Atmospheric Pressure Plasma Deposition of Organosilicon Thin Films by Direct Current and Radio-frequency Plasma Jets
Abstract
1. Introduction
2. Experimental Part
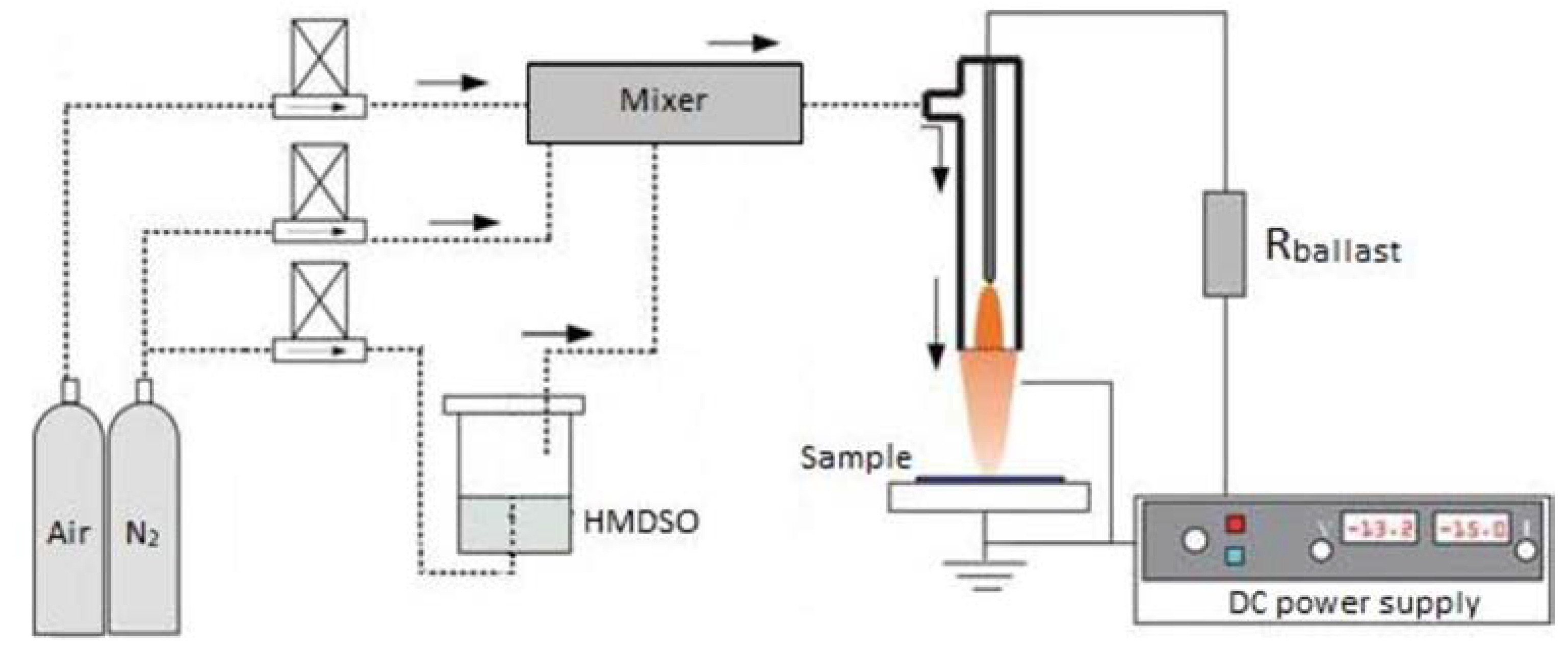
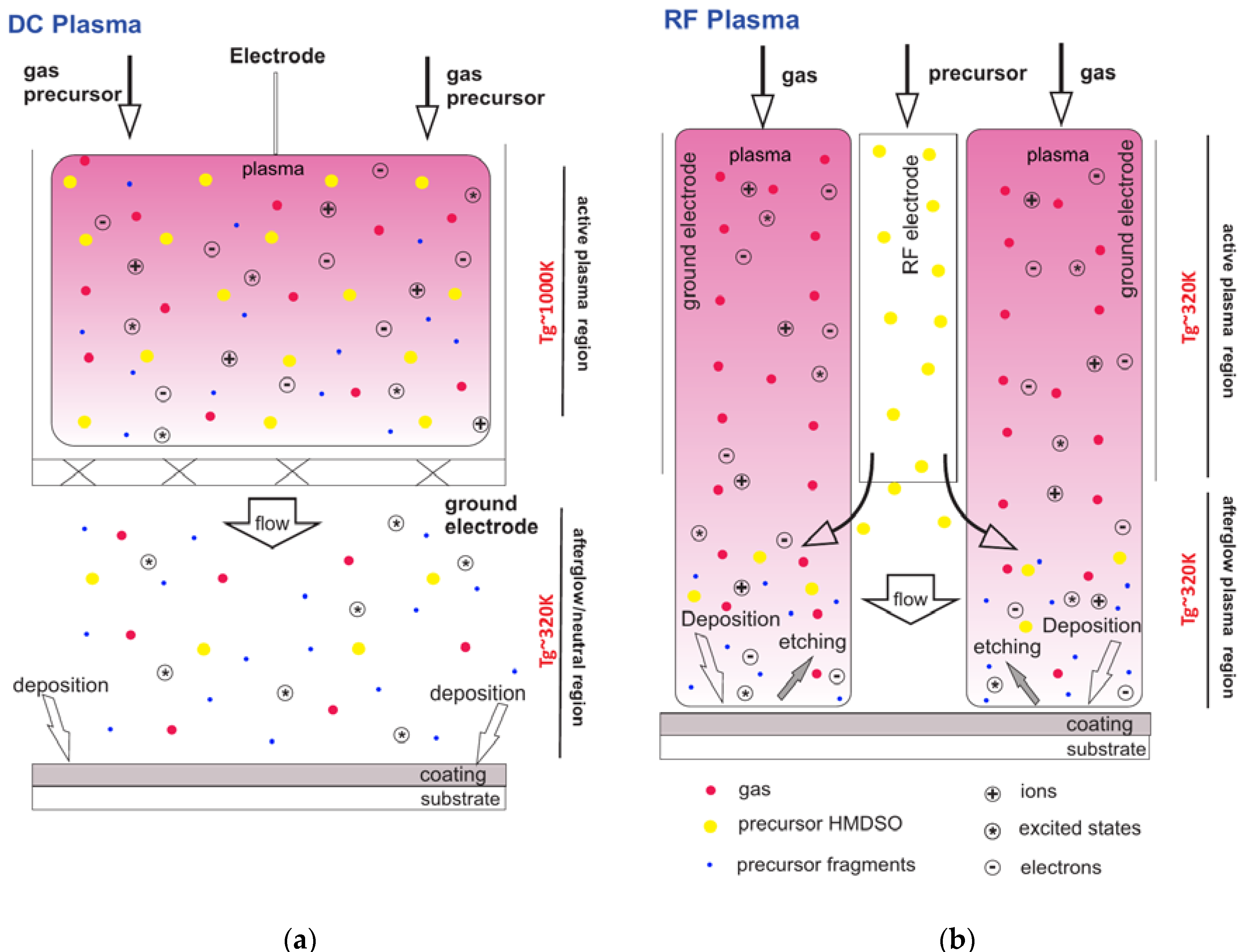
2.1. DC Plasma Jet
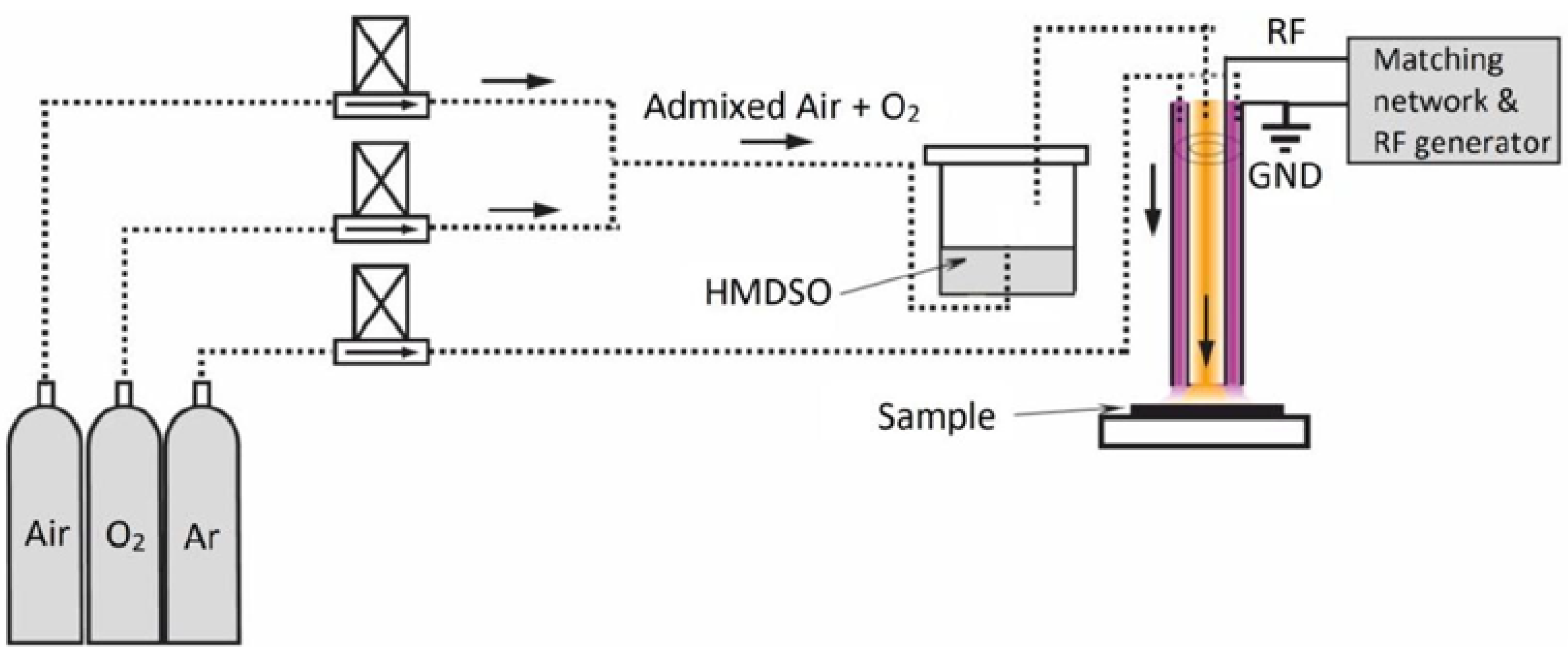
2.2. RF Plasma Jet
2.3. Surface Characterization
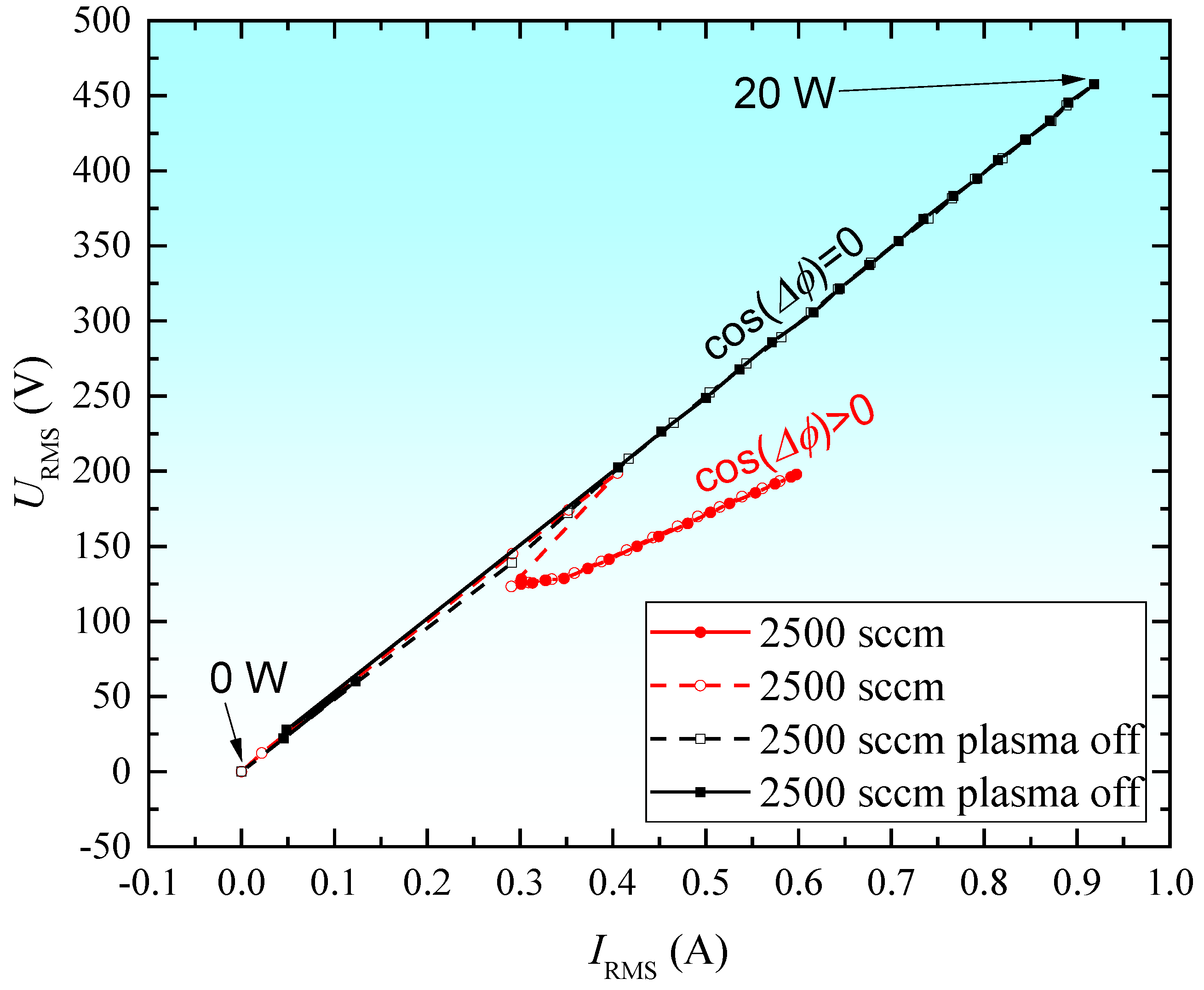
2.4. Discharge Characteristics and Deposition Temperature
3. Results and Discussion
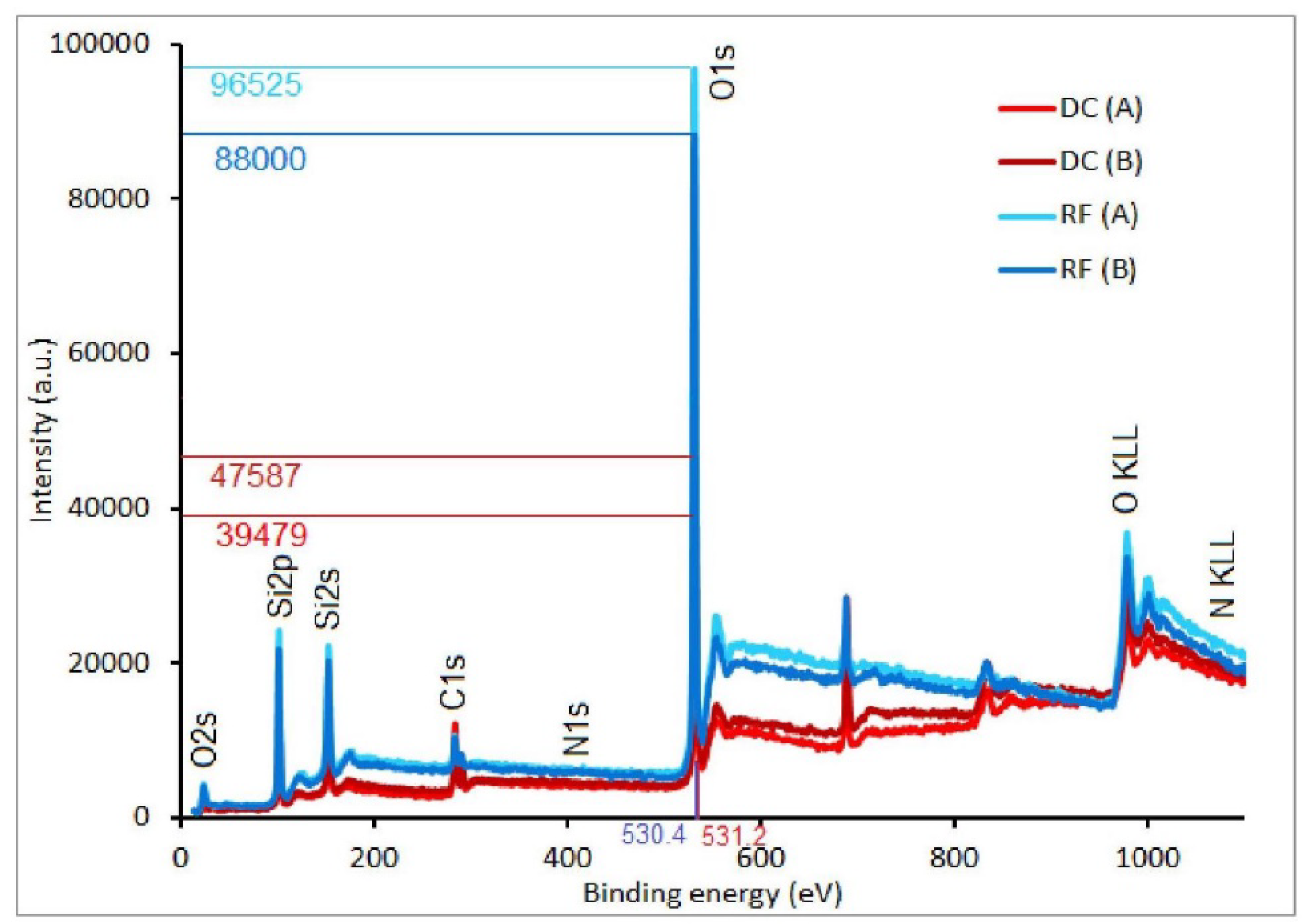
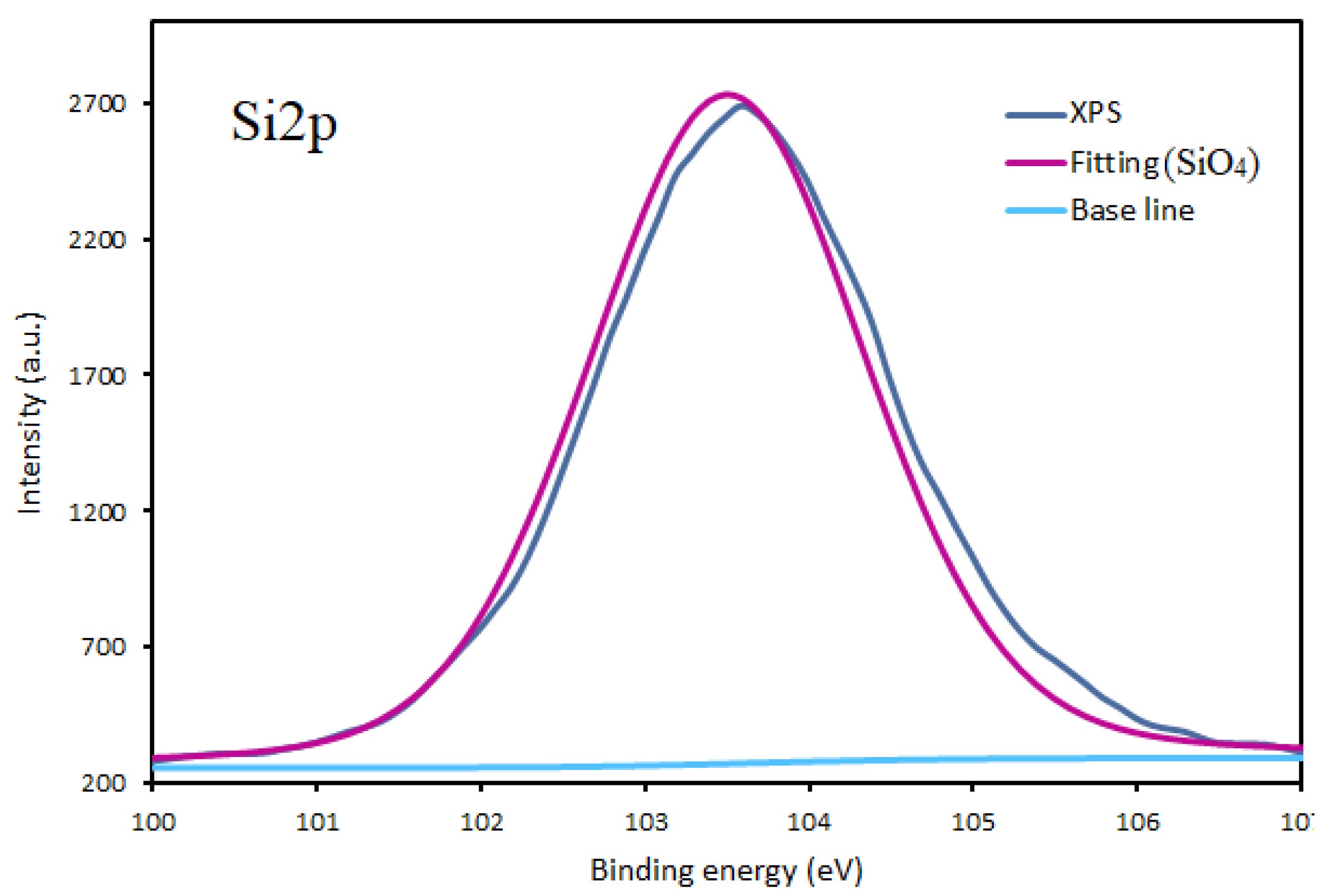
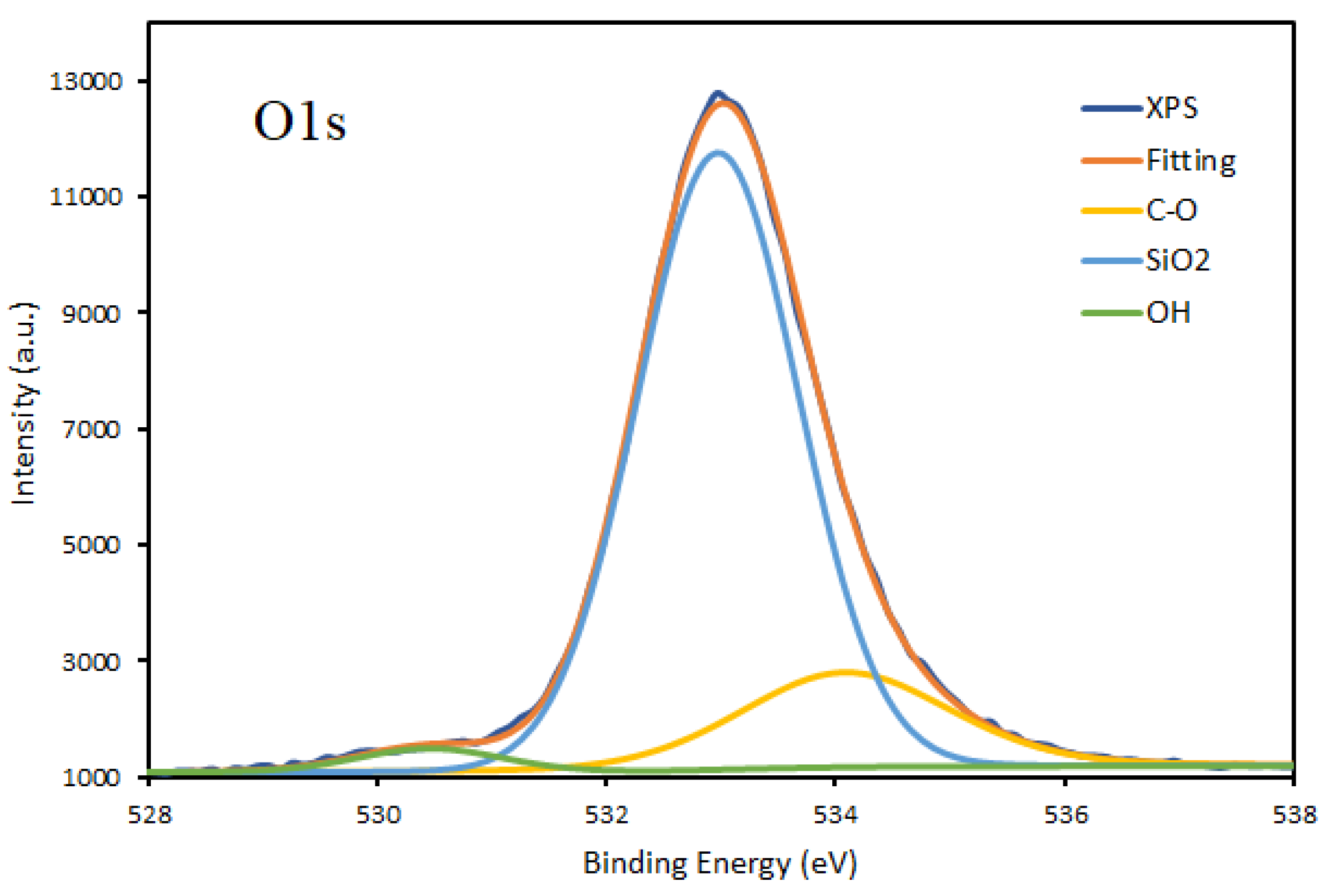
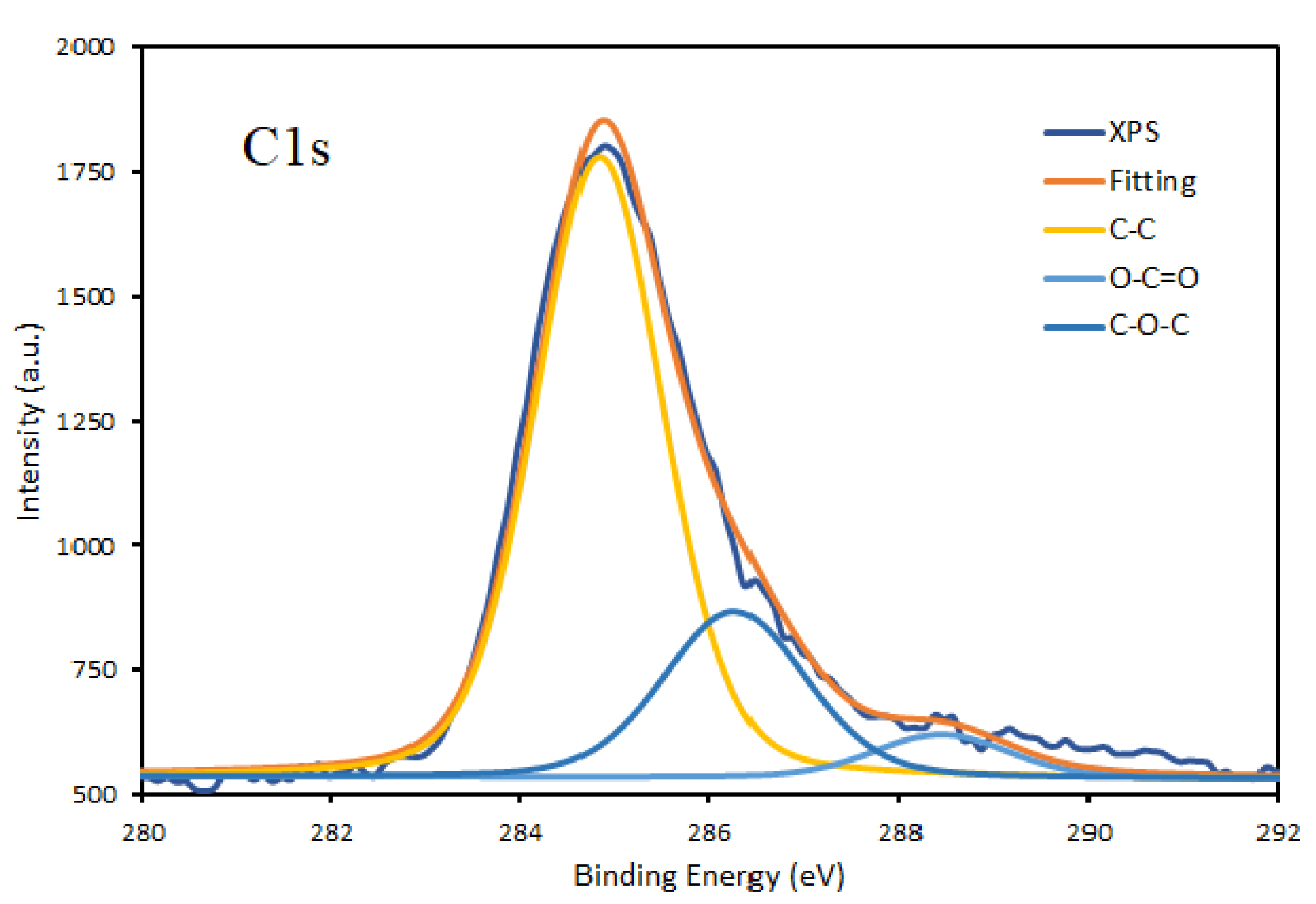
3.1. Chemical Composition
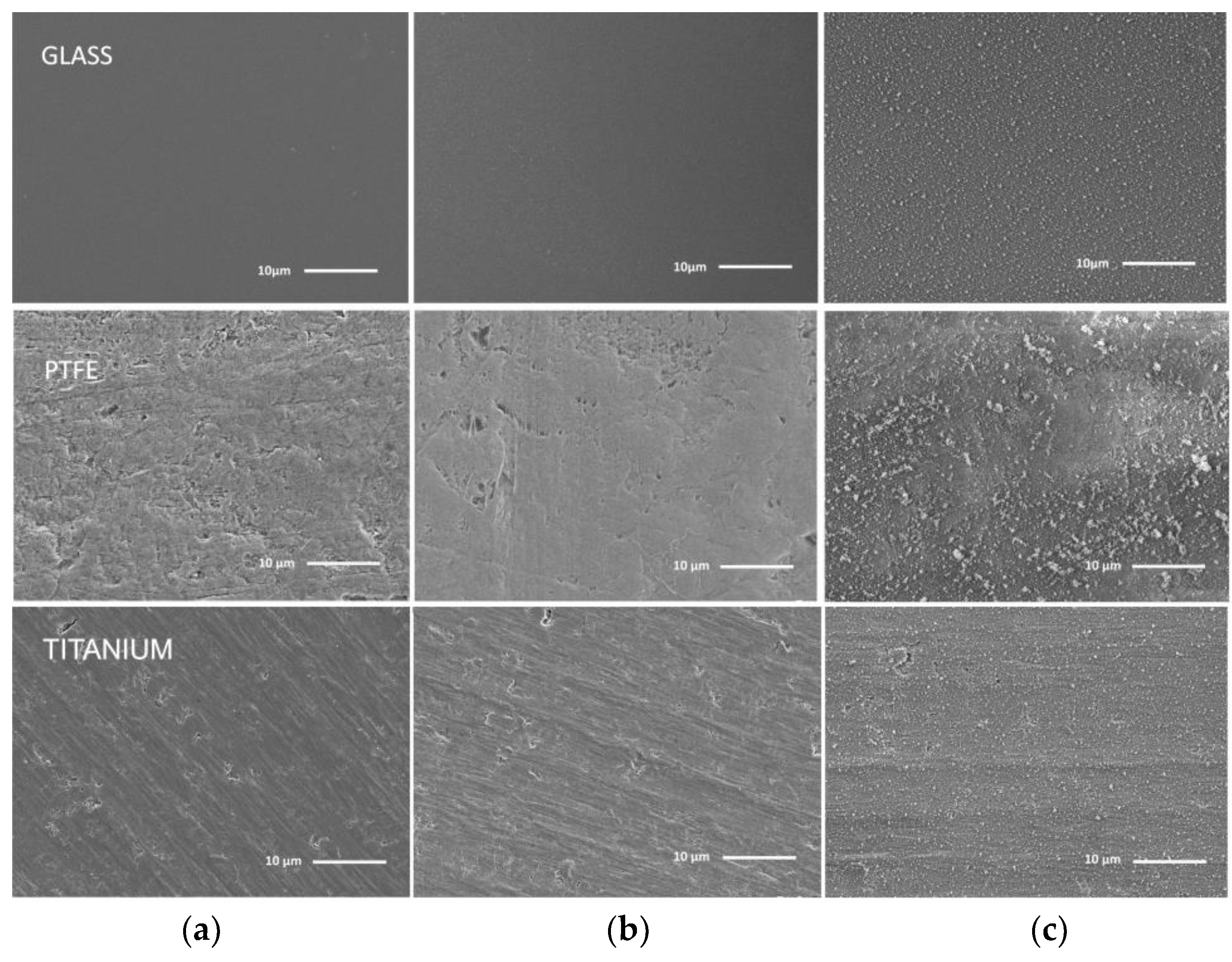
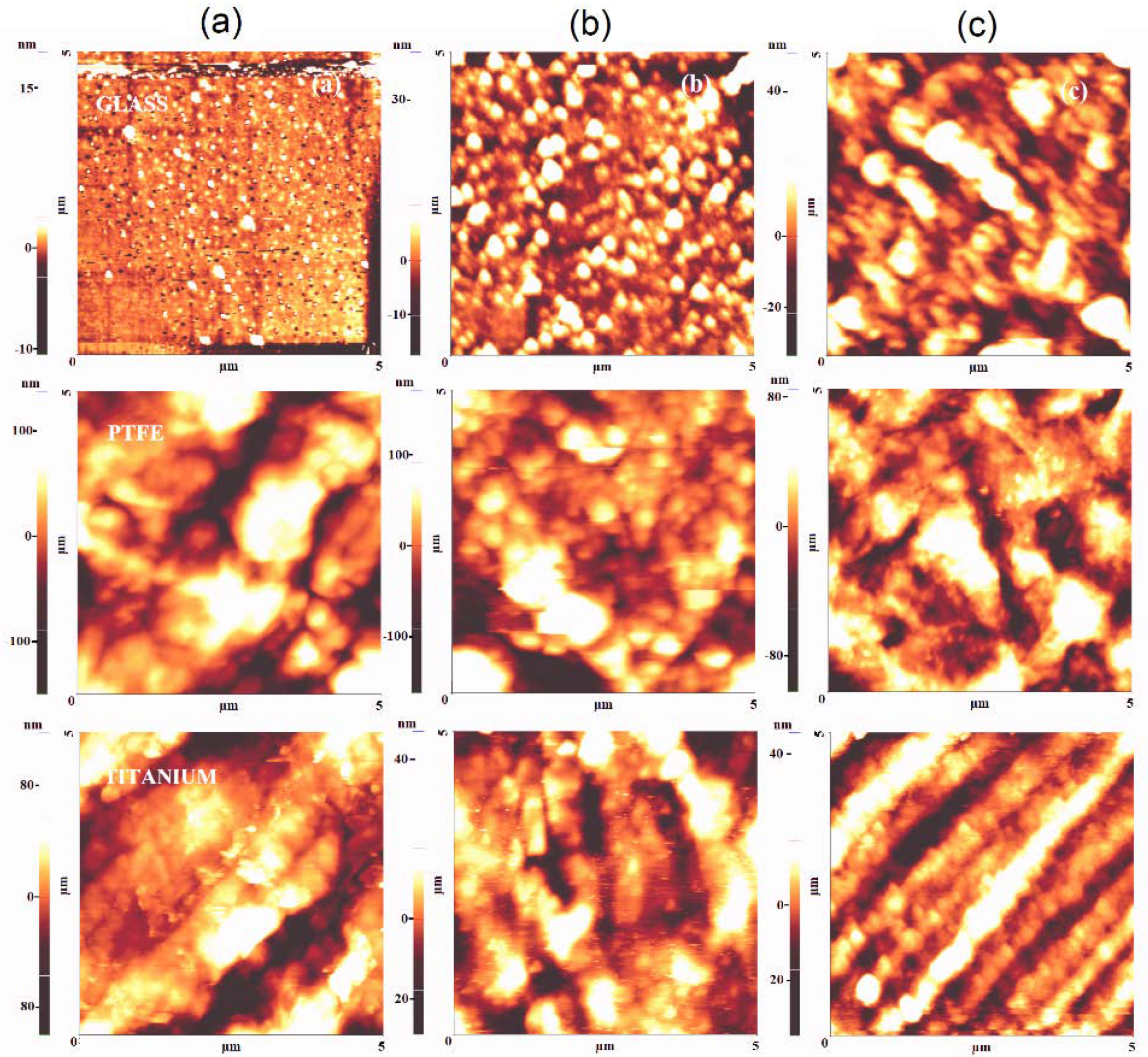
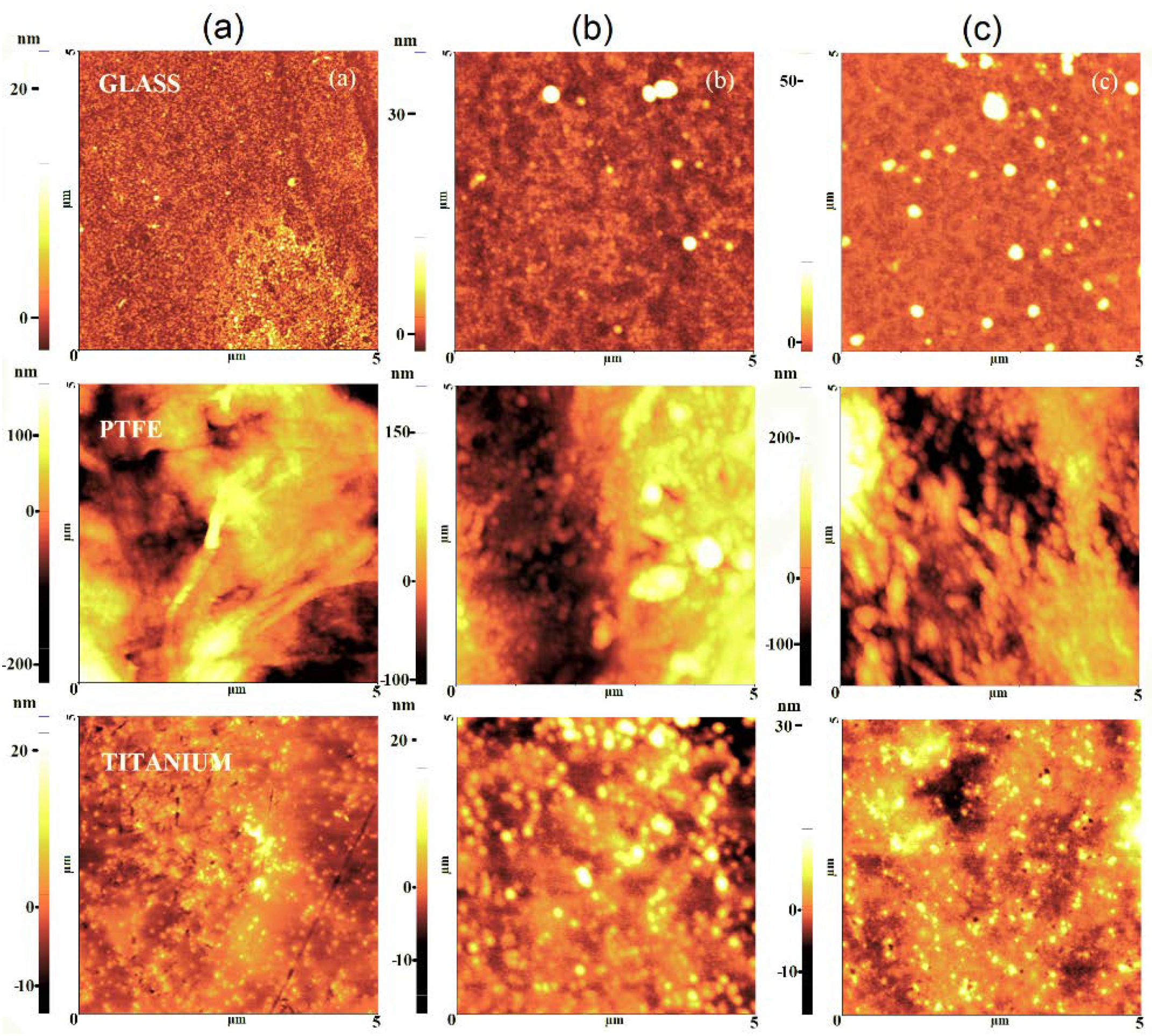
3.2. Films Morphology and Topography
3.2.1. Surface Morphology
3.2.2. Surface Topography
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Plasma Jet | DC | RF |
|---|---|---|
| Current | 18.4 mA | − |
| Voltage | 18.0 kV | − |
| Power | − | 11.4 W |
| Gas Flow | N2–7000 sccm | Ar–2500 sccm |
| Gas Flow (HMDSO) | A–1 sccm, B–5 sccm | A–1.7 sccm, B–7 sccm |
| Distance to substrate | 10 mm | 1 mm |
| Spot size | 10 mm | 12.5 mm |
| DC Plasma Jet | RF Plasma Jet | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Materials | Precursor, sccm | O (%) | C (%) | Si (%) | N (%) | Precursor, sccm | O (%) | C (%) | Si (%) | N (%) |
| Ceramics | 1 | 61 | 16.2 | 22.7 | 0 | 1.7 | 64.8 | 6.3 | 28.2 | 0.6 |
| 5 | 61.2 | 14.3 | 23.6 | 0.83 | 7 | 57.8 | 14.8 | 26.8 | 0.5 | |
| Stainless steel | 1 | 58.7 | 17.3 | 23.5 | 0.47 | 1.7 | 65.4 | 7.4 | 27.2 | 0 |
| 5 | 62.4 | 12.2 | 25.4 | 0 | 7 | 65.4 | 5.8 | 28.7 | 0 | |
| Titanium | 1 | 62.4 | 12.9 | 24 | 0.76 | 1.7 | 63.7 | 7.7 | 28.1 | 0.5 |
| 5 | 61.2 | 12.9 | 25.8 | 0.05 | 7 | 65.2 | 5.8 | 29 | 0 | |
| Glass | 1 | 61.1 | 12 | 26.1 | 0.79 | 1.7 | 64.7 | 7.6 | 27.1 | 0.6 |
| 5 | 61.9 | 11.5 | 25.8 | 0.77 | 7 | 65.1 | 6.5 | 28.3 | 0.2 | |
| PEEK | 1 | 60 | 15.8 | 24.1 | 0 | 1.7 | 66.2 | 5.5 | 28.2 | 0.1 |
| 5 | 62 | 11.6 | 25.9 | 0.47 | 7 | 65 | 6 | 29 | 0 | |
| PTFE | 1 | 54.3 | 22.5 | 23.2 | 0 | 1.7 | 63.93 | 9.16 | 26.81 | 0 |
| 5 | 55.4 | 20.6 | 23.2 | 0.72 | 7 | 62.3 | 11 | 26.7 | 0 | |
| Plasma Jet | DC | RF |
|---|---|---|
| Current | 18.4 mA | − |
| Voltage | 18.0 kV | − |
| Power | − | 11.4 W |
| Gas Flow | N2–7000 sccm | Ar–2500 sccm |
| Gas Flow (HMDSO) | A–1 sccm, B–5 sccm | A–1.7 sccm, B–7 sccm |
| Distance to substrate | 10 mm | 1 mm |
| Spot size | 10 mm | 12.5 mm |
References
- Shahidi, S.; Ghoranneviss, M.; Wiener, J.; Moazzenchi, B.; Mortazavi, H. Effect of hexamethyldisiloxane (HMDSO)/nitrogen plasma polymerisation on the anti felting and dyeability of wool fabric. Fibres Text. East. Eur. 2014, 105, 116–119. [Google Scholar]
- Chifen, A.N.; Jenkins, A.T.A.; Knoll, W.; Förch, R. Adhesion Improvement of Plasma-Polymerized Maleic Anhydride Films on Gold Using HMDSO/O2 Adhesion Layers. Plasma Process. Polym. 2007, 4, 815–822. [Google Scholar] [CrossRef]
- Hegemann, D.; Vohrer, U.; Oehr, C.; Riedel, R. Deposition of SiOx films from O2/HMDSO plasmas. Surf. Coat. Technol. 1999, 116, 1033–1036. [Google Scholar] [CrossRef]
- Vautrin-Ul, C.; Boisse-Laporte, C.; Benissad, N.; Chausse, A.; Leprince, P.; Messina, R. Plasma-polymerized coatings using HMDSO precursor for iron protection. Prog. Org. Coat. 2000, 38, 9–15. [Google Scholar] [CrossRef]
- Zajíčková, L.; Buršıková, V.; Peřina, V.; Macková, A.; Subedi, D.; Janča, J.; Smirnov, S. Plasma modification of polycarbonates. Surf. Coat. Technol. 2001, 142, 449–454. [Google Scholar] [CrossRef]
- Mota, R.P.; Galvão, D.; Durrant, S.F.; de Moraes, M.A.B.; Dantas, S.d.; Cantão, M. HMDSO plasma polymerization and thin film optical properties. Thin Solid Film. 1995, 270, 109–113. [Google Scholar] [CrossRef]
- Lamendola, R.; d’Agostino, R. Process control of organosilicon plasmas for barrier film preparations. Pure Appl. Chem. 1998, 70, 1203–1208. [Google Scholar] [CrossRef][Green Version]
- Deilmann, M.; Theiß, S.; Awakowicz, P. Pulsed microwave plasma polymerization of silicon oxide films: Application of efficient permeation barriers on polyethylene terephthalate. Surf. Coat. Technol. 2008, 202, 1911–1917. [Google Scholar] [CrossRef]
- Deng, X.; Nikiforov, A.Y.; de Geyter, N.; Morent, R.; Leys, C. Deposition of a TMDSO-Based Film by a Non-Equilibrium Atmospheric Pressure DC Plasma Jet. Plasma Process. Polym. 2013, 10, 641–648. [Google Scholar] [CrossRef]
- Sardella, E.; Palumbo, F.; Camporeale, G.; Favia, P. Non-Equilibrium Plasma Processing for the Preparation of Antibacterial Surfaces. Materials 2016, 9, 515. [Google Scholar] [CrossRef]
- Fonseca, J.L.C.; Tasker, S.; Apperley, D.C.; Badyal, J.P.S. Plasma-Enhanced Chemical Vapor Deposition of Organosilicon Materials: A Comparison of Hexamethyldisilane and Tetramethylsilane Precursors. Macromolecules 1996, 29, 1705–1710. [Google Scholar] [CrossRef]
- Guruvenket, S.; Andrie, S.; Simon, M.; Johnson, K.W.; Sailer, R.A. Atmospheric-Pressure Plasma-Enhanced Chemical Vapor Deposition of a-SiCN:H Films: Role of Precursors on the Film Growth and Properties. ACS Appl. Mater. Interfaces 2012, 4, 5293–5299. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Leys, C.; Vujosevic, D.; Vuksanovic, V.; Cvelbar, U.; De Geyter, N.; Morent, R.; Nikiforov, A. Engineering of Composite Organosilicon Thin Films with Embedded Silver Nanoparticles via Atmospheric Pressure Plasma Process for Antibacterial Activity. Plasma Process. Polym. 2014, 11, 921–930. [Google Scholar] [CrossRef]
- Deng, X.; Nikiforov, A.; Vujosevic, D.; Vuksanovic, V.; Mugoša, B.; Cvelbar, U.; De Geyter, N.; Morent, P.; Leys, C. Antibacterial activity of nano-silver non-woven fabric prepared by atmospheric pressure plasma deposition. Mater. Lett. 2015, 149, 95–99. [Google Scholar] [CrossRef]
- Deng, X.; Nikiforov, A.Y.; Coenye, T.; Cools, P.; Aziz, G.; Morent, R.; De Geyter, N.; Leys, C. Antimicrobial nano-silver non-woven polyethylene terephthalate fabric via an atmospheric pressure plasma deposition process. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Deng, X.L.; Nikiforov, A.Y.; Vanraes, P.; Leys, C. Direct current plasma jet at atmospheric pressure operating in nitrogen and air. J. Appl. Phys. 2013, 113, 023305. [Google Scholar] [CrossRef]
- Sigeneger, F.; Schäfer, J.; Weltmann, K.-D.; Foest, R.; Loffhagen, D. Modeling of a Non-Thermal RF Plasma Jet at Atmospheric Pressure. Plasma Process. Polym. 2017, 14, 1600112. [Google Scholar] [CrossRef]
- Selwyn, G.S.; Herrmann, H.W.; Park, J.; Henins, I. Materials Processing Using an Atmospheric Pressure, RF-Generated Plasma Source. Contrib. Plasma Phys. 2001, 41, 610–619. [Google Scholar] [CrossRef]
- Schneider, J.; Akbar, M.I.; Dutroncy, J.; Kiesler, D.; Leins, M.; Schulz, A.; Walker, M.; Schumacher, U.; Stroth, U. Silicon oxide barrier coatings deposited on polymer materials for applications in food packaging industry. Plasma Process. Polym. 2009, 6, 1. [Google Scholar] [CrossRef]
- O’Hare, L.-A.; Parbhoo, B.; Leadley, S.R. Development of a methodology for XPS curve-fitting of the Si 2p core level of siloxane materials. Surf. Interface Anal. 2004, 36, 1427–1434. [Google Scholar] [CrossRef]
- Alexander, M.R.; Short, R.D.; Jones, F.R.; Michaeli, W.; Blomfield, C.J. A study of HMDSO/O2 plasma deposits using a high-sensitivity and -energy resolution XPS instrument: Curve fitting of the Si 2p core level. Appl. Surf. Sci. 1999, 137, 179–183. [Google Scholar] [CrossRef]
- Sonnenfeld, A.; Tun, T.M.; Zajíčková, L.; Kozlov, K.V.; Wagner, H.E.; Behnke, J.F.; Hippler, R. Deposition Process Based on Organosilicon Precursors in Dielectric Barrier Discharges at Atmospheric Pressure—A Comparison. Plasmas Polym. 2001, 6, 237–266. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Kusumoto, K.; Li, C.J. XPS Analysis of SiC Films Prepared by Radio Frequency Plasma Sputtering. Phys. Procedia 2012, 32, 95–102. [Google Scholar] [CrossRef]
- Bashouti, M.Y.; Paska, Y.; Puniredd, S.R.; Stelzner, T.; Christiansen, S.; Haick, H. Silicon nanowires terminated with methyl functionalities exhibit stronger Si-C bonds than equivalent 2D surfaces. Phys. Chem. 2009, 11, 3845–3848. [Google Scholar] [CrossRef] [PubMed]
- Nalwa, H.S. Silicon-Based Materials and Devices; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Premkumar, P.A. High Quality SiO2 -like Layers by Large Area Atmospheric Pressure Plasma Enhanced CVD: Deposition Process Studies by Surface Analysis. Plasma Process. Polym. 2009, 6, 693–702. [Google Scholar] [CrossRef]
- Twomey, B.; Dowling, D.; Byrne, G.; O’Neill, L.; O’Hare, L.-A. Properties of Siloxane Coatings Deposited in a Reel-to-Reel Atmospheric Pressure Plasma System. Plasma Process. Polym. 2007, 4, S450–S454. [Google Scholar] [CrossRef]
- Trinsoutrot, P.; Rabot, C.; Vergnes, H.; Delamoreanu, A.; Zenasni, A.; Caussat, B. High quality graphene synthesized by atmospheric pressure CVD on copper foil. Surf. Coat. Technol. 2013, 230, 87–92. [Google Scholar] [CrossRef]
- Zhang, B.; Lee, W.H.; Piner, R.; Kholmanov, I.; Wu, Y.; Li, H.; Ji, H.; Ruoff, R.S. Low-Temperature Chemical Vapor Deposition Growth of Graphene from Toluene on Electropolished Copper Foils. ACS Nano 2012, 6, 2471–2476. [Google Scholar] [CrossRef]
- Alexander, M.R.; Jones, F.R.; Short, R.D. Mass Spectral Investigation of the Radio-Frequency Plasma Deposition of Hexamethyldisiloxane. J. Phys. Chem. B 1997, 101, 3614–3619. [Google Scholar] [CrossRef]
- Lewis, J. Material challenge for flexible organic devices. Mater. Today 2006, 9, 38–45. [Google Scholar] [CrossRef]
- Gil, E.; Park, J.B.; Oh, J.S.; Yeom, G.Y. Characteristics of SiOx thin films deposited by atmospheric pressure chemical vapor deposition as a function of HMDS/O2 flow rate. Thin Solid Film. 2010, 518, 6403–6407. [Google Scholar] [CrossRef]
- Aresta, G.; Premkumar, P.A.; Starostin, S.A.; de Vries, H.; van de Sanden, M.C.M.; Creatore, M. Optical Characterization of Plasma-Deposited SiO2-Like Layers on Anisotropic Polymeric Substrates. Plasma Process. Polym. 2010, 7, 766–774. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuchakova, I.; Ionita, M.D.; Ionita, E.-R.; Lazea-Stoyanova, A.; Brajnicov, S.; Mitu, B.; Dinescu, G.; De Vrieze, M.; Cvelbar, U.; Zille, A.; et al. Atmospheric Pressure Plasma Deposition of Organosilicon Thin Films by Direct Current and Radio-frequency Plasma Jets. Materials 2020, 13, 1296. https://doi.org/10.3390/ma13061296
Kuchakova I, Ionita MD, Ionita E-R, Lazea-Stoyanova A, Brajnicov S, Mitu B, Dinescu G, De Vrieze M, Cvelbar U, Zille A, et al. Atmospheric Pressure Plasma Deposition of Organosilicon Thin Films by Direct Current and Radio-frequency Plasma Jets. Materials. 2020; 13(6):1296. https://doi.org/10.3390/ma13061296
Chicago/Turabian StyleKuchakova, Iryna, Maria Daniela Ionita, Eusebiu-Rosini Ionita, Andrada Lazea-Stoyanova, Simona Brajnicov, Bogdana Mitu, Gheorghe Dinescu, Mike De Vrieze, Uroš Cvelbar, Andrea Zille, and et al. 2020. "Atmospheric Pressure Plasma Deposition of Organosilicon Thin Films by Direct Current and Radio-frequency Plasma Jets" Materials 13, no. 6: 1296. https://doi.org/10.3390/ma13061296
APA StyleKuchakova, I., Ionita, M. D., Ionita, E.-R., Lazea-Stoyanova, A., Brajnicov, S., Mitu, B., Dinescu, G., De Vrieze, M., Cvelbar, U., Zille, A., Leys, C., & Yu Nikiforov, A. (2020). Atmospheric Pressure Plasma Deposition of Organosilicon Thin Films by Direct Current and Radio-frequency Plasma Jets. Materials, 13(6), 1296. https://doi.org/10.3390/ma13061296







