Colored Tattoo Ink Screening Method with Optical Tissue Phantoms and Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
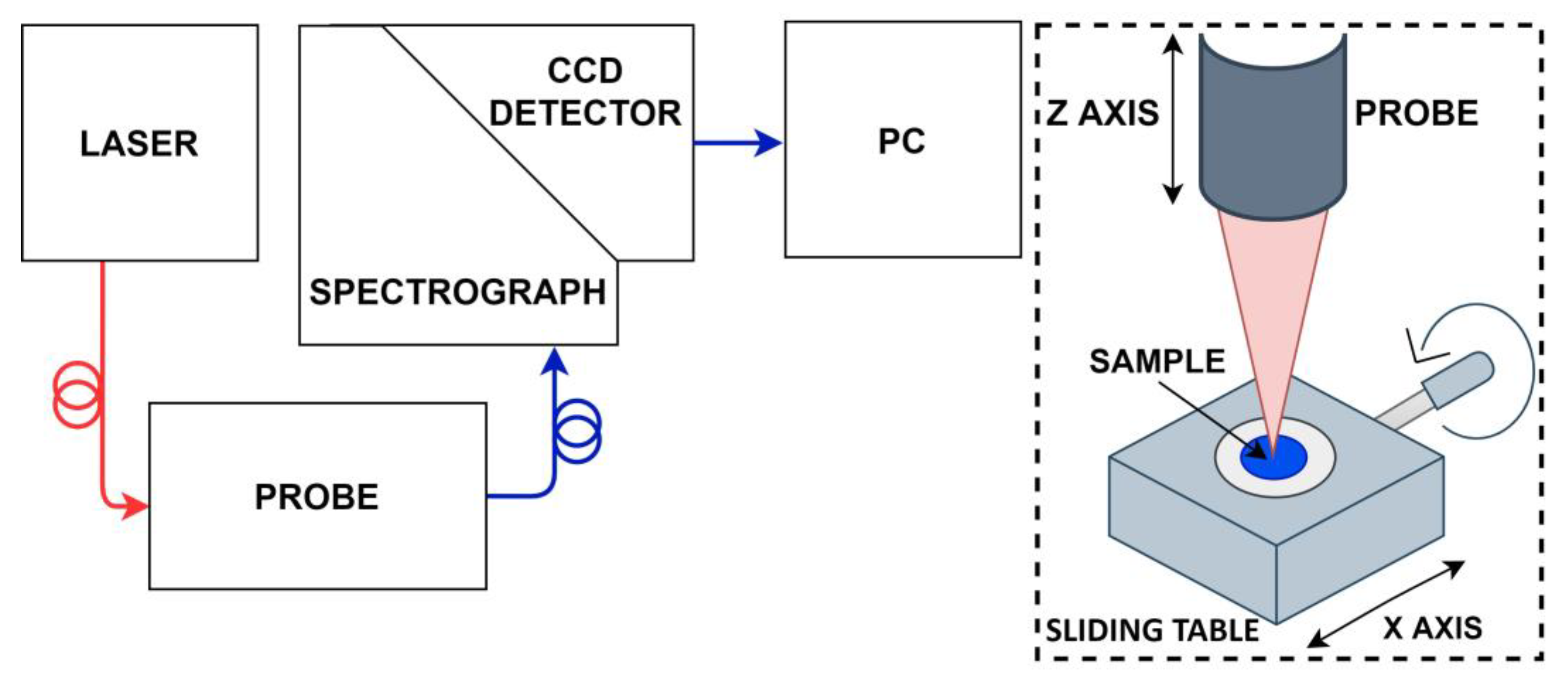
2.1. Raman Spectroscopy
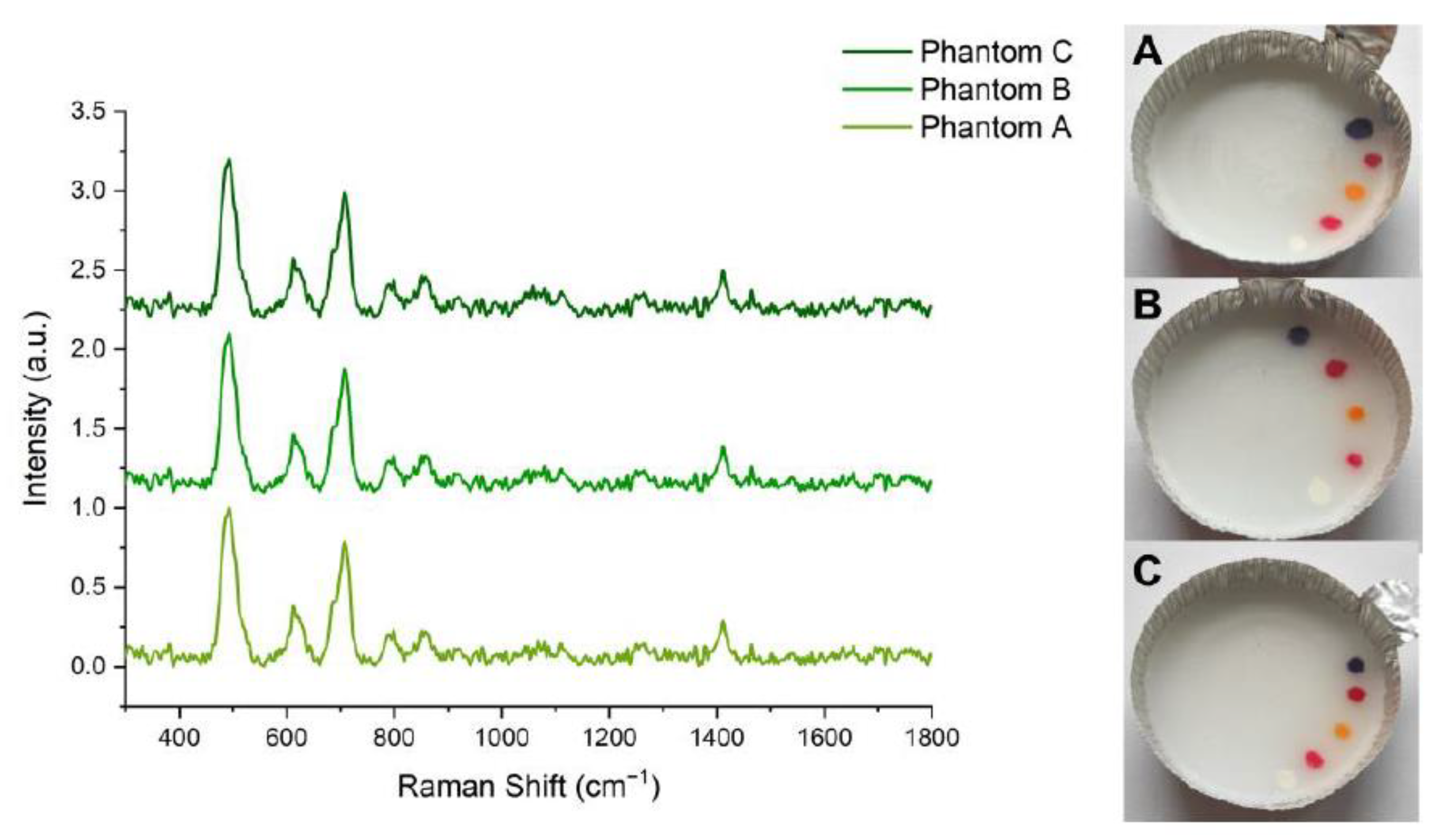
2.2. Optical Tissue Phantoms
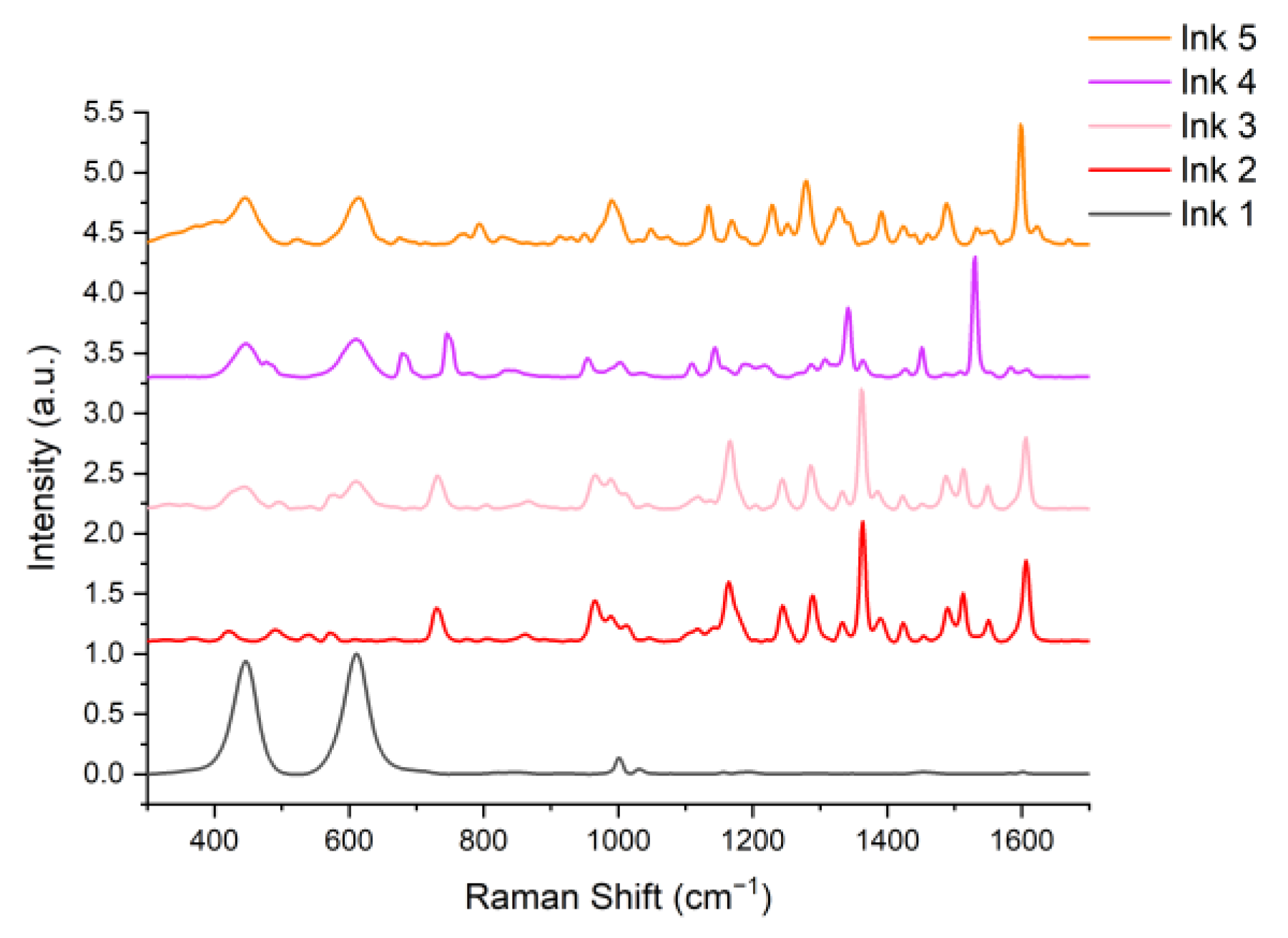
2.3. Tattoo Inks
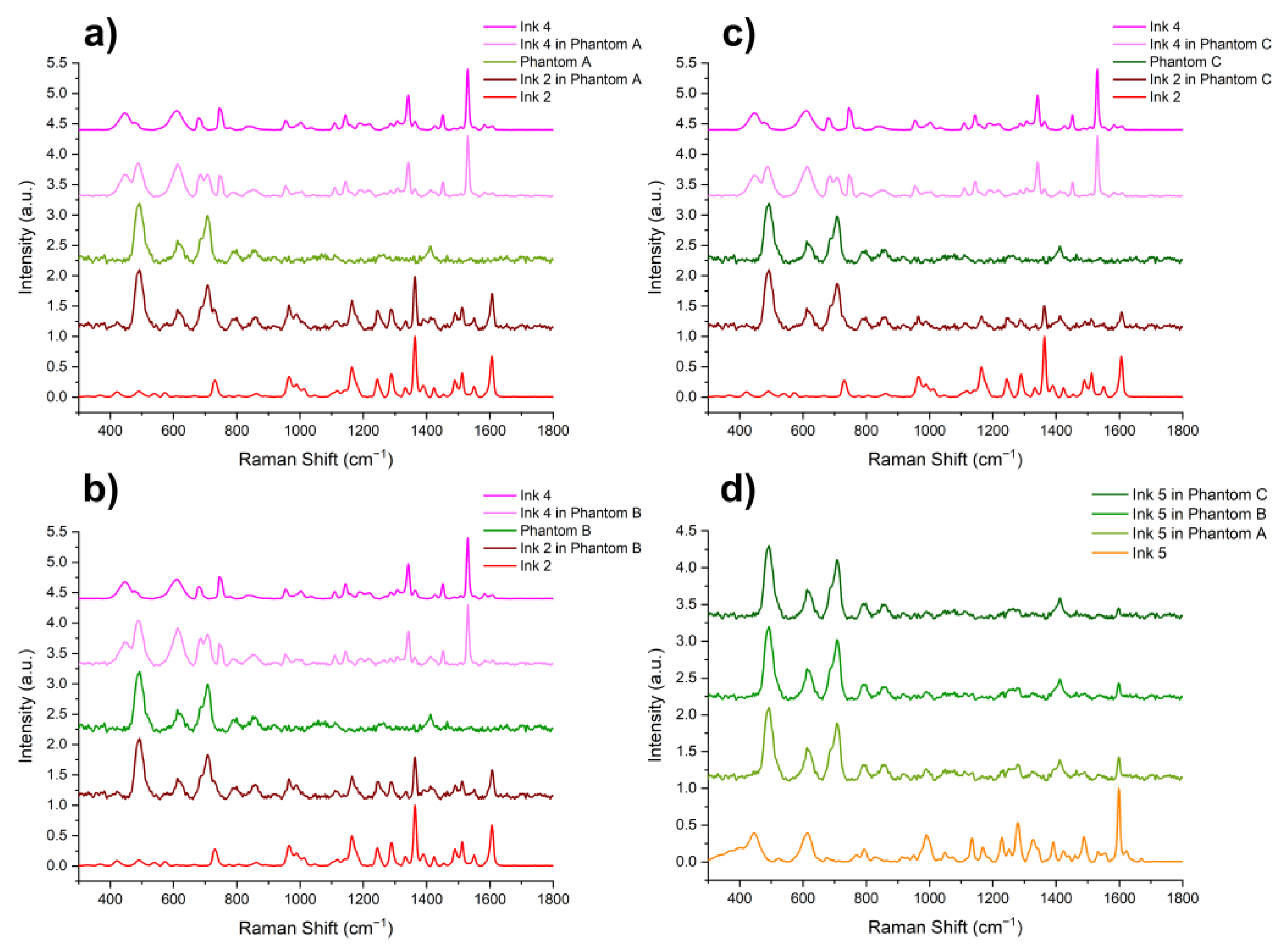
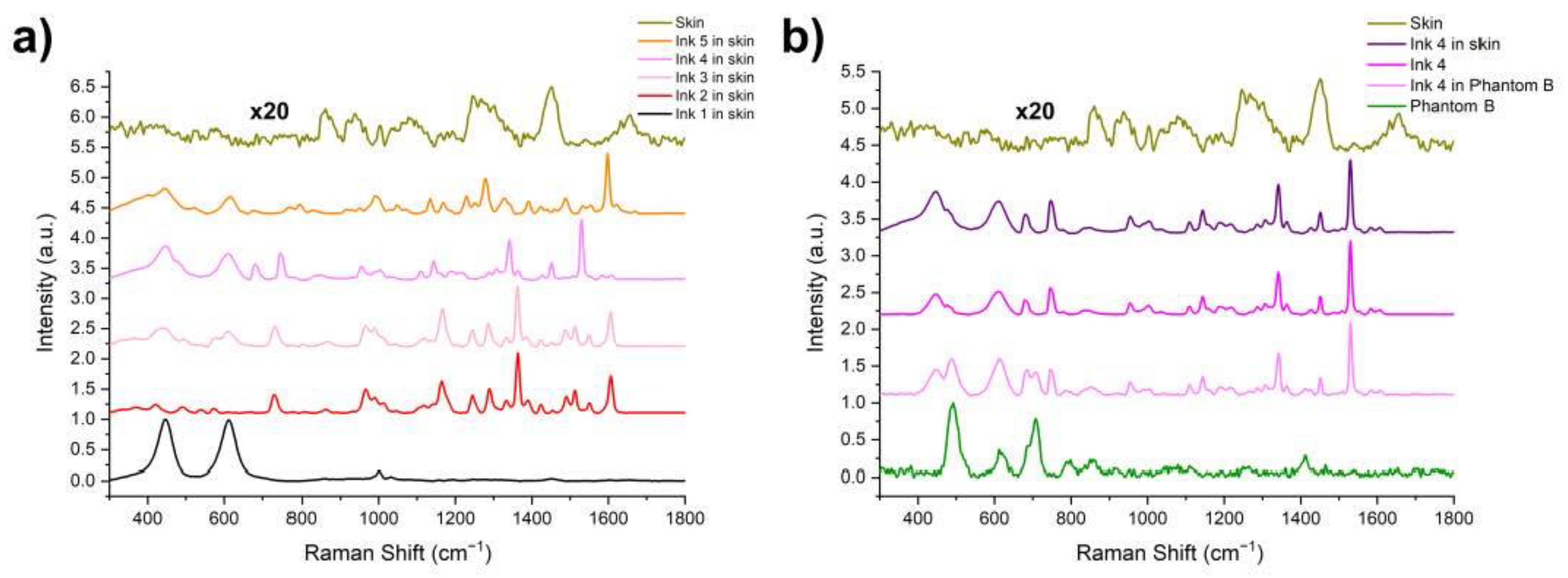
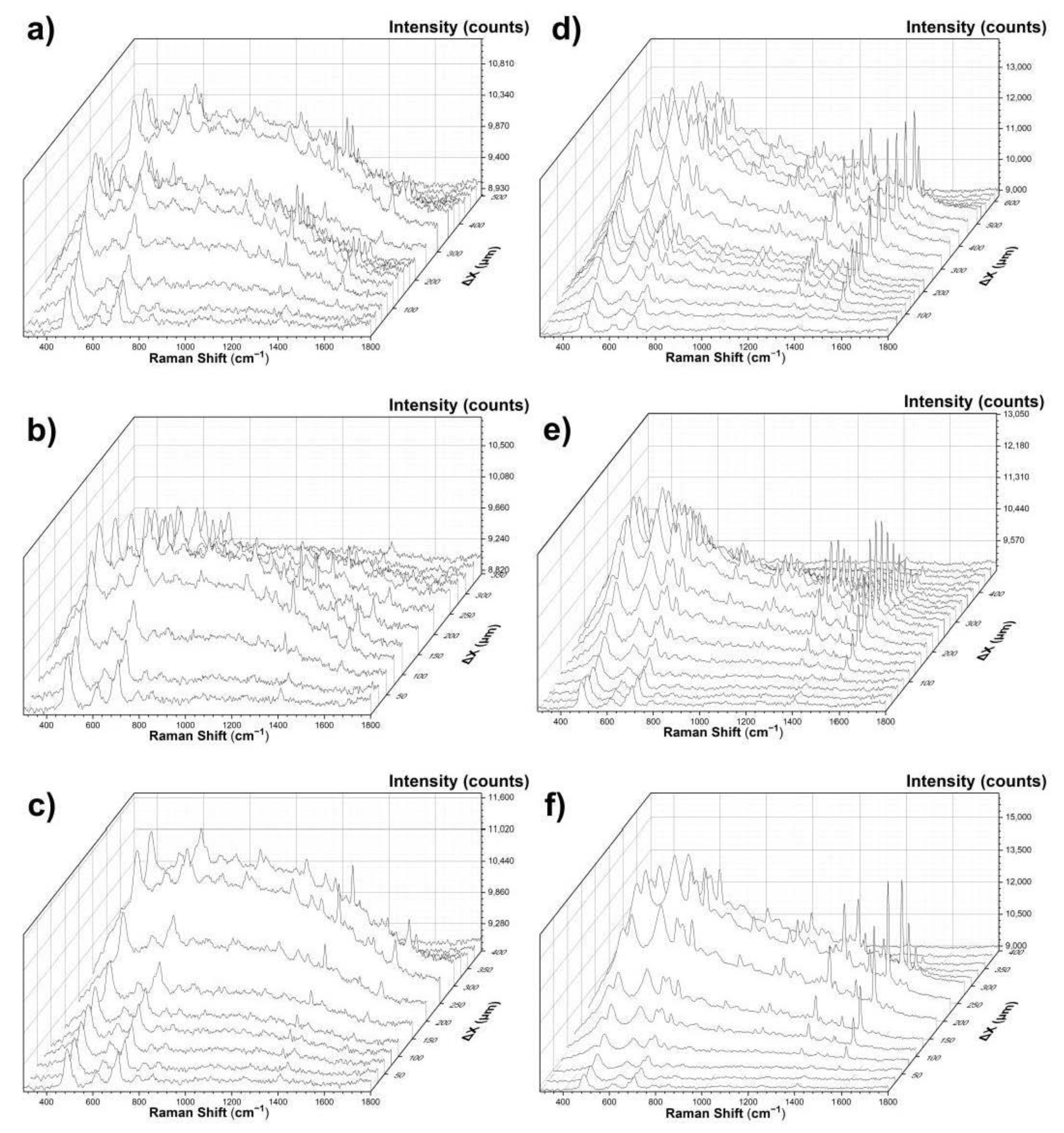
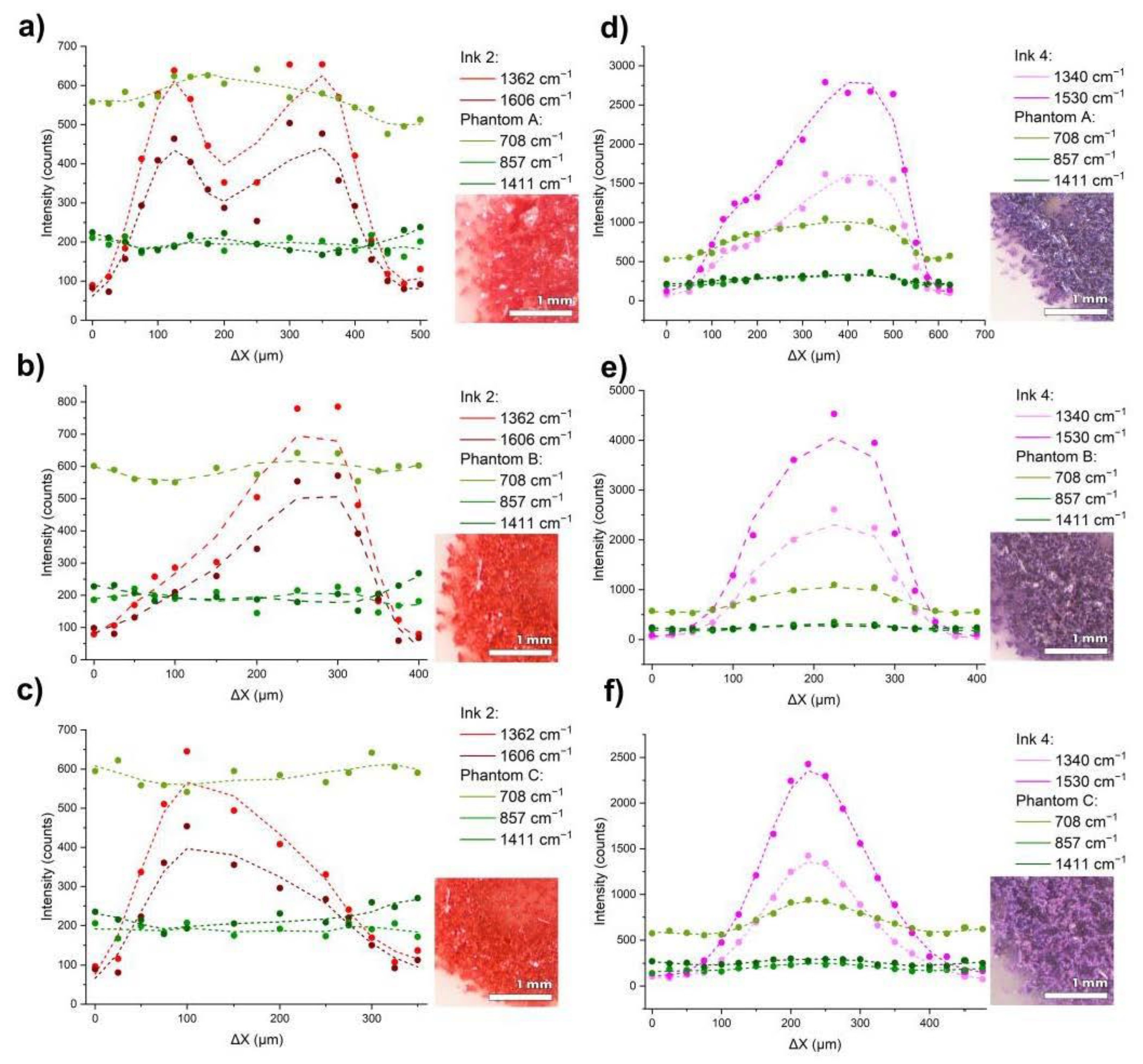
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, P.; Keeter, S. Millennials: Confident Connected Open to Change; Pew Research Center: Philadelphia, PA, USA, 2010. [Google Scholar]
- Three in Ten Americans Have Tattoos, and Most Don’t Stop at Just One. Available online: https://theharrispoll.com/tattoos-can-take-any-number-of-forms-from-animals-to-quotes-to-cryptic-symbols-and-appear-in-all-sorts-of-spots-on-our-bodies-some-visible-in-everyday-life-others-not-so-much-but-one-thi/ (accessed on 19 April 2021).
- Tattoos Aren’t Just for Rebels Anymore. Available online: https://www.foxnews.com/us/fox-news-poll-tattoos-arent-just-for-rebels-anymore (accessed on 19 April 2021).
- Grant, C.A.; Twigg, P.C.; Baker, R.; Tobin, D.J. Tattoo Ink Nanoparticles in Skin Tissue and Fibroblasts. Beilstein J. Nanotechnol. 2015, 6, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Breuner, C.C.; Levine, D.A. Adolescent and Young Adult Tattooing, Piercing, and Scarification. Pediatrics 2017, 140, e20163494. [Google Scholar] [CrossRef]
- World Famous Tattoo Ink Colors. Available online: https://www.worldfamoustattooink.com/pages/world-famous-tattoo-ink-colors (accessed on 19 April 2021).
- Blacklight UV Invisible. Available online: https://www.bloodlinetattooinks.com/product/blacklight-invisible/ (accessed on 19 April 2021).
- Russell, K.; Schleichert, R.; Baum, B.; Villacorta, M.; Hardigan, P.; Thomas, J.; Weiss, E. Ultraviolet-Fluorescent Tattoo Facilitates Accurate Identification of Biopsy Sites. Dermatol. Surg. 2015, 41, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Zirkin, H.J.; Avinoach, I.; Edelwitz, P. A Tattoo and Localized Lymphadenopathy: A Case Report. Cutis 2001, 67, 471–472. [Google Scholar] [PubMed]
- Jack, C.; Adwani, A.; Krishnan, H. Tattoo Pigment in an Axillary Lymph Node Simulating Metastatic Malignant Melanoma. Int. Semin. Surg. Oncol. ISSO 2005, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- McIlwee, B.; Alster, T. Treatment of Cosmetic Tattoos: A Review and Case Analysis. Dermatol. Surg. 2018, 44, 1. [Google Scholar] [CrossRef] [PubMed]
- Regensburger, J.; Lehner, K.; Maisch, T.; Vasold, R.; Santarelli, F.; Engel, E.; Gollmer, A.; König, B.; Landthaler, M.; Bäumler, W. Tattoo Inks Contain Polycyclic Aromatic Hydrocarbons That Additionally Generate Deleterious Singlet Oxygen. Exp. Dermatol. 2010, 19, e275–e281. [Google Scholar] [CrossRef]
- Safety of Tattoos and Permanent Make-up. Adverse Health Effects and Experience with the Council of Europe Resolution 2008, 1. Available online: https://ec.europa.eu/jrc/en/publication/safety-tattoos-and-permanent-make-adverse-health-effects-and-experience-council-europe-resolution (accessed on 19 April 2021).
- Giulbudagian, M.; Schreiver, I.; Singh, A.V.; Laux, P.; Luch, A. Safety of Tattoos and Permanent Make-up: A Regulatory View. Arch. Toxicol. 2020, 94, 357–369. [Google Scholar] [CrossRef]
- World Famous Tattoo Ink, Safety Data Sheet. Available online: https://www.worldfamoustattooink.com/pages/safety-data-sheets (accessed on 26 April 2021).
- Høgsberg, T.; Loeschner, K.; Löf, D.; Serup, J. Tattoo Inks in General Usage Contain Nanoparticles. Br. J. Dermatol. 2011, 165, 1210–1218. [Google Scholar] [CrossRef]
- CICN Groups & Sub-Groups|Colour Index. Available online: https://colour-index.com/cicn-groups-sub-groups (accessed on 19 April 2021).
- Hutton Carlsen, K.; Køcks, M.; Sepehri, M.; Serup, J. Allergic Reactions in Red Tattoos: Raman Spectroscopy for “fingerprint” Detection of Chemical Risk Spectra in Tattooed Skin and Culprit Tattoo Inks. Skin Res. Technol. 2016, 22, 460–469. [Google Scholar] [CrossRef]
- Yakes, B.J.; Michael, T.J.; Perez-Gonzalez, M.; Harp, B.P. Investigation of Tattoo Pigments by Raman Spectroscopy. J. Raman Spectrosc. 2017, 48, 736–743. [Google Scholar] [CrossRef]
- Darvin, M.E.; Schleusener, J.; Parenz, F.; Seidel, O.; Krafft, C.; Popp, J.; Lademann, J. Confocal Raman Microscopy Combined with Optical Clearing for Identification of Inks in Multicolored Tattooed Skin in Vivo. Analyst 2018, 143, 4990–4999. [Google Scholar] [CrossRef] [PubMed]
- Smulko, J.M.; Dingari, N.C.; Soares, J.S.; Barman, I. Anatomy of Noise in Quantitative Biological Raman Spectroscopy. Bioanalysis 2014, 6, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, M.S.; Popov, A.P.; Bykov, A.V.; Tuchin, V.V.; Jędrzejewska-Szczerska, M. Nanoparticle-Free Tissue-Mimicking Phantoms with Intrinsic Scattering. Biomed. Opt. Express 2016, 7, 2088–2094. [Google Scholar] [CrossRef] [PubMed]
- Pardini, P.A.; Serra, M.V.W.; Ranea-Sandoval, H.F.; Pomarico, J.A.; Iriarte, D.I. Study of Inks Used in Biomedical Optics Phantoms: Stability and Ageing. J. Infrared Spectrosc. 2015, 23, 219–225. [Google Scholar] [CrossRef]
- Listewnik, P.; Ronowska, M.; Wasowicz, M.; Tuchin, V.; Szczerska, M. Porous Phantoms Mimicking Tissues—Investigation of Optical Parameters Stability Over Time. Materials 2021, 14, 423. [Google Scholar] [CrossRef]
- Vardaki, M.; Kourkoumelis, N. Tissue Phantoms for Biomedical Applications in Raman Spectroscopy: A Review. Biomed. Eng. Comput. Biol. 2020, 11, 117959722094810. [Google Scholar] [CrossRef]
- Gnyba, M.; Keränen, M.; Maaninen, A.; Suhonen, J.; Szczerska, M.; Kosmowski, B.; Wierzba, P. Raman System for On-Line Monitoring and Optimisation of Hybrid Polymer Gelation. Opto-Electron. Rev. 2005, 13, 9–17. [Google Scholar]
- Culjat, M.O.; Goldenberg, D.; Tewari, P.; Singh, R.S. A Review of Tissue Substitutes for Ultrasound Imaging. Ultrasound Med. Biol. 2010, 36, 861–873. [Google Scholar] [CrossRef]
- Engel, E.; Santarelli, F.; Vasold, R.; Maisch, T.; Ulrich, H.; Prantl, L.; König, B.; Landthaler, M.; Bäumler, W. Modern tattoos cause high concentrations of hazardous pigments in skin. Contact Dermat. 2008, 58, 228–233. [Google Scholar] [CrossRef] [PubMed]
- SpectraBase. Available online: https://spectrabase.com/ (accessed on 26 April 2021).
- Spectral Database Index|IRUG. Available online: http://www.irug.org/search-spectral-database/spectra-index?sortHeader=data_type_raman (accessed on 26 April 2021).
| Label | Phantom A | Phantom B | Phantom C |
|---|---|---|---|
| Proportions of glycerin: PDMS vol.:vol. (mL) | 1.5:11 | 2:11 | 2.5:11 |
| Reduced scattering coefficient (µs’ cm−1) | 1.25 | 1.5 | 1.75 |
| Label (Color) | Ink 1 (White) | Ink 2 (Red) | Ink 3 (Pink) | Ink 4 (Violet) | Ink 5 (Orange) |
|---|---|---|---|---|---|
| Name (producer) | White House (World Famous Tattoo INK) | Paul Rogers Red (World Famous Tattoo INK) | Hot Pink (Eternal) | Forbidden City (World Famous Tattoo INK) | Bright Orange (Eternal) |
| Ingredients | Pigment: C.I.77891, Glycerin, Isopropyl alcohol, Rosin, Hamamelis Virginiana, benzyl alcohol | Water, pigment: C.I.12475, Glycerin, Isopropyl alcohol Rosin, Hamamelis Virginiana, benzyl alcohol | Water, pigment: C.I.77891, C.I.12477, Glycerin, Isopropyl alcohol | Water, pigment: C.I.77891, C.I.12466, C.I.74160, C.I.12475, Glycerin, Isopropyl alcohol, Rosin, Hamamelis Virginiana, DMDM Hydantoin | Water, pigment: C.I.77891, C.I.21160, C.I.21108, Glycerin, Isopropyl alcohol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadura, F.; Wróbel, M.S.; Karpienko, K. Colored Tattoo Ink Screening Method with Optical Tissue Phantoms and Raman Spectroscopy. Materials 2021, 14, 3147. https://doi.org/10.3390/ma14123147
Sadura F, Wróbel MS, Karpienko K. Colored Tattoo Ink Screening Method with Optical Tissue Phantoms and Raman Spectroscopy. Materials. 2021; 14(12):3147. https://doi.org/10.3390/ma14123147
Chicago/Turabian StyleSadura, Filip, Maciej S. Wróbel, and Katarzyna Karpienko. 2021. "Colored Tattoo Ink Screening Method with Optical Tissue Phantoms and Raman Spectroscopy" Materials 14, no. 12: 3147. https://doi.org/10.3390/ma14123147
APA StyleSadura, F., Wróbel, M. S., & Karpienko, K. (2021). Colored Tattoo Ink Screening Method with Optical Tissue Phantoms and Raman Spectroscopy. Materials, 14(12), 3147. https://doi.org/10.3390/ma14123147






