Corrosion Resistance of Shape Recoverable Fe-17Mn-5Si-5Cr Alloy in Concrete Structures
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Microstructure of Test Sample
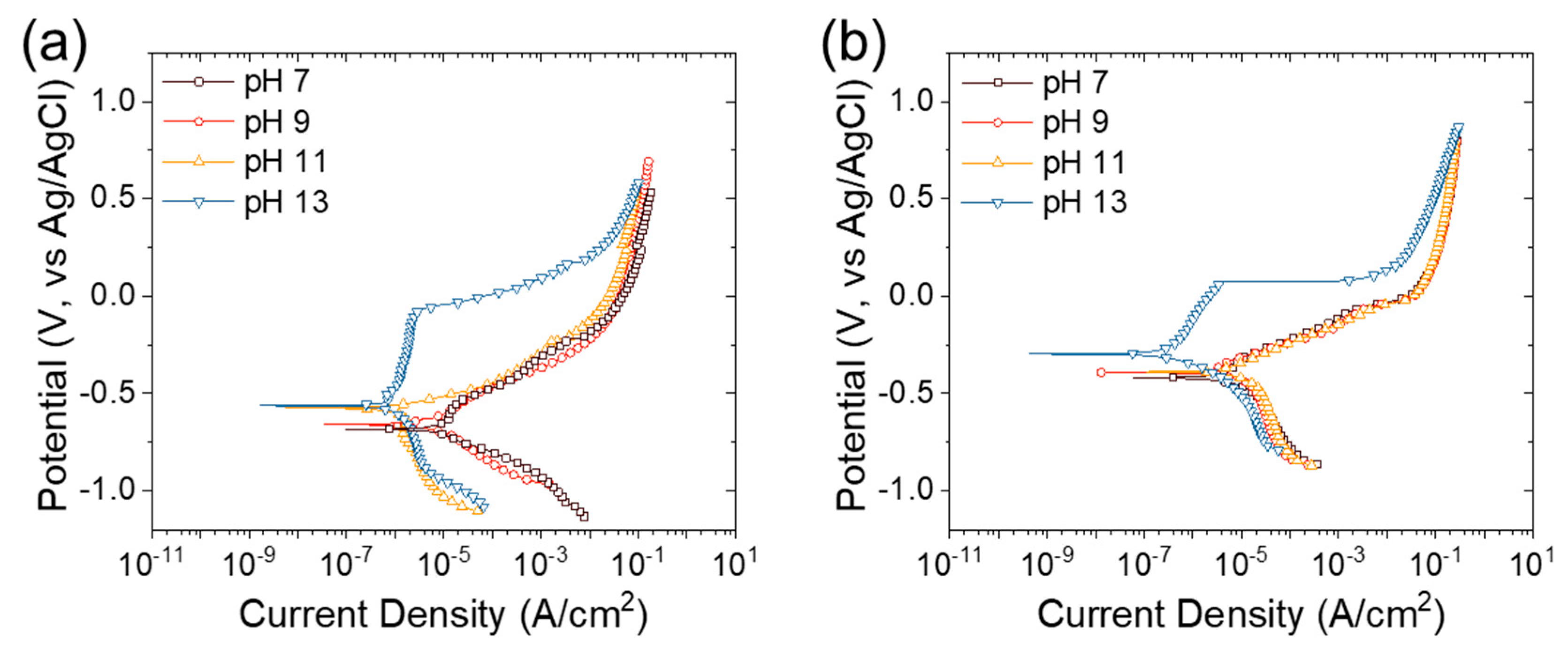
3.2. Corrosion Test without Concrete Preparation
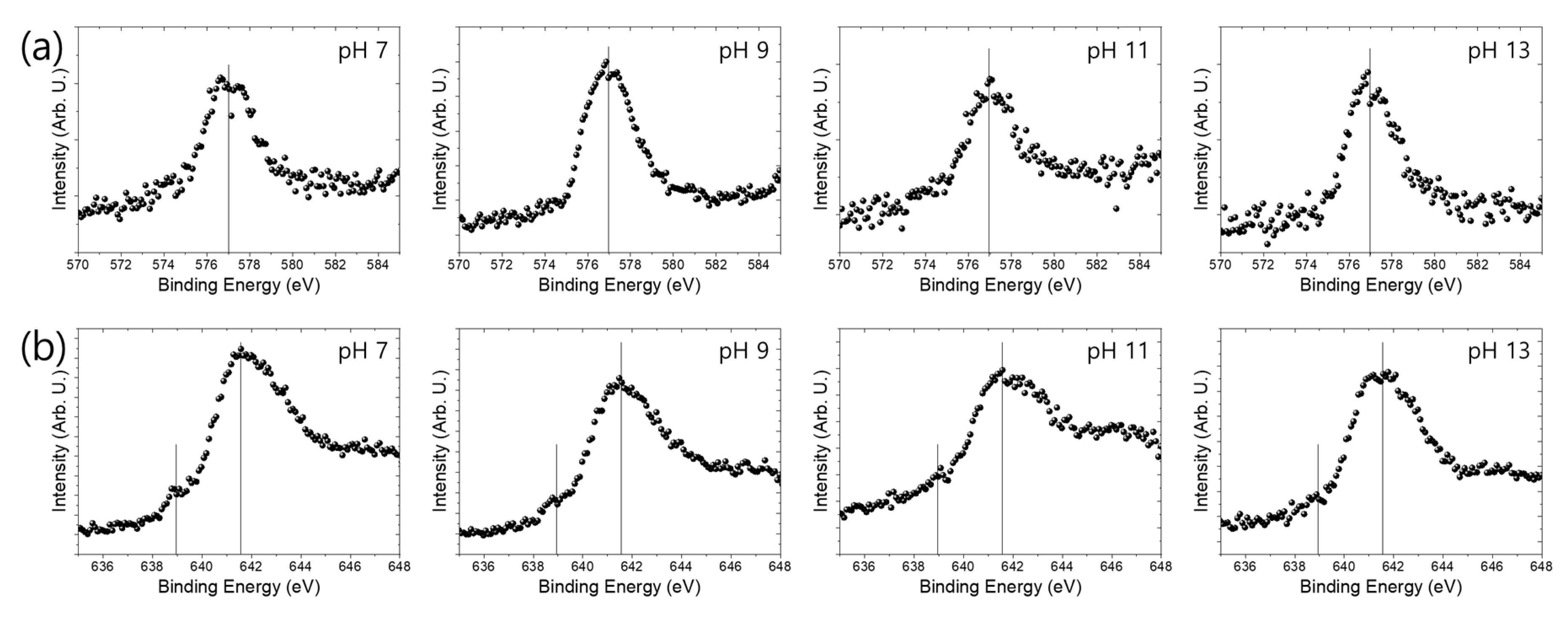
3.3. Passive Oxide Layer
3.4. Short-Term Corrosion Test with Concrete Preparation
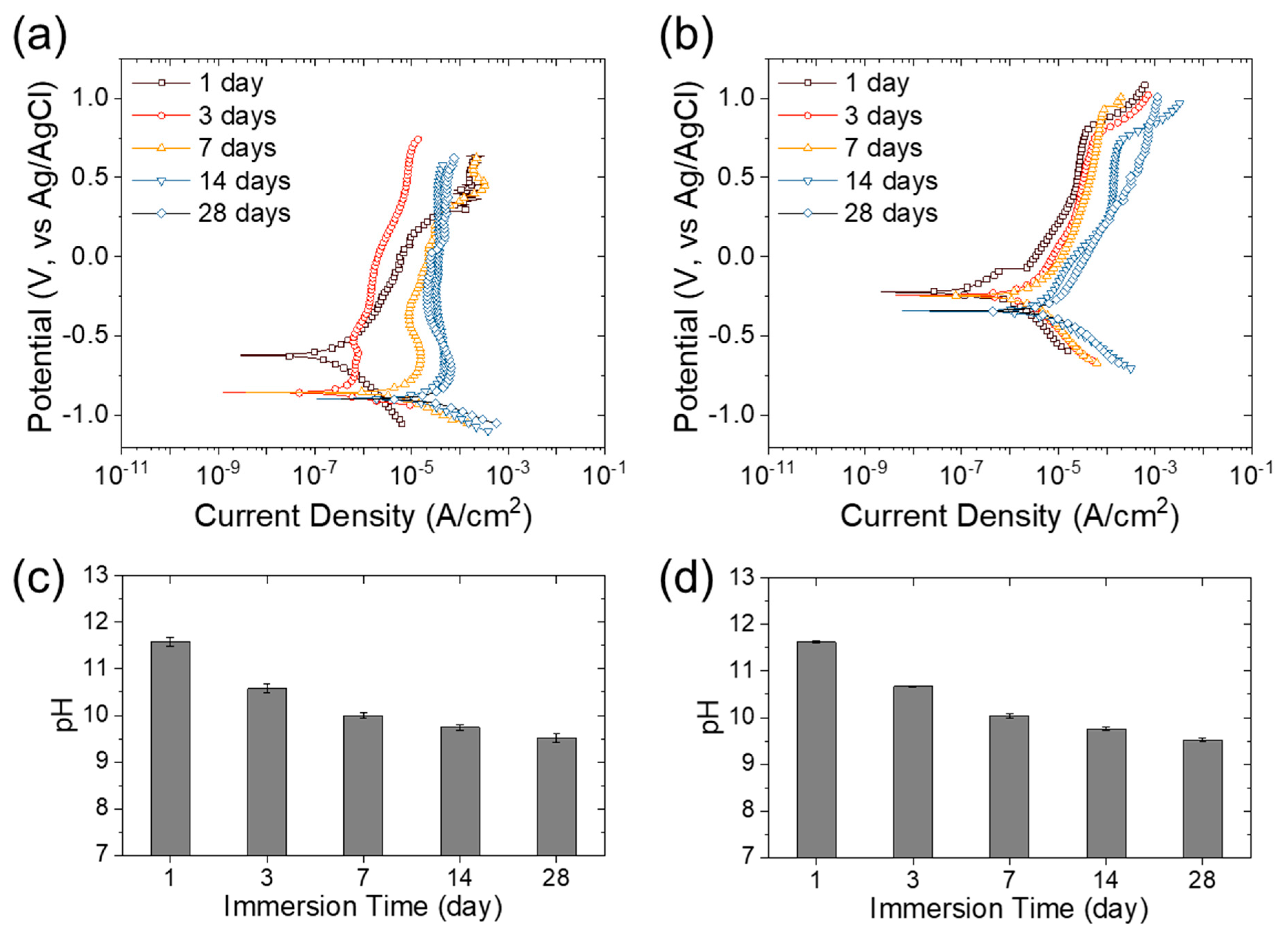
3.5. Long-Term Corrosion Test with Mortar Preparation
4. Conclusions
- Owing to the Mn and Cr, the shape-memorable Fe-17Mn-5Si-5Cr alloy forms a stable passivation oxide layer in alkaline environments, which has a similar pH to concrete.
- This passivation oxide layer enabled the Fe-17Mn-5Si-5Cr alloy to obtain a higher corrosion resistance than S400 carbon steel in salt water (more than 140% in the pH range of 7–13).
- Because of the dipping in salt water for 28 days, the corrosion resistance of the Fe-17Mn-5Si-5Cr alloy decreased by 97.2%, whereas that of the S400 decreased by 99.6%, indicating a lower corrosion sensitivity of the Fe-17Mn-5Si-5Cr alloy to the change in mortar pH.
- The Fe-17Mn-5Si-5Cr alloy showed a higher corrosion resistance than the S400 (more than 150% in the mortar), indicating better chemical stability in the concrete structure.
- These results indicate that the Fe-17Mn-5Si-5Cr shape memory alloy exhibits a higher corrosion resistance than S400 carbon steel (commonly used for reinforcing concrete) and that it is a potential candidate for fabricating structures based on prestressed concrete and reinforced concrete.
Author Contributions
Funding
Conflicts of Interest
References
- Dong, Z.; Kajiwara, S.; Kikuchi, T.; Sawaguchi, T. Effect of pre-deformation at room temperature on shape memory properties of stainless type Fe–15Mn–5Si–9Cr–5Ni–(0.5–1.5) NbC alloys. Acta Mater. 2005, 53, 4009–4018. [Google Scholar] [CrossRef]
- Otsuka, H.; Yamada, H.; Tanahashi, H.; Maruyama, T. Shape memory effect in Fe-Mn-Si-Cr-Ni polycrystalline alloys. Mater. Sci. Forum 1990, 56-58, 655–660. [Google Scholar] [CrossRef]
- Sato, A.; Yamaji, Y.; Mori, T. Physical properties controlling shape memory effect in Fe-Mn-Si alloys. Acta Metall. 1986, 34, 287–294. [Google Scholar] [CrossRef]
- Kim, J.I.; Hwang, K.S. Effect of Annealing Temperature on the Shape Memory Properties of Ti-44.5 Ni-5Cu and Ti-45.2 Ni-5Cu (at%) Alloys. Korean J. Met. Mater. 2019, 57, 562–568. [Google Scholar] [CrossRef]
- Liu, Y. The superelastic anisotropy in a NiTi shape memory alloy thin sheet. Acta Mater. 2015, 95, 411–427. [Google Scholar] [CrossRef]
- Miyazaki, S.; Otsuka, K.; Suzuki, Y. Transformation pseudoelasticity and deformation behavior in a Ti-50.6 at% Ni alloy. Scr. Metall. 1981, 15, 287–292. [Google Scholar] [CrossRef]
- Narayana, P.; Kim, S.-W.; Hong, J.-K.; Reddy, N.; Yeom, J.-T. Estimation of transformation temperatures in Ti–Ni–Pd shape memory alloys. Met. Mater. Int. 2018, 24, 919–925. [Google Scholar] [CrossRef]
- Nishida, M.; Wayman, C.M.; Honma, T. Precipitation processes in near-equiatomic TiNi shape memory alloys. Metall. Trans. A 1986, 17, 1505–1515. [Google Scholar] [CrossRef]
- Hong, K.; Lee, S.; Han, S.; Yeon, Y. Evaluation of Fe-based shape memory alloy (Fe-SMA) as strengthening material for reinforced concrete structures. Appl. Sci. 2018, 8, 730. [Google Scholar] [CrossRef]
- Lagoudas, D.C. Shape Memory Alloys: Modeling and Engineering Applications; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Arabi-Hashemi, A.; Lee, W.; Leinenbach, C. Recovery stress formation in FeMnSi based shape memory alloys: Impact of precipitates, texture and grain size. Mater. Des. 2018, 139, 258–268. [Google Scholar] [CrossRef]
- Choi, E.; Chung, Y.-S.; Choi, J.-H.; Kim, H.-T.; Lee, H. The confining effectiveness of NiTiNb and NiTi SMA wire jackets for concrete. Smart Mater. Struct. 2010, 19, 035024. [Google Scholar] [CrossRef]
- Czaderski, C.; Hahnebach, B.; Motavalli, M. RC beam with variable stiffness and strength. Constr. Build. Mater. 2006, 20, 824–833. [Google Scholar] [CrossRef]
- Lee, W.; Weber, B.; Feltrin, G.; Czaderski, C.; Motavalli, M.; Leinenbach, C. Stress recovery behaviour of an Fe–Mn–Si–Cr–Ni–VC shape memory alloy used for prestressing. Smart Mater.Struct. 2013, 22, 125037. [Google Scholar] [CrossRef]
- Lee, W.; Weber, B.; Leinenbach, C. Recovery stress formation in a restrained Fe–Mn–Si-based shape memory alloy used for prestressing or mechanical joining. Constr. Build. Mater. 2015, 95, 600–610. [Google Scholar] [CrossRef]
- Maji, A.K.; Negret, I. Smart prestressing with shape-memory alloy. J. Eng. Mech. 1998, 124, 1121–1128. [Google Scholar] [CrossRef]
- Dong, Z.; Klotz, U.E.; Leinenbach, C.; Bergamini, A.; Czaderski, C.; Motavalli, M. A Novel Fe-Mn-Si Shape Memory Alloy With Improved Shape Recovery Properties by VC Precipitation. Adv. Eng. Mater. 2009, 11, 40–44. [Google Scholar] [CrossRef]
- Shahverdi, M.; Czaderski, C.; Motavalli, M. Iron-based shape memory alloys for prestressed near-surface mounted strengthening of reinforced concrete beams. Constr. Build. Mater. 2016, 112, 28–38. [Google Scholar] [CrossRef]
- Hong, K.-N.; Yeon, Y.-M.; Shim, W.-B.; Kim, D.-H. Recovery Behavior of Fe-Based Shape Memory Alloys Under Different Restraints. Appl. Sci. 2020, 10, 3441. [Google Scholar] [CrossRef]
- Fontana, M.G. Corrosion Engineering; Tata McGraw-Hill Education: New York, NY, USA, 2005. [Google Scholar]
- Jones, D.A. Principles and Prevention of Corrosion; Macmillan: New York, NY, USA, 1992. [Google Scholar]
- Uhlig, H.H.; King, C. Corrosion and corrosion control. J. Electrochem. Soc. 1972, 119, 327C. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.; Oak, J.-J.; Lee, J.; Park, C.H.; Lee, W.; Park, Y. Effect of Ni, C and Ti Addition on Shape Recovery Behavior and the Mechanical Properties of Fe-17Mn-5Si-5Cr Shape Memory Alloys. Korean J. Met. Mater. 2020, 58, 660–671. [Google Scholar] [CrossRef]
- Amaral, S.; Müller, I. Passivation of pure iron in alkaline solution containing silicate and sulphate—Galvanostatic and Potentiostatic studies. Corros. Sci. 1999, 41, 747–758. [Google Scholar] [CrossRef]
- Bera, S.; Rangarajan, S.; Narasimhan, S. Electrochemical passivation of iron alloys and the film characterisation by XPS. Corros. Sci. 2000, 42, 1709–1724. [Google Scholar] [CrossRef]
- Hancock, P.; Mayne, J. The inhibition of the corrosion of iron in neutral and alkaline solutions. I. J. Appl. Chem. 1959, 9, 345–352. [Google Scholar] [CrossRef]
- Haupt, S.; Strehblow, H. Corrosion, layer formation, and oxide reduction of passive iron in alkaline solution: A combined electrochemical and surface analytical study. Langmuir 1987, 3, 873–885. [Google Scholar] [CrossRef]
- Grosvenor, A.; Kobe, B.; Biesinger, M.; McIntyre, N. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Fajardo, S.; Llorente, I.; Jiménez, J.A.; Bastidas, J.; Bastidas, D.M. Effect of Mn additions on the corrosion behaviour of TWIP Fe-Mn-Al-Si austenitic steel in chloride solution. Corros. Sci. 2019, 154, 246–253. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.G. Effect of manganese on the corrosion behavior of low carbon steel in 10 wt.% sulfuric acid. Int. J. Electrochem. Sci. 2015, 10, 6872–6885. [Google Scholar]
- Nguyen, T.D.; Zhang, J.; Young, D.J. Effects of cerium and manganese on corrosion of Fe–Cr and Fe–Cr–Ni alloys in Ar–20CO2 and Ar–20CO2–20H2O gases at 650 °C. Corros. Sci. 2015, 100, 448–465. [Google Scholar] [CrossRef]
- Wilson, P.; Chen, Z. The effect of manganese and chromium on surface oxidation products formed during batch annealing of low carbon steel strip. Corros. Sci. 2007, 49, 1305–1320. [Google Scholar] [CrossRef]
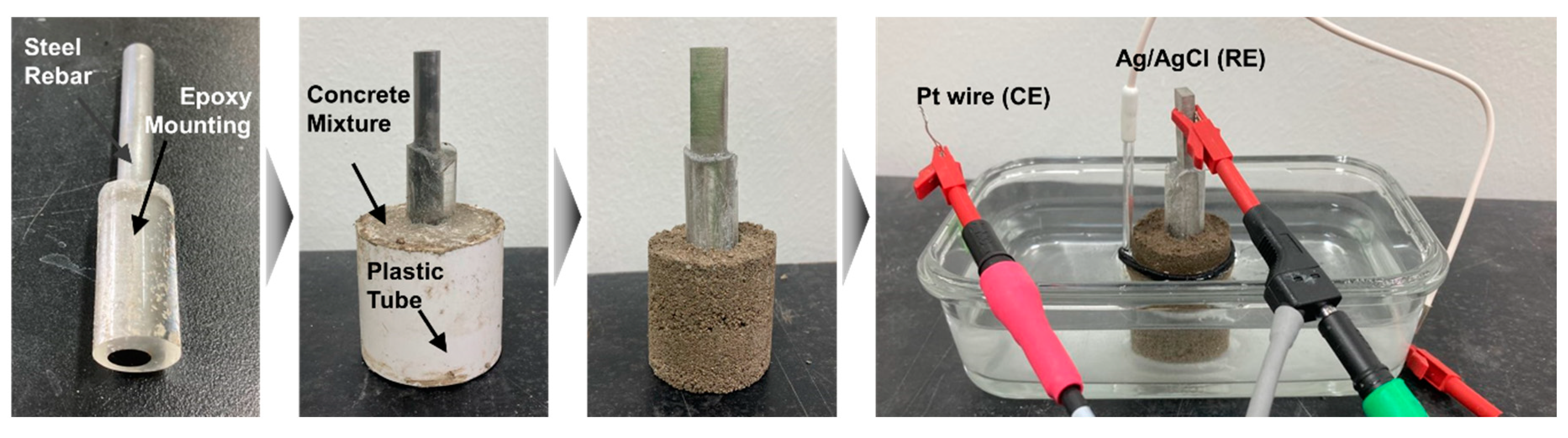



| Name | Fe | Mn | Si | Cr | Ni | Ti | C |
|---|---|---|---|---|---|---|---|
| S400 | Bal. | <1.0 | - | - | - | - | 0.2 |
| FSMA | Bal. | 17 | 5 | 5 | 4 | 1 | 0.3 |
| Reinforcement Material | Mortar | pH Adjustment with CaO | Short-Term Immersion Time | Long-Term Immersion Time |
|---|---|---|---|---|
| S400 | Ⅹ | ○ | 30 min | - |
| ○ | Ⅹ | 10, 60, 120 min | 1, 3, 7, 14, 28 days | |
| FSMA | Ⅹ | ○ | 30 min | - |
| ○ | Ⅹ | 10, 60, 120 min | 1, 3, 7, 14, 28 days |
| Sample | pH | Ecorr (V vs. Ag/AgCl) | icorr (A/cm2) | Rp (kΩ∙cm2) |
|---|---|---|---|---|
| S400 | 7 | −0.685 | 1.7 × 10−5 | 1.8 |
| 9 | −0.659 | 4.3 × 10−6 | 6.8 | |
| 11 | −0.575 | 2.2 × 10−6 | 10.9 | |
| 13 | −0.558 | 7.7 × 10−7 | 76.4 | |
| FSMA | 7 | −0.412 | 4.7 × 10−6 | 8.7 |
| 9 | −0.392 | 3.2 × 10−6 | 11.8 | |
| 11 | −0.387 | 1.6 × 10−6 | 23.2 | |
| 13 | −0.288 | 3.8 × 10−7 | 111.2 |
| Sample | Immersion Time | Ecorr (V vs. Ag/AgCl) | icorr (A/cm2) | Rp (kΩ∙cm2) |
|---|---|---|---|---|
| S400 | 10 min | −0.379 | 6.5 × 10−7 | 72.6 |
| 60 min | −0.305 | 2.3 × 10−7 | 154.7 | |
| 120 min | −0.255 | 2.2 × 10−7 | 267.9 | |
| FSMA | 10 min | −0.312 | 1.9 × 10−7 | 261.1 |
| 60 min | −0.246 | 3.0 × 10−8 | 2107.1 | |
| 120 min | −0.253 | 2.9 × 10−8 | 2118.2 |
| Sample | Immersion Time | Ecorr (V vs. Ag/AgCl) | icorr (A/cm2) | Rp (kΩ∙cm2) |
|---|---|---|---|---|
| S400 | 1 day | −0.621 | 3.3 × 10−7 | 227.4 |
| 3 day | −0.856 | 5.2 × 10−7 | 47.4 | |
| 7 day | −0.858 | 9.2 × 10−6 | 6.4 | |
| 14 day | −0.895 | 1.3 × 10−5 | 4.1 | |
| 28 day | −0.895 | 3.7 × 10−5 | 1.0 | |
| FSMA | 1 day | −0.231 | 2.2 × 10−7 | 350.1 |
| 3 day | −0.241 | 1.3 × 10−6 | 81.9 | |
| 7 day | −0.248 | 3.5 × 10−6 | 37.1 | |
| 14 day | −0.342 | 5.0 × 10−6 | 22.0 | |
| 28 day | −0.341 | 6.9 × 10−6 | 9.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joo, J.; Kang, M.; Shin, D.; Seo, E.; Kim, D.; Yeon, Y.; Hong, K.; Lee, W.; Lee, J. Corrosion Resistance of Shape Recoverable Fe-17Mn-5Si-5Cr Alloy in Concrete Structures. Materials 2020, 13, 5531. https://doi.org/10.3390/ma13235531
Joo J, Kang M, Shin D, Seo E, Kim D, Yeon Y, Hong K, Lee W, Lee J. Corrosion Resistance of Shape Recoverable Fe-17Mn-5Si-5Cr Alloy in Concrete Structures. Materials. 2020; 13(23):5531. https://doi.org/10.3390/ma13235531
Chicago/Turabian StyleJoo, Jaehoon, Minjoo Kang, Dongmin Shin, Eunhye Seo, Dohyung Kim, Yeongmo Yeon, Kinam Hong, Wookjin Lee, and Junghoon Lee. 2020. "Corrosion Resistance of Shape Recoverable Fe-17Mn-5Si-5Cr Alloy in Concrete Structures" Materials 13, no. 23: 5531. https://doi.org/10.3390/ma13235531
APA StyleJoo, J., Kang, M., Shin, D., Seo, E., Kim, D., Yeon, Y., Hong, K., Lee, W., & Lee, J. (2020). Corrosion Resistance of Shape Recoverable Fe-17Mn-5Si-5Cr Alloy in Concrete Structures. Materials, 13(23), 5531. https://doi.org/10.3390/ma13235531





