness (°C) | Refractoriness under Load T0.5 (°C) | Residual MOR (%) |
|---|---|---|---|---|
| FA-S10 | 5.9 | 1694 | 1593 | 21.3 |
| RA-S10 | 6.2 | 1737 | 1664 | 23.0 |
| FA-S15 | 8.5 | 1676 | 1561 | 28.6 |
| RA-S15 | 6.9 | 1742 | 1678 | 35.3 |
| FA-S20 | 11.3 | 1655 | 1532 | 22.5 |
| RA-S20 | 7.4 | 1731 | 1645 | 26.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, M.; Sokolář, R. Corrosion Resistance of Novel Fly Ash-Based Forsterite-Spinel Refractory Ceramics. Materials 2022, 15, 1363. https://doi.org/10.3390/ma15041363
Nguyen M, Sokolář R. Corrosion Resistance of Novel Fly Ash-Based Forsterite-Spinel Refractory Ceramics. Materials. 2022; 15(4):1363. https://doi.org/10.3390/ma15041363
Chicago/Turabian StyleNguyen, Martin, and Radomír Sokolář. 2022. "Corrosion Resistance of Novel Fly Ash-Based Forsterite-Spinel Refractory Ceramics" Materials 15, no. 4: 1363. https://doi.org/10.3390/ma15041363
APA StyleNguyen, M., & Sokolář, R. (2022). Corrosion Resistance of Novel Fly Ash-Based Forsterite-Spinel Refractory Ceramics. Materials, 15(4), 1363. https://doi.org/10.3390/ma15041363






