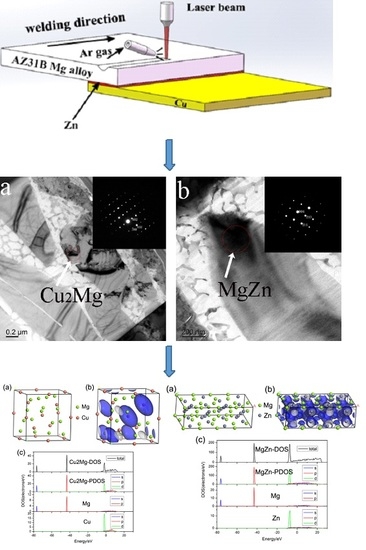
Improvement of the Laser-Welded Lap Joint of Dissimilar Mg Alloy and Cu by Incorporation of a Zn Interlayer
Abstract
1. Introduction
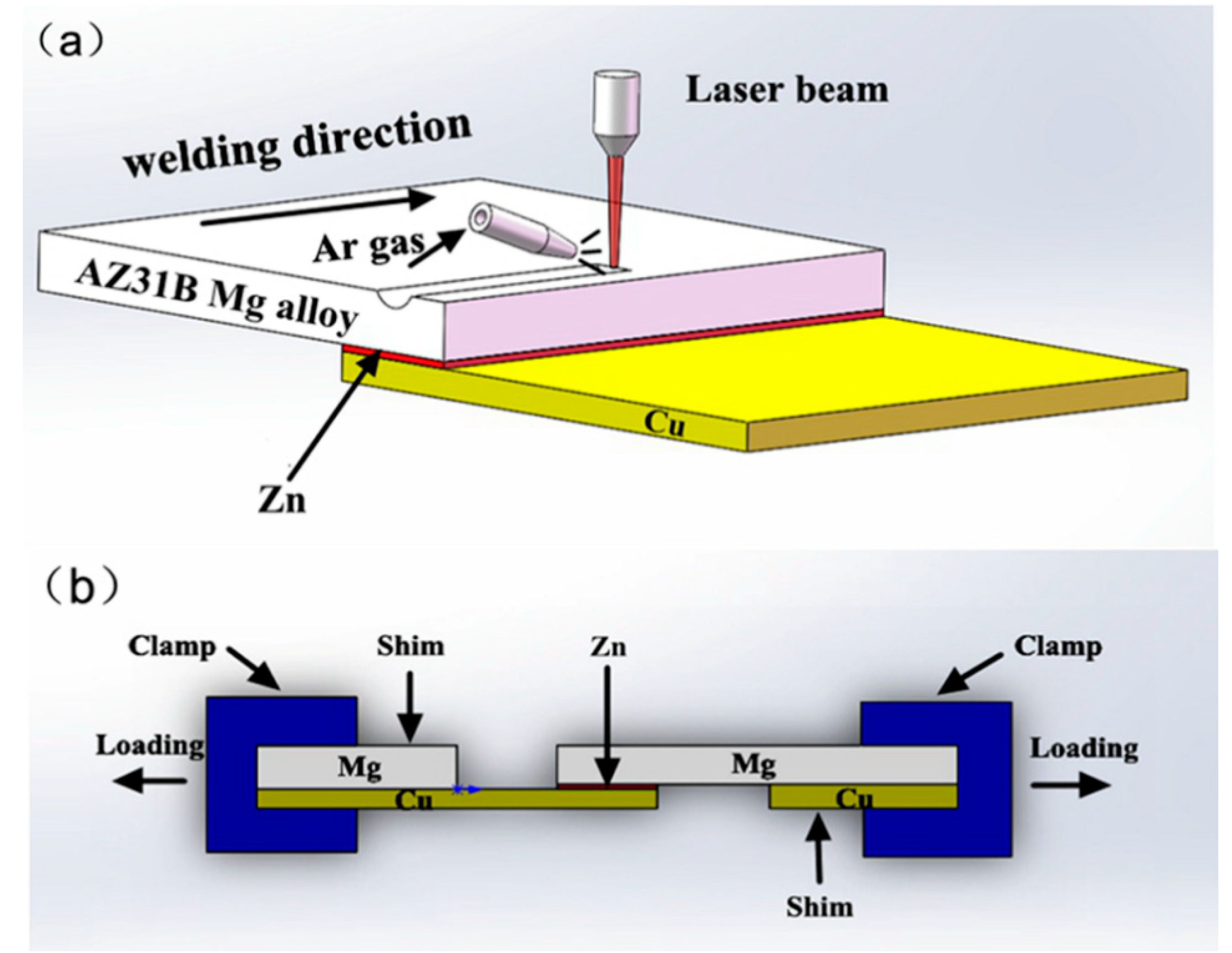
2. Experimental Details
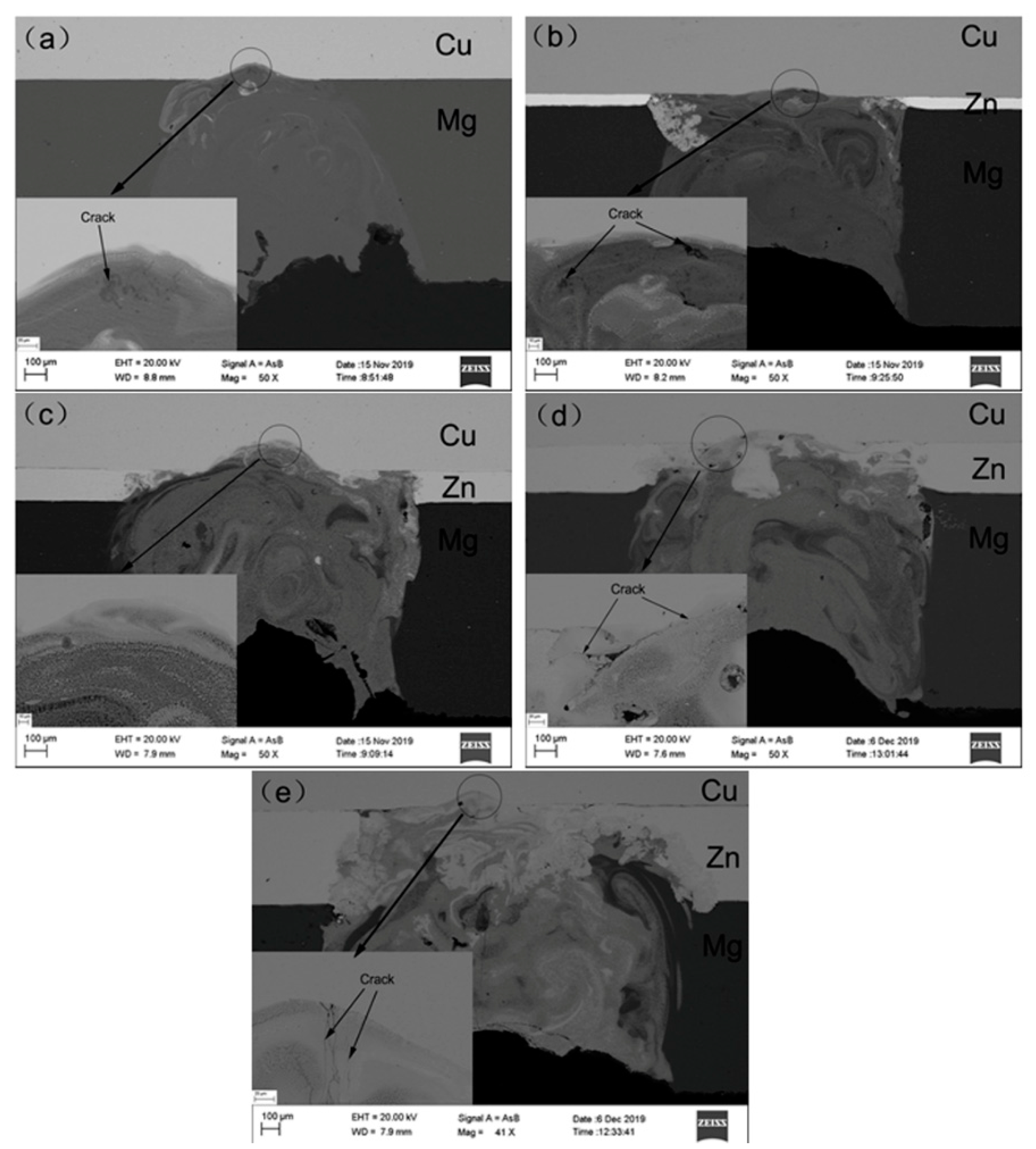
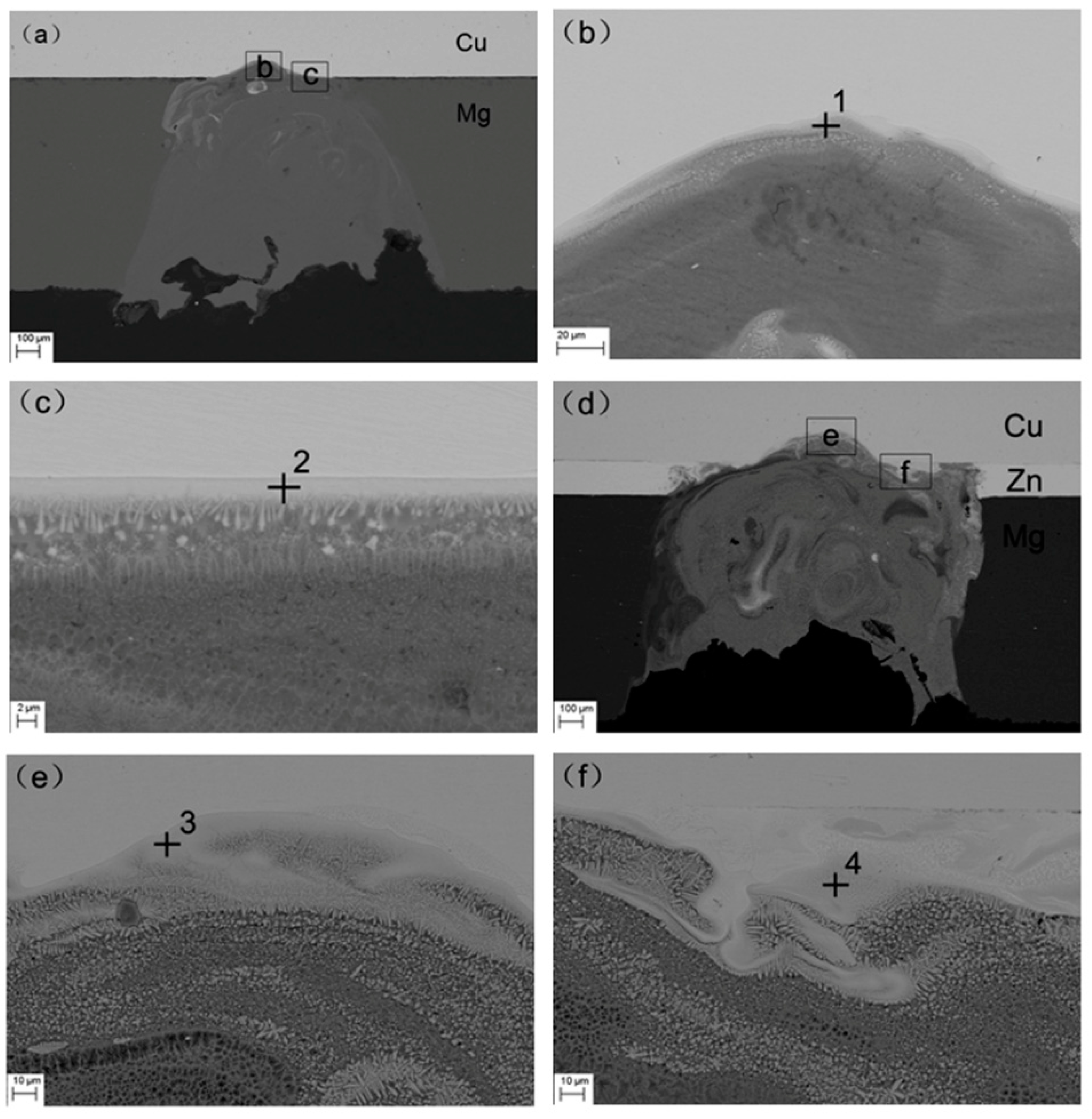
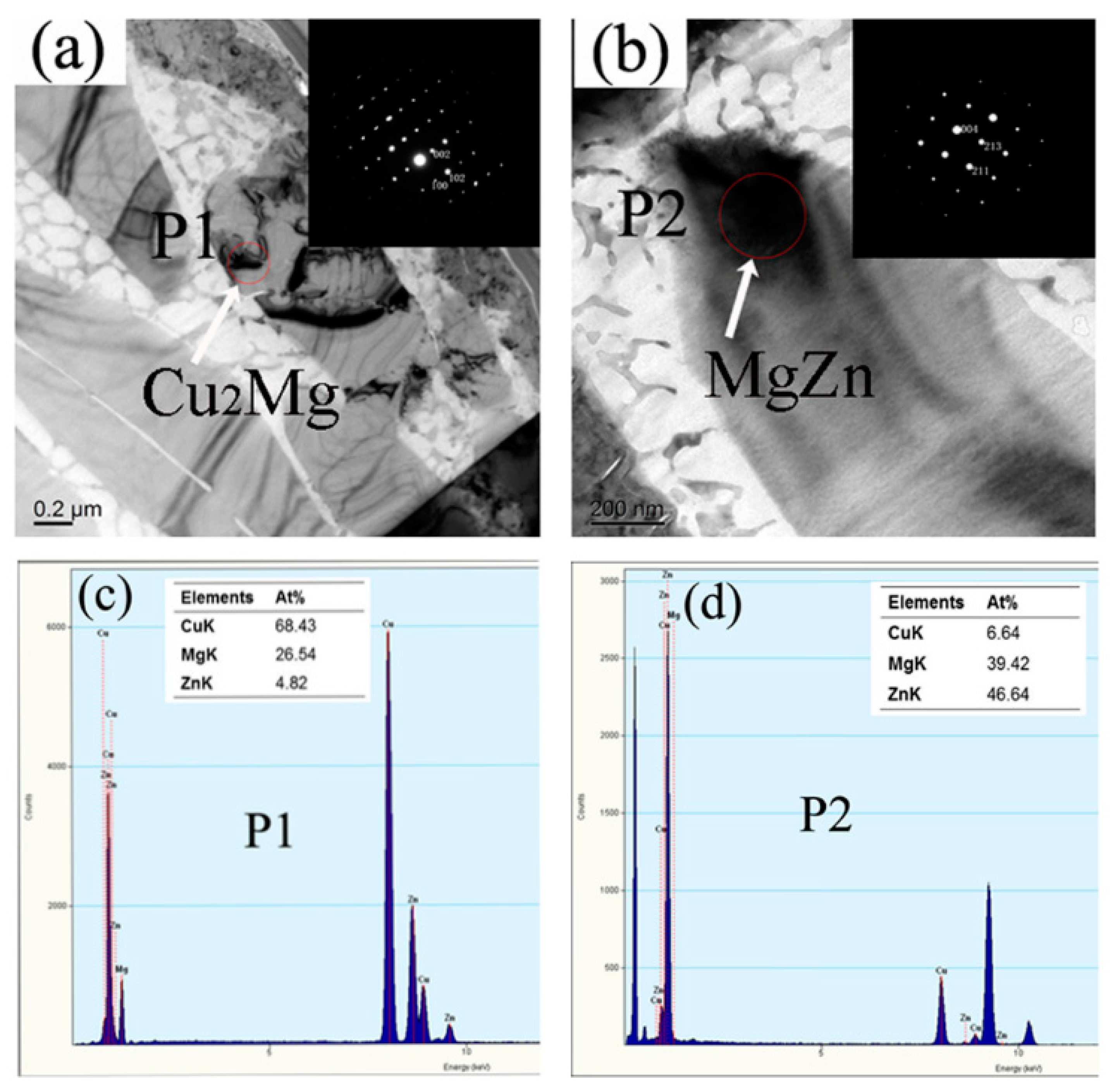
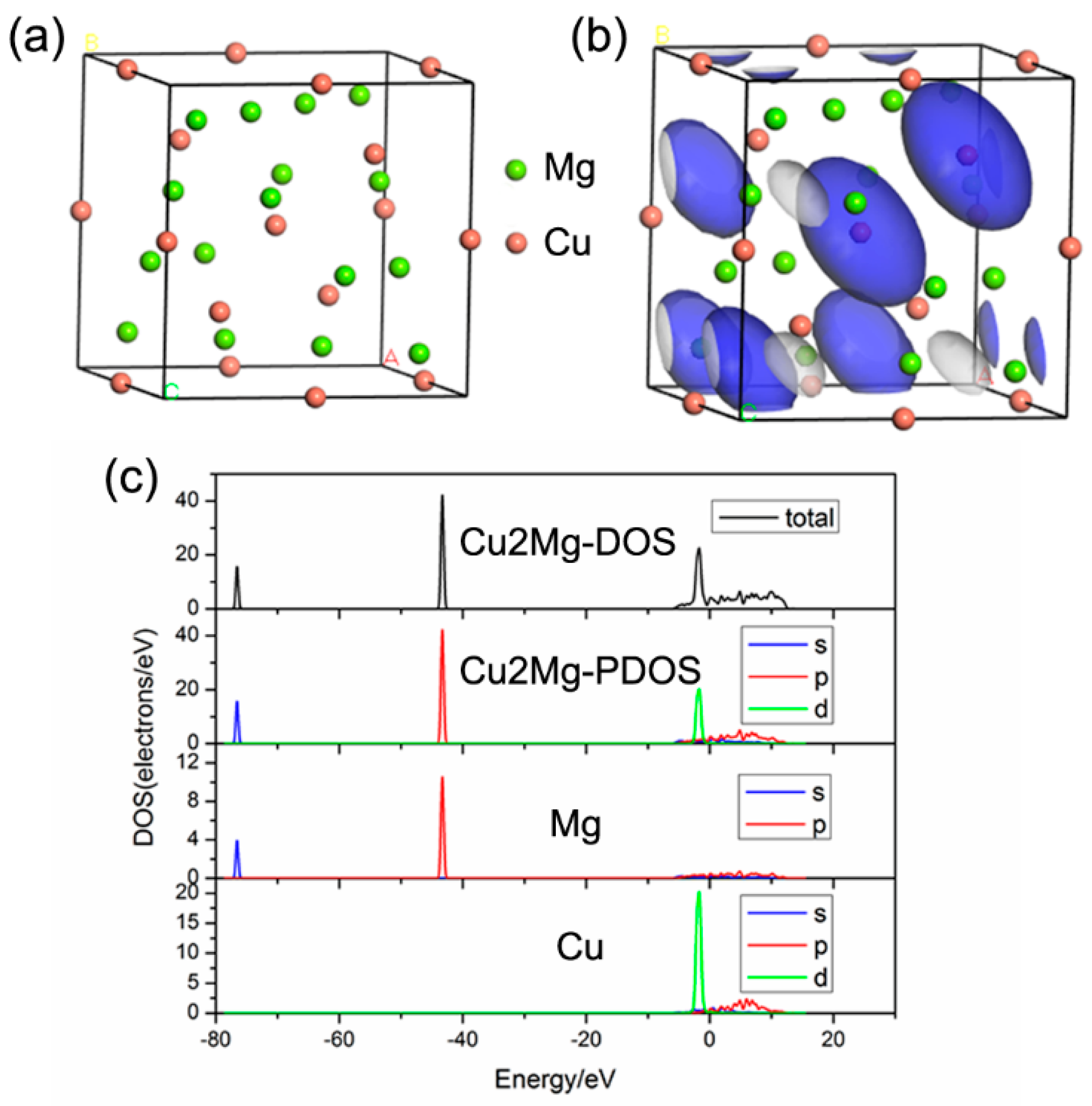
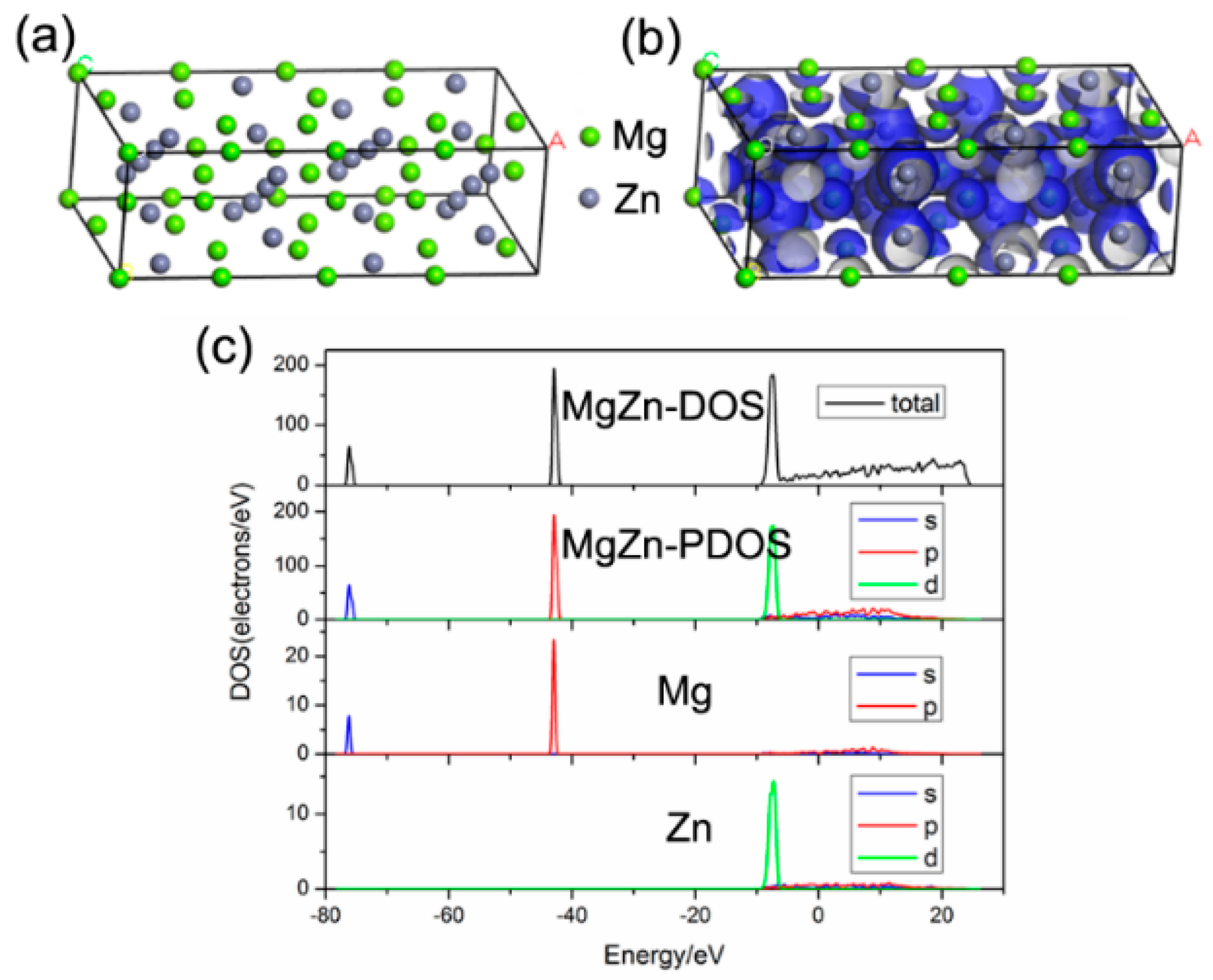
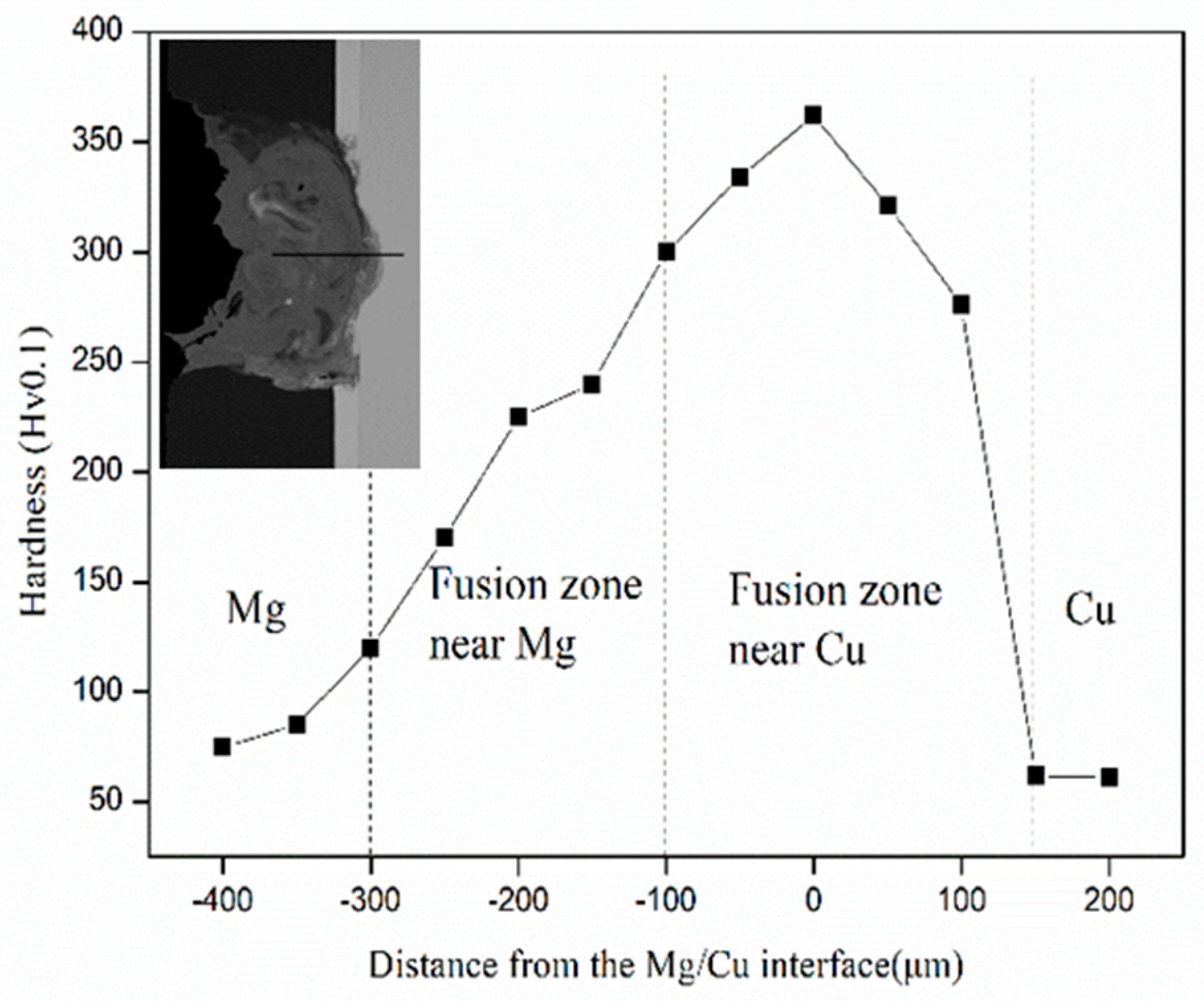
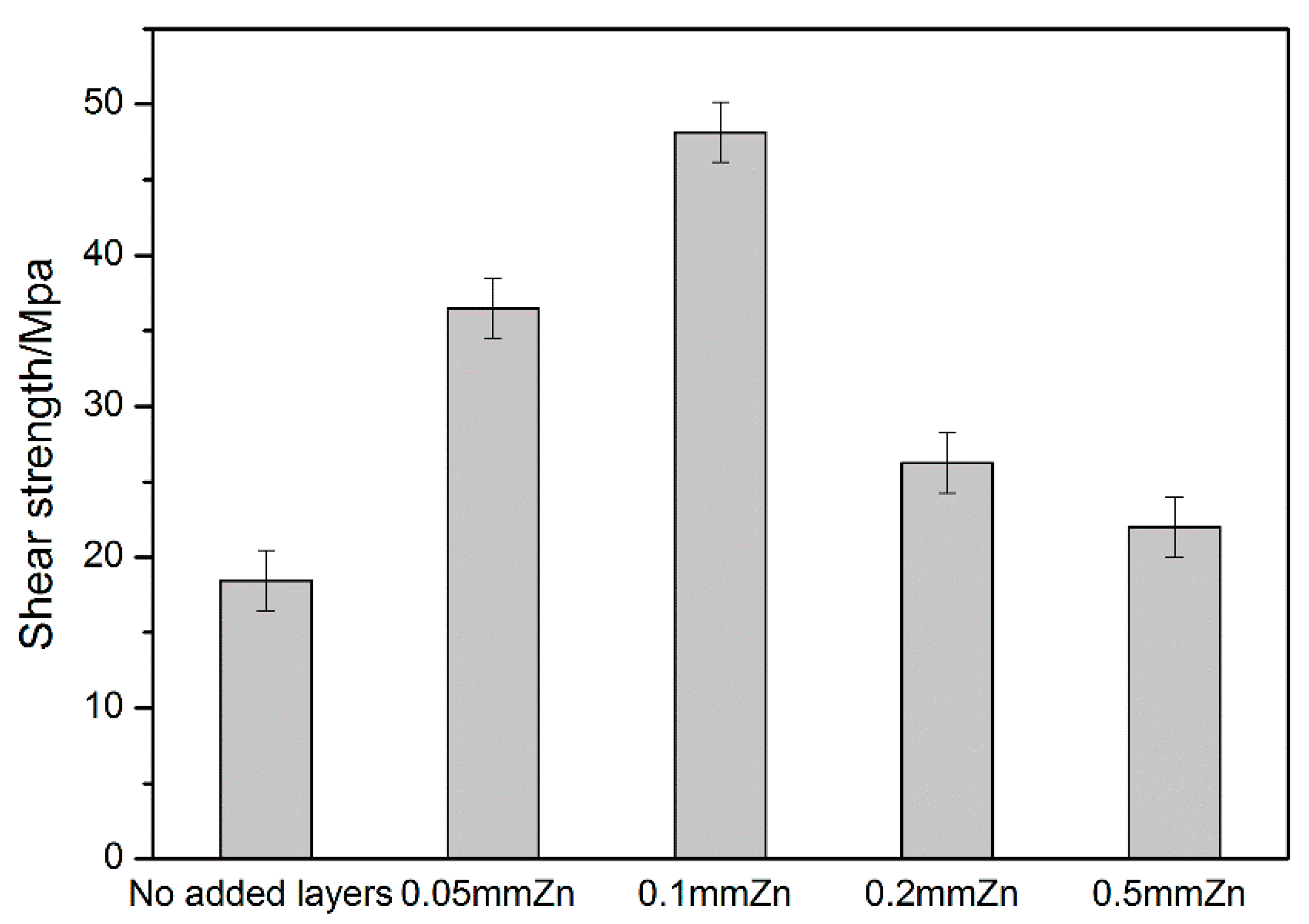
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, X.; Lei, Z.L.; Chen, Y.B.; Han, Y.; Jiang, M.; Tian, Z.; Bi, J.; Lin, S.B.; Jiang, N. Effect of Laser Beam Oscillation on Laser Welding-Brazing of Ti/Al Dissimilar Metals. Materials 2019, 12, 4165. [Google Scholar] [CrossRef] [PubMed]
- Chitturi, V.; Pedapati, S.R.; Awang, M. Effect of Tilt Angle and Pin Depth on Dissimilar Friction Stir Lap Welded Joints of Aluminum and Steel Alloys. Materials 2019, 12, 3901. [Google Scholar] [CrossRef]
- Montemor, M.F.; Simoes, A.M.; Carmezim, M.J. Characterization of rare-earth conversion films formed on the AZ31 magnesium alloy and its relation with corrosion protection. Appl. Surf. Sci. 2007, 253, 6922–6931. [Google Scholar] [CrossRef]
- Li, W.; Zhou, H.; Zhou, W.; Li, W.P.; Wang, M.X. Effect of cooling rate on ignition point of AZ91D–0.98 wt% Ce magnesium alloy. Mater. Lett. 2007, 61, 2772–2774. [Google Scholar] [CrossRef]
- Ishihara, S.; Nan, Z.; Goshima, T. Effect of microstructure on fatigue behavior of AZ31 magnesium alloy. Mater. Sci. Eng. A 2007, 468, 214–222. [Google Scholar] [CrossRef]
- Xie, C.S.; Zhai, Q.M.; Xu, W.Q. Study and Application Development of Strengthening Theory of Copper Alloy with High Strength and High Conductivity. Heat Treat. Met. 2007, 1, 12–19. [Google Scholar]
- Chen, J.; Liu, X.P.; Liang, H. Research progress and application of plasma spraying on copper alloy surface. Heat Treat. Met. 2010, 35, 98–103. [Google Scholar]
- Suetsugu, Y.; Shirai, M.; Ohtsuka, M. Development of copper-alloy Matsumoto–Ohtsuka-type vacuum flanges and its application to accelerator beam pipes. J. Vac. Sci. Technol. A 2009, 27, 1303–1308. [Google Scholar] [CrossRef]
- Sun, Z.; Ion, J. Laser welding of dissimilar metal combinations. J. Mater. Sci. 1995, 30, 4205–4214. [Google Scholar] [CrossRef]
- Mahendran, G.; Balasubramanian, V.; Senthilvelan, T. Influences of diffusion bonding process parameters on bond characteristics of Mg–Cu dissimilar joints. Trans. Nonferr. Met. Soc. 2010, 20, 997–1005. [Google Scholar] [CrossRef]
- Cao, R.; Jing, M.; Feng, Z.; Chen, J.H. Cold metal transfer welding–brazing of magnesium to pure copper. Sci. Technol. Weld. Join. 2014, 19, 451–460. [Google Scholar] [CrossRef]
- Macwan, A.; Chen, D.L. Microstructure and mechanical properties of ultrasonic spot welded copper-to-magnesium alloy joints. Mater. Des. 2015, 84, 261–269. [Google Scholar] [CrossRef]
- Mahendran, G.; Balasubramanian, V.; Senthilvelan, T. Developing diffusion bonding windows for joining AZ31B magnesium and copper alloys. Int. J. Adv. Maunf. Technol. 2009, 42, 689–695. [Google Scholar] [CrossRef]
- Du, S.; Liu, G.; Wang, M. Diffusion Bonding Preparation of AZ31B/Cu Composite and Analysis of Interface Microstructure. Rare Met. Mater. Eng. 2014, 43, 475–480. [Google Scholar]
- Liu, L.M.; Wang, S.X.; Zhu, M.L. Study on TIG welding of dissimilar Mg alloy and Cu with Fe as interlayer. Sci. Technol. Weld. Join. 2006, 11, 523–525. [Google Scholar] [CrossRef]
- Liu, L.M.; Wang, S.X.; Zhao, L.M. Study on the dissimilar magnesium alloy and copper lap joint by TIG welding. Mater. Sci. Eng. A 2008, 476, 206–209. [Google Scholar]
- Zhao, X.Y.; Tan, C.W.; Meng, S.H.; Chen, B.; Song, X.G.; Li, L.Q.; Feng, J.C. Fiber Laser Welding–Brazing Characteristics of Dissimilar Metals AZ31B Mg Alloys to Copper with Mg-Based Filler. J. Mater. Eng. Perform. 2018, 3, 1427–1439. [Google Scholar] [CrossRef]
- Tan, C.W.; He, W.X.; Gong, X.T.; Li, L.Q.; Feng, J.C. Influence of laser power on microstructure and mechanical properties of fiber laser-tungsten inert gas hybrid welded Mg/Cu dissimilar joints. Mater. Des. 2015, 78, 51–62. [Google Scholar] [CrossRef]
- Lang, Q.; Wang, Q.; Han, J.; Zhang, W.J.; Zhang, Y.; Yan, J.C. Microstructure evolution of Mg/Cu dissimilar metal jointed by ultrasonic-induced transient liquid phase bonding with Zn interlayer. Mater. Charact. 2019, 157, 109897. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 11, 2717–2744. [Google Scholar] [CrossRef]
- Yan, P.; Zhou, D.W.; Xu, S.H.; Ping, P.; Liu, J.S. First-Principles Studies of the Effects of Microalloy Elements on Fe/Al Interface for Laser Lap Welding of Steel and Aluminum. Rare Met. Mater. Eng. 2012, 41, 302–306. [Google Scholar]
- Jin, Y.J.; Khan, T.I. Effect of bonding time on microstructure and mechanical properties of transient liquid phase bonded magnesium AZ31 alloy. Mater. Des. 2012, 38, 32–37. [Google Scholar] [CrossRef]
- Xie, R.S.; Chen, X.G.; Lai, Z.W. Microstructure, mechanical properties and mechanism of ultrasound-assisted rapid transient liquid phase bonding of magnesium alloy in air. Mater. Des. 2016, 91, 19–27. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Yang, M.Y.; Zhao, N. Two-Dimensional Transition Metal Dichalcogenides as the Promising Anodes for Potassium Ion Battery from First-principles Prediction. Phys. Chem. Chem. Phys. 2019, 42, 21–25. [Google Scholar] [CrossRef]
- Li, C.M.; Zeng, S.M.; Chen, Z.Q. First-principles calculations of elastic and thermodynamic properties of the four main intermetallic phases in Al–Zn–Mg–Cu alloys. Comput. Mater. Sci 2014, 93, 210–220. [Google Scholar] [CrossRef]

| Alloy | Al | Zn | Mn | Si | Fe | Cu | Ca | Mg |
|---|---|---|---|---|---|---|---|---|
| AZ31B Mg | 3.19 | 0.81 | 0.334 | 0.02 | 0.005 | 0.05 | 0.04 | Bal. |
| Alloy | Fe | O | Ni | S | Pb | Bi | Ti | Cu |
|---|---|---|---|---|---|---|---|---|
| T2 Cu | 0.005 | 0.06 | 0.006 | 0.001 | 0.001 | 0.001 | 0.006 | Bal. |
| Elements | Cu | Mg | Zn |
|---|---|---|---|
| Spectrum 1 | 32.39 | 67.61 | -- |
| Spectrum 2 | 65.95 | 34.05 | -- |
| Spectrum 3 | 54.82 | 33.35 | 11.83 |
| Spectrum 4 | 12.05 | 41.32 | 46.63 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, J.; Yu, B.; Ruan, Q.; Chu, P.K. Improvement of the Laser-Welded Lap Joint of Dissimilar Mg Alloy and Cu by Incorporation of a Zn Interlayer. Materials 2020, 13, 2053. https://doi.org/10.3390/ma13092053
Dai J, Yu B, Ruan Q, Chu PK. Improvement of the Laser-Welded Lap Joint of Dissimilar Mg Alloy and Cu by Incorporation of a Zn Interlayer. Materials. 2020; 13(9):2053. https://doi.org/10.3390/ma13092053
Chicago/Turabian StyleDai, Jun, Banglong Yu, Qingdong Ruan, and Paul K. Chu. 2020. "Improvement of the Laser-Welded Lap Joint of Dissimilar Mg Alloy and Cu by Incorporation of a Zn Interlayer" Materials 13, no. 9: 2053. https://doi.org/10.3390/ma13092053
APA StyleDai, J., Yu, B., Ruan, Q., & Chu, P. K. (2020). Improvement of the Laser-Welded Lap Joint of Dissimilar Mg Alloy and Cu by Incorporation of a Zn Interlayer. Materials, 13(9), 2053. https://doi.org/10.3390/ma13092053