Metal Ions Supported Porous Coatings by Using AC Plasma Electrolytic Oxidation Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Characterization Methods
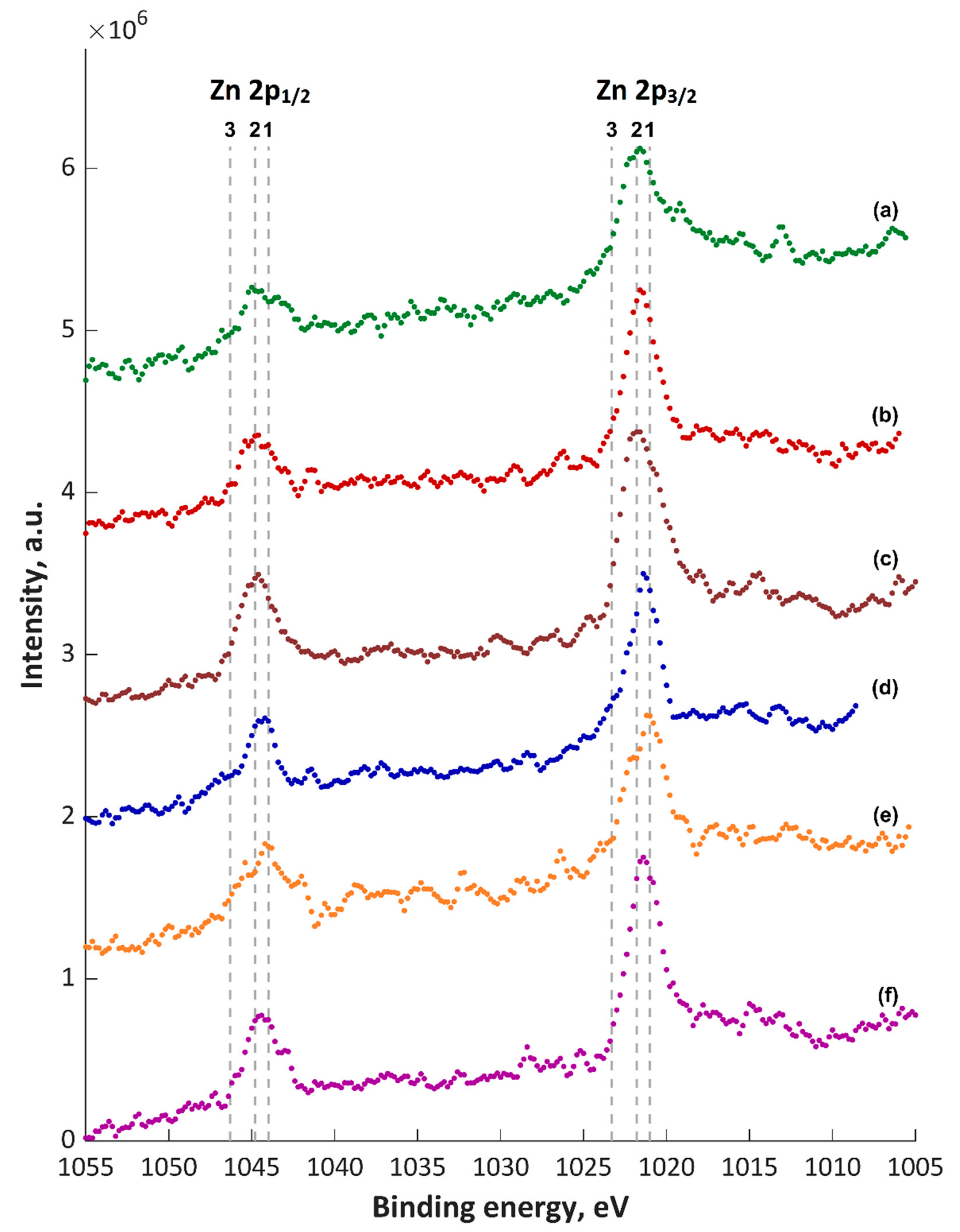
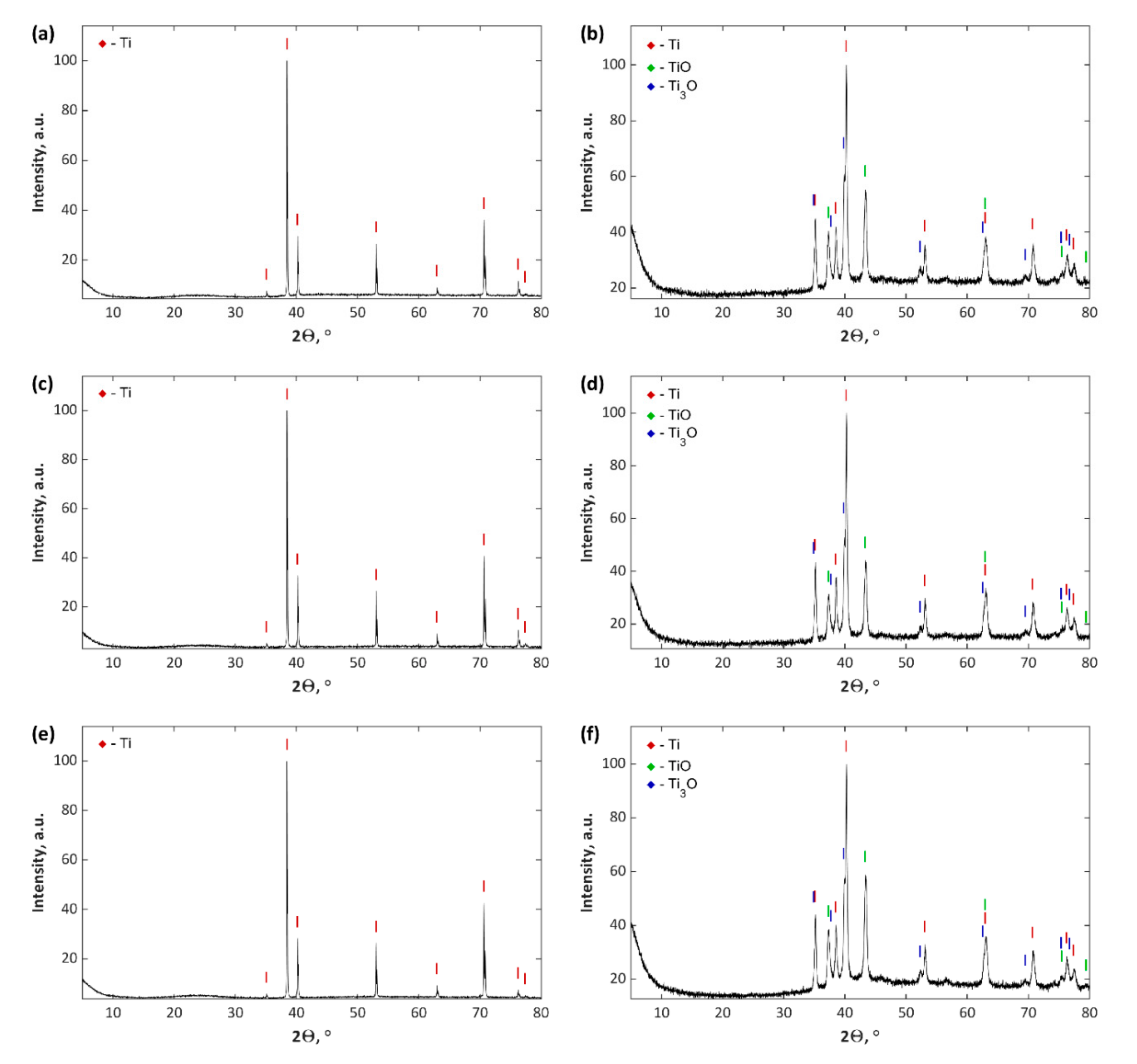
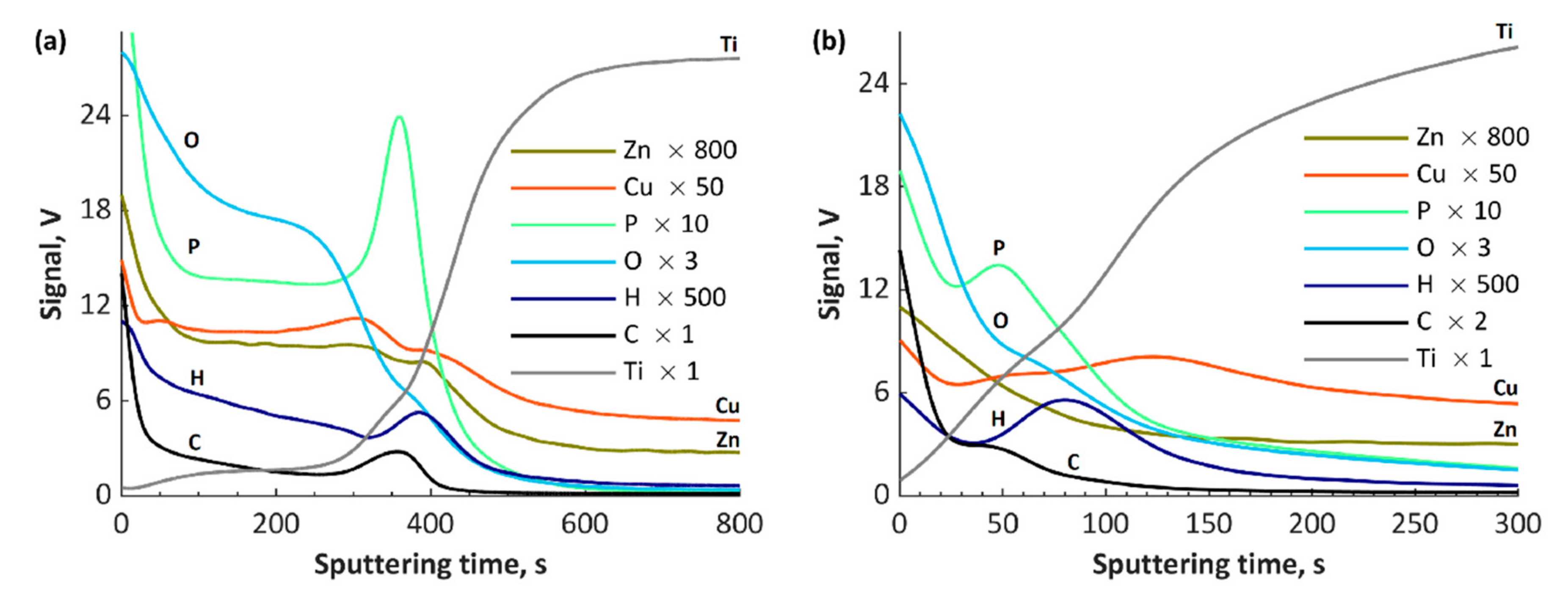
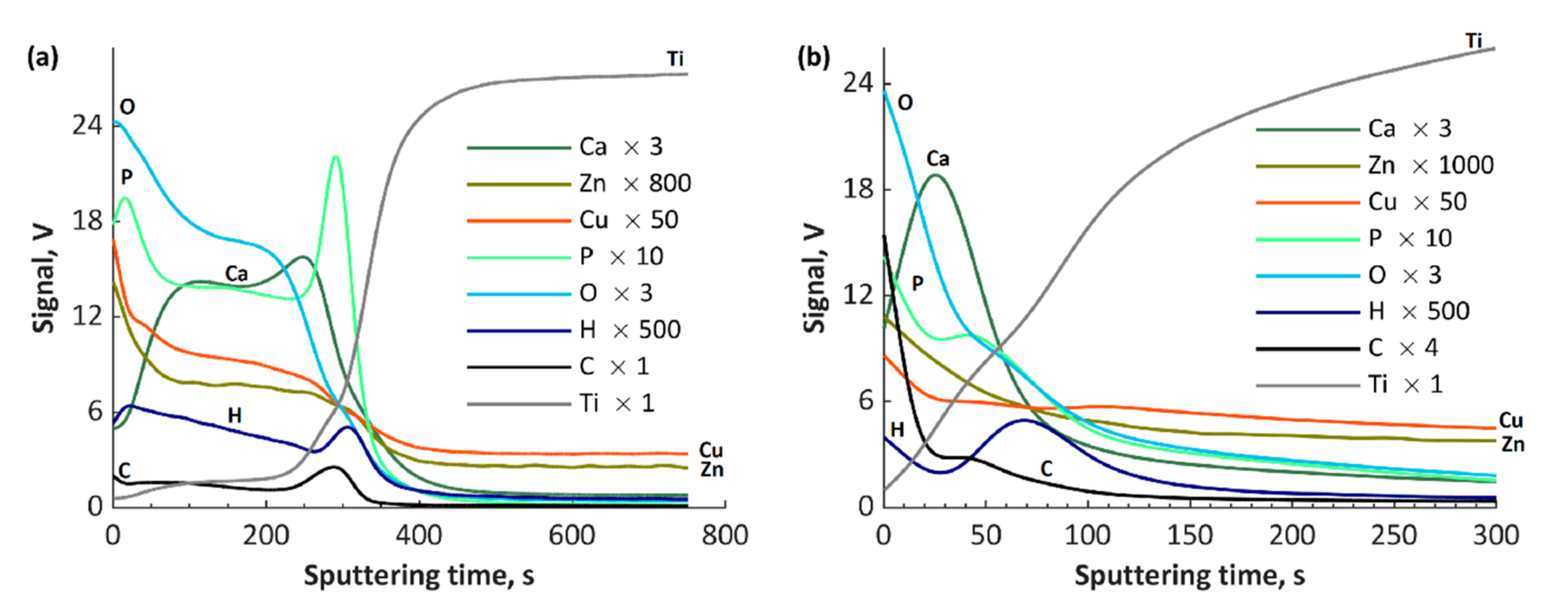
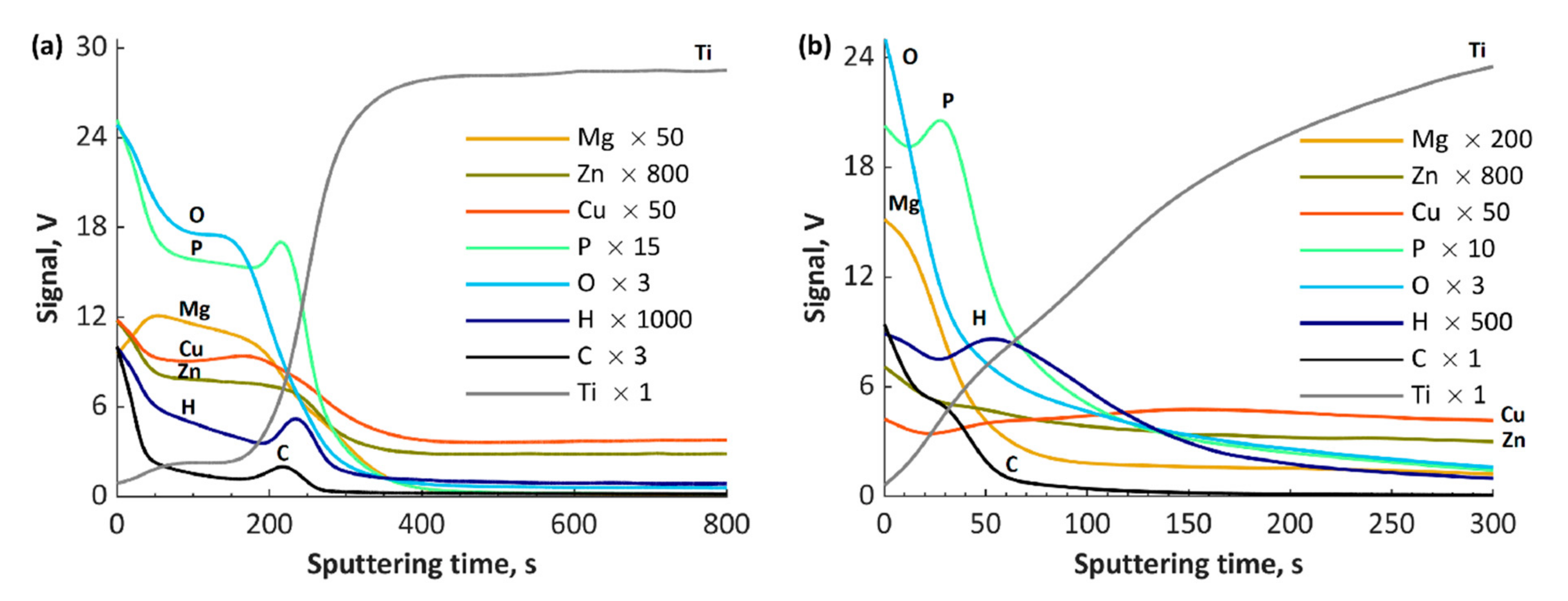
3. Results and Discussion
4. Conclusions
- The AC-PEO process on titanium, performed in electrolytes based on concentrated phosphoric acid with the additions of zinc, copper, calcium and/or magnesium nitrites, using the same anodic voltage of +400 V as well as two cathodic ones, i.e., −35 and −135 V, with a frequency of 5 kHz, results in obtaining coatings with similar chemical composition of top layers, but drastically different morphological properties.
- Increase of negative cathodic voltage in the AC-PEO process from −35 to −135 V results in a significant reduction of coatings thickness and corrosion resistance as well as increase in the proportion of the crystalline-to-amorphous phase in the coatings.
- Atomic concentrations of Ca2+ and Mg2+ ions (5.0–5.4 at% and 5.6–6.5 at%, respectively) in the top layer values differ by one order of the magnitude in comparison to the atomic concentrations of Cu+/Cu2+ and Zn2+ ions (0.3–0.6 at% and 0.4–0.6 at%, respectively) despite the similar concentrations in the electrolyte.
- During the negative voltage pulse in the AC-PEO process, the reductions of some ions, e.g., Cu2+ or Ti4+, can occur, which results in their presence in the top layers.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kaneko, H.; Uchiyama, M.; Nojiri, H.; Kiritani, N. Electrochemical Etching Method. U.S. Patent 5,167,778, 5 August 1992. [Google Scholar]
- Babilas, D.; Urbanczyk, E.; Sowa, M.; Maciej, A.; Korotin, D.M.; Zhidkov, I.S.; Basiaga, M.; Krok-Borkowicze, M.; Szyk-Warszynska, L.; Pamuła, E.; et al. On the electropolishing and anodic oxidation of Ti-15Mo alloy. Electrochim. Acta 2016, 205, 256–265. [Google Scholar] [CrossRef]
- Rokosz, K.; Simon, F.; Hryniewicz, T.; Rzadkiewicz, S. Comparative XPS analysis of passive layers composition formed on AISI 304 L SS after standard and high-current density electropolishing. Surf. Interface Anal. 2015, 47, 87–92. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Raaen, S. XPS analysis of nanolayer formed on AISI 304L SS after high-voltage electropolishing (HVEP). Teh. Vjesn. Tech. Gaz. 2017, 24, 321–326. [Google Scholar]
- Rokosz, K.; Hryniewicz, T.; Raaen, S. Cr/Fe ratio by XPS spectra of magnetoelectropolished AISI 316L SS fitted by gaussian-lorentzian shape lines. Teh. Vjesn. Tech. Gaz. 2014, 21, 533–538. [Google Scholar]
- Rokosz, K. Surface Treatment Technology of Metals and Alloys. Metals 2019, 9, 1134. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Dudek, Ł. Phosphate Porous Coatings Enriched with Selected Elements via PEO Treatment on Titanium and Its Alloys: A Review. Materials 2020, 13, 2468. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Raaen, S. Development of plasma electrolytic oxidation for improved Ti6Al4V biomaterial surface properties. Int. J. Adv. Manuf. Technol. 2016, 85, 2425–2437. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Dudek, L.; Malorny, W. SEM and EDS analysis of nitinol surfaces treated by plasma electrolytic oxidation. Adv. Mater. Sci. 2015, 15, 41–47. [Google Scholar] [CrossRef][Green Version]
- Simka, W.; Krzakała, A.; Korotin, D.M.; Zhidkov, I.S.; Kurmaev, E.Z.; Cholakh, S.O.; Kuna, K.; Dercz, G.; Michalska, J.; Suchanek, K.; et al. Modification of a Ti-Mo alloy surface via plasma electrolytic oxidation in a solution containing calcium and phosphorus. Electrochim. Acta 2013, 96, 180–190. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Raaen, S.; Chapon, P.; Prima, F. Development of copper-enriched porous coatings on ternary Ti-Nb-Zr alloy by plasma electrolytic oxidation. Int. J. Adv. Manuf. Technol. 2017, 89, 2953–2965. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Kacalak, W.; Tandecka, K.; Raaen, S.; Gaiaschi, S.; Chapon, P.; Malorny, W.; Matysek, D.; Pietrzak, K.; et al. Porous Coatings Containing Copper and Phosphorus Obtained by Plasma Electrolytic Oxidation of Titanium. Materials 2020, 13, 828. [Google Scholar] [CrossRef] [PubMed]
- Rokosz, K.; Hryniewicz, T.; Raaen, S.; Matysek, D.; Dudek, L.; Pietrzak, K. SEM, EDS, and XPS characterization of coatings obtained on titanium during AC plasma electrolytic process enriched in magnesium. Adv. Mater. Sci. 2018, 18, 68–78. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Voevodin, A.A.; Lyubimov, V.V.; Zabinski, J.; Donley, M. Plasma electrolytic fabrication of oxide ceramic surface layers for tribotechnical purposes on aluminium alloys. Surf. Coat. Technol. 1998, 110, 140–146. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Kacalak, W.; Tandecka, K.; Raaen, S.; Gaiaschi, S.; Chapon, P.; Malorny, W.; Matysek, D.; Pietrzak, K. Phosphate Coatings Enriched with Copper on Titanium Substrate Fabricated via DC-PEO Process. Materials 2020, 13, 1295. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Gaiaschi, S.; Chapon, P.; Raaen, S.; Matysek, D.; Dudek, L.; Pietrzak, K. Novel Porous Phosphorus-Calcium-Magnesium Coatings on Titanium with Copper or Zinc Obtained by DC Plasma Electrolytic Oxidation: Fabrication and Characterization. Materials 2018, 11, 1680. [Google Scholar] [CrossRef]
- Sowa, M.; Parafiniuk, M.; Mouzelo, C.M.S.; Kazek-Kesik, A.; Zhidkov, I.S.; Kukharenko, A.I.; Cholakh, S.O.; Kurmaev, E.Z.; Simka, W. DC plasma electrolytic oxidation treatment of gum metal for dental implants. Electrochim. Acta 2019, 302, 10–20. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Gaiaschi, S.; Chapon, P.; Raaen, S.; Malorny, W.; Matýsek, D.; Pietrzak, K. Development of Porous Coatings Enriched with Magnesium and Zinc Obtained by DC Plasma Electrolytic Oxidation. Micromachines 2018, 9, 332. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Kacalak, W.; Tandecka, K.; Raaen, S.; Gaiaschi, S.; Chapon, P.; Malorny, W.; Matysek, D.; Dudek, L.; et al. Characterization of Porous Phosphate Coatings Enriched with Calcium, Magnesium, Zinc and Copper Created on CP Titanium Grade 2 by Plasma Electrolytic Oxidation. Metals 2018, 8, 411. [Google Scholar] [CrossRef]
- Torres-Cerón, D.A.; Gordillo-Delgado, F.; Moya-Betancourt, S.N. Effect of the voltage pulse frequency on the structure of TiO2 coatings grown by plasma electrolytic oxidation. J. Phys. Conf. Ser. 2017, 935, 1–7. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Groza, A.; Gaiaschi, S.; Rokosz, K.; Raaen, S.; Negrila, C.C.; Prodan, A.-M.; Costescu, A.; et al. Development of Cerium-Doped Hydroxyapatite Coatings with Antimicrobial Properties for Biomedical Applications. Coatings 2020, 10, 516. [Google Scholar] [CrossRef]
- Lebukhova, N.V.; Rudnev, V.S.; Chigrin, P.G.; Lukiyanchuk, I.V.; Pugachevsky, M.A.; Ustinov, A.Y.; Kirichenko, E.A.; Yarovaya, T.P. The nanostructural catalytic composition CuMoO4/TiO2 + SiO2/Ti for combustion of diesel soot. Surf. Coat. Technol. 2013, 231, 144–148. [Google Scholar] [CrossRef]
- Stojadinovic, S.; Radic, N.; Vasilic, R.; Petkovic, M.; Stefanov, P.; Zekovic, L.; Grbic, B. Photocatalytic properties of TiO2/WO3 coatings formed by plasma electrolytic oxidation of titanium in 12-tungstosilicic acid. Appl. Catal. B Environ. 2012, 126, 334–341. [Google Scholar] [CrossRef]
- Li, L.H.; Kong, Y.M.; Kim, H.W.; Kim, Y.W.; Kim, H.E.; Heo, S.J.; Koak, J.Y. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials 2004, 25, 2867–2875. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.D.; Araujo, J.R.; Landi, S.M.; Kuznetsov, A.; Granjeiro, J.M.; Sena, L.A.D.; Achete, C.A. A study of the physical, chemical and biological properties of TiO2 coatings produced by micro-arc oxidation in a Ca-P-based electrolyte. J. Mater. Sci. 2014, 25, 1769–1780. [Google Scholar] [CrossRef]
- Jin, J.; Li, X.H.; Wu, J.W.; Lou, B.Y. Improving tribological and corrosion resistance of Ti6Al4V alloy by hybrid microarc oxidation/enameling treatments. Rare Met. 2018, 37, 26–34. [Google Scholar] [CrossRef]
- Lebukhov, N.V.; Rudnev, V.S.; Kirichenko, E.A.; Chigrin, P.G.; Lukiyanchuk, I.V.; Karpovicha, N.F.; Pugachevsky, M.A.; Kurjavyj, V.G. The structural catalyst CuMoO4/TiO2/TiO2 + SiO2/Ti for diesel soot combustion. Surf. Coat. Technol. 2015, 261, 344–349. [Google Scholar] [CrossRef]
- Vasilyeva, M.S.; Rudnev, V.S.; Wiedenmann, F.; Wybornov, S.; Yarovaya, T.P.; Jiangd, X. Thermal behavior and catalytic activity in naphthalene destruction of Ce-, Zr- and Mn-containing oxide layers on titanium. Appl. Surf. Sci. 2011, 258, 719–726. [Google Scholar] [CrossRef]
- Lukiyanchuk, I.V.; Rudnev, V.S.; Tyrina, L.M.; Chernykh, I.V. Plasma electrolytic oxide coatings on valve metals and their activity in CO oxidation. Appl. Surf. Sci. 2014, 315, 481–489. [Google Scholar] [CrossRef]
- Luo, Q.; Cai, Q.; Li, X.; Chen, X. Characterization and photocatalytic activity of large-area single crystalline anatase TiO2 nanotube films hydrothermal synthesized on Plasma electrolytic oxidation seed layers. J. Alloys Compd. 2014, 597, 101–109. [Google Scholar] [CrossRef]
- Bayati, M.R.; Golestani-Fard, F.; Moshfegh, A.Z. The effect of growth parameters on photo-catalytic performance of the MAO synthesized TiO2 nano-porous layers. Mater. Chem. Phys. 2010, 120, 582–589. [Google Scholar] [CrossRef]
- Salvador, P. On the Nature of Photogenerated Radical Species Active in the Oxidative Degradation of Dissolved Pollutants with TiO2 Aqueous Suspensions: A Revision in the Light of the Electronic Structure of dsorbed Water. J. Phys. Chem. C 2007, 111, 17038–17043. [Google Scholar] [CrossRef]
- Torres-Cerón, D.A.; Gordillo-Delgado, F.; Plazas-Saldaña, J. Formation of TiO2 nanostructure by plasma electrolytic oxidation for Cr(VI) reduction. J. Phys. Conf. Ser. 2017, 786, 012046. [Google Scholar] [CrossRef]
- Xiang, N.; Zhuang, J.J.; Song, R.G.; Xiang, B.; Xiong, Y.; Su, X.P. Fabrication and photocatalytic activity of MAO–TiO2 films formed on titanium doped with cations. Mater. Technol. Adv. Perform. Mater. 2016, 31, 332–336. [Google Scholar]
- Alsaran, A.; Purcek, G.; Hacisalihoglu, I.; Vangolu, Y.; Bayrak, Ö.; Karaman, I.; Celik, A. Hydroxyapatite production on ultrafine-grained pure titanium by micro-arc oxidation and hydrothermal treatment. Surf. Coat. Technol. 2011, 205, S537–S542. [Google Scholar] [CrossRef]
- Rudnev, V.S.; Morozova, V.P.; Lukiyanchuk, I.V.; Adigamova, M.V. Calcium-Containing Biocompatible Oxide-Phosphate Coatings on Titanium. Russ. J. Appl. Chem. 2010, 83, 671–679. [Google Scholar] [CrossRef]
- Wei, D.; Zhou, Y. Preparation, biomimetic apatite induction and osteoblast proliferation test of TiO2-based coatings containing P with a graded structure. Ceram. Int. 2009, 35, 2343–2350. [Google Scholar] [CrossRef]
- Rizwan, M.; Alias, R.; Zaidi, U.Z.; Mahmoodian, R.; Hamdi, M. Surface modification of valve metals using plasma electrolytic oxidation for antibacterial applications: A review. J. Biomed. Mater. Res. Part. A 2018, 106, 590–605. [Google Scholar] [CrossRef]
- Nie, X.; Leyland, A.; Matthews, A. Deposition of layered bioceramic hydroxyapatite/TiO2 coatings on titanium alloys using a hybrid technique of micro-arc oxidation and electrophoresis. Surf. Coat. Technol. 1999, 125, 407–414. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Rouhaghdam, A.S.; Shahrabi, T. Abrasive wear behaviour of Si3N4/TiO2 nanocomposite coatings fabricated by plasma electrolytic oxidation. Surf. Coat. Technol. 2010, 205, S41–S46. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Sabour Rouhaghdam, A. Wear and coating removal mechanism of alumina/titania nanocomposite layer fabricated by plasma electrolysis. Surf. Coat. Technol. 2011, 205, S57–S62. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Gharabagh, R.S.; Teimouri, M.; Ahmadzadeh, M.; Darband, G.B.; Hasannejad, H. Ceria embedded nanocomposite coating fabricated by plasma electrolytic oxidation on titanium. J. Alloys Compd. 2016, 685, 376–383. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Vovna, V.I.; Gordienko, P.S.; Sinebryukhov, S.L.; Cherednichenko, A.I.; Shchukarev, A.V. Chemical Composition of Antifriction Micro-arc Oxide Coatings on Titanium Alloy BT,’16. Prot. Met. 2001, 37, 168–172. [Google Scholar] [CrossRef]
- Kobata, I.; Toma, I.; Kodera, A.; Suzuki, T.; Makita, Y.; Saito, T. Electrochemical Mechanical Polishing Apparatus Conditioning Method, and Conditioning Solution. U.S. Patent 2008/0188162 A1, 7 August 2008. [Google Scholar]
- Yu, S.; Yang, X.; Yang, L.; Liu, Y.; Yu, Y. Novel Technique for Preparing Ca- and P-Containing Ceramic Coating on Ti-6Al-4V by Micro-Arc Oxidation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 83, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Ceschini, L.; Lanzoni, E.; Martini, C.; Prandstraller, D.; Sambogna, G. Comparison of dry sliding friction and wear of Ti6Al4V alloy treated by plasma electrolytic oxidation and PVD coating. Wear 2008, 264, 86–95. [Google Scholar] [CrossRef]
- Wang, Y.M.; Jiang, B.L.; Lei, T.Q.; Guo, L.X. Microarc oxidation and spraying graphite duplex coating formed on titanium alloy for antifriction purpose. Appl. Surf. Sci. 2005, 246, 214–221. [Google Scholar] [CrossRef]
- Nie, X.; Wilson, A.; Leyland, A.; Matthews, A. Deposition of duplex Al2O3/DLC coatings on Al alloys for tribological applications using a combined micro-arc oxidation and plasma-immersion ion implantation technique. Surf. Coat. Technol. 2000, 131, 506–513. [Google Scholar] [CrossRef]
- Aliasghari, S.; Skeldon, P.; Thompson, G.E. Plasma electrolytic oxidation of titanium in a phosphate/silicateelectrolyte and tribological performance of the coatings. Appl. Surf. Sci. 2014, 316, 463–476. [Google Scholar] [CrossRef]
- Achhab, M.E.; Schierbaum, K. Structure and hydrogen sensing properties of plasma electrochemically oxidized titanium foils. Proc. Eng. 2012, 47, 566–569. [Google Scholar] [CrossRef]
- Achhab, M.E.; Schierbaum, K. Gas sensors based on plasma-electrochemically oxidized titanium foils. J. Sens. Sens. Syst. 2016, 5, 273–281. [Google Scholar] [CrossRef]
- Adigamova, M.V.; Rudnev, V.S.; Lukiyanchuk, I.V.; Morozova, V.P.; Tkachenko, I.A.; Kvach, A.A. The Effect of Fe-Containing Colloid Particles in Electrolyte on the Composition and Magnetic Characteristics of Oxide Layers on Titanium Formed Using the Method of Plasma Electrolytic Oxidation. Prot. Met. Phys. Chem. Surf. 2016, 52, 526–531. [Google Scholar] [CrossRef]
- Boukhvalov, D.W.; Korotin, D.M.; Efremov, A.V.; Kurmaev, E.Z.; Borchers, C.H.; Zhidkov, I.S.; Gunderov, D.V.; Valiev, R.Z.; Gavrilov, N.V.; Cholakh, S.O. Modification of titanium and titanium dioxide surfaces by ion implantation: Combined XPS and DFT study. Phys. Status Solidi B 2015, 252, 748–754. [Google Scholar] [CrossRef]
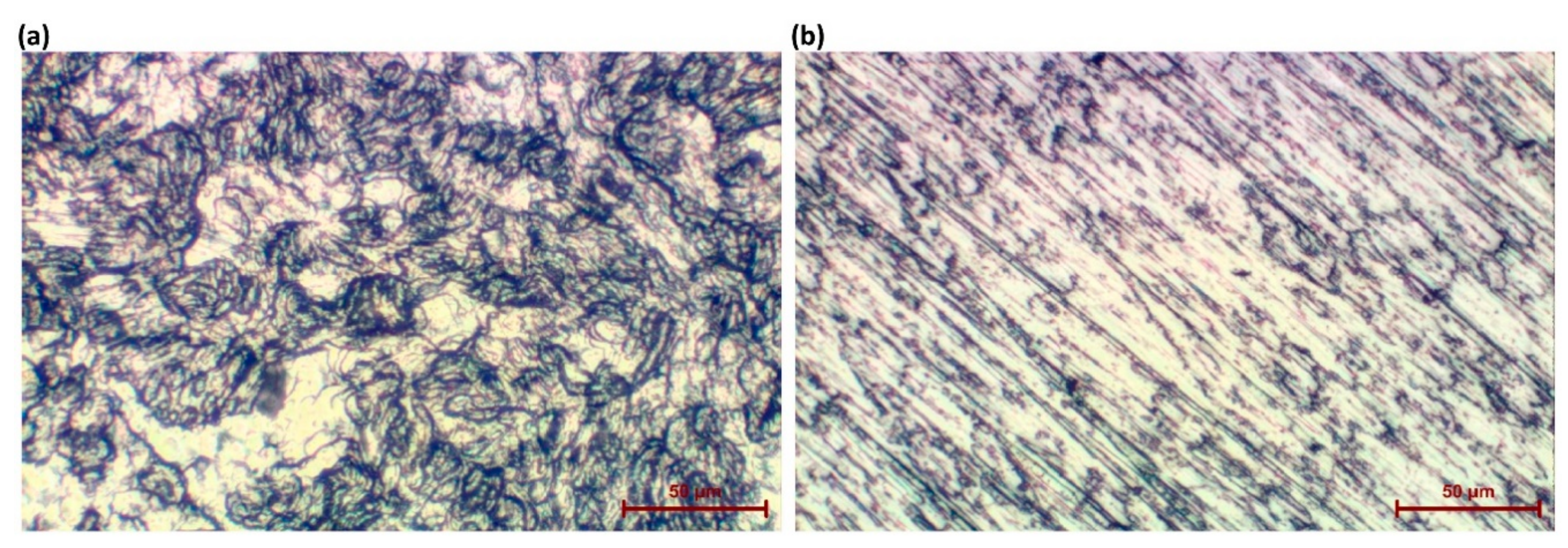

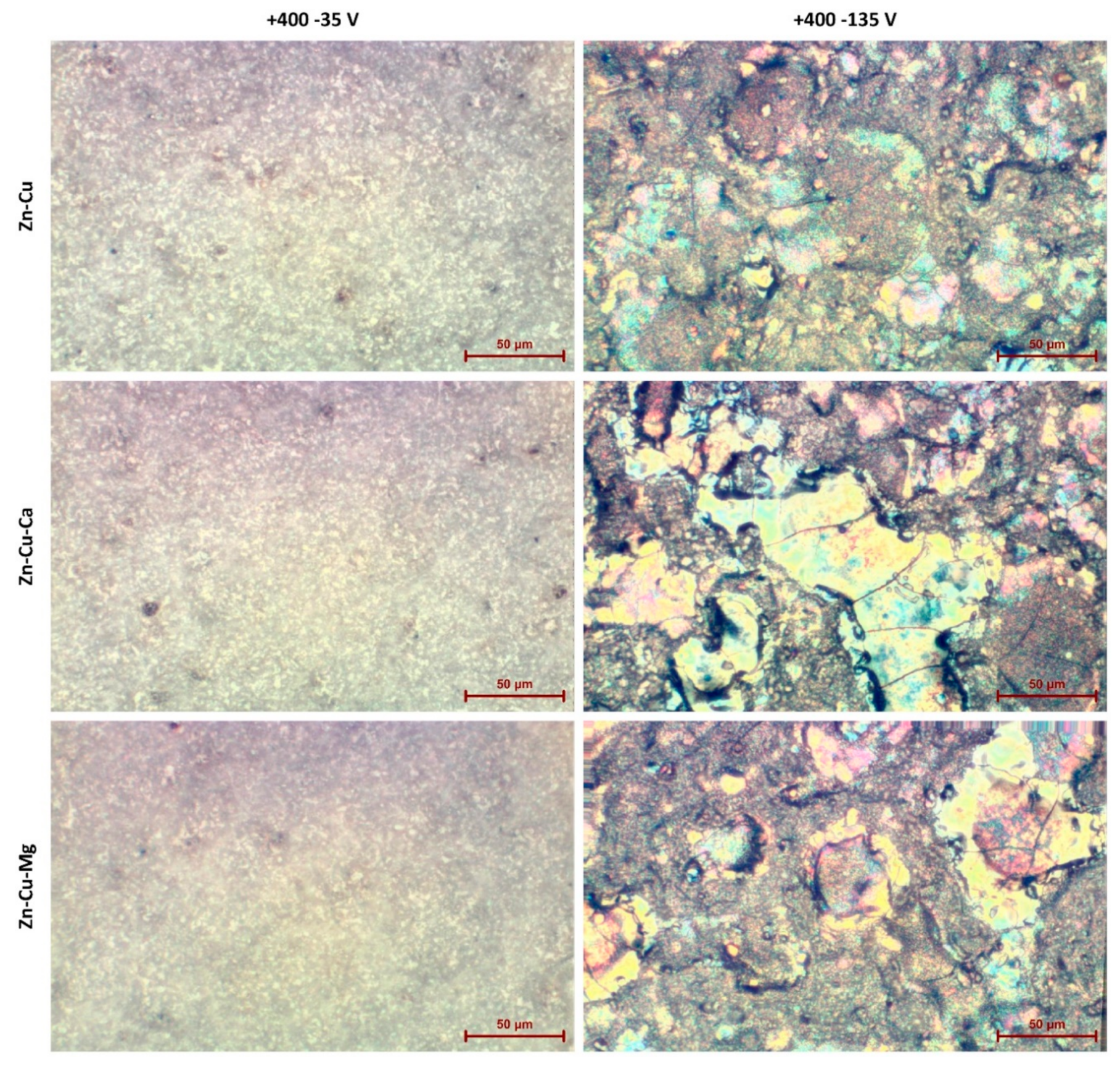
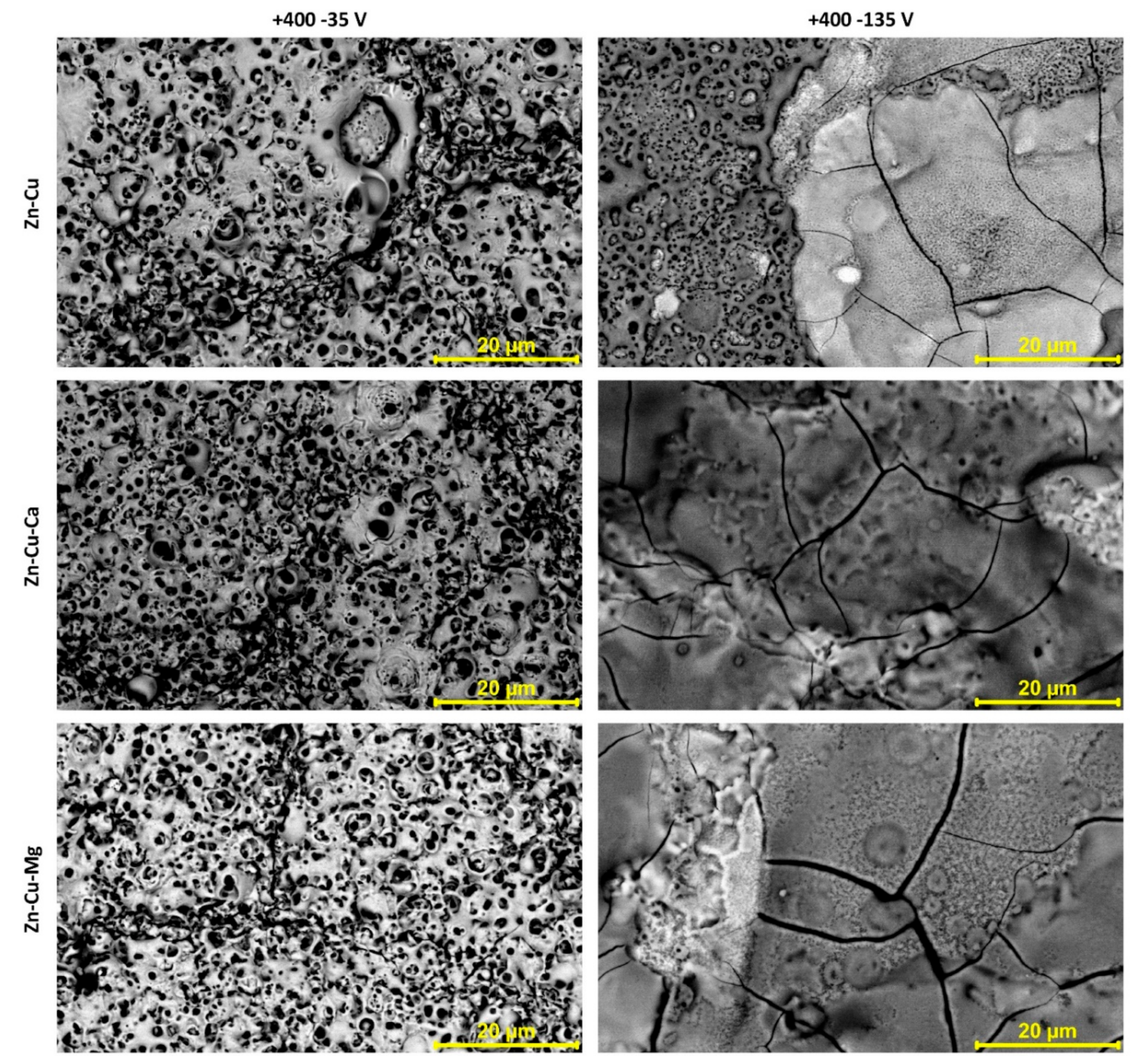

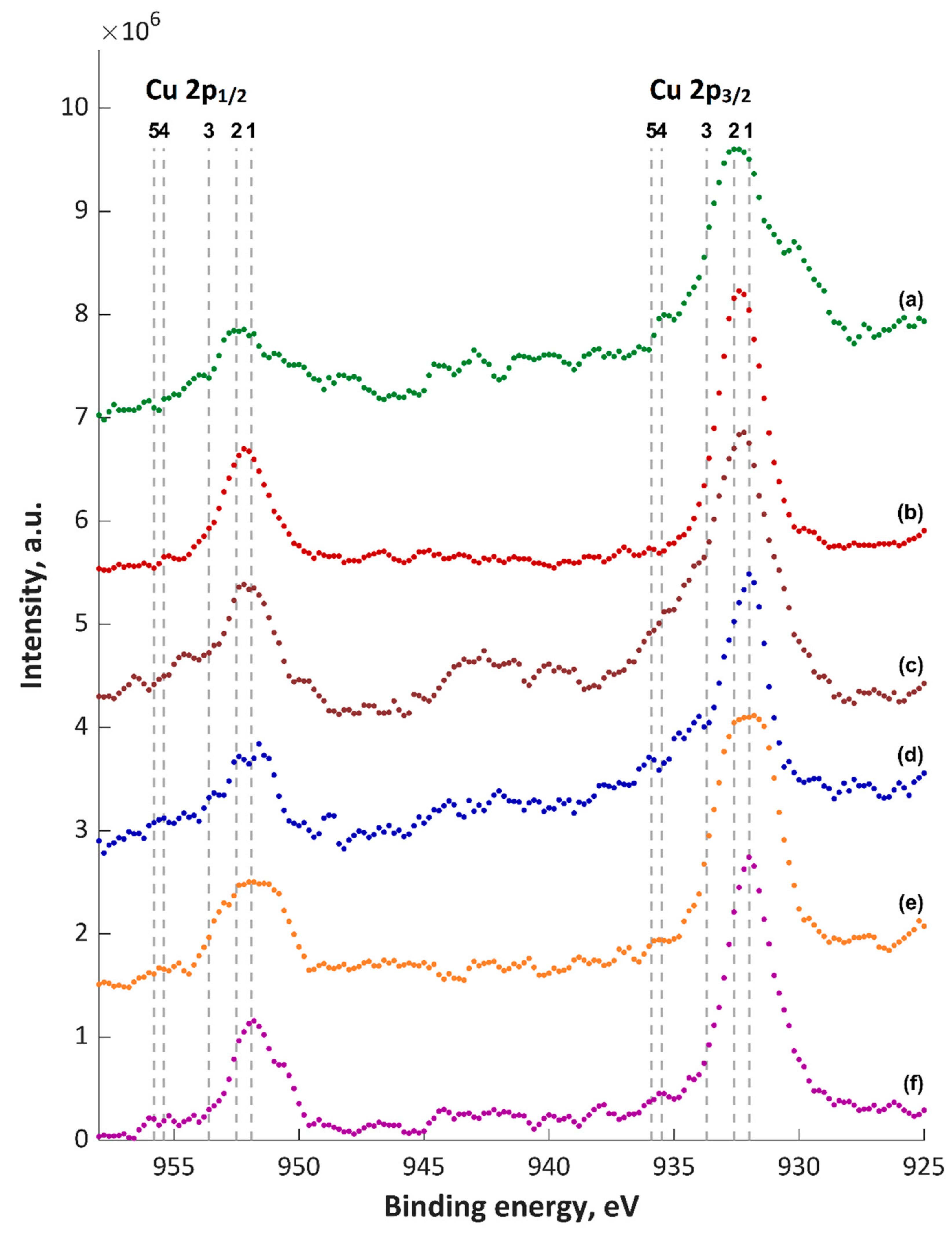

| Content of the Elements, wt% | |||||
|---|---|---|---|---|---|
| N | C | H | Fe | O | Ti |
| <0.03 | <0.10 | <0.015 | <0.30 | <0.25 | balance |
| Content of the Elements, wt% | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cr | Ni | Mo | Mg | Si | Cu | V | Co | C | Ti | P | S | N | Fe |
| 17.07 | 10.26 | 1.9 | 1.68 | 0.64 | 0.19 | 0.11 | 0.04 | 0.03 | 0.03 | 0.02 | 0.04 | 0.04 | balance |
| Abbreviation | Electrolyte Composition | Voltage |
|---|---|---|
| TiZnCu-35 | 1 dm3 H3PO4 (85 wt%) with 250 g Zn(NO3)2∙6H2O and 250 g Cu(NO3)2∙3H2O | +400/−35 V |
| TiZnCu-135 | 1 dm3 H3PO4 (85 wt%) with 250 g Zn(NO3)2∙6H2O and 250 g Cu(NO3)2∙3H2O | +400/−135 V |
| TiZnCuCa-35 | 1 dm3 H3PO4 (85 wt%) with 167.67 g Zn(NO3)2∙6H2O and 166.67 g Cu(NO3)2∙3H2O and 166.67 g Ca(NO3)2∙4H2O | +400/−35 V |
| TiZnCuCa-135 | 1 dm3 H3PO4 (85 wt%) with 167.67 g Zn(NO3)2∙6H2O and 166.67 g Cu(NO3)2∙3H2O and 166.67 g Ca(NO3)2∙4H2O | +400/−135 V |
| TiZnCuMg-35 | 1 dm3 H3PO4 (85 wt%) with 167.67 g Zn(NO3)2∙6H2O and 166.67 g Cu(NO3)2∙3H2O and 166.67 g and 166.67 g Mg(NO3)2∙6H2O | +400/−35 V |
| TiZnCuMg-135 | 1 dm3 H3PO4 (85 wt%) with 167.67 g Zn(NO3)2∙6H2O and 166.67 g Cu(NO3)2∙3H2O and 166.67 g and 166.67 g Mg(NO3)2∙6H2O | +400/−135 V |
| Samples | Elements | ||||||
|---|---|---|---|---|---|---|---|
| O | Ti | P | Zn | Cu | Ca | Mg | |
| Average ± Standard Deviation, at% | |||||||
| TiZnCu-35 | 71.48 ± 0.13 | 15.32 ± 0.22 | 12.08 ± 0.15 | 0.71 ± 0.03 | 0.41 ± 0.03 | ||
| TiZnCu-135 | 61.97 ± 1.23 | 34.35 ± 1.45 | 3.68 ± 0.23 | * | * | ||
| TiZnCuCa-35 | 71.99 ± 0.16 | 14.63 ± 0.06 | 12.05 ± 0.08 | 0.5 ± 0.05 | 0.36 ± 0.05 | 0.47 ± 0.02 | |
| TiZnCuCa-135 | 56.11 ± 1.9 | 41.07 ± 2.03 | 2.82 ± 0.15 | * | * | * | |
| TiZnCuMg-35 | 71.95 ± 0.3 | 14.2 ± 0.32 | 12.37 ± 0.2 | 0.49 ± 0.03 | 0.37 ± 0.04 | 0.62 ± 0.05 | |
| TiZnCuMg-135 | 56.51 ± 1.45 | 40.39 ± 1.51 | 3.1 ± 0.06 | * | * | * | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rokosz, K.; Hryniewicz, T.; Raaen, S.; Gaiaschi, S.; Chapon, P.; Matýsek, D.; Pietrzak, K.; Szymańska, M.; Dudek, Ł. Metal Ions Supported Porous Coatings by Using AC Plasma Electrolytic Oxidation Processing. Materials 2020, 13, 3838. https://doi.org/10.3390/ma13173838
Rokosz K, Hryniewicz T, Raaen S, Gaiaschi S, Chapon P, Matýsek D, Pietrzak K, Szymańska M, Dudek Ł. Metal Ions Supported Porous Coatings by Using AC Plasma Electrolytic Oxidation Processing. Materials. 2020; 13(17):3838. https://doi.org/10.3390/ma13173838
Chicago/Turabian StyleRokosz, Krzysztof, Tadeusz Hryniewicz, Steinar Raaen, Sofia Gaiaschi, Patrick Chapon, Dalibor Matýsek, Kornel Pietrzak, Monika Szymańska, and Łukasz Dudek. 2020. "Metal Ions Supported Porous Coatings by Using AC Plasma Electrolytic Oxidation Processing" Materials 13, no. 17: 3838. https://doi.org/10.3390/ma13173838
APA StyleRokosz, K., Hryniewicz, T., Raaen, S., Gaiaschi, S., Chapon, P., Matýsek, D., Pietrzak, K., Szymańska, M., & Dudek, Ł. (2020). Metal Ions Supported Porous Coatings by Using AC Plasma Electrolytic Oxidation Processing. Materials, 13(17), 3838. https://doi.org/10.3390/ma13173838








