Recent Advances and Trends of Nanofilled/Nanostructured Epoxies
Abstract
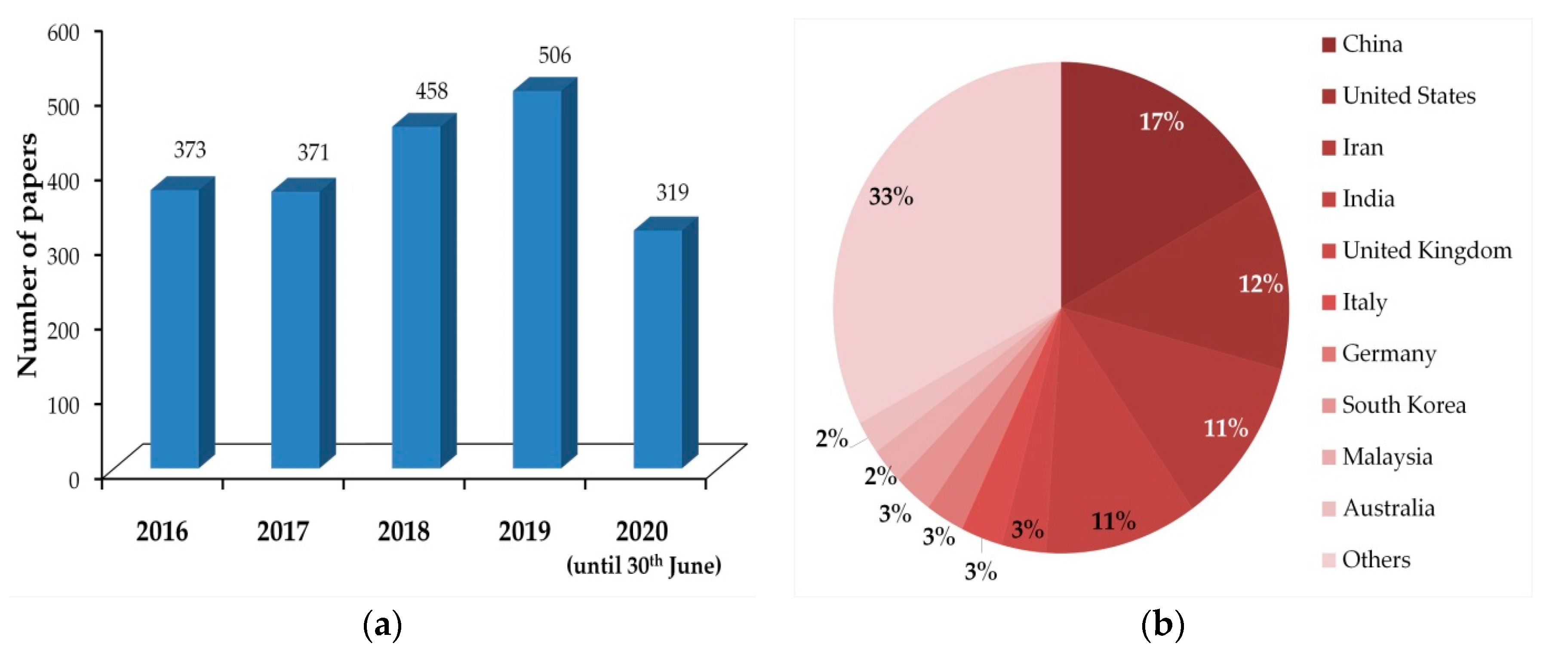
1. Introduction
2. Nanofillers for Epoxy Resins
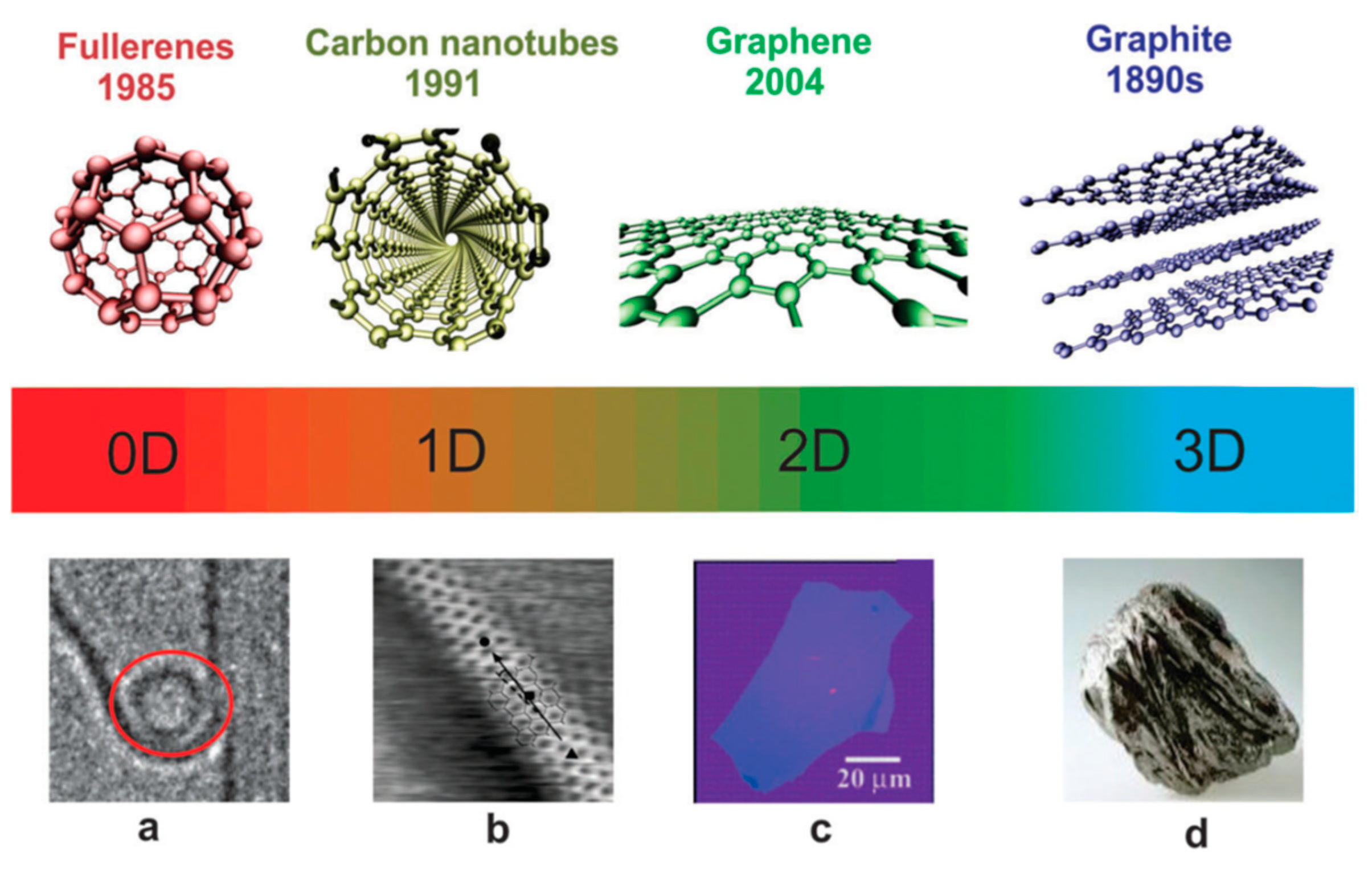
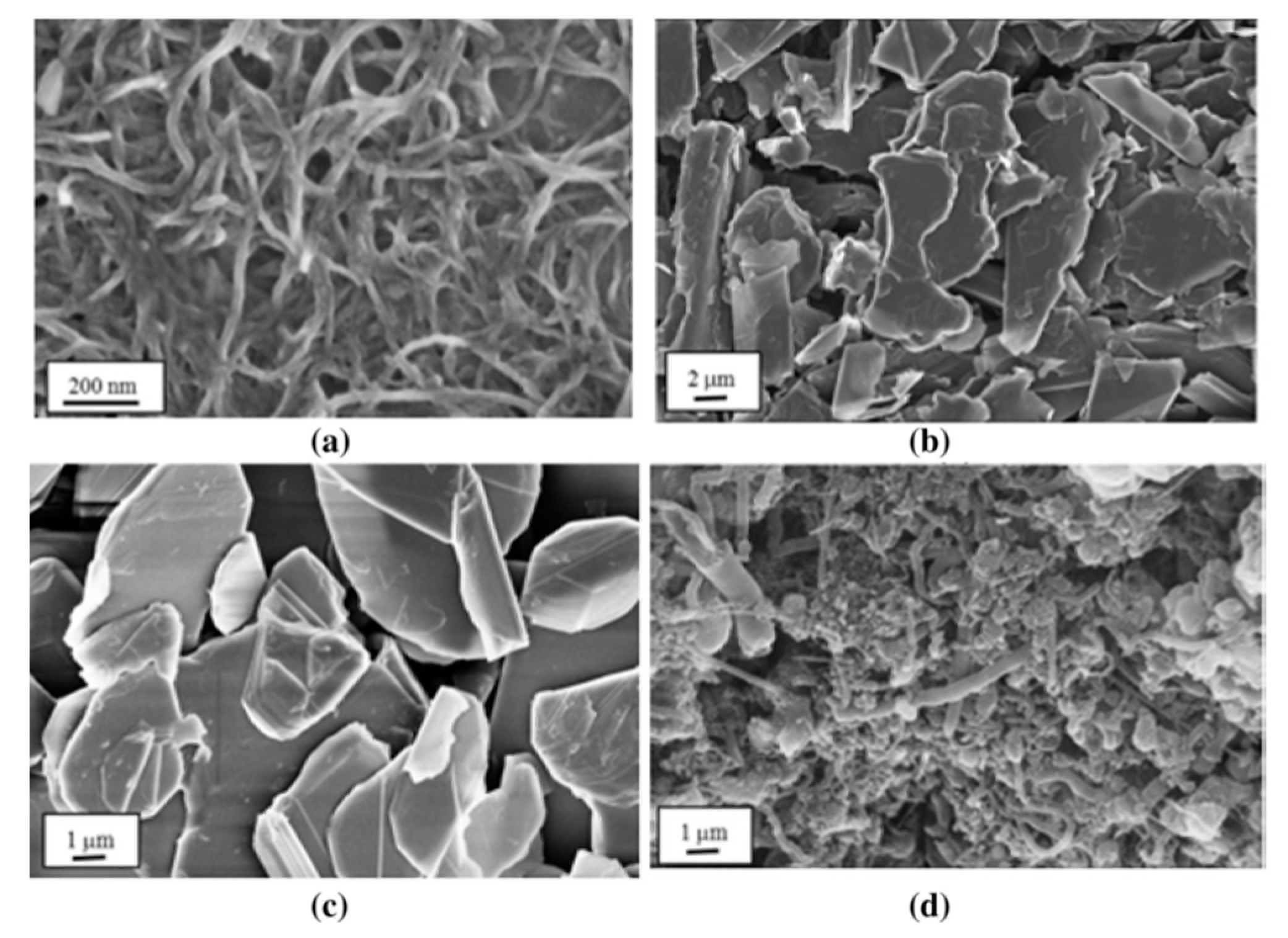
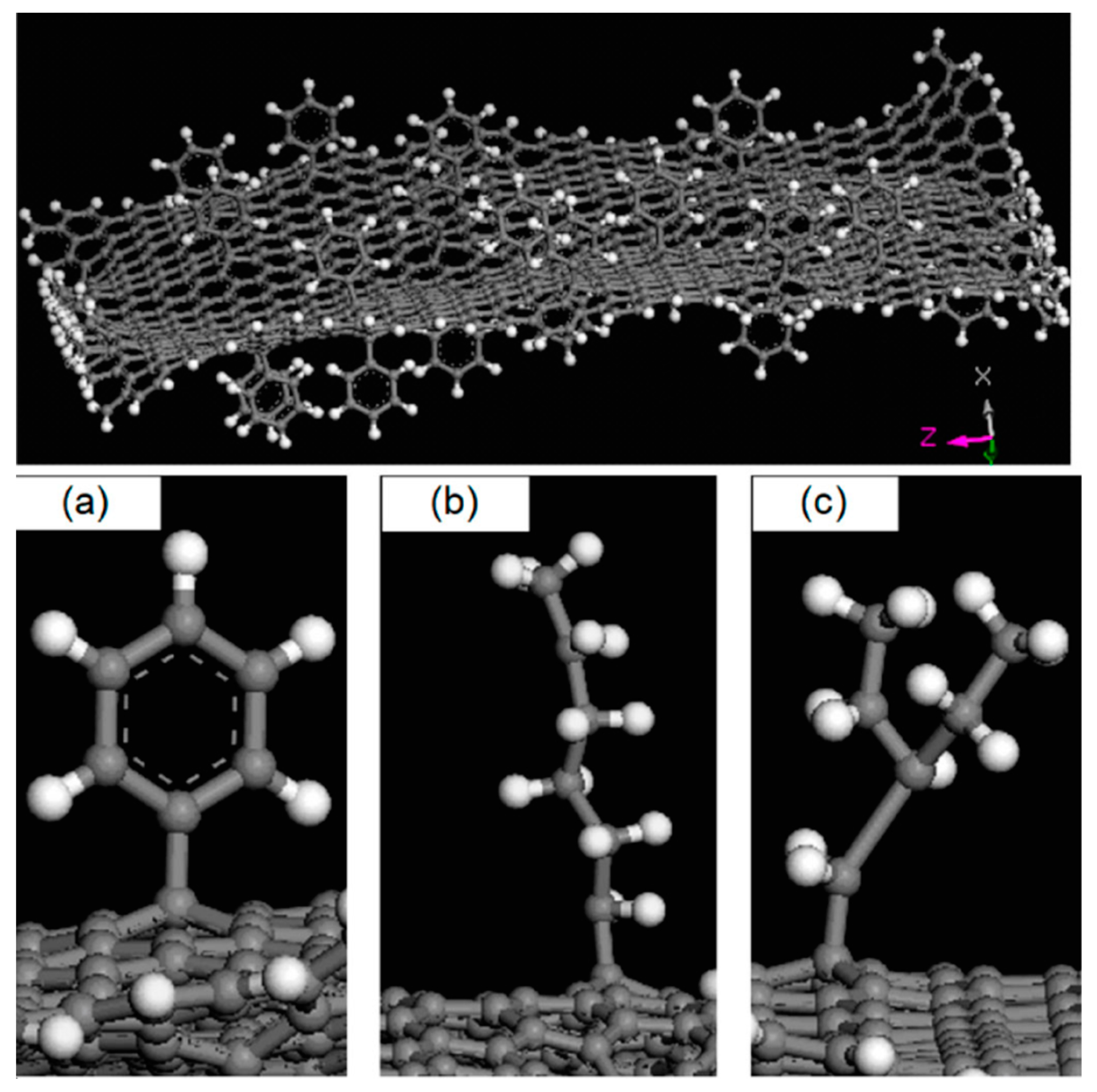
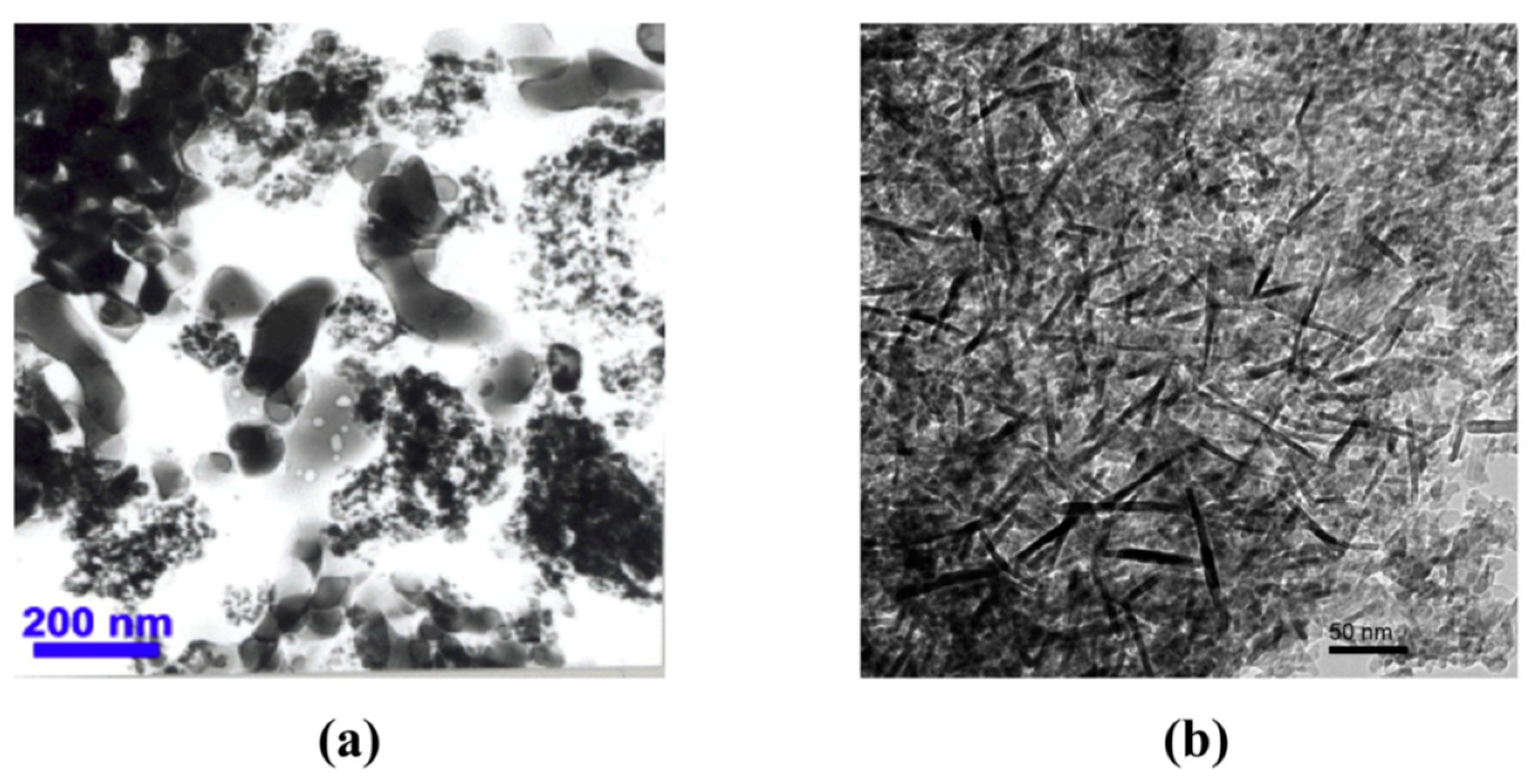
2.1. Carbon-Based Nanofillers
2.2. Metal Oxides and Other Nanooxides: Al2O3, TiO2, SiO2, ZnO, Fe3O4 Nanoparticles
2.3. Nanoclays
2.4. Phosphorus-Based Nanofillers
2.5. Other Nanoparticles
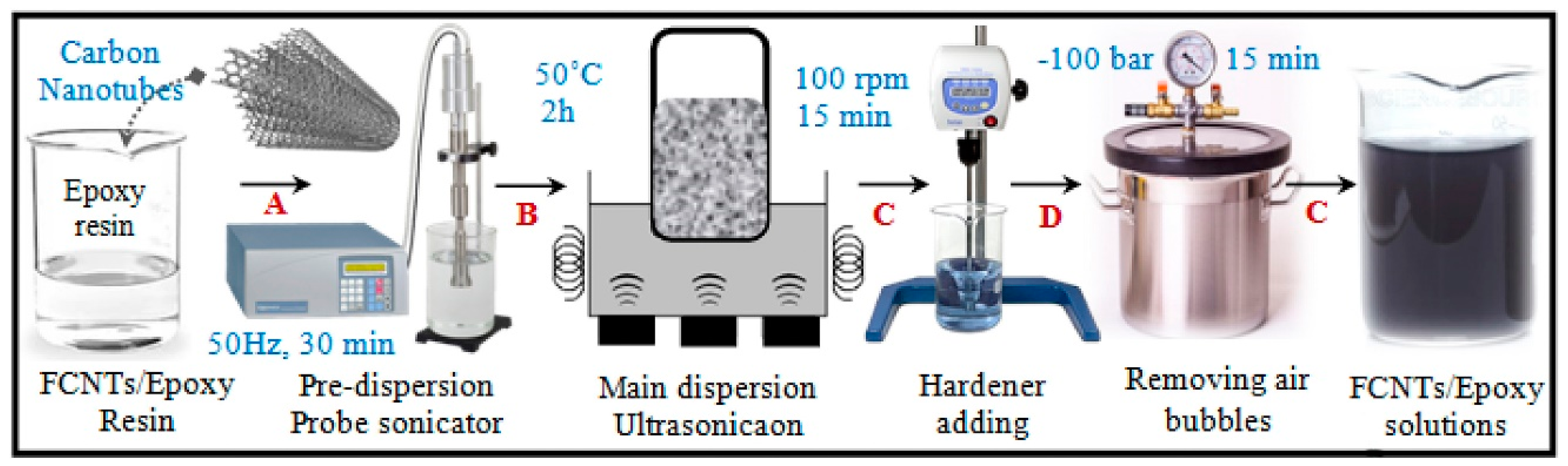
3. Production of Nanocomposites
4. Curing/Cross-Linking Process
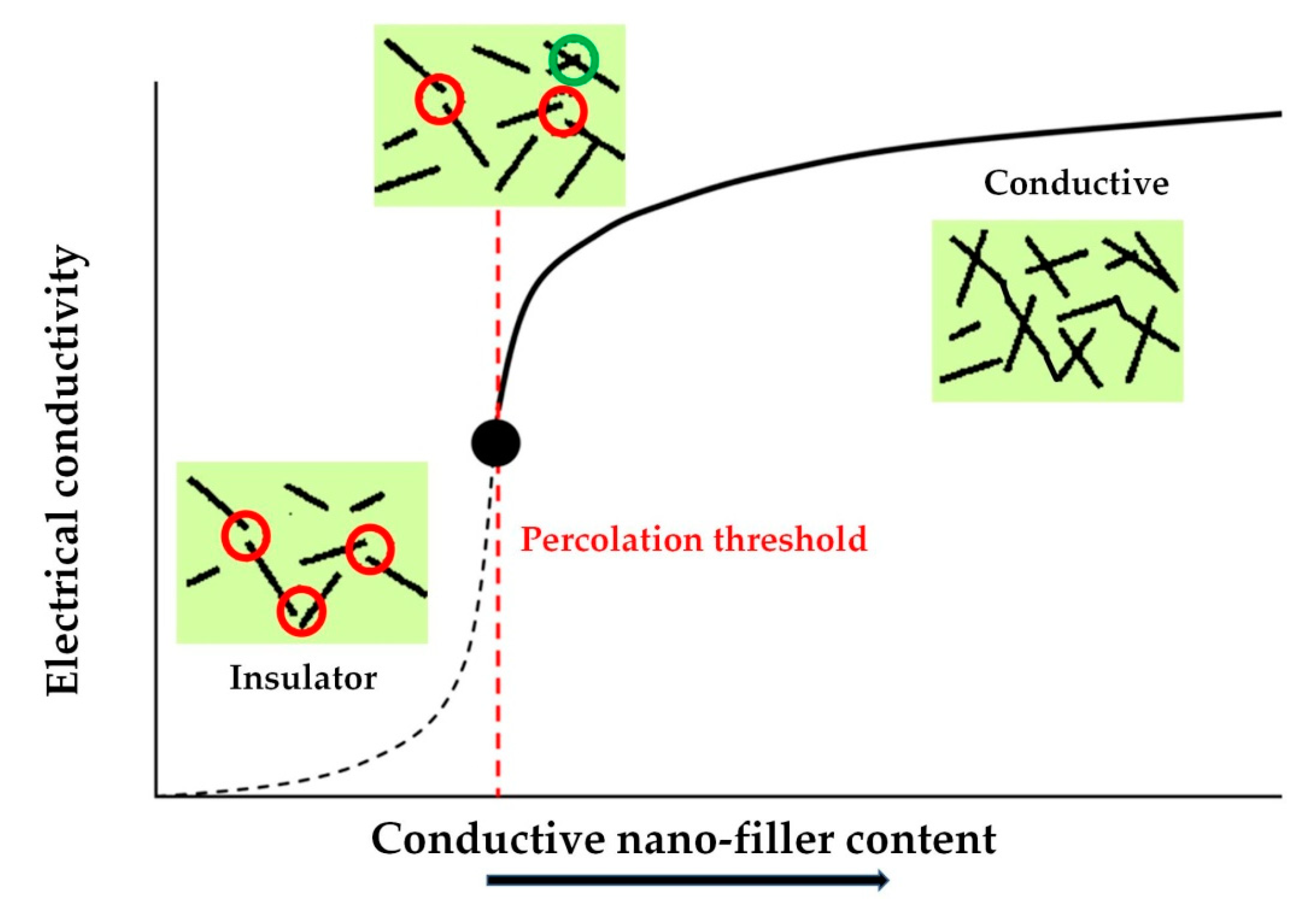
5. Properties of the Epoxy Nanocomposites
6. Applications of Nanofilled/Nanostructured Epoxy Resins
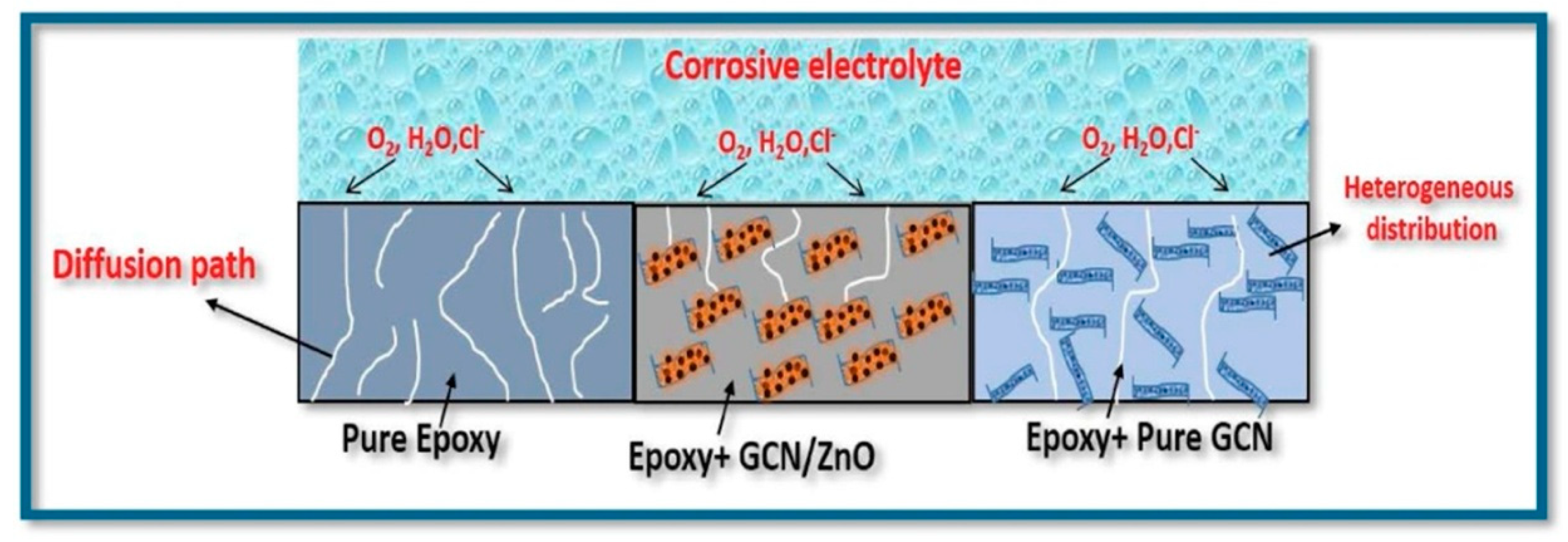
7. Limits in the Production of Epoxy-Based Nanocomposites
8. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- May, C.A. Epoxy resins. In Engineered Materials Handbook; The Materials Information Society, ASM International: Novelty, OH, USA, 1989; Volume 1, pp. 66–77. [Google Scholar]
- Ellis, B. Chemistry and Technology of Epoxy Resins; Springer: Dordrecht, The Netherlands, 1993; ISBN 978-94-011-2932-9. [Google Scholar]
- Mascia, L.; Tang, T. Curing and morphology of epoxy resin-silica hybrids. J. Mater. Chem. 1998, 8, 2417–2421. [Google Scholar] [CrossRef]
- Shehzad, K.; Xu, Y.; Gao, C.; Duan, X. Three-dimensional macro-structures of two-dimensional nanomaterials. Chem. Soc. Rev. 2016, 45, 5541–5588. [Google Scholar] [CrossRef] [PubMed]
- Yetgin, H.; Veziroglu, S.; Aktas, O.C.; Yalçinkaya, T. Enhancing thermal conductivity of epoxy with a binary filler system of h-BN platelets and Al2O3 nanoparticles. Int. J. Adhes. Adhes. 2020, 98, 102540. [Google Scholar] [CrossRef]
- Domun, N.; Paton, K.R.; Blackman, B.R.K.; Kaboglu, C.; Vahid, S.; Zhang, T.; Dear, J.P.; Kinloch, A.J.; Hadavinia, H. On the extent of fracture toughness transfer from 1D/2D nanomodified epoxy matrices to glass fibre composites. J. Mater. Sci. 2020, 55, 4717–4733. [Google Scholar] [CrossRef]
- Jojibabu, P.; Zhang, Y.X.; Prusty, B.G. A review of research advances in epoxy-based nanocomposites as adhesive materials. Int. J. Adhes. Adhes. 2020, 96, 102454. [Google Scholar] [CrossRef]
- Deyab, M.A. Anticorrosion properties of nanocomposites coatings: A critical review. J. Mol. Liq. 2020, 313, 113533. [Google Scholar] [CrossRef]
- Yadav, R.; Tirumali, M.; Wang, X.; Naebe, M.; Kandasubramanian, B. Polymer composite for antistatic application in aerospace. Def. Technol. 2020, 16, 107–118. [Google Scholar] [CrossRef]
- Bahramnia, H.; Mohammadian Semnani, H.; Habibolahzadeh, A.; Abdoos, H. Epoxy/polyurethane nanocomposite coatings for anti-erosion/wear applications: A review. J. Compos. Mater. 2020, 0021998320908299. [Google Scholar] [CrossRef]
- Hou, W.; Gao, Y.; Wang, J.; Blackwood, D.J.; Teo, S. Recent advances and future perspectives for graphene oxide reinforced epoxy resins. Mater. Today Commun. 2020, 23, 100883. [Google Scholar] [CrossRef]
- Gaddam, S.K.; Pothu, R.; Boddula, R. Graphitic carbon nitride (g-C3N4) reinforced polymer nanocomposite systems—A review. Polym. Compos. 2020, 41, 430–442. [Google Scholar] [CrossRef]
- Muralishwara, K.; Kini, U.A.; Sharma, S. Epoxy-clay nanocomposite coatings: A review on synthesis and characterization. Mater. Res. Express 2019, 6, 082007. [Google Scholar] [CrossRef]
- Taherian, R.; Kausar, A. (Eds.) Electrical Conductivity in Polymer-Based Composites; William Andrew Publishing: Norwich, UK, 2019; ISBN 978-0-12-812541-0. [Google Scholar]
- Stavropoulos, S.G.; Sanida, A.; Psarras, G.C. A comparative study on the electrical properties of different forms of carbon allotropes-epoxy nanocomposites. Express Polym. Lett. 2020, 14, 477–490. [Google Scholar] [CrossRef]
- Duan, W.; Chen, Y.; Ma, J.; Wang, W.; Cheng, J.; Zhang, J. High-performance graphene reinforced epoxy nanocomposites using benzyl glycidyl ether as a dispersant and surface modifier. Compos. Part B Eng. 2020, 189, 107878. [Google Scholar] [CrossRef]
- Rafiei Hashjin, R.; Ranjbar, Z.; Yari, H. Modeling of electrical conductive graphene filled epoxy coatings. Prog. Org. Coat. 2018, 125, 411–419. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, Z.; Feng, X.; Zou, Y. Graphene nanoplatelets/epoxy composites with excellent shear properties for construction adhesives. Compos. Part B Eng. 2018, 152, 311–315. [Google Scholar] [CrossRef]
- Jia, Z.; Feng, X.; Zou, Y. Graphene Reinforced Epoxy Adhesive for Fracture Resistance. Compos. Part B Eng. 2018, 155, 457–462. [Google Scholar] [CrossRef]
- Kim, J.; Cha, J.; Chung, B.; Ryu, S.; Hong, S.H. Fabrication and mechanical properties of carbon fiber/epoxy nanocomposites containing high loadings of noncovalently functionalized graphene nanoplatelets. Compos. Sci. Technol. 2020, 192, 108101. [Google Scholar] [CrossRef]
- Huang, H.; Tian, Y.; Xie, Y.; Mo, R.; Hu, J.; Li, M.; Sheng, X.; Jiang, X.; Zhang, X. Modification of graphene oxide with acrylate phosphorus monomer via thiol-Michael addition click reaction to enhance the anti-corrosive performance of waterborne epoxy coatings. Prog. Org. Coat. 2020, 146. [Google Scholar] [CrossRef]
- Yuan, H.; Qi, F.; Zhao, N.; Wan, P.; Zhang, B.; Xiong, H.; Liao, B.; Ouyang, X. Graphene Oxide Decorated with Titanium Nanoparticles to Reinforce the Anti-Corrosion Performance of Epoxy Coating. Coatings 2020, 10, 129. [Google Scholar] [CrossRef]
- Jojibabu, P.; Zhang, Y.X.; Rider, A.N.; Wang, J.; Wuhrer, R.; Prusty, B.G. High-performance epoxy-based adhesives modified with functionalized graphene nanoplatelets and triblock copolymers. Int. J. Adhes. Adhes. 2020, 98, 102521. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Brito, F.S.; Franceschi, W.; Simonetti, E.A.N.; Cividanes, L.S.; Chipara, M.; Lozano, K. Functionalized graphene oxide as reinforcement in epoxy based nanocomposites. Surf. Interfaces 2018, 10, 100–109. [Google Scholar] [CrossRef]
- Xia, Y.; He, Y.; Chen, C.; Wu, Y.; Zhong, F.; Chen, J. Co-modification of polydopamine and KH560 on g-C3N4 nanosheets for enhancing the corrosion protection property of waterborne epoxy coating. React. Funct. Polym. 2020, 146, 104405. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Bai, M.; Shen, Y. Improvement of curing reaction activity of one-component room temperature-curable epoxy adhesive by the addition of functionalized graphene oxide. Int. J. Adhes. Adhes. 2020, 98, 102537. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, K.; Dixit, A.R. A review on the mechanical and thermal properties of graphene and graphene-based polymer nanocomposites: Understanding of modelling and MD simulation. Mol. Simul. 2020, 46, 136–154. [Google Scholar] [CrossRef]
- Jian, W.; Lau, D. Understanding the effect of functionalization in CNT-epoxy nanocomposite from molecular level. Compos. Sci. Technol. 2020, 191, 108076. [Google Scholar] [CrossRef]
- Nobile, M.R.; Naddeo, C.; Raimondo, M.; Guadagno, L. Effect of functionalized carbon nanofillers on the rheological behavior of structural epoxy resins. AIP Conf. Proc. 2019, 2196, 020027. [Google Scholar] [CrossRef]
- Chen, S.; Chen, L.; Wang, Y.; Wang, C.; Miao, M.; Zhang, D. Preparation of nanocomposites with epoxy resins and thiol-functionalized carbon nanotubes by thiol-ene click reaction. Polym. Test. 2019, 77, 105912. [Google Scholar] [CrossRef]
- Chen, S.; Chen, L.; Wang, Y.; Wang, C.; Miao, M.; Zhang, D. Load transfer of thiol-ended hyperbranched polymers to improve simultaneously strength and longation of CNTs/epoxy nanocomposites. Eur. Polym. J. 2019, 120, 109254. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, C.; Lu, R.; Su, S.; Qin, J.; Wang, Y.; Ma, Z.; Wei, H.; Wang, Q. Fracture investigation of functionalized carbon nanotubes-grown carbon fiber fabrics/epoxy composites. Compos. Sci. Technol. 2020, 195, 108161. [Google Scholar] [CrossRef]
- Subadra, S.P.; Yousef, S.; Griskevicius, P.; Makarevicius, V. High-performance fiberglass/epoxy reinforced by functionalized CNTs for vehicle applications with less fuel consumption and greenhouse gas emissions. Polym. Test. 2020, 86. [Google Scholar] [CrossRef]
- Morshed, S.A.; Young, T.J.; Chirdon, W.M.; Zhang, Q.; Tatar, J. Durability of wet lay-up FRP bonded to concrete with nanomodified epoxy adhesives. J. Adhes. 2018, 1–26. [Google Scholar] [CrossRef]
- Li, S.; Yao, Y. Synergistic improvement of epoxy composites with multi-walled carbon nanotubes and hyperbranched polymers. Compos. Part B Eng. 2019, 165, 293–300. [Google Scholar] [CrossRef]
- Glaskova-Kuzmina, T.; Aniskevich, A.; Papanicolaou, G.; Portan, D.; Zotti, A.; Borriello, A.; Zarrelli, M. Hydrothermal Aging of an Epoxy Resin Filled with Carbon Nanofillers. Polymers 2020, 12, 1153. [Google Scholar] [CrossRef] [PubMed]
- Mourad, A.-H.I.; Idrisi, A.H.; Zaaroura, N.; Sherif, M.M.; Fouad, H. Damage assessment of nanofiller-reinforced woven kevlar KM2plus/Epoxy resin laminated composites. Polym. Test. 2020, 86. [Google Scholar] [CrossRef]
- Razavi, S.M.J.; Ayatollahi, M.R.; Nemati Giv, A.; Khoramishad, H. Single lap joints bonded with structural adhesives reinforced with a mixture of silica nanoparticles and multi walled carbon nanotubes. Int. J. Adhes. Adhes. 2018, 80, 76–86. [Google Scholar] [CrossRef]
- Zeng, S.; Shen, M.; Xue, Y.; Zheng, Y.; Zhang, K.; Han, Y.; Yang, L. Controllable mechanical properties of epoxy composites by incorporating self-assembled carbon nanotube-montmorillonite. Compos. Part B Eng. 2019, 164, 368–376. [Google Scholar] [CrossRef]
- Meisak, D.; Macutkevic, J.; Plyushch, A.; Kuzhir, P.; Selskis, A.; Banys, J. Dielectric Relaxation in the Hybrid Epoxy/MWCNT/MnFe2O4 Composites. Polymers 2020, 12, 697. [Google Scholar] [CrossRef]
- Xu, Z.; Deng, N.; Yan, L.; Chu, Z. Functionalized multiwalled carbon nanotubes with monocomponent intumescent flame retardant for reducing the flammability and smoke emission characteristics of epoxy resins. Polym. Adv. Technol. 2018, 29, 3002–3013. [Google Scholar] [CrossRef]
- Kausar, A. Nanocarbon and macrocarbonaceous filler–reinforced epoxy/polyamide: A review. J. Thermoplast. Compos. Mater. 2020. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Shi, W.; Castillo-Rodríguez, M.; Su, D.S.; Wang, D.-Y. Coordinating mechanical performance and fire safety of epoxy resin via functionalized nanodiamond. Diam. Relat. Mater. 2020, 108, 107964. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, G.; Liu, L.; Zhu, B.; Su, D. Promising commercial reinforcement to the nanodiamond/epoxy composite by grafting ammonium ions. J. Mater. Sci. Technol. 2018, 34, 990–994. [Google Scholar] [CrossRef]
- Kim, S.-H.; Rhee, K.Y.; Park, S.-J. Amine-terminated chain-grafted nanodiamond/epoxy nanocomposites as interfacial materials: Thermal conductivity and fracture resistance. Compos. Part B Eng. 2020, 192, 107983. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Zhou, A.; Shen, C.; Dai, Y.; Liu, F.; Chen, J.; Li, P.; Hu, Q. Effects of 2-D transition metal carbide Ti2CTx on properties of epoxy composites. RSC Adv. 2016, 6, 87341–87352. [Google Scholar] [CrossRef]
- Zou, Y.; Fang, L.; Chen, T.; Sun, M.; Lu, C.; Xu, Z. Near-Infrared Light and Solar Light Activated Self-Healing Epoxy Coating having Enhanced Properties Using MXene Flakes as Multifunctional Fillers. Polymers 2018, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, W. High-Thermal-Stability and High-Thermal-Conductivity Ti3C2Tx MXene/Poly(vinyl alcohol) (PVA) Composites. ACS Omega 2018, 3, 2609–2617. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, H.-B.; Luo, J.-Q.; Wang, Q.-W.; Xu, B.; Hong, S.; Yu, Z.-Z. Highly Electrically Conductive Three-Dimensional Ti3C2Tx MXene/Reduced Graphene Oxide Hybrid Aerogels with Excellent Electromagnetic Interference Shielding Performances. ACS Nano 2018, 12, 11193–11202. [Google Scholar] [CrossRef]
- Sliozberg, Y.; Andzelm, J.; Hatter, C.B.; Anasori, B.; Gogotsi, Y.; Hall, A. Interface binding and mechanical properties of MXene-epoxy nanocomposites. Compos. Sci. Technol. 2020, 192. [Google Scholar] [CrossRef]
- Gupta, S.K.; Shukla, D.K. Effect of stress rate on shear strength of aluminium alloy single lap joints bonded with epoxy/nanoalumina adhesives. Int. J. Adhes. Adhes. 2020, 99, 102587. [Google Scholar] [CrossRef]
- Koo, J.-H.; Hwang, J.-S.; Shin, W.-J.; Sakamoto, K.; Oh, D.-H.; Lee, B.-W. Comparison of DC and AC surface breakdown characteristics of GFRP and epoxy nanocomposites in liquid nitrogen. IEEE Trans. Appl. Supercond. 2016, 26. [Google Scholar] [CrossRef]
- Hu, Y.; Du, G.; Chen, N. A novel approach for Al2O3/epoxy composites with high strength and thermal conductivity. Compos. Sci. Technol. 2016, 124, 36–43. [Google Scholar] [CrossRef]
- Mahato, K.K.; Dutta, K.; Chandra Ray, B. Assessment of mechanical, thermal and morphological behavior of nano-Al2O3 embedded glass fiber/epoxy composites at in-situ elevated temperatures. Compos. Part B Eng. 2019, 166, 688–700. [Google Scholar] [CrossRef]
- Pinto, D.; Amaro, A.M.; Bernardo, L. Experimental Study on the Surface Properties of Nanoalumina-Filled Epoxy Resin Nanocomposites. Appl. Sci. 2020, 10, 733. [Google Scholar] [CrossRef]
- Saraç, İ.; Adin, H.; Temiz, Ş. Experimental determination of the static and fatigue strength of the adhesive joints bonded by epoxy adhesive including different particles. Compos. Part B Eng. 2018, 155, 92–103. [Google Scholar] [CrossRef]
- Saraç, İ.; Adin, H.; Temiz, Ş. Investigation of the effect of use of Nano-Al2O3, Nano-TiO2 and Nano-SiO2 powders on strength of single lap joints bonded with epoxy adhesive. Compos. Part B Eng. 2019, 166, 472–482. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Tedim, J.; Ferreira, M.G.S. “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim. Acta 2012, 82, 314–323. [Google Scholar] [CrossRef]
- Nawaz, M.; Yusuf, N.; Habib, S.; Shakoor, R.A.; Ubaid, F.; Ahmad, Z.; Kahraman, R.; Mansour, S.; Gao, W. Development and Properties of Polymeric Nanocomposite Coatings. Polymers 2019, 11, 852. [Google Scholar] [CrossRef]
- Gómez-Laserna, O.; Lando, G.; Kortazar, L.; Martinez-Arkarazo, I.; Monterrubio, I.; Sevillano, E.; Cardiano, P.; Olazabal, M.Á. Eco-friendly nanocomposite products based on BPA-free epoxy–silica hybrid materials for stone conservation. Archaeol. Anthropol. Sci. 2019, 11, 5799–5812. [Google Scholar] [CrossRef]
- Xu, F.; Li, D. Modification of HBA/D230 Polymer for Stone Protection. J. Polym. Environ. 2017, 25, 1304–1312. [Google Scholar] [CrossRef]
- Nayak, R.K.; Mahato, K.K.; Ray, B.C. Water absorption behavior, mechanical and thermal properties of nano TiO2 enhanced glass fiber reinforced polymer composites. Compos. Part A Appl. Sci. Manuf. 2016, 90, 736–747. [Google Scholar] [CrossRef]
- Alam, M.A.; Abdus Samad, U.; Alam, M.; Anis, A.; Al-Zahrani, S.M. Enhancement in Nanomechanical, Thermal, and Abrasion Properties of SiO2 Nanoparticle-Modified Epoxy Coatings. Coatings 2020, 10, 310. [Google Scholar] [CrossRef]
- Alam, M.A.; Samad, U.A.; Sherif, E.-S.M.; Poulose, A.M.; Mohammed, J.A.; Alharthi, N.; Al-Zahrani, S.M. Influence of SiO2 Content and Exposure Periods on the Anticorrosion Behavior of Epoxy Nanocomposite Coatings. Coatings 2020, 10, 118. [Google Scholar] [CrossRef]
- Alsaadi, M.; Bulut, M.; Erkliğ, A.; Jabbar, A. Nano-silica inclusion effects on mechanical and dynamic behavior of fiber reinforced carbon/Kevlar with epoxy resin hybrid composites. Compos. Part B Eng. 2018, 152, 169–179. [Google Scholar] [CrossRef]
- Li, H.; Liu, F.; Tian, H.; Wang, C.; Guo, Z.; Liu, P.; Peng, Z.; Wang, Q. Synergetic enhancement of mechanical and electrical strength in epoxy/silica nanocomposites via chemically-bonded interface. Compos. Sci. Technol. 2018, 167, 539–546. [Google Scholar] [CrossRef]
- Lionetto, F.; Timo, A.; Frigione, M. Cold-Cured Epoxy-Based Organic–Inorganic Hybrid Resins Containing Deep Eutectic Solvents. Polymers 2019, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Frigione, M.; Lettieri, M.; Lionetto, F.; Mascia, L. Experimental Cold-Cured Nanostructured Epoxy-Based Hybrid Formulations: Properties and Durability Performance. Polymers 2020, 12, 476. [Google Scholar] [CrossRef] [PubMed]
- Thipperudrappa, S.; Kini, A.U.; Hiremath, A.; Kumar, K.D. Surface topographical studies of glass fiber reinforced epoxy-ZnO nanocomposites. Mater. Res. Express 2020, 7. [Google Scholar] [CrossRef]
- Kumar, A.M.; Khan, A.; Khan, M.Y.; Suleiman, R.K.; Jose, J.; Dafalla, H. Hierarchical graphitic carbon nitride-ZnO nanocomposite: Viable reinforcement for the improved corrosion resistant behavior of organic coatings. Mater. Chem. Phys. 2020, 251. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, X.; He, X.; Su, Y.; Wang, J.; Yang, J.; Chen, S.; Zheng, Z. Achieving full effective microwave absorption in X band by double-layered design of glass fiber epoxy composites containing MWCNTs and Fe3O4 NPs. Polym. Test. 2020, 86, 106448. [Google Scholar] [CrossRef]
- Sanida, A.; Stavropoulos, S.G.; Speliotis, T.; Psarras, G.C. Probing the magnetoelectric response and energy efficiency in Fe3O4/epoxy nanocomposites. Polym. Test. 2020, 88, 106560. [Google Scholar] [CrossRef]
- Xiong, H.; Qi, F.; Zhao, N.; Yuan, H.; Wan, P.; Liao, B.; Ouyang, X. Effect of organically modified sepiolite as inorganic nanofiller on the anti-corrosion resistance of epoxy coating. Mater. Lett. 2020, 260. [Google Scholar] [CrossRef]
- Bozkurt, Ö.Y.; Bulut, M.; Erkliğ, A.; Faydh, W.A. Axial and lateral buckling analysis of fiber reinforced S-glass/epoxy composites containing nano-clay particles. Compos. Part B Eng. 2019, 158, 82–91. [Google Scholar] [CrossRef]
- Rajaei, M.; Kim, N.K.; Bickerton, S.; Bhattacharyya, D. A comparative study on effects of natural and synthesised nano-clays on the fire and mechanical properties of epoxy composites. Compos. Part B Eng. 2019, 165, 65–74. [Google Scholar] [CrossRef]
- Akbari, V.; Najafi, F.; Vahabi, H.; Jouyandeh, M.; Badawi, M.; Morisset, S.; Ganjali, M.R.; Saeb, M.R. Surface chemistry of halloysite nanotubes controls the curability of low filled epoxy nanocomposites. Prog. Org. Coat. 2019, 135, 555–564. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, D.; Li, Z.; Li, Z.; Peng, X.; Liu, C.; Zhang, Y.; Zheng, P. Recent Developments in the Flame-Retardant System of Epoxy Resin. Materials 2020, 13, 2145. [Google Scholar] [CrossRef] [PubMed]
- Tikhani, F.; Moghari, S.; Jouyandeh, M.; Laoutid, F.; Vahabi, H.; Saeb, M.R.; Dubois, P. Curing Kinetics and Thermal Stability of Epoxy Composites Containing Newly Obtained Nano-Scale Aluminum Hypophosphite (AlPO2). Polymers 2020, 12, 644. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Wu, K.; Jiao, E.; Chen, W.; Hu, Z.; Xu, C.; Shi, J.; Wang, S.; Tan, Z. Surface functionalization of few-layer black phosphorene and its flame retardancy in epoxy resin. Chem. Eng. J. 2020, 382. [Google Scholar] [CrossRef]
- Qiu, S.; Zhou, Y.; Zhou, X.; Zhang, T.; Wang, C.; Yuen, R.K.; Hu, W.; Hu, Y. Air-stable Polyphosphazene-functionalized few-layer black phosphorene for flame Retardancy of epoxy resins. Small 2019, 15, 1805175. [Google Scholar] [CrossRef]
- Gu, L.; Qiu, C.; Qiu, J.; Yao, Y.; Sakai, E.; Yang, L. Preparation and Characterization of DOPO-Functionalized MWCNT and Its High Flame-Retardant Performance in Epoxy Nanocomposites. Polymers 2020, 12, 613. [Google Scholar] [CrossRef]
- Jux, M.; Finke, B.; Mahrholz, T.; Sinapius, M.; Kwade, A.; Schilde, C. Effects of Al(OH)O nanoparticle agglomerate size in epoxy resin on tension, bending, and fracture properties. J. Nanoparticle Res. 2017, 19, 139. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Frigione, M. Cure kinetics and physical characterization of epoxy/modified boehmite nanocomposites. J. Adhes. Sci. Technol. 2017, 31, 645–662. [Google Scholar] [CrossRef]
- Jux, M.; Fankhänel, J.; Daum, B.; Mahrholz, T.; Sinapius, M.; Rolfes, R. Mechanical properties of epoxy/boehmite nanocomposites in dependency of mass fraction and surface modification—An experimental and numerical approach. Polymer 2018, 141, 34–45. [Google Scholar] [CrossRef]
- Ghasem Zadeh Khorasani, M.; Silbernagl, D.; Szymoniak, P.; Hodoroaba, V.-D.; Sturm, H. The effect of boehmite nanoparticles (γ-AlOOH) on nanomechanical and thermomechanical properties correlated to crosslinking density of epoxy. Polymer 2019, 164, 174–182. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Negi, A.; He, J.; Hu, G.; Tian, S.; Liu, J. Synergistic Effects of Boron Nitride (BN) Nanosheets and Silver (Ag) Nanoparticles on Thermal Conductivity and Electrical Properties of Epoxy Nanocomposites. Polymers 2020, 12, 426. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.W.; Kim, J.S.; Memon, M.A.; Baloch, M.M. Hybridization of hexagonal boron nitride nanosheets and multilayer graphene: Enhanced thermal properties of epoxy composites. Compos. Sci. Technol. 2020, 195, 108183. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Chen, C.; Ye, Y.; Zeng, H.; Qu, H.; Liu, J.; Zhou, X.; Long, S.; Xie, X. Highly thermally conductive flame retardant epoxy nanocomposites with multifunctional ionic liquid flame retardant-functionalized boron nitride nanosheets. J. Mater. Chem. A 2018, 6, 20500–20512. [Google Scholar] [CrossRef]
- Dai, C.; Chen, X.; Jiang, T.; Paramane, A.; Tanaka, Y. Improvement of electrical and material properties of epoxy resin/ aluminum nitride nanocomposites for packaging materials. Polym. Test. 2020, 86, 106502. [Google Scholar] [CrossRef]
- Toroń, B.; Szperlich, P.; Kozioł, M. SbSI Composites Based on Epoxy Resin and Cellulose for Energy Harvesting and Sensors—The Influence of SBSI Nanowires Conglomeration on Piezoelectric Properties. Materials 2020, 13, 902. [Google Scholar] [CrossRef]
- Frigione, M.; Lettieri, M. Novel Attribute of Organic–Inorganic Hybrid Coatings for Protection and Preservation of Materials (Stone and Wood) Belonging to Cultural Heritage. Coatings 2018, 8, 319. [Google Scholar] [CrossRef]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.; Bagherzadeh, M.R. Distinctive roles of silane coupling agents on the corrosion inhibition performance of graphene oxide in epoxy coatings. Prog. Org. Coat. 2017, 111, 47–56. [Google Scholar] [CrossRef]
- Abdel Rehim, M.; Turky, G. Silane-functionalized graphene oxide/epoxy resin nanocomposites: Dielectric and thermal studies. J. Appl. Polym. Sci. 2019, 136. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Guo, Z.; Wang, H.; Zhang, Y.; Hong, R.; Peng, Z. Effects of silane coupling agents on the electrical properties of silica/epoxy nanocomposites. In Proceedings of the 2016 IEEE International Conference on Dielectrics (ICD), Montpellier, France, 3–7 July 2016; Volume 2, pp. 1036–1039. [Google Scholar]
- Saeb, M.R.; Najafi, F.; Bakhshandeh, E.; Khonakdar, H.A.; Mostafaiyan, M.; Simon, F.; Scheffler, C.; Mäder, E. Highly curable epoxy/MWCNTs nanocomposites: An effective approach to functionalization of carbon nanotubes. Chem. Eng. J. 2015, 259, 117–125. [Google Scholar] [CrossRef]
- Kwon, D.-J.; Wang, Z.-J.; Choi, J.-Y.; Shin, P.-S.; DeVries, K.L.; Park, J.-M. Interfacial and mechanical properties of epoxy composites containing carbon nanotubes grafted with alkyl chains of different length. Compos. Part A Appl. Sci. Manuf. 2016, 82, 190–197. [Google Scholar] [CrossRef]
- Cha, J.; Jin, S.; Shim, J.H.; Park, C.S.; Ryu, H.J.; Hong, S.H. Functionalization of carbon nanotubes for fabrication of CNT/epoxy nanocomposites. Mater. Des. 2016, 95, 1–8. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Luo, S.; Yu, S.; Huang, H.; Sun, R.; Wong, C.-P. ZnO-Decorated Carbon Nanotube Hybrids as Fillers Leading to Reversible Nonlinear I–V Behavior of Polymer Composites for Device Protection. ACS Appl. Mater. Interfaces 2016, 8, 35545–35551. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Qu, Z.; Wang, Q.; Sun, X.; Cheng, E. Reversible Nonlinear I-V Behavior of ZnO-Decorated Graphene Nanoplatelets/Epoxy Resin Composites. Polymers 2020, 12, 951. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Kuan, H.-C.; Michelmore, A.; Meng, Q.; Qiu, A.; Aakyiir, M.; Losic, D.; Zhu, S.; Ma, J. A new method for preparation of functionalized graphene and its epoxy nanocomposites. Compos. Part B Eng. 2020, 196, 108096. [Google Scholar] [CrossRef]
- Huang, X.; Li, R.; Zeng, L.; Li, X.; Xi, Z.; Wang, K.; Li, Y. A multifunctional carbon nanotube reinforced nanocomposite modified via soy protein isolate: A study on dispersion, electrical and mechanical properties. Carbon 2020, 161, 350–358. [Google Scholar] [CrossRef]
- Zeng, L.; Huang, X.; Li, X.; Li, R.; Li, Y.; Xiong, Y. A gelatin-treated carbon nanofiber/epoxy nanocomposite with significantly improved multifunctional properties. Mater. Today Commun. 2020, 24. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, J.; Chen, S.; Wu, L.; Zhuo, D.; Cheng, X. Green fabrication of graphene oxide/epoxy nanocomposite and its application in diamond abrasive tools. Compos. Part B Eng. 2019, 177, 107383. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, H.; Zhu, R.; Chen, R.; Sheng, X.; Xie, D.; Mei, Y. Green modification of graphene oxide with phytic acid and its application in anticorrosive water-borne epoxy coatings. Prog. Org. Coat. 2020, 143, 105601. [Google Scholar] [CrossRef]
- Mohammadkhani, R.; Ramezanzadeh, M.; Akbarzadeh, S.; Bahlakeh, G.; Ramezanzadeh, B. Graphene oxide nanoplatforms reduction by green plant-sourced organic compounds for construction of an active anti-corrosion coating; experimental/electronic-scale DFT-D modeling studies. Chem. Eng. J. 2020, 397, 125433. [Google Scholar] [CrossRef]
- Domun, N.; Paton, K.R.; Hadavinia, H.; Sainsbury, T.; Zhang, T.; Mohamud, H. Enhancement of fracture toughness of epoxy nanocomposites by combining nanotubes and nanosheets as fillers. Materials 2017, 10, 1179. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, J.; Ohki, Y.; Wang, D.; Liu, G.; Liu, Y.; Tao, K. Dielectric properties of nanocomposites based on epoxy resin and HBP/plasma modified nanosilica. AIP Adv. 2020, 10. [Google Scholar] [CrossRef]
- Wang, X.; Tang, F.; Cao, Q.; Qi, X.; Pearson, M.; Li, M.; Pan, H.; Zhang, Z.; Lin, Z. Comparative study of three carbon additives: Carbon nanotubes, graphene, and fullerene-c60, for synthesizing enhanced polymer nanocomposites. Nanomaterials 2020, 10, 838. [Google Scholar] [CrossRef]
- Glaskova-Kuzmina, T.; Aniskevich, A.; Sevcenko, J.; Zotti, A.; Borriello, A.; Zarrelli, M. Environmental effects on mechanical, thermophysical and electrical properties of epoxy resin filled with carbon nanofillers. AIP Conf. Proc. 2019, 2196, 020004. [Google Scholar] [CrossRef]
- Campo, M.; Redondo, O.; Prolongo, S.G. Barrier properties of thermal and electrical conductive hydrophobic multigraphitic/epoxy coatings. J. Appl. Polym. Sci. 2020. [Google Scholar] [CrossRef]
- Jayan, J.S.; Saritha, A.; Deeraj, B.D.S.; Joseph, K. Triblock copolymer grafted Graphene oxide as nanofiller for toughening of epoxy resin. Mater. Chem. Phys. 2020, 248. [Google Scholar] [CrossRef]
- Narita, F.; Wang, Y.; Kurita, H.; Suzuki, M. Multi-Scale Analysis and Testing of Tensile Behavior in Polymers with Randomly Oriented and Agglomerated Cellulose Nanofibers. Nanomaterials 2020, 10, 700. [Google Scholar] [CrossRef]
- Yan, M.; Jiao, W.; Ding, G.; Chu, Z.; Huang, Y.; Wang, R. High strength and toughness epoxy nanocomposites reinforced with graphene oxide-nanocellulose micro/nanoscale structures. Appl. Surf. Sci. 2019, 497, 143802. [Google Scholar] [CrossRef]
- De Oliveira, J.B.; Guerrini, L.M.; Conejo, L.S.; Rezende, M.C.; Botelho, E.C. Viscoelastic evaluation of epoxy nanocomposite based on carbon nanofiber obtained from electrospinning processing. Polym. Bull. 2019, 76, 6063–6076. [Google Scholar] [CrossRef]
- Glaskova-Kuzmina, T.; Aniskevich, A.; Zotti, A.; Borriello, A.; Zarrelli, M. Flexural properties of the epoxy resin filled with single and hybrid carbon nanofillers. J. Phys. Conf. Ser. 2020. [Google Scholar] [CrossRef]
- Saadatmandi, S.; Ramezanzadeh, B.; Asghari, M.; Bahlakeh, G. Graphene oxide nanoplatform surface decoration by spherical zinc-polypyrrole nanoparticles for epoxy coating properties enhancement: Detailed explorations from integrated experimental and electronic-scale quantum mechanics approaches. J. Alloys Compd. 2020, 816. [Google Scholar] [CrossRef]
- Ribeiro, H.; Trigueiro, J.P.C.; Silva, W.M.; Woellner, C.F.; Owuor, P.S.; Cristian Chipara, A.; Lopes, M.C.; Tiwary, C.S.; Pedrotti, J.J.; Villegas Salvatierra, R.; et al. Hybrid MoS2/h-BN Nanofillers As Synergic Heat Dissipation and Reinforcement Additives in Epoxy Nanocomposites. ACS Appl. Mater. Interfaces 2019, 11, 24485–24492. [Google Scholar] [CrossRef] [PubMed]
- Bagherzadeh, A.; Jamshidi, M.; Monemian, F. Investigating mechanical and bonding properties of micro/nano filler containing epoxy adhesives for anchoring steel bar in concrete. Constr. Build. Mater. 2020, 240, 117979. [Google Scholar] [CrossRef]
- Mucha, M.; Krzyzak, A.; Kosicka, E.; Coy, E.; Kościński, M.; Sterzyński, T.; Sałaciński, M. Effect of MWCNTs on Wear Behavior of Epoxy Resin for Aircraft Applications. Materials 2020, 13, 2696. [Google Scholar] [CrossRef]
- Kausar, A. Performance of corrosion protective epoxy blend-based nanocomposite coatings: A review. Polym.-Plast. Technol. Mat. 2020, 59, 658–673. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, P.; Yang, Q.; He, Y.; Bai, Y. Molybdenum disulfide with poly(dopamine) and epoxy groups as an efficiently anticorrosive reinforcers in epoxy coating. Synth. Met. 2020, 259. [Google Scholar] [CrossRef]
- Di, H.; Yu, Z.; Ma, Y.; Zhang, C.; Li, F.; Lv, L.; Pan, Y.; Shi, H.; He, Y. Corrosion-resistant hybrid coatings based on graphene oxide–zirconia dioxide/epoxy system. J. Taiwan Inst. Chem. Eng. 2016, 67, 511–520. [Google Scholar] [CrossRef]
- Kabeb, S.M.; Hassan, A.; Mohamad, Z.; Sharer, Z.; Mokhtar, M.; Ahmad, F. Synergistic effect of graphene oxide/halloysite in anticorrosion performance and flame retardancy properties of epoxy nanocomposite coating. Chem. Eng. Trans. 2020, 78, 529–534. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Niroumandrad, S.; Ahmadi, A.; Mahdavian, M.; Moghadam, M.H.M. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide. Corros. Sci. 2016, 103, 283–304. [Google Scholar] [CrossRef]
- Miao, X.; Xing, A.; He, L.; Meng, Y.; Li, X. One-step preparation of hyperbranched polyether functionalized graphene oxide for improved corrosion resistance of epoxy coatings. Coatings 2019, 9, 844. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Ahmadi, A.; Mahdavian, M. Enhancement of the corrosion protection performance and cathodic delamination resistance of epoxy coating through treatment of steel substrate by a novel nanometric sol-gel based silane composite film filled with functionalized graphene oxide nanosheets. Corros. Sci. 2016, 109, 182–205. [Google Scholar] [CrossRef]
- Spinelli, G.; Lamberti, P.; Tucci, V.; Guadagno, L.; Vertuccio, L. Damage monitoring of structural resins loaded with carbon fillers: Experimental and theoretical study. Nanomaterials 2020, 10, 434. [Google Scholar] [CrossRef] [PubMed]
- Peretz Damari, S.; Cullari, L.; Laredo, D.; Nadiv, R.; Ruse, E.; Sripada, R.; Regev, O. Graphene and boron nitride nanoplatelets for improving vapor barrier properties in epoxy nanocomposites. Prog. Org. Coat. 2019, 136, 105207. [Google Scholar] [CrossRef]
- Savitha Unnikrishnan, K.; Sunil Jose, T.; Dinoop Lal, S.; Arun, K.J. Glass fiber reinforced bismaleimide/epoxy BaTiO3 nano composites for high voltage applications. Polym. Test. 2020, 87. [Google Scholar] [CrossRef]
- Nagachandrika, P.; Sridharan, K.; Sarathi, R.; Yoshimura, N. Understanding the incipient discharge activity with epoxy/MoS2 nanocomposites. Int. J. Soc. Mater. Eng. Resour. 2018, 23, 195–202. [Google Scholar] [CrossRef]
- Salom, C.; Prolongo, M.G.; Toribio, A.; Martínez-Martínez, A.J.; de Cárcer, I.A.; Prolongo, S.G. Mechanical properties and adhesive behavior of epoxy-graphene nanocomposites. Int. J. Adhes. Adhes. 2018, 84, 119–125. [Google Scholar] [CrossRef]
- Akpinar, I.A.; Gültekin, K.; Akpinar, S.; Akbulut, H.; Ozel, A. Experimental analysis on the single-lap joints bonded by a nanocomposite adhesives which obtained by adding nanostructures. Compos. Part B Eng. 2017, 110, 420–428. [Google Scholar] [CrossRef]
- Kausar, A. Rubber toughened epoxy-based nanocomposite: A promising pathway toward advanced materials. J. Macromol. Sci. Part A Pure Appl. Chem. 2020, 57, 499–511. [Google Scholar] [CrossRef]
- Parhizkar, N.; Shahrabi, T.; Ramezanzadeh, B. A new approach for enhancement of the corrosion protection properties and interfacial adhesion bonds between the epoxy coating and steel substrate through surface treatment by covalently modified amino functionalized graphene oxide film. Corros. Sci. 2017, 123, 55–75. [Google Scholar] [CrossRef]
- Yao, Y.; Sun, H.; Zhang, Y.; Yin, Z. Corrosion protection of epoxy coatings containing 2-hydroxyphosphonocarboxylic acid doped polyaniline nanofibers. Prog. Org. Coat. 2020, 139, 105470. [Google Scholar] [CrossRef]
- Rajitha, K.; Mohana, K.N.S. Synthesis of graphene oxide-based nanofillers and their influence on the anticorrosion performance of epoxy coating in saline medium. Diam. Relat. Mater. 2020, 108, 107974. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Haeri, Z.; Ramezanzadeh, M. A facile route of making silica nanoparticles-covered graphene oxide nanohybrids (SiO2-GO); fabrication of SiO2-GO/epoxy composite coating with superior barrier and corrosion protection performance. Chem. Eng. J. 2016, 303, 511–528. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.; Zhang, M.; Dou, W.; Zhang, X.; Chen, S. The effects of interfacial water and SiO2 surface wettability on the adhesion properties of SiO2 in epoxy nanocomposites. Appl. Surf. Sci. 2020, 502. [Google Scholar] [CrossRef]
- Tallman, T.N.; Hassan, H. A computational exploration of the effect of alignment and aspect ratio on alternating current conductivity in carbon nanofiber–modified epoxy. J. Intell. Mater. Syst. Struct. 2020, 31, 756–770. [Google Scholar] [CrossRef]
- Mutiso, R.M.; Winey, K.I. Electrical Conductivity of Polymer Nanocomposites. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 327–344. ISBN 978-0-08-087862-1. [Google Scholar]
- Haghgoo, M.; Ansari, R.; Hassanzadeh-Aghdam, M.K.; Nankali, M. Analytical formulation for electrical conductivity and percolation threshold of epoxy multiscale nanocomposites reinforced with chopped carbon fibers and wavy carbon nanotubes considering tunneling resistivity. Compos. Part A Appl. Sci. Manuf. 2019, 126, 105616. [Google Scholar] [CrossRef]
- Shkolnik, K.; Chalivendra, V. Numerical studies of electrical contacts of carbon nanotubes-embedded epoxy under tensile loading. Acta Mech. 2018, 229, 99–107. [Google Scholar] [CrossRef]
- Chillu, N.; Jayaganthan, R.; Rao, B.N.; Danikas, M.; Tanaka, T.; Sarathi, R. Investigation on space charge and charge trap characteristics of Al-epoxy nanocomposites. IET Sci. Meas. Technol. 2020, 14, 146–156. [Google Scholar] [CrossRef]
- Sanli, A.; Müller, C.; Kanoun, O.; Elibol, C.; Wagner, M.F.-X. Piezoresistive characterization of multi-walled carbon nanotube-epoxy based flexible strain sensitive films by impedance spectroscopy. Compos. Sci. Technol. 2016, 122, 18–26. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Freuli, F.; Frigione, M. Epoxy/expanded graphite stacks nanocomposites for cold-cured adhesives. J. Adhes. Sci. Technol. 2017, 31, 713–725. [Google Scholar] [CrossRef]
- Khotbehsara, M.M.; Manalo, A.; Aravinthan, T.; Reddy, K.R.; Ferdous, W.; Wong, H.; Nazari, A. Effect of elevated in-service temperature on the mechanical properties and microstructure of particulate-filled epoxy polymers. Polym. Degrad. Stab. 2019, 170, 108994. [Google Scholar] [CrossRef]
- Boomadevi Janaki, G.; Xavier, J.R. Effect of indole functionalized nano-alumina on the corrosion protection performance of epoxy coatings in marine environment. J. Macromol. Sci. Part A 2020, 57, 691–702. [Google Scholar] [CrossRef]
- Han, S.; Meng, Q.; Xing, K.; Araby, S.; Yu, Y.; Mouritz, A.; Ma, J. Epoxy/graphene film for lifecycle self-sensing and multifunctional applications. Compos. Sci. Technol. 2020, 198, 108312. [Google Scholar] [CrossRef]
- Nguyen, H.; Wang, Y.; Ronzello, J.; Chapman, J.; Cao, Y. Discharge behavior of the nanostructured insulation material for high torque density electrical propulsion. In Proceedings of the 2019 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Richland, WA, USA, 20–23 October 2019; pp. 737–740. [Google Scholar]
- Han, Z.J.; Levchenko, I.; Kumar, S.; Yajadda, M.M.A.; Yick, S.; Seo, D.H.; Martin, P.J.; Peel, S.; Kuncic, Z.; Ostrikov, K. Plasma nanofabrication and nanomaterials safety. J. Phys. D Appl. Phys. 2011, 44, 174019. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frigione, M.; Lettieri, M. Recent Advances and Trends of Nanofilled/Nanostructured Epoxies. Materials 2020, 13, 3415. https://doi.org/10.3390/ma13153415
Frigione M, Lettieri M. Recent Advances and Trends of Nanofilled/Nanostructured Epoxies. Materials. 2020; 13(15):3415. https://doi.org/10.3390/ma13153415
Chicago/Turabian StyleFrigione, Mariaenrica, and Mariateresa Lettieri. 2020. "Recent Advances and Trends of Nanofilled/Nanostructured Epoxies" Materials 13, no. 15: 3415. https://doi.org/10.3390/ma13153415
APA StyleFrigione, M., & Lettieri, M. (2020). Recent Advances and Trends of Nanofilled/Nanostructured Epoxies. Materials, 13(15), 3415. https://doi.org/10.3390/ma13153415





