Resistive Switching in Electrodeposited Prussian Blue Layers
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Analysis
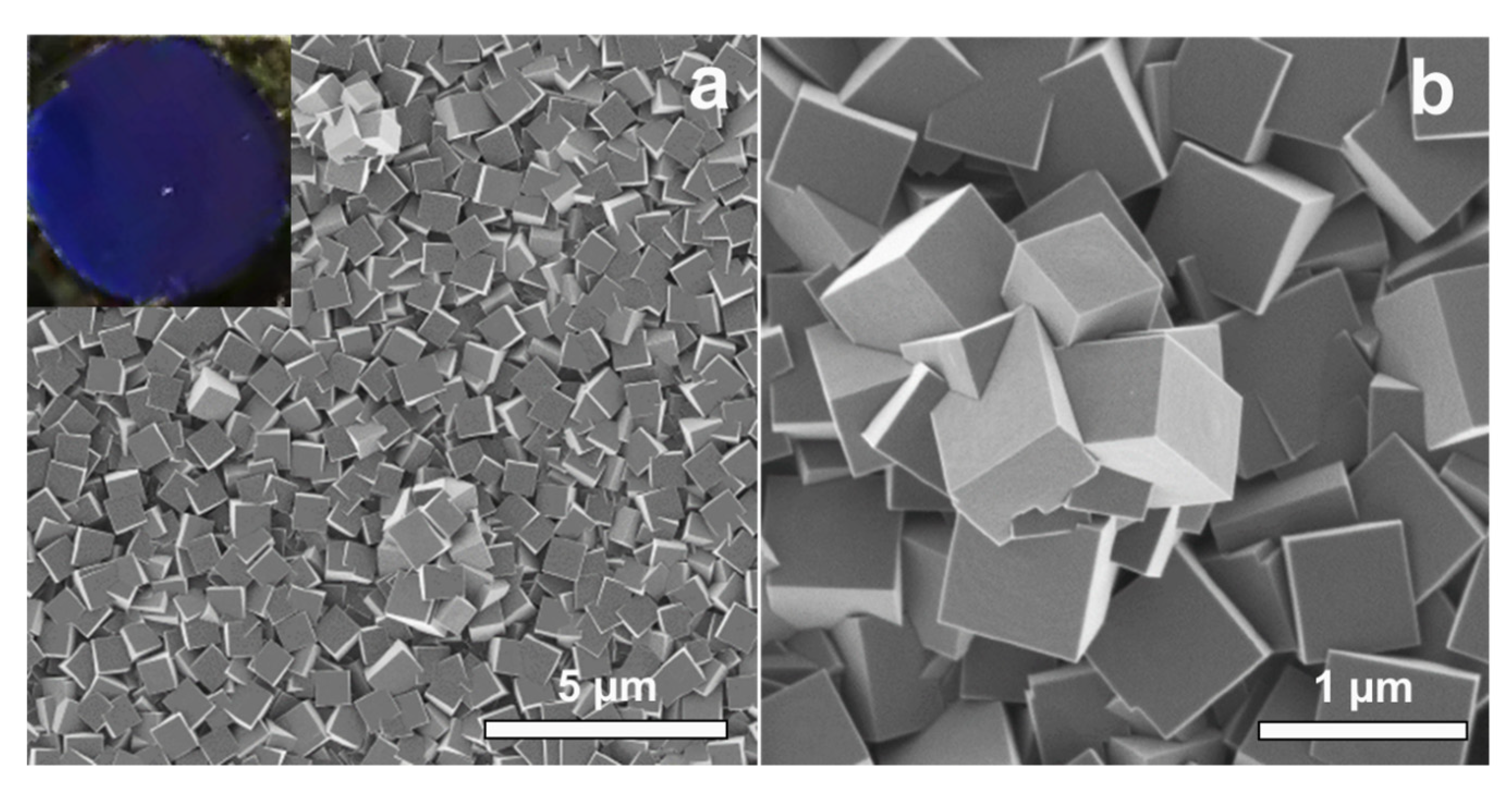
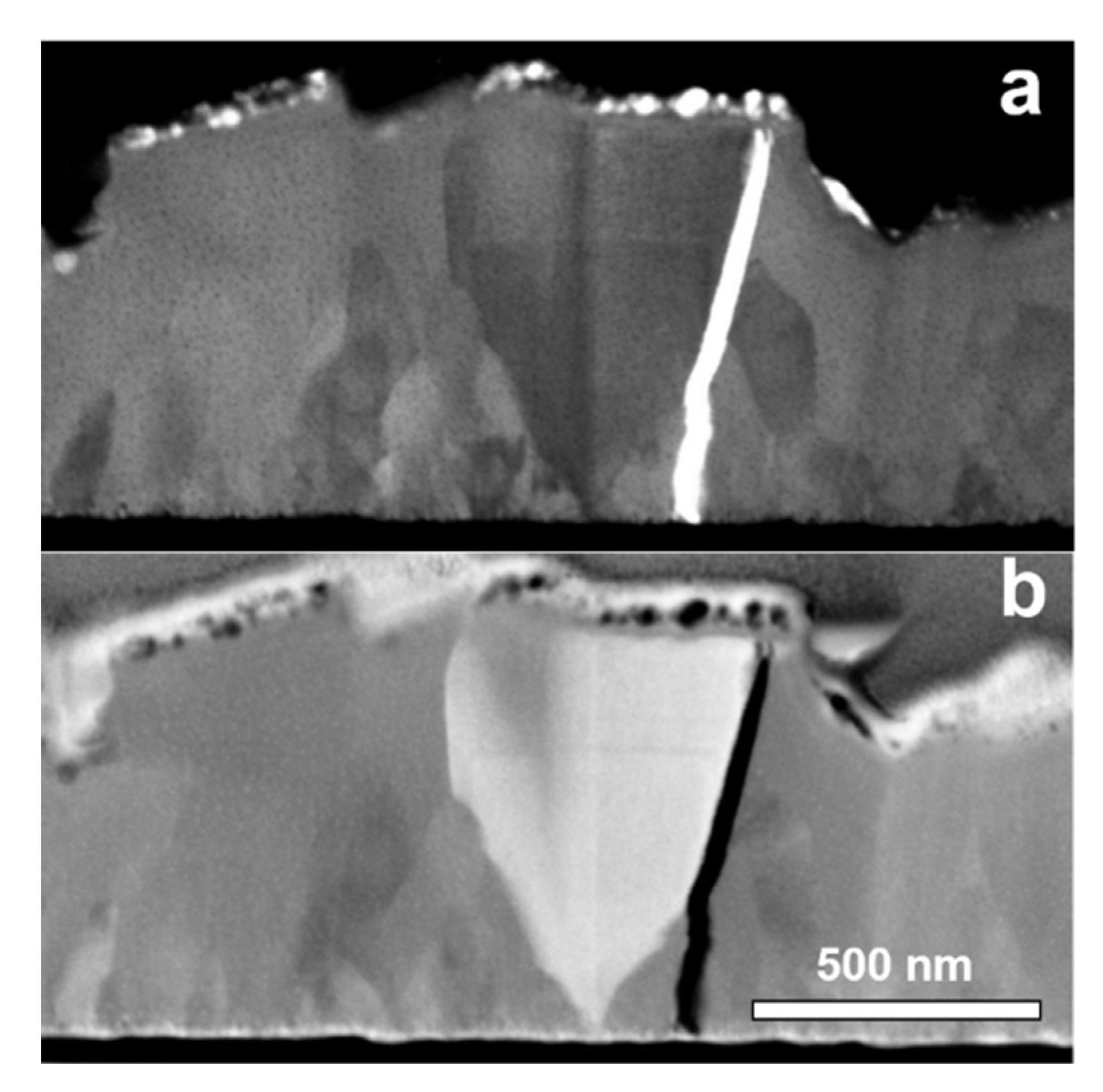
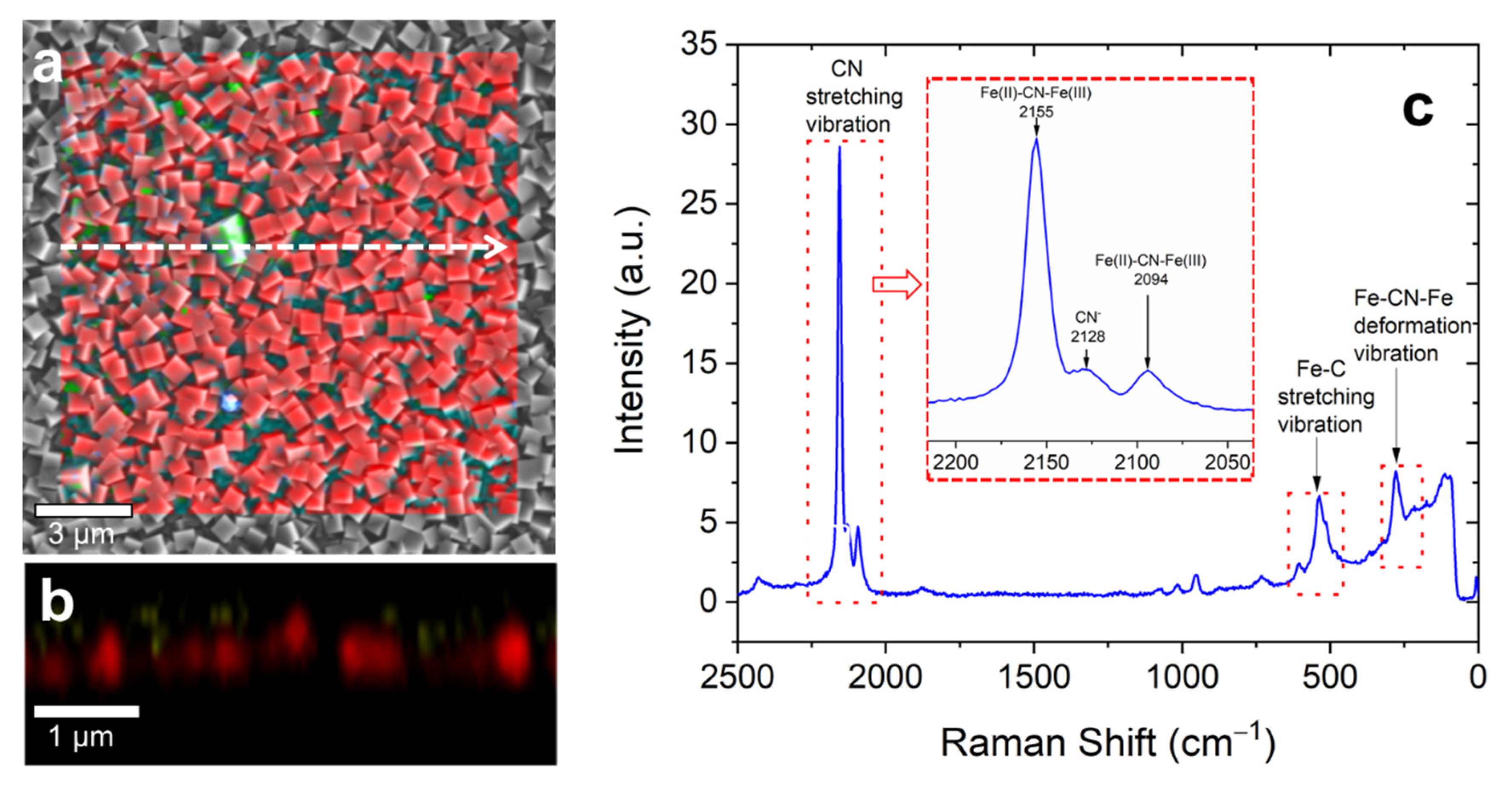
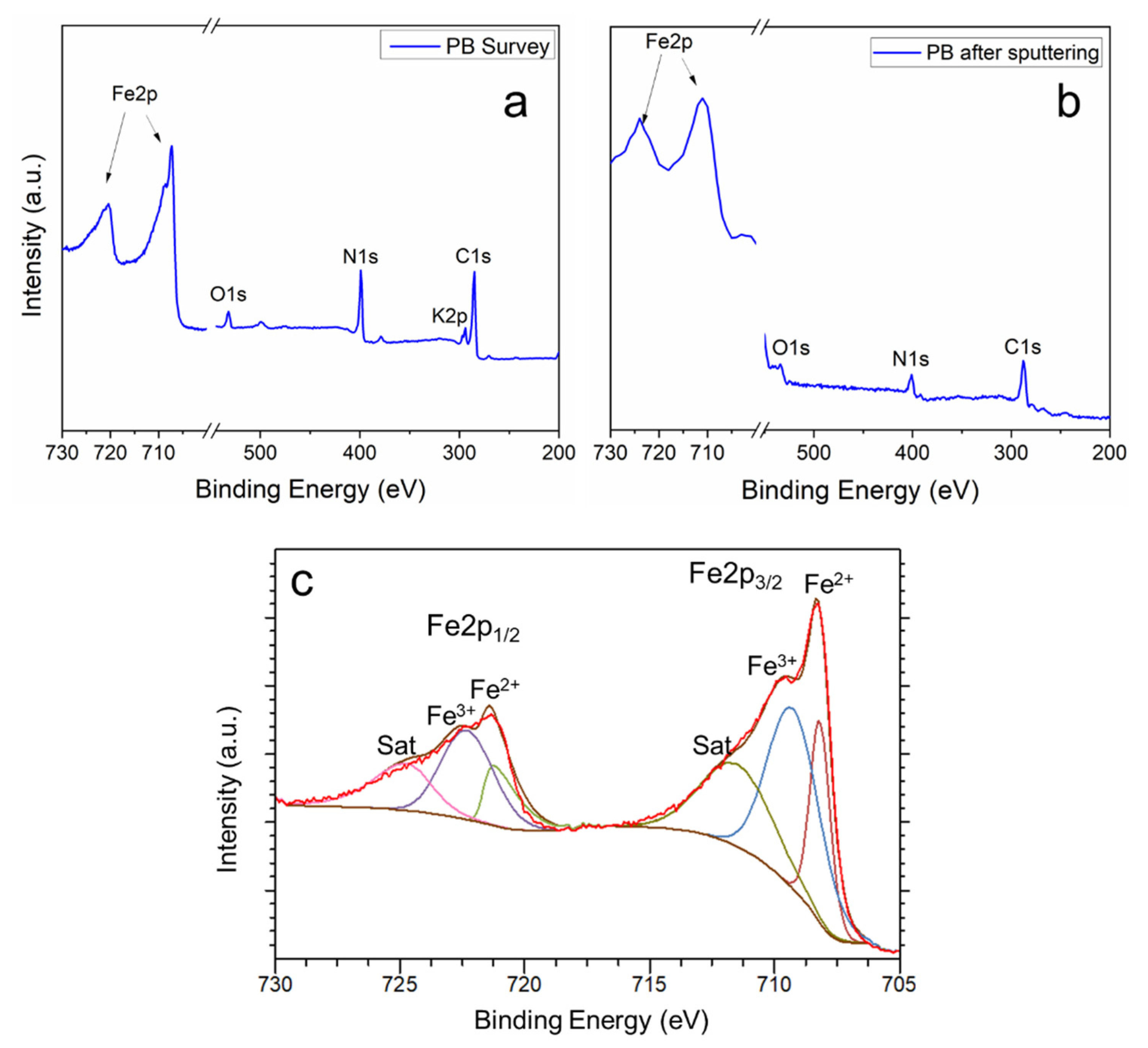
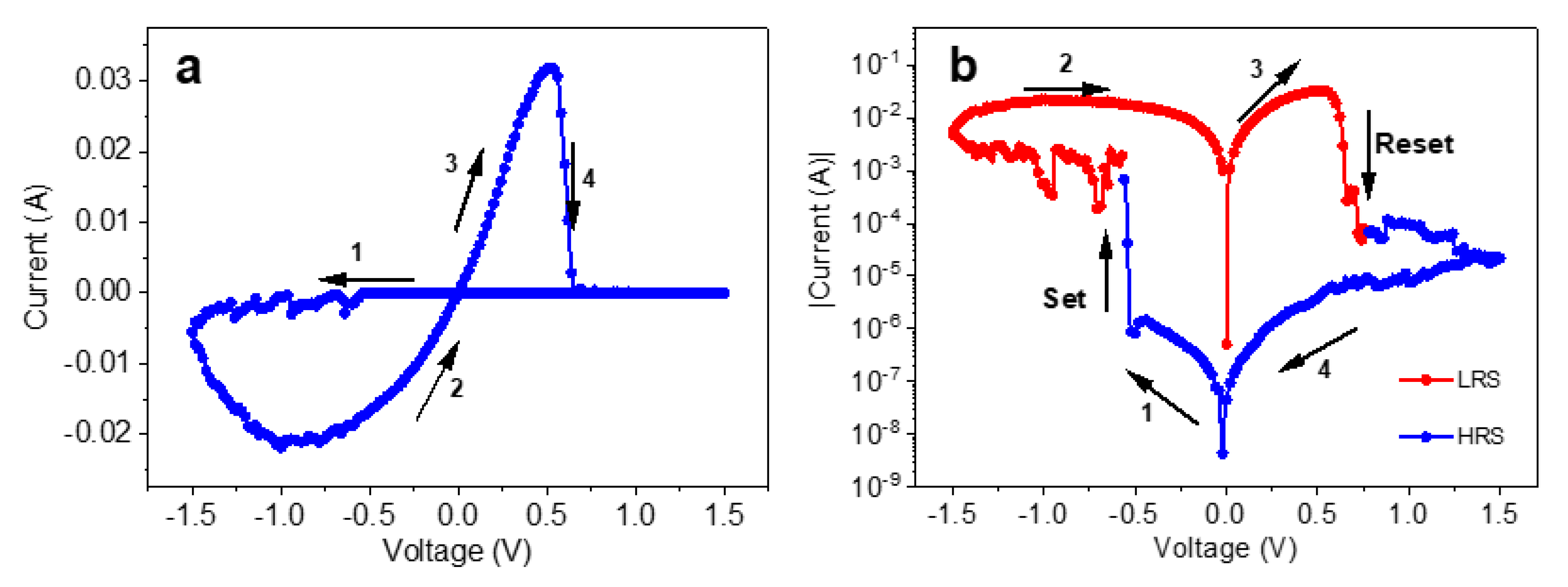
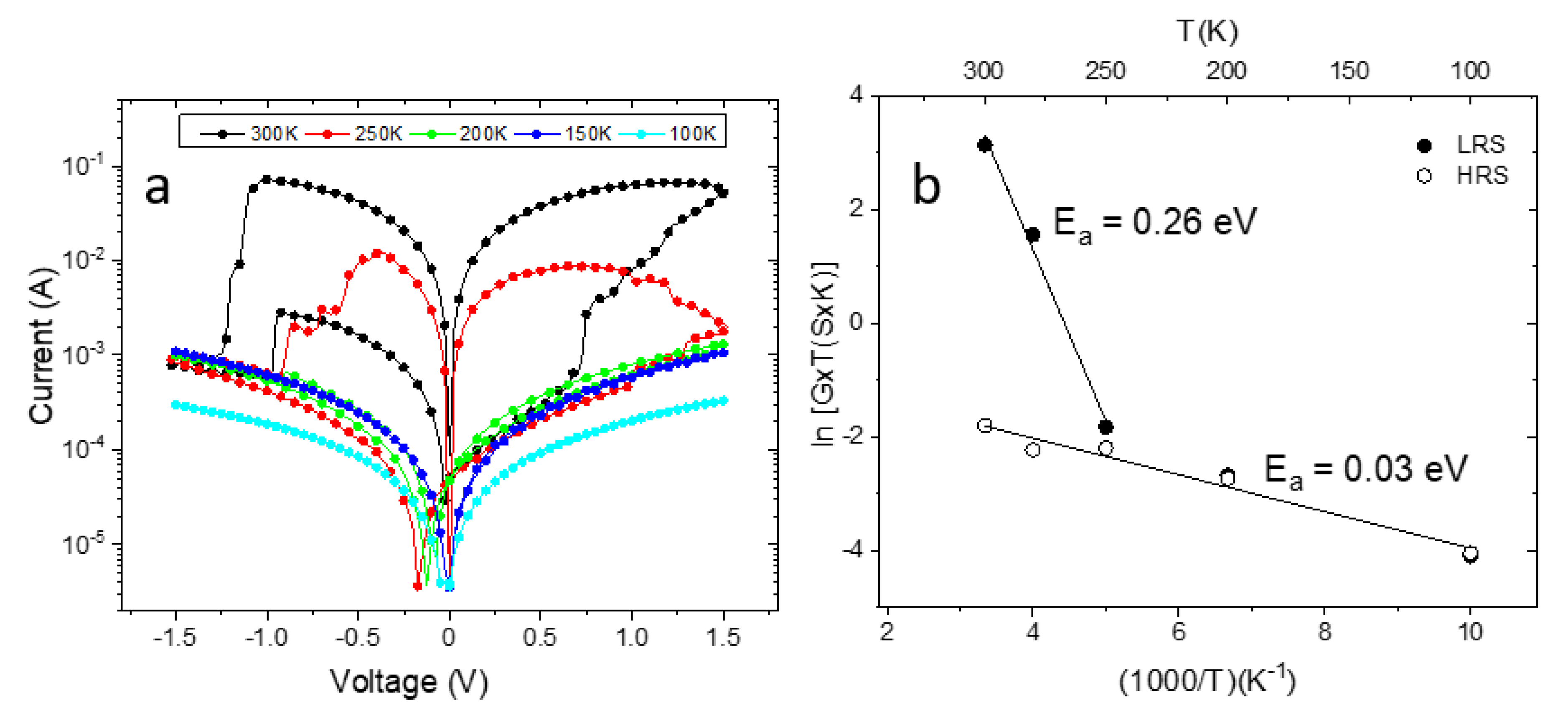
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shalf, J. The future of computing beyond Moore’s Law. Philos. Trans. R. Soc. A 2020, 378, 20190061. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, R.; Liu, X.; Kang, J. The challenges for physical limitations in Si microelectronics. Acta Electron. Sin. 1998, 26, 77–84. [Google Scholar]
- Keyes, R.W. Physical limits of silicon transistors and circuits. Rep. Prog. Phys. 2005, 68, 2701–2746. [Google Scholar] [CrossRef]
- Waser, R.; Aono, M. Nanoionics-based resistive switching memories. Nat. Mater. 2007, 6, 835–840. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, H.; Burr, G.W.; Hwang, C.S.; Wang, K.L.; Xia, Q.; Yang, J.J. Resistive switching materials for information processing. Nat. Rev. Mater. 2020, 5, 173–195. [Google Scholar] [CrossRef]
- Di Ventra, M.; Pershin, Y.V.; Chua, L.O. Circuit Elements With Memory: Memristors, Memcapacitors, and Meminductors. Proc. IEEE 2009, 97, 1717–1724. [Google Scholar] [CrossRef]
- Li, H.B.; Tebikachew, B.E.; Wiberg, C.; Moth-Poulsen, K.; Hihath, J. A Memristive Element Based on an Electrically Controlled Single-Molecule Reaction. Angew. Chem. Int. Ed. 2020. [Google Scholar] [CrossRef]
- Thangkhiew, P.L.; Zulehner, A.; Wille, R.; Datta, K.; Sengupta, I. An efficient memristor crossbar architecture for mapping Boolean functions using Binary Decision Diagrams (BDD). Integration 2020, 71, 125–133. [Google Scholar] [CrossRef]
- Filippov, V.A.; Bobylev, A.N.; Busygin, A.N.; Pisarev, A.D.; Udovichenko, S.Y. A biomorphic neuron model and principles of designing a neural network with memristor synapses for a biomorphic neuroprocessor. Neural Comput. Appl. 2020, 32, 2471–2485. [Google Scholar] [CrossRef]
- Slesazeck, S.; Mikolajick, T. Nanoscale resistive switching memory devices: A review. Nanotechnology 2019, 30, 352003. [Google Scholar] [CrossRef]
- Chua, L.O. Memristor-The Missing Circuit Element. IEEE Trans. Circuit Theory 1971, 18, 507–518. [Google Scholar] [CrossRef]
- Strukov, D.B.; Snider, G.S.; Stewart, D.R.; Williams, R.S. The missing memristor found. Nature 2008, 453, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Buser, H.J.; Schwarzenbach, D.; Petter, W.; Ludi, A. The Crystal Structure of Prussian Blue: Crystal Structure of Prussian Blue. Inorg. Chem. 1977, 16. [Google Scholar] [CrossRef]
- Basset Brown, D.; Shriver, D.F. Structures and Solid-State Reactions of Prussian Blue Analogs Containing Chromium, Manganese, Iron, and Cobalt. Inorg. Chem. 1969, 8, 37–42. [Google Scholar] [CrossRef]
- Katic, V.; Dos Santos, P.L.; Dos Santos, M.F.; Pires, B.M.; Loureiro, H.C.; Lima, A.P.; Queiroz, J.C.M.; Landers, R.; Muñoz, R.A.A.; Bonacin, J.A. 3D Printed Graphene Electrodes Modified with Prussian Blue: Emerging Electrochemical Sensing Platform for Peroxide Detection. ACS Appl. Mater. Interfaces 2019, 11, 35068–35078. [Google Scholar] [CrossRef] [PubMed]
- Isfahani, V.B.; Memarian, N.; Dizaji, H.R.; Arab, A.; Silva, M.M. The physical and electrochromic properties of Prussian Blue thin films electrodeposited on ITO electrodes. Electrochim. Acta 2019, 304, 282–291. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, W.; Walheim, S.; Wang, Z.; Lindemann, P.; Heissler, S.; Liu, J.; Weidler, P.G.; Schimmel, T.; Wöll, C.; et al. Electrochromic switching of monolithic Prussian blue thin film devices. Opt. Express 2015, 23, 13725–13733. [Google Scholar] [CrossRef]
- Goda, E.S.; Lee, S.; Sohail, M.; Yoon, K.R. Prussian blue and its analogues as advanced supercapacitor electrodes. J. Energy Chem. 2020, 50, 206–229. [Google Scholar] [CrossRef]
- Fornasieri, G.; Bordage, A.; Bleuzen, A. Magnetism and Photomagnetism of Prussian Blue Analogue Nanoparticles Embedded in Porous Metal Oxide Ordered Nanostructures. Eur. J. Inorg. Chem. 2018, 2018, 259–271. [Google Scholar] [CrossRef]
- Baggio, B.F.; Vicente, C.; Pelegrini, S.; Plá Cid, C.C.; Brandt, I.S.; Tumelero, M.A.; Pasa, A.A. Morphology and Structure of Electrodeposited Prussian Blue and Prussian White Thin Films. Materials 2019, 12, 1103. [Google Scholar] [CrossRef]
- Lei, S.; Li, Q.-H.; Kang, Y.; Gu, Z.-G.; Zhang, J. Epitaxial growth of oriented prussian blue analogue derived well-aligned CoFe2O4 thin film for efficient oxygen evolution reaction. Appl. Catal. B 2019, 245, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.; Nminibapiel, D.; Shrestha, P.; Liu, J.; Guo, W.; Weidler, P.G.; Baumgart, H.; Wöll, C.; Redel, E. Resistive Switching Nanodevices Based on Metal–Organic Frameworks. ChemNanoMat 2016, 2, 67–73. [Google Scholar] [CrossRef]
- Sato, O.; Kawakami, T.; Kimura, M.; Hishiya, S.; Kubo, S.; Einaga, Y. Electric-field-induced conductance switching in FeCo Prussian blue analogues. J. Am. Chem. Soc. 2004, 126, 13176–13177. [Google Scholar] [CrossRef] [PubMed]
- Alamini, M.F.; da Silva, R.C.; Zoldan, V.C.; Isoppo, E.A.; Rodrigues Filho, U.P.; Aarão Reis, F.D.A.; Klein, A.N.; Pasa, A.A. Normal versus anomalous roughening in electrodeposited Prussian Blue layers. Electrochem. Commun. 2011, 13, 1455–1458. [Google Scholar] [CrossRef]
- Kettle, S.F.A.; Diana, E.; Marchese, E.M.C.; Boccaleri, E.; Stanghellini, P.L. The vibrational spectra of the cyanide ligand revisited: The ν (CN) infrared and Raman spectroscopy of Prussian blue and its analogues. J. Raman Spectrosc. 2011, 42, 2006–2014. [Google Scholar] [CrossRef]
- Kettle, S.F.A.; Diana, E.; Boccaleri, E.; Stanghellini, P.L. The vibrational spectra of the cyanide ligand revisited: Bridging cyanides. Inorg. Chem. 2007, 46, 2409–2416. [Google Scholar] [CrossRef]
- Moretti, G.; Gervais, C. Raman spectroscopy of the photosensitive pigment Prussian blue. J. Raman Spectrosc. 2018, 49, 1198–1204. [Google Scholar] [CrossRef]
- Gervais, C.; Languille, M.A.; Réguer, S.; Gillet, M.; Pelletier, S.; Garnier, C.; Vicenzi, E.P.; Bertrand, L. Why does Prussian blue fade? Understanding the role(s) of the substrate. J. Anal. At. Spectrom. 2013, 28, 1600–1609. [Google Scholar] [CrossRef]
- Mažeikiene, R.; Niaura, G.; Malinauskas, A. Electrochemical redox processes at cobalt hexacyanoferrate modified electrodes: An in situ Raman spectroelectrochemical study. J. Electroanal. Chem. 2014, 719, 60–71. [Google Scholar] [CrossRef]
- Grandjean, F.; Samain, L.; Long, G.J. Characterization and utilization of Prussian blue and its pigments. Dalt. Trans. 2016, 45, 18018–18044. [Google Scholar] [CrossRef]
- Wanger, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, L.E. Handbook of X-ray Photoelectron Spectroscopy; Wiley: Hoboken, NJ, USA, 1979. [Google Scholar]
- Chen, L.; Xu, Y.; Sun, Q.Q.; Liu, H.; Gu, J.J.; Ding, S.J.; Zhang, D.W. Highly uniform bipolar resistive switching with Al2O3 buffer layer in robust NbAlO-based RRAM. IEEE Electron. Device Lett. 2010, 31, 356–358. [Google Scholar] [CrossRef]
- Choi, B.J.; Jeong, D.S.; Kim, S.K.; Rohde, C.; Choi, S.; Oh, J.H.; Kim, H.J.; Hwang, C.S.; Szot, K.; Waser, R.; et al. Resistive switching mechanism of TiO2 thin films grown by atomic-layer deposition. J. Appl. Phys. 2005, 98, 033715. [Google Scholar] [CrossRef]
- Kim, K.M.; Choi, B.J.; Shin, Y.C.; Choi, S.; Hwang, C.S.; Kim, K.M.; Choi, B.J.; Shin, Y.C.; Choi, S.; Seong, C. Anode-interface localized filamentary mechanism in resistive switching of TiO2 thin films. 012907, 9. [CrossRef]
- Peng, H.Y.; Li, G.P.; Ye, J.Y.; Wei, Z.P.; Zhang, Z.; Wang, D.D.; Xing, G.Z.; Wu, T. Electrode dependence of resistive switching in Mn-doped ZnO: Filamentary versus interfacial mechanisms. Appl. Phys. Lett. 2010, 96, 192113. [Google Scholar] [CrossRef]
- Hosseini, P.; Wolkersdörfer, K.; Wark, M.; Redel, E.; Baumgart, H.; Wittstock, G. Morphology and Conductivity of Copper Hexacyanoferrate Films. J. Phys. Chem. C 2020, 124, 16849–16859. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila, L.B.; Müller, C.K.; Hildebrand, D.; Faita, F.L.; Baggio, B.F.; Cid, C.C.P.; Pasa, A.A. Resistive Switching in Electrodeposited Prussian Blue Layers. Materials 2020, 13, 5618. https://doi.org/10.3390/ma13245618
Avila LB, Müller CK, Hildebrand D, Faita FL, Baggio BF, Cid CCP, Pasa AA. Resistive Switching in Electrodeposited Prussian Blue Layers. Materials. 2020; 13(24):5618. https://doi.org/10.3390/ma13245618
Chicago/Turabian StyleAvila, Lindiomar Borges, Christian K. Müller, Dirk Hildebrand, Fabrício L. Faita, Bruna F. Baggio, Cristiani C. Plá Cid, and André A. Pasa. 2020. "Resistive Switching in Electrodeposited Prussian Blue Layers" Materials 13, no. 24: 5618. https://doi.org/10.3390/ma13245618
APA StyleAvila, L. B., Müller, C. K., Hildebrand, D., Faita, F. L., Baggio, B. F., Cid, C. C. P., & Pasa, A. A. (2020). Resistive Switching in Electrodeposited Prussian Blue Layers. Materials, 13(24), 5618. https://doi.org/10.3390/ma13245618





