Bio-Based Polymer Electrolytes for Electrochemical Devices: Insight into the Ionic Conductivity Performance
Abstract
1. Introduction to Bio-Based Polymers
2. Insights to the Polymer Electrolyte
3. Bio-Based Polymers Used as Electrolytes
3.1. Polymers Extracted from Biomass
3.1.1. Starch
3.1.2. Cellulose and Cellulose Derivatives
3.1.3. Chitosan
3.1.4. Gum
Agar
Carrageenan
Pectin
Guar gum and Gum Arabic
3.1.5. Gelatin
3.1.6. Natural Rubber
3.2. Polymers Chemically Synthesized from Bio-Derived Monomers
3.2.1. Poly(lactic acid)
3.2.2. Vegetable Oil-Based Polyurethane
3.3. Polymers Produced by Microorganisms
3.3.1. Bacterial Cellulose
3.3.2. Gellan Gum and Xanthan Gum
3.4. Development of Bio-Based Polymer Electrolyte
3.5. Application of Bio-Based Polymer Electrolytes
4. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AEII | 1-allyl-3-ethylimidadolium iodide |
| AgNO3 | Silver nitrate |
| Al2O3 | Aluminium oxide |
| Al2SiO5 | Aluminium silicate |
| [AmIm][Cl] | 1-allyl-3-methylimidazolium chloride |
| APII | 1-allyl-3-propylimidazolium iodide |
| BaTiO3 | Barium titanate |
| BDG | Diethylene glycol dibutylether |
| BEMA | Bisphenol A ethoxylate dimethacrylate |
| BMATFSI | Butyl-trimethyl ammonium bis(trifluoromethylsulfonyl)imide |
| [BmIm][Cl] | 1-butyl-3-methylimidazolium chloride |
| [BmIm][I] | 1-butyl-3-methylimidazolium iodide |
| [BmIm][OAc] | 1-butyl-3-methylimidazoliumacetate |
| [BmIm][PF6] | 1-butyl-3-methylimidazolium hexafluorophosphate |
| [BmIm][Tf] | 1-butyl-3-methylimidazolium trifluoromethanesulfonate |
| [BmIm][TFSI] | 1-butyl-3-methylimidazolium trifluoromethanesulfonyl imide |
| Bu4NBF4 | Tetrabutylammonium tetrafluoroborat |
| Ce(CF3SO3)3 | Cerium triflate |
| [Ch][OAc] | Trimethyl-ethanolammonium acetate |
| CH3COONH4 | Ammonium acetate |
| CMC | Carboxymethyl cellulose |
| CMCh | Carboxymethyl chitosan |
| CNC | Cellulose nanocrystals |
| CN-HPC | Cyanoethylated hydroxypropyl cellulose |
| Co3O4 | Cobalt oxide |
| DAII | 1-3-diallylimidazolium iodide |
| DES | Deep eutectic solvent |
| DMC | Dimethyl carbonate |
| DPEO | Poly(ethylene oxice) diisocyanate |
| DSSC | Dye sensitized solar cell |
| DTAB | Dodecyltrimethyl ammonium bromide |
| EC | Ethylene carbonate |
| ECD | Electrochromic device |
| EDLC | Electrical double layer capasitor |
| EMC | Ethyl methyl carbonate |
| [EmIm][Br] | 1-butyl-3-methylimidazolium bromide |
| [EmIm][Cl] | 1-ethyl-3-methylimidazolium chloride |
| [EmIm][CF3SO3] | 2-ethyl-3-methylimidazolium trifluoromethanesulfonate |
| [EmIm][C1SO3] | 1-ethyl-3-methylimidazolium methylsulfonate |
| [EmIm][C2SO3] | 1-ethyl-3-methylimidazolium ethylsulfonate |
| [EmIm][C4SO3] | 1-ethyl-3-methylimidazolium butylsulfonate |
| [EmIm][C2SO4] | 1-ethyl-3-methylimidazolium ethylsulfate |
| [EmIm][Eu(SCN)4] | 1-ehtyl-3-methylimidazolium europium(III) tetrathiocyanate |
| [EmIm][OAc] | 1-ethyl-3-methylimidazolium acetate |
| [EmIm][N(CN)2] | 1-ethyl-3-methylimidazolium dicyanamide |
| [EmIm][NO3] | 1-ethyl-3-methylimidazolium nitrate |
| [EmIm][SCN] | 1-ethyl 3-methylimidazolium thiocyanate |
| [EmIm][TFSI] | 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| ENR-25 | 25% epoxidized natural rubber |
| ENR-50 | 50% epoxidized natural rubber |
| EO-EPI | ethylene oxide-epichlorohydrin |
| Er(CF3SO3)3 | Erbium triflate |
| ES | Ethylene sulphite |
| EtC | Ethyl carbonate |
| [Eu(pic)3] | Europium picrate |
| Eu(CF3SO3) 3 | Europium triflate |
| Fe2O3 | Iron (III) oxide |
| Fe3O4 | Iron (II,III) oxide |
| GBL | γ-butyrolactone |
| GO | Graphene oxide |
| GSCN | Guanidinium thiocyanate |
| H2SO4 | Sulphuric acid |
| HCF | Hexacyanoferrate |
| HCl | Hydrochloric acid |
| HEC | Hydroxyethyl cellulose |
| HPC | Hydroxypropyl cellulose |
| HPMC | Hydroxypropyl methyl cellulose |
| I2 | Iodine |
| KCl | Potassium chloride |
| KI | Potassium iodide |
| LENR-50 | Liquid 50% epoxidized natural rubber |
| Li2B4O7 | Lithium tetraborate |
| LiBF4 | Lithium tetrafluoroborate |
| LiBoB | Lithium bis(oxalato) borate |
| LiBs | lithium benzenesulfonate |
| LiCF3SO3 | Lithium trifluoromethanesulfonate |
| LiCl | Lithium chloride |
| LiClO4 | Lithium perchlorate |
| LiI | Lithium iodide |
| LiN(CF3SO2)2 | lithium trifluoromethane sulfonimide/lithium imide |
| LiNO3 | Lithium nitrate |
| LiOAc | Lithium acetate |
| LiPF6 | Lithium hexafluorophosphat |
| LiTFSI | Lithium bis(trifluoromethane sulfonyl)imide |
| MFC | Microfibrillated cellulose |
| MG-30 | 30% methyl methacrylate grafted natural rubber |
| MG-49 | 49% methyl methacrylate grafted natural rubber |
| Mg(C2H3O2)2 | Magnesium acetate |
| Mg(CF3SO3)2 | Magnesium triflat |
| MHII | 1-methyl-3-hexylimidazolium iodide |
| MPII | 1-methyl-3-propylimidazolium iodide |
| [N1112(OH)][NTf2] | N,N,N-trimethyl-N-(2-hydroxyethyl)ammonium bis(trifluoromethylsulfonyl)imide |
| NaCl | Sodium chloride |
| NaClO4 | Sodium perchlorate |
| NADES | Ternary natural deep eutectic solvent (NADES) |
| NaI | Sodium iodide |
| Na2S/S | Poly sulphide solution |
| NH4BF4 | Ammonium tetrafluoroborate |
| NH4Br | Ammonium bromide |
| NH4CF3SO3 | Ammonium triflate |
| NH4Cl | Ammonium chloride |
| (NH4)2CO3 | Ammonium carbonate |
| NH4I | Ammonium iodide |
| NH4NO3 | Ammonium nitrate |
| NH4SCN | Ammonium thiocyanate |
| NiO | Nickel oxide |
| NMBI | N-methylbenzimidazole |
| NMPS | N,N-dimethylene phosphonic acid propylsilane |
| NR | Natural rubber |
| NSB-Chitosan | N-o-sulphonic acid benzyl chitosan |
| NWF | Nonwoven fabric |
| o-H3PO4 | Ortho-phosphoric acid |
| PMMA | poly(methyl methacrylate) |
| PAN | Poly(acrylonitrile) |
| PANI | Polyaniline |
| pAPS | Poly(aminopropyltriethoxysilane) |
| PC | Propylene carbonate |
| PE | Polyethylene |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PEG | Poly(ethylene glycol) |
| PEGMA | Poly(ethylene glycol) methyl ether methacrylate |
| PEO | Poly(ethylene oxide) |
| PHB | Poly(3-hydroxybutyrate) |
| PLA | Poly(lactic acid) |
| PLGA | Poly(lactic-co-glycolic acid) |
| PMII | Propyl-methyl-imidazolium iodide |
| PMMA | Poly(methyl methacrylate) |
| P(AEMIBr) | Poly(1-[2-acryloylethyl]-3-methylimidazolium bromide) |
| POE | Poly(oxyethylene) |
| PSSA | Poly(4-styrene sulfonic acid) (PSSA) |
| PVA | Poly(vinyl alcohol) |
| PVC | Poly(vinyl chloride) |
| PVDF | Poly(vinylidene fluoride) |
| PVDF-HFP | Poly(vinylidene fluoride-hexafluoro propylene) |
| PVP | Poly(vinylpyrrolidone) |
| PVPA | poly(vinylphosphonic acid) |
| Pyr14TFSI | N-butyl-N-methylpyrrolidinium bis(trifluoromethane-sulfonyl) imide |
| SDS | Sodium dodecyl sulphate |
| SiO2 | Silicon diioxide |
| SY | Super yellow |
| TBABF4 | Tetrabutylammonium tetrafluorborate |
| TBAI | Tetrabutylammonium iodide |
| TBP | 4-tert-butylpyridine |
| TEGDME | Tetra(ethylene) glycol dimethyl ether |
| TiO2 | Titanium dioxide |
| Tm(CF3SO3)3 | thulium triflate |
| TPAI | Tetrapropylammonium iodide |
| TW-80 | Polysorbate 80 |
| Zn(CF3SO3)2 | Zinc triflate |
References
- Tanase, E.E.; Rapa, M.; Popa, S. Biopolymers based on renewable resources—A review. Sci. Bull. Ser. F Biotechnol. 2014, 18, 188–195. [Google Scholar]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Natural polymer based cling films for food packaging. Int. J. Pharm. Pharm. Sci. 2015, 7, 10–18. [Google Scholar]
- Imam, S.H.; Bilbao-Sainz, C.; Chiou, B.; Glenn, G.M.; Orts, W.J. Biobased adhesives, gums, emulsions and binders: Current trends and future prospects. J. Adhes. Sci. Technol. 2012, 27, 1–26. [Google Scholar] [CrossRef]
- Kucharski, M.; Łukaszewicz, T.; Mrozek, P. New electrolyte for electrochromic devices. Opto-Electron. Rev. 2004, 12, 175–180. [Google Scholar]
- Hallinan, D.T., Jr.; Balsara, N.P. Polymer electrolytes. Annu. Rev. Mater Res 2013, 43, 503–525. [Google Scholar] [CrossRef]
- Varshney, P.K.; Gupta, S. Natural polymer-based electrolytes for electrochemical devices: A review. Ionics 2011, 17, 479–483. [Google Scholar] [CrossRef]
- Fenton, D.E.; Parker, J.M.; Wright, P.V. Complexes of alkali metal ions with poly(ethylene oxide). Polymer 1973, 14, 589. [Google Scholar] [CrossRef]
- Daud, F.N.; Ahmad, A.; Badri, K.H. An investigation on the properties of palm-based polyurethane solid polymer electrolyte. Int. J. Polym. Sci. 2014, 2014, 1–5. [Google Scholar] [CrossRef]
- Aziz, S.B.; Woo, T.J.; Kadir, M.F.Z.; Ahmed, H.M. A conceptual review on polymer electrolytes and ion transport models. J. Sci. Adv. Mater. Devices 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Liew, C.W.; Ramesh, S. Studies on ionic liquid-based corn starch biopolymer electrolytes coupling with high ionic transport number. Cellulose 2013, 20, 3227–3237. [Google Scholar] [CrossRef]
- Raphael, E.; Avellaneda, C.O.; Manzolli, B.; Pawlicka, A. Agar-based films for application as polymer electrolytes. Electrochim. Acta 2010, 55, 1455–1459. [Google Scholar] [CrossRef]
- Khiar, A.S.A.; Arof, A.K. Conductivity studies of starch-based polymer electrolytes. Ionics 2010, 16, 123–129. [Google Scholar] [CrossRef]
- Kumar, M.; Tiwari, T.; Srivastava, N. Electrical transport behaviour of bio-polymer electrolyte system: Potato starch + ammonium iodide. Carbohydr. Polym. 2012, 88, 54–60. [Google Scholar] [CrossRef]
- Pawlicka, A.; Machado, G.O.; Guimaraes, K.V.; Dragunski, D.C. Solid polymeric electrolytes obtained from modified natural polymers. Proc. SPIE Int. Soc. Opt. Eng. 2002, 5136, 274–279. [Google Scholar]
- Gandini, A.; Lacerda, T.M. From monomers to polymers from renewable resources: Recent advances. Prog. Polym. Sci. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Yahya, M.Z.A.; Arof, A.K. Effect of oleic acid plasticizer on chitosan–lithium acetate solid polymer electrolytes. Eur. Polym. J. 2003, 39, 897–902. [Google Scholar] [CrossRef]
- Majid, S.R.; Arof, A.K. Proton-conducting polymer electrolyte films based on chitosan acetate complexed with NH4NO3 salt. Phys. B Condens. Matter 2005, 355, 78–82. [Google Scholar] [CrossRef]
- Buraidah, M.H.; Teo, L.P.; Majid, S.R.; Arof, A.K. Ionic conductivity by correlated barrier hopping in NH4I doped chitosan solid electrolyte. Phys. B Condens. Matter 2009, 404, 1373–1379. [Google Scholar] [CrossRef]
- Nomanbhay, S.M.; Palanisamy, K. Removal of heavy metal from industrial wastewater using chitosan coated oil palm shell charcoal. Electron. J. Biotechnol. 2005, 8, 43–53. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Physicochemical and bioactivity of cross-linked chitosan-PVA film for food packaging applications. Int. J. Biol. Macromol. 2009, 45, 372–376. [Google Scholar] [CrossRef]
- Osman, Z.; Ibrahim, Z.A.; Arof, A.K. Conductivity enhancement due to ion dissociation in plasticized chitosan based polymer electrolytes. Carbohydr. Polym. 2001, 44, 167–173. [Google Scholar] [CrossRef]
- Mobarak, N.N.; Jumaah, F.N.; Ghani, M.A.; Abdullah, M.P.; Ahmad, A. Carboxymethyl carrageenan based biopolymer electrolytes. Electrochim. Acta 2015, 175, 224–231. [Google Scholar] [CrossRef]
- Sudhakar, Y.N.; Selvakumar, M.; Krishna Bhat, D. Lithium salts doped biodegradable gel polymer electrolytes for supercapacitor application. J. Mater. Environ. Sci. 2015, 6, 1218–1227. [Google Scholar]
- BeMiller, J.N. Gums and related polysaccharides. In Glycoscience: Chemistry and Chemical Biology, 2nd ed.; Fraser-Reid, B.O., Tatsuta, K., Thiem, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1514–1533. [Google Scholar]
- Nwanya, A.C.; Amaechi, C.I.; Udounwa, A.E.; Osuji, R.U.; Maaza, M.; Ezema, F.I. Complex impedance and conductivity of agar-based ion-conducting polymer electrolytes. Appl. Phys. A 2015, 119, 387–396. [Google Scholar] [CrossRef]
- Raphael, E.; Avellaneda, C.O.; Aegerter, M.A.; Silva, M.M. Agar-based gel electrolyte for electrochromic device application. Mol. Cryst. Liq. Cryst. 2012, 554, 264–272. [Google Scholar] [CrossRef]
- Kasem, K.K. Electrochemical behavior of some redox systems pendant in agar gel. J. New Mater. Electrochem. Syst. 2005, 8, 189–195. [Google Scholar]
- Rudhziah, S.; Ahmad, A.; Ahmad, I.; Mohamed, N.S. Biopolymer electrolytes based on blend of kappa-carrageenan and cellulose derivatives for potential application in dye sensitized solar cell. Electrochim. Acta 2015, 175, 162–168. [Google Scholar] [CrossRef]
- Mobarak, N.N.; Ramli, N.; Ahmad, A.; Rahman, M.Y.A. Chemical interaction and conductivity of carboxymethyl κ-carrageenan based green polymer electrolyte. Solid State Ion. 2012, 224, 51–57. [Google Scholar] [CrossRef]
- Rudhziah, S.; Rani, M.S.A.; Ahmad, A.; Mohamed, N.S.; Kaddami, H. Potential of blend of κ-carrageenan and cellulose derivatives for green polymer electrolyte application. Ind. Crop. Prod. 2015, 72, 133–141. [Google Scholar] [CrossRef]
- Endreû, H.; Neuenbu, F.K.G.P.; Christensen, S.H.; Aps, C.P.K. Pectins. In Handbook of Hyrdocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Limited: Sawston, UK, 2009; pp. 274–297. [Google Scholar]
- Smith, D. Jams and preserves. Methods of manufacture. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 3409–3415. [Google Scholar]
- Andrade, J.R.; Raphael, E.; Pawlicka, A. Plasticized pectin-based gel electrolytes. Electrochim. Acta 2009, 54, 6479–6483. [Google Scholar] [CrossRef]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M.Z. Guar gum as a promising starting material for diverse applications: A review. Int. J. Biol. Macromol. 2016, 88, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Del Agua, I.; Mantione, D.; Casado, N.; Sanchez-Sanchez, A.; Malliaras, G.G.; Mecerreyes, D. Conducting polymer iongels based on PEDOT and guar gum. ACS Macro Lett. 2017, 6, 473–478. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef]
- Sudhakar, Y.N.; Selvakumar, M.; Bhat, D.K. Tubular array, dielectric, conductivity and electrochemical properties of biodegradable gel polymer electrolyte. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2014, 180, 12–19. [Google Scholar] [CrossRef]
- Zhang, B.; Sudre, G.; Quintard, G.; Serghei, A.; David, L.; Bernard, J.; Fleury, E.; Charlot, A. Guar gum as biosourced building block to generate highly conductive and elastic ionogels with poly(ionic liquid) and ionic liquid. Carbohydr. Polym. 2017, 157, 586–595. [Google Scholar] [CrossRef]
- Alves, R.; Silva, M.M. The influence of glycerol and formaldehyde in gelatin-based polymer electrolytes. Mol. Cryst. Liq. Cryst. 2014, 591, 64–73. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I. Gelatin. In Handbook of Hyrdocolloidsal, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Sawston, UK, 2009; pp. 142–163. [Google Scholar]
- Pawlicka, A.; Firmino, A.; Vieira, D.; Sentanin, F.; Grote, J.G.; Kajzar, F. Gelatin- and DNA-based ionic conducting membranes for electrochromic devices. Proc. SPIE 2009, 7487, 1–12. [Google Scholar]
- Vieira, D.F.; Avellaneda, O.; Pawlicka, A. Conductivity study of a gelatin-based polymer electrolyte. Electrochim. Acta 2007, 53, 1404–1408. [Google Scholar] [CrossRef]
- Kurian, T.; Mathew, N.M. Natural rubber: Production, properties and applications. In Biopolymers: Biomedical and Environmental Applications; Kalia, S., Avérous, L., Eds.; Scrivener Publishing: Beverly, MA, USA, 2011; pp. 403–436. [Google Scholar]
- Yoshizawa, M.; Marwanta, E.; Ohno, H. Preparation and characteristics of natural rubber/poly(ethylene oxide) salt hybrid mixtures as novel polymer electrolytes. Polymer 2000, 41, 9049–9053. [Google Scholar] [CrossRef]
- Ahmad, H.A.; Ismail, H.; Rashid, A.A. ENR-50 Compatibilized natural rubber/recycled acrylonitrile-butadiene rubber blends. Sains Malays. 2015, 44, 835–842. [Google Scholar] [CrossRef]
- Chew, K.W.; Ng, T.C.; How, Z.H. Conductivity and microstructure study of PLA-based polymer electrolyte salted with lithium perchloride, LiClO4. Int. J. Electrochem. Sci. 2013, 8, 6354–6364. [Google Scholar]
- Chew, K.W. Development of novel bio-degradable electrolyte based on polylactide (PLA) for lithium rechargeable battery. Adv. Mater. Res. 2013, 853, 270–275. [Google Scholar] [CrossRef]
- Osinska-Broniarz, M.; Gieparda, W.; Wesole, D.; Martyla, A.; Przekop, R.; Sierczynska, A. Preparation and characterization of gel polymer electrolytes based on electrospun PLA/PHB membranes for lithium-ion batteries. ECS Trans. 2015, 70, 79–88. [Google Scholar] [CrossRef]
- Osada, I.; Hosseini, S.M.; Jeong, S.; Passerini, S. Novel ternary polymer electrolytes based on poly(lactic acid) from sustainable sources. ChemElectroChem 2017, 4, 463–467. [Google Scholar] [CrossRef]
- Mustapa, S.R.; Aung, M.M.; Ahmad, A.; Mansor, A.; TianKhoon, L. Preparation and characterization of jatropha oil-based polyurethane as non-aqueous solid polymer electrolyte for electrochemical devices. Electrochim. Acta 2016, 222, 293–302. [Google Scholar] [CrossRef]
- Su’ait, M.S.; Ahmad, A.; Badri, K.H.; Mohamed, N.S.; Rahman, M.Y.A.; Azanza Ricardo, C.L.; Scardi, P. The potential of polyurethane bio-based solid polymer electrolyte for photoelectrochemical cell application. Int. J. Hydrogen Energy 2014, 39, 3005–3017. [Google Scholar] [CrossRef]
- Ibrahim, S.; Ahmad, A.; Mohamed, N.S. Characterization of novel castor oil-based polyurethane polymer electrolytes. Polymers 2015, 7, 747–759. [Google Scholar] [CrossRef]
- Esa, F.; Tasirin, S.M.; Rahman, N.A. Overview of bacterial cellulose production and application. Agric. Agric. Sci. Procedia 2014, 2, 113–119. [Google Scholar] [CrossRef]
- Park, J.K.; Jung, J.Y.; Khan, T. Bacterial Cellulose; Woodhead Publishing Limited: Sawston, UK, 2009. [Google Scholar]
- Jiang, G.P.; Zhang, J.; Qiao, J.L.; Jiang, Y.M.; Zarrin, H.; Chen, Z.; Hong, F. Bacterial nanocellulose/Nafion composite membranes for low temperature polymer electrolyte fuel cells. J. Power Sources 2015, 273, 697–706. [Google Scholar] [CrossRef]
- Gadim, T.D.O.; Loureiro, F.J.A.; Vilela, C.; Rosero-Navarro, N.; Silvestre, A.J.D.; Freire, C.S.R.; Figueiredo, F.M.L. Protonic conductivity and fuel cell tests of nanocomposite membranes based on bacterial cellulose. Electrochim. Acta 2017, 233, 52–61. [Google Scholar] [CrossRef]
- Sworn, G. Gellan gum. In Handbook of Hyrdocolloidsal, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Sawston, UK, 2009; pp. 204–227. [Google Scholar]
- Noor, I.S.M.; Majid, S.R.; Arof, A.K.; Djurado, D.; Claro Neto, S.; Pawlicka, A. Characteristics of gellan gum–LiCF3SO3 polymer electrolytes. Solid State Ion. 2012, 225, 649–653. [Google Scholar] [CrossRef]
- Halim, N.F.A.; Majid, S.R.; Arof, A.K.; Kajzar, F.; Pawlicka, A. Gellan gum-LiI gel polymer electrolytes. Mol. Cryst. Liq. Cryst. 2012, 554, 232–238. [Google Scholar] [CrossRef]
- Sworn, G. Xanthan gum. In Handbook of Hyrdocolloidsal, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Sawston, UK, 2009; pp. 186–203. [Google Scholar]
- Park, S.J.; Yoo, K.; Kim, J.-Y.; Kim, J.Y.; Lee, D.-K.; Kim, B.; Kim, H.; Kim, J.H.; Cho, J.; Ko, M.J. Water-based thixotropic polymer gel electrolyte for dye-sensitized solar cells. ACS Nano 2013, 7, 4050–4056. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, U.V.; Pande, S.A. A review of biopolymer chitosan blends in polymer system. Int. Res. J. Sci. Eng. 2013, 1, 13–16. [Google Scholar]
- Ramly, K.; Isa, M.I.N.; Khiar, A.S.A. Conductivity and dielectric behaviour studies of starch/PEO + x wt-%NH4NO3 polymer electrolyte. Mater. Res. Innov. 2011, 15, 82–85. [Google Scholar] [CrossRef]
- Shukur, M.F.; Ithnin, R.; Illias, H.A.; Kadir, M.F.Z. Proton conducting polymer electrolyte based on plasticized chitosan–PEO blend and application in electrochemical devices. Opt. Mater. 2013, 35, 1834–1841. [Google Scholar] [CrossRef]
- Abdullah, D.J.S.A.; Alcázar, J.B.; Barbosa, C.V.; Carreño, N.L.V.; Avellaneda, C.S.O. Influence of the NiO nanoparticles on the ionic conductivity of the agar-based electrolyte. Ciência e Tecnologia 2014, 24, 8–12. [Google Scholar]
- Lopes, L.V.S.; Dragunski, D.C.; Pawlicka, A.; Donoso, J.P. Nuclear magnetic resonance and conductivity study of starch based polymer electrolytes. Electrochim. Acta 2003, 48, 2021–2027. [Google Scholar] [CrossRef]
- Mattos, R.I.; Pawlicka, A.; Tambelli, C.E.; Donoso, J.P. NMR and conductivity study of strach-based polymer electrolytes. Mol. Cryst. Liq. Cryst. 2006, 447, 55–64. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J.; He, K.; Wang, N. The effects of different plasticizers on the properties of thermoplastic starch as solid polymer electrolytes. Macromol. Mater. Eng. 2007, 292, 503–510. [Google Scholar] [CrossRef]
- Pawlicka, A.; Sabadini, A.; Raphael, E.; Dragunski, D. Ionic conductivity thermogravimetry measurements of starch-based polymeric electrolytes. Mol. Cryst. Liq. Cryst. 2008, 485, 804–816. [Google Scholar] [CrossRef]
- Marcondes, R.F.M.S.; D’Agostini, P.S.; Ferreira, J.; Girotto, E.M.; Pawlicka, A.; Dragunski, D.C. Amylopectin-rich starch plasticized with glycerol for polymer electrolyte application. Solid State Ion. 2010, 181, 586–591. [Google Scholar] [CrossRef]
- Ramesh, S.; Shanti, R.; Morris, E.; Durairaj, R. Utilisation of corn starch in production of ’green’ polymer electrolytes. Mater. Res. Innov. 2011, 15, 8–13. [Google Scholar] [CrossRef]
- Ramesh, S.; Liew, C.-W.; Arof, A.K. Ion conducting corn starch biopolymer electrolytes doped with ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate. J. Non. Cryst. Solids 2011, 357, 3654–3660. [Google Scholar] [CrossRef]
- Tiwari, T.; Pandey, K.; Srivastava, N.; Srivastava, P.C. Effects of glutalraldehyde on electrical properties of arrowroot starch + NaI electrolyte system. Polym. Polym. Compos. 2011, 121, 1–7. [Google Scholar]
- Liew, C.W.; Ramesh, S.; Ramesh, K.; Arof, A.K. Preparation and characterization of lithium ion conducting ionic liquid-based biodegradable corn starch polymer electrolytes. J. Solid State Electrochem. 2012, 16, 1869–1875. [Google Scholar] [CrossRef]
- Samsudin, A.S.; Aziz, M.I.A.; Isa, M.I.N. Natural polymer electrolyte system based on sago: Structural and transport behavior characteristics. Int. J. Polym. Anal. Charact. 2012, 17, 600–607. [Google Scholar] [CrossRef]
- Teoh, K.H.; Ramesh, S.; Arof, A.K. Investigation on the effect of nanosilica towards corn starch-lithium perchlorate-based polymer electrolytes. J. Solid State Electrochem. 2012, 16, 3165–3170. [Google Scholar] [CrossRef]
- Ramesh, S.; Shanti, R.; Morris, E. Studies on the thermal behavior of CS:LiTFSI:[Amim] Cl polymer electrolytes exerted by different [Amim] Cl content. Solid State Sci. 2012, 14, 182–186. [Google Scholar] [CrossRef]
- Ramesh, S.; Shanti, R.; Morris, E. Studies on the plasticization efficiency of deep eutectic solvent in suppressing the crystallinity of corn starch based polymer electrolytes. Carbohydr. Polym. 2012, 87, 701–706. [Google Scholar] [CrossRef]
- Sudhakar, Y.N.; Selvakumar, M. Lithium perchlorate doped plasticized chitosan and starch blend as biodegradable polymer electrolyte for supercapacitors. Electrochim. Acta 2012, 78, 398–405. [Google Scholar] [CrossRef]
- Shukur, M.F.; Ithnin, R.; Sonsudin, F.; Yahya, R.; Ahmad, Z.; Kadir, M.F.Z. Conduction mechanism and dielectric properties of solid biopolymer electrolyte incorporated with silver nitrate. Adv. Mater. Res. 2013, 701, 115–119. [Google Scholar] [CrossRef]
- Shukur, M.F.; Ibrahim, F.M.; Majid, N.A.; Ithnin, R.; Kadir, M.F.Z. Electrical analysis of amorphous corn starch-based polymer electrolyte membranes doped with LiI. Phys. Scr. 2013, 88, 1–9. [Google Scholar] [CrossRef]
- Shukur, M.F.; Sonsudin, F.; Yahya, R.; Ahmad, Z.; Ithnin, R. Electrical properties of starch based silver ion conducting solid biopolymer electrolyte. Adv. Mater. Res. 2013, 701, 120–124. [Google Scholar] [CrossRef]
- Khanmirzaei, M.H.; Ramesh, S. Ionic transport and FTIR properties of lithium iodide doped biodegradable rice starch based polymer electrolytes. Int. J. Electrochem. Sci. 2013, 8, 9977–9991. [Google Scholar]
- Sudhakar, Y.N.; Selvakumar, M. Ionic conductivity studies and dielectric studies of poly(styrene sulphonic acid)/starch blend polymer electrolyte containing LiClO4. J. Appl. Electrochem. 2013, 43, 21–29. [Google Scholar] [CrossRef]
- Tiwari, T.; Kumar, M.; Srivastava, N.; Srivastava, P.C. Electrical transport study of potato starch-based electrolyte system-II. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2014, 182, 6–13. [Google Scholar] [CrossRef]
- Shukur, M.F.; Ithnin, R.; Kadir, M.F.Z. Electrical characterization of corn starch-LiOAc electrolytes and application in electrochemical double layer capacitor. Electrochim. Acta 2014, 136, 204–216. [Google Scholar] [CrossRef]
- Singh, R.; Baghel, J.; Shukla, S.; Bhattacharya, B.; Rhee, H.-W.; Singh, P.K. Detailed electrical measurements on sago starch biopolymer solid electrolyte. Phase Transit. 2014, 87, 1237–1245. [Google Scholar] [CrossRef]
- Khanmirzaei, M.H.; Ramesh, S. Nanocomposite polymer electrolyte based on rice starch/ionic liquid/TiO2 nanoparticles for solar cell application. Measurement 2014, 58, 68–72. [Google Scholar] [CrossRef]
- Liew, C.W.; Ramesh, S. Comparing triflate and hexafluorophosphate anions of ionic liquids in polymer electrolytes for supercapacitor applications. Materials 2014, 7, 4019–4033. [Google Scholar] [CrossRef]
- Teoh, K.H.; Lim, C.-S.; Ramesh, S. Lithium ion conduction in corn starch based solid polymer electrolytes. Measurement 2014, 48, 87–95. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Majid, N.A.; Kasmani, R.M.; Illias, H.A.; Kadir, M.F.Z. The effect of plasticization on conductivity and other properties of starch/chitosan blend biopolymer electrolyte incorporated with ammonium iodide. Mol. Cryst. Liq. Cryst. 2014, 603, 73–88. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Shukur, M.F.; Illias, H.A.; Kadir, M.F.Z. Conductivity and electrical properties of corn starch-chitosan blend biopolymer electrolyte incorporated with ammonium iodide. Phys. Scr. 2014, 89, 1–11. [Google Scholar] [CrossRef]
- Shukur, M.F.; Ithnin, R.; Kadir, M.F.Z. Electrical properties of proton conducting solid biopolymer electrolytes based on starch-chitosan blend. Ionics 2014, 20, 977–999. [Google Scholar] [CrossRef]
- Shukur, M.F.; Ithnin, R.; Kadir, M.F.Z. Protonic transport analysis of starch-chitosan blend based electrolytes and application in electrochemical device. Mol. Cryst. Liq. Cryst. 2014, 603, 52–65. [Google Scholar] [CrossRef]
- Teoh, K.H.; Lim, C.-S.; Liew, C.-W.; Ramesh, S. Electric double-layer capacitors with corn starch-based biopolymer electrolytes incorporating silica as filler. Ionics 2015, 21, 2061–2068. [Google Scholar] [CrossRef]
- Khanmirzaei, M.H.; Ramesh, S.; Ramesh, K. Effect of different iodide salts on ionic conductivity and structural and thermal behavior of rice-starch-based polymer electrolytes for dye-sensitized solar cell application. Ionics 2015, 21, 2383–2391. [Google Scholar] [CrossRef]
- Khanmirzaei, M.H.; Ramesh, S.; Ramesh, K. Polymer electrolyte based dye-sensitized solar cell with rice starch and 1-methyl-3-propylimidazolium iodide ionic liquid. Mater. Des. 2015, 85, 833–837. [Google Scholar] [CrossRef]
- Shukur, M.F.; Kadir, M.F.Z. Electrical and transport properties of NH4Br-doped cornstarch-based solid biopolymer electrolyte. Ionics 2015, 21, 111–124. [Google Scholar] [CrossRef]
- Liew, C.W.; Ramesh, S. Electrical, structural, thermal and electrochemical properties of corn starch-based biopolymer electrolytes. Carbohydr. Polym. 2015, 124, 222–228. [Google Scholar] [CrossRef]
- Chatterjee, B.; Kulshrestha, N.; Gupta, P.N. Electrical properties of starch-PVA biodegradable polymer blend. Phys. Scr. 2015, 90, 1–9. [Google Scholar] [CrossRef]
- Navaratnam, S.; Sanusi, A.; Ahmad, A.H.; Ramesh, S.; Ramesh, K.; Othman, N. Conductivity studies of biopolymer electrolyte based on potato starch/chitosan blend doped with LICF3SO3. J. Teknol. 2015, 7, 1–5. [Google Scholar] [CrossRef]
- Amran, N.N.A.; Manan, N.S.A.; Kadir, M.F.Z. The effect of LiCF3SO3 on the complexation with potato starch-chitosan blend polymer electrolytes. Ionics 2016, 22, 1647–1658. [Google Scholar] [CrossRef]
- Teoh, K.H.; Lim, C.S.; Liew, C.W.; Ramesh, S. Preparation and performance analysis of barium titanate incorporated in corn starch-based polymer electrolytes for electric double layer capacitor application. J. Appl. Polym. Sci. 2016, 133, 1–8. [Google Scholar] [CrossRef]
- Lin, Y.; Li, J.; Liu, K.; Liu, Y.; Liu, J.; Wang, X. Unique starch polymer electrolyte for high capacity all-solid-state lithium sulfur battery. Green Chem. 2016, 18, 3796–3803. [Google Scholar] [CrossRef]
- Shukur, M.F.; Ithnin, R.; Kadir, M.F.Z. Ionic conductivity and dielectric properties of potato starch-magnesium acetate biopolymer electrolytes: The effect of glycerol and 1-butyl-3-methylimidazolium chloride. Ionics 2016, 22, 1113–1123. [Google Scholar] [CrossRef]
- Chatterjee, B.; Kulshrestha, N.; Gupta, P.N. Nano composite solid polymer electrolytes based on biodegradable polymers starch and poly vinyl alcohol. Measurement 2016, 82, 490–499. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Kadir, M.F.Z. Electrochemical characterizations and the effect of glycerol in biopolymer electrolytes based on methylcellulose-potato starch blend. Mol. Cryst. Liq. Cryst. 2016, 627, 220–233. [Google Scholar] [CrossRef]
- Kulshrestha, N.; Gupta, P.N. Structural and electrical characterizations of 50:50 PVA:starch blend complexed with ammonium thiocyanate. Ionics 2016, 22, 671–681. [Google Scholar] [CrossRef]
- Khiar, A.S.A.; Anuar, M.R.S.; Md Parid, M.A. Effect of 1-ethyl-3-methylimidazolium nitrate on the electrical properties of starch/chitosan blend polymer electrolyte. Mater. Sci. Forum 2016, 846, 510–516. [Google Scholar] [CrossRef]
- Railanmaa, A.; Lehtimäki, S.; Lupo, D. Comparison of starch and gelatin hydrogels for non-toxic supercapacitor electrolytes. Appl. Phys. A Mater. Sci. Process. 2017, 123, 1–8. [Google Scholar] [CrossRef]
- Vernon-Carter, E.J.; Alvarez-Ramirez, J.; Bello-Perez, L.A.; Roldan-Cruz, C.; Garcia-Hernandez, A.; Huerta, L. The order of addition of corn starch/lithium perchlorate/glycerol affects the optical, mechanical, electrical properties of a solid polymer electrolyte. Ionics 2017, 23, 1–13. [Google Scholar] [CrossRef]
- Azli, A.A.; Manan, N.S.A.; Kadir, M.F.Z. The development of Li+ conducting polymer electrolyte based on potato starch/graphene oxide blend. Ionics 2017, 23, 411–425. [Google Scholar] [CrossRef]
- Chauhan, J.K.; Kumar, M.; Yadav, M.; Tiwari, T.; Srivastava, N. Effect of NaClO4 concentration on electrolytic behaviour of corn starch film for supercapacitor application. Ionics 2017, 23, 2943–2949. [Google Scholar] [CrossRef]
- Selvanathan, V.; Azzahari, A.D.; Adyani, A.A.; Yahya, R. Ternary natural deep eutectic solvent (NADES) infused phthaloyl starch as cost efficient quasi-solid gel polymer electrolyte. Carbohydr. Polym. 2017, 167, 210–218. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Shukur, M.F.; Kadir, M.F.Z. The effect of NH4NO3 towards the conductivity enhancement and electrical behavior in methyl cellulose-starch blend based ionic conductors. Ionics 2017, 23, 1137–1154. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Shukur, M.F.; Kadir, M.F.Z. NH4NO3 as charge carrier contributor in glycerolized potato starch-methyl cellulose blend-based polymer electrolyte and the application in electrochemical double-layer capacitor. Ionics 2017, 23, 1–25. [Google Scholar] [CrossRef]
- Regiani, A.M.; De Oliveira-Machado, G.; LeNest, J.F.; Gandini, A.; Pawlicka, A. Cellulose derivatives as solid electrolyte matrixes. Macromol. Symp. 2001, 175, 45–53. [Google Scholar] [CrossRef]
- Tambelli, C.E.; Donoso, J.P.; Regiani, A.M.; Pawlicka, A.; Gandini, A.; LeNest, J.F. Nuclear magnetic resonance and conductivity study of HEC/polyether-based polymer electrolytes. Electrochim. Acta 2001, 46, 1665–1672. [Google Scholar] [CrossRef]
- Yue, Z.; McEwen, I.J.; Cowie, J.M.G. Ion conducting behaviour and morphology of solid polymer electrolytes based on a regioselectively substituted cellulose ether with PEO side chains. J. Mater. Chem. 2002, 12, 2281–2285. [Google Scholar] [CrossRef]
- Yue, Z.; McEwen, I.J.; Cowie, J.M.G. Novel gel polymer electrolytes based on a cellulose ester with PEO side chains. Solid State Ion. 2003, 156, 155–162. [Google Scholar] [CrossRef]
- Schroers, M.; Kokil, A.; Weder, C. Solid polymer electrolytes based on nanocomposites of ethylene oxide-epichlorohydrin copolymers and cellulose whiskers. J. Appl. Polym. Sci. 2004, 93, 2883–2888. [Google Scholar] [CrossRef]
- Azizi Samir, M.A.S.; Mateos, A.M.; Alloin, F.; Sanchez, J.Y.; Dufresne, A. Plasticized nanocomposite polymer electrolytes based on poly(oxyethylene) and cellulose whiskers. Electrochim. Acta 2004, 49, 4667–4677. [Google Scholar] [CrossRef]
- Machado, G.O.; Pawlicka, A.; Yonashiro, M. Solid polymeric electrolytes networks of hydroxypropyl cellulose with jeffamine. Nonlinear Opt. Quantum Opt. 2004, 32, 141–148. [Google Scholar]
- Azizi Samir, M.A.S.; Alloin, F.; Sanchez, J.Y.; Dufresne, A. POE-based nanocomposite polymer electrolytes reinforced with cellulose whiskers. Electrochim. Acta 2005, 50, 3897–3903. [Google Scholar] [CrossRef]
- Azizi Samir, M.A.S.; Alloin, F.; Dufresne, A. High performance nanocomposite polymer electrolytes. Compos. Interfaces 2006, 13, 545–559. [Google Scholar] [CrossRef]
- Selvakumar, M.; Bhat, D.K. LiClO4 doped cellulose acetate as biodegradable polymer electrolyte for supercapacitors. J. Appl. Polym. Sci. 2008, 110, 594–602. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Wang, C.-M.; Hsieh, P.-T.; Chen, Y.-C.; Liu, C.-C.; Shih, S.-C. A novel gel polymer electrolyte based on lithium salt with an ethyl cellulose. Colloid Polym. Sci. 2009, 287, 1355–1358. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, Y.; Sun, K.; Zhou, X.; Zhang, N. A microporous gel electrolyte based on poly(vinylidene fluoride-co-hexafluoropropylene)/fully cyanoethylated cellulose derivative blend for lithium-ion battery. Electrochim. Acta 2009, 54, 1888–1892. [Google Scholar] [CrossRef]
- Johari, N.A.; Kudin, T.I.T.; Ali, A.M.M.; Winie, T.; Yahya, M.Z.A. Studies on cellulose acetate-based gel polymer electrolytes for proton batteries. Mater. Res. Innov. 2009, 13, 232–234. [Google Scholar] [CrossRef]
- Lee, J.M.; Nguyen, D.Q.; Lee, S.B.; Kim, H.; Ahn, B.S.; Lee, H.; Kim, H.S. Cellulose triacetate-based polymer gel electrolytes. J. Appl. Polym. Sci. 2010, 115, 32–36. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Fa, W.; Zhang, Y.; Huang, B. Synthesis of a grafted cellulose gel electrolyte in an ionic liquid ([Bmim]I) for dye-sensitized solar cells. Carbohydr. Polym. 2011, 86, 1216–1220. [Google Scholar] [CrossRef]
- Ali, R.M.; Harun, N.I.; Ali, A.M.M.; Yahya, M.Z.A. Conductivity studies on plasticised cellulose acetate–ammonium iodide based polymer electrolytes. Mater. Res. Innov. 2011, 15, 39–42. [Google Scholar] [CrossRef]
- Johari, N.A.; Kudin, T.I.T.; Ali, A.M.M.; Yahya, M.Z.A. Effects of TiO2 on conductivity performance of cellulose acetate based polymer gel electrolytes for proton batteries. Mater. Res. Innov. 2011, 15, 229–231. [Google Scholar] [CrossRef]
- Harun, N.I.; Ali, R.M.; Ali, A.M.M.; Yahya, M.Z.A. Conductivity studies on cellulose acetate-tetrafluoroborate based polymer electrolytes. Mater. Res. Innov. 2011, 15, 39–42. [Google Scholar] [CrossRef]
- Harun, N.I.; Ali, R.M.; Ali, A.M.M.; Yahya, M.Z.A. Dielectric behaviour of cellulose acetate-based polymer electrolytes. Ionics 2011, 18, 599–606. [Google Scholar] [CrossRef]
- Harun, N.I.; Md Ali, R.; Ali, A.M.M.; Yahya, M.Z.A. Effects of ammonium tetrafluoroborate on conductivity and thermal studies on cellulose acetate based polymer electrolytes. Adv. Mater. Res. 2013, 667, 150–154. [Google Scholar] [CrossRef]
- Samsudin, A.S.; Khairul, W.M.; Isa, M.I.N. Characterization on the potential of carboxy methylcellulose for application as proton conducting biopolymer electrolytes. J. Non. Cryst. Solids 2012, 358, 1104–1112. [Google Scholar] [CrossRef]
- Asghar, A.; Abdul Samad, Y.; Singh Lalia, B.; Hashaikeh, R. PEG based quasi-solid polymer electrolyte: Mechanically supported by networked cellulose. J. Memb. Sci. 2012, 421–422, 85–90. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Deng, J.; Yi, B.; Yu, X.; Shen, P.; Tan, S. A novel polymer gel electrolyte based on cyanoethylated cellulose for dye-sensitized solar cells. Electrochim. Acta 2012, 80, 219–226. [Google Scholar] [CrossRef]
- Ramesh, S.; Shanti, R.; Morris, E. Discussion on the influence of DES content in CA-based polymer electrolytes. J. Mater. Sci. 2012, 47, 1787–1793. [Google Scholar] [CrossRef]
- Ramesh, S.; Shanti, R.; Morris, E. Characterization of conducting cellulose acetate based polymer electrolytes doped with ’green’ ionic mixture. Carbohydr. Polym. 2013, 91, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Shanti, R.; Morris, E. Plasticizing effect of 1-allyl-3-methylimidazolium chloride in cellulose acetate based polymer electrolytes. Carbohydr. Polym. 2012, 87, 2624–2629. [Google Scholar] [CrossRef]
- Sahli, N.B.; Bin Ali, A.M. Effect of lithium triflate salt concentration in methyl cellulose-based solid polymer electrolytes. In Proceedings of the 2012 IEEE Colloquium on Humanities, Science and Engineering (CHUSER), Kota Kinabalu, Malaysia, 3–4 December 2012; pp. 739–742. [Google Scholar]
- Shuhaimi, N.E.A.; Teo, L.P.; Woo, H.J.; Majid, S.R.; Arof, A.K. Electrical double-layer capacitors with plasticized polymer electrolyte based on methyl cellulose. Polym. Bull. 2012, 69, 807–826. [Google Scholar] [CrossRef]
- Mustafa, M.F.; Ridwan, N.I.M.; Hatta, F.F.; Yahya, M.Z.A. Effect of dimethyl carbonate plasticizer on ionic cnductivity of methyl cellulose-based polymer electrolyte. Malays. J. Anal. Sci. 2012, 16, 283–289. [Google Scholar]
- Liu, J.; Li, W.; Zuo, X.; Liu, S.; Li, Z. Polyethylene-supported polyvinylidene fluoride-cellulose acetate butyrate blended polymer electrolyte for lithium ion battery. J. Power Sources 2013, 226, 101–106. [Google Scholar] [CrossRef]
- Bella, F.; Nair, J.R.; Gerbaldi, C. Towards green, efficient and durable quasi-solid dye-sensitized solar cells integrated with a cellulose-based gel-polymer electrolyte optimized by a chemometric DoE approach. RSC Adv. 2013, 3, 15993–16001. [Google Scholar] [CrossRef]
- Abidin, S.Z.Z.; Ali, A.M.M.; Hassan, O.H.; Yahya, M.Z.A. Electrochemical studies on cellulose acetate-LiBOB polymer gel electrolytes. Int. J. Electrochem. Sci. 2013, 8, 7320–7326. [Google Scholar]
- Samad, Y.A.; Asghar, A.; Lalia, B.S.; Hashaikeh, R. Networked cellulose entrapped and reinforced PEO-based solid polymer electrolyte for moderate temperature applications. J. Appl. Polym. Sci. 2013, 129, 2998–3006. [Google Scholar] [CrossRef]
- Ran, Y.; Yin, Z.; Ding, Z.; Guo, H.; Yang, J. A polymer electrolyte based on poly(vinylidene fluoride-hexafluoropylene)/hydroxypropyl methyl cellulose blending for lithium-ion battery. Ionics 2013, 19, 757–762. [Google Scholar] [CrossRef]
- Ramesh, S.; Shanti, R.; Morris, E. Employment of [Amim] Cl in the effort to upgrade the properties of cellulose acetate based polymer electrolytes. Cellulose 2013, 20, 1377–1389. [Google Scholar] [CrossRef]
- Kiristi, M.; Bozduman, F.; Gulec, A.; Teke, E.; Oksuz, L.; Oksuz, A.U.; Deligoz, H. Complementary all solid state electrochromic devices using carboxymethyl cellulose based electrolytes. J. Macromol. Sci. Part A 2014, 51, 481–487. [Google Scholar] [CrossRef]
- Arof, A.K.; Amirudin, S.; Yusof, S.Z.; Noor, I.M. A method based on impedance spectroscopy to determine transport properties of polymer electrolytes. Phys. Chem. Chem. Phys. 2014, 16, 1856–1867. [Google Scholar] [CrossRef]
- Chiappone, A.; Bella, F.; Nair, J.R.; Meligrana, G.; Bongiovanni, R.; Gerbaldi, C. Structure-performance correlation of nanocellulose-based polymer electrolytes for efficient quasi-solid DSSCs. ChemElectroChem 2014, 1, 1350–1358. [Google Scholar] [CrossRef]
- Xiao, S.Y.; Wang, F.X.; Chang, Z.; Wu, Y.P. An environmentally friendly and economic membrane based on cellulose as a gel polymer electrolyte for lithium ion batteries. J. Power Sources 2014, 270, 53–58. [Google Scholar] [CrossRef]
- Ng, W.F.; Chai, M.N.; Isa, M.I.N. Proton conducting carboxy methyl cellulose solid polymer electrolytes doped with citric acid. Adv. Mater. Res. 2014, 895, 130–133. [Google Scholar] [CrossRef]
- Jafirin, S.; Ahmad, I.; Ahmad, A. Composite polymer electrolyte based on MG-49 and carboxymethyl cellulose from kenaf. AIP Conf. Proc. 2014, 1571, 822–828. [Google Scholar]
- Abiddin, J.F.Z.; Ahmad, A.H. Fourier transform infrared spectroscopy and electrical characterization of methylcellulose based solid polymer electrolyte doped with sodium iodide. Jurnal Teknologi 2015, 3, 41–45. [Google Scholar]
- Abiddin, J.F.Z.; Ahmad, A.H. Conductivity study and fourier transform infrared (FTIR) characterization of methyl cellulose solid polymer electrolyte with sodium iodide conducting ion. AIP Conf. Proc. 2015, 1674, 1–6. [Google Scholar]
- Li, M.; Wang, X.; Wang, Y.; Chen, B.; Wu, Y.; Holze, R. A gel polymer electrolyte based on composite of nonwoven fabric and methyl cellulose with good performance for lithium ion batteries. RSC Adv. 2015, 5, 52382–52387. [Google Scholar] [CrossRef]
- Leng, L.; Zeng, X.; Chen, P.; Shu, T.; Song, H.; Fu, Z.; Wang, H.; Liao, S. A novel stability-enhanced lithium-oxygen battery with cellulose-based composite polymer gel as the electrolyte. Electrochim. Acta 2015, 176, 1108–1115. [Google Scholar] [CrossRef]
- Colò, F.; Bella, F.; Nair, J.R.; Destro, M.; Gerbaldi, C. Cellulose-based novel hybrid polymer electrolytes for green and efficient Na-ion batteries. Electrochim. Acta 2015, 174, 185–190. [Google Scholar] [CrossRef]
- Razalli, S.M.M.; Sheikh Mohd Saaid, S.I.Y.; Marwan Ali, A.M.; Hassan, O.H.; Yahya, M.Z.A. Cellulose acetate-lithium bis(trifluoromethanesulfonyl)imide solid polymer electrolyte: ATR-FTIR and ionic conductivity behavior. Funct. Mater. Lett. 2015, 8, 1–4. [Google Scholar]
- Sudhakar, Y.N.; Selvakumar, M.; Bhat, D.K. Preparation and characterization of phosphoric acid-doped hydroxyethyl cellulose electrolyte for use in supercapacitor. Mater. Renew. Sustain. Energy 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Xiao, S.Y.; Li, M.X.; Chang, Z.; Wang, F.X.; Gao, J.; Wu, Y.P. Natural macromolecule based carboxymethyl cellulose as a gel polymer electrolyte with adjustable porosity for lithium ion batteries. J. Power Sources 2015, 288, 368–375. [Google Scholar] [CrossRef]
- Ledwon, P.; Andrade, J.R.; Lapkowski, M.; Pawlicka, A. Hydroxypropyl cellulose-based gel electrolyte for electrochromic devices. Electrochim. Acta 2015, 159, 227–233. [Google Scholar] [CrossRef]
- Samsi, N.S.; Zakaria, R.; Hassan, O.H.; Yahya, M.Z.A.; Ali, A.M.M. Optical behavior of NH4I doped cellulose acetate membrane for dye sensitized solar cell. Malays. J. Anal. Sci. 2015, 19, 760–765. [Google Scholar]
- Rani, M.S.A.; Dzulkurnain, N.A.; Ahmad, A.; Mohamed, N.S. Conductivity and dielectric behavior studies of carboxymethyl cellulose from kenaf bast fiber incorporated with ammonium acetate-BMATFSI biopolymer electrolytes. Int. J. Polym. Anal. Charact. 2015, 20, 250–260. [Google Scholar] [CrossRef]
- Li, M.X.; Wang, X.W.; Yang, Y.Q.; Chang, Z.; Wu, Y.P.; Holze, R. A dense cellulose-based membrane as a renewable host for gel polymer electrolyte of lithium ion batteries. J. Memb. Sci. 2015, 476, 112–118. [Google Scholar] [CrossRef]
- Ahmad, N.H.B.; Isa, M.I.N. Proton conducting solid polymer electrolytes based carboxymethyl cellulose doped ammonium chloride: Ionic conductivity and transport studies. Int. J. Plast. Technol. 2015, 19, 47–55. [Google Scholar] [CrossRef]
- Sohaimy, M.I.H.; Isa, M.I.N. Effect of ammonium carbonate salt concentration on structural and ionic conductivity of cellulose based solid polymer electrolytes. Fibers Polym. 2015, 16, 1031–1034. [Google Scholar] [CrossRef]
- Ramlli, M.A.; Isa, M.I.N. Structural and ionic transport properties of protonic conducting solid biopolymer electrolytes based on carboxymethyl cellulose doped with ammonium fluoride. J. Phys. Chem. B 2016, 120, 11567–11573. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.Y.; Liew, C.W.; Numan, A.; Yugal, K.; Ramesh, K.; Ng, H.M.; Chong, T.V.; Ramesh, S. Effects of ionic liquid on the hydroxylpropylmethyl cellulose (HPMC) solid polymer electrolyte. Ionics 2016, 22, 2421–2430. [Google Scholar] [CrossRef]
- Chai, M.N.; Isa, M.I.N. Novel proton conducting solid bio-polymer electrolytes based on carboxymethyl cellulose doped with oleic acid and plasticized with glycerol. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef]
- Ngamaroonchote, A.; Chotsuwan, C. Performance and reliability of cellulose acetate-based gel electrolyte for electrochromic devices. J. Appl. Electrochem. 2016, 46, 575–582. [Google Scholar] [CrossRef]
- Samsi, N.S.; Zakaria, R.; Hassan, O.H.; Azhan Yahya, M.Z.; Ali, A.M.M. X-ray diffraction and infrared studies on plasticized cellulose acetate complexed with ammonium iodide for solid polymer electrolyte. Mater. Sci. Forum 2016, 846, 523–527. [Google Scholar] [CrossRef]
- Zhao, M.; Zuo, X.; Wang, C.; Xiao, X.; Liu, J.; Nan, J. Preparation and performance of the polyethylene-supported polyvinylidene fluoride/cellulose acetate butyrate/nano-SiO2 particles blended gel polymer electrolyte. Ionics 2016, 22, 2123–2132. [Google Scholar] [CrossRef]
- Monisha, S.; Mathavan, T.; Selvasekarapandian, S.; Benial, A.M.F.; Latha, M.P. Preparation and characterization of cellulose acetate and lithium nitrate for advanced electrochemical devices. Ionics 2016, 23, 1–10. [Google Scholar] [CrossRef]
- Monisha, S.; Selvasekarapandian, S.; Mathavan, T.; Milton Franklin Benial, A.; Manoharan, S.; Karthikeyan, S. Preparation and characterization of biopolymer electrolyte based on cellulose acetate for potential applications in energy storage devices. J. Mater. Sci. Mater. Electron. 2016, 27, 9314–9324. [Google Scholar] [CrossRef]
- Razalli, S.M.M.; Saaid, S.I.Y.S.M.; Kudin, T.I.T.; Yahya, M.Z.A.; Hassan, O.H.; Ali, A.M.M. Electrochemical properties of glyme based plasticizer on gel polymer electrolytes doped with lithium bis(trifluoromethanesulfonyl)imide. Mater. Sci. Forum 2016, 846, 534–538. [Google Scholar] [CrossRef]
- Song, A.; Huang, Y.; Zhong, X.; Cao, H.; Liu, B.; Lin, H.; Wang, M.; Li, X. Gel polymer electrolyte with high performances based on pure natural polymer matrix of potato starch composite lignocellulose. Electrochim. Acta 2017, 245, 981–992. [Google Scholar] [CrossRef]
- Song, A.; Huang, Y.; Liu, B.; Cao, H.; Zhong, X.; Lin, Y.; Wang, M.; Li, X.; Zhong, W. Gel polymer electrolyte based on polyethylene glycol composite lignocellulose matrix with higher comprehensive performances. Electrochim. Acta 2017, 247, 505–515. [Google Scholar] [CrossRef]
- Gaur, S.S.; Dhar, P.; Sonowal, A.; Sharma, A.; Kumar, A.; Katiyar, V. Thermo-mechanically stable sustainable polymer based solid electrolyte membranes for direct methanol fuel cell applications. J. Memb. Sci. 2017, 526, 348–354. [Google Scholar] [CrossRef]
- Gupta, S.; Varshney, P.K. Effect of plasticizer concentration on structural and electrical properties of hydroxyethyl cellulose (HEC)-based polymer electrolyte. Ionics 2017, 23, 1613–1617. [Google Scholar] [CrossRef]
- Monisha, S.; Mathavan, T.; Selvasekarapandian, S.; Benial, A.M.F.; Aristatil, G.; Mani, N.; Premalatha, M.; Pandi, D.V. Investigation of bio polymer electrolyte based on cellulose acetate-ammonium nitrate for potential use in electrochemical devices. Carbohydr. Polym. 2017, 157, 38–47. [Google Scholar] [CrossRef]
- Sohaimy, M.I.H.; Isa, M.I.N. Ionic conductivity and conduction mechanism studies on cellulose based solid polymer electrolytes doped with ammonium carbonate. Polym. Bull. 2017, 74, 1371–1386. [Google Scholar] [CrossRef]
- Mohamed, N.S.; Subban, R.H.Y.; Arof, A.K. Polymer batteries fabricated from lithium complexed acetylated chitosan. J. Power Sources 1995, 56, 153–156. [Google Scholar] [CrossRef]
- Subban, R.H.Y.; Arof, A.K. Sodium iodide added chitosan electrolyte film for polymer batteries. Phys. Scr. 1996, 53, 382. [Google Scholar] [CrossRef]
- Subban, R.H.Y.; Arof, A.K.; Radhakrishna, S. Polymer batteries with chitosan electrolyte mixed with sodium perchlorate. Mater. Sci. Eng. B 1996, 38, 156–160. [Google Scholar] [CrossRef]
- Velazquez-Morales, P.; Le Nest, J.-F.; Gandini, A. Polymer electrolytes derived from chitosan/polyether networks. Electrochim. Acta 1998, 43, 1275–1279. [Google Scholar] [CrossRef]
- Osman, Z.; Arof, A.K. FTIR studies of chitosan acetate based polymer electrolytes. Electrochim. Acta 2003, 48, 993–999. [Google Scholar] [CrossRef]
- De Vasconcelos, C.L.; Rocha, A.N.L.; Pereira, M.R.; Fonseca, J.L.C. Electrolyte diffusion in a chitosan membrane. Polym. Int. 2001, 50, 309–312. [Google Scholar] [CrossRef]
- Kamarulzaman, N.; Osman, Z.; Muhamad, M.R.; Ibrahim, Z.A. Performance characteristics of LiMn2O4/polymer/carbon electrochemical cells. J. Power Sources 2001, 97–98, 722–725. [Google Scholar] [CrossRef]
- Yahya, M.Z.A.; Arof, A.K. Characteristics of chitosan-lithium acetate-palmitic acid complexes. J. New Mater. Electrochem. Syst. 2002, 5, 123–128. [Google Scholar]
- Yahya, M.Z.A.; Arof, A.K. Studies on lithium acetate doped chitosan conducting polymer system. Eur. Polym. J. 2002, 38, 1191–1197. [Google Scholar] [CrossRef]
- Yahya, M.Z.A.; Arof, A.K. Conductivity and X-ray photoelectron studies on lithium acetate doped chitosan films. Carbohydr. Polym. 2004, 55, 95–100. [Google Scholar] [CrossRef]
- Ali, A.M.M.; Yahya, M.Z.A.; Mustaffa, M.; Ahmad, A.H.; Subban, R.H.Y.; Harun, M.K.; Mohamad, A.A. Electrical properties of plasticized chitosan-lithium imide with oleic acid-based polymer electrolytes for lithium rechargeable batteries. Ionics 2005, 11, 460–463. [Google Scholar] [CrossRef]
- Wan, Y.; Creber, K.A.M.; Peppley, B.; Tam Bui, V.; Halliop, E. New solid polymer electrolyte membranes for alkaline fuel cells. Polym. Int. 2005, 54, 5–10. [Google Scholar] [CrossRef]
- Majid, S.R.; Idris, N.H.; Hassan, M.F.; Winie, T.; Khiar, A.S.A.; Arof, A.K. Transport studies on filler-doped chitosan based polymer electrolyte. Ionics 2005, 11, 451–455. [Google Scholar] [CrossRef]
- Winie, T.; Majid, S.R.; Hassan, M.F.; Arof, A.K. Characterization of plasticized hexanoyl chitosan-based polymer electrolytes and application in LiCoO2/MCMB cells. Mater. Sci. Forum 2006, 517, 85–88. [Google Scholar] [CrossRef]
- Winie, T.; Arof, A.K. FT-IR studies on interactions among components in hexanoyl chitosan-based polymer electrolytes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 63, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Khiar, A.S.A.; Majid, S.R.; Idris, N.H.; Hassan, M.F.; Puteh, R.; Arof, A.K. Ionic hopping transport in chitosan-based polymer electrolytes. Mater. Sci. Forum 2006, 517, 237–241. [Google Scholar] [CrossRef]
- Yahya, M.Z.A.; Ali, A.M.M.; Mohammat, M.F.; Hanafiah, M.A.K.M.; Mustaffa, M.; Ibrahim, S.C.; Darus, Z.M.; Harun, M.K. Ionic conduction model in salted chitosan membranes plasticized with fatty acid. J. Appl. Sci. 2006, 6, 1287–1291. [Google Scholar]
- Winie, T.; Majid, S.R.; Khiar, A.S.A.; Arof, A.K. Ionic conductivity of chitosan membranes and application for electrochemical devices. Polym. Adv. Technol. 2006, 17, 523–527. [Google Scholar] [CrossRef]
- Wan, Y.; Peppley, B.; Creber, K.A.M.; Bui, V.T.; Halliop, E. Preliminary evaluation of an alkaline chitosan-based membrane fuel cell. J. Power Sources 2006, 162, 105–113. [Google Scholar] [CrossRef]
- Ng, L.S.; Mohamad, A.A. Protonic battery based on a plasticized chitosan-NH4NO3 solid polymer electrolyte. J. Power Sources 2006, 163, 382–385. [Google Scholar] [CrossRef]
- Winie, T.; Arof, A.K. Transport properties of hexanoyl chitosan-based gel electrolyte. Ionics 2006, 12, 149–152. [Google Scholar] [CrossRef]
- Idris, N.H.; Senin, H.B.; Arof, A.K. Dielectric spectra of LiTFSI-doped chitosan/PEO blends. Ionics 2007, 13, 213–217. [Google Scholar] [CrossRef]
- Majid, S.R.; Arof, A.K. Mobility and density of ions in chitosan-orthophosphoric acid-ammonium nitrate electrolytes. Phys. Status Solidi Appl. Mater. Sci. 2007, 204, 2396–2401. [Google Scholar] [CrossRef]
- Mohamad, S.A.; Ali, M.H.; Yahya, R.; Ibrahim, Z.A.; Arof, A.K. Photovoltaic activity in a ZnSe/PEO-chitosan blend electrolyte junction. Ionics 2007, 13, 235–240. [Google Scholar] [CrossRef]
- Fuentes, S.; Retuert, P.J.; González, G. Lithium ion conductivity of molecularly compatibilized chitosan-poly(aminopropyltriethoxysilane)-poly(ethylene oxide) nanocomposites. Electrochim. Acta 2007, 53, 1417–1421. [Google Scholar] [CrossRef]
- Pawlicka, A.; Danczuk, M.; Wieczorek, W.; Zygadło-Monikowska, E. Influence of plasticizer type on the properties of polymer electrolytes based on chitosan. J. Phys. Chem. A 2008, 112, 8888–8895. [Google Scholar] [CrossRef] [PubMed]
- Göktepe, F.; Çelik, S.U.; Bozkurt, A. Preparation and the proton conductivity of chitosan/poly(vinyl phosphonic acid) complex polymer electrolytes. J. Non-Cryst. Solids 2008, 354, 3637–3642. [Google Scholar]
- Ng, L.S.; Mohamad, A.A. Effect of temperature on the performance of proton batteries based on chitosan-NH4NO3-EC membrane. J. Membr. Sci. 2008, 325, 653–657. [Google Scholar] [CrossRef]
- Idris, N.K.; Aziz, N.A.N.; Zambri, M.S.M.; Zakaria, N.A.; Isa, M.I.N. Ionic conductivity studies of chitosan-based polymer electrolytes doped with adipic acid. Ionics 2009, 15, 643–646. [Google Scholar] [CrossRef]
- Majid, S.R.; Arof, A.K. Conductivity studies and performance of chitosan based polymer electrolytes in H2/air fuel cell. Polym. Adv. Technol. 2009, 20, 524–528. [Google Scholar] [CrossRef]
- Kadir, M.F.Z.A.; Teo, L.P.; Majid, S.R.; Arof, A.K. Conductivity studies on plasticised PEO/chitosan proton conducting polymer electrolyte. Mater. Res. Innov. 2009, 13, 259–262. [Google Scholar] [CrossRef]
- Du, J.F.; Bai, Y.; Pan, D.A.; Chu, W.Y.; Qiao, L.J. Characteristics of proton conducting polymer electrolyte based on chitosan acetate complexed with CH3COONH4. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 549–554. [Google Scholar] [CrossRef]
- Muhammad, F.H.; Subban, R.H.Y.; Majid, S.R.; Winie, T.; Arof, A.K. Characterisation of Al2O3 doped hexanoyl chitosan–LiCF3SO3–EC polymer electrolytes. Mater. Res. Innov. 2009, 13, 249–251. [Google Scholar] [CrossRef]
- Muhammad, F.H.; Subban, R.H.Y.; Winie, T. Electrical studies on hexanoyl chitosan-based nanocomposite polymer electrolytes. AIP Conf. Proc. 2009, 1136, 61–65. [Google Scholar]
- Rosli, N.H.A.; Muhammad, F.H.; Subban, R.H.Y.; Winie, T. Structural and electrical studies of hexanoyl chitosan based electrolyte system. Mater. Res. Innov. 2011, 15, 1–4. [Google Scholar] [CrossRef]
- Winie, T.; Ramesh, S.; Arof, A.K. Studies on the structure and transport properties of hexanoyl chitosan-based polymer electrolytes. Phys. B Condens. Matter 2009, 404, 4308–4311. [Google Scholar] [CrossRef]
- Kumar, M.S.; Bhat, D.K. LiClO4-doped plasticized chitosan as biodegradable polymer gel electrolyte for supercapacitors. J. Appl. Polym. Sci. 2009, 114, 2445–2454. [Google Scholar] [CrossRef]
- Arof, A.K.; Buraidah, M.H.; Teo, L.P.; Majid, S.R.; Yahya, R.; Taha, R.M. Characterizations of chitosan-based polymer electrolyte photovoltaic cells. Int. J. Photoenergy 2010, 2010, 1–7. [Google Scholar]
- Buraidah, M.H.; Teo, L.P.; Majid, S.R.; Arof, A.K. Characteristics of TiO2/solid electrolyte junction solar cells with redox couple. Opt. Mater. 2010, 32, 723–728. [Google Scholar] [CrossRef]
- Du, J.F.; Bai, Y.; Chu, W.Y.; Qiao, L.J. Synthesis and performance of proton conducting chitosan/NH4Cl electrolyte. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 260–266. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abidin, Z.H.Z.; Arof, A.K. Influence of silver ion reduction on electrical modulus parameters of solid polymer electrolyte based on chitosansilver triflate electrolyte membrane. Express Polym. Lett. 2010, 4, 300–310. [Google Scholar] [CrossRef]
- Singh, P.K.; Bhattacharya, B.; Nagarale, R.K.; Kim, K.-W.; Rhee, H.-W. Synthesis, characterization and application of biopolymer-ionic liquid composite membranes. Synth. Met. 2010, 160, 139–142. [Google Scholar] [CrossRef]
- Kadir, M.F.Z.; Majid, S.R.; Arof, A.K. Plasticized chitosan–PVA blend polymer electrolyte based proton battery. Electrochim. Acta 2010, 55, 1475–1482. [Google Scholar] [CrossRef]
- Jaafar, N.K.; Lepit, A.; Aini, N.A.; Saat, A.; Ali, A.M.M.; Yahya, M.Z.A. Effects of lithium salt on chitosan-g-PMMA based polymer electrolytes. Mater. Res. Innov. 2011, 15, 202–205. [Google Scholar] [CrossRef]
- Buraidah, M.H.; Teo, L.P.; Yusuf, S.N.F.; Noor, M.M.; Kufian, M.Z.; Careem, M.A.; Majid, S.R.; Taha, R.M.; Arof, A.K. TiO2/chitosan-NH4I(+I2)-BMII-based dye-sensitized solar cells with anthocyanin dyes extracted from black rice and red cabbage. Int. J. Photoenergy 2011, 2011, 1–11. [Google Scholar] [CrossRef]
- Aziz, N.A.; Majid, S.R.; Yahya, R.; Arof, A.K. Conductivity, structure, thermal properties of chitosan-based polymer electrolytes with nanofillers. Polym. Adv. Technol. 2011, 22, 1345–1348. [Google Scholar] [CrossRef]
- Winie, T.; Han, C.C.; Subban, R.H.Y. Ac conductivity and dielectric properties of hexanoyl chitosan-LiClO4-TiO2 composite polymer electrolytes. Adv. Mater. Res. 2011, 335–336, 873–880. [Google Scholar] [CrossRef]
- Kadir, M.F.Z.; Aspanut, Z.; Yahya, R.; Arof, A.K. Chitosan–PEO proton conducting polymer electrolyte membrane doped with NH4NO3. Mater. Res. Innov. 2011, 15, 164–167. [Google Scholar] [CrossRef]
- Kadir, M.F.Z.; Arof, A.K. Application of PVA-chitosan blend polymer electrolyte membrane in electrical double layer capacitor. Mater. Res. Innov. 2011, 15, 217–220. [Google Scholar] [CrossRef]
- Navaratnam, S.; Ramesh, K.; Basirun, W.J. Investigation of ion conducting behaviour of composite chitosan based polymer electrolytes. Mater. Res. Innov. 2011, 152, 184–186. [Google Scholar] [CrossRef]
- Buraidah, M.H.; Arof, A.K. Characterization of chitosan/PVA blended electrolyte doped with NH4I. J. Non. Cryst. Solids 2011, 357, 3261–3266. [Google Scholar] [CrossRef]
- Mattos, R.I.; Raphael, E.; Majid, S.R.; Arof, A.K.; Pawlicka, A. Enhancement of electrical conductivity in plasticized chitosan based membranes. Mol. Cryst. Liq. Cryst. 2012, 554, 150–159. [Google Scholar] [CrossRef]
- Aziz, N.A.; Majid, S.R.; Arof, A.K. Synthesis and characterizations of phthaloyl chitosan-based polymer electrolytes. J. Non. Cryst. Solids 2012, 358, 1581–1590. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, H.; Wu, C.; Wang, R. Preparation and characterization of conductive chitosan-ionic liquid composite membranes. Polym. Adv. Technol. 2012, 23, 1429–1434. [Google Scholar] [CrossRef]
- Winie, T.; Muhammad, F.H.; Rosli, N.H.A. Effect of anion size on the conductivity behaviour of hexanoyl chitosan-based polymer electrolytes. Adv. Mater. Res. 2012, 545, 317–320. [Google Scholar] [CrossRef]
- Shukur, M.F.; Kadir, M.F.Z.; Ahmad, Z.; Ithnin, R. Dielectric studies of proton conducting polymer electrolyte based on chitosan/PEO blend doped with NH4NO3. Adv. Mater. Res. 2012, 488–489, 583–587. [Google Scholar] [CrossRef]
- Shukur, M.F.; Kadir, M.F.Z.; Ahmad, Z.; Ithnin, R. Transport properties of chitosan/PEO blend based proton conducting polymer electrolyte. Adv. Mater. Res. 2012, 488–489, 114–117. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Thanikaikarasan, S.; Antony, R.; Balakumar, S.; Shajan, X.S. Effect of nanochitosan on electrochemical, interfacial and thermal properties of composite solid polymer electrolytes. Ionics 2012, 18, 737–745. [Google Scholar] [CrossRef]
- Alaei, I.; Al-Bat’hi, S.A.M. TiO2/solid state polymer junction for photovoltaic application. Adv. Mater. Res. 2012, 576, 623–625. [Google Scholar] [CrossRef]
- Mobarak, N.N.; Ramli, N.; Ahmad, A.; Abdullah, M.P. Development of carboxymethyl chitosan based green polymer electrolyte. J. Biobased Mater. Bioenergy 2013, 7, 267–268. [Google Scholar] [CrossRef]
- Mobarak, N.N.; Ahmad, A.; Abdullah, M.P.; Ramli, N.; Rahman, M.Y.A. Conductivity enhancement via chemical modification of chitosan based green polymer electrolyte. Electrochim. Acta 2013, 92, 161–167. [Google Scholar] [CrossRef]
- Sudhakar, Y.N.; Selvakumar, M.; Bhat, D.K. LiClO4-doped plasticized chitosan and poly(ethylene glycol) blend as biodegradable polymer electrolyte for supercapacitors. Ionics 2013, 19, 277–285. [Google Scholar] [CrossRef]
- Winie, T.; Hanif, N.S.M.; Rosli, N.H.A.; Subban, R.H.Y. Ac conductivity study of hexanoyl chitosan-LiCF3SO3-EC-Al2O3 nanocomposite polymer electrolytes. Adv. Mater. Res. 2013, 667, 93–98. [Google Scholar] [CrossRef]
- Shukur, M.F.; Azmi, M.S.; Zawawi, S.M.M.; Majid, N.A.; Illias, H.A.; Kadir, M.F.Z. Conductivity studies of biopolymer electrolytes based on chitosan incorporated with NH4Br. Phys. Scr. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Hassan, F.; Woo, H.J.; Aziz, N.A.; Kufian, M.Z.; Majid, S.R. Synthesis of Al2TiO5 and its effect on the properties of chitosan-NH4SCN polymer electrolytes. Ionics 2013, 19, 483–489. [Google Scholar] [CrossRef]
- Hamdan, K.Z.; Khiar, A.S.A. Conductivity and dielectric studies of methylcellulose/chitosan-NH4CF3SO3 polymer electrolyte. Key Eng. Mater. 2014, 594–595, 818–822. [Google Scholar]
- Mobarak, N.N.; Ramli, N.; Abdullah, M.P.; Ahmad, A. Spectroscopic studies of carboxymethyl chitosan-ammonium triflate (NH4CF3SO3) based solid polymer electrolytes. AIP Conf. Proc. 2014, 1571, 843–849. [Google Scholar]
- Leones, R.; Sentanin, F.; Esperança, J.M.S.S.; Pawlicka, A.; De Zea Bermudez, V.; Silva, M.M. Chitosan and ionic liquid based solid polymer electrolytes: The anion alkyl chain length effect. ECS Trans. 2014, 61, 51–59. [Google Scholar] [CrossRef]
- Rosli, N.H.A.; Muhammad, F.H.; Han, C.; Winie, T. Effect of filler type on the electrical properties of hexanoyl chitosan- based polymer electrolytes. Adv. Mater. Res. 2014, 832, 224–227. [Google Scholar] [CrossRef]
- Jaafar, N.K.; Lepit, A.; Aini, N.A.; Ali, A.M.M.; Saat, A.; Yahya, M.Z.A. Structural and electrical properties of plasticized radiation induced chitosan grafted poly(methylmethacrylate) polymer electrolytes. Int. J. Electrochem. Sci. 2014, 9, 821–829. [Google Scholar]
- Taib, N.U.; Idris, N.H. Plastic crystal-solid biopolymer electrolytes for rechargeable lithium batteries. J. Membr. Sci. 2014, 468, 149–154. [Google Scholar] [CrossRef]
- Hafiza, M.N.; Bashirah, A.N.A.; Bakar, N.Y.; Isa, M.I.N. Electrical properties of carboxyl methylcellulose/chitosan dual-blend green polymer doped with ammonium bromide. Int. J. Polym. Anal. Charact. 2014, 19, 151–158. [Google Scholar] [CrossRef]
- Winie, T.; Jamal, A.; Hanif, N.S.M.; Shahril, N.S.M. Hexanoyl chitosan-polystyrene blend based composite polymer electrolyte with surface treated TiO2 fillers. Key Eng. Mater. 2014, 594–595, 656–660. [Google Scholar]
- Winie, T.; Hanif, N.S.M.; Chan, C.H.; Arof, A.K. Effect of the surface treatment of the TiO2 fillers on the properties of hexanoyl chitosan/polystyrene blend-based composite polymer electrolytes. Ionics 2014, 20, 347–352. [Google Scholar] [CrossRef]
- Winie, T.; Mohd Shahril, N.S. Conductivity enhancement by controlled percolation of inorganic salt in multiphase hexanoyl chitosan/polystyrene polymer blends. Front. Mater. Sci. 2015, 9, 132–140. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Illias, H.A.; Kadir, M.F.Z. Incorporation of NH4Br in PVA-chitosan blend-based polymer electrolyte and its effect on the conductivity and other electrical properties. Ionics 2014, 20, 1235–1245. [Google Scholar] [CrossRef]
- Begum, S.N.S.; Pandian, R.; Aswal, V.K.; Ramasamy, R.P. Chitosan-gold-lithium nanocomposites as solid polymer electrolyte. J. Nanosci. Nanotechnol. 2014, 14, 5761–5773. [Google Scholar] [CrossRef]
- Fauzi, I.; Arcana, I.M. Solid polymer electrolyte from phosphorylated chitosan. AIP Conf. Proc. 2014, 1589, 154–158. [Google Scholar]
- Fadzallah, I.A.; Majid, S.R.; Careem, M.A.; Arof, A.K. Relaxation process in chitosan-oxalic acid solid polymer electrolytes. Ionics 2014, 20, 969–975. [Google Scholar] [CrossRef]
- Fadzallah, I.A.; Majid, S.R.; Careem, M.A.; Arof, A.K. A study on ionic interactions in chitosan-oxalic acid polymer electrolyte membranes. J. Memb. Sci. 2014, 463, 65–72. [Google Scholar] [CrossRef]
- Fauzi, I.; Arcana, I.M.; Wahyuningrum, D. Synthesis and characterization of solid polymer electrolyte from N-succinyl chitosan and lithium perchlorate. Adv. Mater. Res. 2014, 896, 58–61. [Google Scholar] [CrossRef]
- Fauzi, I.; Wahyuningrum, D.; Arcana, I.M. The influence of succinyl groups and lithium perchlorate on chitosan membranes as electrolyte polymers. Macromol. Symp. 2015, 353, 185–190. [Google Scholar] [CrossRef]
- Muhammad, F.H.; Fadzil, A.F.M.; Tan, W. FTIR and electrical studies of hexanoyl chitosan-based nanocomposite polymer electrolytes. Adv. Mater. Res. 2014, 1043, 36–39. [Google Scholar] [CrossRef]
- Muhammad, F.H.; Subban, R.H.Y.; Winie, T. Structural and electrical characterization of hexanoyl chitosan-LiClO4-TiO2-DMC polymer electrolytes. Key Eng. Mater. 2014, 594–595, 608–612. [Google Scholar]
- Khiar, A.S.A.; Radzi, S.M.; Razak, N.A. Effect of LiCF3SO3 on L-chitosan/PMMA blend polymer electrolytes. Mol. Cryst. Liq. Cryst. 2014, 603, 66–72. [Google Scholar] [CrossRef]
- Pandey, R.P.; Shahi, V.K. A N-o-sulphonic acid benzyl chitosan (NSBC) and N,N-dimethylene phosphonic acid propylsilane graphene oxide (NMPSGO) based multi-functional polymer electrolyte membrane with enhanced water retention and conductivity. RSC Adv. 2014, 4, 57200–57209. [Google Scholar] [CrossRef]
- Arifin, N.A.; Khiar, A.S.A. Effect of BMITFSI to the electrical properties of methycelloluse/chitosan/NH4TF-based polymer electrolyte. Proc. SPIE Int. Soc. Opt. Eng. 2015, 9668, 1–7. [Google Scholar]
- Shukur, M.F.; Kadir, M.F.Z. Hydrogen ion conducting starch-chitosan blend based electrolyte for application in electrochemical devices. Electrochim. Acta 2015, 158, 152–165. [Google Scholar] [CrossRef]
- Chupp, J.; Shellikeri, A.; Palui, G.; Chatterjee, J. Chitosan-based gel film electrolytes containing ionic liquid and lithium salt for energy storage applications. J. Appl. Polym. Sci. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Shamsudin, I.J.; Ahmad, A.; Hassan, N.H.; Kaddami, H. Bifunctional ionic liquid in conductive biopolymer based on chitosan for electrochemical devices application. Solid State Ion. 2015, 278, 11–19. [Google Scholar] [CrossRef]
- Muhammad, F.H.; Azmar, A.; Winie, T. Transport properties of hexanoyl chitosan-LiClO4-TiO2 composite polymer electrolyte. AIP Conf. Proc. 2015, 1674, 1–6. [Google Scholar]
- Datta, R.S.; Said, S.M.; Shahrir, S.R.; Abdullah, N.; Sabri, M.F.M.; Balamurugan, S.; Miyazaki, Y.; Hayashi, K.; Hashim, N.A.; Habiba, U.; et al. Ionic liquid entrapment by electrospun polymer nanofibre matrix as a high conductivity polymer electrolyte. RSC Adv. 2015, 5, 48217–48223. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abidin, Z.H.Z. Ion-transport study in nanocomposite solid polymer electrolytes based on chitosan: Electrical and dielectric analysis. J. Appl. Polym. Sci. 2015, 132, 1–10. [Google Scholar] [CrossRef]
- Abu Bakar, N.Y.; Muhamaruesa, N.H.M.; Aniskari, N.A.B.; Isa, M.I.N.M. Electrical studies of carboxy methycellulose-chitosan blend biopolymer doped dodecyltrimethyl ammonium bromide solid electrolytes. Am. J. Appl. Sci. 2015, 12, 40–46. [Google Scholar] [CrossRef]
- Buraidah, M.H.; Teo, L.P.; Au Yong, C.M.; Shah, S.; Arof, A.K. Performance of polymer electrolyte based on chitosan blended with poly(ethylene oxide) for plasmonic dye-sensitized solar cell. Opt. Mater. 2016, 57, 202–211. [Google Scholar] [CrossRef]
- Sudaryanto; Yulianti, E.; Patimatuzzohrah. Structure and properties of solid polymer electrolyte based on chitosan and ZrO2 nanoparticle for lithium ion battery. AIP Conf. Proc. 2016, 1710, 1–10. [Google Scholar]
- Kalaiselvimary, J.; Pradeepa, P.; Sowmya, G.; Edwinraj, S.; Ramesh Prabhu, M. Electrical characterization of proton conducting polymer electrolyte based on bio polymer with acid dopant. AIP Conf. Proc. 2016, 1728, 1–4. [Google Scholar]
- Yusuf, S.N.F.; Azzahari, A.D.; Yahya, R.; Majid, S.R.; Careem, M.A.; Arof, A.K. From crab shell to solar cell: A gel polymer electrolyte based on N-phthaloylchitosan and its application in dye-sensitized solar cells. RSC Adv. 2016, 6, 27714–27724. [Google Scholar] [CrossRef]
- Fadzallah, I.A.; Noor, I.M.; Careem, M.A.; Arof, A.K. Investigation of transport properties of chitosan-based electrolytes utilizing impedance spectroscopy. Ionics 2016, 22, 1635–1645. [Google Scholar] [CrossRef]
- Shirdast, A.; Sharif, A.; Abdollahi, M. Effect of the incorporation of sulfonated chitosan/sulfonated graphene oxide on the proton conductivity of chitosan membranes. J. Power Sources 2016, 306, 541–551. [Google Scholar] [CrossRef]
- Aziz, S. Role of dielectric constant on ion transport: Reformulated Arrhenius equation. Adv. Mater. Sci. Eng. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Alves, R.; Sentanin, F.; Sabadini, R.C.; Pawlicka, A.; Silva, M.M. Influence of cerium triflate and glycerol on electrochemical performance of chitosan electrolytes for electrochromic devices. Electrochim. Acta 2016, 217, 108–116. [Google Scholar] [CrossRef]
- Alves, R.; Donoso, J.P.; Magon, C.J.; Silva, I.D.A.; Pawlicka, A.; Silva, M.M. Solid polymer electrolytes based on chitosan and containing europium triflate. J. Rare Earths 2016, 432, 307–312. [Google Scholar]
- Aziz, S.; Abdullah, O.G.; Rasheed, M.A.; Ahmed, H.M. Effect of high salt concentration (HSC) on structural, morphological, electrical characteristics of chitosan based solid polymer electrolytes. Polymers 2017, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Pasini Cabello, S.D.; Ochoa, N.A.; Takara, E.A.; Mollá, S.; Compañ, V. Influence of pectin as a green polymer electrolyte on the transport properties of chitosan-pectin membranes. Carbohydr. Polym. 2017, 157, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, S.; Gao, S.; Ravi, M.; Liu, R.; Ma, Q. Mg-ion conducting gel polymer electrolyte membranes containing biodegradable chitosan: Preparation, structural, electrical and electrochemical properties. Polym. Test. 2017, 62, 278–286. [Google Scholar] [CrossRef]
- Muhammad, F.H.; Jamal, A.; Winie, T. Study on factors governing the conductivity performance of acylated chitosan-NaI electrolyte system. Ionics 2017, 23, 3045–3056. [Google Scholar] [CrossRef]
- Aziz, S.; Rasheed, M.A.; Abidin, Z.H.Z. Optical and electrical characteristics of silver ion conducting nanocomposite solid polymer electrolytes based on chitosan. J. Electron. Mater. 2017, 46, 6119–6130. [Google Scholar] [CrossRef]
- Alves, R.; Sentanin, F.; Sabadini, R.C.; Pawlicka, A.; Silva, M.M. Innovative electrolytes based on chitosan and thulium for solid state applications: Synthesis, structural, thermal characterization. J. Electroanal. Chem. 2017, 788, 156–164. [Google Scholar] [CrossRef]
- Leones, R.; Reis, P.M.; Sabadini, R.C.; Ravaro, L.P.; Silva, I.D.A.; Camargo, A.S.S.D.; Donoso, J.P.; Magon, C.J.; Esperanca, J.M.S.S.; Pawlicka, A.; et al. A luminescent europium ionic liquid to improve the performance of chitosan polymer electrolytes. Electrochim. Acta 2017, 240, 474–485. [Google Scholar] [CrossRef]
- Leones, R.; Sabadini, R.C.; Esperança, J.M.S.S.; Pawlicka, A.; Silva, M.M. Effect of storage time on the ionic conductivity of chitosan-solid polymer electrolytes incorporating cyano-based ionic liquids. Electrochim. Acta 2017, 232, 22–29. [Google Scholar] [CrossRef]
- Leones, R.; Sabadini, R.C.; Esperança, J.M.S.S.; Pawlicka, A.; Silva, M.M. Playing with ionic liquids to uncover novel polymer electrolytes. Solid State Ion. 2017, 300, 46–52. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, H.; Zhou, C.H.; Xu, S.; Sebo, B.; Zhao, X.Z. Novel agarose polymer electrolyte for quasi-solid state dye-sensitized solar cell. J. Power Sources 2011, 196, 2410–2415. [Google Scholar] [CrossRef]
- Wang, W.; Guo, X.; Yang, Y. Lithium iodide effect on the electrochemical behavior of agarose based polymer electrolyte for dye-sensitized solar cell. Electrochim. Acta 2011, 56, 7347–7351. [Google Scholar] [CrossRef]
- Lima, E.; Raphael, E.; Sentanin, F.; Rodrigues, L.C.; Ferreira, R.A.S.; Carlos, L.D.; Silva, M.M.; Pawlicka, A. Photoluminescent polymer electrolyte based on agar and containing europium picrate for electrochemical devices. Mater. Sci. Eng. B 2012, 177, 488–493. [Google Scholar] [CrossRef]
- Leones, R.; Sentanin, F.; Rodrigues, L.C.; Marrucho, I.M.; Esperanca, J.M.S.S.; Pawlicka, A.; Silva, M.M. Investigation of polymer electrolytes based on agar and ionic liquids. Express Polym. Lett. 2012, 6, 1007–1016. [Google Scholar] [CrossRef]
- Yang, Y.; Yi, P.; Zhou, C.; Cui, J.; Zheng, X.; Xiao, S.; Guo, X.; Wang, W. Magnetic field processed solid-state dye-sensitized solar cells with nickel oxide modified agarose electrolyte. J. Power Sources 2013, 243, 919–924. [Google Scholar] [CrossRef]
- Guo, X.Y.; Yi, P.F.; Wang, W.J.; Xiao, S.; Yang, Y. Effects of polyethylene glycol on agarose-based magnetic polymer electrolyte for dye-sensitized solar cell. Adv. Mater. Res. 2013, 652–654, 860–864. [Google Scholar] [CrossRef]
- Guo, X.; Yi, P.; Yang, Y.; Cui, J.; Xiao, S.; Wang, W. Effects of surfactants on agarose-based magnetic polymer electrolyte for dye-sensitized solar cells. Electrochim. Acta 2013, 90, 524–529. [Google Scholar] [CrossRef]
- Koh, J.C.H.; Ahmad, Z.A.; Mohamad, A.A. Bacto agar-based gel polymer electrolyte. Ionics 2012, 18, 359–364. [Google Scholar] [CrossRef]
- Alves, R.D.; Rodrigues, L.C.; Andrade, J.R.; Pawlicka, A.; Pereira, L.; Martins, R.; Fortunato, E.; Silva, M.M. Study and characterization of a novel polymer electrolyte based on agar doped with magnesium triflate. Mol. Cryst. Liq. Cryst. 2013, 570, 1–11. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, J.; Yi, P.; Zheng, X.; Guo, X.; Wang, W. Effects of nanoparticle additives on the properties of agarose polymer electrolytes. J. Power Sources 2014, 248, 988–993. [Google Scholar] [CrossRef]
- Boopathi, G.; Pugalendhi, S.; Selvasekarapandian, S.; Premalatha, M.; Monisha, S.; Aristatil, G. Development of proton conducting biopolymer membrane based on agar-agar for fuel cell. Ionics 2016, 23, 1–10. [Google Scholar] [CrossRef]
- Raphael, E.; Jara, D.H.; Schiavon, M.A. Optimizing photovoltaic performance in CuInS2 and CdS quantum dot-sensitized solar cells by using an agar-based gel polymer electrolyte. RSC Adv. 2017, 7, 6492–6500. [Google Scholar] [CrossRef]
- Selvalakshmi, S.; Vijaya, N.; Selvasekarapandian, S.; Premalatha, M. Biopolymer agar-agar doped with NH4SCN as solid polymer electrolyte for electrochemical cell application. J. Appl. Polym. Sci. 2017, 134, 1–10. [Google Scholar] [CrossRef]
- Selvalakshmi, S.; Mathavan, T.; Selvasekarapandian, S.; Premalatha, M. Study on NH4I composition effect in agar-agar-based biopolymer electrolyte. Ionics 2017, 23, 1–7. [Google Scholar] [CrossRef]
- Nadia, S.R.; Khanmirzaei, M.H.; Ramesh, S.; Ramesh, K. Quasi-solid-state agar-based polymer electrolytes for dye-sensitized solar cell applications using imidazolium-based ionic liquid. Ionics 2017, 23, 1585–1590. [Google Scholar] [CrossRef]
- Shamsudin, I.J.; Ahmad, A.; Hassan, N.H.; Kaddami, H. Solid biopolymer electrolyte based on κ-Carrageenan for electrochemical devices application. Asian J. Chem. 2014, 26, 77–80. [Google Scholar] [CrossRef]
- Jumaah, F.N.; Mobarak, N.N.; Ahmad, A.; Ghani, M.A.; Rahman, M.Y.A. Derivative of iota-carrageenan as solid polymer electrolyte. Ionics 2015, 21, 1311–1320. [Google Scholar] [CrossRef]
- Rudhziah, S.; Ahmad, A.; Ahmad, I.; Mohamed, N.S. Biopolymer electrolytes based on blend of kappa-carrageenan and cellulose derivatives for potential application in dye sensitized solar cell. Electrochim. Acta 2015, 175, 1–7. [Google Scholar] [CrossRef]
- Chan, S.; Bantang, J.P.; Camacho, D. Influence of nanomaterial fillers in biopolymer electrolyte system for squaraine-based dye-sensitized solar cells. Int. J. Electrochem. Sci. 2015, 10, 7696–7706. [Google Scholar]
- Rudhziah, S.; Ahmad, A.; Mohamed, N.S. The effect of lithium iodide to the properties of carboxymethyl κ-carrageenan/carboxymethyl cellulose polymer electrolyte and dye-sensitized solar cell performance. Polymers 2016, 8, 1–10. [Google Scholar]
- Karthikeyan, S.; Selvasekarapandian, S.; Premalatha, M.; Monisha, S.; Boopathi, G.; Aristatil, G.; Arun, A.; Madeswaran, S. Proton-conducting I-Carrageenan-based biopolymer electrolyte for fuel cell application. Ionics 2016, 23, 1–6. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Estrany, F.; Armelin, E.; Díaz, D.D.; Alemán, C. Towards sustainable solid-state supercapacitors: Electroactive conducting polymers combined with biohydrogels. J. Mater. Chem. A 2016, 4, 1792–1805. [Google Scholar] [CrossRef]
- Moniha, V.; Alagar, M.; Selvasekarapandian, S.; Sundaresan, B.; Boopathi, G. Conductive bio-polymer electrolyte iota-carrageenan with ammonium nitrate for application in electrochemical devices. J. Non-Cryst. Solids 2018, 481, 424–434. [Google Scholar] [CrossRef]
- Mishra, R.K.; Datt, M.; Banthia, A.K.; Majeed, A.B.A. Development of novel pectin based membranes as proton conducting material. Int. J. Plast. Technol. 2012, 16, 80–88. [Google Scholar] [CrossRef]
- Leones, R.; Botelho, M.B.S.; Sentanin, F.; Cesarino, I.; Pawlicka, A.; Camargo, A.S.S.; Silva, M.M. Pectin-based polymer electrolytes with Ir(III) complexes. Mol. Cryst. Liq. Cryst. 2014, 604, 117–125. [Google Scholar] [CrossRef]
- Vijaya, N.; Selvasekarapandian, S.; Sornalatha, M.; Sujithra, K.S.; Monisha, S. Proton-conducting biopolymer electrolytes based on pectin doped with NH4X (X = Cl, Br). Ionics 2016, 23, 1–10. [Google Scholar] [CrossRef]
- Mendes, J.P.; Esperança, J.M.S.S.; Medeiros, M.J.; Pawlicka, A.; Silva, M.M. Structural, morphological, ionic conductivity, thermal properties of pectin-based polymer electrolytes. Mol. Cryst. Liq. Cryst. 2017, 643, 266–273. [Google Scholar] [CrossRef]
- Khalid, M.; Hartono, A.M.B. Ionically conducting and environmentally safe gum Arabic as a high-performance gel-like electrolyte for solid-state supercapacitors. J. Solid State Electrochem. 2017, 21, 2443–2447. [Google Scholar] [CrossRef]
- Mattos, R.I.; Pawlicka, A.; Tambelli, C.E.; Donoso, J.P. NMR and conductivity study of gelatin-based polymer electrolytes. Mol. Cryst. Liq. Cryst. 2008, 483, 120–129. [Google Scholar] [CrossRef]
- Silva, M.M.; Barbosa, P.C.; Rodrigues, L.C. New developments in conducting polymers based on commercial gelatin. ECS Trans. 2009, 16, 413–419. [Google Scholar]
- Silva, M.M.; Barbosa, P.C.; Rodrigues, L.C.; Goncalves, A.; Costa, C.; Fortunato, E. Gelatin in electrochromic devices. Opt. Mater. 2010, 32, 719–722. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Tang, Z.; Xiao, Y.; Huang, M.; Lin, J. Application of poly(acrylic acid-g-gelatin)/polypyrrole gel electrolyte in flexible quasi-solid-state dye-sensitized solar cell. Electrochim. Acta 2010, 55, 2777–2781. [Google Scholar] [CrossRef]
- Mattos, R.I.; Pawlicka, A.; Lima, J.F.; Tambelli, C.E.; Magon, C.J.; Donoso, J.P. Magnetic resonance and conductivity study of gelatin-based proton conductor polymer electrolytes. Electrochim. Acta 2010, 55, 1396–1400. [Google Scholar] [CrossRef]
- Vieira, D.F.; Pawlicka, A. Optimization of performances of gelatin/LiBF4-based polymer electrolytes by plasticizing effects. Electrochim. Acta 2010, 55, 1489–1494. [Google Scholar] [CrossRef]
- Lima, E.; Mattos, R.; Sentanin, F.; Rodrigues, L.C.; Silva, M.M.; Ferreira, R.A.S.; Carlos, L.D.; Pawlicka, A. Functional novel polymer electrolytes containing europium picrate. Mater. Res. Innov. 2011, 15, 1–6. [Google Scholar] [CrossRef]
- Pawlicka, A.; Mattos, R.I.; Lima, J.F.; Tambelli, C.E.; Magon, C.J.; Donoso, J.P. Magnetic resonance and conductivity study of a gelatin-based polymer gel electrolyte. Electrochim. Acta 2011, 57, 187–191. [Google Scholar] [CrossRef]
- Leones, R.; Sentanin, F.; Rodrigues, L.C.; Ferreira, R.A.S.; Marrucho, I.M.; Esperanca, J.M.S.S.; Pawlicka, A.; Carlos, L.D.; Silva, M.M. Novel polymer electrolytes based on gelatin and ionic liquids. Opt. Mater. 2012, 35, 187–195. [Google Scholar] [CrossRef]
- Ponez, L.; Sentanin, F.C.; Majid, S.R.; Arof, A.K.; Pawlicka, A. Ion-conducting membranes based on gelatin and containing LiI/I2 for electrochromic devices. Mol. Cryst. Liq. Cryst. 2012, 554, 239–251. [Google Scholar] [CrossRef]
- Basu, T.; Goswami, M.M.; Middya, T.R.; Tarafdar, S. Morphology and ion-conductivity of gelatin-LiClO4 films: Fractional diffusion analysis. J. Phys. Chem. B 2012, 116, 11362–11369. [Google Scholar] [CrossRef]
- Basu, T.; Giri, A.; Tarafdar, S.; Das, S. Electrical impedance response of gamma irradiated gelatin based solid polymer electrolytes analyzed using a generalized calculus formalism. J. Electroanal. Chem. 2015, 755, 52–62. [Google Scholar] [CrossRef]
- Basu, T.; Middya, T.R.; Tarafdar, S. Ion-conductivity study and anomalous diffusion analysis of plasticized gelatin films. J. Appl. Polym. Sci. 2013, 130, 3018–3024. [Google Scholar] [CrossRef]
- Alves, R.D.; Rodrigues, L.C.; Andrade, J.R.; Fernandes, M.; Pinto, J.V.; Pereira, L.; Pawlicka, A.; Martins, R.; Fortunato, E.; Bermudez, V.D.Z.; et al. GelatinnZn(CF3SO3)2 polymer electrolytes for electrochromic devices. Electroanalysis 2013, 25, 1483–1490. [Google Scholar] [CrossRef]
- Ramadan, R.; Kamal, H.; Hashem, H.M.; Abdel-Hady, K. Gelatin-based solid electrolyte releasing Li+ for smart window applications. Sol. Energy Mater. Sol. Cells 2014, 127, 147–156. [Google Scholar] [CrossRef]
- Sharma, S.; Khannam, M.; Boruah, M.; Nath, B.C.; Dolui, S.K. Development of dye-sensitized solar cells based on gold/gelatin gel electrolyte: Effect of different aspect ratio of gold nanocrystals. IEEE J. Photovolt. 2015, 5, 1665–1673. [Google Scholar] [CrossRef]
- Leones, R.; Sabadini, R.C.; Sentanin, F.C.; Esperança, J.M.S.S.; Pawlicka, A.; Silva, M.M. Polymer electrolytes for electrochromic devices through solvent casting and sol-gel routes. Sol. Energy Mater. Sol. Cells 2017, 169, 98–106. [Google Scholar] [CrossRef]
- Idris, R.; Glasse, M.D.; Latham, R.J.; Linford, R.G.; Schlindwein, W.S. Polymer electrolytes based on modified natural rubber for used in rechargeable lithium batteries. J. Power Sources 2001, 94, 206–211. [Google Scholar] [CrossRef]
- Glasse, M.D.; Idris, R.; Latham, R.J.; Linford, R.G.; Schlindwein, W.S. Polymer electrolytes based on modified natural rubber. Solid State Ion. 2002, 147, 289–294. [Google Scholar] [CrossRef]
- Kumutha, K.; Alias, Y.; Said, R. FTIR and thermal studies of modified natural rubber based polymer electrolytes. Ionics 2005, 11, 472–476. [Google Scholar] [CrossRef]
- Aziz, M.; Latif, F.; Chew, C.L.; Katun, N. The impedance spectroscopy studies of PVC/ENR 50/LiCF3SO3. Solid State Phenom. 2006, 111, 67–70. [Google Scholar] [CrossRef]
- Ali, A.M.M.; Yahya, M.Z.A.; Bahron, H.; Subban, R.H.Y. Electrochemical studies on polymer electrolytes based on poly(methyl methacrylate)-grafted natural rubber for lithium polymer battery. Ionics 2006, 12, 303–307. [Google Scholar] [CrossRef]
- Latif, F.; Aziz, M.; Ali, A.M.M.; Yahya, M.Z.A. The coagulation impact of 50% epoxidised natural rubber chain in ethylene carbonate-plasticized solid electrolytes. Macromol. Symp. 2009, 277, 62–68. [Google Scholar] [CrossRef]
- Kamisan, A.S.; Kudin, T.I.T.; Ali, A.M.M.; Yahya, M.Z.A. Gel polymer electrolyte based on methyl-grafted natural rubber for proton batteries. Mater. Res. Innov. 2009, 13, 263–265. [Google Scholar] [CrossRef]
- Su’ait, M.S.; Ahmad, A.; Hamzah, H.; Rahman, M.Y.A. Preparation and characterization of PMMA-MG49-LiClO4 solid polymeric electrolyte. J. Phys. D. Appl. Phys. 2009, 42, 1–5. [Google Scholar]
- Low, S.P.; Ahmad, A.; Rahman, M.Y.A. Effect of ethylene carbonate plasticizer and TiO2 nanoparticles on 49% poly(methyl methacrylate) grafted natural rubber-based polymer electrolyte. Ionics 2010, 16, 821–826. [Google Scholar] [CrossRef]
- Kamisan, A.S.; Kudin, T.I.T.; Ali, A.M.M.; Yahya, M.Z.A. Electrical and physical studies on 49% methyl-grafted natural rubber-based composite polymer gel electrolytes. Electrochim. Acta 2011, 57, 207–211. [Google Scholar] [CrossRef]
- Ahmad, A.; Rahman, M.Y.A.; Su’ait, M.S.; Hamzah, H. Study of MG49-PMMA based solid polymer electrolyte. Open Mater. Sci. J. 2011, 5, 170–177. [Google Scholar] [CrossRef]
- Kamisan, A.S.; Kudin, T.I.T.; Ali, A.M.M.; Yahya, M.Z.A. Polymer gel electrolytes based on 49% methyl-grafted natural rubber. Sains Malays. 2011, 40, 49–54. [Google Scholar]
- Zaki, N.H.M.; Mahmud, Z.S.; Adam, N.I.; Ahmad, A.H.; Ali, A.M.M.; Yahya, M.Z.A. Characterization of plasticized grafted natural rubber-30% poly(methyl methacrylate) (MG30) based polymer electrolytes. In Proceedings of the 2012 IEEE Symposium on Business, Engineering and Industrial Applications, Bandung, Indonesia, 23–26 September 2012; pp. 705–708. [Google Scholar]
- Rahman, M.Y.A.; Ahmad, A.; Lee, T.K.; Farina, Y.; Dahlan, H.M. LiClO4 salt concentration effect on the properties of PVC-modified low molecular weight LENR50-based solid polymer electrolyte. Polym. Polym. Compos. 2012, 124, 2227–2233. [Google Scholar] [CrossRef]
- Zainal, N.; Mohamed, N.S.; Idris, R. Properties of ENR-50 based electrolyte system. Sains Malays. 2013, 42, 481–485. [Google Scholar]
- Jafirin, S.; Ahmad, I.; Ahmad, A. Potential use of cellulose from kenaf in polymer electrolytes based on MG49 rubber composites. BioResources 2013, 8, 5947–5964. [Google Scholar] [CrossRef]
- Ali, A.M.M.; Subban, R.H.Y.; Bahron, H.; Yahya, M.Z.A.; Kamisan, A.S. Investigation on modified natural rubber gel polymer electrolytes for lithium polymer battery. J. Power Sources 2013, 244, 636–640. [Google Scholar] [CrossRef]
- Ataollahi, N.; Ahmad, A.; Hamzah, H.; Rahman, M.Y.; Mohamed, N.S. Effects of LiCF3SO3 on PVDF-HFP/MG49 (70/30) based blend polymer electrolyte. Appl. Mech. Mater. 2013, 313–314, 117–120. [Google Scholar] [CrossRef]
- Ataollahi, N.; Ahmad, A.; Hamzah, H.; Rahman, M.Y.A.; Mohamed, N.S. Ionic conduction of blend poly(vinylidene fluoride-hexafluoro propylene) and poly(methyl methacrylate)-grafted natural rubber based solid polymer electrolyte. Int. J. Electrochem. Sci. 2013, 8, 7875–7884. [Google Scholar]
- TianKhoon, L.; Ataollahi, N.; Hassan, N.H.; Ahmad, A. Studies of porous solid polymeric electrolytes based on poly(vinylidene fluoride) and poly(methyl methacrylate) grafted natural rubber for applications in electrochemical devices. J. Solid State Electrochem. 2016, 20, 203–213. [Google Scholar] [CrossRef]
- Idris, R.; Bujang, N.H. Epoxidised natural rubber based polymer electrolyte systems for electrochemical device applications. Adv. Mater. Res. 2014, 896, 62–65. [Google Scholar] [CrossRef]
- Ataollahi, N.; Ahmad, A.; Lee, T.K.; Abdullah, A.R.; Rahman, M.Y.A. Preparation and characterization of PVDF-MG49-NH4CF3SO3 based solid polymer electrolyte. e-Polymers 2014, 14, 115–120. [Google Scholar] [CrossRef]
- Jafirin, S.; Ahmad, I.; Ahmad, A. Carboxymethyl cellulose from kenaf reinforced composite polymer electrolytes based 49% poly(methyl methacrylate) -grafted natural rubber. Malays. J. Anal. Sci. 2014, 18, 376–384. [Google Scholar]
- Tiankhoon, L.; Hassan, N.H.; Rahman, M.Y.A.; Vedarajan, R.; Matsumi, N.; Ahmad, A. One-pot synthesis nano-hybrid ZrO2-TiO2 fillers in 49% poly(methyl methacrylate) grafted natural rubber (MG49) based nano-composite polymer electrolyte for lithium ion battery application. Solid State Ion. 2015, 276, 72–79. [Google Scholar] [CrossRef]
- Nazir, K.; Aziz, A.F.; Adam, N.I.; Yahya, M.Z.A.; Ali, A.M.M. Effect of epoxidation on 30% poly(methyl methacrylate)-grafted natural rubber polymer electrolytes. AIP Conf. Proc. 2015, 1674, 1–6. [Google Scholar]
- Chan, C.H.; Kammer, H.W. Impedance spectroscopy of polymer electrolytes based on epoxidized natural rubber with 50 mol% epoxide content. Polym. Eng. Sci. 2015, 55, 2250–2255. [Google Scholar] [CrossRef]
- Jamal, A.; Muhammad, F.H.; Ali, A.M.M.; Winie, T. Blends of hexanoyl chitosan/epoxidized natural rubber doped with EMImTFSI. Ionics 2017, 23, 357–366. [Google Scholar] [CrossRef]
- Chan, C.H.; Kammer, H.-W. Impedance spectra of polymer electrolytes. Ionics 2017, 23, 2327–2337. [Google Scholar] [CrossRef]
- Zimmermann, J.; Jürgensen, N.; Morfa, A.J.; Wang, B.; Tekoglu, S.; Hernandez-Sosa, G. Poly(lactic-co-glycolic acid) (PLGA) as ion-conducting polymer for biodegradable light-emitting electrochemical cells. ACS Sustain. Chem. Eng. 2016, 4, 7050–7055. [Google Scholar] [CrossRef]
- Neto, M.J.; Sentanin, F.; Esperanca, J.M.S.S.; Medeiros, M.J.; Pawlicka, A.; Bermudez, V.D.Z.; Silva, M.M. Gellan gum—Ionic liquid membranes for electrochromic device application. Solid State Ion. 2015, 274, 64–70. [Google Scholar] [CrossRef]
- Singh, R.; Bhattacharya, B.; Rhee, H.W.; Singh, P.K. Solid gellan gum polymer electrolyte for energy application. Int. J. Hydrogen Energy 2015, 40, 9365–9372. [Google Scholar] [CrossRef]
- Sudhakar, Y.N.; Selvakumar, M.; Krishna Bhat, D. Effect of acid dopants in biodegradable gel polymer electrolyte and the performance in an electrochemical double layer capacitor. Phys. Scr. 2015, 90, 1–12. [Google Scholar] [CrossRef]
- Singh, R.; Polu, A.R.; Bhattacharya, B.; Rhee, H.W.; Varlikli, C.; Singh, P.K. Perspectives for solid biopolymer electrolytes in dye sensitized solar cell and battery application. Renew. Sustain. Energy Rev. 2016, 65, 1098–1117. [Google Scholar] [CrossRef]
- O’Regan, B.; Gratzel, M. A low-cost, high-efficiency solar cell based on dye sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Wu, J.; Lan, Z.; Hao, S.; Li, P.; Lin, J.; Huang, M.; Fang, L.; Huang, Y. Progress on the electrolytes for dye-sensitized solar cells. Pure Appl. Chem 2008, 80, 2241–2258. [Google Scholar] [CrossRef]
- Adedokun, O.; Titilope, K.; Awodugba, A.O. Review on natural dye-sensitized solar cells (DSSCs). Int. J. Eng. Technol. 2016, 2, 34–41. [Google Scholar] [CrossRef]
- Alias, S.S.; Mohamad, A.A. Effect of NH4I and I2 concentration on agar gel polymer electrolyte properties for a dye-sensitized solar cell. Ionics 2013, 19, 1185–1194. [Google Scholar] [CrossRef]
- Hsu, H.L.; Tien, C.F.; Yang, Y.T.; Leu, J. Dye-sensitized solar cells based on agarose gel electrolytes using allylimidazolium iodides and environmentally benign solvents. Electrochim. Acta 2013, 91, 208–213. [Google Scholar] [CrossRef]
- Singh, R.; Jadhav, N.A.; Majumder, S.; Bhattacharya, B.; Singh, P.K. Novel biopolymer gel electrolyte for dye-sensitized solar cell application. Carbohydr. Polym. 2013, 91, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Ahmad, A.; Mohamed, N.S. Comprehensive studies on polymer electrolyte and dye-sensitized solar cell developed using castor oil-based polyurethane. J. Solid State Electrochem. 2018, 22, 461–470. [Google Scholar] [CrossRef]
- Su’ait, M.S.; Jumaah, F.N.; Faizzi, H.M.; Mamat, S.; Ludin, N.A.; Farhan, W.A.; Haron, A.; Atifah, N.; Latif, M.N.; Badri, K.H.; et al. Palm-based polyurethane-ionic liquid gel polymer electrolyte for quasi-solid state dye sensitized solar cell. Ind. Crop. Prod. 2018, 113, 406–413. [Google Scholar] [CrossRef]

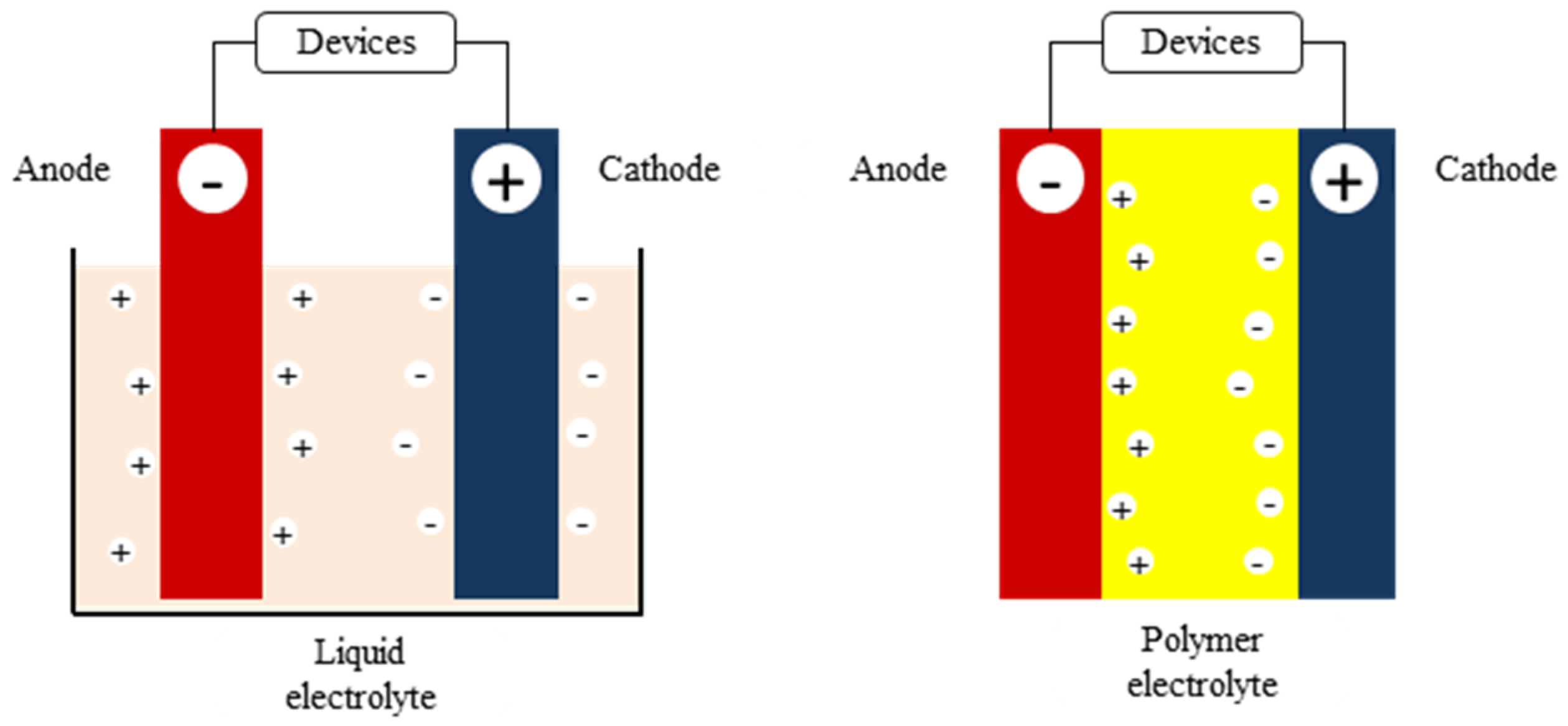

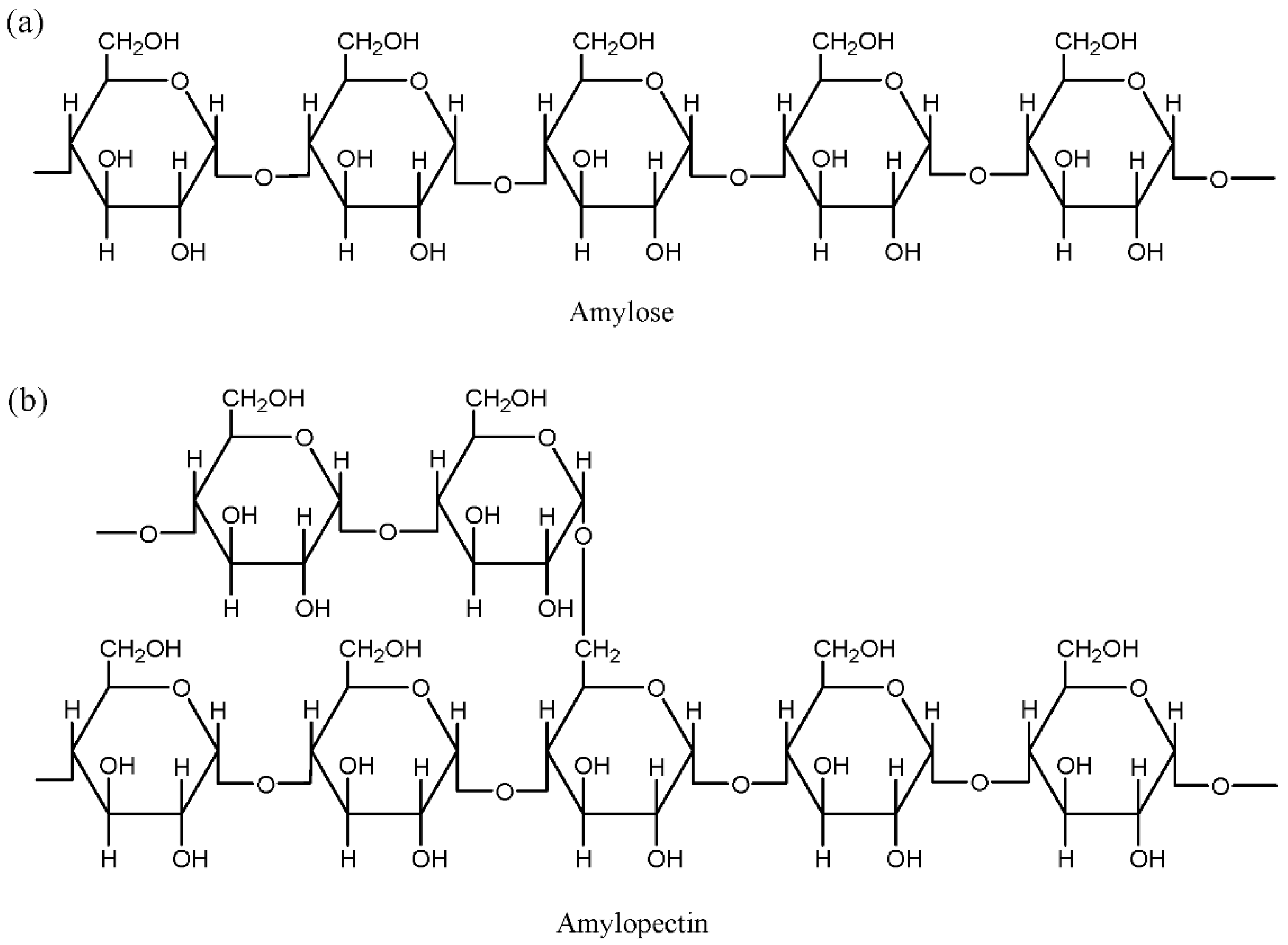
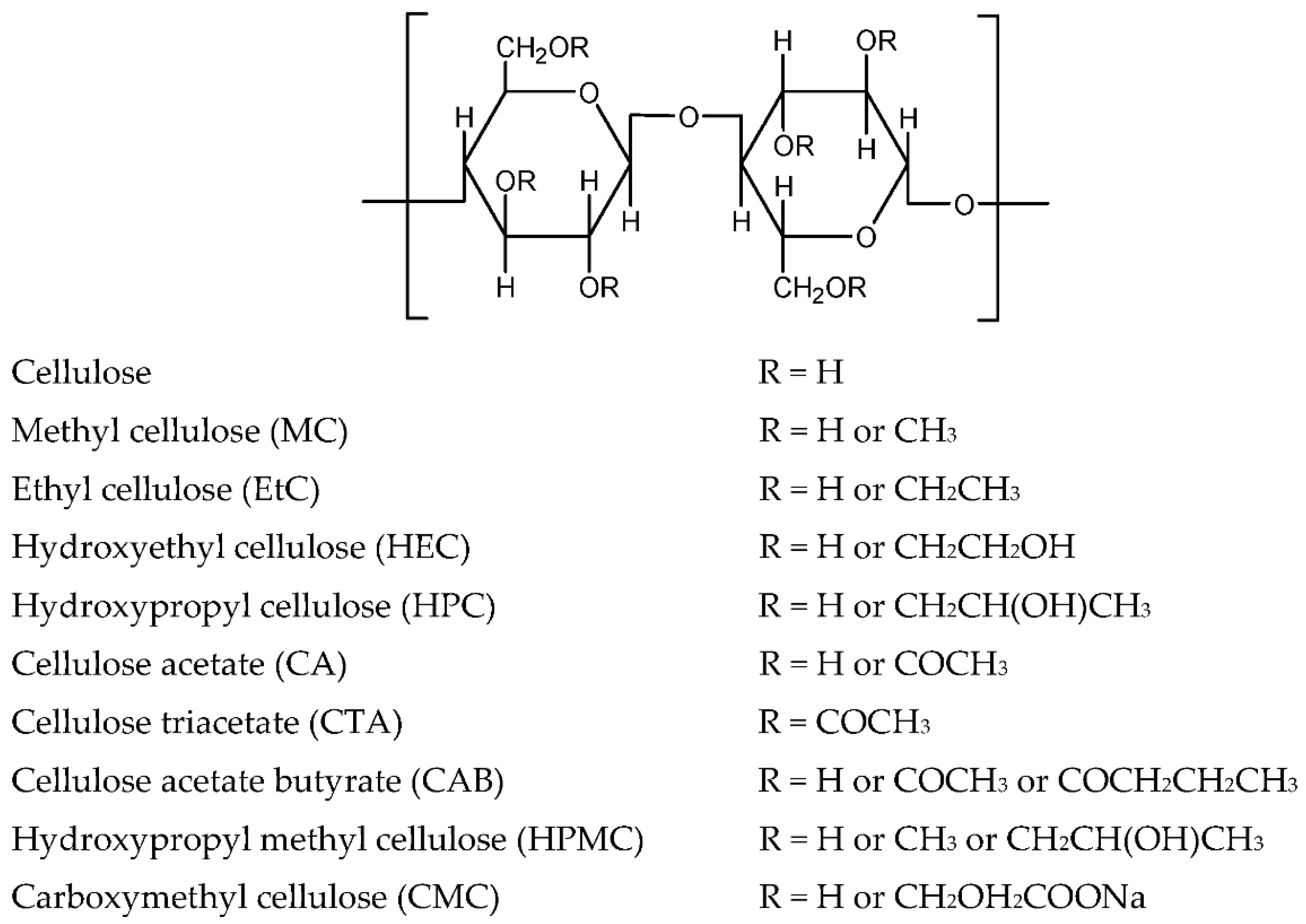
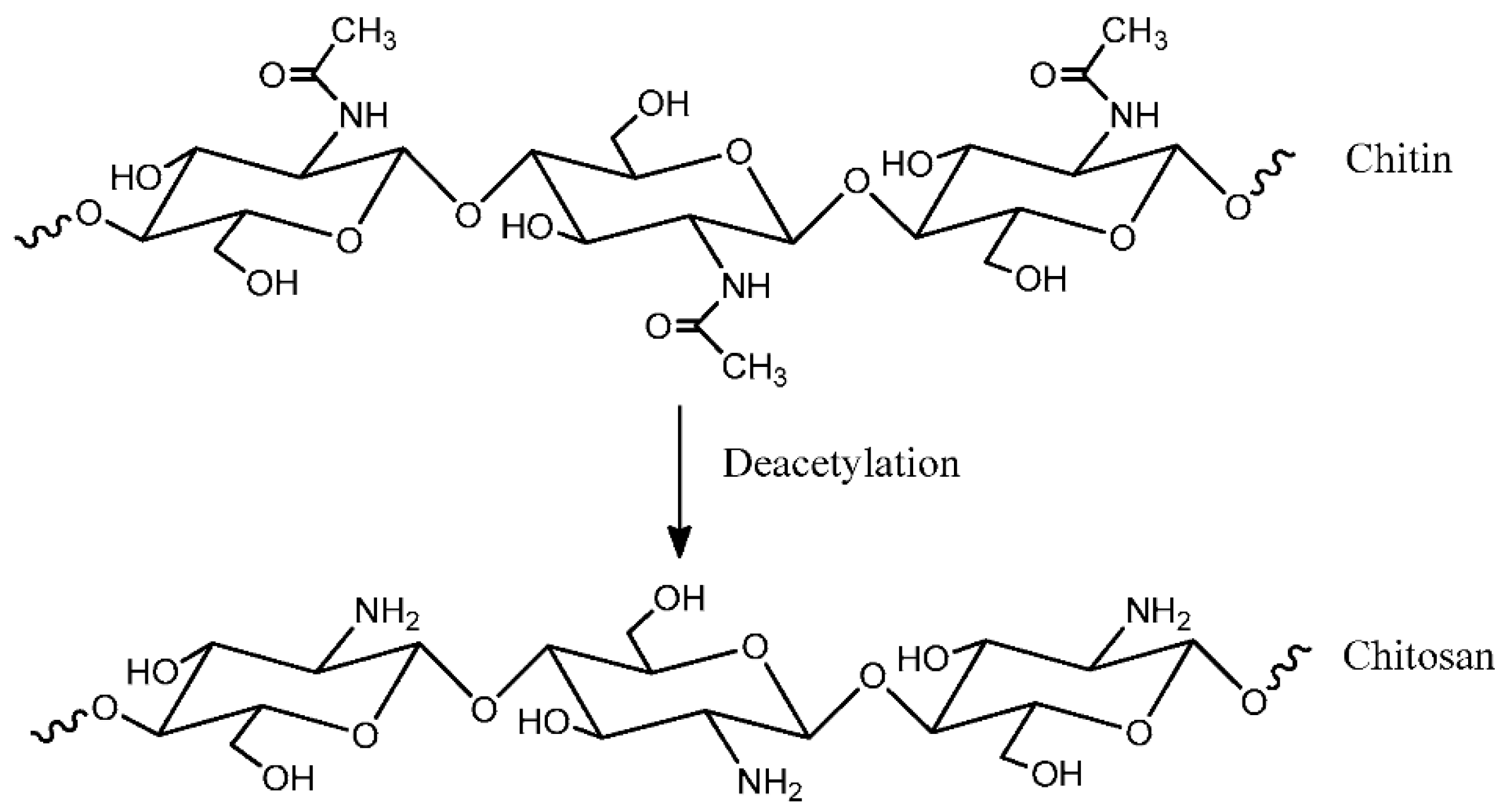
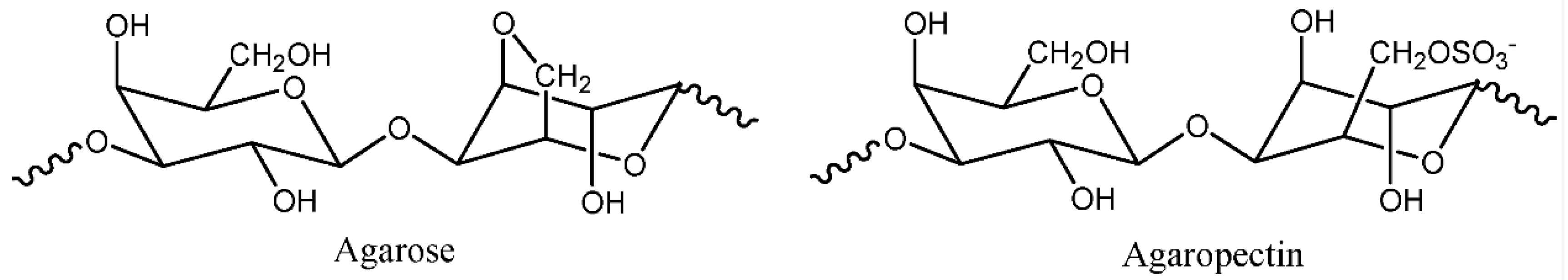
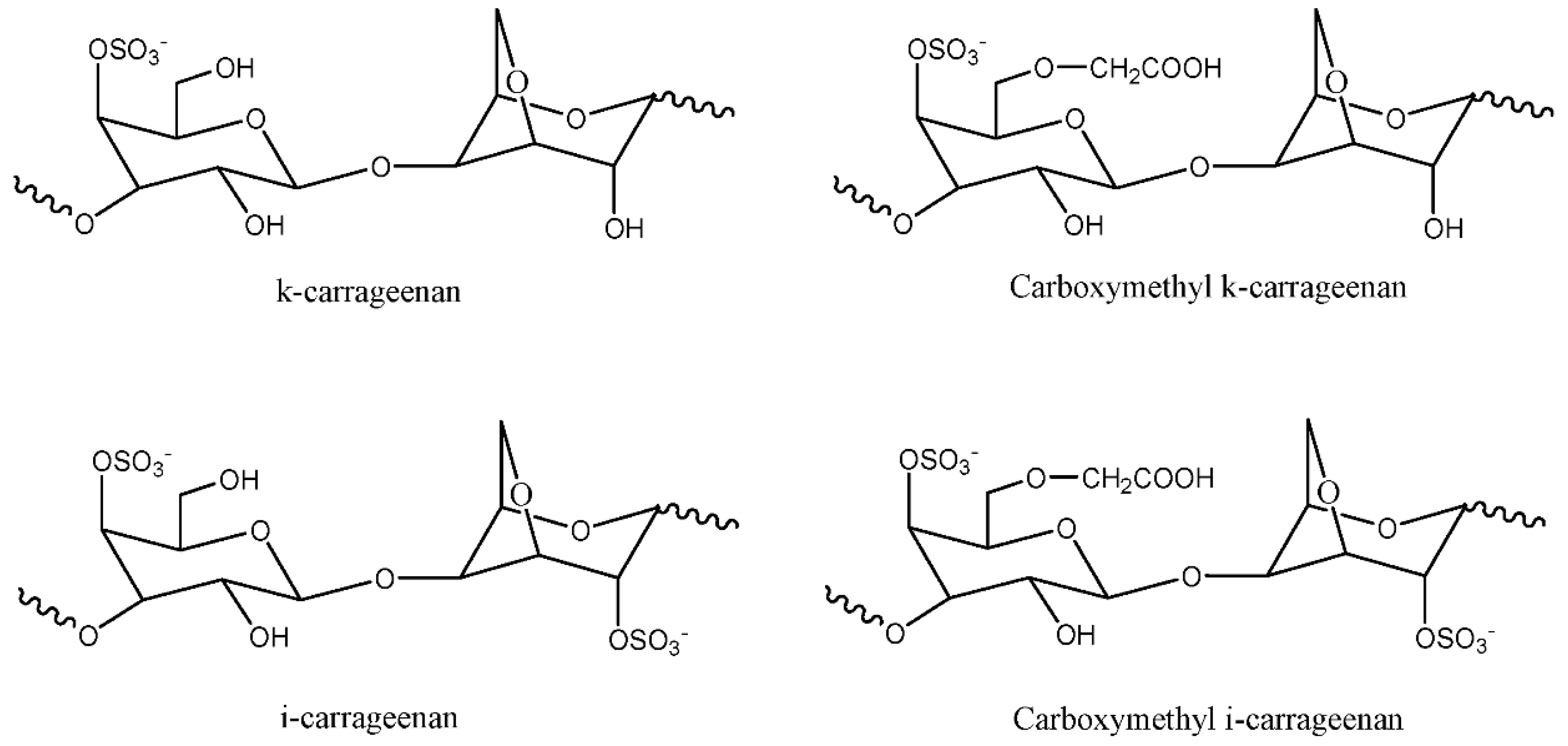
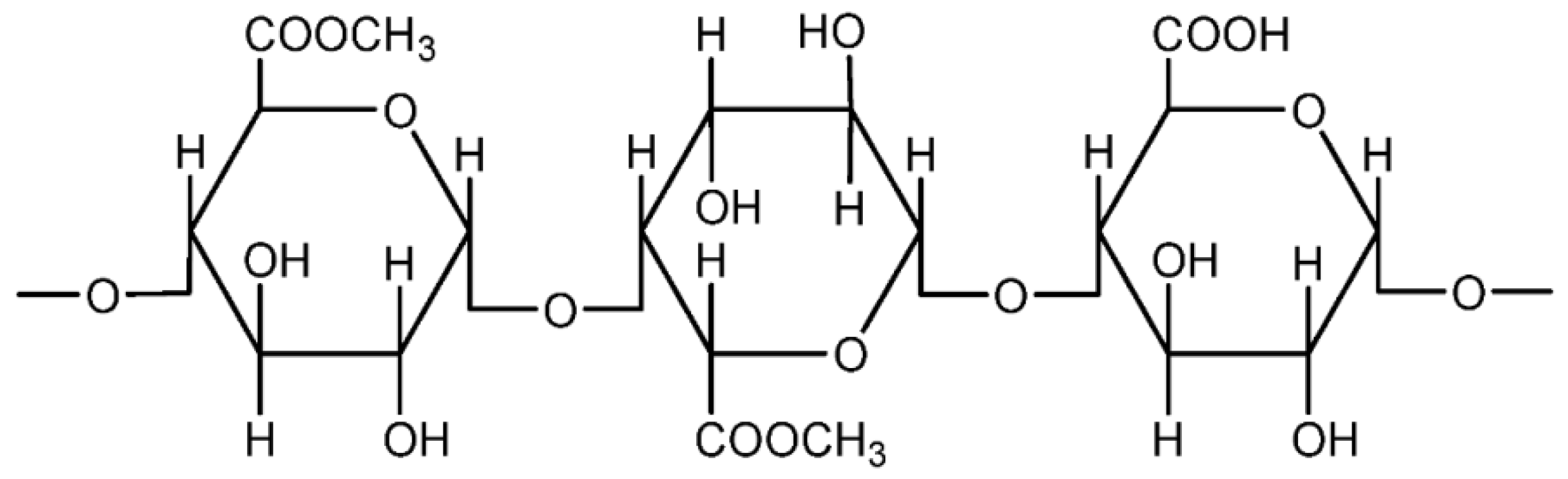

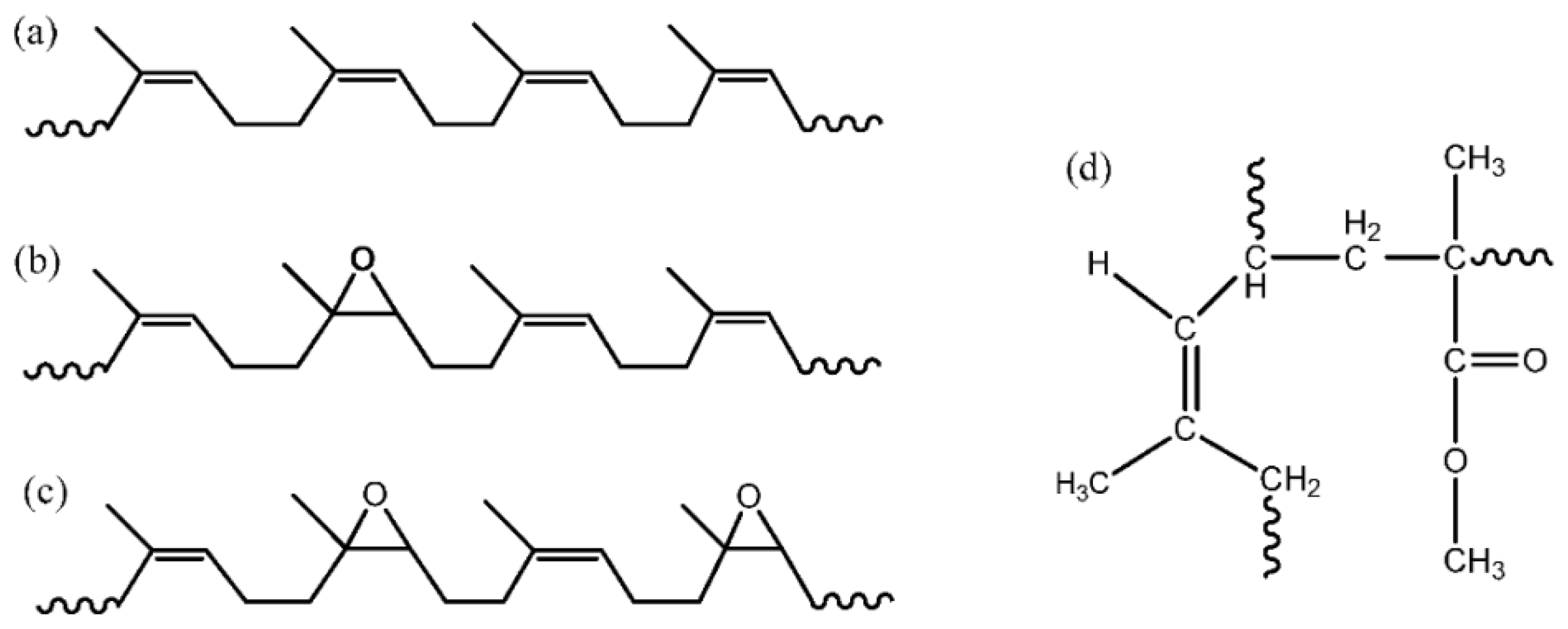
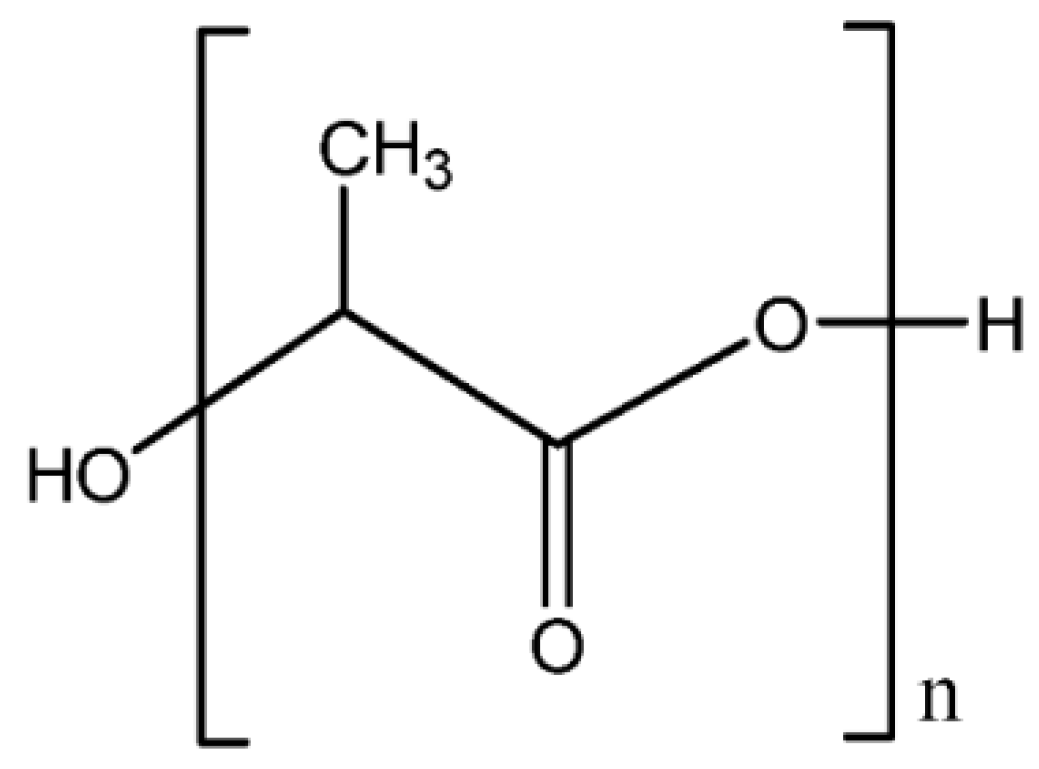
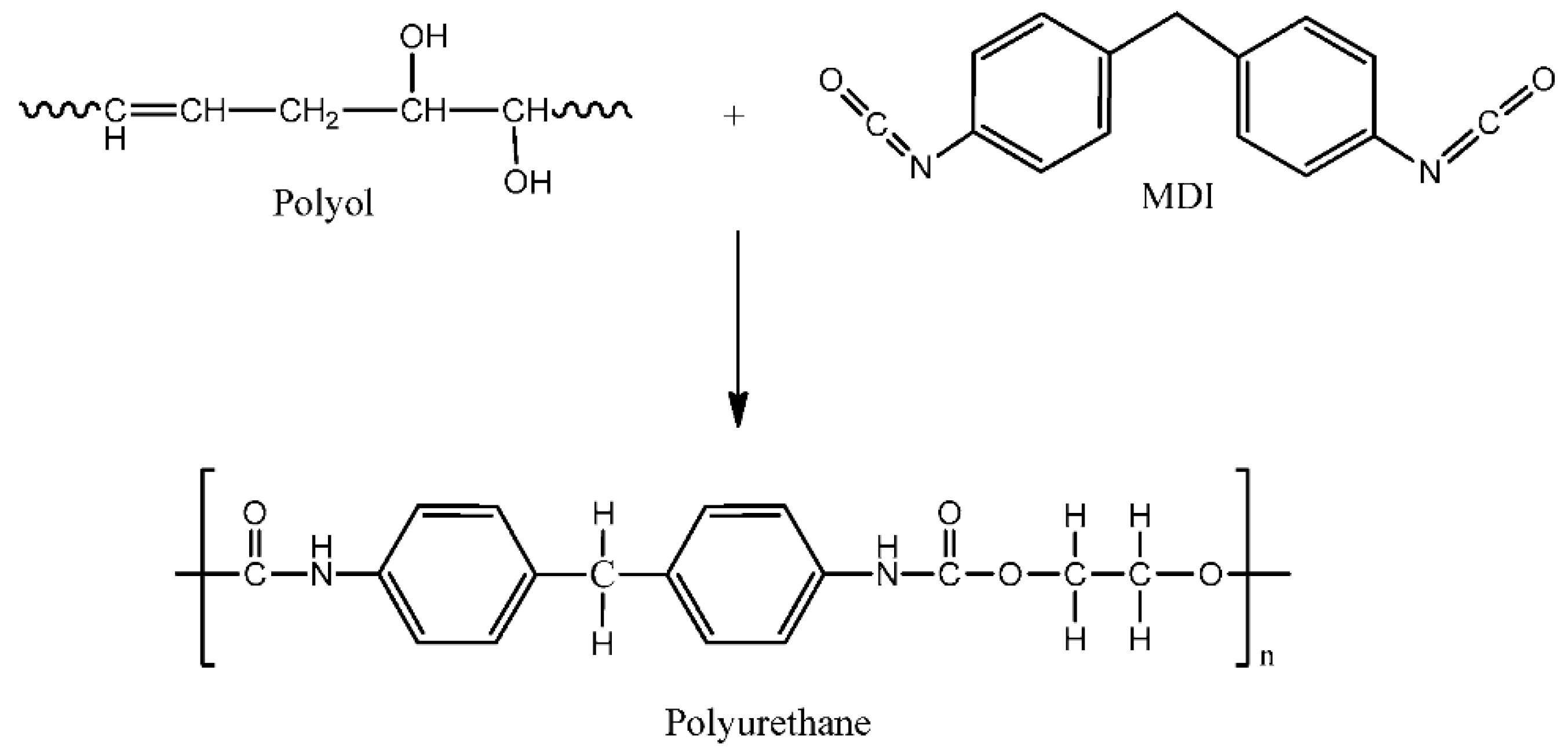
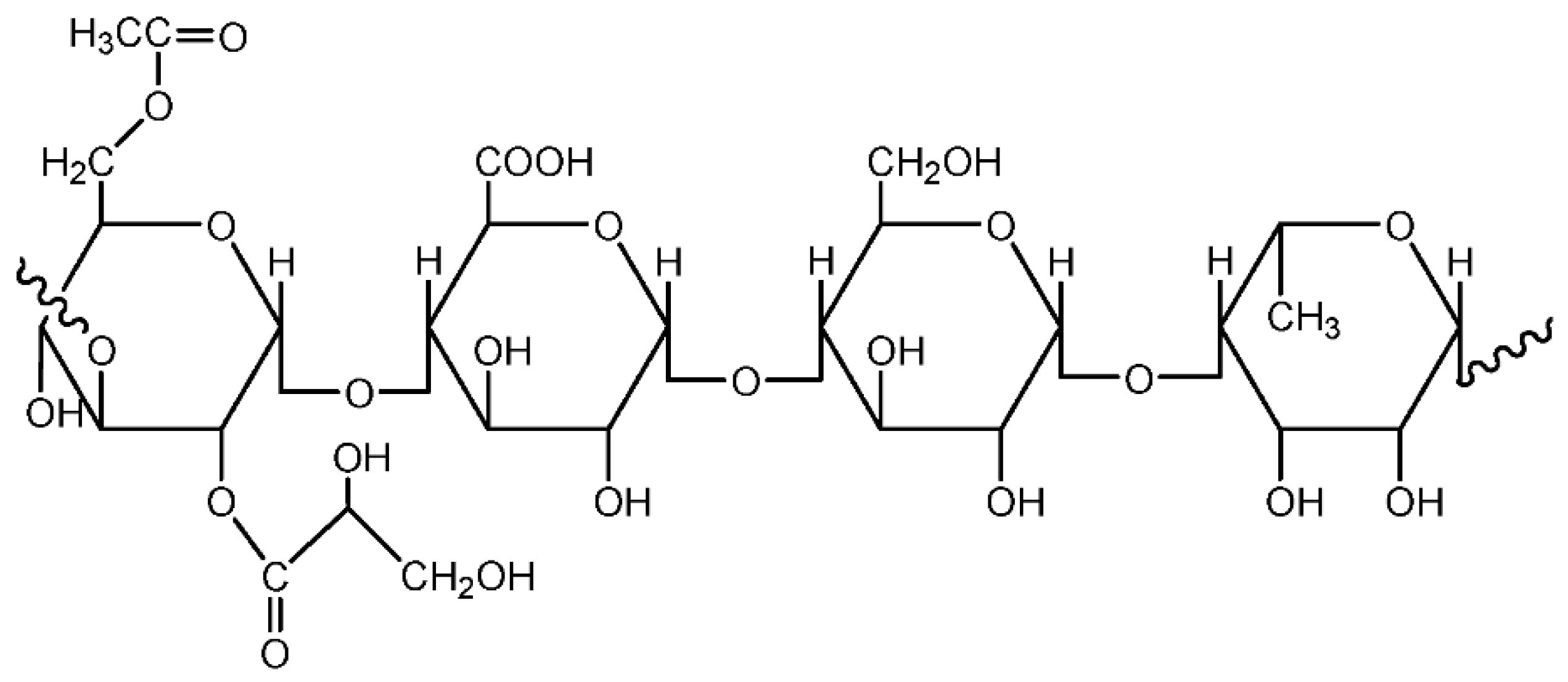
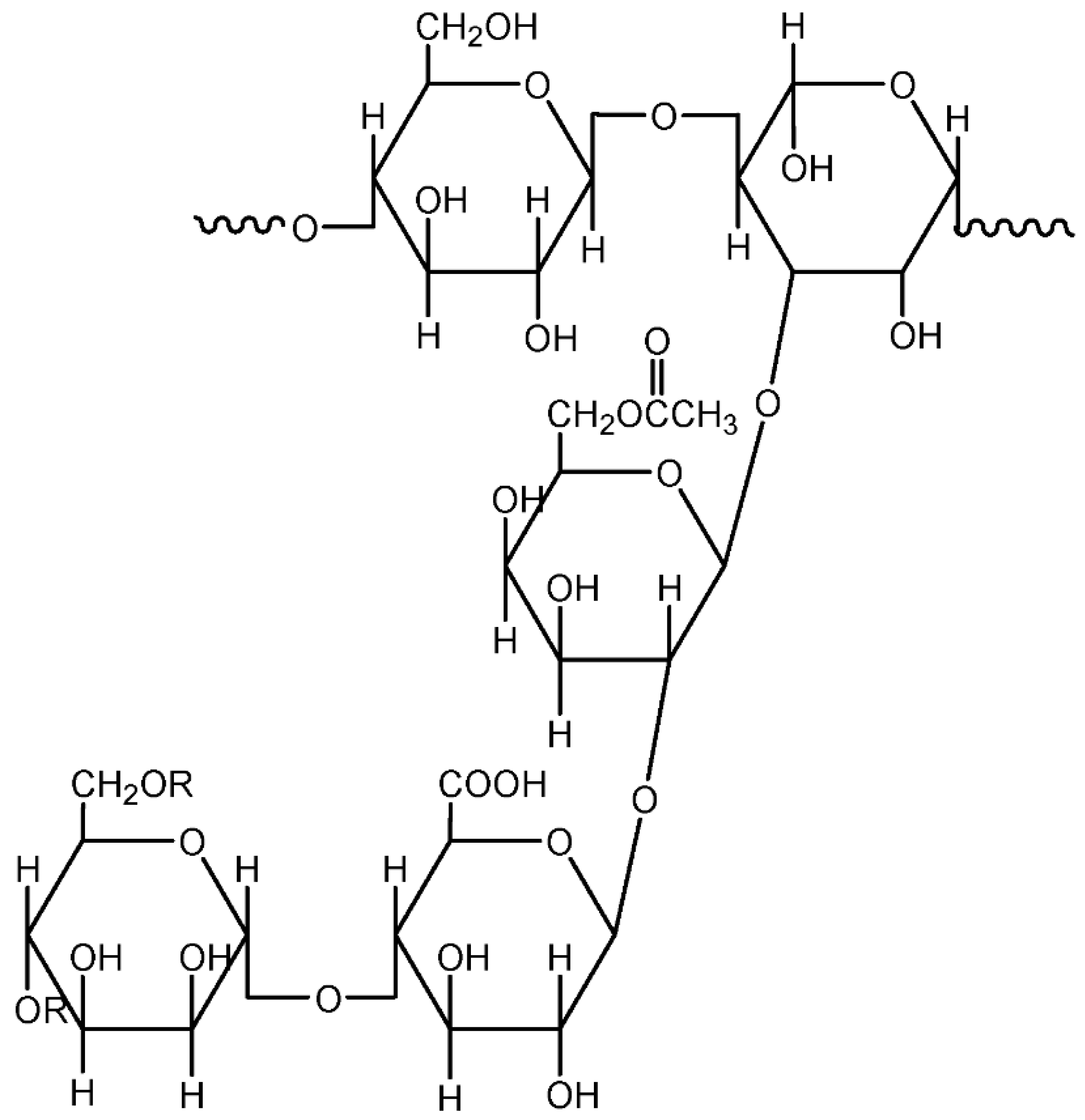
| Bio-Based Polymer | Source | Uses |
|---|---|---|
| Starch | Sago, corn, tapioca, potato, rice | Adhesives, thickener and stabilizer in foods, and bio-plastics |
| Cellulose | Plants, bacterial | Paper, textile, and wood manufacturing |
| Chitin/chitosan | Shrimp, crab, lobster, shell fish | Cosmetics, foods, pharmaceutics |
| Oils | Palm oil, castor oil, soybean oil, canola oil | Resins, coatings, and adhesives |
| Pectin | Citrus fruits | Additives in food industry, pharmaceutics |
| Latex | Rubber tree, guayule shrubs | Medical, adhesives |
| Source | Gum | Structure | |
|---|---|---|---|
| Marine algae | Agar, alginates, carrageenan | Linear, un-branched molecules | |
| Higher plants | Extracts | Pectin | Linear, un-branched molecules |
| Seeds | Guar gum | Linear molecules with short branches | |
| Exudates | Gum Arabic | Branch-on-branch molecules | |
| Microorganism | Gellan, Xanthan | Linear molecules with short branches | |
| Polymer | Electrolyte System | State | Electrochemical Properties | Physical Properties | Device | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| σ (s/cm) | I-TN | Stability (V) | Ea | Tg (°C) | Structural | |||||
| Starch | Corn starch–LiClO4–glycerol | Solid | 7.9 × 10−5 | – | – | – | −58 | Amorph | – | [14] |
| Corn starch–LiClO4–glycerol | Solid | 5.0 × 10−5 | – | – | – | – | – | – | [66] | |
| Corn starch–LiClO4–glycerol | Gel | 10−4 | – | – | 0.35 eV | – | – | – | [67] | |
| Corn starch–NaCl–glucose | Solid | - | – | – | – | – | – | – | [68] | |
| Corn starch–NaCl–glycerol | - | – | – | – | – | – | – | |||
| Corn starch–NaCl–sorbitol | - | – | – | – | – | – | – | |||
| Corn starch–NaCl–urea | - | – | – | – | – | – | – | |||
| Corn starch–NaCl–formamide | 10−3 | – | – | – | – | – | – | |||
| Corn starch–LiClO4–glycerol | Solid | 6.1 × 10−5 | – | – | – | – | – | – | [69] | |
| Cassava starch–LiClO4–glycerol | 8.4 × 10−5 | – | – | – | – | – | – | |||
| Starch–NH4NO3 | Solid | 2.8 × 10−5 | – | – | 0.41 eV | – | – | – | [12] | |
| Corn starch–LiClO4–glycerol | Solid | 1.1 × 10−4 | – | – | – | −75 | – | – | [70] | |
| Corn starch–LiTFSI–AmIm][Cl] | Solid | 4.2 × 10−4 | – | – | – | – | – | – | [71] | |
| Corn starch–LiPF6–[BmIm][PF6] | Solid | 1.5 × 10−4 | – | – | – | – | – | – | [72] | |
| Arrowroot starch–NaI–glutaraldehyde | Solid | 6.7 × 10−4 | 0.95 | – | – | – | – | – | [73] | |
| Tapioca starch/PEO–NH4NO3 | Solid | 2.8 × 10−7 | – | – | – | – | Semi-cr | – | [63] | |
| Corn starch–LiPF6–[BmIm][Tf] | Solid | 6.0 × 10−4 | – | – | 0.01 eV | −29 | Amorph | – | [74] | |
| Sago starch–NH4Br | Solid | 6.9 × 10−9 | – | – | 0.07 eV | - | – | – | [75] | |
| Corn starch–LiClO4–SiO2 | Solid | 1.2 × 10−4 | – | – | – | 87.1 | – | – | [76] | |
| Corn starch–LiTFSI–[AmIm][Cl] | Gel | 5.7 × 10−2 | – | – | 4.8 kJ/mol | – | – | – | [77] | |
| Corn starch–LiTFSI–DES | Solid | 1.0 × 10−3 | – | – | – | – | – | – | [78] | |
| Potato starch–NH4I | Solid | 2.4 × 10−4 | 0.95 | – | – | – | – | – | [13] | |
| Starch/chitosan–LiClO4–glycerol | Solid | 3.7 × 10−4 | – | – | 0.52 eV | – | – | – | [79] | |
| Corn starch–AgNO3 | Solid | - | – | – | 0.71 eV | – | Amorph | – | [80] | |
| Corn starch–LiI–glycerol | Solid | 9.6 × 10−4 | – | – | 0.16 eV | – | Amorph | – | [81] | |
| Corn starch–AgNO3 | Solid | 1.0 × 10−9 | – | – | – | – | – | – | [82] | |
| Rice starch–LiI | Solid | 4.7 × 10−5 | – | – | 0.41 eV | – | – | – | [83] | |
| Poly(styrene sulphonic acid)/starch–LiClO4–glycerol | Solid | 5.7 × 10−3 | – | – | – | – | – | – | [84] | |
| Potato starch–NaI–glutaraldehyde–PEG | Solid | 1.8 × 10−4 | 0.99 | – | – | 75 | – | – | [85] | |
| Corn starch–LiOAc–glycerol | Solid | 1.0 × 10−3 | – | 2.1 | 0.14 eV | – | Amorph | – | [86] | |
| Sago starch–KI–I2 | Solid | 3.4 × 10−4 | – | – | – | – | – | – | [87] | |
| Rice starch–LiI–MPII–TiO2 | Solid | 3.6 × 10−4 | – | – | 0.22 eV | – | Amorph | DSSC | [88] | |
| Corn starch–LiPF6–[BmIm][PF6] | Solid | 1.5 × 10−4 | – | 2.9 | – | – | – | Super-capacitor | [89] | |
| Corn starch–LiPF6–[BmIm][Tf] | 3.2 × 10−4 | – | 3.1 | – | – | – | ||||
| Corn starch–LiClO4 | Solid | 1.6 × 10−6 | – | – | 0.64 eV | 64 | – | – | [90] | |
| Corn starch/chitosan–NH4I–glycerol | Solid | 1.3 × 10−3 | 0.99 | 1.9 | 0.18 eV | – | – | – | [91] | |
| Starch/chitosan–NH4I | Solid | 3.0 × 10−4 | – | – | 0.20 eV | – | Amorph | – | [92] | |
| Starch/chitosan–NH4Cl–glycerol | Solid | 5.1 × 10−4 | – | – | 0.19 eV | −0.37 | – | – | [93] | |
| Starch/chitosan–NH4Br–EC | Solid | 1.4 × 10−3 | 0.92 | 1.8 | 0.17 eV | – | Amorph | EDLC | [94] | |
| Corn starch–LiClO4–SiO2 | Solid | 1.2 × 10−4 | – | 3.0 | 0.25 eV | – | – | EDLC | [95] | |
| Rice starch–LiI | Solid | 4.7 × 10−5 | – | – | – | −12 | Amorph | DSSC | [96] | |
| Rice starch–NH4I | 1.4 × 10−4 | – | – | – | −38 | Amorph | ||||
| Rice starch–NaI | 4.8 × 10−4 | – | – | – | −42 | Amorph | ||||
| Rice starch–NaI–MPII | Solid | 1.2 × 10−3 | – | – | – | −58 | Amorph | DSSC | [97] | |
| Corn starch–NH4Br–glycerol | Solid | 1.8 × 10−3 | 0.98 | 1.6 | 0.11 eV | – | Amorph | – | [98] | |
| Corn starch–LiPF6–[BmIm][PF6] | Solid | 2.0 × 10−4 | – | 2.9 | – | – | Amorph | – | [99] | |
| Potato starch/PVA–KCl–glycerol | Solid | 5.4 × 10−5 | 0.97 | – | 0.12 eV | – | Amorph | – | [100] | |
| Potato starch/chitosan–LiCF3SO3 | Solid | 7.1 × 10−7 | – | – | – | – | – | – | [101] | |
| Potato starch/chitosan–LiCF3SO3–glycerol | Solid | 1.3 × 10−3 | – | – | 0.11 eV | – | Amorph | – | [102] | |
| Corn starch–LiClO4–BaTiO3 | Solid | 1.8 × 10−4 | – | 3.1 | – | 17.2 | – | EDLC | [103] | |
| Corn starch–LiTFSI | Solid | 3.4 × 10−4 | – | – | – | – | – | Battery | [104] | |
| Potato starch–Mg(C2H3O2)2–[BmIm][Cl]–glycerol | Solid | 1.1 × 10−5 | 0.92 | – | – | – | – | – | [105] | |
| Potato starch/Poly(vinyl alcohol)–LiBr–glycerol | Solid | 10−3 | – | – | – | – | – | – | [106] | |
| Potato starch/methylcellulose–LiClO4–glycerol | Solid | 4.3 × 10−4 | – | – | – | – | Amorph | – | [107] | |
| Starch/PVA-NH4SCN | Solid | 1.3 × 10−4 | – | – | – | – | Amorph | – | [108] | |
| Tapioca starch/chitosan–NH4NO3–[EmIm][NO3] | Solid | 7.4 × 10−5 | – | – | – | – | – | – | [109] | |
| Starch–NaCl | Gel | 6.2 × 10−2 | – | – | – | – | – | – | [110] | |
| Corn starch–LiClO4–glycerol | Solid | 9.0 × 10−3 | – | – | – | – | Amorph | – | [111] | |
| Potato starch–LiCF3SO3–[BmIm][Cl]–GO | Solid | 4.8 × 10−4 | – | – | – | – | Amorph | – | [112] | |
| Corn starch–NaClO4–glutaraldehyde | Solid | 10−2 | – | 2.4 | – | – | - | Super-capacitor | [113] | |
| Potato starch–NADES | Solid | 2.9 × 10−3 | – | – | – | – | Amorph | – | [114] | |
| Potato starch/methyl cellulose–NH4NO3–glycerol | Solid | 1.3 × 10−3 | 0.98 | 1.8 | – | −27.5 | Amorph | EDLC | [115,116] | |
| Cellulose | HEC/DPEO–LiClO4 | Solid | 2.1 × 10−5 | – | – | 0.17 eV | – | – | – | [117,118] |
| HEC–LiClO4–glycerol | Solid | 9.5 × 10−5 | – | – | – | −60 | Amorph | – | [14] | |
| Cellulose/PEO–LiCF3SO3 | Solid | 10−7 | – | – | 53 kJ/mol | – | – | – | [119] | |
| HPC/PEO–LiCF3SO3–PC | Gel | 10−3 | – | – | 16 kJ/mol | – | – | – | [120] | |
| EO-EPI/nano-cellulose–LiClO4 | Solid | 1.6 × 10−4 | – | – | – | – | – | – | [121] | |
| POE/nano-cellulose–LiTFSI–TEGDME | Solid | 10−6 | – | – | – | – | – | – | [122] | |
| HPC/Jeffamine–LiClO4 | Solid | 1.3 × 10−5 | – | – | – | – | – | – | [123] | |
| POE/nano-cellulose–LiTFSI | Solid | 10−7 | – | – | – | – | – | – | [124,125] | |
| Cellulose acetate–LiClO4 | Solid | 4.9 × 10−3 | – | – | – | – | – | Super-capacitor | [126] | |
| Ethyl cellulose–LiClO4-PC | Gel | 6.5 × 10−3 | – | – | 0.18 eV | – | – | – | [127] | |
| PVDF–HPF/cellulose–LiPF6–EC/DMC | Solid | 4.4 × 10−3 | – | 4.8 | – | – | Semi-cr | – | [128] | |
| Cellulose acetate–NH4BF4–SiO2 | Gel | 7.9 × 10−3 | – | – | – | – | – | Battery | [129] | |
| Cellulose triacetate–LiTFSI–Pyr1,3TFSI | Gel | 10−4 | – | – | – | – | – | – | [130] | |
| Cellulose–acrylic acid–[BmIm][I] | Gel | 7.3 × 10−3 | – | – | – | – | – | DSSC | [131] | |
| Cellulose acetate–NH4I–PC | Solid | 1.2 × 10−4 | – | – | – | – | – | – | [132] | |
| Cellulose acetate–NH4BF4–TiO2 | Gel | 1.4 × 10−2 | – | – | – | – | – | Battery | [133] | |
| Cellulose acetate–NH4BF4–PEG | Solid | 1.4 × 10−5 | – | – | – | – | – | – | [134,135,136] | |
| CMC–DTAB | Solid | 7.7 × 10−4 | 0.92 | – | 0.09 eV | – | Amorph | – | [137] | |
| PEG/network cellulose–LiClO4 | Gel | 10−4 | – | 4.7 | – | – | Amorph | – | [138] | |
| CN-HPC–LiI–I2–MHII | Gel | 2.5 × 10−3 | – | – | – | – | Amorph | DSSC | [139] | |
| Cellulose acetate–LiTFSI–DES | Gel | 2.6 × 10−3 | – | – | 4.23 kJ/mol | – | Amorph | – | [140,141] | |
| Cellulose acetate–LiTFSI–[AmIm][Cl] | Solid | 1.8 × 10−3 | – | – | – | – | Amorph | – | [142] | |
| Methyl cellulose–LiCF3SO3 | Solid | 2.1 × 10−5 | – | – | – | – | Amorph | – | [143] | |
| Methyl cellulose–PEG–NH4NO3 | Solid | 10−6 | – | 2.4 | – | – | Amorph | EDLC | [144] | |
| Methyl cellulose–KOH–DMC | Solid | 10−5 | – | – | – | – | – | – | [145] | |
| PE/PVDF/Cellulose acetate butyrate–LiPF6–EC/EMC | Gel | 2.5 × 10−3 | – | – | – | – | – | Battery | [146] | |
| PEO/CMC–NaI–I2–MPII | Gel | – | – | – | – | – | – | DSSC | [147] | |
| Cellulose acetate–LiBOB–GBL | Gel | 5.4 × 10−3 | – | 4.7 | – | – | – | – | [148] | |
| PEO/Network cellulose–LiClO4 | Solid | 8.0 × 10−7 | – | 5.0 | – | – | Semi-cr | – | [149] | |
| PVDF–HFP/HPMC–LiPF6 | Gel | 3.8 × 10−4 | – | 5.0 | – | – | Amorph | – | [150] | |
| Cellulose acetate–LiTFSI–[Amim][Cl] | Solid | 4.7 × 10−2 | – | – | 1.25 kJ/mol | – | – | - | [151] | |
| CMC–LiClO4–PC | Gel | – | – | – | – | – | – | ECD | [152] | |
| MC–LiBOB | Solid | – | – | – | – | – | – | – | [153] | |
| MFC/BEMA/PEGMA–NaI–I2 | Gel | – | – | – | – | – | Amorph | DSSC | [154] | |
| PVDF/Methyl cellulose–LiPF6–EC/EMC | Gel | 2.0 × 10−4 | – | – | – | – | – | Battery | [155] | |
| CMC–Citric acid | Solid | 4.4 × 10−7 | 0.89 | – | – | – | – | – | [156] | |
| MG-49/CMC–LiCF3SO3 | Solid | 3.3 × 10−7 | – | – | – | – | Amorph | – | [157] | |
| Methyl cellulose–NaI | Solid | 2.7 × 10−5 | – | – | – | – | – | – | [158,159] | |
| MC–NWF–LiPF6–EC/DMC/EMC | Gel | 2.9 × 10−4 | – | – | – | – | – | Battery | [160] | |
| Cellulose acetate/PVDF–HFP–LiTFSI–TEGDME | Gel | 5.5 × 10−4 | – | 4.7 | – | – | – | Battery | [161] | |
| PEO/CMC–NaClO4 | Solid | – | – | – | – | – | – | Battery | [162] | |
| Cellulose acetate–LiTFSI | Solid | 5.6 × 10−4 | – | – | – | – | Amorph | – | [163] | |
| HEC–H3PO4 | Solid | 4.1 × 10−3 | – | – | 0.12 eV | – | – | Super-capacitor | [164] | |
| CMC–LiPF6–EC/DMC/DEC | Gel | 4.8 × 10−4 | – | – | 25.5 kJ/mol | – | – | Battery | [165] | |
| HPC–Bu4NBF4–PEG | Gel | 3.5 × 10−5 | – | – | – | −37 | – | ECD | [166] | |
| Cellulose acetate–NH4I | Solid | 10−4 | – | – | – | – | – | DSSC | [167] | |
| CMC–CH3COONH4–BMATFSI | Solid | 2.2 × 10−3 | – | – | 0.06 eV | – | – | - | [168] | |
| HEC–LiPF6–EC/DMC/DEC | Gel | 1.8 × 10−4 | – | – | 3.57 kJ/mol | – | – | Battery | [169] | |
| CMC–NH4Cl | Solid | 1.4 × 10−3 | – | – | – | – | – | – | [170] | |
| CMC–(NH4)2CO3 | Solid | 7.7 × 10−6 | – | – | – | – | Amorph | – | [171] | |
| CMC–NH4F | Solid | – | – | – | – | – | Semi-cr | – | [172] | |
| HPMC–Mg(CF3SO3)2–[BmIm][Tf] | Solid | 2.4 × 10−4 | – | – | 1.28 eV | 27.5 | Amorph | – | [173] | |
| CMC–Oleic acid–glycerol | Solid | 1.6 × 10−4 | – | – | – | – | – | – | [174] | |
| Cellulose acetate–LiClO4–PC | Gel | 5.3 × 10−3 | – | – | – | – | – | ECD | [175] | |
| Cellulose acetate–NH4I–EC | Solid | 10−3 | – | – | – | – | Amorph | – | [176] | |
| PVDF/cellulose acetate butyrate/PE–LiPF6–EC/DMC/EMC–SiO2 | Gel | 2.9 × 10−3 | – | 5.2 | – | – | – | Battery | [177] | |
| Cellulose acetate–LiNO3 | Solid | 1.9 × 10−3 | – | 4.1 | 0.16 eV | – | Amorph | ECD | [178] | |
| Cellulose acetate–NH4SCN | Solid | 3.3 × 10−3 | 0.99 | – | 0.15 eV | 113.7 | Amorph | Battery | [179] | |
| Cellulose acetate–LiTFSI-BDG | Gel | 2.9 × 10−3 | – | 3.8 | – | – | Amorph | – | [180] | |
| Lignocellulose/potato starch–LiPF6–EC/DMC/EMC | Gel | 1.3 × 10−3 | – | – | 12.7 kJ/mol | – | – | Battery | [181] | |
| Lignocellulose–PEG | Gel | 3.2 × 10−3 | – | – | – | – | – | Battery | [182] | |
| PVA/chitosan/CNC–Acetic acid | Solid | 6.4 × 10−4 | – | – | – | – | Amorph | Fuel cell | [183] | |
| HEC–Li2B4O7–glycerol | Solid | 4.6 × 10−3 | – | – | – | – | Amorph | – | [184] | |
| Cellulose acetate–NH4NO3 | Solid | 1.0 × 10−3 | 0.97 | 4.3 | 0.05 eV | 111.6 | Amorph | ECD | [185] | |
| CMC–(NH4)2CO3 | Solid | 7.7 × 10−6 | 0.98 | – | 0.21 eV | - | - | – | [186] | |
| Chitosan | Acetylated chitosan–LiNO3 | Solid | 10−4 | – | – | – | – | Amorph | Battery | [187] |
| Chitosan acetate–NaI | Solid | 4.9 × 10−5 | – | – | – | – | – | Battery | [188] | |
| Chitosan–NaClO4 | Solid | 4.6 × 10−2 | – | – | – | – | – | Battery | [189] | |
| Oxipropylated chitosan/polyether–LiTFSI | Solid | – | – | – | – | – | – | – | [190] | |
| Chitosan acetate–LiCF3SO3–EC | Solid | 10−5 | – | – | – | – | – | – | [21,191] | |
| Chitosan–KCl | Solid | – | – | – | – | – | – | – | [192] | |
| Chitosan acetate–LiCF3SO3–EC | Solid | 1.3 × 10−5 | – | – | – | – | – | Battery | [193] | |
| Chitosan acetate–LiOAc–palmitic acid | Solid | 5.5 × 10−6 | – | – | – | – | – | – | [194] | |
| Chitosan–LiCF3SO3–EC | Solid | 5.5 × 10−6 | – | – | 0.44 eV | – | Amorph | – | [195] | |
| Chitosan–LiOAc–oleic acid | Solid | 10−5 | – | – | – | – | Amorph | – | [16] | |
| Chitosan–LiOAc–EC | Solid | 7.6 × 10−6 | – | – | – | – | – | – | [196] | |
| Chitosan acetate–LiN(CF3SO2)2–oleic acid | Solid | 3.4 × 10−6 | – | – | – | – | Amorph | – | [197] | |
| Chitosan–KOH | Solid | 10−2 | – | – | – | – | – | Fuel cell | [198] | |
| Chitosan acetate–NH4NO3 | Solid | 2.5 × 10−5 | – | – | 0.45 eV | – | Amorph | – | [17] | |
| Chitosan acetate–NH4NO3–Al2SiO3 | Solid | 2.1 × 10−5 | – | – | – | – | – | – | [199] | |
| Hexanoyl chitosan–LiCF3SO3–EC/PC | Solid | 1.1 × 10−4 | – | – | – | – | – | Battery | [200,201] | |
| Chitosan acetate–NH4CF3SO3–DMC | Solid | 10−6 | – | – | 0.60 eV | – | – | – | [202] | |
| Chitosan–LiOAc–oleic acid | Solid | 1.1 × 10−5 | – | – | 0.29 eV | – | – | – | [203] | |
| Chitosan–LiOAc–palmitic acid | 5.5 × 10−6 | – | – | 0.45 eV | – | – | ||||
| Chitosan acetate–LiCF3SO3 | Solid | – | – | – | 0.38 eV | – | – | Super-capacitor | [204] | |
| Chitosan acetate–H3PO4 | – | – | 0.49 eV | – | – | |||||
| Chitosan/glutaraldehyde–KOH | Solid | 10−2 | – | – | – | – | – | Fuel cell | [205] | |
| Chitosan acetate–NH4NO3–EC | Solid | 9.9 × 10−3 | – | – | – | – | – | Battery | [206] | |
| Hexanoyl chitosan–LiCF3SO3–EC | Gel | 2.8 × 10−5 | – | – | – | – | – | – | [207] | |
| Chitosan/PEO–LiTFSI | Solid | 1.4 × 10−6 | – | – | 0.64 eV | – | – | – | [208] | |
| Chitosan acetate–NH4NO3–o-H3PO4 | Solid | – | – | – | – | – | – | – | [209] | |
| Chitosan/PEO–NH4I–I2 | Solid | 4.3 × 10−6 | – | – | – | – | – | DSSC | [210] | |
| Chitosan/PEO/pAPS–LiClO4 | Solid | 1.7 × 10−5 | – | – | – | – | – | – | [211] | |
| Chitosan–HCl–glycerol | Solid | 2.2 × 10−5 | – | – | 16.6 kJ/mol | −87 | Amorph | – | [212] | |
| Chitosan–PVPA | Solid | – | – | – | 0.32 eV | – | Amorph | – | [213] | |
| Chitosan acetate–NH4NO3–EC | Solid | 10−5 | – | 1.8 | 0.10 eV | – | – | – | [214] | |
| Chitosan acetate–apidic acid | Solid | 1.4 × 10−9 | – | – | 0.52 eV | – | – | – | [215] | |
| Chitosan acetate–NH4NO3–H3PO4–Al2SiO3 | Solid | 1.8 × 10−4 | – | – | – | – | Amorph | Fuel cell | [216] | |
| Chitosan acetate/PEO–NH4NO3–ES | Solid | 10−4 | – | – | 0.02 eV | – | – | – | [217] | |
| Chitosan acetate–CH3COONH4 | Solid | 2.9 × 10−4 | – | – | 0.19 eV | – | Amorph | – | [218] | |
| Hexanoyl chitosan–LiCF3SO3–EC–Al2O3 | Solid | 1.0 × 10−4 | – | – | – | – | – | – | [219] | |
| Hexanoyl chitosan–LiClO4–TiO2 | Solid | 3.1 × 10−4 | – | – | – | – | Amorph | – | [220,221] | |
| Hexanoyl chitosan–LiCF3SO3–DEC/EC | Solid | 4.3 × 10−5 | – | – | 0.11 eV | – | Semi-cr | – | [222] | |
| Chitosan acetate–NH4I–EC | Solid | 7.6 × 10−6 | – | – | 0.21 eV | – | – | – | [18] | |
| Chitosan–LiClO4–EC/PC | Gel | 5.5 × 10−3 | – | – | – | – | – | Super-capacitor | [223] | |
| Chitosan acetate–NH4I–[BmIm][I] | Solid | 3.4 × 10−5 | – | – | – | – | – | DSSC | [224,225] | |
| Chitosan acetate–NH4Cl | Solid | 5.4 × 10−3 | – | – | 0.1 eV | – | Semi-cr | – | [226] | |
| Chitosan acetate–AgCF3SO3 | Solid | – | – | – | 1.16 eV | – | – | – | [227] | |
| Chitosan acetate–NaI-I2–[EmIm][SCN] | Solid | 2.6 × 10−4 | – | – | – | – | Amorph | DSSC | [228] | |
| PVA/chitosan acetate–NH4NO3 | Solid | 1.6 × 10−3 | – | – | 0.14 eV | – | Amorph | Battery | [229] | |
| Chitosan-g-PMMA–LiCF3SO3 | Solid | 4.1 × 10−5 | – | – | – | 110 | – | – | [230] | |
| Chitosan/PEO–NH4I–I2–[BmIm][I] | gel | 5.5 × 10−4 | – | – | – | – | – | DSSC | [231] | |
| Chitosan acetate–NH4SCN–Al2O3 | Solid | 5.9 × 10−4 | – | – | – | 190 | Semi-cr | – | [232] | |
| Hexanoyl chitosan–LiClO4–TiO2 | Solid | – | – | – | – | – | – | – | [233] | |
| Chitosan acetate/PEO–NH4NO3 | Solid | 1.0 × 10−4 | – | – | – | – | Semi-cr | – | [234] | |
| PVA/Chitosan–NH4NO3–EC | Solid | 1.6 × 10−3 | – | 1.7 | – | – | – | EDLC | [235] | |
| Chitosan–LiCF3SO3–EC/PC–SiO2 | Solid | 4.4 × 10−5 | – | – | 0.26 eV | – | – | – | [236] | |
| Chitosan/PVA–NH4I | Solid | 1.8 × 10−6 | – | – | 0.38 eV | – | Amorph | – | [237] | |
| Chitosan acetate–glycerol | Solid | 1.1 × 10−5 | – | – | – | −70 | – | – | [238] | |
| Phthaloyl chitosan–NH4SCN | Solid | 2.4 × 10−5 | – | 2.1 | 0.08 eV | – | Amorph | – | [239] | |
| Chitosan–[CBIm][Cl]–I2 | Solid | 9.1 × 10−3 | – | – | – | – | – | – | [240] | |
| Hexanoyl chitosan–LiClO4 | Solid | 4.2 × 10−7 | – | – | – | – | – | – | [241] | |
| Hexanoyl chitosan–LiCF3SO3 | 4.1 × 10−6 | |||||||||
| Chitosan/PEO–NH4NO3 | Solid | – | – | – | 0.29 eV | – | – | – | [242,243] | |
| Nano-chitosan/PEO–LiCF3SO3 | Solid | 10−3 | – | – | – | – | Semi-cr | – | [244] | |
| PEO/Chitosan–NH4I–I2 | Solid | 1.2 × 10−5 | – | – | – | – | – | DSSC | [245] | |
| CMCh–ClCH2COOH | Solid | 2 × 10−7 | – | – | – | – | – | – | [246,247] | |
| Chitosan/PEO–LiClO4–EC/PC | Solid | 1.1 × 10−4 | – | – | 0.12 eV | – | – | Super-capacitor | [248] | |
| Hexanoyl chitosan–LiCF3SO3–EC–Al2O3 | Solid | – | – | – | – | – | – | – | [249] | |
| Chitosan–NH4Br–glycerol | Solid | 2.2 × 10−4 | – | – | 0.20 eV | – | Amorph | – | [250] | |
| Chitosan–NH4SCN–Al2TiO5 | Solid | 2.1 × 10−4 | – | – | – | – | Amorph | – | [251] | |
| Chitosan/PEO–NH4NO3–EC | Solid | 2.1 × 10−3 | – | 1.75 | 0.18 eV | – | Amorph | EDLC | [64] | |
| Methyl cellulose/chitosan–NH4CF3SO3 | Solid | 5.0 × 10−6 | – | – | – | – | – | – | [252] | |
| CMCh–NH4CF3SO3 | Solid | 8.9 × 10−6 | – | 0.8 | – | – | – | – | [253] | |
| Chitosan–[EmIm][C1SO3]–glycerol | Solid | 7.8 × 10−4 | – | – | 12.1 kJ/mol | – | – | – | [254] | |
| Chitosan–[EmIm][C2SO3]–glycerol | 4.2 × 10−4 | – | – | 14.3 kJ/mol | – | – | ||||
| Chitosan–[EmIm][C4SO3]–glycerol | 1.5 × 10−4 | – | – | 16.7 kJ/mol | – | – | ||||
| Hexanoyl chitosan–LiClO4–TiO2 | Solid | 3.1 × 10−4 | – | – | 0.08 eV | – | – | – | [255] | |
| Hexanoyl chitosan–LiClO4–SiO2 | 2.0 × 10−4 | – | – | 0.12 eV | – | – | ||||
| Chitosan-g-PMMA–LiCF3SO3–EC | Solid | 2.2 × 10−4 | – | – | – | – | – | – | [256] | |
| Chitosan–LiTFSI–succinonitrile | Solid | 0.4 ×10−3 | – | 4.7 | – | – | Amorph | Battery | [257] | |
| CMC/chitosan–NH4Br | Solid | 1.2 × 10−5 | – | – | – | – | – | – | [258] | |
| Hexanoyl chitosan/polystyrene–LiCF3SO3–TiO2 | Solid | 2.8 × 10−4 | – | – | – | – | Amorph | – | [259,260,261] | |
| PVA/chitosan–NH4Br | Solid | 7.7 × 10−4 | – | 1.6 | 0.15 eV | – | Amorph | – | [262] | |
| Corn starch/chitosan–NH4I | Solid | 3.0 × 10−4 | – | – | 0.20 eV | – | Amorph | – | [92] | |
| Chitosan/gold–LiClO4 | Solid | 7.2 × 10−7 | – | – | – | – | Amorph | – | [263] | |
| Phosphorylated chitosan–LiClO4 | Solid | 1.4 × 10−3 | – | – | – | – | – | – | [264] | |
| Chitosan–Oxalic acid | Solid | 5.0 × 10−7 | – | – | 0.61 eV | – | Amorph | – | [265,266] | |
| N-Succinyl chitosan–LiClO4 | Solid | 8.0 × 10−3 | – | – | – | – | – | – | [267,268] | |
| Hexanoyl chitosan–LiClO4–DMC | Solid | 10−4 | – | – | 0.06 eV | – | – | – | [269] | |
| Hexanoyl chitosan–LiClO4–DMC–TiO2 | Solid | 4.1 × 10−4 | – | – | – | – | Amorph | – | [270] | |
| Lauroyl chitosan/PMMA–LiCF3SO3–EC | Solid | 7.6 × 10−4 | – | – | – | – | Amorph | – | [271] | |
| NSB–Chitosan–NMPS–GO | Solid | 8.9 × 10−2 | – | – | 4.57 kJ/mol | – | – | – | [272] | |
| Methyl cellulose/chitosan–NH4CF3SO3–[BmIm][TFSI] | Solid | 4.0 × 10−4 | – | – | – | – | – | – | [273] | |
| Starch/chitosan–NH4Cl–glycerol | Solid | 5.1 × 10−4 | 0.97 | 1.65 | – | – | Amorph | Battery | [274] | |
| Chitosan acetate–LiCl | Gel | 2.9 × 10−3 | – | – | 0.20 eV | – | – | – | [275] | |
| Chitosan–[BmIm][OAc] | Solid | 2.4 × 10−3 | 0.75 | 3.4 | 0.29 eV | 35 | Amorph | – | [276] | |
| Hexanoyl chitosan–LiClO4/TiO2 | Solid | 3.0 × 10−4 | – | – | – | – | – | – | [277] | |
| PVA/chitosan–[BmIm][Br] | Solid | 4.2 × 10−2 | 0.65 | – | – | – | – | – | [278] | |
| PVA/chitosan–[EmIm][Cl] | 5.5 × 10−2 | 0.70 | – | – | – | – | ||||
| Chitosan–NaCF3SO3–Al2O3 | Solid | – | – | – | – | – | Amorph | – | [279] | |
| CMCh–DTAB | Solid | 1.9 × 10−6 | – | – | – | – | – | – | [280] | |
| Chitosan/PEO–NH4I | Solid | 3.7 × 10−6 | 0.85 | – | – | – | Amorph | DSSC | [281] | |
| Chitosan–LiClO4–ZrO2 | Solid | 3.6 × 10−4 | 0.55 | – | – | – | Amorph | – | [282] | |
| Chitosan–perchloric acid | Solid | 5.9 × 10−4 | – | – | – | – | – | – | [283] | |
| N-phthaloyl chitosan–TPAI–I2–EC | Solid | 5.5 × 10−3 | – | – | 0.11 eV | – | Amorph | DSSC | [284] | |
| Chitosan–oxalic acid | Solid | 4.1 × 10−5 | – | – | – | – | – | – | [285] | |
| Sulfonated chitosan–sulfonated GO | Solid | 7.2 × 10−3 | – | – | – | – | – | – | [286] | |
| Chitosan–LiCF3SO3–Al2O3 | Solid | 10−6 | – | – | – | – | Amorph | – | [287] | |
| Chitosan–Ce(CF3SO3)3–glycerol | Solid | 1.7 × 10−5 | – | – | – | – | Amorph | – | [288] | |
| Chitosan–Eu(CF3SO3)3–glycerol | Solid | 1.5 × 10−6 | – | – | – | – | Amorph | – | [289] | |
| Chitosan–NaCF3SO3 | Solid | 2.4 × 10−4 | – | – | 0.3 eV | – | Amorph | – | [290] | |
| Chitosan/pectin–HCl | Solid | 2.4 × 10−3 | – | – | – | – | – | – | [291] | |
| Chitosan–Mg(CF3SO3)2–[EmIm][CF3SO3] | Solid | 3.6 × 10−5 | 0.98 | 4.15 | 0.72 eV | – | – | – | [292] | |
| Hexanoyl chitosan–NaI | Solid | 1.3 × 10−6 | – | – | – | −24 | Amorph | – | [293] | |
| Lauroyl chitosan–NaI | 1.1 × 10−8 | – | – | – | −10 | Amorph | ||||
| Chitosan–AgCF3SO3–Al2O3 | Solid | – | – | – | – | – | – | – | [294] | |
| Chitosan–Tm(CF3SO3)3–glycerol | Solid | 10−5 | – | – | – | – | Amorph | ECD | [295] | |
| Chitosan–[EmIm][Eu(SCN)4] | Solid | 1.3 × 10−5 | – | – | – | – | Semi-cr | – | [296] | |
| Chitosan–[EmIm][SCN] | Solid | 1.6 × 10−3 | – | 4.0 | – | – | Amorph | – | [297,298] | |
| Agar | Agar–Acetic acid | Solid | 1.1 × 10−4 | – | – | 33.5 kJ/mol | – | Amorph | – | [11] |
| Agar–LiI–I2–TiO2 | Gel | 5.1 × 10−4 | – | – | – | – | – | DSSC | [299] | |
| Agar–LiI–I2–TiO2 | Gel | 4.0 × 10−4 | – | – | – | – | – | DSSC | [300] | |
| Agar–Eu(pic)3–glycerol | Solid | 1.6 × 10−5 | – | – | – | – | Amorph | ECD | [301] | |
| Agar–LiClO4–glycerol | Gel | 6.5 × 10−5 | – | – | – | – | Amorph | ECD | [26] | |
| Agar–[EmIm][C2SO4]–glycerol | Solid | – | – | – | – | – | Amorph | ECD | [302] | |
| Agar–[EmIm][OAc]–glycerol | 2.4 × 10−5 | – | – | 24.3 kJ/mol | – | Amorph | ||||
| Agar–[Ch][OAc]–glycerol | – | – | – | – | – | Amorph | ||||
| Agar–LiI–I2–NiO | Gel | – | – | – | – | – | – | DSSC | [303] | |
| Agar–LiI–I2–Fe3O4–PEG | Gel | 2.9 × 10−3 | – | – | – | – | – | DSSC | [304] | |
| Agar–LiI–I2–Fe3O4–SDS | Gel | – | – | – | – | – | – | DSSC | [305] | |
| Agar–LiI–I2–Fe3O4–PVP | – | – | – | – | – | – | ||||
| Agar–LiI–I2–Fe3O4–TW-80 | 3.0 × 10−3 | – | – | – | – | – | ||||
| Bacto agar–NaI–I2 | Gel | 1.2 × 10−3 | – | 2.0 | – | – | Amorph | – | [306] | |
| Agar–Mg(CF3SO3)2–glycerol | Solid | 1.0 × 10−6 | – | – | – | – | Amorph | ECD | [307] | |
| Agar–LiI–I2–TiO2 | Gel | 2.7 × 10−3 | – | – | – | – | – | DSSC | [308] | |
| Agar–LiI–I2–Co3O4 | 4.4 × 10−3 | – | – | – | – | – | ||||
| Agar–LiI–I2–NiO | 3.3 × 10−3 | – | – | – | – | – | ||||
| Agar–NiO–glycerol–acetic acid | Solid | 5.2 × 10−5 | – | – | – | – | Amorph | – | [65] | |
| Agar–LiClO4–glycerol | Solid | 6.5 × 10−8 | – | – | 0.1 eV | – | Amorph | – | [25] | |
| Agar–KClO4–glycerol | 9.1 × 10−8 | – | – | 0.1 eV- | – | Amorph | ||||
| Agar–Acetic acid–glycerol | 3.5 × 10−8 | – | – | 0.1 eV | – | Amorph | ||||
| Agar–Lactic acid–glycerol | 2.2 × 10−8 | – | – | 0.1 eV | – | Amorph | ||||
| Agar–NH4NO3 | Solid | 6.6 × 10−4 | 0.99 | – | 0.12 eV | – | Amorph | Fuel cell | [309] | |
| Agar–Na2S/S–glycerol | Gel | 1.8 × 10−3 | – | – | – | – | – | DSSC | [310] | |
| Agar–NH4SCN | Solid | 1.0 × 10−3 | 0.97 | – | 0.25 eV | 55 | Amorph | – | [311] | |
| Agar–NH4I | Solid | 1.1 × 10−4 | – | – | 0.43 eV | – | Amorph | – | [312] | |
| Agar–KI–MPII | Gel | 1.5 × 10−3 | – | – | – | – | Amorph | DSSC | [313] | |
| Carrageenan | Carboxymethyl κ-carrageenan–acetic acid | Solid | 2.0 × 10−4 | – | – | – | – | – | – | [29] |
| κ-carrageenan–[Bmim]Cl | Solid | 2.4 × 10−3 | – | – | – | – | Amorph | – | [314] | |
| Carboxymethyl ɩ-carrageenan–acetic acid | Solid | 4.9 × 10−6 | – | – | – | – | Amorph | – | [315] | |
| Carboxymethyl κ-carrageenan/CMC–NH4I | Solid | 2.4 × 10−3 | 0.99 | 2.0 | 0.01 eV | – | – | DSSC | [316] | |
| κ-carrageenan–TBAI–I2–TiO2 | Solid | - | – | - | – | – | – | DSSC | [317] | |
| κ-carrageenan–TBAI–I2–Fe2O3 | - | – | - | – | – | – | ||||
| κ-carrageenan–TBAI–I2–halloysite clay | - | – | - | – | – | – | ||||
| Carboxymethyl κ-carrageenan/CMC–acetic acid | Solid | 3.3 × 10−4 | – | 2.75 | – | −13.5 | – | – | [30] | |
| Carboxymethyl κ-carrageenan–LiNO3 | Solid | 5.9 × 10−3 | – | 3.1 | 0.18 eV | – | – | – | [22] | |
| Carboxymethyl ɩ-carrageenan–LiNO3 | 5.5 × 10−3 | – | 3.0 | 0.38 eV | – | – | ||||
| Carboxymethyl κ-carrageenan/CMC–LiI–I2 | Solid | 3.9 × 10−3 | – | - | 0.01 eV | −43.0 | – | DSSC | [318] | |
| ɩ-carrageenan–NH4Br | Solid | 1.1 × 10−3 | – | 2.1 | 0.18 eV | - | Amorph | Fuel cell | [319] | |
| k-carrageenan/PEDOT–PANI | Gel | – | – | – | – | – | – | Super-capacitor | [320] | |
| ɩ-carrageenan–NH4NO3 | Solid | 1.5 × 10−3 | 0.95 | 2.46 | 0.14 eV | 64 | – | ECD | [321] | |
| Pectin | Pectin–LiClO4 | Solid | 4.7 × 10−4 | – | – | – | – | Amorph | – | [33] |
| Pectin–HCl–glutaraldehyde | Solid | 2.5 × 10−2 | – | – | – | – | Amorph | – | [322] | |
| Pectin–KCl–glycerol | Solid | 1.5 × 10−3 | – | – | – | – | Amorph | – | [323] | |
| Pectin–KCl–Ir(III)–glycerol | 5.4 × 10−5 | – | – | – | – | Amorph | ||||
| Pectin–NH4Cl | Solid | 4.5 × 10−4 | – | – | – | – | Amorph | – | [324] | |
| Pectin–NH4Br | 1.1 × 10−3 | – | – | – | – | Amorph | ||||
| Pectin–[N1112(OH)][NTf2]–glycerol | Solid | 1.4 × 10−6 | – | – | – | – | Amorph | – | [325] | |
| Guar gum and gum arabic | Guar gum–LiClO4–glycerol | Solid | 2.2 × 10−3 | – | – | 0.18 eV | – | – | – | [37] |
| Guar gum–[BmIm][Cl]–PEDOT | Gel | 10−2 | – | – | – | – | – | – | [35] | |
| Guar gum–[BmIm][Cl]–P(AEMIBr) | Gel | 10−4 | – | – | – | – | – | – | [38] | |
| Gum Arabic–o-H3PO4 | Gel | 1.8 × 10−2 | – | – | – | – | – | Super-capacitor | [326] | |
| Gelatin | Gelatin–glycerol–formaldehyde–acetic acid | Solid | 4.5 × 10−5 | – | – | 32.6 kJ/mol | – | – | – | [42] |
| Gelatin–LiClO4–glycerol | Solid | 10−4 | – | – | 0.35 eV | – | – | – | [327] | |
| Gelatin–LiBF4–glycerol | Gel | 2.3 × 10−5 | – | – | – | – | – | – | [41] | |
| Gelatin–LiClO4–glycerol | Gel | 3.2 × 10−5 | – | – | – | – | – | |||
| Gelatin–HCl–glycerol | Gel | 5.4 × 10−5 | – | – | – | – | – | |||
| Gelatin–Acetic acid–glycerol | Gel | 8.7 × 10−4 | – | – | – | – | – | |||
| Gelatin–LiClO4–EC/PC | Solid | 2.0 × 10−9 | – | – | – | – | – | – | [328] | |
| Gelatin–LiClO4 | Solid | – | – | – | – | – | – | ECD | [329] | |
| Poly(acrylic acid-g-gelatin)/polypyrrole–KI–I2 | Gel | 1.4 × 10−2 | – | – | 10.3 kJ/mol | – | – | DSSC | [330] | |
| Gelatin–Acetic acid–glycerol | Solid | 2 × 10−5 | – | – | 0.22 eV | – | – | – | [331] | |
| Gelatin–LiBF4–glycerol | Gel | 1.5 × 10−5 | – | – | 43.1 kJ/mol | – | Amorph | – | [332] | |
| Gelatin–[Eu(pic)3]–glycerol | Solid | – | – | – | – | – | Amorph | ECD | [333] | |
| Gelatin–HCl–glycerol | Solid | 4.0 × 10−5 | – | – | 23 kJ/mol | – | – | – | [334] | |
| Gelatin–[EmIm][OAc] | Solid | 1.2 × 10−4 | – | – | 16.7 kJ/mol | – | Amorph | ECD | [335] | |
| Gelatin–LiI–I2 | Solid | 5 × 10−5 | – | – | 8 kJ/mol | −76 | Amorph | ECD | [336] | |
| Gelatin–LiClO4–glycerol | Solid | 10−4 | – | – | – | – | – | – | [337,338] | |
| Gelatin–Glycerol | Solid | 9.1 × 10−3 | – | – | – | – | Amorph | – | [339] | |
| Gelatin–Zn(CF3SO3 )2 | Solid | 3.1 × 10−10 | – | – | – | – | Amorph | – | [340] | |
| Gelatin–LiClO4–glycerol | Solid | 1.1 × 10−4 | – | – | 9.37 kJ/mol | – | Amorph | – | [341] | |
| Gelatin–LiCl–glycerol | 2.0 × 10−4 | – | – | 6.31 kJ/mol | – | Amorph | ||||
| Gelatin–Mg(CF3SO3)2–glycerol | Solid | 3.8 × 10−10 | – | – | 49.0 kJ/mol | – | Amorph | – | [39] | |
| Gelatin/Au–LiI–I2 | Gel | 2.2 × 10−2 | – | – | – | 77 | – | DSSC | [342] | |
| Gelatin–[EmIm][N(CN)2]–glycerol | Solid | 2.4 × 10−3 | – | – | – | – | Amorph | ECD | [343] | |
| Gelatin–NaCl | Gel | 8.5 × 10−2 | – | – | – | – | – | – | [110] | |
| Natural rubber | NR/PEO–LiBs-PEO1000 | Solid | 10−6 | – | – | – | – | – | – | [44] |
| ENR-25–LiCF3SO3–EC/PC | Solid | 2.9 × 10−4 | – | – | – | −43 | – | – | [344] | |
| ENR-50–LiCF3SO3–EC/PC | 1.3 × 10−4 | – | – | – | −35 | – | ||||
| MG-49–LiCF3SO3–EC/PC | 4.3 × 10−4 | – | – | – | – | – | ||||
| ENR-25/PEO–LiCF3SO3–EC/PC | Solid | 10−4 | – | – | – | – | – | – | [345] | |
| ENR-50/PEO–LiCF3SO3–EC/PC | 10−4 | – | – | – | – | – | ||||
| MG-30–LiCF3SO3–EC–Al2SiO5 | Solid | – | – | – | – | −41 | – | – | [346] | |
| PVC/ENR-50–LiCF3SO3 | Solid | 3.6 × 10−5 | – | – | – | – | – | – | [347] | |
| PMMA/ENR-50–LiCF3SO3 | 5.1 × 10−5 | – | – | – | – | – | ||||
| MG-30–LiCF3SO3 | Gel | 8.4 × 10−4 | – | 4.3 | – | – | – | – | [348] | |
| MG-30–LiCF3SO3–EC | – | – | – | – | – | – | ||||
| MG-30–LiCF3SO3–PC | – | – | – | – | – | – | ||||
| PMMA/ENR-50–LiCF3SO3–EC | Solid | – | – | – | – | – | – | – | [349] | |
| MG-49–NH4CF3SO3–PC | Gel | 3.3 × 10−2 | – | – | – | – | – | Battery | [350] | |
| MG-49–LiBF4 | Solid | 2.3 × 10−7 | – | – | – | – | Amorph | – | [351] | |
| MG-49–LiClO4 | 4.0 × 10−8 | – | – | – | – | Amorph | ||||
| MG-49–LiClO4–EC–TiO2 | Solid | 1.1 × 10−3 | – | – | – | – | – | – | [352] | |
| MG-49–NH4CF3SO3–SiO3 | Gel | 7.6 × 10−3 | – | – | – | – | – | – | [353] | |
| PMMA/MG-49–LiBF4 | Solid | 8.3 × 10−6 | – | – | – | – | Amorph | – | [354] | |
| MG-49–NH4CF3SO3–PC | Gel | 2.9 × 10−2 | – | – | – | – | – | – | [355] | |
| MG-30–NH4CF3SO3–EC | Solid | 10−4 | – | – | – | – | Amorph | – | [356] | |
| PVC/LENR-50–LiClO4 | Solid | 2.3 × 10−8 | – | – | – | – | Amorph | – | [357] | |
| ENR-50–Li2NH | Solid | 3.5 × 10−5 | – | – | – | – | – | – | [358] | |
| MG-49/Cellulose–LiCF3SO3 | Solid | 5.3 × 10−7 | – | – | – | – | Amorph | – | [359] | |
| MG-30–LiCF3SO3–EC | Gel | 9.0 × 10−3 | – | 4.2 | 0.14 eV | −77.2 | – | Battery | [360] | |
| PVDF–HFP/MG-49–LiCF3SO3 | Solid | 2.0 × 10−4 | – | 3.0 | 0.14 eV | – | Semi-cr | – | [361,362,363] | |
| ENR-50–LiN(SO2CF3)2–EC/PC | Solid | 2.6 × 10−4 | – | – | – | −46.8 | – | – | [364] | |
| PVDF/MG-49–NH4CF3SO3 | Solid | 6.3 × 10−4 | – | 4.0 | – | – | Semi-cr | – | [365] | |
| MG-49/CMC–LiCF3SO3 | Solid | 3.3 × 10−7 | – | 2.7 | – | – | – | – | [366] | |
| MG-49–LiCF3SO3–ZrO2/TiO2 | Solid | 1.2 × 10−5 | – | – | 0.10 eV | – | Amorph | Battery | [367] | |
| MG-30–LiCF3SO3 | Solid | 5.6 × 10−3 | – | – | – | −43.9 | – | – | [368] | |
| ENR-50–LiClO4 | Solid | 10−5 | – | – | – | −22 | – | – | [369] | |
| ENR-25/hexanoyl chitosan–LiN(CF3SO2)2–[EmIm][TFSI] | Solid | 1.3 × 10−6 | – | – | – | −31 | – | – | [370] | |
| ENR-25–LiClO4 | Solid | – | – | – | – | −42 | – | – | [371] | |
| Poly(lactic acid) | PLA–LiClO4–EC–Al2O3 | Solid | 2.1 × 10−5 | – | – | – | – | Amorph | Battery | [47] |
| PLA–LiClO4–EC–SiO2 | Solid | 1.3 × 10−5 | – | – | – | – | Amorph | – | [46] | |
| PLA/PHB/PC–LiPF6–EC | Gel | 1.5 × 10−2 | – | 4.4 | – | 69 | Semi-cr | Battery | [48] | |
| PLGA/SY–TBABF4 | Solid | 10−10 | – | – | – | – | – | LEC | [372] | |
| PLA–LiTFSI–Pyr14TFSI | Solid | – | – | – | – | 0.1 | Amorph | Battery | [49] | |
| Vegetable oil-based polyurethane | Palm-based PU–LiCF3SO3 | Solid | 1.6 × 10−5 | – | – | – | – | Amorph | – | [8] |
| Palm-based PU–LiI–I2–EC | Solid | 7.6 × 10−4 | – | – | 0.11 eV | – | Amorph | DSSC | [51] | |
| Castor oil-based PU–LiI | Solid | 1.4 × 10−6 | 0.99 | 2.0 | 0.13 eV | −27.5 | – | – | [52] | |
| Castor oil-based PU–NaI | 4.3 × 10−7 | 0.98 | 1.8 | 0.22 eV | −26.1 | – | ||||
| Jatropha oil-based PU–LiClO4–EC | Solid | 1.3 × 10−4 | 0.83 | 4.8 | 2.8 meV | – | Amorph | – | [50] | |
| Bacterial cellulose | Bacterial cellulose–Nafion | Solid | 5.6 × 10−2 | – | – | – | – | – | Fuel cell | [55] |
| Bacterial cellulose/PSSA–HCl | Solid | 10−3 | – | – | – | – | – | Fuel cell | [56] | |
| Gellan and Xanthan gum | Gellan–LiCF3SO3 | Solid | 5.4 × 10−4 | – | 5.4 | 14.6 kJ/mol | – | Semi-cr | – | [58] |
| Gellan–LiI–Glycerol | Solid | 1.5 × 10−3 | – | – | 2.4 kJ/mol | – | – | – | [59] | |
| Gellan–[N1112(OH)][NTf2]–Er(CF3SO3)3 | Solid | 5.2 × 10−6 | – | 3.5 | – | – | Semi-cr | ECD | [373] | |
| Gellan–KI–I2 | Solid | 2.5 × 10−2 | – | – | 0.24 eV | – | Amorph | DSSC | [374] | |
| Gellan–o-H3PO4 | Gel | 5.1 × 10−3 | – | – | 0.14 meV | – | – | EDLC | [375] | |
| Gellan–H2SO4 | 1.5 × 10−3 | – | – | 0.17 meV | – | – | ||||
| Gellan–HCl | 3.7 × 10−4 | – | – | 0.19 meV | – | – | ||||
| Xanthan–PMII–I2–TBP–GSCN | Gel | – | – | – | – | – | – | DSSC | [61] | |
| Xanthan–LiClO4–glycerol | Gel | 2.6 × 10−3 | – | – | – | – | – | Super-capacitor | [23] | |
| Xanthan–Li2B4O7–glycerol | 2.7 × 10−2 | – | – | – | – | – | ||||
| Polymer | Electrolyte System | State | σ (s/cm) | JSC (mA/cm2) | VOC (V) | FF | η (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Starch | Rice starch–LiI–MPII–TiO2 | Solid | 3.6 × 10−4 | 0.49 | 0.45 | 0.75 | 0.17 | [88] |
| Rice starch–LiI | Solid | 4.7 × 10−5 | – | – | – | – | [96] | |
| Rice starch–NH4I | 1.4 × 10−4 | – | – | – | – | |||
| Rice starch–NaI | 4.5 × 10−4 | 2.40 | 0.48 | 0.67 | 0.78 | |||
| Rice starch–NaI–MPII | Solid | 1.2 × 10−3 | 4.78 | 0.57 | 0.76 | 2.09 | [97] | |
| Cellulose | Cellulose-g-acrylic acid–[BmIm][I] | Gel | 7.3 × 10−3 | 12.65 | 0.71 | 0.611 | 5.51 | [131] |
| CN–HPC–LiI–I2–MHII | Gel | 2.5 × 10−3 | 14.40 | 0.76 | 0.70 | 7.55 | [139] | |
| PEO/CMC–NaI–I2–MPII | Gel | - | 10.03 | 0.75 | 0.69 | 5.18 | [147] | |
| MFC/BEMA/PEGMA–NaI–I2 | Gel | - | 15.20 | 0.76 | 0.61 | 7.03 | [154] | |
| Chitosan | Chitosan/PEO–NH4I | Solid | 3.7 × 10−6 | 2.71 | 0.58 | 0.50 | 0.78 | [281] |
| N-phthaloyl chitosan–TPAI–I2–EC | Solid | 5.5 × 10−3 | 12.72 | 0.60 | 0.66 | 5.00 | [284] | |
| Agar | Agar–LiI–I2–TiO2 | Gel | - | 10.96 | 0.54 | 0.57 | 4.74 | [299] |
| Agar–NH4I–I2–Glycerol | Gel | 4.9 × 10−3 | 0.007 | 0.29 | – | – | [380] | |
| Agar–LiI–I2–NiO | Gel | – | – | – | – | 2.95 | [303] | |
| Agar–MPII–PC–GuSCN–NMBI–I2 | Gel | – | 11.73 | 0.70 | 0.64 | 5.25 | [381] | |
| Agar–AEII–PC–GuSCN–NMBI–I2 | 11.71 | 0.72 | 0.65 | 5.45 | ||||
| Agar–DAII–PC–GuSCN–NMBI–I2 | 11.53 | 0.70 | 0.62 | 4.97 | ||||
| Agar–APII–PC–GuSCN–NMBI–I2 | 11.84 | 0.70 | 0.60 | 4.96 | ||||
| Agar–LiI–I2–Fe3O4–SDS | Gel | – | 3.18 | 0.66 | 0.62 | 1.29 | [305] | |
| Agar–LiI–I2–Fe3O4–PVP | – | 3.00 | 0.67 | 0.59 | 1.19 | |||
| Agar–LiI–I2–Fe3O4–TW-80 | 3.0 × 10−3 | 5.00 | 0.70 | 0.53 | 1.83 | |||
| Agar–KI–I2 | Gel | 9.0 × 10−3 | 3.27 | 0.67 | 0.24 | 0.54 | [382] | |
| Agar–LiI–I2–TiO2 | Gel | 2.7 × 10−3 | 5.28 | 0.61 | 0.55 | 1.71 | [308] | |
| Agar–LiI–I2–Co3O4 | 4.4 × 10−3 | 7.24 | 0.63 | 0.46 | 2.11 | |||
| Agar–LiI–I2–NiO | 3.3 × 10−3 | 6.20 | 0.62 | 0.52 | 2.02 | |||
| Agar–Na2S/S–glycerol | Gel | – | 10.75 | 0.58 | 0.47 | 2.97 | [310] | |
| Agar–KI–MPII | Gel | 1.5 × 10−3 | 9.28 | 0.46 | 0.50 | 2.16 | [313] | |
| Carrageenan | Carboxymethyl κ-carrageenan/CMC–NH4I | Solid | 2.4 × 10−3 | 0.49 | 0.60 | 0.64 | 0.13 | [316] |
| κ-carrageenan–TBAI–I2–TiO2 | Solid | – | 1.83 | 0.75 | 0.55 | 0.76 | [317] | |
| κ-carrageenan–TBAI–I2–Fe2O3 | – | 3.96 | 0.68 | 0.07 | 0.20 | |||
| κ-carrageenan–TBAI–I2–halloysite clay | – | 1.39 | 0.74 | 0.50 | 0.51 | |||
| Carboxymethyl κ-carrageenan/CMC–LiI–I2 | Solid | 3.9 × 10−3 | 0.40 | 0.49 | 0.57 | 0.11 | [318] | |
| Gelatine | Poly(acrylic acid-g-gelatine)/polypyrrole–KI–I2 | Gel | 1.4 × 10−2 | 2.76 | 0.66 | 0.70 | 1.28 | [330] |
| Gelatine/Au–LiI–I2 | Gel | 2.2 × 10−2 | 4.94 | 0.65 | 0.60 | 1.97 | [342] | |
| Vegetable oil-based polyurethane | Palm-based PU–LiI–I2–EC | Solid | 7.6 × 10−4 | 0.06 | 0.14 | - | - | [51] |
| Castor oil-based PU–NaI | Solid | 4.3 × 10−7 | 3.60 | 0.49 | 0.46 | 0.80 | [383] | |
| Palm-based PU–MPII | Gel | 9.1 × 10−4 | 3.30 | 0.71 | 0.36 | 1.00 | [384] | |
| Gellan and Xanthan gum | Gellan–KI–I2 | Solid | 2.5 × 10−2 | 3.20 | 0.57 | 0.90 | 1.47 | [374] |
| Xanthan–PMII–I2–TBP–GSCN | Gel | – | – | – | – | 4.78 | [61] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rayung, M.; Aung, M.M.; Azhar, S.C.; Abdullah, L.C.; Su’ait, M.S.; Ahmad, A.; Jamil, S.N.A.M. Bio-Based Polymer Electrolytes for Electrochemical Devices: Insight into the Ionic Conductivity Performance. Materials 2020, 13, 838. https://doi.org/10.3390/ma13040838
Rayung M, Aung MM, Azhar SC, Abdullah LC, Su’ait MS, Ahmad A, Jamil SNAM. Bio-Based Polymer Electrolytes for Electrochemical Devices: Insight into the Ionic Conductivity Performance. Materials. 2020; 13(4):838. https://doi.org/10.3390/ma13040838
Chicago/Turabian StyleRayung, Marwah, Min Min Aung, Shah Christirani Azhar, Luqman Chuah Abdullah, Mohd Sukor Su’ait, Azizan Ahmad, and Siti Nurul Ain Md Jamil. 2020. "Bio-Based Polymer Electrolytes for Electrochemical Devices: Insight into the Ionic Conductivity Performance" Materials 13, no. 4: 838. https://doi.org/10.3390/ma13040838
APA StyleRayung, M., Aung, M. M., Azhar, S. C., Abdullah, L. C., Su’ait, M. S., Ahmad, A., & Jamil, S. N. A. M. (2020). Bio-Based Polymer Electrolytes for Electrochemical Devices: Insight into the Ionic Conductivity Performance. Materials, 13(4), 838. https://doi.org/10.3390/ma13040838







