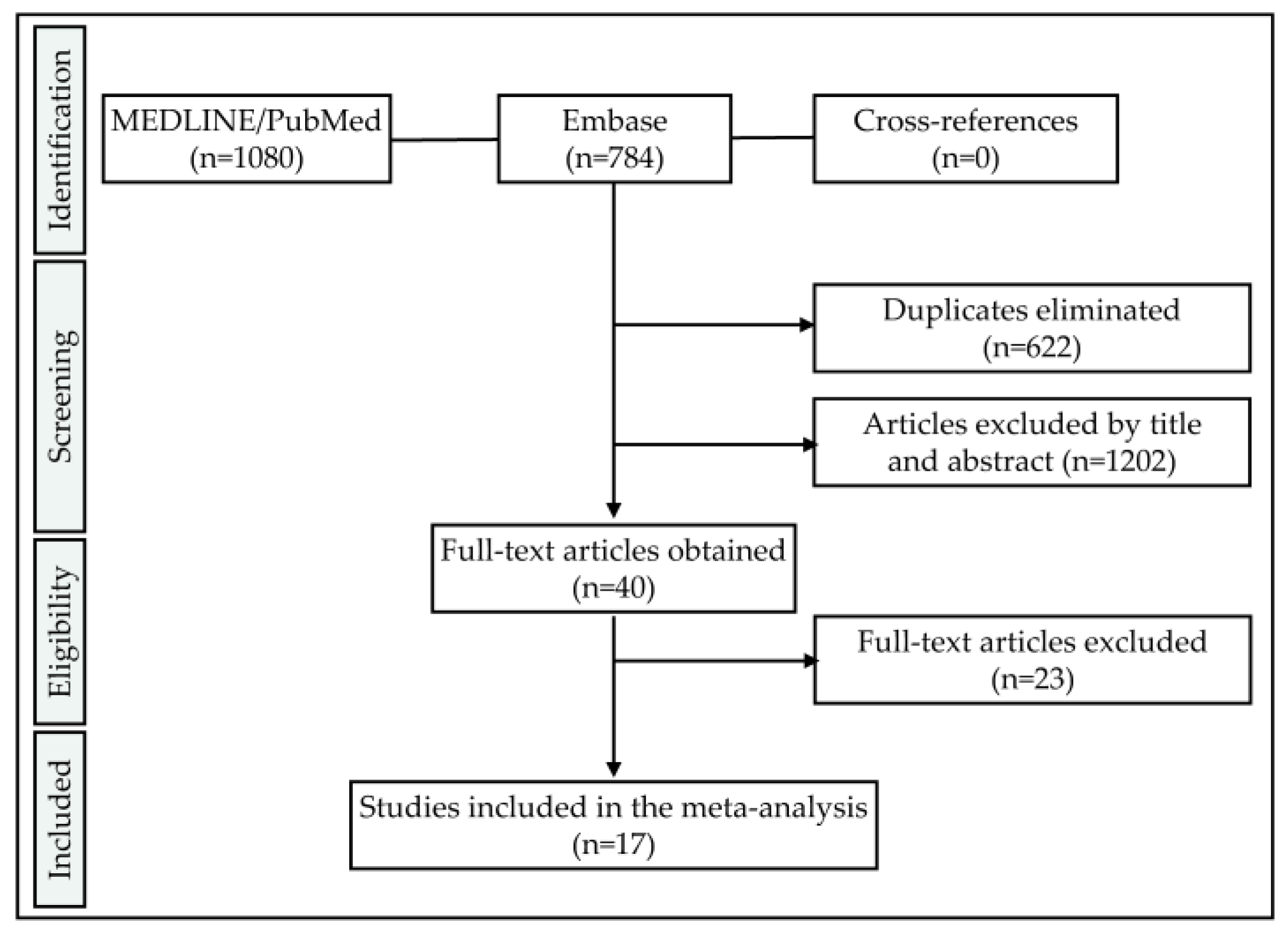
1. Introduction
To date, titanium can be considered the gold standard material in oral implantology [
1]. However, due to increasing esthetic standards and a discussed impact of metal/titanium particle release on the pathogenesis of peri-implant bone loss [
2,
3], a renaissance of ceramic oral implants can be observed in dental media. Nowadays, the market share of zirconia oral implants seems to be increasing, even if still comparatively small compared to conventional titanium implants.
Nonetheless, the superiority of ceramic oral implants regarding esthetics and biocompatibility, or, as an example, the frequently claimed patients’ demand for metal-free implantology are still not soundly scientifically evidenced. Nevertheless, the majority of dental experts are of the opinion that zirconia oral implants will be coexistent with titanium implants in the near future [
4].
When zirconium dioxide (zirconia, ZrO
2) was introduced as ceramic implant material, research focused to evaluate and improve its osseointegrative potential by creating a microroughened surface topography [
5]. In the first instance, parameters like bone-to-implant contact (BIC), push-in values and removal torque were assessed in animal experiments. As a result, zirconia implants with various surface modifications (additive by sintering a porous ceramic layer, subtractive by sandblasting and/or acid-etching or, for example, by texturing the inner surface of a mold in case of an injection-molded implant) can nowadays be considered comparable to titanium implants by means of osseointegration in preclinical studies [
6]. This finding was confirmed in clinical trials, however limited to short- and mid-term observation periods and the replacement of up to three adjacent missing teeth (single-tooth restorations and three-unit fixed dental prostheses) using one-piece ceramic implants [
7].
From a technical point of view, such a 1-piece design, comprising the abutment and endosseous part in a single piece, might benefit from increased fracture resistance and reduced susceptibility for low-temperature degradation or so-called “aging” (by exposing a reduced total surface area to aging by inducing oral fluids), compared to 2-piece ceramic implants. Furthermore, 1-piece implants do not have a micro-gap in between the assembled implant and abutment. One might consider the absence of such a micro-gap beneficial, since it is capable in hosting bacteria, potentially resulting in marginal inflammation and consecutive bone resorption [
8]. However, no advantage of a monobloc design was found for “seamless”, 1-piece implants made from titanium [
9]. Moreover, from a practitioner’s point of view, a 1-piece implant design is associated with several surgical and prosthodontic shortcomings [
10]. As an example, submerged implant healing is hardly possible, since the transmucosal part of a 1-piece implant cannot be detached. If no sufficient primary stability can be attained or guided bone regeneration is necessary, a missing option for wound closure might be considered disadvantageous. Furthermore, there is only a limited potential to compensate for mal-positioned implants with the provisional and final restoration. When trying to remove subsections in case of misaligned implants to support a bridge, intra-oral grinding of the zirconia abutment is necessary [
11]. This, however, might have an impact upon the osseointegration (due to potential heat development or the displacement of zirconia particles in surrounding tissues) and fracture resistance of the implant [
12]. Therefore, a two-piece design represents the favorable option for daily clinical use. Today, several two-piece zirconia implants are available on the market. In these systems, implant-abutment assembly is mostly realized by either luting the abutment to the implant or by screw-retention [
13]. Luting the abutment to the implant seals the micro-gap, and allows for initial but irreversible correction of the implant angulation, but misses flexibility for future restorations of the implant. On the other hand, when going for screw-retention, several ceramic implants are still assembled with a titanium screw, and therefore, still not metal-free in the proper sense.
Even if the market share of zirconia dental implants increases, concerns regarding their fracture resistance are still present, and standardized testing protocols for zirconia implants adequately addressing the aging behavior of the final product are still missing [
14]. To overcome this, different treatments were proposed to mimic intraoral conditions to the extent possible for the evaluation of ceramic implants. These treatments included thermal aging (high-temperature conditions or thermal cycling) [
15,
16] and/or dynamic loading procedures (various exposure times and different applied loading modes) [
12,
17]. Zirconia implants evaluated regarding their fracture resistance in the literature comprised a heterogeneous range of features like material selection (yttria-stabilized tetragonal zirconia polycrystal, Y-TZP or alumina-toughened zirconia, ATZ) [
18], design (1- or 2-piece) [
13], manufacturing (subtractive or by ceramic injection molding, CIM) [
19], restoration (anatomical crown, hemisphere or no restoration) [
20,
21], abutment preparation (in the case of 1-piece implants) [
22], or assembly (in the case of 2-piece implants) [
13].
Therefore, the objective of the present systematic review was to evaluate the influence of the aforementioned treatments and features on the fracture resistance of zirconia oral implants in different preclinical studies. The null hypothesis supposed no distinction between treatments and features in relation to bending moment when statically loading the implant to fracture.
2. Materials and Methods
2.1. Study Design
To determine a selection of comparable studies on the question of zirconia implant fracture resistance, the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement of 2009 was applied [
23]. Therefore, this report takes the appropriate Enhancing the Quality and Transparency of health Research (EQUATOR) (
http://www.equator-network.org) guidelines into account.
2.2. Focused Question
Is there a variable significantly affecting the fracture resistance of 1- and 2-piece zirconia implants in preclinical in-vitro studies?
2.3. Search Strategy
Two databases, namely the Medical Literature Analysis and Retrieval System Online (MEDLINE) (PubMed) and Embase (accessed via Ovid), were screened for relevant articles. The database specific search strategies consisted of a combination of subject headings and free text words. Data was extracted from the databases on 3rd December 2019 without applying any time restrictions. Thereafter, references of included articles were screened for further records satisfying the inclusion criteria (cross-referencing). In case of the availability of the full methodological procedures in the literature and accessibility of information regarding the included samples, unpublished data of the authors of the present review was likewise included. The resulting studies were imported and stored in a reference managing program (EndNote X9; Clarivate Analytics, Philadelphia, PA, USA). Articles written in English and the German language were considered.
2.4. Screening Process
To build up the search terms, three categories addressing the samples (dental implants), materials (zirconia ceramics) and outcome (fracture load) were combined (“AND”). These categories consisted of combinations (“OR”) of free text words and indexed vocabulary (MEDLINE: MeSH terms, Embase: Emtree terms). An asterisk was used in combination with some free text words as a truncation symbol (e. g. “ceramic *”) to allow for the so-called “wildcard search”.
Pubmed search term:
| ((((dental implant [MeSH Terms]) OR ((((oral) AND ((implant) OR implants))) OR ((dental) AND ((implant) OR implants))))) AND (((zircon *) OR ceramic *) OR ceramics[MeSH Terms])) AND (((((ageing) OR aging) OR artificial mouth) OR fracture resistance) OR load *) |
Embase search term:
| (‘tooth implant’/exp OR (oral AND implant) OR (dental AND implant)) AND (zircon * OR ceramic * OR ‘ceramics’/exp) AND (ageing OR aging OR (artificial AND mouth) OR (fracture AND resistance) OR load *) |
2.5. Eligibility Criteria
Studies to be included in this systematic review needed to fulfill the following inclusion criteria:
- -
Language: English or German
- -
Samples: Screw-shaped, ceramic oral implants containing a minimum of 50% v/v ZrO2 within the bulk material
- -
Outcome: Static loading to fracture
- -
Outcome measure: Bending moment [Ncm or Nmm] or fracture load [N] allowing to calculate the bending moment (e.g., by adopting ISO 14801 or providing data to calculate the lever arm) was provided
- -
Sample size: Minimum of five samples tested
2.6. Selection of Studies
Concerning the inclusion criteria, both the first author and the senior author of this manuscript (A.B. and B.C.S.) independently screened the titles and abstracts of the extracted data in the reference management program. If sufficient information needed for inclusion or exclusion was not provided within the title or abstract, the corresponding full texts were read. In case of disagreement, a third author (S.P.) was consulted for final decision making.
2.7. Data Extraction
Besides the total number of samples within one study, the number of implants made from different materials (Y-TZP, ATZ), processing routes (subtractive, injection molding), design (1- and 2-piece) and diameters were retrieved. Further features like restoration mode (anatomical crown, hemisphere or no reconstruction), abutment preparation (yes/no in case of 1-piece implants), implant-abutment connection (screwed/bonded in case of 2-piece implants), thermal aging (thermal cycling, high temperature, no aging) or dynamic loading (yes/no), dynamic loading conditions (exerted load and amount of cycles), crosshead speed during static fracture, and angulation, were likewise extracted. This allowed us to group the implants finally subjected to static loading within the included studies in cohorts. For standardization purposes, the bending moment at the time point of fracture [Ncm] was considered the outcome measure of interest, and the corresponding authors of the articles to be included were contacted by email in case of solely providing fracture load values [N] without mentioning the lever arm. Extracted cohorts were subdivided into groups subjected to comparable treatments:
No dynamic loading
1–1.2 million loading cycles (50 N)
1–1.2 million loading cycles (100 N)
3.5–5 million loading cycles (100 N)
5 million loading cycles (>500 N)
10 million loading cycles (100 N)
2.8. Statistical Analysis
From the included nineteen studies/datasets, two to twelve observations were extracted each. One observation consisted of the mean bending moment and standard deviation (at the time point of fracture) and/or mean fracture load and standard deviation (including additional information allowing us to calculate the bending moment) of a specific cohort of implants (comprising the same type of implant subjected to the same treatment) extracted from one included study. These observations had sample sizes of 2 to 12 implants. To analyze the effect of specific treatments of features (as indicated in 2.7) on the bending moment, a multilevel mixed-effects generalized linear model was used for each outcome, with each investigation as random effect to cluster observations by the respective studies. The Šidák method was used to correct for multiple testing. The level of significance was set at p < 0.05.
In order to compare the aforementioned groups (1–6, depending on load and cycles) for heterogeneity of the data, both inter- and intra-standard deviations with 95% confidence intervals (Cis) were computed. In addition, the cohort-specific standard error of the bending moment was used for weighting. Furthermore, box plots were created for visualization of the data. The data were analyzed with STATA 16.1 (StataCorp LLC, Texas, TX, USA).
4. Discussion
The present systematic review and meta-analysis included the data of 17 studies and two unpublished datasets. To be finally able to compare the outcomes of the included data, it was necessary to extract or calculate the bending moment at the time point of implant fracture [Ncm], since the mostly reported fracture load values [N] do not respect the leverage (length of the lever arm) and are therefore, if not considering a rigorously standardized embedding procedure as described in ISO 14801, not comparable to each other. Of the included 19 investigations/datasets, three studies reported the bending moment individually calculated for each included implant [
13,
20,
22], whereas six studies [
15,
17,
21,
24,
25,
26] and the two included unpublished datasets fully respected ISO 14801 for embedding. Fully respecting this ISO implies the fixation of the endosseous part in a rigid clamping device or embedding in a material with a modulus of elasticity higher than 3 GPa. Moreover, the embedding/clamping level should respect a distance of 3.0 ± 0.5 mm apically from the nominal bone level, as specified in the manufacturer’s instructions for use. Furthermore, implant abutments need to be equipped with a non-anatomical hemisphere designed to realize a distance of
= 11.0 ± 0.5 mm from the center of the hemisphere to the embedding level (
Figure 11).
When loading such samples with an angle of
= 30° to the vertical, the lever arm (
) or bending moment (
) for this configuration can be calculated with the reported fracture load (
) by using Equation (1).
This results in
= 0.55 cm when embedding according to ISO 14801. For the aforementioned publications/datasets fully respecting ISO 14801 for embedding and reporting the fracture load values [N], the bending moment was therefore calculated by multiplying the fracture load with 0.55. Interestingly, some of the included investigations reported embedding according to ISO 14801, but solely adopted the embedding level (simulation of a bony recession of 3 mm), and sometimes the angulation (30°), but did not use a loading hemisphere, finally resulting in a lever arm different to 5.5 mm, as proposed by the ISO standard [
16,
19,
27,
28]. In most cases, anatomical crowns (maxillary premolars or incisors) made from ceramic materials were used instead of the hemisphere, finally resulting in altered lever arms and loading conditions. In the investigations of one group, the crown design and embedding procedure were described in detail (
,
and
were reported), allowing us to calculate
and
[
19,
27,
28]. To calculate the bending moment for the remaining studies, authors needed to provide the necessary data upon request or standardized photographs provided in the publications, or by the authors needed to allow the approximation of the lever arm by using an image analysis software (ImageJ, National Institutes of Health, Bethesda, MD, USA) [
12,
16,
18,
29,
30]. In order to be able to compare the outcome of preclinical studies evaluating the fracture resistance of dental implants, it is therefore recommended to either fully adopt an ISO standard for the embedding procedure or to provide the bending moment additionally to the fracture load. Considering different lever arms due to different embedding procedures for the implants included in this systematic review and meta-analysis, one needs to keep in mind that dynamic loading prior to static loading to fracture can result in altered fatigue, even if the applied load was the same.
The heterogeneity of the included samples comprising a mixture of market-available products (finally sterilized and incorporating a micro-roughened surface) [
15,
16,
22,
24,
26] but also prototype implants (e.g., with or without any surface post-processing) [
13,
19,
21,
25,
28,
30] represents a major limitation of the present systematic review and meta-analysis. However, it was shown that, for example, surface modifications like micro-roughening to enhance osseointegration or steam-sterilization can significantly compromise fracture strength and ageing kinetics [
31,
32].
Another shortcoming of this systematic review presents the fact that of the 19 included datasets, more than half (nine published and two unpublished studies) were at least partially authored by the collaborates of the current paper. This might be considered a reasonable risk of bias. However, the present review was conducted according to standardized guidelines, and the available literature was systematically screened on the basis of predefined search terms and inclusion criteria. Modifying the search strategy, outcome measure or inclusion criteria in consequence of unexpected or homogeneously authored findings would likewise present a source of bias.
Regarding the treatments, the included samples have been subjected to prior to loading, and six groups (representing different categories of loading conditions as indicated in
Section 2.7) have been evaluated for heterogeneity of the outcome. As a result, no heterogeneity of the bending moments for groups 1–6 was found (
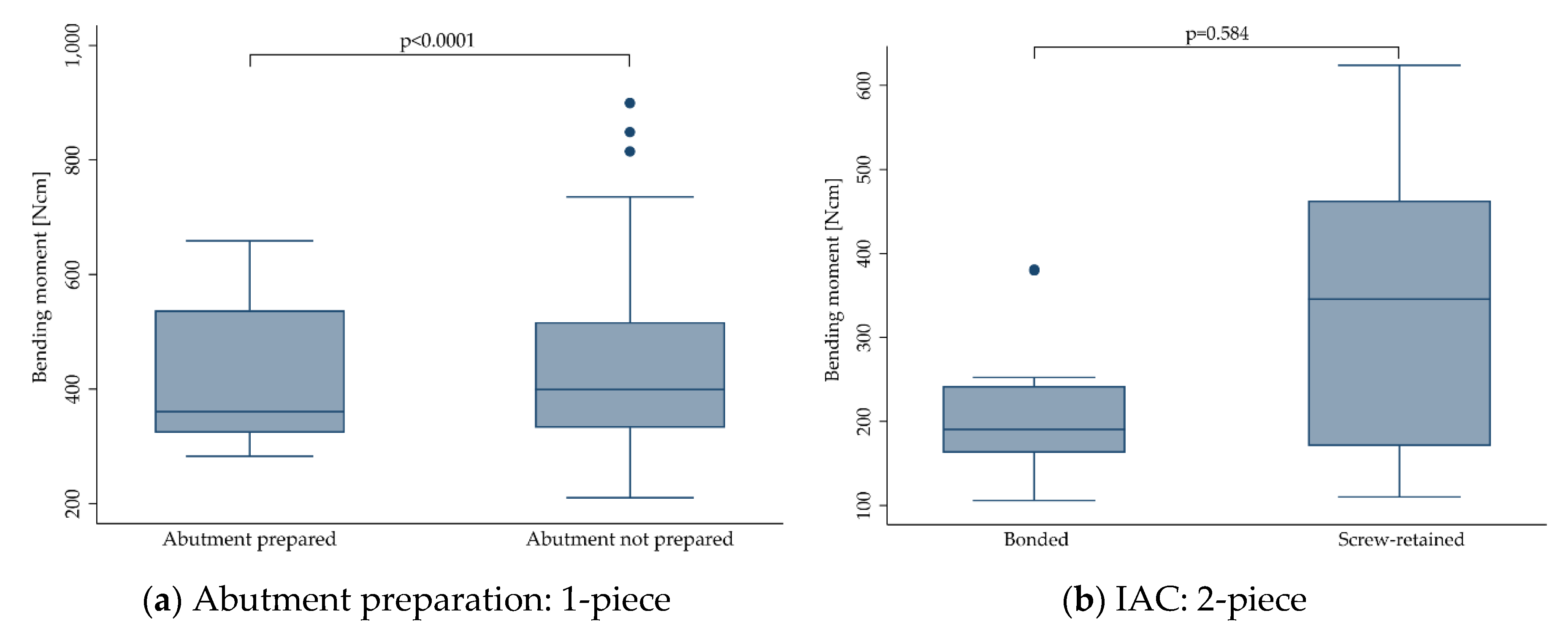
p = 0.612). This did not change when stratifying the implants according to their design (1-piece:
p = 0.951; 2-piece:
p = 0.056). Therefore, it was decided to pool the data of all groups for any further calculations, and yet still, one can hardly generalize the present findings and apply them to a specific zirconia implant system.
No statistically significant influence of hydrothermal aging on the fracture resistance of zirconia implants was calculated in the present review. It is important to note that aging or so-called low-temperature degradation (LTD) can, depending upon the sample quality and surface conditions, result in both increased [
21,
25] and decreased [
33] fracture load. This might be explained by the following: Assuming a zirconia sample surface with various process-related defects/impurities, the largest defects/impurities are thought to act as “locus minoris resistentiae”, and can thereby be considered representative for the fracture resistance of this sample. Increased fracture load of such zirconia samples after a hydrothermal aging procedure is thought to be attributed to a transformed layer at the sample surface, inducing a compressive stress on the surface, tending to close a potential advancing crack at such existing defects/impurities located on the surface. This phenomenon is liable to cause an increase in the strength of the material, and was described for the first time three decades ago [
34]. On the other side, at some point when the degradation process penetrates deeper into the material, the contribution from the aging may instead cause the strength of the same sample to be decreased, since once transformed to the monoclinic, zirconia grains cannot exhibit stress-induced phase transformation toughening anymore [
33]. As an example, in the included investigation of Monzavi and co-workers [
15] the effect of artificial aging on the mechanical resistance and micromechanical properties of commercially- and noncommercially-available zirconia dental implants was evaluated. In this study, the bending moment was significantly increased after aging for three of six groups, whereas two groups showed no influence of the aging procedure, and one group was negatively affected in terms of fracture resistance by the treatment [
15]. When pooling the outcomes of the included studies showing positive, negative or no effects of LTD on the fracture resistance of zirconia implants in one dataset, as happened in the present meta-analyses, no effect of hydrothermal aging on the bending moment at the time point of fracture was calculated (
p > 0.446). This, however, might be misleading, since several of the included studies indeed showed that aging can significantly affect the fracture resistance. However, due to the explanation given at the beginning of this paragraph, both in a negative or positive way. Therefore, missing significance, as calculated for pooled data in this review, should not be interpreted as an argument to refrain from aging tests of a zirconia implant system prior to market release. Therefore, pooling the data from different studies using the different conditions of thermal aging needs to be considered a limitation of the present review. It is discussed in the literature that the present amount of transformation to the monoclinic on the surface of as-delivered zirconia implants can be decisive for the ongoing fracture resistance after further hydrothermal aging procedures. In detail, implants showing no or very limited transformation to the monoclinic when released to the market (e.g., due to final temperature annealing [
35] or manufacturing by ceramic injection-molding [
21,
25]) were observed to be less fracture-resistant in the original as-delivered state, but significantly gained fracture resistance due to increasing compressive stress at the sample surface after transformation to the monoclinic occurred. In contrast, samples already revealing a transformed layer of several micrometers (e.g., due to subtractive manufacturing or post-processing steps like sandblasting in order to roughen the surface to enhance osseointegration [
26]) mostly do not benefit from further aging by means of an increased fracture resistance. Besides the amount of already transformed grains, implant surface topography showed to have a significant impact on aging susceptibility and its impact on fracture resistance [
32,
36]. As an example, implants structured with porous or alveolar surfaces were more likely to be negatively affected by aging procedures due to interconnected porosities in the surface layer, offering a path for the transformation to start at every surface accessible by water [
25]. Finally, a layer structured in this way can be transformed in a shorter period of time.
Of the implants included in the present investigation, 209 of 731 were restored with anatomically-shaped crowns [
16,
19,
20,
27,
28,
29,
30]. Most of these crowns were designed as maxillary central incisors, and were manufactured from: lithium disilicate [
20], veneered [
29] or monolithic [
19,
27,
28] zirconia, or porcelain fused to metal [
30]. Another included study restored the implants with maxillary first premolar restorations made from lithium disilicate [
16], whereas Joda and collaborates restored the implants with non-anatomical hemispheres likewise made from lithium disilicate [
24].
Most of the included studies not restoring the implants with anatomically-shaped crowns were conducted by adopting ISO 14801. According to this standard, the loading force shall be applied to the hemispherical loading surface, by a loading device with a plane surface normal to the loading direction of the machine, without additional horizontal loading forces. In contrast, especially incisor crowns present an inclined plane when loaded during the dynamic and finally static loading procedure, resulting in an increased shear force. Additionally, some investigations applied horizontal forces during the dynamic loading procedure (as it happens in the oral cavity), causing further fatigue of the sample [
20,
29,
30]. Therefore, not the restoration itself, but the altered investigational setup, resulting in increased shear forces and fatigue during static loading, and in some cases, precedent chewing simulation might be considered responsible for decreased fracture resistance. Nonetheless, this finding should be taken into account when drafting international standards in order to guarantee clinical safety, since the anatomical reconstruction of zirconia oral implants and horizontal shear forces during loading represent clinical reality. Regarding the nature or location of failure, 1-piece implants mostly fractured at the embedding level or slightly below, with crack initiation on the tensile side of the implant. As described in the included studies, it seems that the fracture mode was not affected by crown supply. In 2-piece implants, fracture modes were generally observed to be highly heterogeneous, depending on the mode of assembly and the materials used.
When it comes to clinical reality, the fracture resistance of a zirconia implant should finally withstand the maximum voluntary bite forces of the patients. Nonetheless, one cannot find the definition of any indication specific (e.g., for implants installed in anterior or posterior regions) minimum value for the fracture strength of a zirconia implant in ISO 14801. This, as an example, is provided in detail in ISO 6872 for ceramic materials used for reconstructions (e.g., crowns, bridges) in dentistry [
37]. Taking the highest bending moment measured in vivo (95 Ncm) with the help of strain gauge abutments into account [
38], and applying a safety buffer of 100%, one might consider a minimum fracture resistance of 200 Ncm sufficient to guarantee clinical safety. When applying this requirement to the included studies, mostly 2-piece prototype implants and implants with a reduced diameter (≤ 3.3 mm) did not meet this demand [
19,
24,
27,
28,
30].
Of the zirconia implants included in the present investigation, 577 were manufactured from Y-TZP and 154 from ATZ. Overall, implant stability was significantly affected by the material, in favor of ATZ (
p = 0.002). When evaluating 1- and 2-piece implants separately, however, only 1-piece implants made from ATZ performed better (
p = 0.001), whereas 2-piece implants performed the same, regardless of the material selection (
p = 0.282). This might be explained by the fact that 1-piece zirconia implants or even, as an example, 2-piece titanium implants are mostly made from one single material (in the case of titanium: the implant, the abutment and the abutment screw are mostly fabricated from titanium). In contrast, most of the available 2-piece zirconia systems represent a multi-material complex comprising at least two or sometimes even three different materials. In some cases, only the implant body is manufactured from zirconia, whereas the screw (e.g., titanium or PEEK) and/or abutment (e.g., glass-fiber or polyetherketoneketone/PEKK) might be manufactured from different materials revealing different aging or degradation behavior during treatments (hydrothermal aging, dynamic loading), precedent to final static loading to fracture. To date, sound correlations to approximate intraoral aging conditions in an accelerated way in the dental laboratory are mostly available for zirconia ceramics, but missing for screw and abutment materials prone to degradation in aqueous environments, like e.g., polyetherketones [
39,
40]. In consequence, no standardized testing procedures were proposed to the present date, sufficiently evaluating multi-material, 2-piece implants regarding their fracture resistance, and individually respecting the degradation behavior of several included components. Regrettably, the sample size and heterogeneity of the extracted data gathered from 2-piece implants included in the present review did not allow for the statistical evaluation of a potential impact of the screw or abutment material on the fracture resistance of 2-piece zirconia implants. In one of the included studies, the aim was to measure the abutment rotation and fracture load of 2-piece zirconia implants screwed with three different abutment screw materials [
16]. Implants and abutments of the included system were assembled with screws made from gold, titanium and PEEK.
As a result, no significant differences were found for these three materials, even if PEEK screws showed inferior results. When choosing PEEK as an abutment screw material, the incorporation of continuous carbon fibers proved to positively affect the maximum tensile strength of the screw [
41]. However, a strengthening effect on the entire implant-abutment complex in case of zirconia implants still needs to be evidenced. In one of the included studies [
26], a 2-piece ATZ implant system assembled with a carbon-fiber-reinforced abutment screw showed to be non-inferior compared to a market-established 2-piece titanium implant of a highly comparable design regarding its fracture resistance.