NiYAl-Derived Nanoporous Catalysts for Dry Reforming of Methane
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Nanoporous Catalysts
2.2. Preparation of Conventional Ni Catalysts
2.3. Microstructural Characterization
2.4. Catalytic Studies
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Song, Y.; Ozdemir, E.; Ramesh, S.; Adishev, A.; Subramanian, S.; Harale, A.; Albuali, M.; Fadhel, B.A.; Jamal, A.; Moon, D.; et al. Dry reforming of methane by stable Ni-Mo nanocatalysts on single-crystalline MgO. Science 2020, 367, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Muraza, O.; Galadima, A. A review on coke management during dry reforming of methane. Int. J. Energy Res. 2015, 39, 1196–1216. [Google Scholar] [CrossRef]
- Nair, M.M.; Kaliaguine, S. Structured catalysts for dry reforming of methane. New J. Chem. 2016, 40, 4049–4060. [Google Scholar] [CrossRef]
- Lavoie, J.M. Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front. Chem. 2014, 2, 81. [Google Scholar] [CrossRef] [PubMed]
- Kathiraser, Y.; Thisartarn, W.; Sutthiumporn, K.; Kawi, S. Inverse NiAl2O4 on LaAlO3−Al2O3: Unique catalytic structure for stable CO2 reforming of methane. J. Phys. Chem. C 2013, 117, 8120–8130. [Google Scholar] [CrossRef]
- Ashok, J.; Bian, Z.; Wang, Z.; Kawi, S. Ni-phyllosilicate structure derived Ni–SiO2–MgO catalysts for bi-reforming applications: Acidity, basicity and thermal stability. Catal. Sci. Technol. 2018, 8, 1730–1742. [Google Scholar] [CrossRef]
- Ni, J.; Chen, L.; Lin, J.; Kawi, S. Carbon deposition on borated alumina supported nano-sized Ni catalysts for dry reforming of CH4. Nano Energy 2012, 1, 674–686. [Google Scholar] [CrossRef]
- Bian, Z.; Suryawinata, I.Y.; Kawi, S. Highly carbon resistant multicore-shell catalyst derived from Ni-Mg phyllosilicate nanotubes@silica for dry reforming of methane. Appl. Catal. B 2016, 195, 1–8. [Google Scholar] [CrossRef]
- Bian, Z.; Kawi, S. Sandwich-like Silica@Ni@Silica multicore–shell catalyst for the low-temperature dry reforming of methane: Confinement effect against carbon formation. ChemCatChem 2018, 10, 320–328. [Google Scholar] [CrossRef]
- Shoji, S.; Peng, X.; Imai, T.; Kumar, P.S.M.; Higuchi, K.; Yamamoto, Y.; Tokunaga, T.; Arai, S.; Ueda, S.; Hashimoto, A.; et al. Topologically immobilized catalysis centre for long-term stable carbon dioxide reforming of methane. Chem. Sci. 2019, 10, 3701–3705. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Ueda, S.; Nagao, S.; Hirata, H.; Deepthi, K.R.; Abe, H. N2O-emission-free exhaust remediation by Rh-NbOx nanocomposites developed from Rh3Nb alloy precursor. RSC Adv. 2017, 7, 9628–9631. [Google Scholar] [CrossRef]
- Tanabe, T.; Imai, T.; Tokunaga, T.; Arai, S.; Yamamoto, Y.; Ueda, S.; Ramesh, G.V.; Nagao, S.; Hirata, H.; Matsumoto, S.; et al. Nanophase-separated Ni3Nb as an automobile exhaust catalyst. Chem. Sci. 2017, 8, 3374–3378. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Peng, X.; Yamaguchi, A.; Cho, Y.; Zhang, Y.; Higuchi, K.; Yamamoto, Y.; Tokunaga, T.; Arai, S.; Miyauchi, M.; et al. Nanoporous nickel composite catalyst for the dry reforming of methane. ACS Omega 2018, 3, 16651–16657. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Abe, H.; Tanabe, T.; Ito, Y.; Tokunaga, T.; Arai, S.; Yamamoto, Y.; Hirata, A.; Chen, M.W. Earth-abundant and durable nanoporous catalyst for exhaust-gas conversion. Adv. Funct. Mater. 2016, 26, 1609–1616. [Google Scholar] [CrossRef]
- Huang, J.; Yang, B.; Chen, H.; Wang, H. Thermodynamic optimisation of the Ni-Al-Y ternary system. J. Phase Equilib. Diffus. 2015, 36, 357–365. [Google Scholar] [CrossRef]
- Li, Q.; Feng, C.; Jiao, Q.; Guo, L.; Liu, C.; Xu, H.B. Shape-controlled synthesis of yttria nanocrystals under hydrothermal conditions. Phys. Stat. Sol. (a) 2004, 201, 3055–3059. [Google Scholar] [CrossRef]

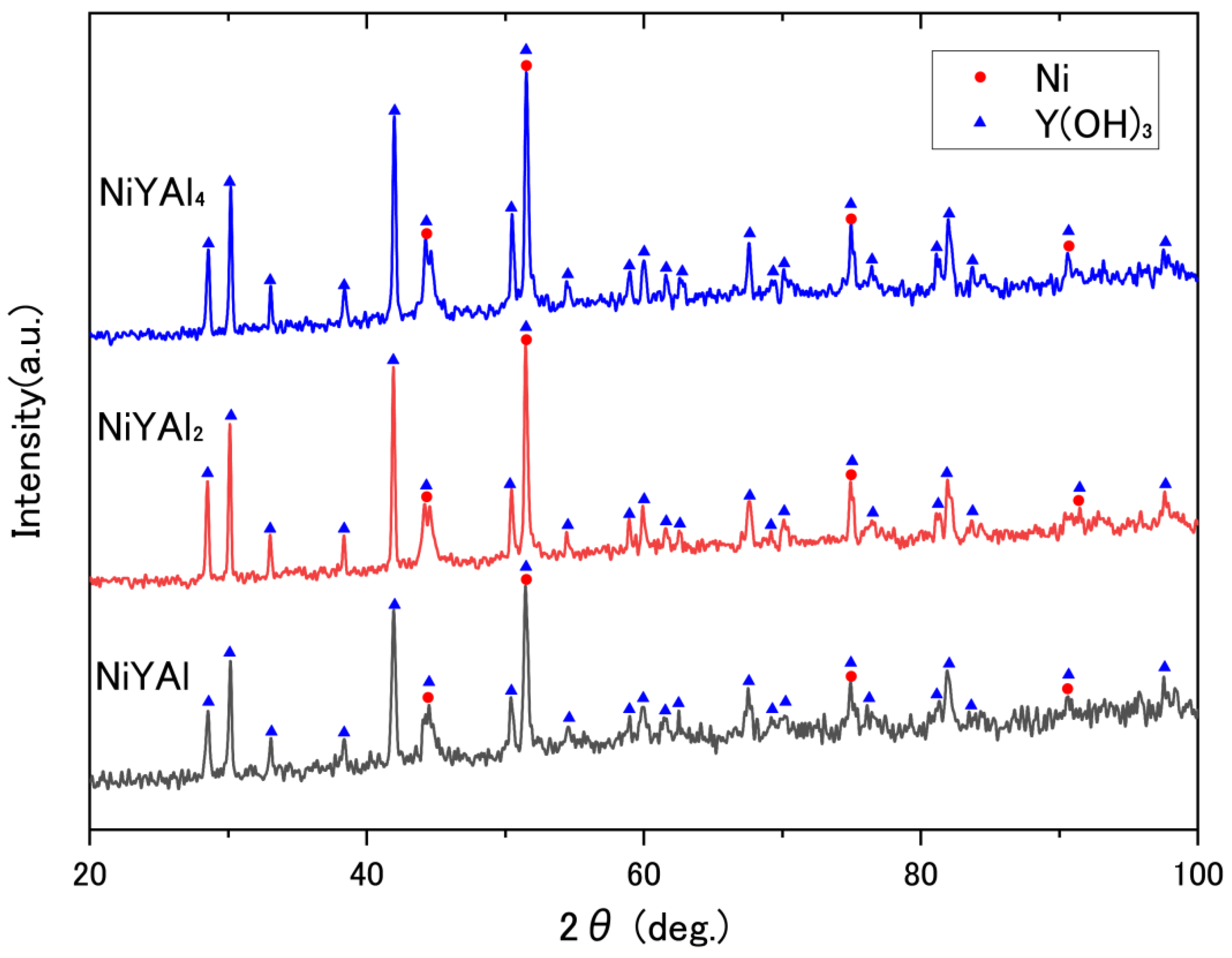
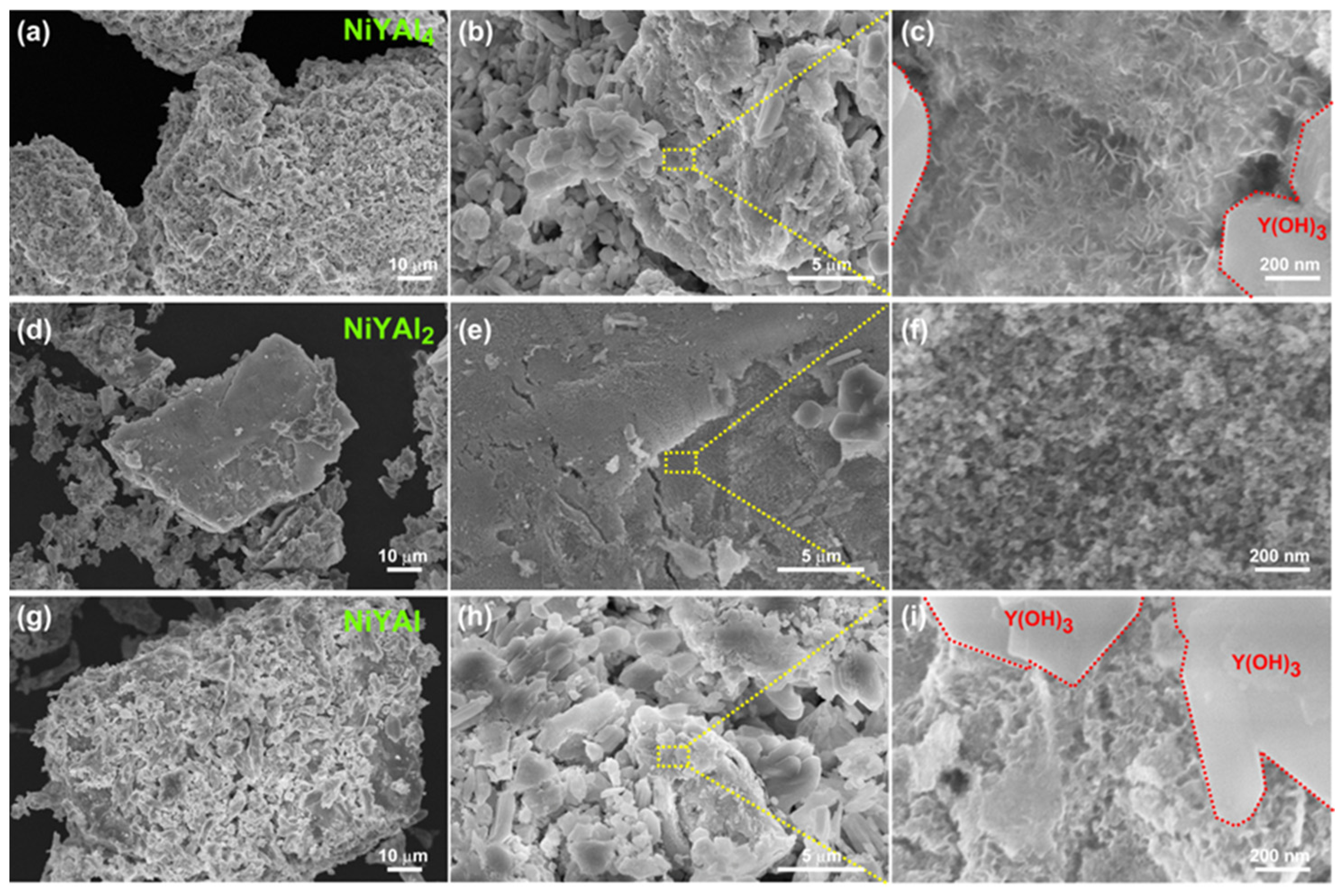
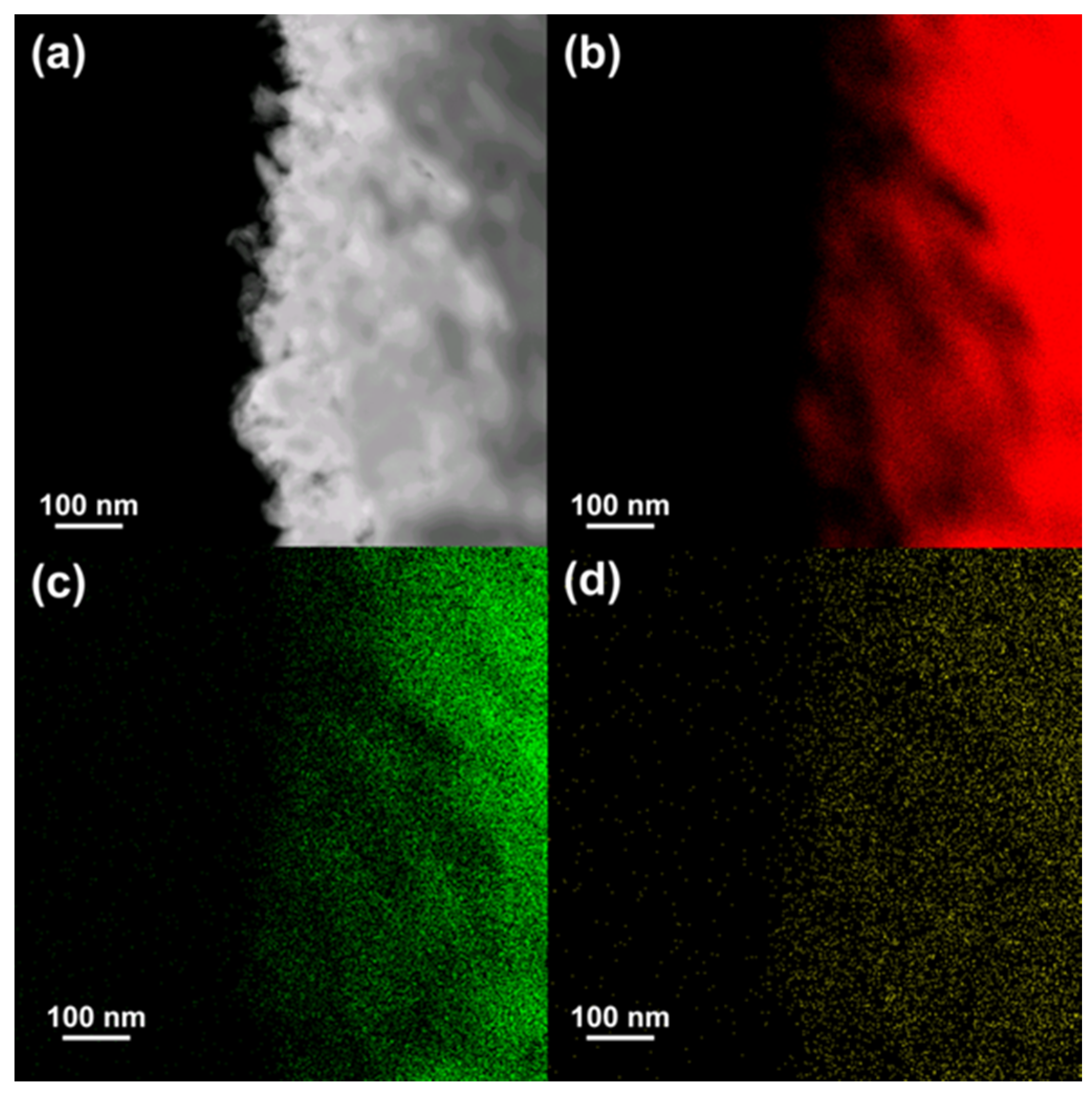

| Sample | CH4 Conv. (%) | CO2 Conv. (%) | CH4 Consumption Rate (mmol h−1) | CO2 Consumption Rate (mmol h−1) | H2 Formation Rate (mmol h−1) | CO Formation Rate (mmol h−1) | H2/CO Ratio |
|---|---|---|---|---|---|---|---|
| NiYAl4 | 12 | 19 | 3.3 | 5.1 | 5.7 | 8.1 | 0.7 |
| NiYAl2 | 45 | 33 | 12 | 8.8 | 18 | 8.5 | 2.2 |
| NiYAl | 32 | 29 | 8.5 | 7.9 | 14 | 9.8 | 1.4 |
| NiYAl4 (CO + O2) | 0 | 0 | - | - | - | - | - |
| Ni/Al2O3 | 56 | 37 | 15 | 10 | 18 | 7.7 | 2.3 |
| Raney Ni | 33 | 31 | 8.9 | 8.5 | 17 | 13 | 1.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imada, S.; Peng, X.; Cai, Z.; Najib, A.S.B.M.; Miyauchi, M.; Abe, H.; Fujita, T. NiYAl-Derived Nanoporous Catalysts for Dry Reforming of Methane. Materials 2020, 13, 2044. https://doi.org/10.3390/ma13092044
Imada S, Peng X, Cai Z, Najib ASBM, Miyauchi M, Abe H, Fujita T. NiYAl-Derived Nanoporous Catalysts for Dry Reforming of Methane. Materials. 2020; 13(9):2044. https://doi.org/10.3390/ma13092044
Chicago/Turabian StyleImada, Syota, Xiaobo Peng, Zexing Cai, Abdillah Sani Bin Mohd Najib, Masahiro Miyauchi, Hideki Abe, and Takeshi Fujita. 2020. "NiYAl-Derived Nanoporous Catalysts for Dry Reforming of Methane" Materials 13, no. 9: 2044. https://doi.org/10.3390/ma13092044
APA StyleImada, S., Peng, X., Cai, Z., Najib, A. S. B. M., Miyauchi, M., Abe, H., & Fujita, T. (2020). NiYAl-Derived Nanoporous Catalysts for Dry Reforming of Methane. Materials, 13(9), 2044. https://doi.org/10.3390/ma13092044






