Effect of Polishing on Electrochemical Behavior and Passive Layer Composition of Different Stainless Steels
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
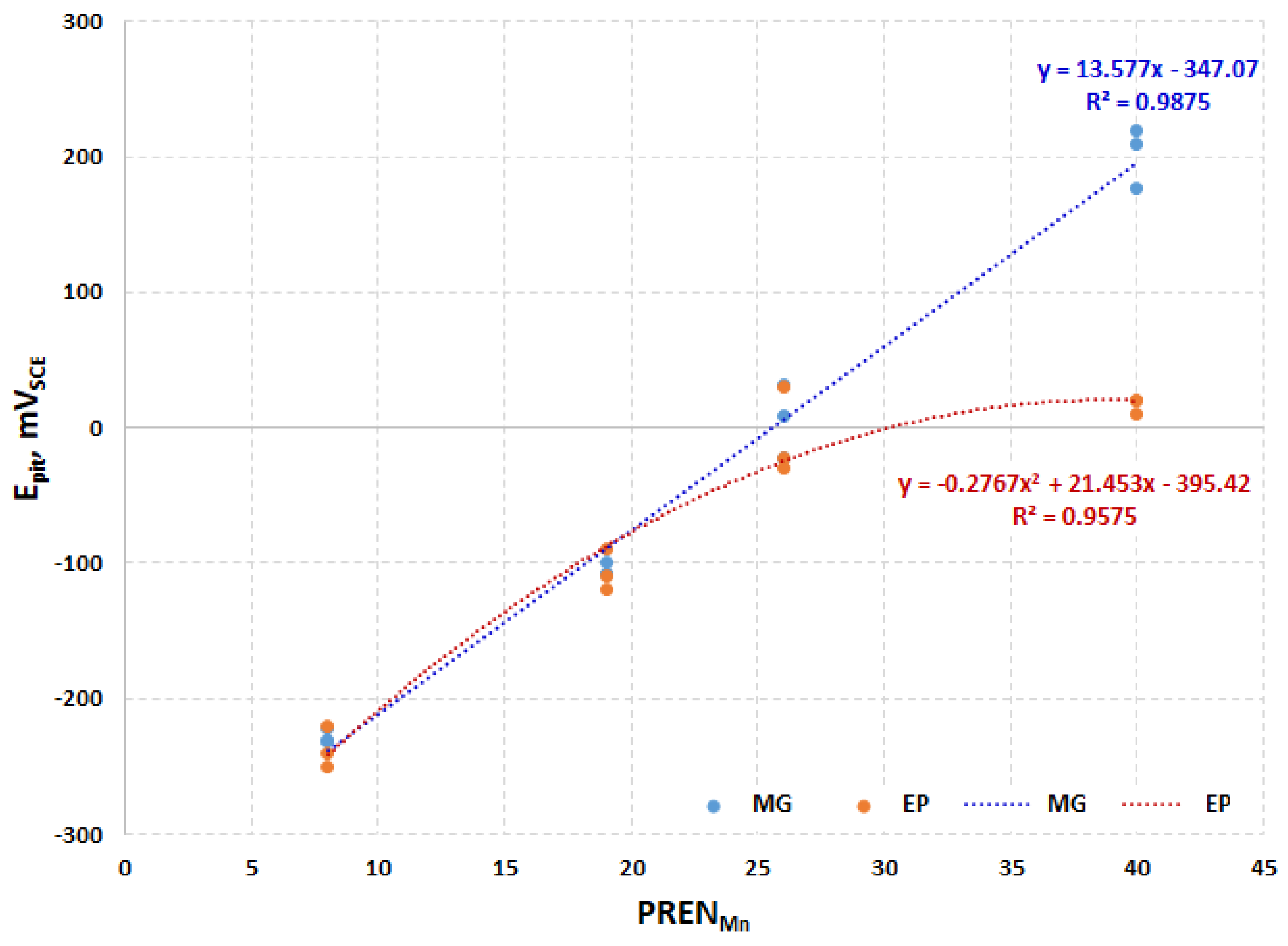
- CrNiMo austenites show a substantially better corrosion behavior than CrMnN austenites;
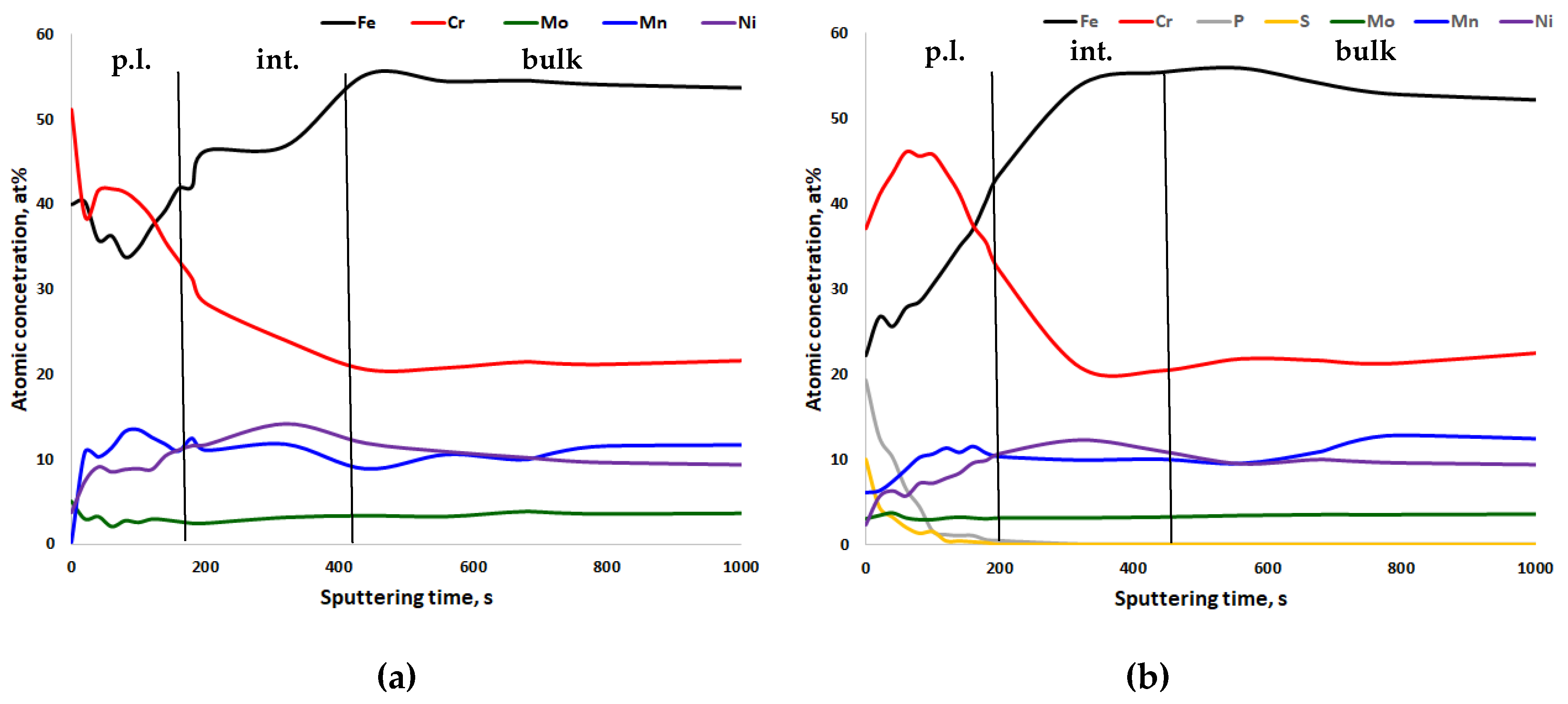
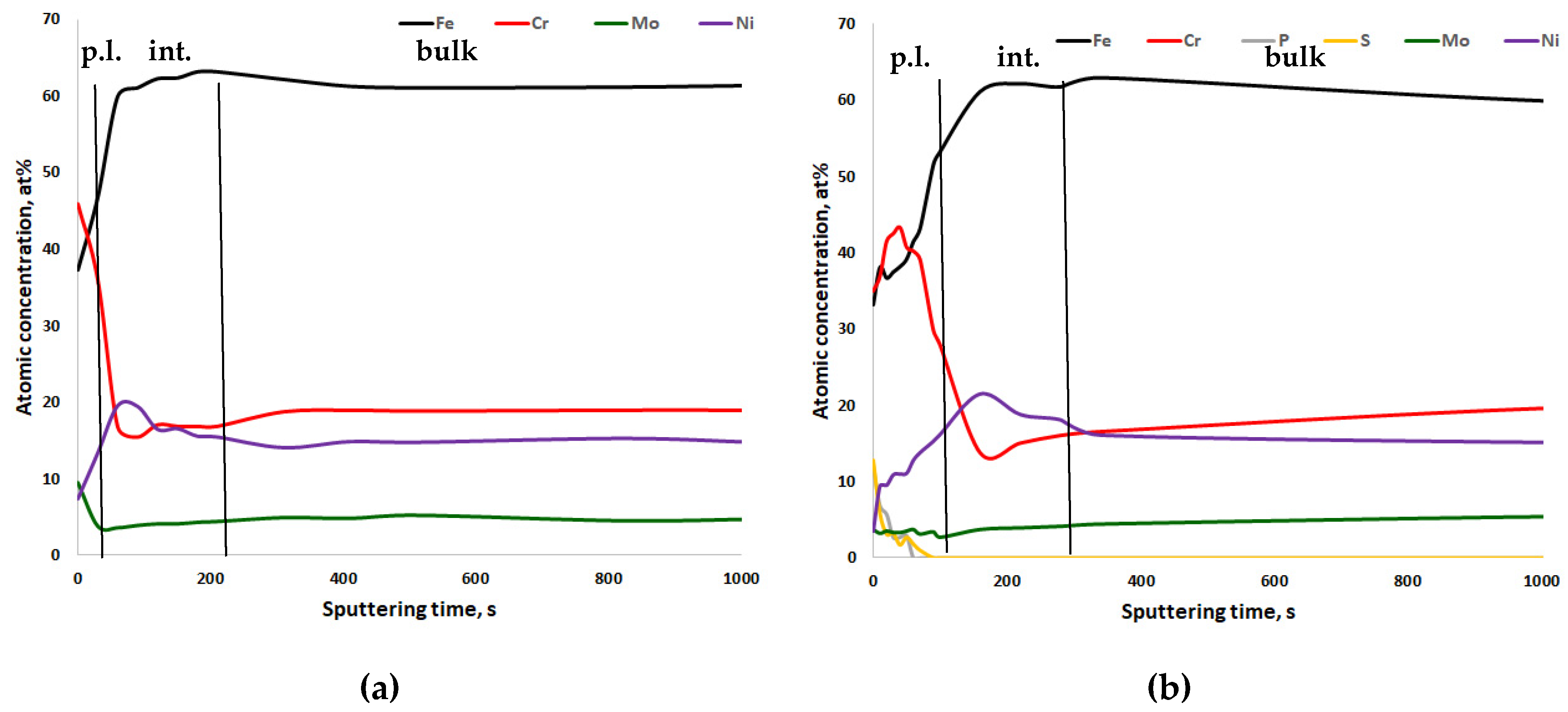
- For all steels, chromium enrichment was noted in the passive layers;
- For CrNiMo austenites, there is nickel enrichment underneath the passive layer, while for CrMnN austenites, manganese tends to be enriched in the passive layer;
- After electropolishing, sulfate and phosphate are present in the passive layers. The poor corrosion properties of electropolished specimens, as compared to mechanically ground ones, can be explained by this.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, S.-J.; Lai, J.-J. The effects of electropolishing (EP) process parameters on corrosion resistance of 316L stainless steel. J. Mater. Process. Technol. 2003, 140, 206–210. [Google Scholar] [CrossRef]
- Hryniewicz, T.; Rokosz, K. Corrosion resistance of magnetoelectropolished AISI 316L SS biomaterial. Anti-Corros. Methods Mater. 2014, 61, 57–64. [Google Scholar] [CrossRef]
- Rokosz, K.; Simon, F.; Hryniewicz, T.; Rzadkiewicz, S. Comparative XPS analysis of passive layers composition formed on AISI 304 L SS after standard and high-current density electropolishing. Surf. Interface Anal. 2015, 47, 87–92. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Raaen, S. Cr/Fe ratio by XPS spectra of magnetoelectropolished AISI 316L SS fitted by gaussian-lorentzian shape lines. Teh. Vjesn. Tech. Gaz. 2014, 21, 533–538. [Google Scholar]
- Rokosz, K.; Hryniewicz, T.; Raaen, S. Characterization of Passive Film Formed on AISI 316L Stainless Steel after Magnetoelectropolishing in a Broad Range of Polarization Parameters. Steel Res. Int. 2012, 83, 910–918. [Google Scholar] [CrossRef]
- Sutow, E.J. The influence of electropolishing on the corrosion resistance of 316L stainless steel. J. Biomed. Mater. Res. 1980, 14, 587–595. [Google Scholar] [CrossRef]
- Lee, E.-S. Machining Characteristics of the Electropolishing of Stainless Steel (STS316L). Int. J. Adv. Manuf. Tech. 2000, 16, 591–599. [Google Scholar] [CrossRef]
- Han, Y.; Mei, J.; Peng, Q.; Han, E.-H.; Ke, W. Effect of electropolishing on corrosion of nuclear grade 316L stainless steel in deaerated high temperature water. Corros. Sci. 2016, 112, 625–634. [Google Scholar] [CrossRef]
- Sojitra, P.; Engineer, C.; Kothwala, D.; Raval, A.; Kotadia, H.; Mehta, G. Electropolishing of 316LVM Stainless Steel Cardiovascular Stents: An Investigation of Material Removal, Surface Roughness and Corrosion Behaviour. Trends Biomater. Artif. Organs 2010, 23, 115–121. [Google Scholar]
- Rokosz, K.; Hryniewicz, T. Pitting Corrosion Resistance of AISI 316L Stainless Steel in Ringer’s Solution after Magnetoelectrochemical Polishing. Corrosion 2010, 66, 035004-035004-11. [Google Scholar] [CrossRef]
- Ziemniak, S.E.; Hanson, M.; Sander, P.C. Electropolishing effects on corrosion behavior of 304 stainless steel in high temperature, hydrogenated water. Corros. Sci. 2008, 50, 2465–2477. [Google Scholar] [CrossRef]
- Berge, P. Importance of surface preparation for corrosion control in nuclear power stations. Mater. Perform. 1997, 36, 56–62. [Google Scholar]
- Robertson, J. The mechanism of high temperature aqueous corrosion of stainless steels. Corros. Sci. 1991, 32, 443–465. [Google Scholar] [CrossRef]
- Maekawa, T.; Kagawa, M.; Nakajima, N. Corrosion Behaviors of Stainless Steel in High-Temperature Water and Superheated Steam. Trans. Jpn. I. Met. 1968, 9, 130–136. [Google Scholar] [CrossRef]
- Warzee, M.; Hennaut, J.; Maurice, M.; Sonnen, C.; Waty, J.; Berge, P. Effect of Surface Treatment on the Corrosion of Stainless Steels in High-Temperature Water and Steam. J. Electrochem. Soc. 1965, 112, 670. [Google Scholar] [CrossRef]
- Guinard, L.; Kerrec, O.; Noel, D.; Gardey, S.; Coulet, F. Influence of the initial surface condition on the release of nickel alloys in the primary circuit of PWRs (EDF--97-NB-00045). Nucl. Energy 1997, 36, 19–27. [Google Scholar]
- Holzleitner, S.; Kranister, W.; Mori, G.; Schmuki, P. Applying A Multi Method Approach To Chloride Induced Scc Of Two Austenitic Cr-Ni-Mo-N Stainless Steels, Corrosion 2008, New Orleans, LA, USA, 16–18 March 2008; NACE International: Houston, TX, USA, 2008; pp. 1–18. [Google Scholar]
- Mori, G.; Sonnleitner, R.; Holzleitner, S.; Panzenböck, M.; Pippan, R. Spannungs- und Schwingungsrisskorrosion - Gemeinsamkeiten und Unterschiede. Mater. Test. 2010, 52, 42–51. [Google Scholar] [CrossRef]
- Elsener, B.; Rossi, A. The passive film on stainless steels—Results of XPS surface analysis. In Korrosions nichtrostender Stähle—Auf die Oberfläche kommt es an! Proceedings of the 3-Länder-Korrosionstagung 2008, 24–25 April 2008; Technical University of Vienna: Vienna, Austria, 2008; pp. 13–22. [Google Scholar]
- Uggowitzer, P.J.; Magdowski, R.; Speidel, M.O. High Nitrogen Steels. Nickel Free High Nitrogen Austenitic Steels. Isij Int. 1996, 36, 901–908. [Google Scholar] [CrossRef]
- Speidel, M.O. Nitrogen Containing Austenitic Stainless Steels. Materialwiss. Werkst. 2006, 37, 875–880. [Google Scholar] [CrossRef]
- Holzleitner, S.; Mori, G.; Falk, H.; Eglsaeer, S. Electrochemical and SCC Behavior Of Highly Alloyed Austenitic Stainless Steels In Different Chloride Containing Media, Corrosion 2007, Nashville, TN, USA, 11–15 March 2007; NACE International: Houston, TX, USA, 2007; pp. 1–17. [Google Scholar]
- Vichytil, C.; Sonnleitner, R.; Mori, G.; Panzenböck, M.; Fluch, R. Corrosion Fatigue Investigations on Austenitic Stainless Steels with Different Alloying Concepts; Corrosion 2010; NACE International: Houston, TX, USA, 2020. [Google Scholar]
- Forsén, O.; Aromaa, J.; Tavi, M. Examples of Electrochemical Testing of Stainless Steels in Industrial Environments. Mater. Sci. Forum 1995, 192–194, 41–52. [Google Scholar] [CrossRef]
- Merello, R.; Botana, F.J.; Botella, J.; Matres, M.V.; Marcos, M. Influence of chemical composition on the pitting corrosion resistance of non-standard low-Ni high-Mn–N duplex stainless steels. Corros. Sci. 2003, 45, 909–921. [Google Scholar] [CrossRef]
- Mori, G.; Bauernfeind, D. Pitting and crevice corrosion of superaustenitic stainless steels. Mater. Corros. 2004, 55, 164–173. [Google Scholar] [CrossRef]
- Rondelli, G.; Vicentini, B. Heat exchanger Technologies for the global Environment. In Proceedings of the 1994 International Joint Power Generation Conference, 2–6 October 1994; Maurer, J.R., Ed.; ASME Orders: Fairfield, NJ, USA, 1994; Volume 25, p. 61. [Google Scholar]
- Speidel, M.O. Ultra High Strength Austenitic Stainless Steels, 2nd Stainless Steel World 2001, 13–15 November 2001, The Hague, The Netherlands; KCI Publishing BV: Zutphen, The Nederlands, 2001; pp. 98–106. [Google Scholar]
- Speidel, M.O.; Zhend-Cui, M.L.; Kowanda, C.; Speidel, H.; Diener, M. High-nitrogen austenitic stainless steels - Future materials for the chemical industries. Trans. Ind. Inst. Met. 2003, 56, 281–286. [Google Scholar]
- Speidel, M.O.; Zheng-Cui, M. High-Nitrogen Austenitic Stainless Steels, High Nitrogen Steels, HNS 2003, 26–28 March 2003; Speidel, M.O., Ed.; vdf Hochschulverlag AG: Zurich, Switzerland, 2003; pp. 63–73. [Google Scholar]
- Jargelius-Pettersson, R.F.A. Application of the Pitting Resistance Equivalent Concept to Some Highly Alloyed Austenitic Stainless Steels. Corrosion 1998, 54, 162–168. [Google Scholar] [CrossRef]
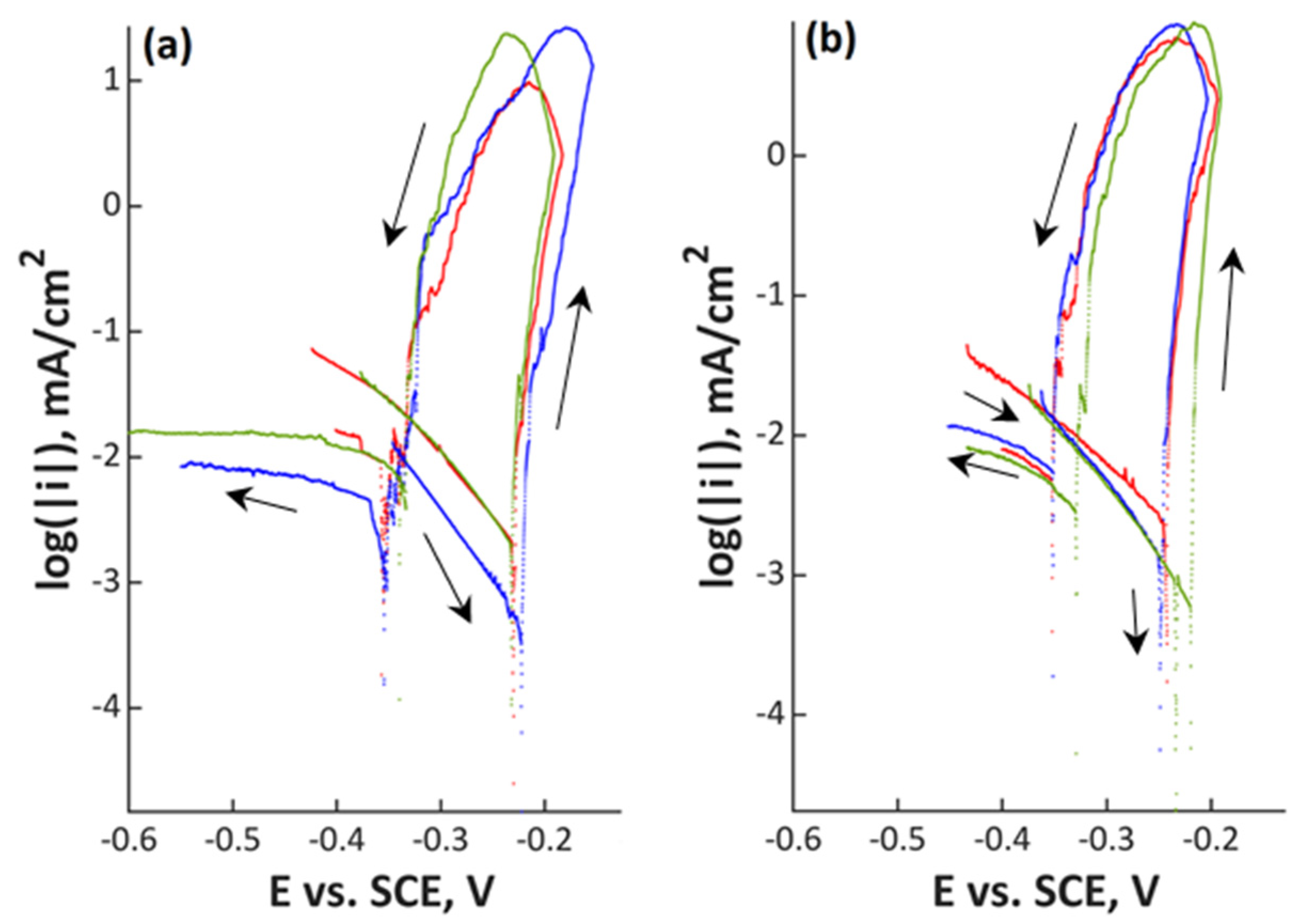
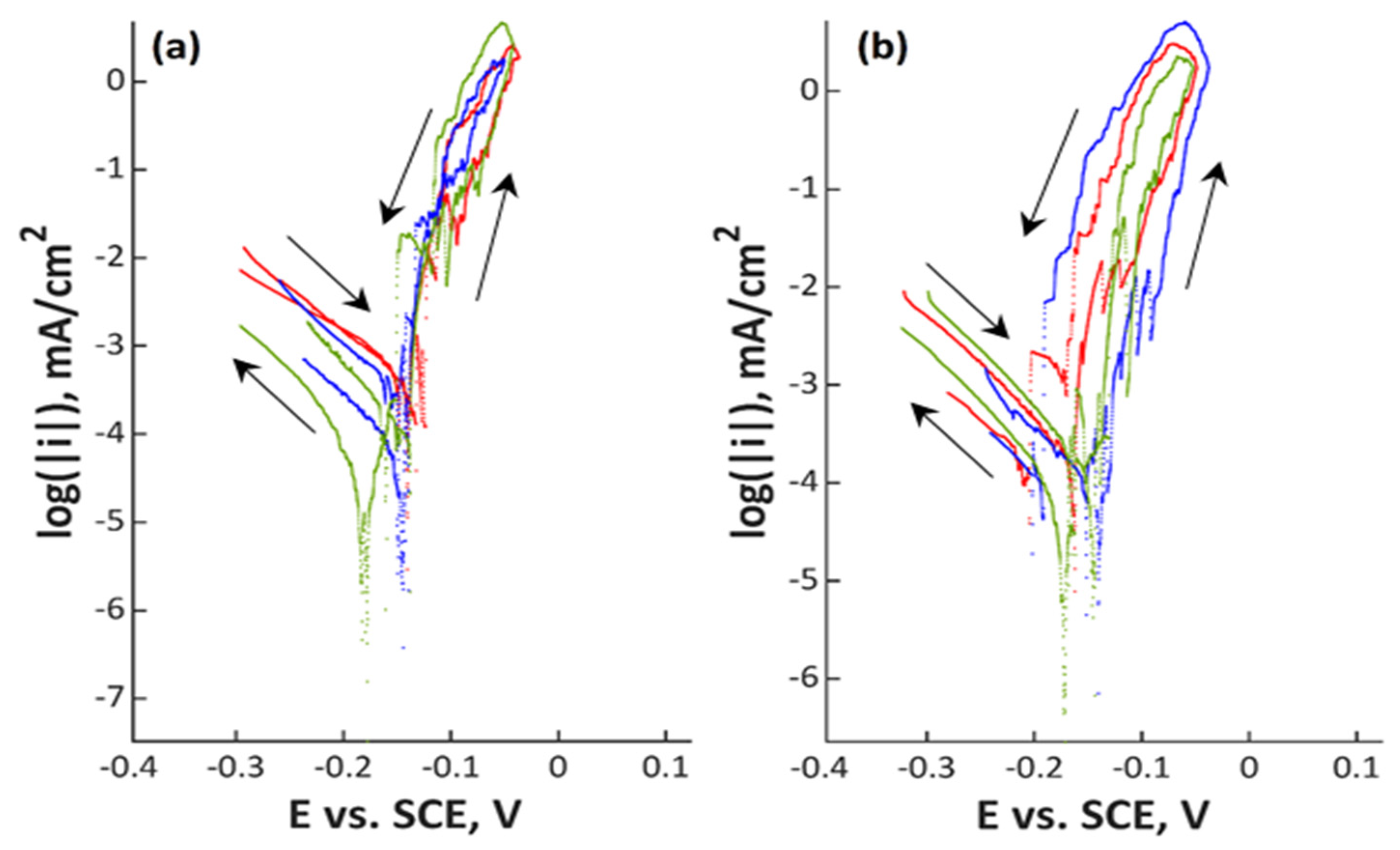

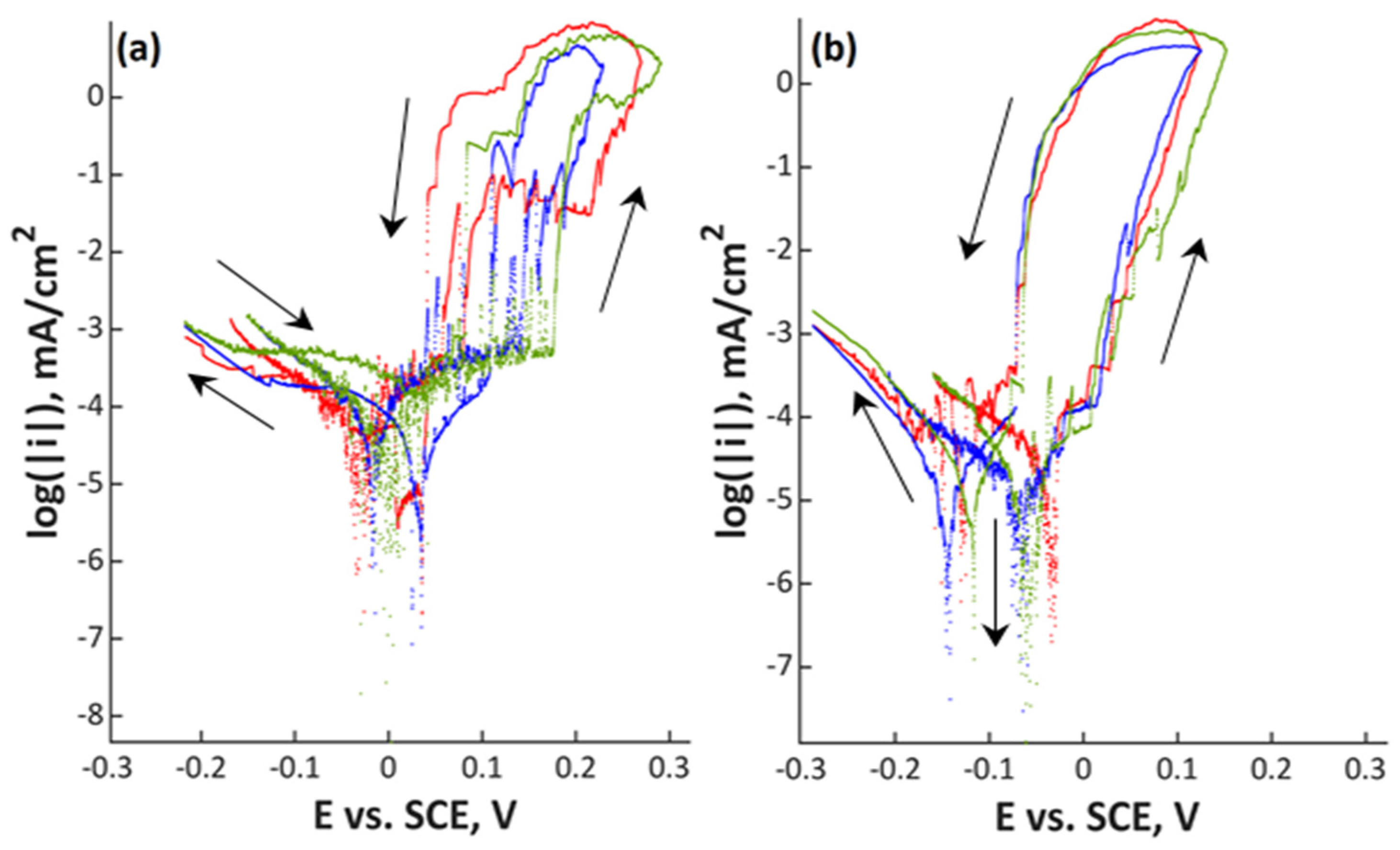
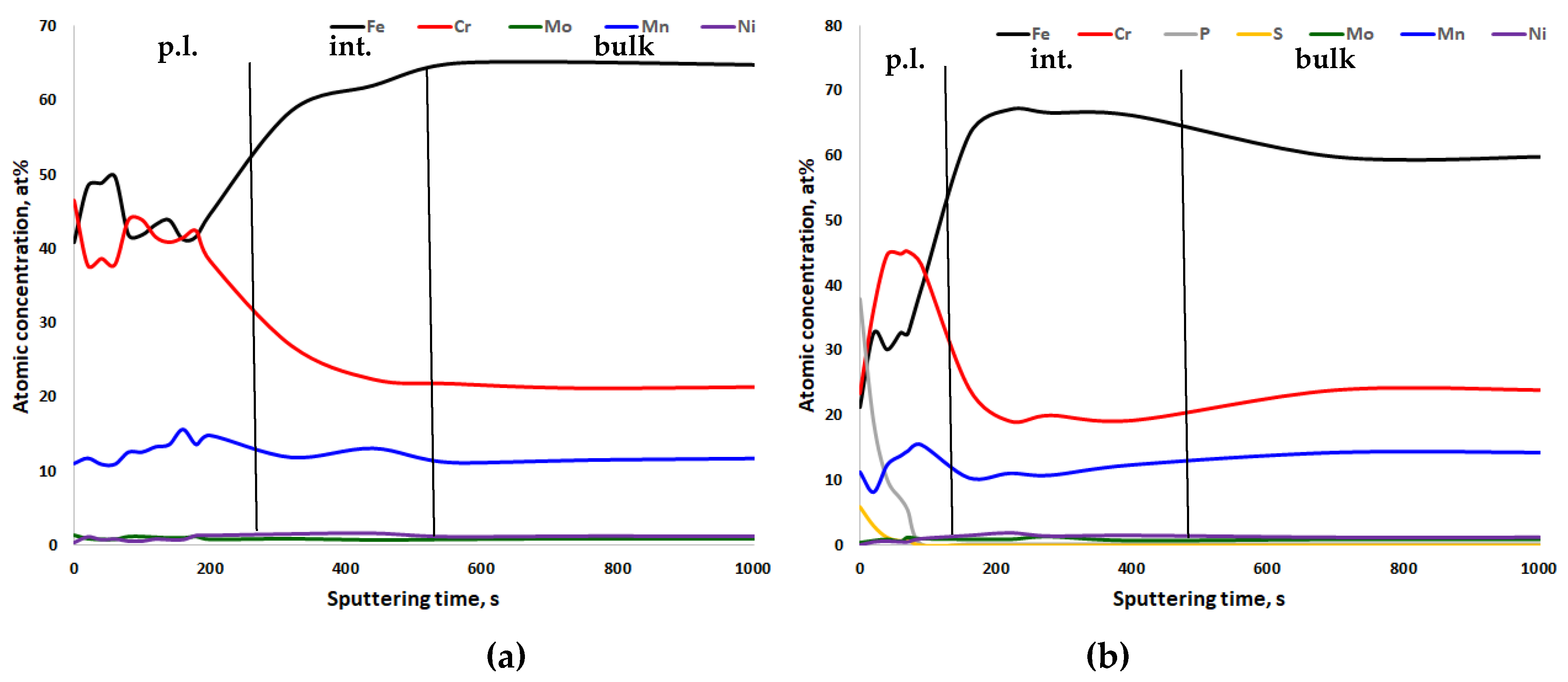

| Material | C | Si | Mn | Cr | Ni | Mo | N | PREN | PRENMn |
|---|---|---|---|---|---|---|---|---|---|
| 18Cr21Mn2NiN | <0.06 | 0.1 | 21.2 | 18.2 | 1.7 | 0.5 | 0.6 | 29 | 8 |
| 20Cr20Mn7Ni2MoN | <0.03 | 20.0 | 20.0 | 7.0 | 2.3 | 0.7 | 39 | 19 | |
| 18Cr15Ni3Mo (S31603) | <0.03 | 0.3 | 1.8 | 17.5 | 14.7 | 2.8 | 0.1 | 28 | 26 |
| 27Cr29Ni3Mo (N08028) | <0.03 | 0.3 | 2.8 | 27.4 | 29.4 | 3.3 | 0.3 | 43 | 40 |
| Material | Yield Strength [MPa] | Tensile Strength [MPa] | Fracture Elongation [%] |
|---|---|---|---|
| 18Cr21Mn2NiN | 560 | 920 | 65 |
| 20Cr20Mn7Ni2MoN | 510 | 900 | 54 |
| 18Cr15Ni3Mo (S31603) | 290 | 560 | 65 |
| 27Cr29Ni3Mo (N08028) | 380 | 790 | 64 |
| Material | After Mechanical Grinding | After Electrochemical Polishing | ||||||
|---|---|---|---|---|---|---|---|---|
| Ecorr mVSCE | icorr mA/cm2 | Epit mVSCE | Erep mVSCE | Ecorr mVSCE | icorr mA/cm2 | Epit mVSCE | Erep mVSCE | |
| 18Cr21Mn2NiN | −230 | 8.21 × 10−4 | −230 | −331 | −240 | 9.74 × 10−4 | −240 | −350 |
| −222 | 2.21 × 10−4 | −222 | −322 | −250 | 5.13 × 10−4 | −250 | −350 | |
| −232 | 8.21 × 10−4 | −232 | −329 | −220 | 2.36 × 10−4 | −220 | −320 | |
| 20Cr20Mn7Ni2MoN | −145 | 1.44 × 10−4 | −100 | −100 | −160 | 1.06 × 10−4 | −120 | −200 |
| −138 | 6.82 × 10−5 | −108 | −108 | −140 | 4.70 × 10−5 | −90 | −190 | |
| −140 | 2.58 × 10−4 | −108 | −113 | −140 | 7.58 × 10−5 | −110 | −130 | |
| 18Cr15Ni3Mo (UNS S31603) | −195 | 5.13 × 10−5 | 32 | −104 | −130 | 2.26 × 10−5 | −30 | −150 |
| −111 | 1.79 × 10−5 | −22 | −124 | −120 | 9.74 × 10−6 | 30 | −140 | |
| −179 | 5.13 × 10−5 | 9 | −76 | −90 | 4.72 × 10−5 | −22 | −130 | |
| 27Cr29Ni3Mo (UNS N08028) | −32 | 1.23 × 10−5 | 219 | 41 | −30 | 1.08 × 10−5 | 20 | −70 |
| −97 | 1.90 × 10−5 | 209 | −10 | −60 | 5.13 × 10−6 | 20 | −70 | |
| −10 | 2.15 × 10−5 | 176 | −82 | −50 | 5.13 × 10−6 | 10 | −60 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rokosz, K.; Solecki, G.; Mori, G.; Fluch, R.; Kapp, M.; Lahtinen, J. Effect of Polishing on Electrochemical Behavior and Passive Layer Composition of Different Stainless Steels. Materials 2020, 13, 3402. https://doi.org/10.3390/ma13153402
Rokosz K, Solecki G, Mori G, Fluch R, Kapp M, Lahtinen J. Effect of Polishing on Electrochemical Behavior and Passive Layer Composition of Different Stainless Steels. Materials. 2020; 13(15):3402. https://doi.org/10.3390/ma13153402
Chicago/Turabian StyleRokosz, Krzysztof, Grzegorz Solecki, Gregor Mori, Rainer Fluch, Marianne Kapp, and Jouko Lahtinen. 2020. "Effect of Polishing on Electrochemical Behavior and Passive Layer Composition of Different Stainless Steels" Materials 13, no. 15: 3402. https://doi.org/10.3390/ma13153402
APA StyleRokosz, K., Solecki, G., Mori, G., Fluch, R., Kapp, M., & Lahtinen, J. (2020). Effect of Polishing on Electrochemical Behavior and Passive Layer Composition of Different Stainless Steels. Materials, 13(15), 3402. https://doi.org/10.3390/ma13153402






