Thermal Conductivity and Cure Kinetics of Epoxy-Boron Nitride Composites—A Review
Abstract
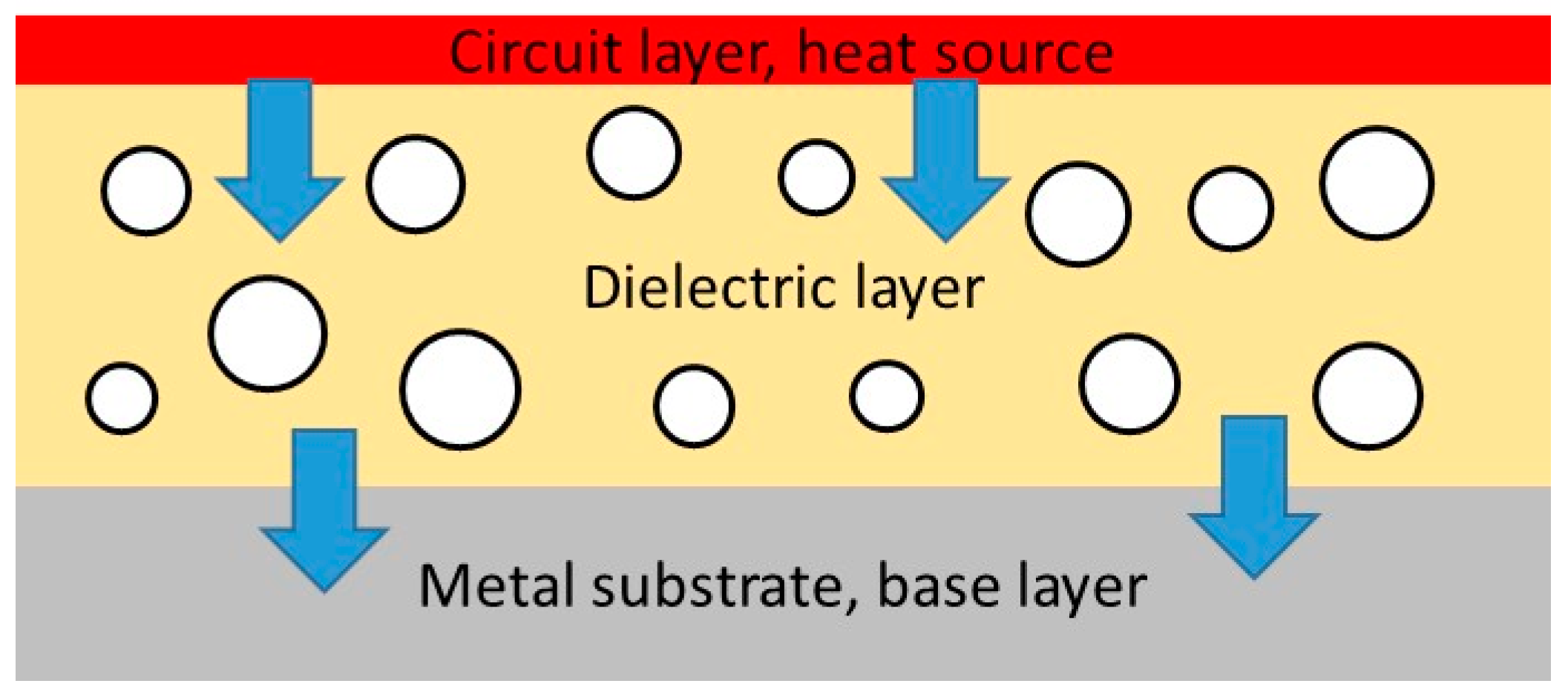
1. Introduction
2. Cure Kinetics
2.1. General Aspects
2.2. Epoxy Composites
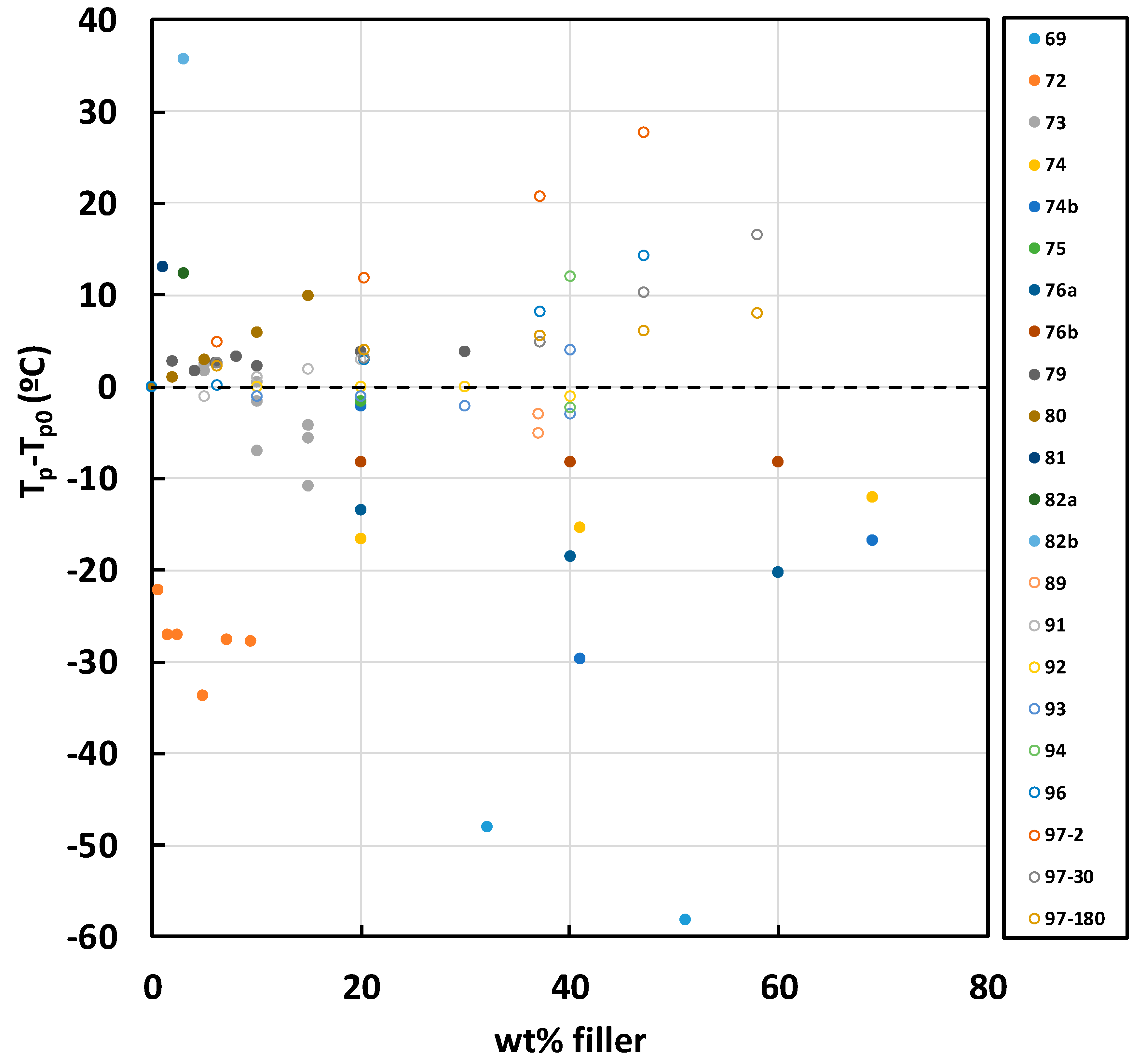
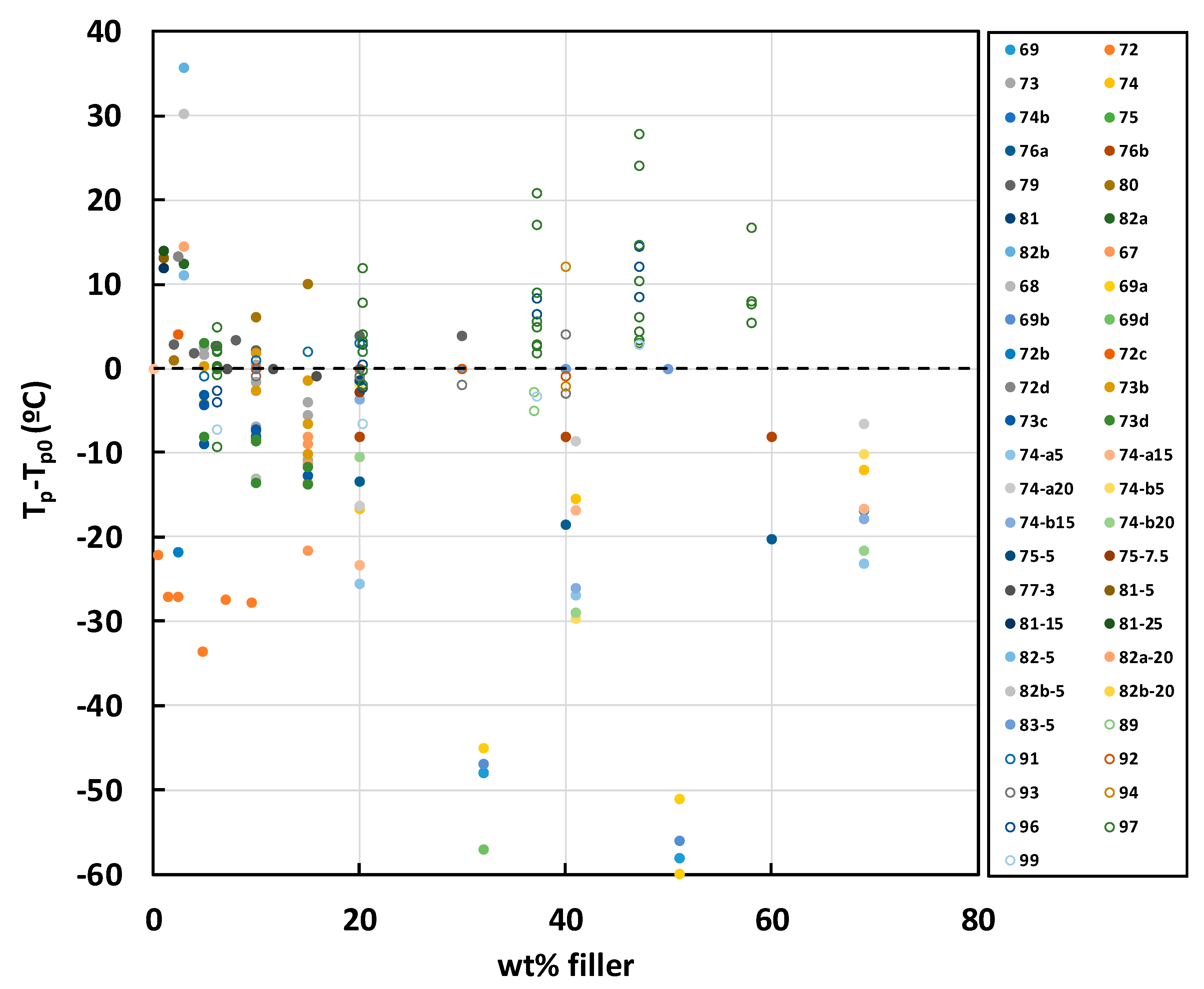
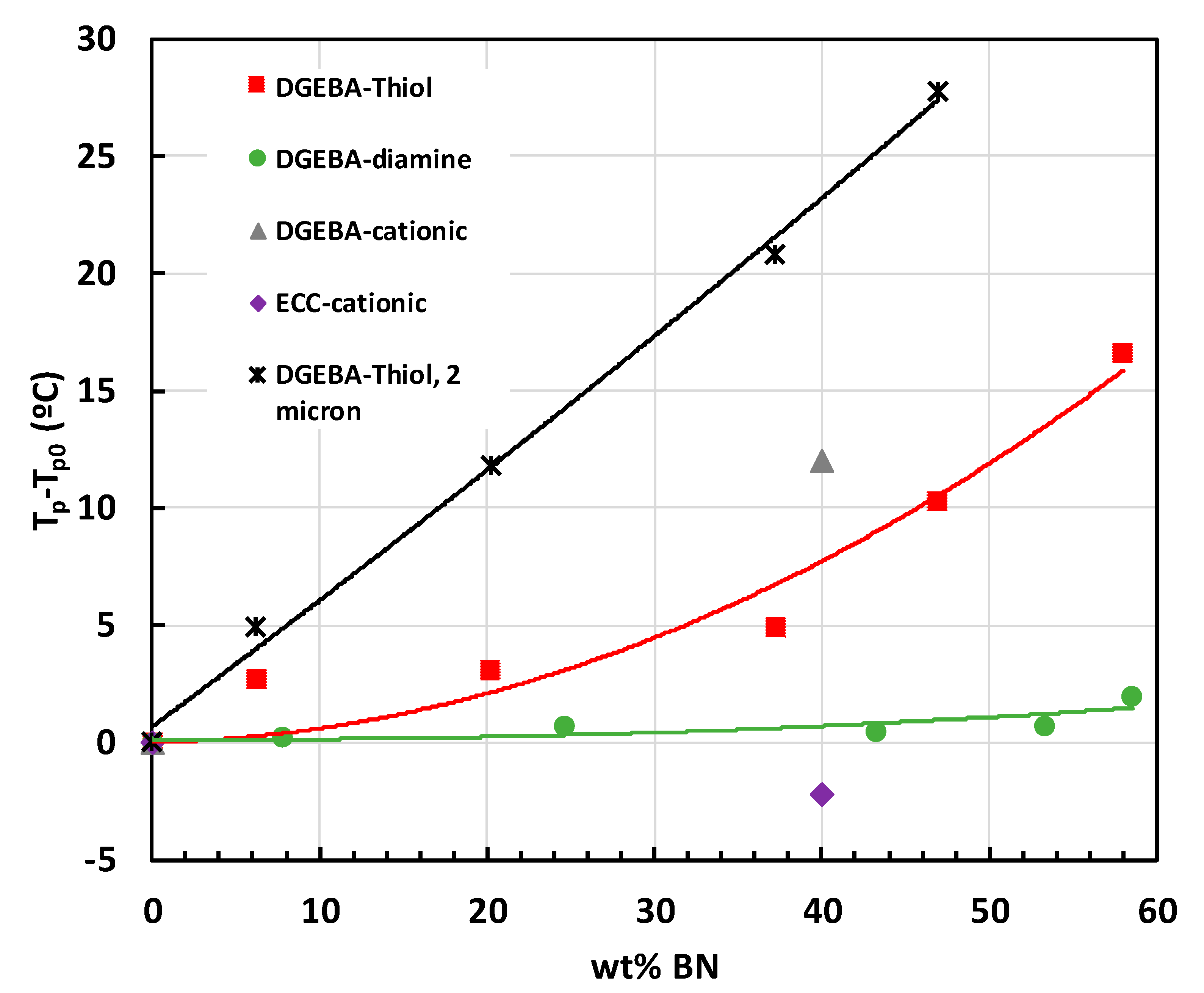
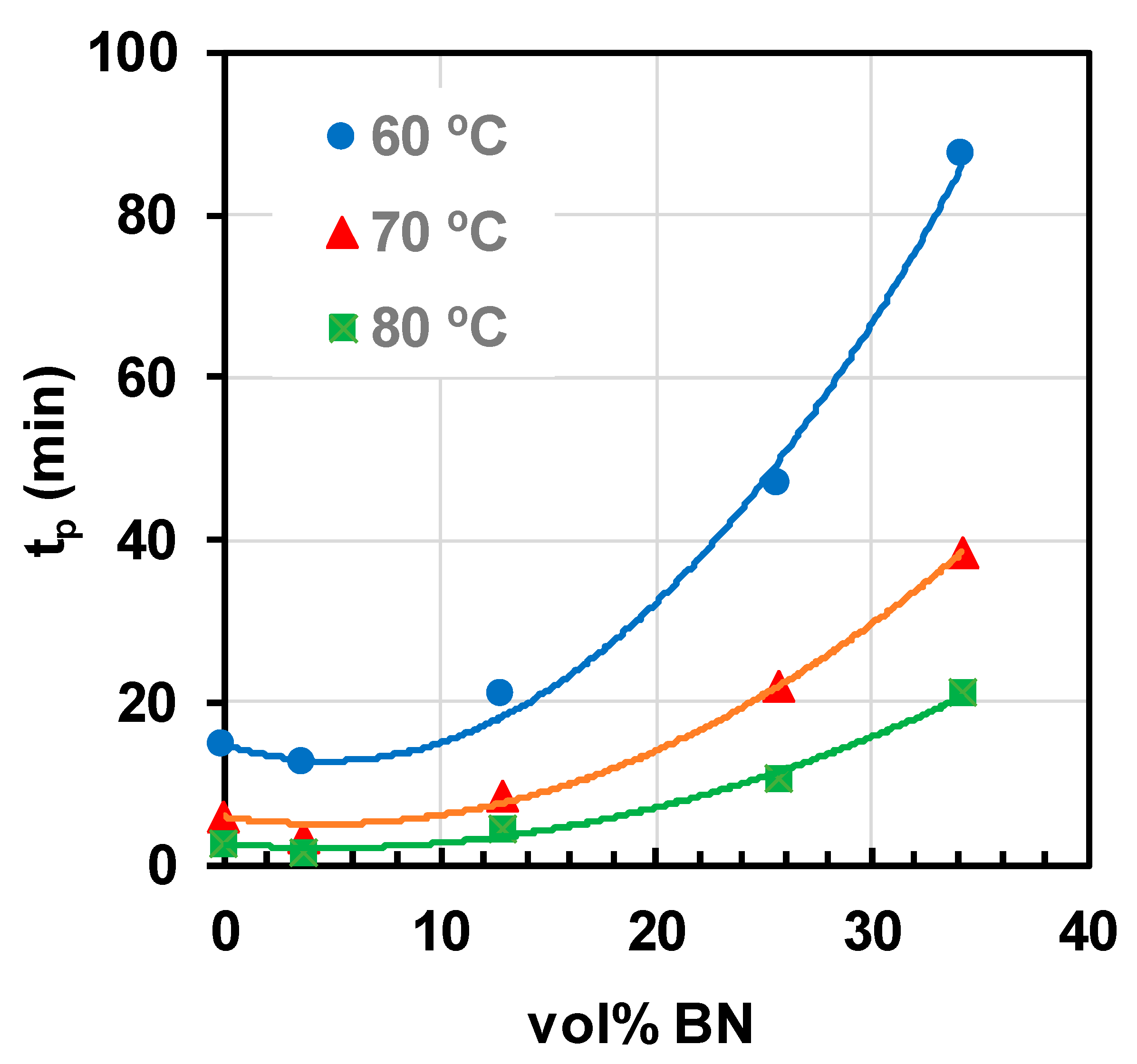
2.3. Epoxy-BN Composites
3. Thermal Conductivity
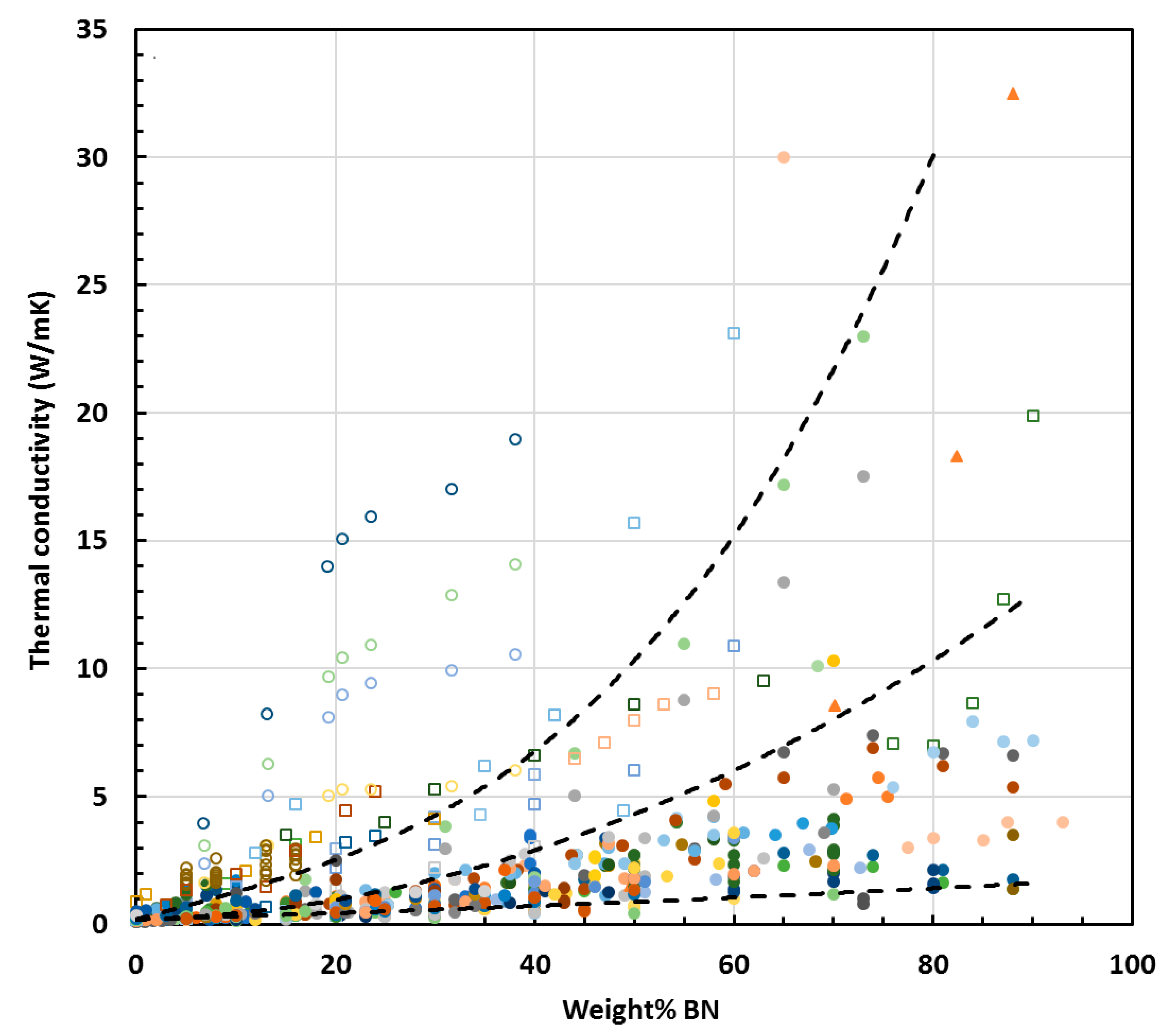
3.1. Effect of BN Content
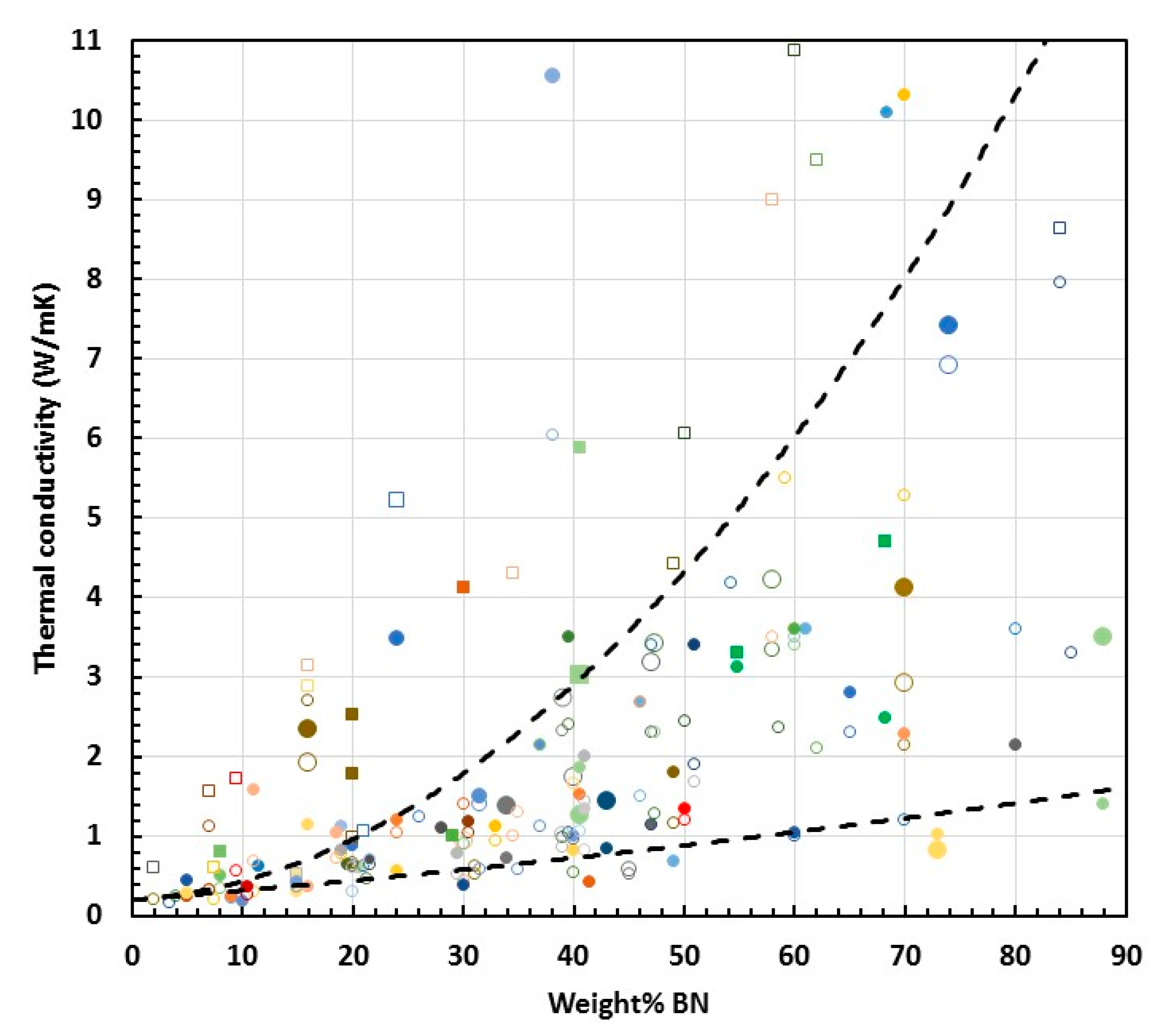
3.2. Effect of Surface Treatments and Coupling Agents
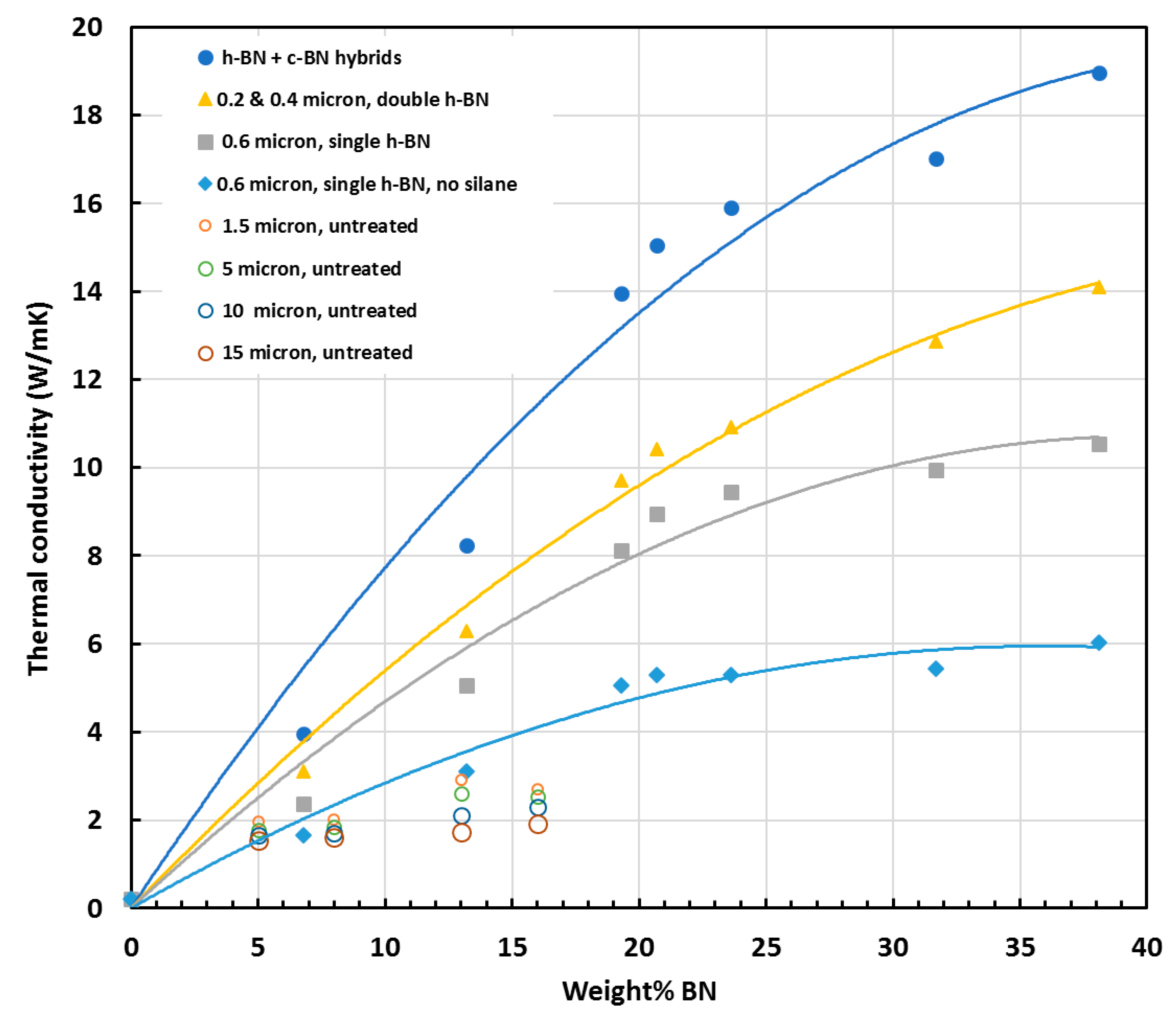
3.3. Effect of BN Particle Size and Shape
4. Special Procedures
4.1. Orientation
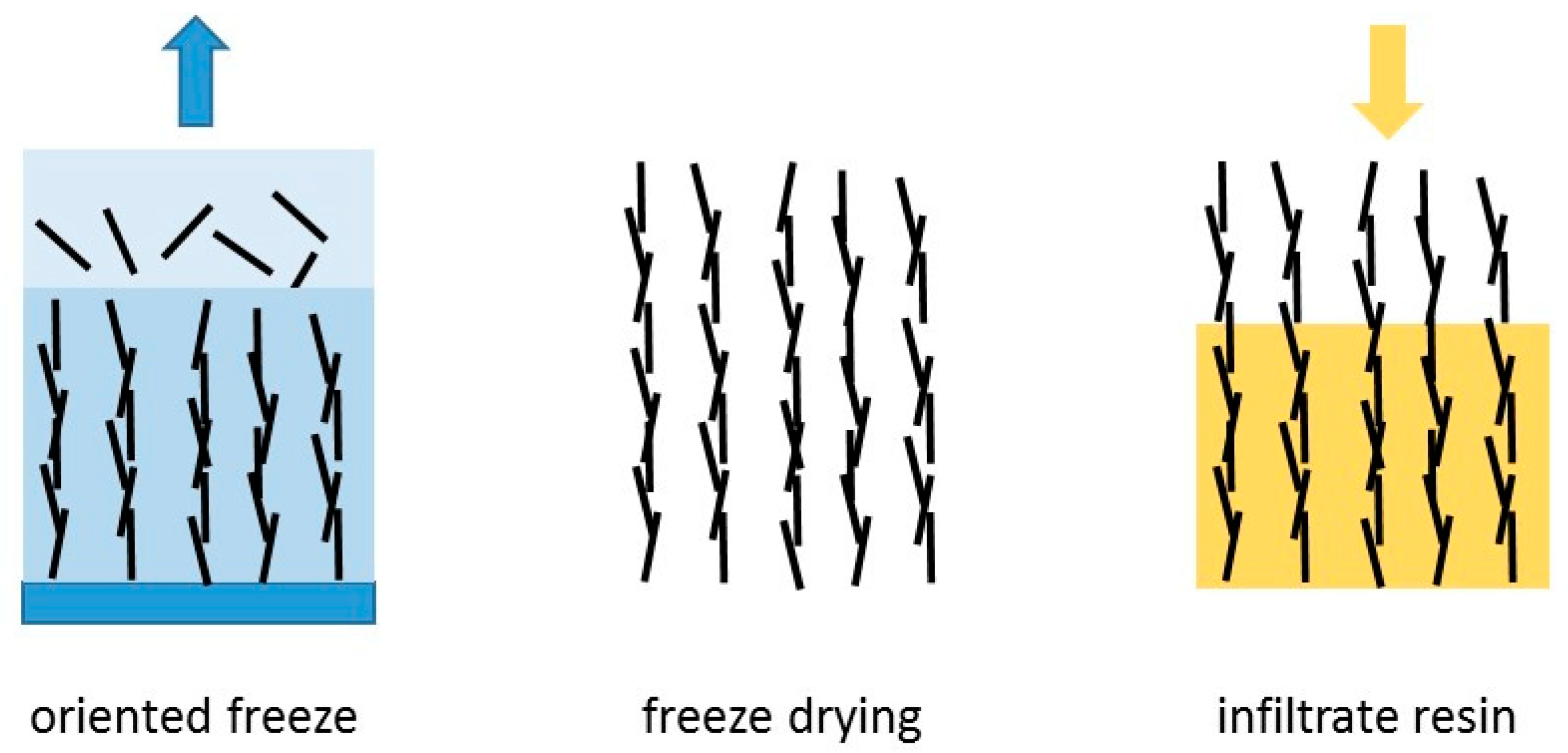
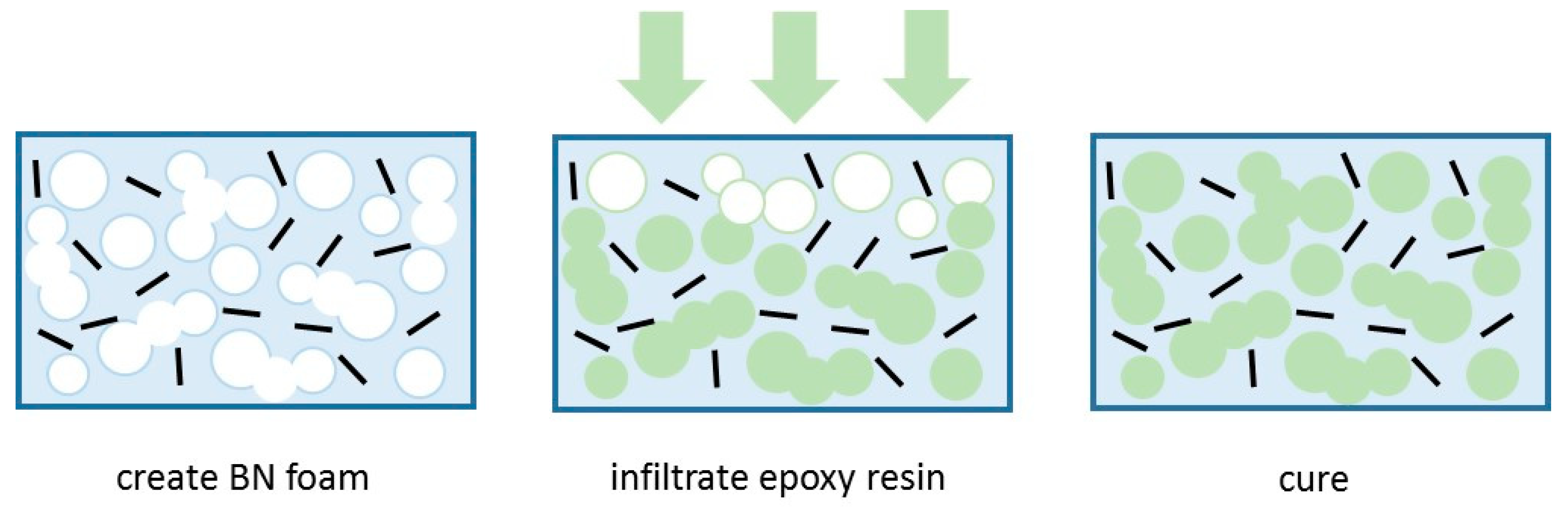
4.2. Three-Dimensional Structures
4.3. Surface Decoration
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bergquist. Selection Guide. Thermal Management for LED Applications. Available online: https://dm.henkel-dam.com/is/content/henkel/14919_Bergquist_LED_Thermal_Solutions_v4_LRpdf (accessed on 31 July 2020).
- Insulated Metal Substrate Copper Clads & Pre-Preg. Available online: https://www.aitechnology.com/products/insulated-metal-substrates/thermclads/ (accessed on 31 July 2020).
- Technology by MOS, Printed Circuit Board Technology for the Future. Available online: http://www.mos-electronic.com/en/downloads/english/Technology_en_07_2016.pdf (accessed on 31 July 2020).
- IKM (Insulated Metal Substrate). Available online: https://www.technoboards-kc.com/en/products-services/technologies/ims-insulated-metal-substrate.html (accessed on 31 July 2020).
- Tec-Thermal/Thermal Management & IMS. Available online: http://www.ventec-group.com/products/tec-thermal-thermal-management-ims/ (accessed on 31 July 2020).
- Zhou, T.; Wang, X.; Liu, X.; Xiong, D. Improved thermal conductivity of epoxy composites using a hybrid multi-walled carbon nanotube/micro-SiC filler. Carbon 2010, 48, 1171–1176. [Google Scholar] [CrossRef]
- Choi, S.; Kim, J. Thermal conductivity of epoxy composites with binary-particle system of aluminum oxide and aluminum nitride fillers. Compos. Part B 2013, 51, 140–147. [Google Scholar] [CrossRef]
- Heo, Y.; Im, H.; Kim, J.; Kim, J. The influence of Al(OH)3-coated graphene oxide on improved thermal conductivity and maintained electrical resistivity of Al2O3/epoxy composites. J. Nanopart. Res. 2012, 14, 1196. [Google Scholar] [CrossRef]
- Yamane, T.; Nagai, N.; Katayama, S.; Todoki, M. Measurement of thermal conductivity of silicon dioxide thin films using a 3ω method. J. Appl. Phys. 2002, 91, 9772–9776. [Google Scholar] [CrossRef]
- Jeong, J.; Li, C.; Kwon, Y.; Lee, J.; Kim, S.H.; Yun, R. Particle shape effect on the viscosity and thermal conductivity of ZnO nanofluids. Int. J. Refrig. 2013, 36, 2233–2241. [Google Scholar] [CrossRef]
- Chisholm, N.; Mahfuz, H.; Rangari, V.K.; Ashfaq, A.; Jeelani, S. Fabrication and mechanical characterization of carbon/SiC-epoxy nanocomposites. Compos. Struct. 2005, 67, 115–124. [Google Scholar] [CrossRef]
- Xie, H.; Wang, J.; Xi, T.; Liu, Y. Thermal conductivity of suspensions containing nanosized SiC particles. Int. J. Thermophys. 2002, 23, 571–580. [Google Scholar] [CrossRef]
- Shi, Z.; Radwan, M.; Kirihara, S.; Miyamoto, Y.; Jin, Z. Enhanced thermal conductivity of polymer composites filled with three-dimensional brushlike AlN nanowhiskers. Appl. Phys. Lett. 2009, 95, 224104. [Google Scholar] [CrossRef]
- Zhu, X.W.; Zhou, Y.; Hirao, K. Effect of processing method and additive composition on microstructure and thermal conductivity of Si3N4 ceramics. J. Eur. Ceram. Soc. 2006, 26, 711–718. [Google Scholar] [CrossRef]
- BN Boron Nitride. Thermal Properties. Available online: http://www.ioffe.ru/SVA/NSM/Semicond/BN/thermal.html (accessed on 31 July 2020).
- Boron Nitride. Available online: https://en.wikipedia.org/wiki/Boron_nitride (accessed on 31 July 2020).
- Saint-Gobain. CarboThermTM Thermal Management Fillers. Available online: https://www.bn.saint-gobain.com/sites/imdf.bn.com/files/carbotherm-bn-thermal-fillers-ds_0.pdf (accessed on 31 July 2020).
- Montserrat, S.; Flaqué, C.; Pagés, P.; Malek, J. Effect of the cross-linking degree on curing kinetics of an epoxy-anhydride system. J. Appl. Polym. Sci. 1995, 56, 1413–1421. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Mechanism and kinetics of epoxy-amine cure studied by differential scanning calorimetry. Macromolecules 1996, 29, 1867–1873. [Google Scholar] [CrossRef]
- Montserrat, S.; Andreu, G.; Cortés, P.; Calventus, Y.; Colomer, P.; Hutchinson, J.M.; Malek, J. Addition of a reactive diluent to a catalyzed epoxy-anhydride system. 1. Influence on the cure kinetics. J. Appl. Polym. Sci. 1995, 61, 1663–1674. [Google Scholar] [CrossRef]
- Karkanas, P.I.; Partridge, I.K. Cure modeling and monitoring of epoxy/amine resin systems. I. Cure kinetics modeling. J. Appl. Polym. Sci. 1995, 77, 1419–1431. [Google Scholar] [CrossRef]
- Rosu, D.; Cascaval, C.N.; Mustata, F.; Ciobanu, C. Cure kinetics of epoxy resins studied by non-isothermal DSC data. Thermochim. Acta 2002, 383, 119–127. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N.; Vyazovkin, S.; Mititelu, A.; Sladic, C.; Vincent, L. A study of epoxy-amine cure kinetics by combining isoconversional analysis with temperature modulated DSC and dynamic rheometry. Macromol. Chem. Phys. 2003, 204, 1815–1821. [Google Scholar] [CrossRef]
- Hardis, R.; Jessop, J.L.P.; Peters, F.E.; Kessler, M.R. Cure kinetics characterization and monitoring of an epoxy resin using DSC, Raman spectroscopy, and DEA. Comp. Part A Appl. Sci. Manuf. 2013, 49, 100–108. [Google Scholar] [CrossRef]
- Filyanov, Y.M. Effect of a filler on the glass transition temperature of an epoxy resin and its relation to the filled polymer properties. Polym. Sci. USSR 1978, 20, 2074–2078. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Moon, K.; Wong, C.P. Glass transition and relaxation behavior of epoxy nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3849–3858. [Google Scholar] [CrossRef]
- Stevens, G.C.; Richardson, M.J. Factors influencing the glass transition of DGEBA-anhydride epoxy resins. Polymer 1983, 24, 851–858. [Google Scholar] [CrossRef]
- Allaoui, A.; El Bounia, N. How carbon nanotubes affect the cure kinetics and glass transition temperature of their epoxy composites? A review. Express Polym. Lett. 2009, 3, 588–594. [Google Scholar] [CrossRef]
- Linec, M.; Music, B. The effects of silica-based fillers on the properties of epoxy molding compounds. Materials 2019, 12, 1811. [Google Scholar] [CrossRef] [PubMed]
- Dorigato, A.; Pegoretti, A. Shape memory epoxy nanocomposites with carbonaceous fillers and in-situ generated silver nanoparticles. Polym. Eng. Sci. 2019, 59, 694–703. [Google Scholar] [CrossRef]
- Vijayan, P.; Tanvir, A.; Mrlik, M.; Urbanek, M.; Al-Maardeed, M. TiO2/halloysite hybrid filler reinforced epoxy nanocomposites. Polym. Comp. 2018, 39, E2426–E2435. [Google Scholar] [CrossRef]
- Kang, S.; Hong, S.I.; Choe, C.R.; Park, M.; Rim, S.; Kim, J. Preparation and characterization of epoxy composites filled with functionalized nanosilica particles obtained via sol–gel process. Polymer 2001, 42, 879–887. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Shim, M.; Kim, S. Autocatalytic cure kinetics of natural zeolite filled epoxy composites. Mater. Chem. Phys. 1997, 48, 36–40. [Google Scholar] [CrossRef]
- Lu, M.; Shim, M.; Kim, S. Effect of filler on cure behavior of an epoxy system: Cure modeling. Polym. Eng. Sci. 1999, 39, 274–285. [Google Scholar] [CrossRef]
- Wu, J.; Chung, D.D.L. Calorimetric study of the effect of carbon fillers on the curing of epoxy. Carbon 2004, 42, 3039–3042. [Google Scholar] [CrossRef]
- Erdogan, B.C.; Seyhan, A.T.; Ocak, Y.; Tanoglu, M.; Balköse, D.; Ülkü, S. Cure kinetics of epoxy resin-natural zeolite composites. J. Therm. Anal. Calorim. 2008, 94, 743–747. [Google Scholar] [CrossRef]
- Haman, K.; Badrinaryanan, P.; Kessler, M.R. Effect of a zirconium tungstate filler on the cure behavior of a cyanate ester resin. Appl. Mater. Interfaces 2009, 1, 1190–1195. [Google Scholar] [CrossRef]
- Barghamadi, M. Kinetics and thermodynamics of isothermal curing reaction of epoxy-4, 4′-diaminoazobenzene reinforced with nanosilica and nanoclay particles. Polym. Comp. 2010, 31, 1442–1448. [Google Scholar] [CrossRef]
- Baller, J.; Thomassey, M.; Ziehmer, M.; Sanctuary, R. The catalytic influence of alumina nanoparticles on epoxy curing. Thermochim. Acta 2011, 517, 34–39. [Google Scholar] [CrossRef]
- Yu, J.H.; Duan, J.K.; Peng, W.Y.; Wang, L.C.; Peng, P.; Jiang, P.K. Influence of nano-AlN particles on thermal conductivity, thermal stability and cure behavior of cycloaliphatic epoxy/trimethacrylate system. Express Polym. Lett. 2011, 5, 132–141. [Google Scholar] [CrossRef]
- Saad, G.R.; Ezz, A.A.; Ahmed, H.A. Cure kinetics, thermal stability, and dielectric properties of epoxy/barium ferrite/polyaniline composites. Thermochim. Acta 2015, 599, 84–94. [Google Scholar] [CrossRef]
- Bi, Q.; Hao, L.; Zhang, Q.; Wang, P.; Xu, P.; Ding, Y. Study on the effect of amino-functionalized alumina on the curing kinetics of epoxy composites. Thermochim. Acta 2019, 678, 178302. [Google Scholar] [CrossRef]
- Nascimento, L.F.C.; da Luz, F.S.; Costa, U.O.; Braga, F.D.; Lima, E.P.; Monteiro, S.N. Curing kinetic parameters of epoxy composite reinforced with mallow fibers. Materials 2019, 12, 3939. [Google Scholar] [CrossRef]
- Olivier, P.; Ioualalen, K.; Cottu, J.P. Dynamic mechanical spectrometry analysis of modifications in the cure kinetics of polyepoxy composites with particulate fillers. J. Appl. Polym. Sci. 1997, 63, 745–760. [Google Scholar] [CrossRef]
- Sanctuary, R.; Baller, J.; Zielinski, B.; Becker, N.; Krüger, J.K.; Philipp, M.; Müller, U.; Ziehmer, M. Influence of Al2O3 nanoparticles on the isothermal cure of an epoxy resin. J. Phys. Condens. Matter 2009, 21, 035118. [Google Scholar] [CrossRef]
- Dutta, A.; Ryan, M.E. Effect of fillers on kinetics of epoxy cure. J. Appl. Polym. Sci. 1979, 24, 635–649. [Google Scholar] [CrossRef]
- De Miranda, M.I.G.; Tomedi, C.; Bica, C.I.D.; Samios, D. A d.s.c. kinetic study on the effect of filler concentration on crosslinking of diglycidylether of bisphenoI-A with 4,4’-diaminodiphenylmethane. Polymer 1997, 38, 1017–1020. [Google Scholar] [CrossRef]
- Omrani, A.; Rostami, A.A.; Sedaghat, E. Kinetics of cure for a coating system including DGEBA (n = 0)/1,8-NDA and barium carbonate. Thermochim. Acta 2010, 497, 21–26. [Google Scholar] [CrossRef]
- Zabihi, O.; Khodabandeh, A.; Mostafavi, S.M. Preparation, optimization and thermal characterization of a novel conductive thermoset nanocomposite containing polythiophene nanoparticles using dynamic thermal analysis. Polym. Degrad. Stab. 2012, 97, 3–13. [Google Scholar] [CrossRef]
- Karasinski, E.N.; da Luz, M.G.; Lepienski, C.M.; Coelho, L.A.F. Nanostructured coating based on epoxy/metal oxides: Kinetic curing and mechanical properties. Thermochim. Acta 2013, 569, 167–176. [Google Scholar] [CrossRef]
- Tarrío-Saavedra, J.; López-Beceiro, J.; Naya, S.; Gracia, C.; Artiaga, R. Controversial effects of fumed silica on the curing and thermomechanical properties of epoxy composites. Express Polym. Lett. 2010, 6, 382–395. [Google Scholar] [CrossRef]
- Ghaffari, M.; Ehsani, M.; Vandalvand, M.; Avazverdi, E.; Askari, A.; Goudarzi, A. Studying the effect of micro- and nano-sized ZnO particles on the curing kinetic of epoxy/polyaminoamide system. Prog. Org. Coat. 2015, 89, 277–283. [Google Scholar] [CrossRef]
- Harsch, M.; Karger-Kocsis, J.; Holst, M. Influence of fillers and additives on the cure kinetics of an epoxy/anhydride resin. Eur. Polym. J. 2007, 43, 1168–1178. [Google Scholar] [CrossRef]
- Yung, K.C.; Liem, H. Enhanced thermal conductivity of boron nitride epoxy-matrix composite through multi-modal particle size mixing. J. Appl. Polym. Sci. 2007, 106, 3587–3591. [Google Scholar] [CrossRef]
- Abbasi, S.; Aravamudhan, S. Effect of boron nitride (hBN) filler on thermal properties of underfill epoxy. In Proceedings of the 16th IEEE ITherm Conference, Orlando, FL, USA, 30 May–2 June 2017; pp. 251–259. [Google Scholar]
- Singh, A.K.; Panda, B.P.; Mohanty, S.; Nayak, K.S.; Gupta, M.K. Synergistic effect of hybrid graphene and boron nitride on the cure kinetics and thermal conductivity of epoxy adhesives. Polym. Adv. Technol. 2017, 28, 1851–1864. [Google Scholar] [CrossRef]
- Teng, C.; Ma, C.M.; Chiou, K.; Lee, T.; Shih, Y. Synergetic effect of hybrid boron nitride and multi-walled carbon nanotubes on the thermal conductivity of epoxy composites. Mater. Chem. Phys. 2011, 126, 722–728. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, Z.; Sun, Y.; Li, H.; Wang, Y.; Zhang, C.; Gong, X.; Wang, Y.; Yang, X.; Liu, Y. Boron nitride nanoparticles with high specific surface area: Preparation by a calcination method and application in epoxy resin. J. Inorg. Organomet. Polym. 2017, 27, 1142–1147. [Google Scholar] [CrossRef]
- Isarn, I.; Massagués, L.; Ramis, X.; Serra, A.; Ferrando, F. New BN-epoxy composites obtained by thermal latent cationic curing with enhanced thermal conductivity. Compos. Part A 2017, 103, 35–47. [Google Scholar] [CrossRef]
- Isarn, I.; Gamardella, F.; Massagués, L.; Fernàndez-Francos, X.; Serra, A.; Ferrando, F. New epoxy composite thermosets with enhanced thermal conductivity and high Tg obtained by cationic homopolymerization. Polym. Compos. 2018, 39, 1760–1769. [Google Scholar] [CrossRef]
- Isarn, I.; Ramis, X.; Ferrando, F.; Serra, A. Thermoconductive thermosetting composites based on boron nitride fillers and thiol-epoxy matrices. Polymers 2018, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Isarn, I.; Bonnaud, L.; Massagués, L.; Serra, A.; Ferrando, F. Study of the synergistic effect of boron nitride and carbon nanotubes in the improvement of thermal conductivity of epoxy composites. Polym. Int. 2020, 69, 280–290. [Google Scholar] [CrossRef]
- Hutchinson, J.M.; Román, F.; Cortés, P.; Calventus, Y. Epoxy composites filled with boron nitride and aluminium nitride for improved thermal conductivity. Polimery 2017, 62, 560–566. [Google Scholar] [CrossRef]
- Hutchinson, J.M.; Román, F.; Folch, A. Epoxy-thiol systems filled with boron nitride for high thermal conductivity applications. Polymers 2018, 10, 340. [Google Scholar] [CrossRef]
- Moradi, S.; Calventus, Y.; Román, F.; Hutchinson, J.M. Achieving high thermal conductivity in epoxy composites: Effect of boron nitride particle size and matrix-filler interface. Polymers 2019, 11, 1156. [Google Scholar] [CrossRef]
- Daneshmehr, S.; Román, F.; Hutchinson, J.M. The surface modification of boron nitride particles. J. Thermal Anal. Calorim. 2019. [Google Scholar] [CrossRef]
- Moradi, S.; Calventus, Y.; Román, F.; Ruiz, P.; Hutchinson, J.M. Epoxy composites filled with boron nitride: Cure kinetics and the effect of particle shape on the thermal conductivity. J. Thermal Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- Agrawal, A.; Chandrakar, S. Influence of particulate surface treatment on physical, mechanical, thermal, and dielectric behavior of epoxy/hexagonal boron nitride composites. Polym. Compos. 2020, 41, 1574–1583. [Google Scholar] [CrossRef]
- Bian, W.; Yao, T.; Chen, M.; Zhang, C.; Shao, T.; Yang, Y. The synergistic effects of the micro-BN and nano-Al2O3 in micro-nano composites on enhancing the thermal conductivity for insulating epoxy resin. Comp. Sci. Technol. 2018, 168, 420–428. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Zhu, Y.; Jiang, P. Cellulose nanofiber supported 3D interconnected BN nanosheets for epoxy nanocomposites with ultrahigh thermal management capability. Adv. Funct. Mater. 2017, 27, 1604754. [Google Scholar] [CrossRef]
- Chen, C.; Xue, Y.; Li, Z.; Wen, Y.; Li, X.; Wu, F.; Li, X.; Shi, D.; Xue, Z.; Xie, X. Construction of 3D boron nitride nanosheets/silver networks in epoxy-based composites with high thermal conductivity via in-situ sintering of silver nanoparticles. Chem. Eng. J. 2019, 369, 1150–1160. [Google Scholar] [CrossRef]
- Chung, S.; Lin, J. Thermal conductivity of epoxy resin composites filled with combustion synthesized h-BN particles. Molecules 2016, 21, 670. [Google Scholar] [CrossRef] [PubMed]
- Donnay, M.; Tzavalas, S.; Logakis, E. Boron nitride filled epoxy with improved thermal conductivity and dielectric breakdown strength. Comp. Sci. Technol. 2015, 110, 152–158. [Google Scholar] [CrossRef]
- Fang, L.; Wu, C.; Qian, R.; Xie, L.; Yang, K.; Jiang, P. Nano-micro structure of functionalized boron nitride and aluminum oxide for epoxy composites with enhanced thermal conductivity and breakdown strength. RSC Adv. 2014, 4, 21010–21017. [Google Scholar] [CrossRef]
- Feng, Y.; Han, G.; Wang, B.; Zhou, X.; Ma, J.; Ye, Y.; Liu, C.; Xie, X. Multiple synergistic effects of graphene-based hybrid and hexagonal born nitride in enhancing thermal conductivity and flame retardancy of epoxy. Chem. Eng. J. 2020, 379, 122402. [Google Scholar] [CrossRef]
- Firdaus, S.M.; Mariatti, M. Nano-sized boron nitride epoxy composites for underfill application: Effect of diluent and filler loading. J. Mater. Sci. Mater. Electron. 2015, 26, 774–783. [Google Scholar] [CrossRef]
- Fu, Y.; He, Z.; Mo, D.; Lu, S. Thermal conductivity enhancement with different fillers for epoxy resin adhesives. Appl. Therm. Eng. 2014, 66, 493–498. [Google Scholar] [CrossRef]
- Fu, C.; Yan, C.; Ren, L.; Zeng, X.; Du, G.; Sun, R.; Xu, J.; Wong, C. Improving thermal conductivity through welding boron nitride nanosheets onto silver nanowires via silver nanoparticles. Comp. Sci. Technol. 2019, 177, 118–126. [Google Scholar] [CrossRef]
- Fu, X.; Guo, Y.; Du, Q.; Guan, L.; He, S. Improved dielectric stability of epoxy composites with ultralow boron nitride loading. RSC Adv. 2019, 9, 4344–4350. [Google Scholar] [CrossRef]
- Fu, J.; Shi, L.; Zhang, D.; Zhong, Q.; Chen, Y. Effect of nanoparticles on the performance of thermally conductive epoxy adhesives. Polym. Eng. Sci. 2010, 50, 1809–1819. [Google Scholar] [CrossRef]
- Gaska, K.; Rybak, A.; Kapusta, C.; Sekula, R.; Siwek, A. Enhanced thermal conductivity of epoxy-matrix composites with hybrid fillers. Polym. Adv. Technol. 2015, 26, 26–31. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, Q.; Dang, J.; Xie, C. Thermal conductivity epoxy resin composites filled with boron nitride. Polym. Adv. Technol. 2012, 23, 1025–1028. [Google Scholar] [CrossRef]
- Han, S.; Meng, Q.; Qiu, Z.; Osman, A.; Cai, R.; Yu, Y.; Liu, T.; Araby, S. Mechanical, toughness and thermal properties of 2D material-reinforced epoxy composites. Polymer 2019, 184, 121884. [Google Scholar] [CrossRef]
- Han, Y.; Shi, X.; Yang, X.; Guo, Y.; Zhang, J.; Kong, J.; Gu, J. Enhanced thermal conductivities of epoxy nanocomposites via incorporating in-situ fabricated hetero-structured SiC-BNNS fillers. Comp. Sci. Technol. 2020, 187, 107944. [Google Scholar] [CrossRef]
- He, Y.; Wang, Q.; Liu, W.; Liu, Y. Functionalization of boron nitride nanoparticles and their utilization in epoxy composites with enhanced thermal conductivity. Phys. Stat. Sol. A 2014, 211, 677–684. [Google Scholar] [CrossRef]
- Hong, J.; Yoon, S.; Hwang, T.; Lee, Y.; Won, S.; Nam, J. Interphase control of boron nitride/epoxy composites for high thermal conductivity. Korea Aust. Rheol. J. 2010, 22, 259–264. [Google Scholar]
- Hong, J.; Yoon, S.; Hwang, T.; Oh, J.; Hong, S.; Lee, Y.; Nam, J. High thermal conductivity epoxy composites with bimodal distribution of aluminum nitride and boron nitride fillers. Thermochim. Acta 2012, 537, 70–75. [Google Scholar] [CrossRef]
- Hou, J.; Li, G.; Yang, N.; Qin, L.; Grami, M.E.; Zhang, Q.; Wang, N.; Qu, X. Preparation and characterization of surface modified boron nitride epoxy composites with enhanced thermal conductivity. RSC Adv. 2014, 4, 44282–44290. [Google Scholar] [CrossRef]
- Hu, J.; Huang, Y.; Yao, Y.; Pan, G.; Sun, J.; Zeng, X.; Sun, R.; Xu, J.; Song, B.; Wong, C. Polymer composite with improved thermal conductivity by constructing a hierarchically ordered three-dimensional interconnected network of BN. ACS Appl. Mater. Interfaces 2017, 9, 13544–13553. [Google Scholar] [CrossRef]
- Hu, J.; Huang, Y.; Zeng, X.; Li, Q.; Ren, L.; Sun, R.; Xu, J.; Wong, C. Polymer composite with enhanced thermal conductivity and mechanical strength through orientation manipulating of BN. Comp. Sci. Technol. 2018, 160, 127–137. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, P.; Li, G.; Zhou, F.; Lu, D.; Sun, R.; Wong, C. Spherical and flake-like BN filled epoxy composites: Morphological effect on the thermal conductivity, thermo-mechanical and dielectric properties. J. Mater. Sci. Mater. Electron. 2015, 26, 3564–3572. [Google Scholar] [CrossRef]
- Huang, T.; Zeng, X.; Yao, Y.; Sun, R.; Meng, F.; Xue, J.; Wong, C. Boron nitride@graphene oxide hybrids for epoxy composites with enhanced thermal conductivity. RSC Adv. 2016, 6, 35847–35854. [Google Scholar] [CrossRef]
- Huang, T.; Zeng, X.; Yao, Y.; Sun, R.; Meng, F.; Xue, J.; Wong, C. A novel h-BN-RGO hybrids for epoxy resin composites achieving enhanced high thermal conductivity and energy density. RSC Adv. 2017, 7, 23355–23362. [Google Scholar] [CrossRef]
- Ishida, H.; Rimdusit, S. Very high thermal conductivity obtained by boron nitride-filled polybenzoxazine. Thermochim. Acta 1998, 320, 177–186. [Google Scholar] [CrossRef]
- Islam, A.M.; Lim, H.; You, N.; Ahn, S.; Goh, M.; Hahn, J.R.; Yeo, H.; Jang, S.G. Enhanced thermal conductivity of liquid crystalline epoxy resin using controlled linear polymerization. ACS Macro. Lett. 2018, 7, 1180–1185. [Google Scholar] [CrossRef]
- Jang, I.; Shin, K.; Yang, I.; Kim, H.; Kim, J.; Kim, W.; Jeon, S.; Kim, J. Enhancement of thermal conductivity of BN/epoxy composite through surface modification with silane coupling agents. Colloids Surf. A Physicochem. Eng. Asp. 2017, 518, 64–72. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, X.; Feng, Y.; Li, S.; Zhou, X.; Xie, X. Enhanced thermal conductivity and ideal dielectric properties of epoxy composites containing polymer modified hexagonal boron nitride. Compos. Part A 2018, 107, 657–664. [Google Scholar] [CrossRef]
- Kargar, F.; Barani, Z.; Salgado, R.; Debnath, B.; Lewis, J.S.; Aytan, E.; Lake, R.K.; Balandin, A.A. Thermal percolation threshold and thermal properties of composites with high loading of graphene and boron nitride fillers. ACS Appl. Mater. Interfaces 2018, 10, 37555–37565. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J. Fabrication of Fe3O4 coated boron nitride nanoplatelets by liquid-phase exfoliation for thermally enhanced epoxy composites via magnetic alignment. Comp. Sci. Technol. 2020, 188, 107961. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J. Exfoliated boron nitride nanosheet/MWCNT hybrid composite for thermal conductive material via epoxy wetting. Compos. Part B 2018, 140, 9–15. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J. Fabrication of thermally conductive composite with surface modified boron nitride by epoxy wetting method. Ceram. Int. 2014, 40, 5181–5189. [Google Scholar] [CrossRef]
- Kim, K.; Kim, M.; Hwang, Y.; Kim, J. Chemically modified boron nitride-epoxy terminated dimethylsiloxane composite for improving the thermal conductivity. Ceram. Int. 2014, 40, 2047–2056. [Google Scholar] [CrossRef]
- Kim, K.; Kim, M.; Kim, J. Thermal and mechanical properties of epoxy composites with a binary particle filler system consisting of aggregated and whisker type boron nitride particles. Comp. Sci. Technol. 2014, 103, 72–77. [Google Scholar] [CrossRef]
- Kim, K.; Oh, H.; Kim, J. Fabrication of covalently linked exfoliated boron nitride nanosheet/multi-walled carbon nanotube hybrid particles for thermal conductive composite materials. RSC Adv. 2018, 8, 33506–33515. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, H.; Kim, J. Enhanced thermal conductivity of epoxy composites using boron nitride nanoplatelets prepared by Fe3O4 assisted liquid-phase exfoliation. Ceram. Int. 2019, 45, 24121–24126. [Google Scholar] [CrossRef]
- Kochetov, R.; Andritsch, T.; Lafont, U.; Morshuis, P.H.F.; Picken, S.J.; Smit, J.J. Thermal behaviour of epoxy resin filled with high thermal conductivity nanopowders. In Proceedings of the 2009 IEEE Electrical Insulation Conference, Montreal, QC, Canada, 31 May–3 June 2009. [Google Scholar]
- Lee, W.S.; Yu, J. Comparative study of thermally conductive fillers in underfill for the electronic components. Diam. Relat. Mater. 2005, 14, 1647–1653. [Google Scholar]
- Lee, D.; Lee, S.; Byun, S.; Paik, K.; Song, S.H. Novel dielectric BN/epoxy nanocomposites with enhanced heat dissipation performance for electronic packaging. Compos. Part A 2018, 107, 217–223. [Google Scholar] [CrossRef]
- Lee, J.; Shin, H.; Rhee, K.Y. Surface functionalization of boron nitride platelets via a catalytic oxidation/silanization process and thermomechanical properties of boron nitride-epoxy composites. Compos. Part B 2019, 157, 276–282. [Google Scholar] [CrossRef]
- Lei, H.; Shi, Q.; Chen, J. Research on BNNTs/epoxy/silicone ternary composite systems for high thermal conductivity. IOP Conf. Ser. Mater. Sci. Eng. 2018, 381, 012076. [Google Scholar] [CrossRef]
- Lei, Y.; Han, Z.; Ren, D.; Pan, H.; Xu, M.; Liu, X. Design of h-BN-filled cyanate/epoxy thermal conductive composite with stable dielectric properties. Macromol. Res. 2018, 26, 602–608. [Google Scholar] [CrossRef]
- Lewis, J.S.; Barani, Z.; Magana, A.S.; Kargar, F.; Balandin, A.A. Thermal and electrical conductivity control in hybrid composites with graphene and boron nitride fillers. Mater. Res. Express 2019, 6, 085325. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Chen, C.; Ye, Y.; Zeng, H.; Qu, H.; Liu, J.; Zhou, X.; Long, S.; Xie, X. Highly thermally conductive flame retardant epoxy nanocomposites with multifunctional ionic liquid nanosheets. J. Mater. Chem. A 2018, 6, 20500–20512. [Google Scholar] [CrossRef]
- Lim, H.S.; Oh, J.W.; Kim, S.Y.; Yoo, M.; Park, S.; Lee, W.S. Anisotropically alignable magnetic boron nitride platelets decorated with iron oxide nanoparticles. Chem. Mater. 2013, 25, 3315–3319. [Google Scholar] [CrossRef]
- Lin, Z.; Mcnamara, A.; Liu, Y.; Moon, K.; Wong, C. Exfoliated hexagonal boron nitride-based polymer nanocomposite with enhanced thermal conductivity for electronic encapsulation. Comp. Sci. Technol. 2014, 90, 123–128. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Liu, X. Novel functionalized BN nanosheets/epoxy composites with advanced thermal conductivity and mechanical properties. ACS Appl. Mater. Interfaces 2020, 12, 6503–6515. [Google Scholar] [CrossRef]
- Liu, J.; Guo, Y.; Weng, C.; Zhang, H.; Zhang, Z. High thermal conductive epoxy based composites fabricated by multi-material direct ink writing. Compos. Part A 2020, 129, 105684. [Google Scholar] [CrossRef]
- Mun, S.Y.; Lim, H.M.; Lee, S. Thermal and electrical properties of epoxy composite with expanded graphite-ceramic core-shell hybrids. Mater. Res. Bull. 2018, 97, 19–23. [Google Scholar] [CrossRef]
- Na, T.; Liu, X.; Jiang, H.; Zhao, L.; Zhao, C. Enhanced thermal conductivity of fluorinated epoxy resins by incorporating inorganic filler. React. Functional Polym. 2018, 128, 84–90. [Google Scholar] [CrossRef]
- Owais, M.; Zhao, J.; Imani, A.; Wang, G.; Zhang, H.; Zhang, Z. Synergetic effect of hybrid fillers of boron nitride, graphene nanoplatelets and short carbon fibers for enhanced thermal conductivity and electrical resistivity of epoxy nanocomposites. Compos. Part A 2019, 117, 11–22. [Google Scholar] [CrossRef]
- Pawelski, C.; Kang, E.; Bakis, G.; Altstädt, V. Effect of filler type and particle size distribution on thermal properties of bimodal and hybrid—BN/boehmite-filled EP-Novolac composites. AIP Conf. Proc. 2019, 2055, 050007. [Google Scholar] [CrossRef]
- Permal, A.; Devarajan, M.; Hung, H.L.; Zahner, T.; Lacey, D.; Ibrahim, K. Thermal and mechanical properties of epoxy composite filled with binary particle system of polygonal aluminum oxide and boron nitride platelets. J. Mater. Sci. 2016, 51, 7415–7426. [Google Scholar] [CrossRef]
- Qu, T.; Yang, N.; Hou, J.; Li, G.; Yao, Y.; Zhang, Q.; He, L.; Wua, D.; Qu, X. Flame retarding epoxy composites with poly(phosphazene-co-bisphenol A)-coated boron nitride to improve thermal conductivity and thermal stability. RSC Adv. 2017, 7, 6140–6151. [Google Scholar] [CrossRef]
- Ryu, S.; Oh, T.; Kim, J. Surface modification of a BN/ETDS composite with aniline trimer for high thermal conductivity and excellent mechanical properties. RSC Adv. 2018, 8, 22846–22852. [Google Scholar] [CrossRef]
- Salehirad, M.; Nikje, M.M.A.; Ahmadian-Alam, L. Synthesis and characterization of functionalized Fe3O4/boron nitride as magnetically alignable 2D-nanofiller to improve the thermal conductivity of epoxy nanocomposites. Ind. Eng. Chem. Res. 2018, 57, 1803–1814. [Google Scholar] [CrossRef]
- Song, W.; Wang, P.; Cao, L.; Anderson, A.; Meziani, M.J.; Farr, A.J.; Sun, Y. Polymer/boron nitride nanocomposite materials for superior thermal transport performance. Angew. Chem. Int. Ed. 2012, 51, 6498–6501. [Google Scholar] [CrossRef]
- Su, Z.; Wang, H.; He, J.; Guo, Y.; Qu, Q.; Tian, X. Fabrication of thermal conductivity enhanced polymer composites by constructing an oriented three-dimensional staggered interconnected network of boron nitride platelets and carbon nanotubes. ACS Appl. Mater. Interfaces 2018, 10, 36342–36345. [Google Scholar] [CrossRef]
- Sun, J.; Wang, D.; Yao, Y.; Zeng, X.; Pan, G.; Huang, Y.; Hu, J.; Sun, R.; Xu, J.; Wong, C. Boron nitride microsphere/epoxy composites with enhanced thermal conductivity. High Volt. 2017, 2, 147–153. [Google Scholar] [CrossRef]
- Tanaka, T.; Wang, Z.; Iizuka, T.; Kozako, M.; Ohki, Y. High thermal conductivity epoxy/BN composites with sufficient dielectric breakdown strength. In Proceedings of the IEEE 2011 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Cancun, Mexico, 16–19 October 2011; Volumes 1 and 2, pp. 691–694. [Google Scholar]
- Tang, Y.; Zhang, P.; Zhu, M.; Li, J.; Li, Y.; Wang, Z.; Huang, L. Temperature effects on the dielectric properties and breakdown performance of h-BN/epoxy composites. Materials 2019, 12, 4112. [Google Scholar] [CrossRef]
- Tang, D.; Su, J.; Kong, M.; Zhao, Z.; Yang, Q.; Huang, Y.; Liao, X.; Niu, Y. Preparation and properties of epoxy/BN highly thermal conductive composites reinforced with SiC whisker. Polym. Comp. 2016, 37, 2611–2621. [Google Scholar] [CrossRef]
- Tian, Z.; Sun, J.; Wang, S.; Zeng, X.; Bai, S.; Zhao, N.; Wong, C. A thermal interface material based on foam-templated three-dimensional hierarchical porous boron nitride. J. Mater. Chem. A 2018, 6, 17540–17547. [Google Scholar] [CrossRef]
- Voo, R.; Mariattia, M.; Sim, L.C. Thermal properties and moisture absorption of nanofillers-filled epoxy composite thin film for electronic application. Polym. Adv. Technol. 2012, 23, 1620–1627. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, P. 3D vertically aligned BNNS network with long-range continuous channels for achieving a highly thermally conductive composite. ACS Appl. Mater. Interfaces 2019, 11, 28943–28952. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, P. Melamine foam-supported 3D interconnected boron nitride nanosheets network encapsulated in epoxy to achieve significant thermal conductivity enhancement at an ultralow filler loading. Chem. Eng. J. 2018, 348, 723–731. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Cheng, Y.; Chen, S.; Yang, M.; Huang, J.; Wang, H.; Wu, G.; Wu, H. Alignment of boron nitride nanofibers in epoxy composite films for thermal conductivity and dielectric breakdown strength improvement. Nanomaterials 2018, 8, 242. [Google Scholar] [CrossRef]
- Wattanakul, K.; Manuspiya, H.; Yanumet, N. The adsorption of cationic surfactants on BN surface: Its effects on the thermal conductivity and mechanical properties of BN-epoxy composite. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 369, 203–210. [Google Scholar] [CrossRef]
- Wattanakul, K.; Manuspiya, H.; Yanumet, N. Effective surface treatments for enhancing the thermal conductivity of BN-filled epoxy composite. J. Appl. Polym. Sci. 2011, 119, 3234–3243. [Google Scholar] [CrossRef]
- Wattanakul, K.; Manuspiya, H.; Yanumet, N. Thermal conductivity and mechanical properties of BN-filled epoxy composite: Effects of filler content, mixing conditions, and BN agglomerate size. J. Comp. Mater. 2011, 45, 1967–1980. [Google Scholar] [CrossRef]
- Weng, L.; Wang, H.; Zhang, X.; Liu, L.; Zhang, H. Preparation and properties of boron nitride/epoxy composites with high thermal conductivity and electrical insulation. J. Mater. Sci. Mater. Electron. 2018, 29, 14267–14276. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Negi, A.; He, J.; Hu, G.; Tian, S.; Liu, J. Synergistic effects of boron nitride (BN) nanosheets and silver (Ag) nanoparticles on thermal conductivity and electrical properties of epoxy nanocomposites. Polymers 2020, 12, 426. [Google Scholar] [CrossRef]
- Xia, C.; Garcia, A.C.; Shi, S.Q.; Qiu, Y.; Warner, N.; Wu, Y.; Cai, L.; Rizvi, H.R.; D’Souza, N.A.; Nie, X. Hybrid boron nitride-natural fiber composites for enhanced thermal conductivity. Sci. Rep. 2016, 6, 34726. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Guo, Y.; Tang, Y.; Ding, J.; Zhang, X.; Zheng, K.; Tian, X. Epoxy composite with significantly improved thermal conductivity by constructing a vertically aligned three-dimensional network of silicon carbide nanowires/ boron nitride nanosheets. Compos. Part B 2020, 187, 107855. [Google Scholar] [CrossRef]
- Xu, Y.; Chung, D.D.L. Increasing the thermal conductivity of boron nitride and aluminum nitride particle epoxy-matrix composites by particle surface treatments. Compos. Interfaces 2000, 7, 243–256. [Google Scholar] [CrossRef]
- Yadav, A.K. Thermal Characteristics of Boron Nitride Filled Epoxy Composites. Master’s Thesis, National Institute of Technology, Rourkela, India, June 2013. [Google Scholar]
- Yetgin, H.; Veziroglu, S.; Aktas, O.C.; Yalçinkaya, T. Enhancing thermal conductivity of epoxy with a binary filler system of h-BN platelets and Al2O3 nanoparticles. Int. J. Adhes. Adhes. 2020, 98, 102540. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, J.; Li, Z.; Tian, W.; Wang, L.; Luo, J.; Li, Q.; Fan, X.; Yao, Y. Enhanced through-plane thermal conductivity of boron nitride/epoxy composites. Compos. Part A 2017, 98, 25–31. [Google Scholar] [CrossRef]
- Yu, J.; Huang, X.; Wu, C.; Wu, X.; Wang, G.; Jiang, P. Interfacial modification of boron nitride nanoplatelets for epoxy composites with improved thermal properties. Polymer 2012, 53, 471–480. [Google Scholar] [CrossRef]
- Yung, K.C.; Wang, J.; Yue, T.M. Thermal management for boron nitride filled metal core printed circuit board. J. Comp. Mater. 2008, 42, 2615–2627. [Google Scholar] [CrossRef]
- Yung, K.C.; Zhu, B.L.; Yue, T.M.; Xie, C.S. Development of epoxy-matrix composite with both high-thermal conductivity and low-dielectric constant via hybrid filler systems. J. Appl. Polym. Sci. 2010, 116, 518–527. [Google Scholar] [CrossRef]
- Yung, K.C.; Liem, H.; Choy, H.S. Prerequisite for maximizing thermal conductivity of epoxy laminate using filler. J. Mater. Sci. Mater. Electron. 2013, 24, 1095–1104. [Google Scholar] [CrossRef]
- Zeng, X.; Yao, Y.; Gong, Z.; Wang, F.; Sun, R.; Xu, J.; Wong, C. Ice-templated assembly strategy to construct 3D boron nitride nanosheet networks in polymer composites for thermal conductivity improvement. Small 2015, 11, 6205–6213. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, J.; Ren, L.; Yao, Y.; Wang, M.; Zeng, X.; Sun, R.; Xua, J.; Wong, C. Nacre-inspired polymer composites with high thermal conductivity and enhanced mechanical strength. Compos. Part A 2019, 121, 92–99. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, R.; Li, Y.; Li, H.; Wu, Z.; Huang, J.; Yu, B.; Gao, X.; Li, J.; Li, L. Optimization of boron nitride sphere loading in epoxy: Enhanced thermal conductivity and excellent electrical insulation. Polymers 2019, 11, 1335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, W.; Li, Y.; Zhao, D.; Yin, H. Hybrid fillers of hexagonal and cubic boron nitride in epoxy composites for thermal management applications. RSC Adv. 2019, 9, 7388–7399. [Google Scholar] [CrossRef]
- Zhou, W.; Zuo, J.; Zhang, X.; Zhou, A. Thermal, electrical, and mechanical properties of hexagonal boron nitride-reinforced epoxy composites. J. Comp. Mater. 2014, 48, 2517–2526. [Google Scholar] [CrossRef]
- Zhu, B.L.; Ma, J.; Wu, J.; Yung, K.C.; Xie, C.S. Study on the properties of the epoxy-matrix composites filled with thermally conductive AlN and BN ceramic particles. J. Appl. Polym. Sci. 2010, 118, 2754–2764. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, P.; Lv, P.; Xu, T.; Zheng, J.; Ma, C.; Yu, K.; Feng, W.; Wei, W.; Chen, L. Densely packed polymer/boron nitride composite for superior anisotropic thermal conductivity. Polym. Comp. 2018, 39, E1653–E1658. [Google Scholar] [CrossRef]
- Zivkovic, I.; Murk, A. Boron nitride loading for thermal conductivity improvement of composite microwave absorbers. Electron. Lett. 2012, 48, 1130–1131. [Google Scholar] [CrossRef]
- Rimdusit, S.; Jubsilp, C.; Tiptipakorn, S. High thermal conductivity of BN-filled polybenzoxazines. In Alloys and Composites of Polybenzoxazines: Properties and Applications; Rimdusit, S., Jubsilp, C., Tiptipakorn, S., Eds.; Springer: Singapore, 2013; ISBN 978-981-4451-76-5. [Google Scholar]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hutchinson, J.M.; Moradi, S. Thermal Conductivity and Cure Kinetics of Epoxy-Boron Nitride Composites—A Review. Materials 2020, 13, 3634. https://doi.org/10.3390/ma13163634
Hutchinson JM, Moradi S. Thermal Conductivity and Cure Kinetics of Epoxy-Boron Nitride Composites—A Review. Materials. 2020; 13(16):3634. https://doi.org/10.3390/ma13163634
Chicago/Turabian StyleHutchinson, John M., and Sasan Moradi. 2020. "Thermal Conductivity and Cure Kinetics of Epoxy-Boron Nitride Composites—A Review" Materials 13, no. 16: 3634. https://doi.org/10.3390/ma13163634
APA StyleHutchinson, J. M., & Moradi, S. (2020). Thermal Conductivity and Cure Kinetics of Epoxy-Boron Nitride Composites—A Review. Materials, 13(16), 3634. https://doi.org/10.3390/ma13163634




