Titanium Porous Coating Using 3D Direct Energy Deposition (DED) Printing for Cementless TKA Implants: Does It Induce Chronic Inflammation?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Manufacturing of Specimens
2.2. Sample Preparation and Test Groups
2.3. In Vitro Preparation
2.3.1. Viability Analysis on Culture of Fibroblasts
2.3.2. Inflammatory Multiplex Cytokine Assay
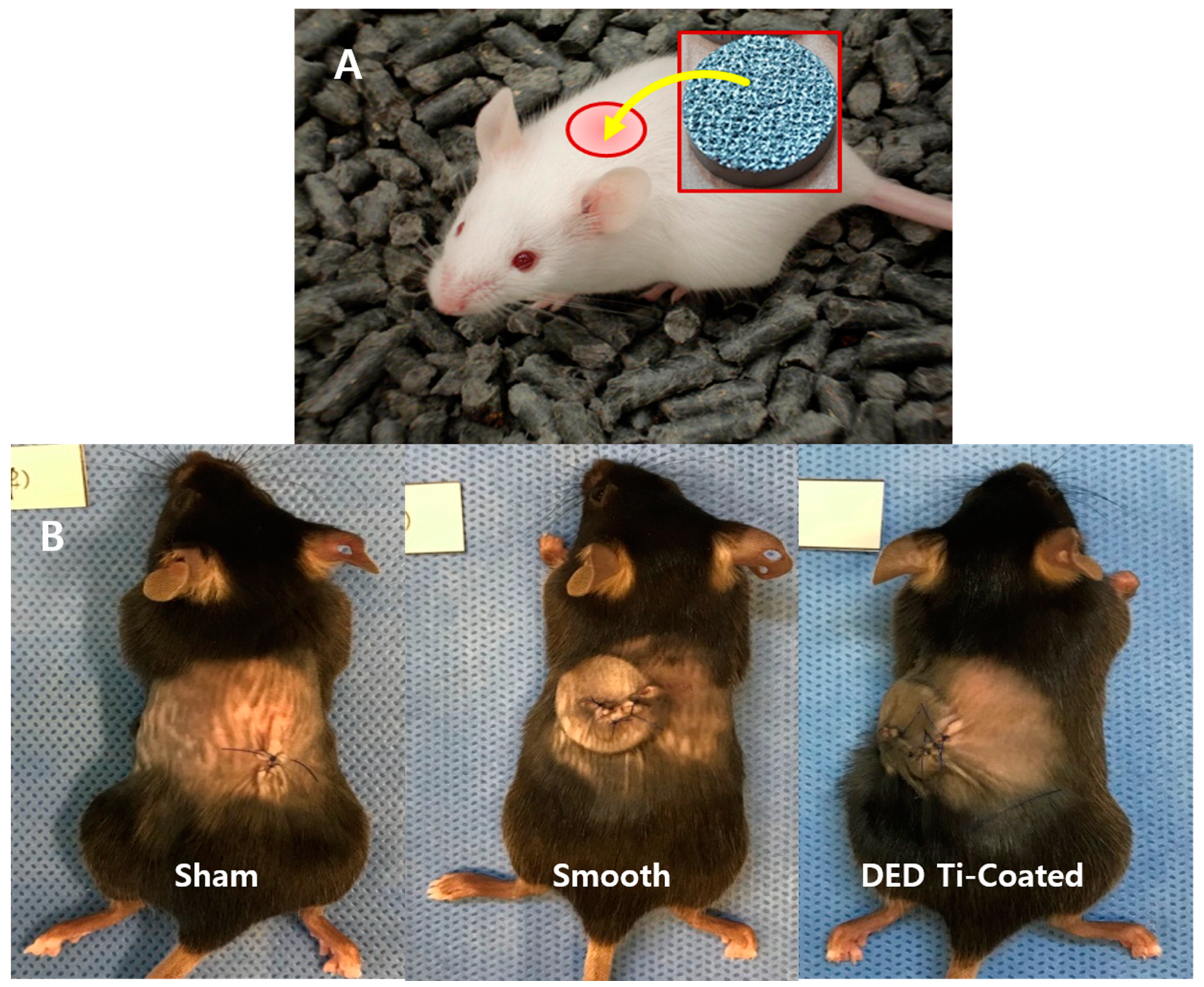
2.4. In Vivo Preparation
2.4.1. Immuno Profiling and Inflammatory Multiplex Cytokine Assay
2.4.2. Tissue Histomorphometry
2.5. Statistical Analysis
3. Results
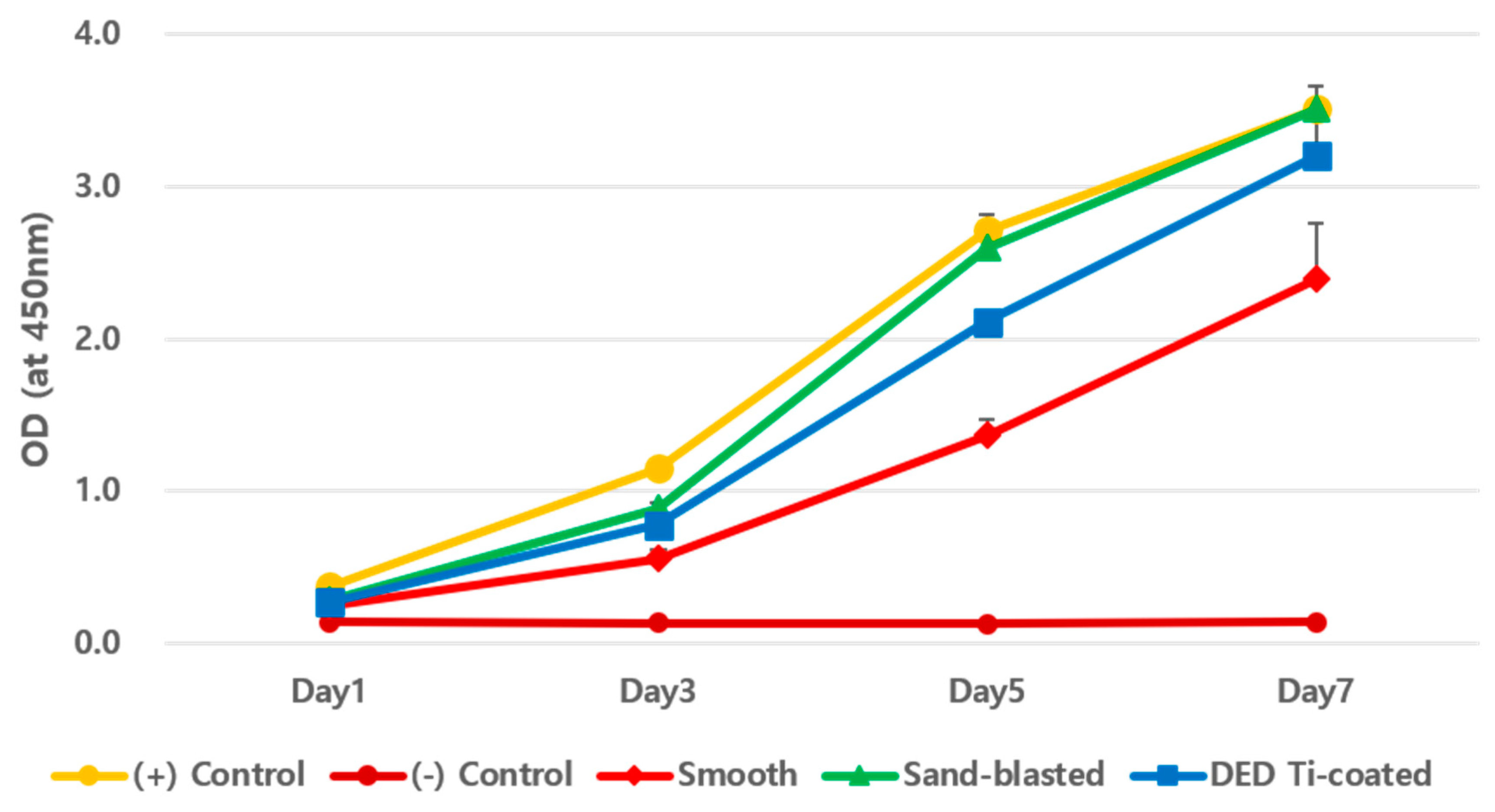
3.1. In Vitro CCK-8 Assay
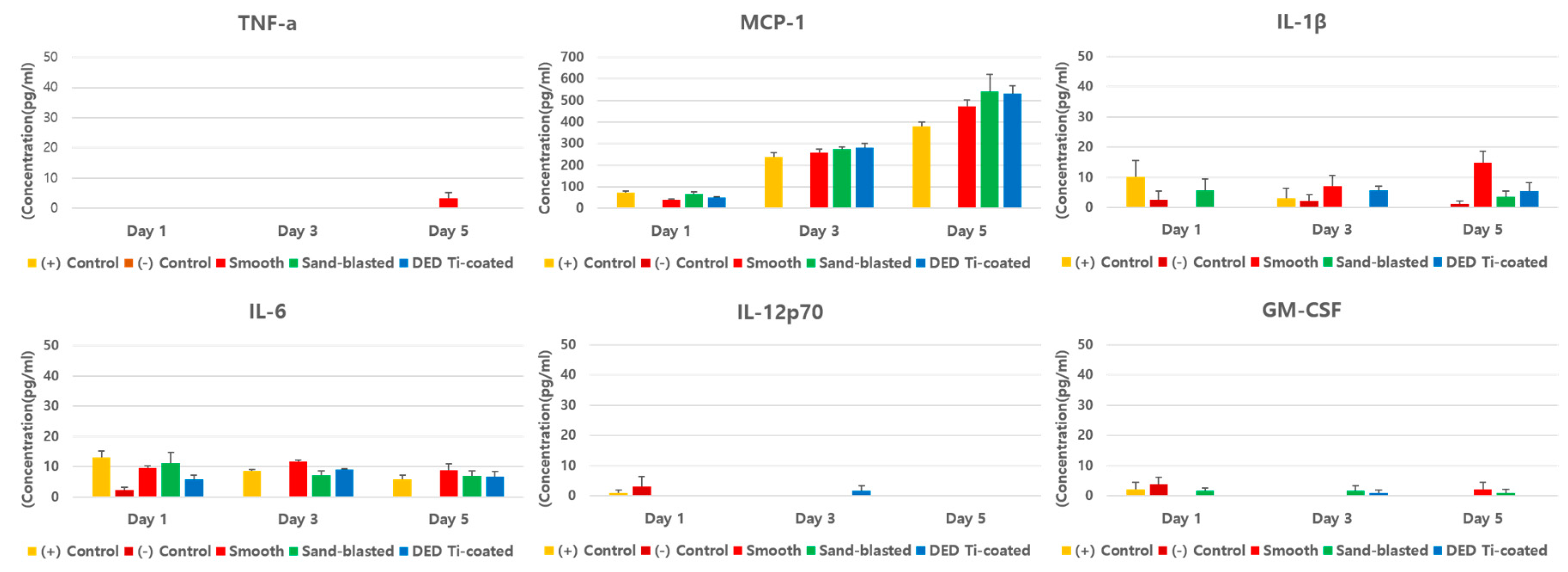
3.2. In Vitro Inflammatory Cytokine Assay
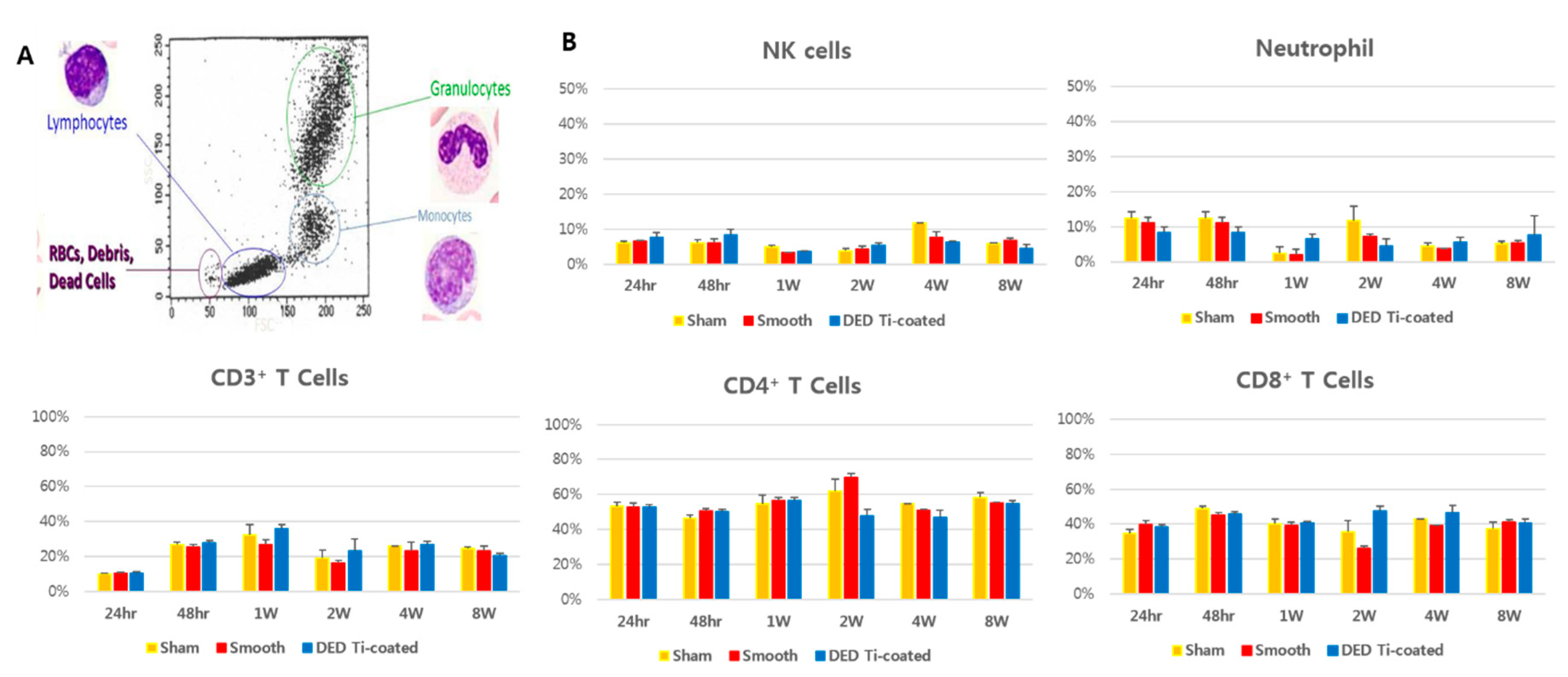
3.3. In Vivo Immune Profiling
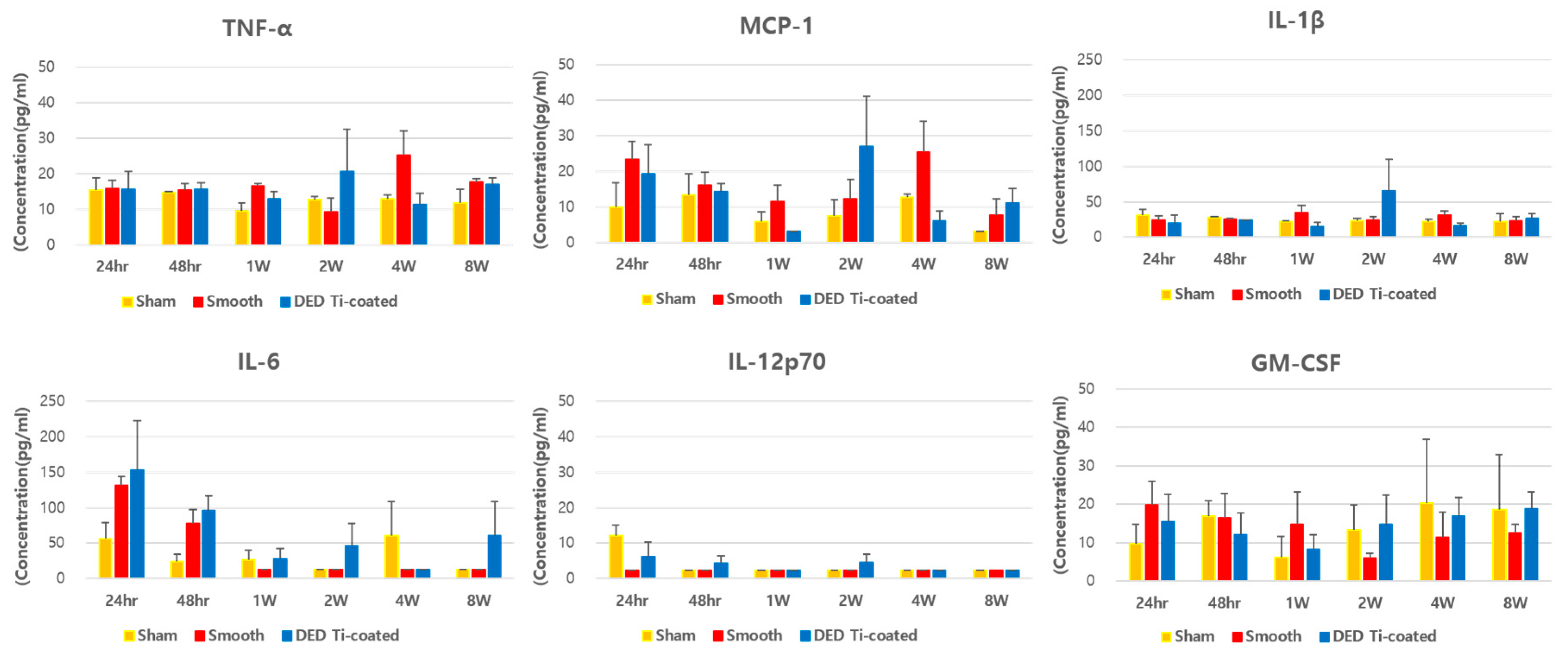
3.4. In Vivo Inflammatory Cytokine Assay
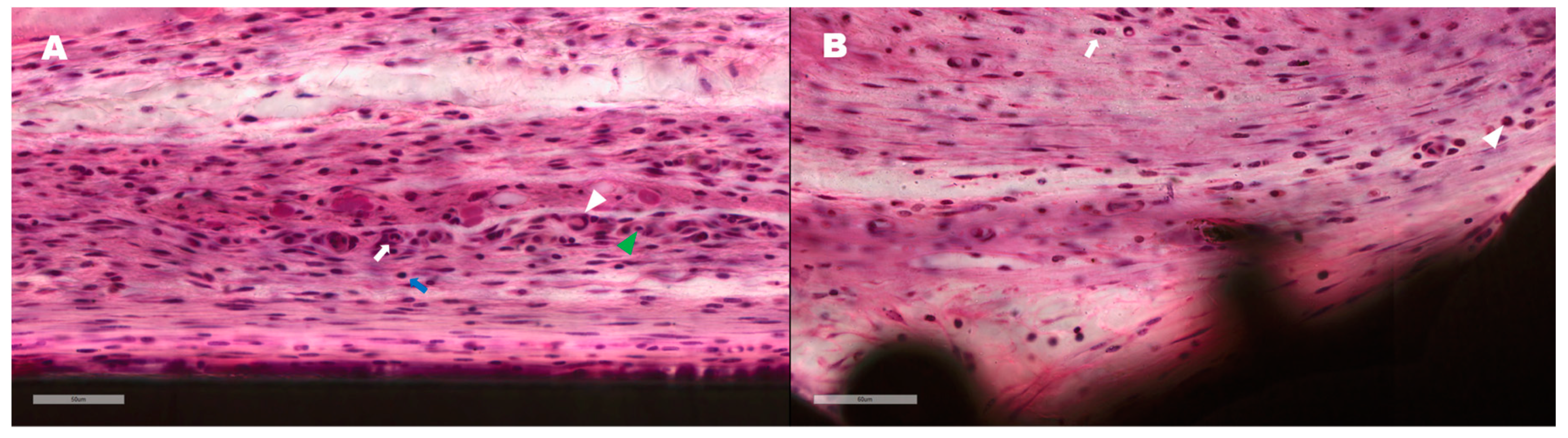
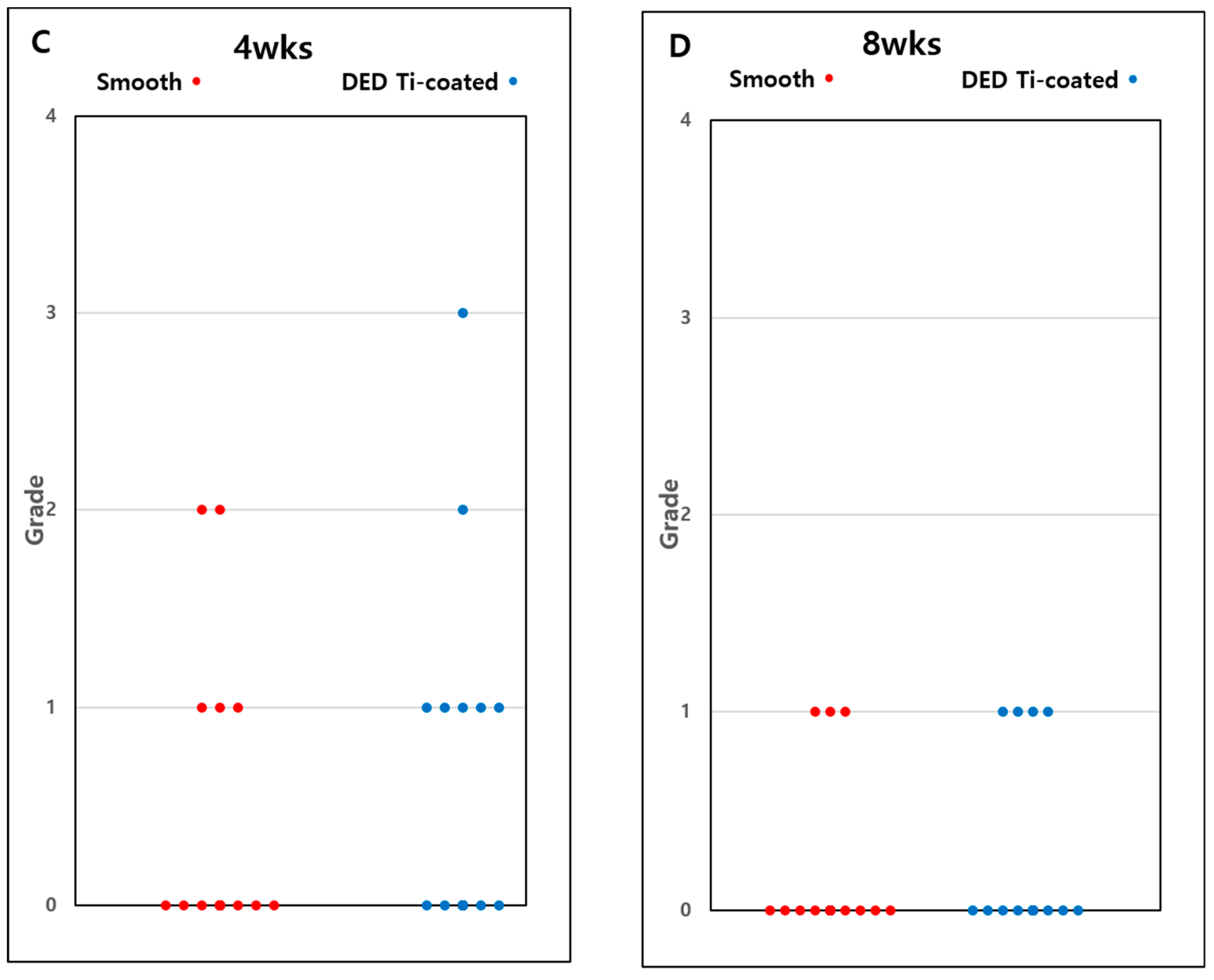
3.5. In Vivo Histomorphometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prudhon, J.-L.; Verdier, R. Cemented or cementless total knee arthroplasty? SICOT J. 2017, 3, 70. [Google Scholar] [CrossRef] [PubMed]
- Karachalios, T.; Komnos, G.; Amprazis, V.; Antoniou, I.; Athanaselis, S. A 9-Year Outcome Study Comparing Cancellous Titanium-Coated Cementless to Cemented Tibial Components of a Single Knee Arthroplasty Design. J. Arthroplast. 2018, 33, 3672–3677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo, J.; Goodman, S.B.; Konttinen, Y.T.; Wimmer, M.A.; Holinka, M. Osteolysis around Total Knee Arthroplasty: A Review of Pathogenetic Mechanisms. Acta Biomater. 2013, 9, 8046–8058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Rourke, M.R.; Callaghan, J.J.; Goetz, D.D.; Sullivan, P.M.; Johnston, R.C. Osteolysis Associated with a Cemented Modular Posterior-Cruciate-Substituting Total Knee Design: Five to Eight-Year Follow-Up. J. Bone Joint. Surg. Am. 2002, 84, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Naudie, D.D.R.; Ammeen, D.J.; Engh, G.A.; Rorabeck, C.H. Wear and Osteolysis around Total Knee Arthroplasty. J. Am. Acad. Orthop. Surg. 2007, 15, 53–64. [Google Scholar] [CrossRef]
- Boyle, K.K.; Nodzo, S.R.; Ferraro, J.T.; Augenblick, D.J.; Pavlesen, S.; Phillips, M.J. Uncemented vs Cemented Cruciate Retaining Total Knee Arthroplasty in Patients with Body Mass Index Greater Than 30. J. Arthroplast. 2018, 33, 1082–1088. [Google Scholar] [CrossRef]
- Ingham, E.; Green, T.R.; Stone, M.H.; Kowalski, R.; Watkins, N.; Fisher, J. Production of TNF-Alpha and Bone Resorbing Activity by Macrophages in Response to Different Types of Bone Cement Particles. Biomaterials 2000, 21, 1005–1013. [Google Scholar] [CrossRef]
- Meneghini, R.M.; Hanssen, A.D. Cementless Fixation in Total Knee Arthroplasty: Past, Present, and Future. J. Knee Surg. 2008, 21, 307–314. [Google Scholar] [CrossRef]
- Lombardi, A.V.; Berasi, C.C.; Berend, K.R. Evolution of Tibial Fixation in Total Knee Arthroplasty. J. Arthroplast. 2007, 22, 25–29. [Google Scholar] [CrossRef]
- Illgen, R.; Tueting, J.; Enright, T.; Schreibman, K.; McBeath, A.; Heiner, J. Hybrid Total Knee Arthroplasty: A Retrospective Analysis of Clinical and Radiographic Outcomes at Average 10 Years Follow-Up. J. Arthroplast. 2004, 19, 95–100. [Google Scholar] [CrossRef]
- Rand, J.A.; Trousdale, R.T.; Ilstrup, D.M.; Harmsen, W.S. Factors Affecting the Durability of Primary Total Knee Prostheses. J. Bone Joint. Surg. Am. 2003, 85, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Robertsson, O.; Bizjajeva, S.; Fenstad, A.M.; Furnes, O.; Lidgren, L.; Mehnert, F.; Odgaard, A.; Pedersen, A.B.; Havelin, L.I. Knee Arthroplasty in Denmark, Norway and Sweden: A Pilot Study from the Nordic Arthroplasty Register Association. Acta Orthop. 2010, 81, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Saldívar-García, A.J.; López, H.F. Microstructural Effects on the Wear Resistance of Wrought and As-Cast Co-Cr-Mo-C Implant Alloys. J. Biomed. Mater. Res. A 2005, 74, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Jo, W.L.; Kim, Y.S.; Kwon, S.Y.; Cho, Y.S.; Lim, Y.W. Titanium Powder Coating Using Metal 3D Printing: A Novel Coating Technology for Cobalt–Chromium Alloy Implants. Tissue Eng. Regen. Med. 2019, 16, 11–18. [Google Scholar] [CrossRef]
- Grandfield, K.; Palmquist, A.; Gonçalves, S.; Taylor, A.; Taylor, M.; Emanuelsson, L.; Thomsen, P.; Engqvist, H. Free Form Fabricated Features on CoCr Implants with and without Hydroxyapatite Coating in vivo: A Comparative Study of Bone Contact and Bone Growth Induction. J. Mater. Sci. Mater. Med. 2011, 22, 899–906. [Google Scholar] [CrossRef]
- Sotereanos, N.G.; Engh, C.A.; Glassman, A.H.; Macalino, G.E.; Engh, C.A. Cementless Femoral Components should be Made from Cobalt Chrome. Clin. Orthop. Relat. Res. 1995, 313, 146–153. [Google Scholar]
- Franceschetti, E.; Torre, G.; Palumbo, A.; Papalia, R.; Karlsson, J.; Ayeni, O.R.; Samuelsson, K.; Franceschi, F. No Difference Between Cemented and Cementless Total Knee Arthroplasty in Young Patients: A Review of the Evidence. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 1749–1756. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, H.; Li, J.; Wang, H.; Zhou, Z.; Pei, F. No Difference in Implant Survivorship and Clinical Outcomes between Full-Cementless and Full-Cemented Fixation in Primary Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. Int. J. Surg. 2018, 53, 312–319. [Google Scholar] [CrossRef]
- Nam, D.; Lawrie, C.M.; Salih, R.; Nahhas, C.R.; Barrack, R.L.; Nunley, R.M. Cemented Versus Cementless Total Knee Arthroplasty of the Same Modern Design: A Prospective, Randomized Trial. J. Bone Jt. Surg. Am. 2019, 101, 1185. [Google Scholar] [CrossRef]
- Shah, F.A.; Omar, O.; Suska, F.; Snis, A.; Matic, A.; Emanuelsson, L.; Norlindh, B.; Lausmaa, J.; Thomsen, P.; Palmquist, A. Long-Term Osseointegration of 3D Printed CoCr Constructs with an Interconnected Open-Pore Architecture Prepared by Electron Beam Melting. Acta Biomater. 2016, 36, 296–309. [Google Scholar] [CrossRef] [Green Version]
- Murr, L.E.; Gaytan, S.M.; Martinez, E.; Medina, F.; Wicker, R.B. Next Generation Orthopaedic Implants by Additive Manufacturing Using Electron Beam Melting. Int. J. Biomater. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.V.; Muller-Kamskii, G.; Kovalevsky, A.; Dzhenzhera, G.; Strokin, E.; Kolomiets, A.; Ramon, J. Design and 3D-Printing of Titanium Bone Implants: Brief Review of Approach and Clinical Cases. Biomed. Eng. Lett. 2018, 8, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Khosroshahi, M.E. Surface Morphology Characterization of Laser-Induced Titanium Implants: Lesson to Enhance Osseointegration Process. Biomed. Eng. Lett. 2018, 8, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Sukur, E.; Akman, Y.E.; Ozturkmen, Y.; Kucukdurmaz, F. Particle Disease: A Current Review of the Biological Mechanisms in Periprosthetic Osteolysis After Hip Arthroplasty. Open Orthop. J. 2016, 10, 241–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bressan, E.; Ferroni, L.; Gardin, C.; Bellin, G.; Sbricoli, L.; Sivolella, S.; Brunello, G.; Schwartz-Arad, D.; Mijiritsky, E.; Penarrocha, M.; et al. Metal Nanoparticles Released from Dental Implant Surfaces: Potential Contribution to Chronic Inflammation and Peri-Implant Bone Loss. Materials 2019, 12, 2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amirhosseini, M.; Andersson, G.; Aspenberg, P.; Fahlgren, A. Mechanical Instability and Titanium Particles Induce Similar Transcriptomic Changes in a Rat Model for Periprosthetic Osteolysis and Aseptic Loosening. Bone Rep. 2017, 7, 17–25. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, Z.; Shi, J.; Wang, L.; Hou, Z.; Guo, X.; Tao, Y.; Wu, X.; Zhou, W.; Liu, Y.; et al. Titanium Particle-Induced Osteogenic Inhibition and Bone Destruction are Mediated by the GSK-3 β/β -Catenin Signal Pathway. Cell Death Dis. 2017, 8, e2878. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Guo, C.-C.; Gao, Z.-Y.; Wang, C.-Y.; Zhang, H.-N.; Lv, C.-Y.; Yin, Z.-Y.; Wang, Y.-Z. Micrometer-Sized Titanium Particles Induce Aseptic Loosening in Rabbit Knee. Biomed. Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Shin, T.; Kim, Y.-S.; Kim, J.; Lee, K.-Y.; Lee, S.-J.; Sun, D.; Lim, Y.-W.; Lim, D. Applicability Evaluation of Direct Metal Tooling-Based Additive Manufacturing for Reducing Ceramic Liner Fracture in Total Hip Arthroplasty. Surf. Coat. Technol. 2018, 347, 313–319. [Google Scholar] [CrossRef]
- Shin, T.; Park, S.-J.; Kang, K.S.; Kim, J.S.; Kim, Y.; Lim, Y.; Lim, D. A Laser-Aided Direct Metal Tooling Technology for Artificial Joint Surface Coating. Int. J. Precis. Eng. Manuf. 2017, 18, 233–238. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Vu, N.B.; Truong, N.H.; Dang, L.T.; Phi, L.T.; Ho, N.T.-T.; Pham, T.N.; Phan, T.P.; Pham, P.V. In Vitro and In Vivo Biocompatibility of Ti-6Al-4V Titanium Alloy and UHMWPE Polymer for Total Hip Replacement. Biomed. Res. Ther. 2016, 3, 567–577. [Google Scholar] [CrossRef]
- Lehmann, J.S.; Zhao, A.; Sun, B.; Jiang, W.; Ji, S. Multiplex Cytokine Profiling of Stimulated Mouse Splenocytes Using a Cytometric Bead-Based Immunoassay Platform. J. Vis. Exp. 2017, 129, e56440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Yang, P.; Guo, X.; Huang, N.; Shen, R. An In Vitro Evaluation of Inflammation Response of Titanium Functionalized with Heparin/Fibronectin Complex. Cytokine 2011, 56, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Pajarinen, J.; Nabeshima, A.; Lin, T.-H.; Sato, T.; Gibon, E.; Jämsen, E.; Lu, L.; Nathan, K.; Yao, Z.; Goodman, S.B. Murine Model of Progressive Orthopedic Wear Particle-Induced Chronic Inflammation and Osteolysis. Tissue Eng. Part C Methods 2017, 23, 1003–1011. [Google Scholar] [CrossRef]
- Miron, R.J.; Sculean, A.; Shuang, Y.; Bosshardt, D.D.; Gruber, R.; Buser, D.; Chandad, F.; Zhang, Y. Osteoinductive Potential of a Novel Biphasic Calcium Phosphate Bone Graft in Comparison with Autographs, Xenografts, and DFDBA. Clin. Oral Implants Res. 2016, 27, 668–675. [Google Scholar] [CrossRef]
- Schwartz, Z.; Hyzy, S.L.; Moore, M.A.; Hunter, S.A.; Ronholdt, C.J.; Sunwoo, M.; Boyan, B.D. Osteoinductivity of Demineralized Bone Matrix Is Independent of Donor Bisphosphonate Use. J. Bone Jt. Surg. Am. 2011, 93, 2278–2286. [Google Scholar] [CrossRef] [Green Version]
- Sampatanukul, T.; Serichetaphongse, P.; Pimkhaokham, A. Histological Evaluations and Inflammatory Responses of Different Dental Implant Abutment materials: A Human Histology Pilot Study. Clin. Implant Dent. Relat. Res. 2018, 20, 160–169. [Google Scholar] [CrossRef]
- Li, B.; Cao, H.; Zhao, Y.; Cheng, M.; Qin, H.; Cheng, T.; Hu, Y.; Zhang, X.; Liu, X. In Vitro and In Vivo Responses of Macrophages to Magnesium-Doped Titanium. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Shigematsu, H.; Kumagai, K.; Kobayashi, H.; Eguchi, T.; Kitaura, K.; Suzuki, S.; Horikawa, T.; Matsutani, T.; Ogasawara, K.; Hamada, Y.; et al. Accumulation of Metal-Specific T Cells in Inflamed Skin in a Novel Murine Model of Chromium-Induced Allergic Contact Dermatitis. PLoS ONE 2014, 9, e85983. [Google Scholar] [CrossRef]
- Schuler, M.; Trentin, D.; Textor, M.; Tosatti, S.G.P. Biomedical Interfaces: Titanium Surface Technology for Implants and Cell Carriers. Nanomedicine 2006, 1, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.J.; Jacobs, J.J. Biologic Effects of Implant Debris. Bull. NYU Hosp. Jt. Dis. 2009, 67, 182–188. [Google Scholar] [PubMed]
- Grosse, S.; Haugland, H.K.; Lilleng, P.; Ellison, P.; Hallan, G.; Høl, P.J. Wear Particles and Ions from Cemented and Uncemented Titanium-Based Hip Prostheses—A Histological and Chemical Analysis of Retrieval Material. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 709–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haleem-Smith, H.; Argintar, E.; Bush, C.; Hampton, D.; Postma, W.F.; Chen, F.H.; Rimington, T.; Lamb, J.; Tuan, R.S. Biological Responses of Human Mesenchymal Stem Cells to Titanium Wear Debris Particles. J. Orthop. Res. 2012, 30, 853–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salou, L.; Hoornaert, A.; Louarn, G.; Layrolle, P. Enhanced Osseointegration of Titanium Implants with Nanostructured Surfaces: An experimental Study in Rabbits. Acta Biomater. 2015, 11, 494–502. [Google Scholar] [CrossRef]
- Popov, V.V.; Muller-Kamskii, G.; Katz-Demyanetz, A.; Kovalevsky, A.; Usov, S.; Trofimcow, D.; Dzhenzhera, G.; Koptyug, A. Additive Manufacturing to Veterinary Practice: Recovery of Bony Defects after the Osteosarcoma Resection in Canines. Biomed. Eng. Lett. 2019, 9, 97–108. [Google Scholar] [CrossRef]
- Agarwal, R.; García, A.J. Biomaterial Strategies for Engineering Implants for Enhanced Osseointegration and Bone Repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Seemann, S.; Zohles, F.; Lupp, A. Comprehensive Comparison of Three Different Animal Models for Systemic Inflammation. J. Biomed. Sci. 2017, 24, 60. [Google Scholar] [CrossRef]
- Tao, L.; Reese, T.A. Making Mouse Models that Reflect Human Immune Responses. Trends Immunol. 2017, 38, 181–193. [Google Scholar] [CrossRef] [Green Version]
- Bracken, M.B. Why Animal Studies are Often Poor Predictors of Human Reactions to Exposure. J. R. Soc. Med. 2009, 102, 120–122. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, D.J.; Sonn, C.-H.; Hong, D.H.; Kwon, K.B.; Park, S.J.; Ban, H.Y.; Kwak, T.Y.; Lim, D.; Wang, J.H. Titanium Porous Coating Using 3D Direct Energy Deposition (DED) Printing for Cementless TKA Implants: Does It Induce Chronic Inflammation? Materials 2020, 13, 472. https://doi.org/10.3390/ma13020472
Ryu DJ, Sonn C-H, Hong DH, Kwon KB, Park SJ, Ban HY, Kwak TY, Lim D, Wang JH. Titanium Porous Coating Using 3D Direct Energy Deposition (DED) Printing for Cementless TKA Implants: Does It Induce Chronic Inflammation? Materials. 2020; 13(2):472. https://doi.org/10.3390/ma13020472
Chicago/Turabian StyleRyu, Dong Jin, Chung-Hee Sonn, Da Hee Hong, Kyeu Back Kwon, Sang Jun Park, Hun Yeong Ban, Tae Yang Kwak, Dohyung Lim, and Joon Ho Wang. 2020. "Titanium Porous Coating Using 3D Direct Energy Deposition (DED) Printing for Cementless TKA Implants: Does It Induce Chronic Inflammation?" Materials 13, no. 2: 472. https://doi.org/10.3390/ma13020472




