Novel Microwave-Assisted Method of Y2Ti2O7 Powder Synthesis
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Pyrochlore Obtained in Calcining Furnace
3.2. Microwave-Assisted Pyrochlore Synthesis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lepicka, M.; Gradzka-Dahlke, M.; Pieniak, D.; Pasierbiewicz, K.; Krynska, K.; Niewczas, A. Tribological performance of titanium nitride coatings: A comparative study on TiN-coated stainless steel and titanium alloy. Wear 2019, 422, 68–80. [Google Scholar] [CrossRef]
- Szala, M.; Hejwowski, T. Cavitation erosion resistance and wear mechanism model of flame-sprayed Al2O3-40%TiO2/NiMoAl cermet coatings. Coatings 2018, 8, 254. [Google Scholar] [CrossRef]
- Stańczyk, M.; Figlus, T. The effect of selected parameters of vibro-abrasive processing on the surface quality of products made of 6082 aluminium alloy. Materials 2019, 12, 4117. [Google Scholar] [CrossRef]
- Winiarski, G.; Gontarz, A.; Samołyk, G. Flange formation in aluminium alloy EN AW 6060 tubes by radial extrusion with the use of a limit ring. Arch. Civ. Mech. Eng. 2019, 19, 1020–1028. [Google Scholar] [CrossRef]
- Cortés, P.; Cantwell, W.J. The fracture properties of a fibre–metal laminate based on magnesium alloy. Compos. Part B 2006, 37, 163–170. [Google Scholar] [CrossRef]
- Dziubińska, A.; Gontarz, A.; Zagórski, I. Qualitative research on AZ31 magnesium alloy aircraft brackets with a triangular rib produced by a new forging method. Aircr. Eng. Aerosp. Technol. 2018, 90, 482–488. [Google Scholar] [CrossRef]
- Amacher, R.; Cugnoni, J.; Botsis, J.; Sorensen, L.; Smith, W.; Dransfeld, C. Thin ply composites: Experimental characterization and modeling of size-effects. Compos. Sci. Technol. 2014, 101, 121–132. [Google Scholar] [CrossRef]
- Dadej, K.; Bienias, J.; Valvo, P.S. Experimental testing and analytical modeling of asymmetric end-notched flexure tests on glass-fiber metal laminates. Metals 2020, 10, 56. [Google Scholar] [CrossRef]
- Figlus, T.; Koziol, M.; Kuczynski, L. The effect of selected operational factors on the vibroactivity of upper gearbox housings made of composite materials. Sensors 2019, 19, 4240. [Google Scholar] [CrossRef] [PubMed]
- Gardynski, L.; Caban, J.; Barta, D. Research of composite materials used in the construction of vehicle bodywork. Adv. Sci. Technol. Res. J. 2018, 12, 181–187. [Google Scholar] [CrossRef]
- Żebrowski, R.; Walczak, M.; Korga, A.; Iwan, M.; Szala, M. Effect of shot peening on the mechanical properties and cytotoxicity behaviour of titanium implants produced by 3D printing technology. J. Healthc. Eng. 2019, 2019, 8169538. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Awais, M.; Jabeen, S.; Farooq, M. Recent Trends in Preparation and Applications of Biodegradable Polymer Composites. J. Renew. Mater. 2020, 8, 1305–1326. [Google Scholar] [CrossRef]
- Zdanowska, P.; Florczak, I.; Sloma, J.; Tucki, K.; Orynycz, O.; Wasiak, A.; Swic, A. An evaluation of the quality and microstructure of biodegradable composites as contribution towards better management of food industry wastes. Sustainability 2019, 11, 1504. [Google Scholar] [CrossRef]
- Blatnicky, M.; Dizo, J.; Gerlici, J.; Saga, M.; Lack, T.; Kuba, E. Design of a robotic manipulator for handling products of automotive industry. Int. J. Adv. Robot. Syst. 2020, 17, 1. [Google Scholar] [CrossRef]
- Dizo, J.; Blatnicky, M.; Saga, M.; Harusinec, J.; Gerlici, J.; Legutko, S. Development of a New System for Attaching the Wheels of the Front Axle in the Cross-Country Vehicle. Symmetry 2020, 12, 1156. [Google Scholar] [CrossRef]
- Dizo, J.; Harusinec, J.; Blatnicky, M. Structural Analysis of a Modified Freight Wagon Bogie Frame. Matec Web Conf. 2019, 134, 00010. [Google Scholar] [CrossRef]
- Dziubińska, A.; Gontarz, A.; Dziubiński, M.; Barszcz, M. The forming of magnesium alloy forgings for aircraft and automotive applications. Adv. Sci. Technol. Res. J. 2016, 10, 158–168. [Google Scholar] [CrossRef]
- Pavelcik, V.; Barta, D.; Sapieta, M. Proposal of a Mechanism for Car Seat Movement. Adv. Sci. Technol. Res. J. 2020, 14, 50–57. [Google Scholar] [CrossRef]
- Gronostajski, Z.; Pater, Z.; Madej, L.; Gontarz, A.; Lisiecki, L.; Łukaszek-Sołek, A.; Łuksza, J.; Mróz, S.; Muskalski, Z.; Muzykiewicz, W.; et al. Recent development trends in metal forming. Arch. Civ. Mech. Eng. 2019, 19, 898–941. [Google Scholar] [CrossRef]
- Caesarendra, W.; Pratama, M.; Kosasih, B.; Tjahjowidodo, T.; Głowacz, A. Parsimonious Network Based on a Fuzzy Inference System (PANFIS) for Time Series Feature Prediction of Low Speed Slew Bearing Prognosis. Appl. Sci. 2018, 8, 2656. [Google Scholar] [CrossRef]
- Głowacz, A. Fault Detection of Electric Impact Drills and Coffee Grinders Using Acoustic Signals. Sensors 2019, 19, 269. [Google Scholar] [CrossRef] [PubMed]
- Plechawska-Wojcik, M.; Wolszczak, P.; Cechowicz, R.; Lygas, K. Construction of Neural Nets in Brain-Computer Interface for Robot Arm Steering. In Proceedings of the 9th International Conference on Human System Interactions (HSI), Portsmouth, UK, 6–8 July 2016; pp. 348–354. [Google Scholar]
- Tropp, M.; Tomasikova, M.; Bastovansky, R.; Krzywonos, L.; Brumercik, F.; Krzysiak, Z. Transient thermal simulation of working components of mechatronic system for deep drawing of molybdenum sheets. In Proceedings of the 58th International Conference of Machine Design Departments (ICMD 2017), Prague, Czech Republic, 6–8 September 2017; pp. 408–413. [Google Scholar]
- Aimar, A.; Palermo, A.; Innocenti, B. The role of 3D printing in medical applications: A state of the art. J. Healthc. Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef] [PubMed]
- Karpinski, R.; Jaworski, L.; Zubrzycki, J. The design and structural analysis of the endoprosthesis of the shoulder joint. Itm Web Conf. 2017, 15, 07015. [Google Scholar] [CrossRef]
- Walczak, M.; Caban, J.; Galuszko, K. Studies of remelted nickel-based alloy in a simulated chemical environment. Przem. Chem 2017, 96, 1329–1332. (In Polish) [Google Scholar] [CrossRef]
- Ambrozkiewicz, B.; Litak, G.; Wolszczak, P. Modelling of Electromagnetic Energy Harvester with Rotational Pendulum Using Mechanical Vibrations to Scavenge Electrical Energy. Appl. Sci. 2020, 10, 671. [Google Scholar] [CrossRef]
- Lei, H.; Wen, Q.; Yu, F.; Li, D. AlN film based piezoelectric large-aperture MEMS scanning micromirror integrated with angle sensors. J. Micromech. Microeng. 2018, 28, 115012. [Google Scholar] [CrossRef]
- Mikielewicz, D.; Kosowski, K.; Tucki, K.; Piwowarski, M.; Stepien, R.; Orynycz, O.; Wlodarski, W. Influence of Different Biofuels on the Efficiency of Gas Turbine Cycles for Prosumer and Distributed Energy Power Plants. Energies 2019, 12, 3173. [Google Scholar] [CrossRef]
- Orynycz, O.; Tucki, K. Technology Management Leading to a Smart System Solution Assuring a Decrease of Energy Consumption in Recreational Facilities. Energies 2020, 13, 3425. [Google Scholar] [CrossRef]
- Syta, A.; Litak, G.; Friswell, M.I.; Adhikari, S. Multiple solutions and corresponding power output of a nonlinear bistable piezoelectric energy harvester. Eur. Phys. J. B 2016, 89, 99. [Google Scholar] [CrossRef]
- Wang, J.L.; Gu, S.H.; Zhang, C.Y.; Hu, G.B.; Chen, G.; Yang, K.; Li, H.; Lai, Y.Y.; Litak, G.; Yurchenko, D. Hybrid wind energy scavenging by coupling vortex-induced vibrations and galloping. Energy Convers. Manag. 2020, 213, 112835. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; Ning, H.; Williams, C.A.; London, A.J.; Dawson, K.; Hong, Z.; Gorley, M.J.; Grovenor, C.R.M.; Tatlock, G.J.; et al. Processing and microstructure characterisation of oxide dispersion strengthened Fe–14Cr–0.4Ti–0.25Y2O3 ferritic steels fabricated by spark plasma sintering. J. Nucl. Mater. 2015, 464, 61–68. [Google Scholar] [CrossRef]
- Brocq, M.; Radiguet, B.; Poissonnet, S.; Cuvilly, F.; Pareige, P.; Legendre, F. Nanoscale characterization and formation mechanism of nanoclusters in an ODS steel elaborated by reactive- inspired ball-milling and annealing. J. Nucl. Mater. 2011, 409, 80–85. [Google Scholar] [CrossRef]
- He, L.F.; Shirahata, J.; Nakayama, T.; Suzuki, T.; Suematsu, H.; Ihara, I.; Bao, Y.W.; Komatsu, T.; Niihara, K. Mechanical properties of Y2Ti2O7. Scr. Mater. 2011, 64, 548–551. [Google Scholar] [CrossRef]
- Lu, C.; Lu, Z.; Wang, X.; Xie, R.; Li, Z.; Higgins, M.; Liu, C.; Gao, F.; Wang, L. Enhanced Radiation-tolerant Oxide Dispersion Strengthened Steel and its Microstructure Evolution under Helium-implantation and Heavy-ion Irradiation. Sci. Rep. 2017, 7, 40343. [Google Scholar] [CrossRef] [PubMed]
- Dawson, K.; Tatlock, G.J. Characterisation of nanosized oxides in ODM401 oxide dispersion strengthened steel. J. Nucl. Mater. 2014, 444, 252–260. [Google Scholar] [CrossRef]
- Shinaoka, H.; Motome, Y.; Miyake, T.; Ishibashi, S.; Werner, P. First-principles studies of spin- orbital physics in pyrochlore oxides. J. Phys. Condens. Matter 2014, 31, 323001. [Google Scholar] [CrossRef]
- Saenko, S.Y.; Shkuropatenko, V.A.; Tarasov, R.V.; Surkov, A.E.; Lobach, K.V.; Ulybkina, E.A.; Litvinenko, L.M.; Mironova, A.G. Obtaining corrosion-resistant zirconate pyrochlore as a material for immobilizing actinides. Zbornik Nauk. Pr. Pat Ukrndi Vognetriviv Im. A. S. Berezhny 2016, 116, 133–144. (In Russian) [Google Scholar]
- Norby, T. Fast oxygen ion conductors—From doped to ordered systems. J. Mater. Chem. 2001, 11, 11–18. [Google Scholar] [CrossRef]
- Shi, F.W.; Meng, X.J.; Wang, G.S.; Lin, T.; Ma, J.H.; Li, Y.W.; Chu, J.H. The third-order optical nonlinearity of the pyrochlore phase 0.7Pb(Mg1/3Nb2/3)O-3-0.3PbTiO(3) thin film on quartz. Phys. B Condens. Matter 2005, 370, 277–280. [Google Scholar] [CrossRef]
- Lumpkin, G.R.; Pruneda, M.; Rios, S.; Smith, K.L.; Trachenko, K.; Whittle, K.R.; Zaluzec, N.J. Nature of the chemical bond and prediction of radiation tolerance in pyrochlore and defect fluorite compounds. J. Solid State Chem. 2007, 180, 1512–1518. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Nakayama, T.; Suematsu, H.; Suzuki, T.; Takeda, M.; Niihara, K. Low thermal conductivity Y2Ti2O7 as a candidate material for thermal/environmental barrier coatings. Ceram. Int. 2016, 42, 11314–11323. [Google Scholar] [CrossRef]
- Ren, W.; Trolier-McKinstry, S.; Randall, C.A.; Shrout, T.R. Bismuth zinc niobate pyrochlore dielectric thin films for capacitive applications. J. Appl. Phys. 2001, 89, 767–774. [Google Scholar] [CrossRef]
- Wuensch, B.J.; Eberman, K.W.; Heremans, C.; Ku, E.M.; Onnerud, P.; Yeo, E.M.; Haile, S.M.; Stalick, J.K.; Jorgensen, J.D. Connection between oxygen-ion conductivity of pyrochlore fuel-cell materials and structural change with composition and temperature. Solid State Ion. 2000, 129, 111–133. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, G.; Jiang, D.; Wang, S. Recent development of A2B2O7 system transparent ceramics. J. Adv. Ceram. 2018, 7, 289–306. [Google Scholar] [CrossRef]
- Matteucci, F.; Cruciani, G.; Dondi, M.; Baldi, G.; Barzanti, A. Crystal structural and optical properties of Cr-doped Y2Ti2O7 and Y2Sn2O7 pyrochlores. Acta Mater. 2007, 55, 2229–2238. [Google Scholar] [CrossRef]
- Gong, W.; Li, D.; Chen, Z.; Zheng, F.; Liu, Y.; Du, Y.; Huang, B. Phase equilibria of the TiO2–Y2O3 system. Calphad 2009, 33, 624–627. [Google Scholar] [CrossRef]
- Mietła, N.; Gubernat, A. Synthesis and properties of inorganic pigments based on titanate pyrochlore compounds. Szkło i Ceramika 2013, 64, 13–17. (In Polish) [Google Scholar]
- Chen, Z.S.; Gong, W.P.; Chen, T.F.; Li, S.L. Synthesis and characterization of pyrochlore-type yttrium titanate nanoparticles by modified sol–gel method. Bull. Mater. Sci. 2011, 34, 429–434. [Google Scholar] [CrossRef]
- Mahapatra, A.; Subudhi, S.; Swain, S.; Sahu, R.; Negi, R.R.; Samanta, B.; Kumar, P. Electrical and Optical Properties of Yttrium Titanate Thin Films Synthesized by Sol-Gel Technique. Integr. Ferroelectr. 2019, 203, 43–51. [Google Scholar] [CrossRef]
- Gill, J.K.; Pandey, O.P.; Singh, K. Role of sintering temperature on thermal, electrical and structural properties of Y2Ti2O7 pyrochlores. Int. J. Hydrog. Energy 2011, 36, 14943–14947. [Google Scholar] [CrossRef]
- Singh, M.; Gill, J.K.; Kumar, S.; Singh, K. Preparation of Y2Ti2O7 pyrochlore using high-energy ball milling and their structural, thermal and conducting properties. Ionics 2012, 18, 479–486. [Google Scholar] [CrossRef]
- Milićević, B.; Marinović-Cincović, M.; Dramićanin, M.D. Non-isothermal crystallization kinetics of Y2Ti2O7. Powder Technol. 2017, 310, 67–73. [Google Scholar] [CrossRef]
- Chen, C.L.; Zeng, Y. Synthesis and characteristics of W–Ti alloy dispersed with Y2Ti2O7 oxides. Int. J. Refract. Met. Hard Mater. 2016, 56, 104–109. [Google Scholar] [CrossRef]
- Liu, T.; Wang, L.; Wang, C.; Shen, H.; Zhang, H. Feasibility of using Y2Ti2O7 nanoparticles to fabricate high strength oxide dispersion strengthened Fe–Cr–Al steels. Mater. Des. 2015, 88, 862–870. [Google Scholar] [CrossRef]
- Simondon, E.; Giroux, P.F.; Chaffron, L.; Fitch, A.; Castany, P.; Gloriant, T. Mechanical synthesis of nanostructured Y2Ti2O7 pyrochlore oxides. Solid State Sci. 2018, 85, 54–59. [Google Scholar] [CrossRef]
- Kakihana, M.; Milanova, M.M.; Arima, M.; Okubo, T.; Yashima, M.; Yoshimura, M. Polymerized Complex Route to Synthesis of Pure Y2Ti2O7 at 750 °C Using Yttrium-Titanium Mixed-Metal Citric Acid Complex. J. Am. Ceram. Soc. 1996, 79, 1673–1676. [Google Scholar] [CrossRef]
- Gadipelly, T.; Dasgupta, A.; Ghosh, C.; Krupa, V.; Sornadurai, D.; Sahu, B.K.; Dhara, S. Synthesis and structural characterisation of Y2Ti2O7 using microwave hydrothermal route. J. Alloy. Compd. 2020, 814, 152273. [Google Scholar] [CrossRef]
- Karthick, G.; Karati, A.; Murty, B.S. Low temperature synthesis of nanocrystalline Y2Ti2O7, Y2Zr2O7, Y2Hf2O7 with exceptional hardness by reverse co-precipitation technique. J. Alloy. Compd. 2020, 837, 155491. [Google Scholar] [CrossRef]
- International standard GOST 23619-79. Refractory Heat-Insulating Mullite-Silica Glass-Fibrous Materials and Products. Specifications; EASC: Moscow, Russia, 1997. (In Russian)
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Cryst. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Arévalo, A.M.Z.; Castellar, G.C.; Lozada, W.V.; Ariza, I.P.; Ríos, J.S.V.; Bautista, M.M.C. Conceptual approach to thermal analysis and its main applications. Prospectiva 2017, 15, 117–125. [Google Scholar] [CrossRef]
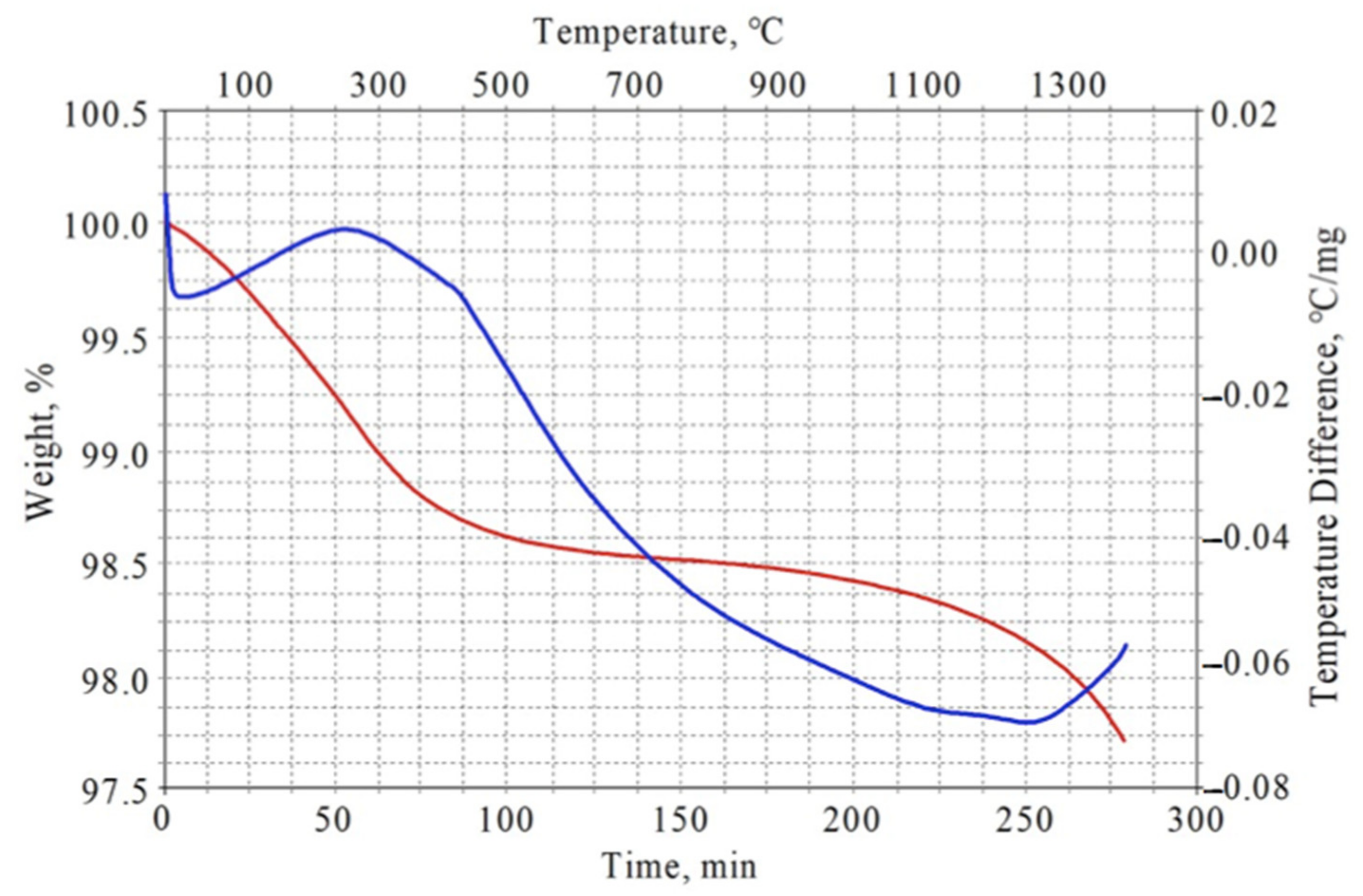
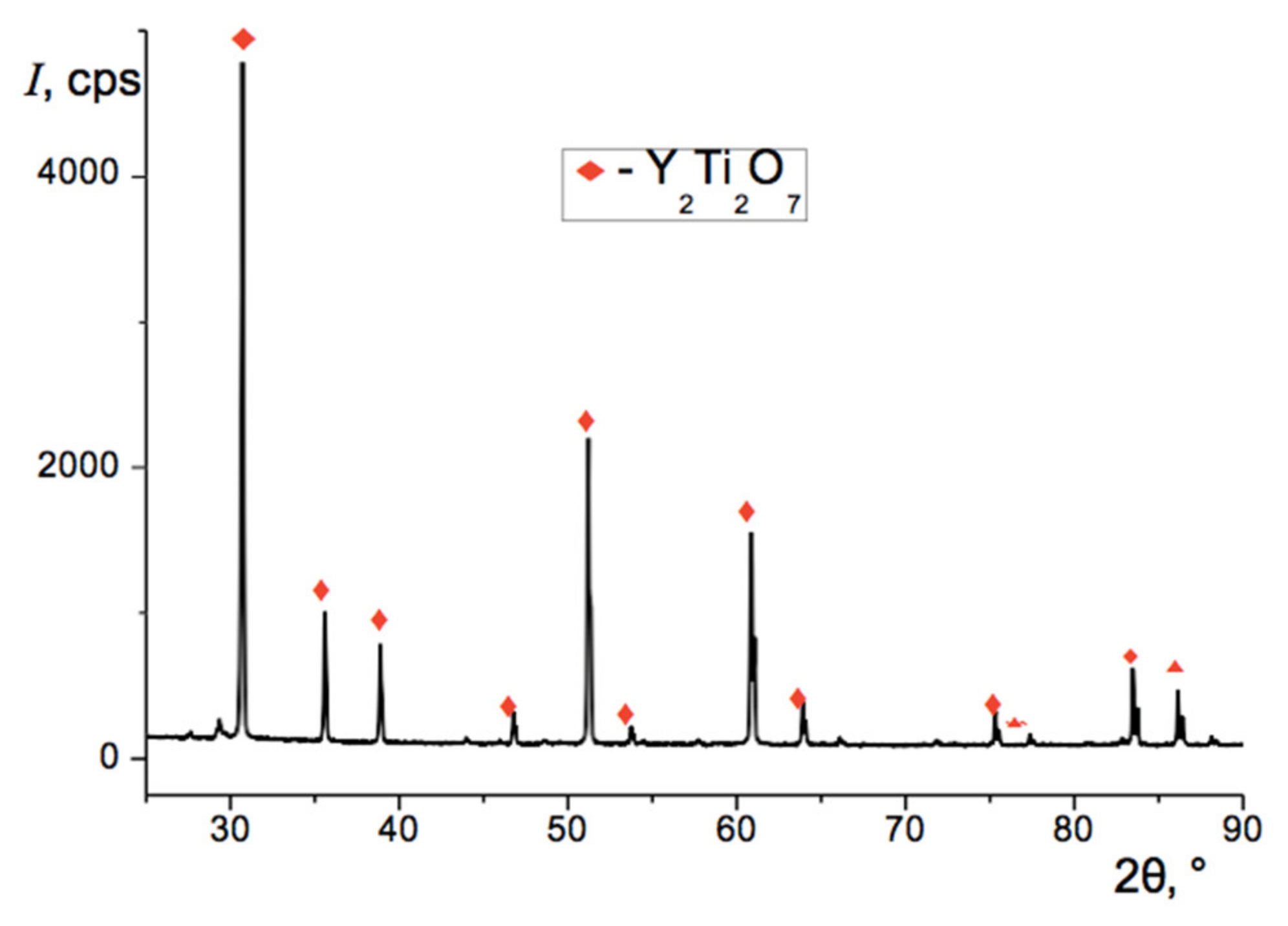

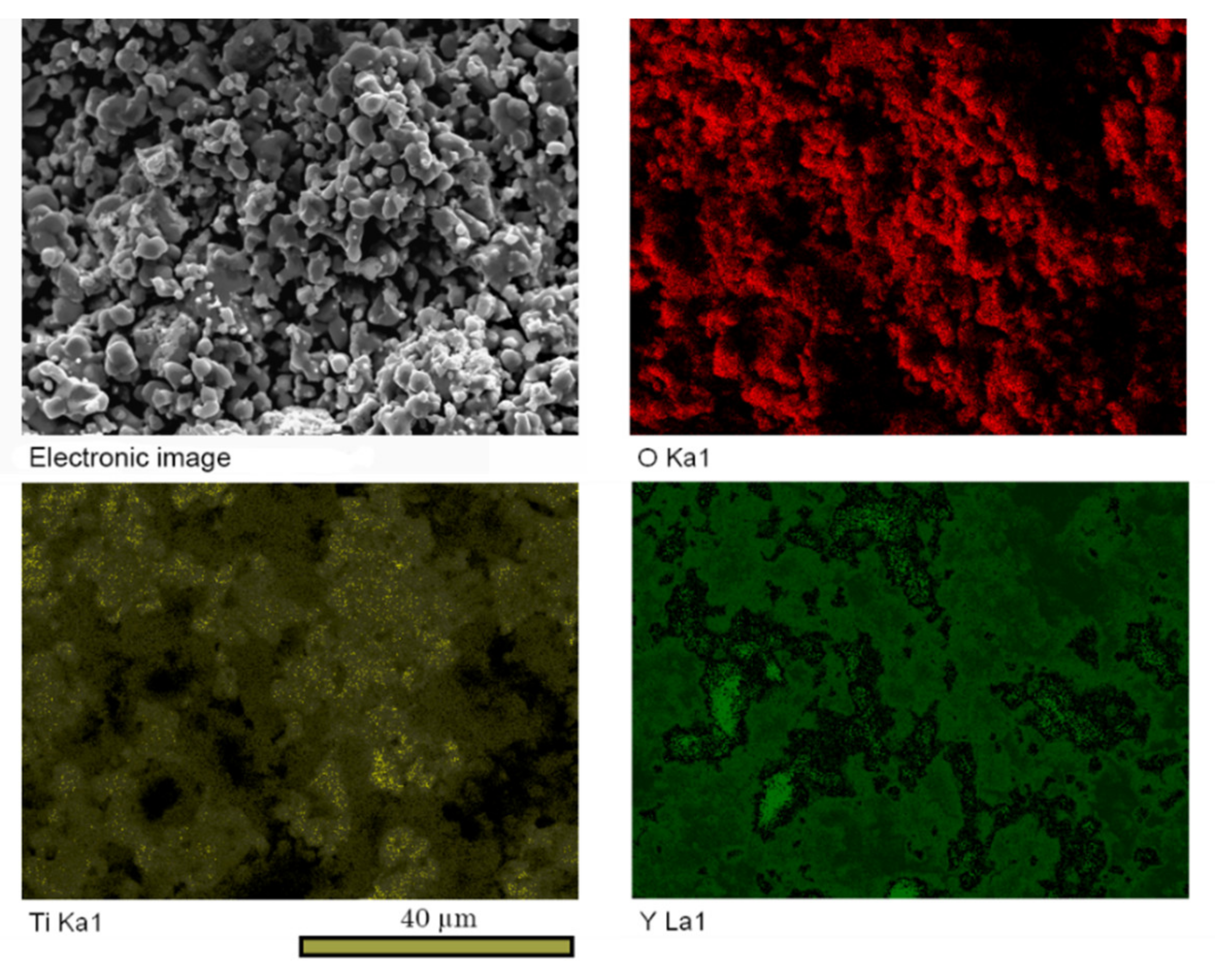

| No. | Y2Ti2O7 Content, wt.% | Total Calcining Time, h |
|---|---|---|
| 1 | 20 | 7 |
| 2 | 96.8 | 17 |
| 3 | 98.0 | 25 |
| Sample | Y2Ti2O7 Content, wt.% | Total Calcining Time in the Conventional Furnace, h | Total Calcining Time in the Microwave Oven, h |
|---|---|---|---|
| #1 | 51 | - | 1 |
| #2 | 87 | 7 | 1 |
| #3 | 99 | 7 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chishkala, V.; Lytovchenko, S.; Mazilin, B.; Gevorkyan, E.; Shkuropatenko, V.; Voyevodin, V.; Rucki, M.; Siemiątkowski, Z.; Matijošius, J.; Dudziak, A.; et al. Novel Microwave-Assisted Method of Y2Ti2O7 Powder Synthesis. Materials 2020, 13, 5621. https://doi.org/10.3390/ma13245621
Chishkala V, Lytovchenko S, Mazilin B, Gevorkyan E, Shkuropatenko V, Voyevodin V, Rucki M, Siemiątkowski Z, Matijošius J, Dudziak A, et al. Novel Microwave-Assisted Method of Y2Ti2O7 Powder Synthesis. Materials. 2020; 13(24):5621. https://doi.org/10.3390/ma13245621
Chicago/Turabian StyleChishkala, Vladimir, Serhiy Lytovchenko, Bohdan Mazilin, Edwin Gevorkyan, Vladimir Shkuropatenko, Viktor Voyevodin, Mirosław Rucki, Zbigniew Siemiątkowski, Jonas Matijošius, Agnieszka Dudziak, and et al. 2020. "Novel Microwave-Assisted Method of Y2Ti2O7 Powder Synthesis" Materials 13, no. 24: 5621. https://doi.org/10.3390/ma13245621
APA StyleChishkala, V., Lytovchenko, S., Mazilin, B., Gevorkyan, E., Shkuropatenko, V., Voyevodin, V., Rucki, M., Siemiątkowski, Z., Matijošius, J., Dudziak, A., Caban, J., & Kilikevičius, A. (2020). Novel Microwave-Assisted Method of Y2Ti2O7 Powder Synthesis. Materials, 13(24), 5621. https://doi.org/10.3390/ma13245621









