Effects of Milling Time, Zirconia Addition, and Storage Environment on the Radiopacity Performance of Mechanically Milled Bi2O3/ZrO2 Composite Powders
Abstract
1. Introduction
2. Experimental Procedures
3. Results and Discussion
3.1. Effect of Milling Time and Zirconia Addition
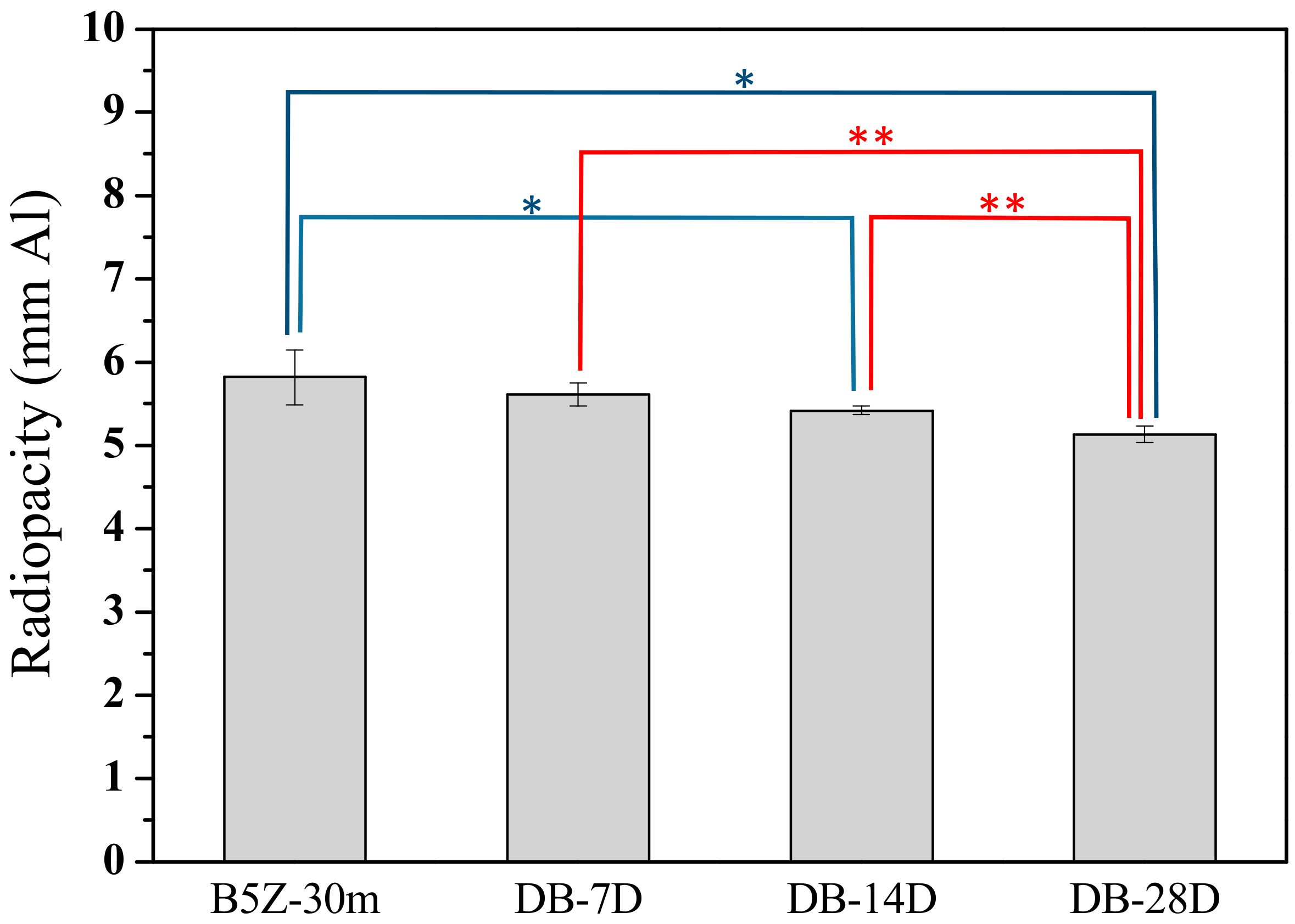
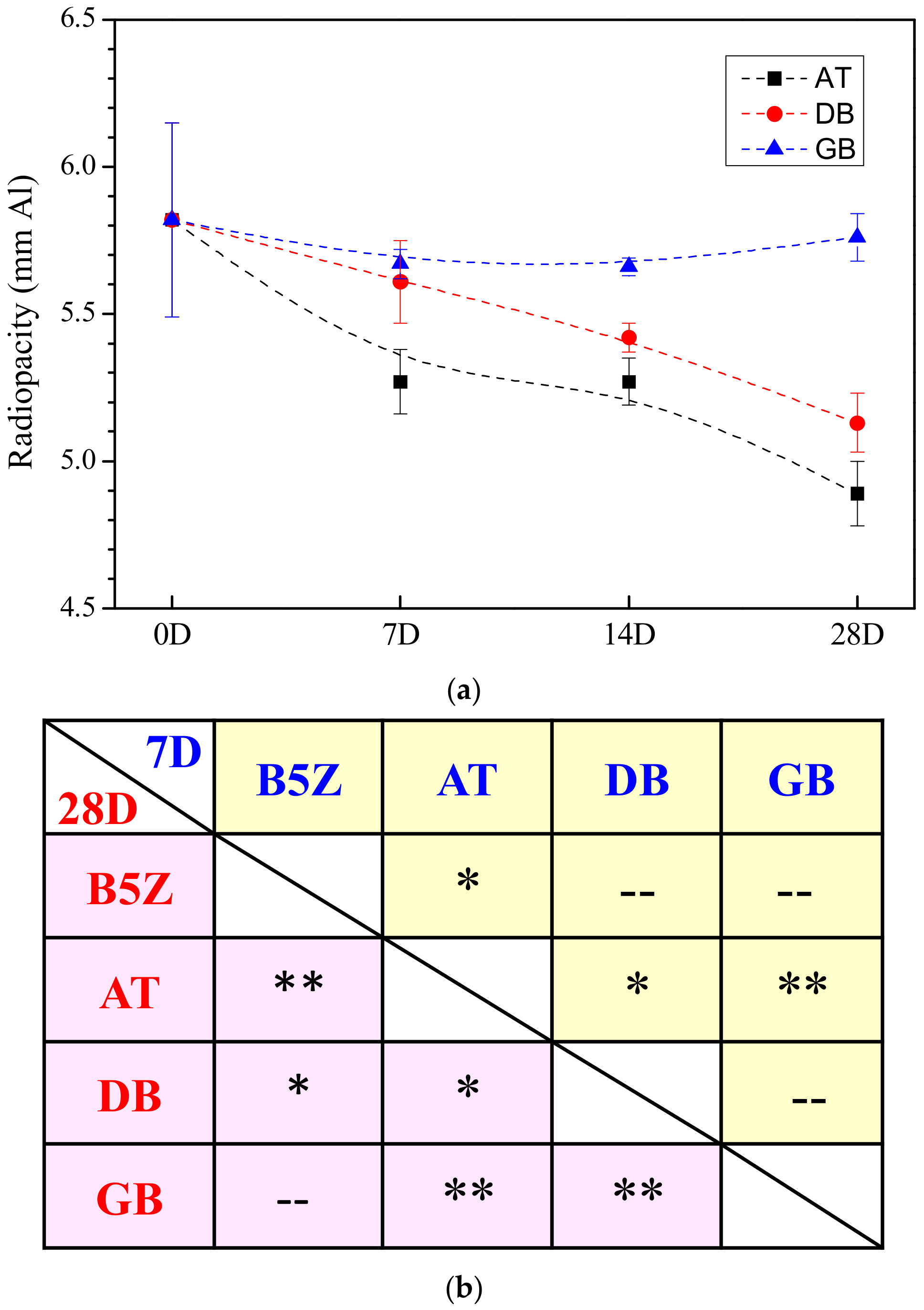
3.2. Effect of Storage Environment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, S.J.; Monsef, M.; Torabinejad, M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J. Endod. 1993, 19, 541–544. [Google Scholar] [CrossRef]
- Torabinejad, M.; Hong, C.; McDonald, F.; Pittford, T.; Ford, T.P. Physical and chemical properties of a new root-end filling material. J. Endod. 1995, 21, 349–353. [Google Scholar] [CrossRef]
- Camilleri, J.; Montesin, F.E.; Brady, K.; Sweeney, R.; Curtis, R.V.; Ford, T.R.P. The constitution of mineral trioxide aggregate. Dent. Mater. 2005, 21, 297–303. [Google Scholar] [CrossRef] [PubMed]
- ISO 6876. Dental Root Canal Sealing Materials; ISO International Organization for Standardization: Geneva, Switzerland, 2001. [Google Scholar]
- Lee, J.C.; Um, S.H.; Rhee, S.H. Synthesis of a mineral trioxide aggregate by spray-pyrolysis. Ceram. Int. 2016, 42, 2263–2270. [Google Scholar] [CrossRef]
- Ji, D.Y.; Wu, H.D.; Hsieh, S.C.; Teng, N.C.; Chen, C.C.; Ke, E.S.; Lin, Y.C.; Lee, S.Y.; Yang, J.C. Effects of a Novel Hydration Accelerant on the Biological and Mechanical Properties of White Mineral Trioxide Aggregate. J. Endod. 2011, 37, 851–855. [Google Scholar] [CrossRef]
- Camilleri, J.; Sorrentino, F.; Damidot, D. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dent. Mater. 2013, 29, 580–593. [Google Scholar] [CrossRef]
- Khalil, I.; Naaman, A.; Camilleri, J. Investigation of a novel mechanically mixed mineral trioxide aggregate (MM-MTATM). Int. Endod. J. 2015, 48, 757–767. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, D.; Cho, S.; Jang, J.H.; Kim, S.G.; Kim, S.Y. Improvement of the Bonding Properties of Mineral Trioxide Aggregate by Elastin-Like Polypeptide Supplementation. Scanning 2019, 2019, 34843968. [Google Scholar] [CrossRef]
- Nekoofar, M.H.; Aseeley, Z.; Dummer, P.M.H. The effect of various mixing techniques on the surface microhardness of mineral trioxide aggregate. Int. Endod. J. 2010, 43, 312–320. [Google Scholar] [CrossRef]
- Shahi, S.; Rahimi, S.; Yavari, H.R.; Samiei, M.; Janani, M.; Bahari, M.; Abdolrahimi, M.; Pakdel, F.; Aghbali, A. Effects of Various Mixing Techniques on Push-out Bond Strengths of White Mineral Trioxide Aggregate. J. Endod. 2012, 38, 501–504. [Google Scholar] [CrossRef]
- Basturk, F.B.; Nekoofar, M.H.; Gunday, M.; Dummer, P.M.H. X-ray Diffraction Diffraction Analysis of MTA Mixed and Placed with Various Techniques. Clin. Oral Investig. 2018, 22, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Cutajar, A.; Mallia, B.; Abela, S.; Camilleri, J. Replacement of radiopacifier in mineral trioxide aggregate; characterization and determination of physical properties. Dent. Mater. 2011, 27, 879–891. [Google Scholar] [CrossRef]
- Li, Q.; Coleman, N.J. Impact of Bi2O3 and ZrO2 Radiopacifiers on the Early Hydration and C-S-H Gel Structure of White Portland Cement. J. Funct. Biomater. 2019, 10, 46. [Google Scholar] [CrossRef]
- Giudice, G.L.; Giudice, R.L.; Matarese, G.; Isola, G.; Cicciù, M.; Terranova, A.; Palaia, G.; Romeo, U. Valutazione dei sistemi di ingrandimento in odontoiatria conservativa e restaurativa. Studio in vitro. Dent. Cadmos 2015, 83, 296–305. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zhang, M.M.; Wang, J.; Jiang, L.; Liang, Y.H. Outcomes of Endodontic Microsurgery Using a Microscope and Mineral Trioxide Aggregate: A Prospective Cohort Study. J. Endod. 2017, 43, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J. Mineral trioxide aggregate: Present and future developments. Endod. Top. 2015, 32, 31–46. [Google Scholar] [CrossRef]
- Ha, W.N.; Nicholson, T.; Kahler, B.; Walsh, L.J. Mineral Trioxide Aggregate—A Review of Properties and Testing Methodologies. Materials 2017, 10, 1261. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part I: Vital pulp therapy. Int. Endod. J. 2018, 51, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Parirokh, M.; Dummer, P. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part II: Other clinical applications and complications. Int. Endod. J. 2018, 51, 284–317. [Google Scholar] [CrossRef]
- Hosoya, N.; Takigawa, T.; Horie, T.; Maeda, H.; Yamamoto, Y.; Momoi, Y.; Yamamoto, K.; Okiji, T. A review of the literature on the efficacy of mineral trioxide aggregate in conservative dentistry. Dent. Mater. J. 2019, 38, 693–700. [Google Scholar] [CrossRef]
- Cavenago, B.C.; Pereira, T.C.; Duarte, M.A.; Ordinola-Zapata, R.; Marciano, M.A.; Bramante, C.M.; Bernardineli, N. Influence of powder-to-water ratio on radiopacity, setting time, pH, calcium ion release and a micro-CT volumetric solubility of white mineral trioxide aggregate. Int. Endod. J. 2014, 47, 120–126. [Google Scholar] [CrossRef]
- Coomaraswamy, K.S.; Lumley, P.J.; Hofmann, M.P. Effect of Bismuth Oxide Radioopacifier Content on the Material Properties of an Endodontic Portland Cement–based (MTA-like) System. J. Endod. 2007, 33, 295–298. [Google Scholar] [CrossRef]
- Camilleri, J. Staining Potential of Neo MTA Plus, MTA Plus, and Biodentine Used for Pulpotomy Procedures. J. Endod. 2015, 41, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Parirokh, M.; Eghbal, M.J.; Brink, F. Chemical differences between white and gray mineral trioxide aggregate. J. Endod. 2005, 31, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Shuk, P.; Wiemhofer, H.D.; Guth, U.; Gopel, W.; Greenblatt, M. Oxide ion conducting solid electrolytes based on Bi2O3. Solid State Ionics 1996, 89, 179–196. [Google Scholar] [CrossRef]
- Fruth, V.; Ianculescu, A.; Berger, D.; Preda, S.; Voicu, G.; Tenea, E.; Popa, M. Synthesis, structure and properties of doped Bi2O3. J. Eur. Ceram. Soc. 2006, 26, 3011–3016. [Google Scholar] [CrossRef]
- Aurivillius, B.; Sillén, L.G.; Aubivillius, B. Polymorphy of Bismuth Trioxide. Nature 1945, 155, 305–306. [Google Scholar] [CrossRef]
- Harwig, H.A.; Gerards, A.G. The polymorphism of bismuth sesquioxide. Thermochim. Acta 1979, 28, 121–131. [Google Scholar] [CrossRef]
- Zhou, W. Structural chemistry and physical properties of some ternary oxides in the Bi2O3–Ta2O5 system. J. Solid State Chem. 1992, 101, 1–17. [Google Scholar] [CrossRef]
- Jardiel, T.; Calatayud, D.G.; Rodriguez, M.; Fernandez-Hevia, D.; Caballero, A.C. Synthesis of metastable Bi6Ti5WO22 phase by the mechanochemical method. Mater. Lett. 2013, 94, 58–60. [Google Scholar] [CrossRef]
- Asryan, N.A.; Kol’Tsova, T.N.; Alikhanyan, A.S.; Nipan, G.D. Thermodynamics and Phase Diagram of the Bi2O3–SnO2 System. Inorg. Mater. 2002, 38, 1141–1147. [Google Scholar] [CrossRef]
- Zdujic, M.; Poleti, D.; Jovalekic, C.; Karanović, L. Mechanochemical synthesis and electrical conductivity of nanocrystalline δ-Bi2O3 stabilized by HfO2 and ZrO2. J. Serbian Chem. Soc. 2009, 74, 1401–1411. [Google Scholar] [CrossRef]
- Lin, S.E.; Wei, W.C.J. Long-term degradation of Ta2O5-doped Bi2O3 systems. J. Eur. Ceram. Soc. 2011, 31, 3081–3086. [Google Scholar] [CrossRef]
- Saito, T.; Miida, R. Crystal Structure and Ionic Conductivity in Bi2O3-Rich Bi2O3–Ta2O5 Sintered Oxides. Jpn. J. Appl. Phys. 1999, 38, 4838–4842. [Google Scholar] [CrossRef]
- Ling, C.D.; Withers, R.L.; Schmid, S.; Thompson, J.G. A Review of Bismuth-Rich Binary Oxides in the Systems Bi2O3–Nb2O5, Bi2O3–Ta2O5, Bi2O3–MoO3, and Bi2O3–WO3. J. Solid State Chem. 1998, 137, 42–61. [Google Scholar] [CrossRef]
- Chen, M.S.; Chen, S.H.; Lai, F.C.; Chen, C.Y.; Hsieh, M.Y.; Chang, W.J.; Yang, J.C.; Lin, C.K. Sintering Temperature-Dependence on Radiopacity of Bi(2-x)ZrxO(3+x/2) Powders Prepared by Sol-Gel Process. Materials 2018, 11, 1685. [Google Scholar] [CrossRef]
- Chen, M.S.; Yang, J.C.; Lai, F.C.; Chen, C.Y.; Hsieh, M.Y.; Fang, A.; Chen, S.H.; Lin, C.K. Radiopacity performances of precipitated ZrO2-doped Bi2O3 powders and the influences of dopant concentrations and sintering temperatures. Ceram. Int. 2017, 43, 14008–14014. [Google Scholar] [CrossRef]
- Tsuzuki, T.; McCormick, P.G. Nanopowders synthesized by mechanochemical processing. J. Mater. Sci. 2004, 39, 5143–5146. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, X.; Xiao, Q.; Luo, W.; Zhang, H.; Wang, C.; Zhang, T.; Li, S.; Kong, L.B. Bismuth lanthanum titanate ceramics from amorphous precursors activated by using mechanochemical treatment. Ceram. Int. 2018, 44, 13106–13112. [Google Scholar] [CrossRef]
- Lin, H.N.; Chen, M.S.; Chang, Y.H.; Lee, P.Y.; Lin, C.K. Effect of Oxygen Concentration and Tantalum Addition on the Formation of High Temperature Bismuth Oxide Phase by Mechanochemical Reaction. Materials 2019, 12, 1947. [Google Scholar] [CrossRef]
- Benjamin, J.S. Mechanical Alloying. Sci. Am. 1976, 234, 40–49. [Google Scholar] [CrossRef]
- Murty, B.S.; Ranganathan, S. Novel materials synthesis by mechanical alloying/milling. Int. Mater. Rev. 1998, 43, 101–141. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]


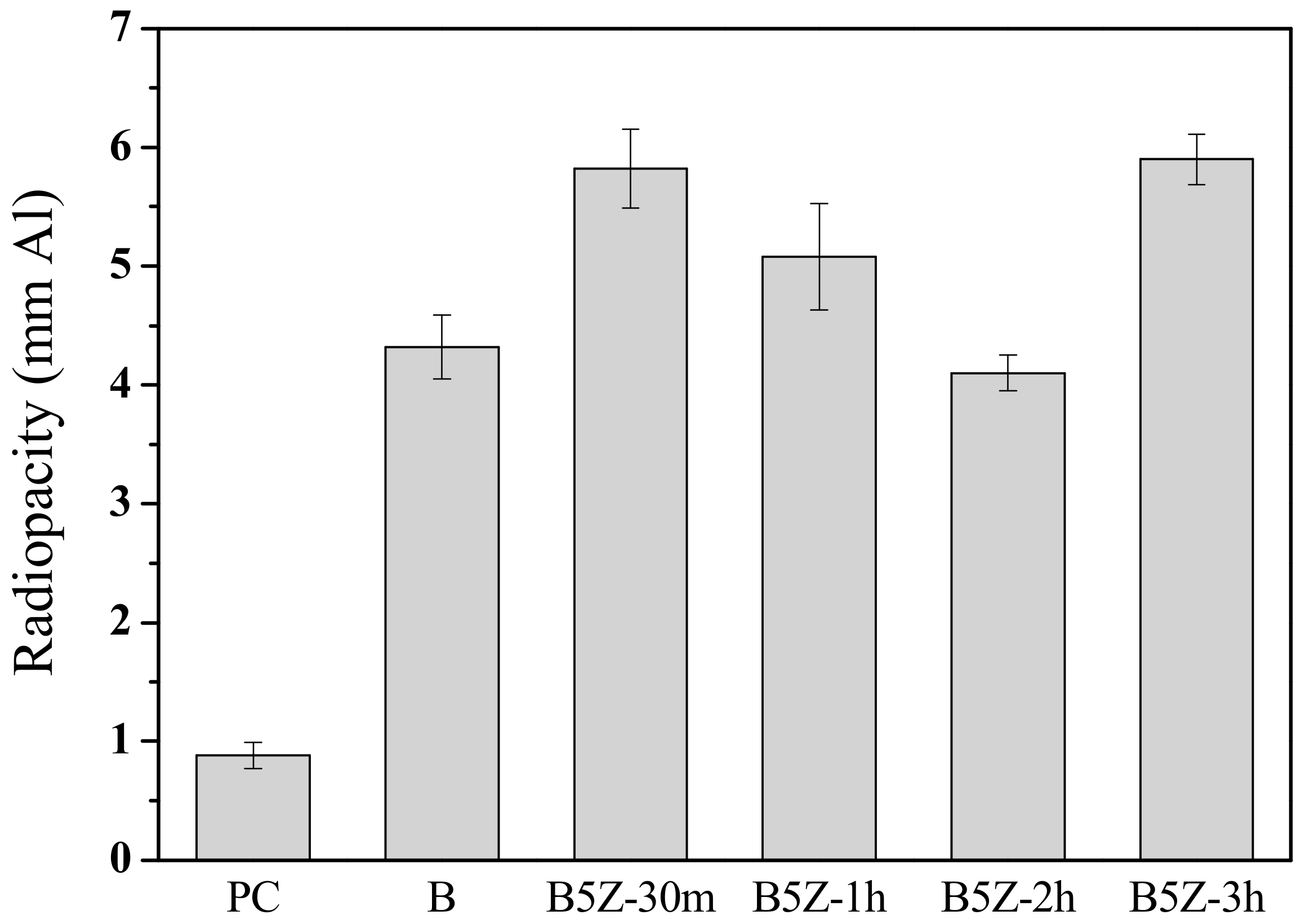
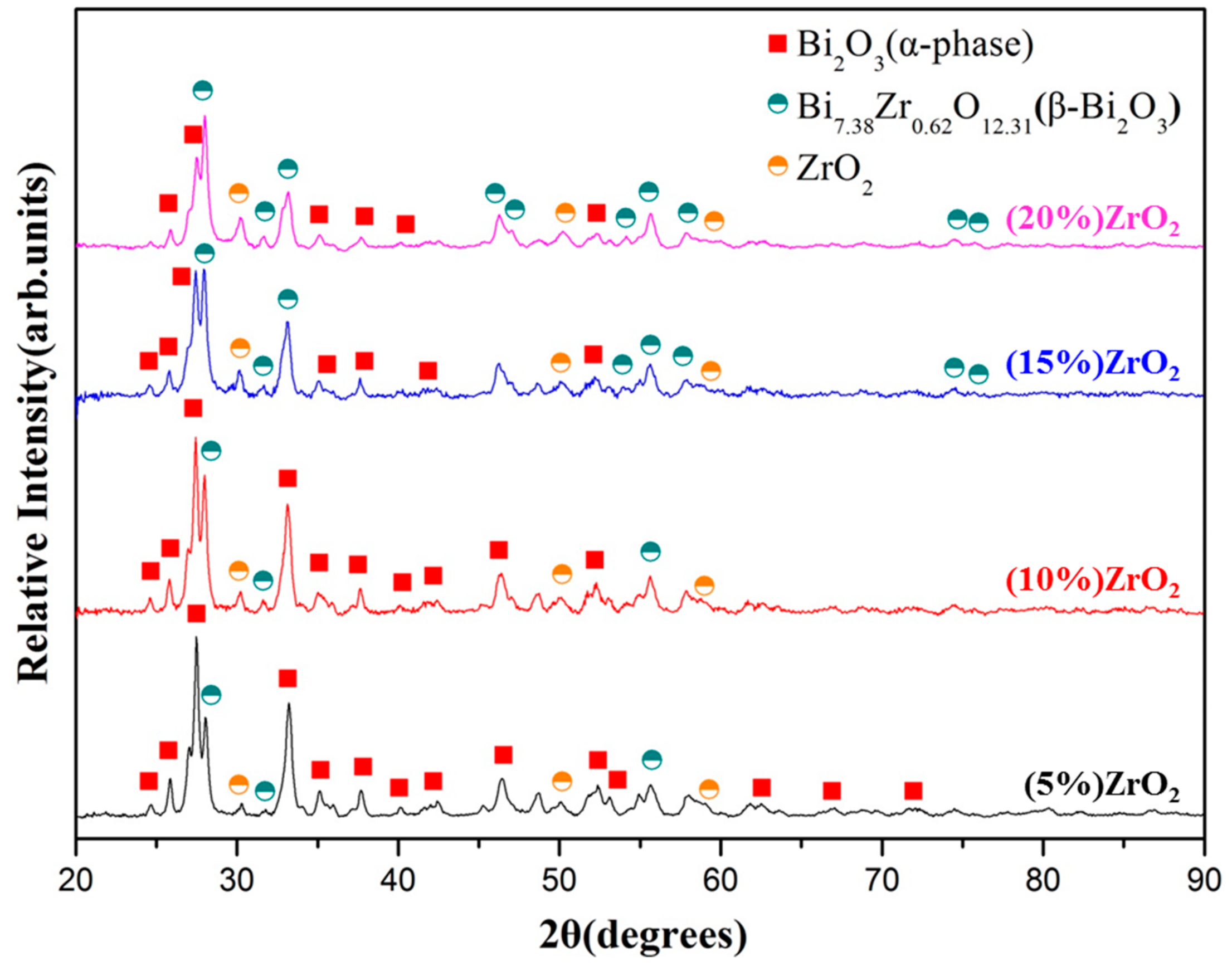



| Milling Condition | Milling Time | Crystalline Phases * |
|---|---|---|
| Inside Ar-filled glove box | 5 min | α-Bi2O3 (67.2%) + δ-Bi7.38Zr0.62O2.31 (28.7%) + ZrO2 (4.1%) |
| 15 min | α-Bi2O3 (60.4%) + δ-Bi7.38Zr0.62O2.31 (35.9%) + ZrO2 (3.7%) | |
| 30 min | α-Bi2O3 (52.1%) + δ-Bi7.38Zr0.62O2.31 (44.2%) + ZrO2 (3.7%) | |
| 1 h | α-Bi2O3 (30.7%) + δ-Bi7.38Zr0.62O2.31 (67.7%) + ZrO2 (1.6%) | |
| 2 h | α-Bi2O3 (20.2%) + δ-Bi7.38Zr0.62O2.31 (78.7%) + ZrO2 (1.1%) | |
| 3 h | α-Bi2O3 (16.0%) + δ-Bi7.38Zr0.62O2.31 (82.9%) + ZrO2 (1.1%) |
| Sample Condition | Storage Period # | Radiopacity * |
|---|---|---|
| B5Z-30m | 0D | 5.82 ± 0.33 |
| AT | 7D | 5.27 ± 0.11 |
| 14D | 5.27 ± 0.08 | |
| 28D | 4.89 ± 0.10 | |
| DB | 7D | 5.61 ± 0.14 |
| 14D | 5.42 ± 0.05 | |
| 28D | 5.13 ± 0.10 | |
| GB | 7D | 5.67 ± 0.05 |
| 14D | 5.66 ± 0.03 | |
| 28D | 5.76 ± 0.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.-S.; Lin, H.-N.; Cheng, Y.-C.; Fang, A.; Chen, C.-Y.; Lee, P.-Y.; Lin, C.-K. Effects of Milling Time, Zirconia Addition, and Storage Environment on the Radiopacity Performance of Mechanically Milled Bi2O3/ZrO2 Composite Powders. Materials 2020, 13, 563. https://doi.org/10.3390/ma13030563
Chen M-S, Lin H-N, Cheng Y-C, Fang A, Chen C-Y, Lee P-Y, Lin C-K. Effects of Milling Time, Zirconia Addition, and Storage Environment on the Radiopacity Performance of Mechanically Milled Bi2O3/ZrO2 Composite Powders. Materials. 2020; 13(3):563. https://doi.org/10.3390/ma13030563
Chicago/Turabian StyleChen, May-Show, Hsiu-Na Lin, Yu-Chun Cheng, Alex Fang, Chin-Yi Chen, Pee-Yew Lee, and Chung-Kwei Lin. 2020. "Effects of Milling Time, Zirconia Addition, and Storage Environment on the Radiopacity Performance of Mechanically Milled Bi2O3/ZrO2 Composite Powders" Materials 13, no. 3: 563. https://doi.org/10.3390/ma13030563
APA StyleChen, M.-S., Lin, H.-N., Cheng, Y.-C., Fang, A., Chen, C.-Y., Lee, P.-Y., & Lin, C.-K. (2020). Effects of Milling Time, Zirconia Addition, and Storage Environment on the Radiopacity Performance of Mechanically Milled Bi2O3/ZrO2 Composite Powders. Materials, 13(3), 563. https://doi.org/10.3390/ma13030563








