Metal-Organic Framework (MOF)/Epoxy Coatings: A Review
Abstract
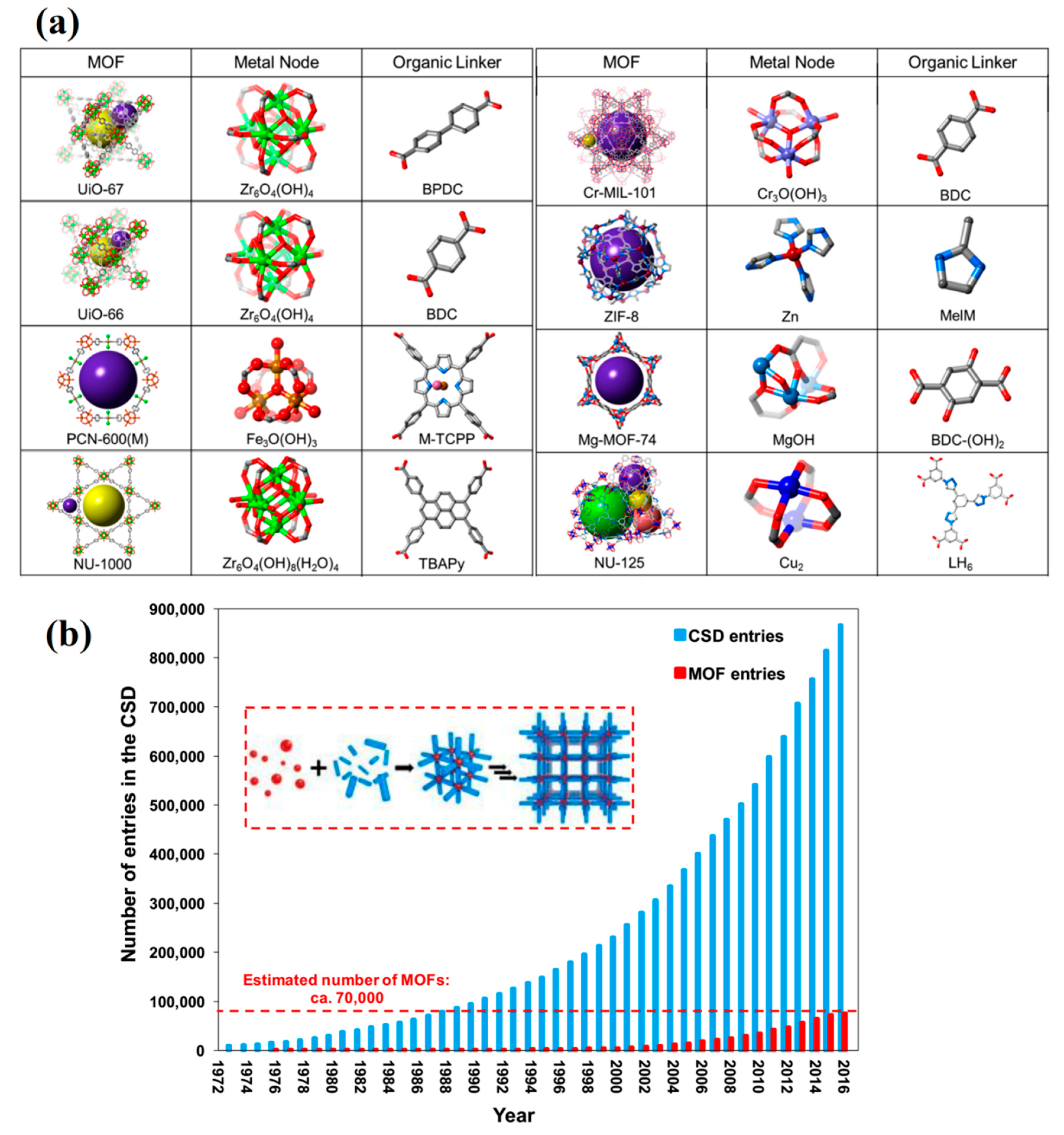
:1. Introduction
2. MOFs in Epoxy Coatings
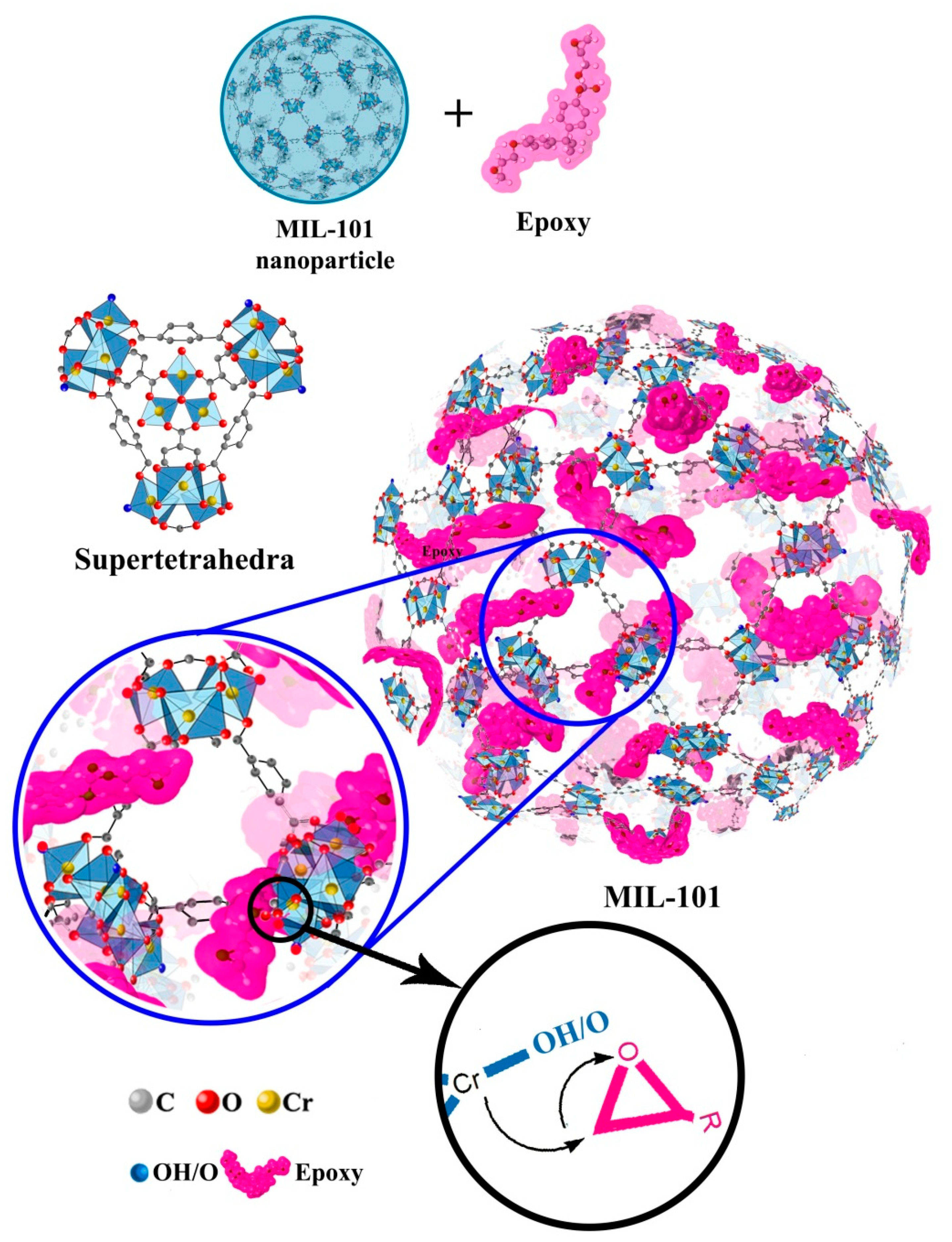
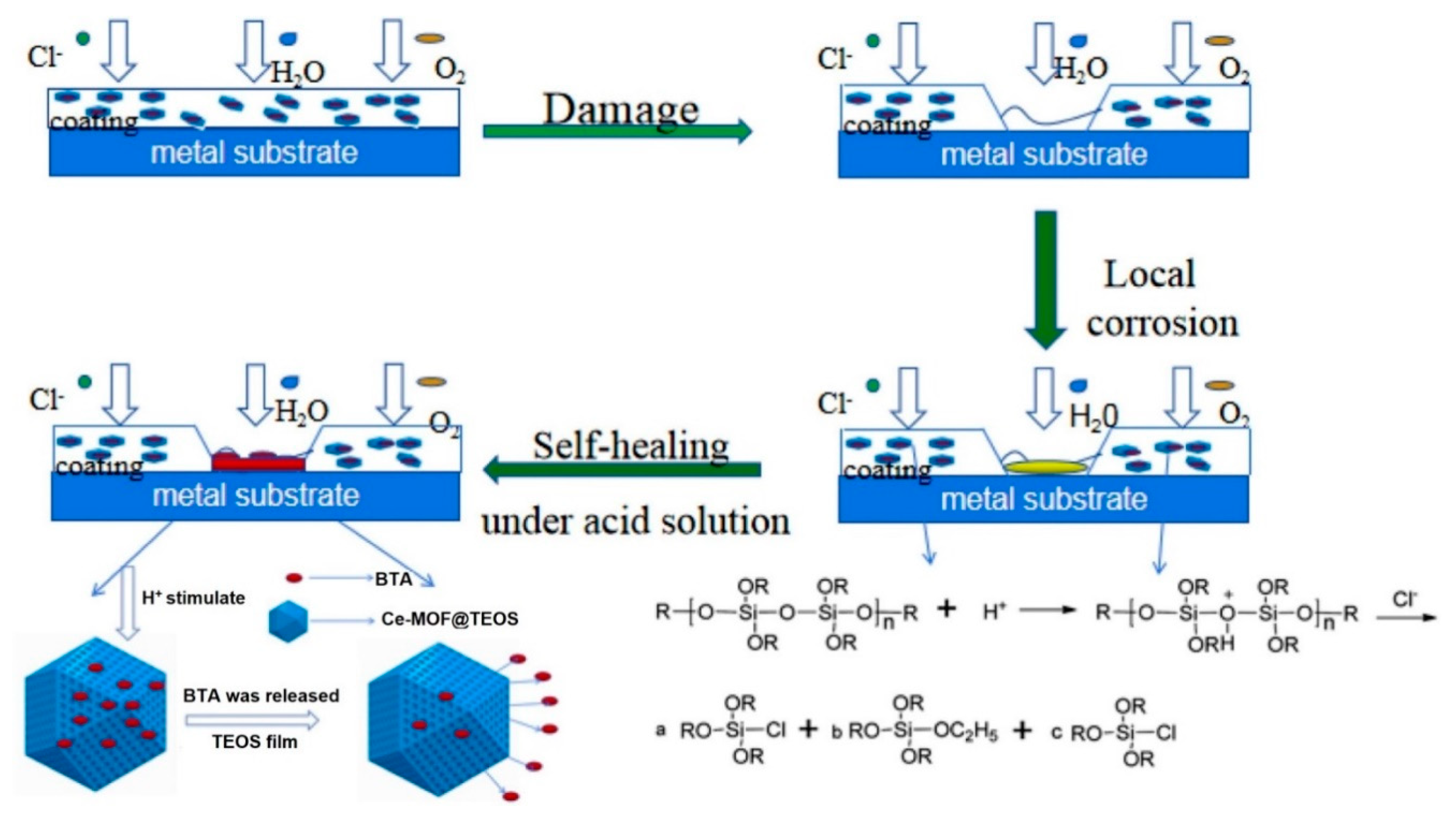
2.1. Anti-Corrosion Properties
2.2. Flame-Retardant Properties
2.3. Other Properties
3. Future Ahead of MOFs for Coating Applications
Author Contributions
Funding
Conflicts of Interest
References
- Mosser, M.F.; Greaser, J.H. Coating for Aerospace Aluminum Parts. European Patent EP0781861A1, 2 July 1997. [Google Scholar]
- Winrow, T.L.; Lohrman, M.E.; Wu, J.B. Coating for Sports Implements. U.S. Patent 5851158A, 22 December 1998. [Google Scholar]
- Ye, Y.; Yang, D.; Zhang, D.; Chen, H.; Zhao, H.; Li, X.; Wang, L. POSS-tetraaniline modified graphene for active corrosion protection of epoxy-based organic coating. Chem. Eng. J. 2020, 383, 123160. [Google Scholar] [CrossRef]
- Richards, C.; McMurray, H.; Williams, G. Smart-release inhibition of corrosion driven organic coating failure on zinc by cationic benzotriazole based pigments. Corros. Sci. 2019, 154, 101–110. [Google Scholar] [CrossRef]
- Ai, Y.-F.; Xia, L.; Pang, F.-Q.; Xu, Y.-L.; Zhao, H.-B.; Jian, R.-K. Mechanically strong and flame-retardant epoxy resins with anti-corrosion performance. Compos. Eng. 2020, 193, 108019. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Yazdi, M.K.; Vahabi, H.; Moghadam, P.N.; Saeb, M.R. Towards advanced flame retardant organic coatings: Expecting a new function from polyaniline. Prog. Org. Coatings 2019, 130, 144–148. [Google Scholar] [CrossRef]
- Rad, E.R.; Vahabi, H.; De Anda, A.R.; Saeb, M.R.; Thomas, S. Bio-epoxy resins with inherent flame retardancy. Prog. Org. Coatings 2019, 135, 608–612. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Tikhani, F.; Hampp, N.; Akbarzadeh Yazdi, D.; Zarrintaj, P.; Reza Ganjali, M.; Reza Saeb, M. Highly curable self-healing vitrimer-like cellulose-modified halloysite nanotube/epoxy nanocomposite coatings. Chem. Eng. J. 2020, 396, 125196. [Google Scholar] [CrossRef]
- Jiang, C.-C.; Cao, Y.-K.; Xiao, G.-Y.; Zhu, R.-F.; Lu, Y.-P. A review on the application of inorganic nanoparticles in chemical surface coatings on metallic substrates. RSC Adv. 2017, 7, 7531–7539. [Google Scholar] [CrossRef] [Green Version]
- Bahlakeh, G.; Ramezanzadeh, B.; Saeb, M.R.; Terryn, H.; Ghaffari, M. Corrosion protection properties and interfacial adhesion mechanism of an epoxy/polyamide coating applied on the steel surface decorated with cerium oxide nanofilm: Complementary experimental, molecular dynamics (MD) and first principle quantum mechanics (QM) simulation methods. Appl. Surf. Sci. 2017, 419, 650–669. [Google Scholar] [CrossRef]
- Tikhani, F.; Moghari, S.; Jouyandeh, M.; Laoutid, F.; Vahabi, H.; Saeb, M.R.; Dubois, P. Curing Kinetics and Thermal Stability of Epoxy Composites Containing Newly Obtained Nano-Scale Aluminum Hypophosphite (AlPO2). Polymers 2020, 12, 644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouyandeh, M.; Ganjali, M.; Ali, J.A.; Aghazadeh, M.; Paran, S.M.R.; Naderi, G.; Saeb, M.R.; Thomas, S. Curing epoxy with polyvinylpyrrolidone (PVP) surface-functionalized ZnxFe3−xO4 magnetic nanoparticles. Prog. Org. Coatings 2019, 136, 105227. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Jazani, O.M.; Navarchian, A.H.; Shabanian, M.; Vahabi, H.; Saeb, M.R. Surface engineering of nanoparticles with macromolecules for epoxy curing: Development of super-reactive nitrogen-rich nanosilica through surface chemistry manipulation. Appl. Surf. Sci. 2018, 447, 152–164. [Google Scholar] [CrossRef]
- Saeb, M.R.; Najafi, F.; Bakhshandeh, E.; Khonakdar, H.A.; Mostafaiyan, M.; Simon, F.; Scheffler, C.; Mäder, E. Highly curable epoxy/MWCNTs nanocomposites: An effective approach to functionalization of carbon nanotubes. Chem. Eng. J. 2015, 259, 117–125. [Google Scholar] [CrossRef]
- Ghiyasi, S.; Sari, M.G.; Shabanian, M.; Hajibeygi, M.; Zarrintaj, P.; Rallini, M.; Torre, L.; Puglia, D.; Vahabi, H.; Jouyandeh, M.; et al. Hyperbranched poly(ethyleneimine) physically attached to silica nanoparticles to facilitate curing of epoxy nanocomposite coatings. Prog. Org. Coatings 2018, 120, 100–109. [Google Scholar] [CrossRef]
- Tikhani, F.; Jouyandeh, M.; Jafari, S.H.; Chabokrow, S.; Ghahari, M.; Gharanjig, K.; Klein, F.; Hampp, N.; Ganjali, M.R.; Formela, K.; et al. Cure Index demonstrates curing of epoxy composites containing silica nanoparticles of variable morphology and porosity. Prog. Org. Coatings 2019, 135, 176–184. [Google Scholar] [CrossRef]
- Habibzadeh, S.; Rahmani, E.; Saeb, M.R.; Ganjali, M.; Chaouki, J. Multilayer Thin Films on Fine Particles. In Multilayer Thin Films—Versatile Applications for Materials Engineering; IntechOpen: London, UK, 2020. [Google Scholar]
- Li, F.; Du, M.; Zheng, Q. Dopamine/Silica Nanoparticle Assembled, Microscale Porous Structure for Versatile Superamphiphobic Coating. ACS Nano 2016, 10, 2910–2921. [Google Scholar] [CrossRef]
- Deng, X.; Mammen, L.; Zhao, Y.; Lellig, P.; Müllen, K.; Li, C.; Butt, H.-J.; Vollmer, D. Transparent, Thermally Stable and Mechanically Robust Superhydrophobic Surfaces Made from Porous Silica Capsules. Adv. Mater. 2011, 23, 2962–2965. [Google Scholar] [CrossRef]
- Shi, X.; Sitharaman, B.; Pham, Q.P.; Liang, F.; Wu, K.; Billups, W.E.; Wilson, L.J.; Mikos, A.G. Fabrication of porous ultra-short single-walled carbon nanotube nanocomposite scaffolds for bone tissue engineering. Biomaterials 2007, 28, 4078–4090. [Google Scholar] [CrossRef] [Green Version]
- Javidparvar, A.A.; Naderi, R.; Ramezanzadeh, B. Manipulating graphene oxide nanocontainer with benzimidazole and cerium ions: Application in epoxy-based nanocomposite for active corrosion protection. Corros. Sci. 2020, 165, 108379. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2011, 112, 1105–1125. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J.; Taghizadeh, M.; Taghizadeh, A.; Hayati, B.; Shekarchi, A.A.; Vossoughi, M. Activated carbon/metal-organic framework nanocomposite: Preparation and photocatalytic dye degradation mathematical modeling from wastewater by least squares support vector machine. J. Environ. Manag. 2019, 233, 660–672. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, M.; Taghizadeh, A. Ultrasound-assisted green synthesis and application of recyclable nanoporous chromium-based metal-organic framework. Korean J. Chem. Eng. 2018, 36, 287–298. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular chemistry: Secondary building units as a basis for the design of highly porous and robust metal-organic carboxylate frameworks. Accounts Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, M.; Taghizadeh, A. Activated carbon/metal-organic framework composite as a bio-based novel green adsorbent: Preparation and mathematical pollutant removal modeling. J. Mol. Liq. 2019, 277, 310–322. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, A.; Taghizadeh, M.; Abdi, J. In situ deposition of Ag/AgCl on the surface of magnetic metal-organic framework nanocomposite and its application for the visible-light photocatalytic degradation of Rhodamine dye. J. Hazard. Mater. 2019, 378, 120741. [Google Scholar] [CrossRef]
- Li, J.-R.J.; Sculley, J.; Zhou, H.-C. Metal–Organic Frameworks for Separations. Chem. Rev. 2011, 112, 869–932. [Google Scholar] [CrossRef]
- McKinlay, A.C.; Morris, R.E.; Horcajada, P.; Férey, G.; Gref, R.; Couvreur, P.; Serre, C.; Patrick, C. BioMOFs: Metal-Organic Frameworks for Biological and Medical Applications. Angew. Chem. Int. Ed. 2010, 49, 6260–6266. [Google Scholar] [CrossRef]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydin, A.O.; Hupp, J.T. Metal–Organic Framework Materials with Ultrahigh Surface Areas: Is the Sky the Limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Farha, O.K.; Roberts, J.M.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal–organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, M.; Taghizadeh, A.; Abdi, J.; Hayati, B.; Shekarchi, A.A. Bio-based magnetic metal-organic framework nanocomposite: Ultrasound-assisted synthesis and pollutant (heavy metal and dye) removal from aqueous media. Appl. Surf. Sci. 2019, 480, 288–299. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Oveisi, M.; Taghizadeh, A.; Taghizadeh, M. Synthesis of pearl necklace-like ZIF-8@chitosan/PVA nanofiber with synergistic effect for recycling aqueous dye removal. Carbohydr. Polym. 2019, 227, 115364. [Google Scholar] [CrossRef]
- Schoedel, A.; Li, M.; Li, D.; O’Keeffe, M.; Yaghi, O.M. Structures of Metal–Organic Frameworks with Rod Secondary Building Units. Chem. Rev. 2016, 116, 12466–12535. [Google Scholar] [CrossRef]
- Royuela, S.; Gil-San-Millan, R.; Real, M.J.M.; Ramos, M.M.; Segura, J.L.; Navarro, J.A.R.; Zamora, F. Catalytically Active Imine-based Covalent Organic Frameworks for Detoxification of Nerve Agent Simulants in Aqueous Media. Materials 2019, 12, 1974. [Google Scholar] [CrossRef] [Green Version]
- Devic, T.; Serre, C. High valence 3p and transition metal based MOFs. Chem. Soc. Rev. 2014, 43, 6097–6115. [Google Scholar] [CrossRef]
- Valizadeh, B.; Nguyen, T.N.; Stylianou, K.C. Shape engineering of metal–organic frameworks. Polyhedron 2018, 145, 1–15. [Google Scholar] [CrossRef]
- Howarth, A.J.; Peters, A.W.; Vermeulen, N.A.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Best Practices for the Synthesis, Activation, and Characterization of Metal–Organic Frameworks. Chem. Mater. 2016, 29, 26–39. [Google Scholar] [CrossRef]
- Gładysiak, A.; Nguyen, T.N.; Navarro, J.A.R.; Rosseinsky, M.J.; Stylianou, K.C. A Recyclable Metal-Organic Framework as a Dual Detector and Adsorbent for Ammonia. Chemistry 2017, 23, 13602–13606. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Wang, C.; Wang, S.; Wu, L.; Zhang, X. Light-harvesting metal-organic framework nanoprobes for ratiometric fluorescence energy transfer-based determination of pH values and temperature. Microchim. Acta 2019, 186, 476. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Zou, R.; Xu, Q. Metal–organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 2015, 8, 1837–1866. [Google Scholar] [CrossRef]
- Wang, L.; Han, Y.; Feng, X.; Zhou, J.; Qi, P.; Wang, B. Metal–organic frameworks for energy storage: Batteries and supercapacitors. Co-ord. Chem. Rev. 2016, 307, 361–381. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [Green Version]
- Deibert, B.J.; Li, J. A distinct reversible colorimetric and fluorescent low pH response on a water-stable zirconium–porphyrin metal–organic framework. Chem. Commun. 2014, 50, 9636–9639. [Google Scholar] [CrossRef]
- Ding, Z.; Tan, J.; Feng, G.; Yuan, Z.; Wu, C.; Zhang, X. Nanoscale metal–organic frameworks coated with poly (vinyl alcohol) for ratiometric peroxynitrite sensing through FRET. Chem. Sci. 2017, 8, 5101–5106. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): A review. J. Hazard. Mater. 2013, 244, 444–456. [Google Scholar] [CrossRef]
- Zhang, H.; Nai, J.; Yu, L.; Lou, X.W. (David) Metal-Organic-Framework-Based Materials as Platforms for Renewable Energy and Environmental Applications. Joule 2017, 1, 77–107. [Google Scholar] [CrossRef] [Green Version]
- Merí-Bofí, L.; Royuela, S.; Zamora, F.; Ruiz-González, M.L.; Segura, J.L.; Muñoz-Olivas, R.; Mancheño, M.J. Thiol grafted imine-based covalent organic frameworks for water remediation through selective removal of Hg( ii ). J. Mater. Chem. A 2017, 5, 17973–17981. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C.; Patrick, C. Metal–Organic Frameworks in Biomedicine. Chem. Rev. 2011, 112, 1232–1268. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2009, 9, 172–178. [Google Scholar] [CrossRef]
- Llewellyn, P.L.; Bourrelly, S.; Serre, C.; Vimont, A.; Daturi, M.; Hamon, L.; De Weireld, G.; Chang, J.-S.; Hong, D.-Y.; Hwang, Y.K.; et al. High Uptakes of CO2 and CH4 in Mesoporous Metal—Organic Frameworks MIL-100 and MIL-101. Langmuir 2008, 24, 7245–7250. [Google Scholar] [CrossRef]
- Shi, X.; Nguyen, T.A.; Suo, Z.; Liu, Y.; Avci, R. Effect of nanoparticles on the anticorrosion and mechanical properties of epoxy coating. Surf. Coatings Technol. 2009, 204, 237–245. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Zarrintaj, P.; Ganjali, M.R.; Ali, J.A.; Karimzadeh, I.; Aghazadeh, M.; Ghaffari, M.; Saeb, M.R. Curing epoxy with electrochemically synthesized GdxFe3-xO4 magnetic nanoparticles. Prog. Org. Coatings 2019, 136, 105245. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Aghazadeh, M.; Stadler, F.J.; Saeb, M.R. Curing epoxy with electrochemically synthesized NixFe3-xO4 magnetic nanoparticles. Prog. Org. Coatings 2019, 136, 105198. [Google Scholar] [CrossRef]
- Wetzel, B.; Haupert, F.; Zhang, M.Q. Epoxy nanocomposites with high mechanical and tribological performance. Compos. Sci. Technol. 2003, 63, 2055–2067. [Google Scholar] [CrossRef]
- Williams, J.; Graddage, N.; Rahatekar, S. Effects of plasma modified carbon nanotube interlaminar coating on crack propagation in glass epoxy composites. Compos. Part A Appl. Sci. Manuf. 2013, 54, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Perreux, D.; Suri, C. A study of the coupling between the phenomena of water absorption and damage in glass/epoxy composite pipes. Compos. Sci. Technol. 1997, 57, 1403–1413. [Google Scholar] [CrossRef]
- Zilg, C.; Thomann, R.; Finter, J.; Mülhaupt, R. The influence of silicate modification and compatibilizers on mechanical properties and morphology of anhydride-cured epoxy nanocomposites. Macromol. Mater. Eng. 2000, 280, 41–46. [Google Scholar] [CrossRef]
- Ahmadi, Z. Epoxy in nanotechnology: A short review. Prog. Org. Coat. 2019, 132, 445–448. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran, S.M.R.; Shabanian, M.; Ghiyasi, S.; Vahabi, H.; Badawi, M.; Formela, K.; Puglia, D.; Saeb, M.R. Curing behavior of epoxy/Fe3O4 nanocomposites: A comparison between the effects of bare Fe3O4, Fe3O4/SiO2/chitosan and Fe3O4/SiO2/chitosan/imide/phenylalanine-modified nanofillers. Prog. Org. Coatings 2018, 123, 10–19. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Shabanian, M.; Khaleghi, M.; Paran, S.; Ghiyasi, S.; Vahabi, H.; Formela, K.; Puglia, D.; Saeb, M.R. Acid-aided epoxy-amine curing reaction as reflected in epoxy/Fe3O4 nanocomposites: Chemistry, mechanism, and fracture behavior. Prog. Org. Coatings 2018, 125, 384–392. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Ali, J.A.; Ganjali, M.R.; Aghazadeh, M.; Paran, S.M.R.; Naderi, G.; Puglia, D.; Saeb, M.R. Epoxy/layered double hydroxide (LDH) nanocomposites: Synthesis, characterization, and Excellent cure feature of nitrate anion intercalated Zn-Al LDH. Prog. Org. Coatings 2019, 136. [Google Scholar] [CrossRef]
- Moghadam, P.Z.; Li, A.; Wiggin, S.; Tao, A.; Maloney, A.G.P.; Wood, P.A.; Ward, S.C.; Fairen-Jimenez, D. Development of a Cambridge Structural Database Subset: A Collection of Metal–Organic Frameworks for Past, Present, and Future. Chem. Mater. 2017, 29, 2618–2625. [Google Scholar] [CrossRef]
- Beg, S.; Rahman, M.; Jain, A.; Saini, S.; Midoux, P.; Pichon, C.; Ahmad, F.J.; Akhter, S. Nanoporous metal organic frameworks as hybrid polymer–metal composites for drug delivery and biomedical applications. Drug Discov. Today 2017, 22, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Giliopoulos, D.; Zamboulis, A.; Giannakoudakis, D.A.; Bikiaris, D.N.; Triantafyllidis, K.S. Polymer/Metal Organic Framework (MOF) Nanocomposites for Biomedical Applications. Molecules 2020, 25, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahid, S.; Nijmeijer, K.; Nehache, S.; Vankelecom, I.; Deratani, A.; Quémener, D. MOF-mixed matrix membranes: Precise dispersion of MOF particles with better compatibility via a particle fusion approach for enhanced gas separation properties. J. Membr. Sci. 2015, 492, 21–31. [Google Scholar] [CrossRef]
- Tanaka, K.; Oda, S.; Shiro, M. ChemInform Abstract: A Novel Chiral Porous Metal-Organic Framework: Asymmetric Ring Opening Reaction of Epoxide with Amine in the Chiral Open Space. Chem. Commun. 2008, 39, 820–822. [Google Scholar] [CrossRef] [Green Version]
- Doitomi, K.; Xu, K.; Hirao, H. The mechanism of an asymmetric ring-opening reaction of epoxide with amine catalyzed by a metal–organic framework: Insights from combined quantum mechanics and molecular mechanics calculations. Dalton Trans. 2017, 46, 3470–3481. [Google Scholar] [CrossRef] [Green Version]
- Jouyandeh, M.; Tikhani, F.; Shabanian, M.; Movahedi, F.; Moghari, S.; Akbari, V.; Gabrion, X.; Laheurte, P.; Vahabi, H.; Saeb, M.R. Synthesis, characterization, and high potential of 3D metal–organic framework (MOF) nanoparticles for curing with epoxy. J. Alloys Compd. 2020, 829, 154547. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran, S.M.R.; Jannesari, A.; Puglia, D.; Saeb, M.R. Protocol for nonisothermal cure analysis of thermoset composites. Prog. Org. Coatings 2019, 131, 333–339. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran, S.M.R.; Jannesari, A.; Saeb, M.R. “Cure Index” for thermoset composites. Prog. Org. Coatings 2019, 127, 429–434. [Google Scholar] [CrossRef]
- Akbari, V.; Najafi, F.; Vahabi, H.; Jouyandeh, M.; Badawi, M.; Morisset, S.; Ganjali, M.R.; Saeb, M.R. Surface chemistry of halloysite nanotubes controls the curability of low filled epoxy nanocomposites. Prog. Org. Coatings 2019, 135, 555–564. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Moini Jazani, O.; Navarchian, A.; Saeb, M. Epoxy coatings physically cured with hydroxyl-contained silica nanospheres and halloysite nanotubes. Prog. Color Colorants Coat. 2018, 11, 199–207. [Google Scholar]
- Jouyandeh, M.; Karami, Z.; Jazani, O.M.; Formela, K.; Paran, S.; Jannesari, A.; Saeb, M.R. Curing epoxy resin with anhydride in the presence of halloysite nanotubes: The contradictory effects of filler concentration. Prog. Org. Coatings 2019, 126, 129–135. [Google Scholar] [CrossRef]
- Zhang, Y.; Bo, X.; Luhana, C.; Wang, H.; Li, M.; Guo, L. Facile synthesis of a Cu-based MOF confined in macroporous carbon hybrid material with enhanced electrocatalytic ability. Chem. Commun. 2013, 49, 6885. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M.; Oveisi, M.; Taghizadeh, A.; Taghizadeh, M. Novel magnetic amine functionalized carbon nanotube/metal-organic framework nanocomposites: From green ultrasound-assisted synthesis to detailed selective pollutant removal modelling from binary systems. J. Hazard. Mater. 2019, 368, 746–759. [Google Scholar] [CrossRef]
- Xiao, T.; Liu, D. Progress in the synthesis, properties and applications of ZIF-7 and its derivatives. Mater. Today Energy 2019, 14, 100357. [Google Scholar] [CrossRef]
- Xiong, Y.; Ye, F.; Shen, S.; Zhao, S.; Su, L.; Chen, S.; Zhang, C. Synthesis of a mixed valence state Ce-MOF as an oxidase mimetic for the colorimetric detection of biothiols. Chem. Commun. 2015, 51, 4635–4638. [Google Scholar] [CrossRef]
- Biserčić, M.S.; Marjanović, B.; Zasońska, B.A.; Stojadinović, S.; Ćirić-Marjanović, G.N. Novel microporous composites of MOF-5 and polyaniline with high specific surface area. Synth. Met. 2020, 262, 116348. [Google Scholar] [CrossRef]
- Zhoua, C.; Lia, Z.; Lia, J.; Yuana, T.; Chena, B.; Mac, X.; Jiangd, D.; Luo, X.; Chen, D.; Liu, Y. Epoxy composite coating with excellent anticorrosion and self-healing performances based on multifunctional zeolitic imidazolate framework derived nanocontainers. Chem. Eng. J. 2020, 385, 123835. [Google Scholar] [CrossRef]
- Renabc, B.; Chenac, Y.; Liabcd, Y.; Li, W.; Gao, S.; Liac, H.; Cao, R.; Li, Y.; Li, H.F. Rational design of metallic anti-corrosion coatings based on zinc gluconate@ZIF-8. Chem. Eng. J. 2020, 384, 123389. [Google Scholar] [CrossRef]
- Cao, K.; Yu, Z.; Yin, D. Preparation of Ce-MOF@TEOS to enhance the anti-corrosion properties of epoxy coatings. Prog. Org. Coatings 2019, 135, 613–621. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, J.; Zhang, D.; Qi, T.; Li, G.L. pH-responsive self-healing anticorrosion coatings based on benzotriazole-containing zeolitic imidazole framework. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 561, 1–8. [Google Scholar] [CrossRef]
- Yin, D. Enhancement of the Anti-Corrosion Performance of Composite Epoxy Coatings in Presence of BTA-loaded Copper-Based Metal-Organic Frameworks. Int. J. Electrochem. Sci. 2019, 14, 4240–4253. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Mao, W.; Zhang, D.; Guo, Y.; Song, Y.; Wang, J.-P.; Qi, T.; Li, G.L. pH-Responsive zeolitic imidazole framework nanoparticles with high active inhibitor content for self-healing anticorrosion coatings. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 555, 18–26. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Y.; Chen, J.; Zhang, J.; Fang, Q. Dopamine modified metal-organic frameworks on anti-corrosion properties of waterborne epoxy coatings. Prog. Org. Coatings 2017, 109, 126–134. [Google Scholar] [CrossRef]
- Wang, H.; Fan, Y.; Tian, L.; Zhao, J.; Ren, L. Colorimetric/fluorescent dual channel sensitive coating for early detection of copper alloy corrosion. Mater. Lett. 2020, 265, 127419. [Google Scholar] [CrossRef]
- Ramezanzadeha, M.; Ramezanzadeha, B.; Mahdavian, M.; Bahlakehb, G. Development of metal-organic framework (MOF) decorated graphene oxide nanoplatforms for anti-corrosion epoxy coatings. Carbon 2020, 161, 231–251. [Google Scholar] [CrossRef]
- Zhong, J.; Kankala, R.K.; Wang, S.-B.; Chen, A. Recent Advances in Polymeric Nanocomposites of Metal-Organic Frameworks (MOFs). Polymers 2019, 11, 1627. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Ma, L.; Wang, L.-L.; Sun, Y.; Liu, Y. Insights into the Use of Metal–Organic Framework As High-Performance Anticorrosion Coatings. ACS Appl. Mater. Interfaces 2018, 10, 2259–2263. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Zhao, H.; Xue, Q.; Wang, L. Polydopamine coated graphene oxide for anticorrosive reinforcement of water-borne epoxy coating. Chem. Eng. J. 2018, 335, 255–266. [Google Scholar] [CrossRef]
- Vahabi, H.; Jouyandeh, M.; Cochez, M.; Khalili, R.; Vagner, C.; Ferriol, M.; Movahedifar, E.; Ramezanzadeh, B.; Rostami, M.; Ranjbar, Z.; et al. Short-lasting fire in partially and completely cured epoxy coatings containing expandable graphite and halloysite nanotube additives. Prog. Org. Coatings 2018, 123, 160–167. [Google Scholar] [CrossRef]
- Movahedifar, E.; Vahabi, H.; Saeb, M.R.; Thomas, S. Saeb Flame Retardant Epoxy Composites on the Road of Innovation: An Analysis with Flame Retardancy Index for Future Development. Molecules 2019, 24, 3964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, F.; Fang, Z.; Li, Z.; Wang, M.; Wang, W.; Zhao, D.; Xu, W.; Liu, Y.; Zhang, J.; Guo, X. Porous Co3O4 materials prepared by solid-state thermolysis of a novel Co-MOF crystal and their superior energy storage performances for supercapacitors. J. Mater. Chem. A 2013, 1, 7235. [Google Scholar] [CrossRef]
- Guo, H.; Su, S.; Liu, Y.; Ren, X.; Guo, W. Enhanced catalytic activity of MIL-101(Fe) with coordinatively unsaturated sites for activating persulfate to degrade organic pollutants. Environ. Sci. Pollut. Res. 2020, 27, 17194–17204. [Google Scholar] [CrossRef]
- Hou, Y.; Hu, W.; Gui, Z.; Hu, Y. A novel Co(II)–based metal-organic framework with phosphorus-containing structure: Build for enhancing fire safety of epoxy. Compos. Sci. Technol. 2017, 152, 231–242. [Google Scholar] [CrossRef]
- Zheng, Y.; Lu, Y.; Zhou, K. A novel exploration of metal–organic frameworks in flame-retardant epoxy composites. J. Therm. Anal. Calorim. 2019, 138, 905–914. [Google Scholar] [CrossRef]
- Huang, R.; Guo, X.; Ma, S.; Xie, J.; Xu, J.; Ma, J. Novel Phosphorus-Nitrogen-Containing Ionic Liquid Modified Metal-Organic Framework as an Effective Flame Retardant for Epoxy Resin. Polymers 2020, 12, 108. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Hu, W.; Gui, Z.; Hu, Y. Preparation of Metal–Organic Frameworks and Their Application as Flame Retardants for Polystyrene. Ind. Eng. Chem. Res. 2017, 56, 2036–2045. [Google Scholar] [CrossRef]
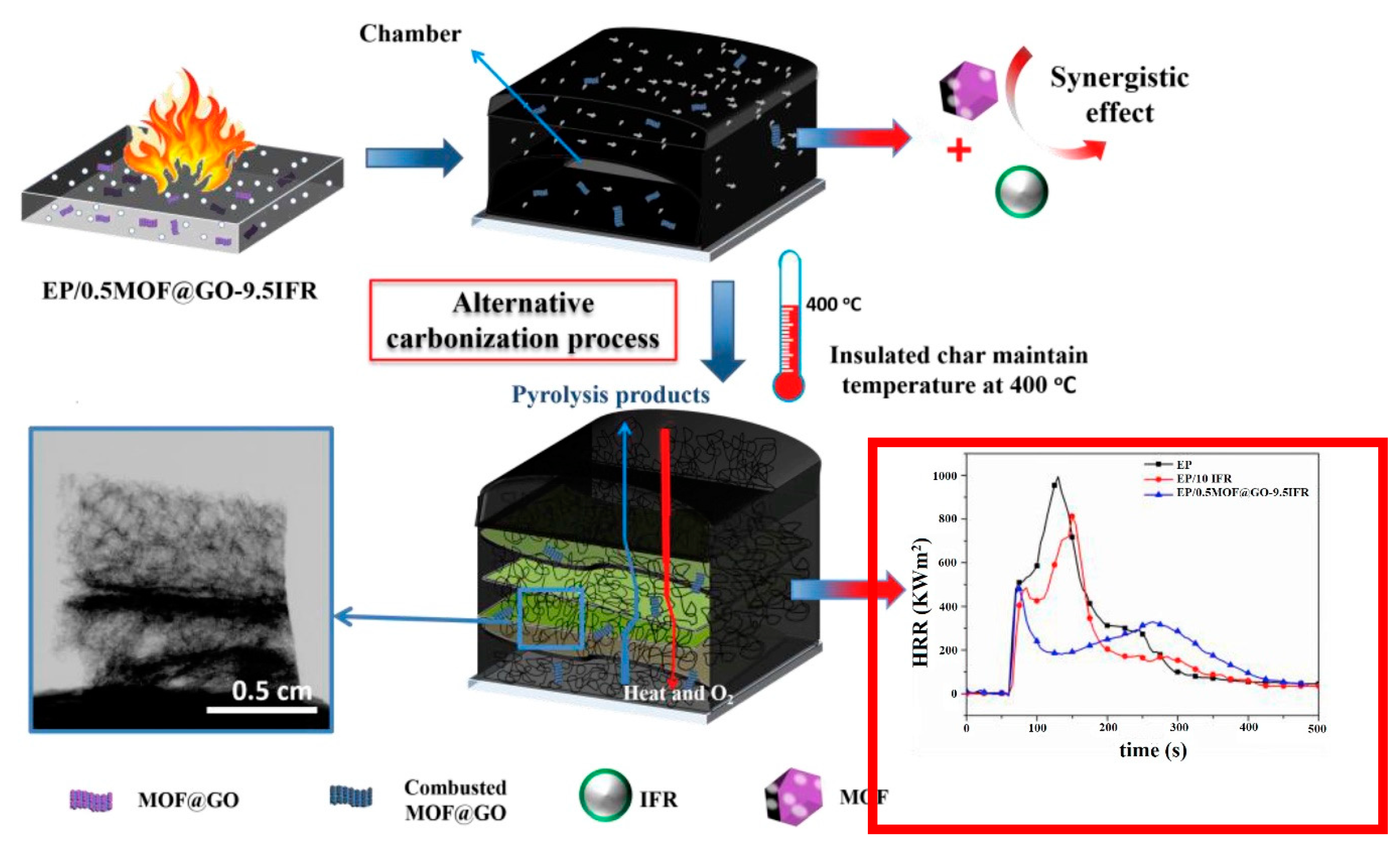
- Zhang, J.; Li, Z.; Zhang, L.; Molleja, J.G.; Wang, D. Bimetallic metal-organic frameworks and graphene oxide nano-hybrids for enhanced fire retardant epoxy composites: A novel carbonization mechanism. Carbon 2019, 153, 407–416. [Google Scholar] [CrossRef]
- Smith, Z.P.; Bachman, J.E.; Li, T.; Gludovatz, B.; Kusuma, V.A.; Xu, T.; Hopkinson, D.P.; Ritchie, R.O.; Long, J.R. Increasing M2(dobdc) Loading in Selective Mixed-Matrix Membranes: A Rubber Toughening Approach. Chem. Mater. 2018, 30, 1484–1495. [Google Scholar] [CrossRef]
- Hu, C.; Xiao, J.-D.; Mao, X.; Song, L.-L.; Yang, X.-Y.; Liu, S.-J. Toughening mechanisms of epoxy resin using aminated metal-organic framework as additive. Mater. Lett. 2019, 240, 113–116. [Google Scholar] [CrossRef]
- Roy, P.K.; Ramanan, A. Toughening of epoxy resin using Zn4O(1, 4-benzenedicarboxylate)3 metal–organic frameworks. RSC Adv. 2014, 4, 52338–52345. [Google Scholar]
- Tan, J.-C.; Cheetham, A.K. Mechanical properties of hybrid inorganic–organic framework materials: Establishing fundamental structure–property relationships. Chem. Soc. Rev. 2011, 40, 1059. [Google Scholar] [CrossRef] [PubMed]
- Kuc, A.B.; Enyashin, A.; Seifert, G. Metal−Organic Frameworks: Structural, Energetic, Electronic, and Mechanical Properties. J. Phys. Chem. B 2007, 111, 8179–8186. [Google Scholar] [CrossRef]
- Liu, C.; Mullins, M.; Hawkins, S.; Kotaki, M.; Sue, H. Epoxy Nanocomposites Containing Zeolitic Imidazolate Framework-8. ACS Appl. Mater. Interfaces 2017, 10, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, G.; Zhou, Z.; Li, Q. Metal-organic frameworks filled epoxy composites: Exploring new chances for low-κ polymer dielectrics. Polym. Technol. Mater. 2019, 59, 184–194. [Google Scholar] [CrossRef]
- Bariki, R.; Majhi, D.; Das, K.; Behera, A.; Mishra, B.G. Facile synthesis and photocatalytic efficacy of UiO-66/CdIn2S4 nanocomposites with flowerlike 3D-microspheres towards aqueous phase decontamination of triclosan and H2 evolution. Appl. Catal. B Environ. 2020, 270, 118882. [Google Scholar] [CrossRef]
- Li, H.; Liu, F.; Tian, H.; Wang, C.; Guo, Z.; Liu, P.; Peng, Z.; Wang, Q. Synergetic enhancement of mechanical and electrical strength in epoxy/silica nanocomposites via chemically-bonded interface. Compos. Sci. Technol. 2018, 167, 539–546. [Google Scholar] [CrossRef]
- Kim, S.; Ando, S.; Wang, X. Ternary composites of linear and hyperbranched polyimides with nanoscale silica for low dielectric constant, high transparency, and high thermal stability. RSC Adv. 2015, 5, 40046–40054. [Google Scholar] [CrossRef] [Green Version]
- Eslava, S.; Zhang, L.; Esconjauregui, S.; Yang, J.; Vanstreels, K.; Baklanov, M.R.; Saiz, E. Metal-Organic Framework ZIF-8 Films As Low-κ Dielectrics in Microelectronics. Chem. Mater. 2012, 25, 27–33. [Google Scholar] [CrossRef]
- Usman, M.; Lu, K.-L. Metal–organic frameworks: The future of low-κ materials. NPG Asia Mater. 2016, 8, e333. [Google Scholar] [CrossRef]
- Zagorodniy, K.; Seifert, G.; Hermann, H. Metal-organic frameworks as promising candidates for future ultralow-k dielectrics. Appl. Phys. Lett. 2010, 97, 251905. [Google Scholar] [CrossRef]

| Epoxy/MOF Composite-Based Materials for Corrosion Inhibition | |||||
|---|---|---|---|---|---|
| No | MOFs | Corrosion Inhibitor | Corrosive Media | Main Findings | Reference |
| 1 | ZIF-8 | Hollow mesoporous silica nanoparticles (HMSN)-BTA | 3.5 wt.% NaCl solution | After 30 days of immersion in the corrosive solution, the values of film resistance (Rf) of the epoxy coatings with HMSN-BTA@ZIF-8 were much higher than 108 Ω cm2, while values decreased to only about 106 Ω cm2 for the neat epoxy. | [81] |
| 2 | ZIF-8 | Zinc Gluconate (ZnG) | 3.5 wt.% NaCl solution | Incorporation of ZnG@ZIF-8 as a corrosion inhibitor revealed no signs of corrosion in the epoxy, even after 40 days of immersion. | [82] |
| 3 | Ce-MOF | BTA | 3.5 wt.% NaCl solution | Incorporation of 3 wt.% BI loaded Ce-MOF@ tetraethyl orthosilicate (TEOS) into the epoxy coating improved the charge transfer resistance by 1.5% after 2 h of immersion. | [83] |
| 4 | ZIF-7 | BTA | 0.1 M HCl solution | The epoxy coating doped with ZIF-7@BTA exhibited a superior barrier performance, which provided 99.4% inhibition efficiency. | [84] |
| 5 | Cu-MOF | BTA | 3.5 wt.% NaCl solution | Incorporation of 2 wt.% BTA-Cu-MOF into epoxy exhibited a corrosion potential of 0.492 V, which was ca. 26.5 times the value of the polarization resistance of the blank epoxy. | [85] |
| 6 | ZIF-7 | - | 0.1 M HCl solution | The coating resistance of 1.7% ZIF incorporated epoxy was ca. 2 times of that of the blank epoxy after 72 h of immersion. | [86] |
| 7 | MOF-5 | - | 3.5 wt.% NaCl solution | Dopamine@MOF-5 effectively delayed penetration of corrosive solution into the coating for 480 h. | [87] |
| 8 | ZIF-8 | - | 5 wt.% NaCl solution | RHS *@ZIF-8 structure improved early-stage corrosion inhibition of epoxy with no essential damage in the coating. | [88] |
| 9 | ZIF-8 | - | 3.5 wt.% NaCl solution | By addition of graphene oxide (GO)@ZIF-8, the cathodic delamination resistance, and wet adhesion strength were improved by about 73% and 60%, respectively. | [89] |
| Epoxy-MOF Composite-Based Materials for Flame Retardancy | |||||
|---|---|---|---|---|---|
| No | MOFs | Flame Retardant | Flame Tests | Main Findings | Reference |
| 1 | Co-MOF | Di (para-aminobenzoic acid) phenyl phosphate amide | CC 1 SSTF 2 | Decrease in pHRR and THR by 28% and 18.6%, respectively, by incorporating 2 wt.% of phosphorus-Co-MOF. | [97] |
| 2 | ZIF-8 ZIF-67 MIL-101 (Fe) | - | CC | 2 wt.% of Co-MOF/epoxy, Zn-MOF/epoxy, and Fe-MOF/epoxy composites burnt relatively slowly, and the reduction in pHRR in the composites was 31.3%, 27.8%, and 18.6%, respectively. | [98] |
| 3 | NH2-MIL-101 (Al) | Phosphorus-nitrogen-containing ionic liquid (IL@NH2) | LOI 3 CC | Addition of 3 wt.% IL modified-MOF (IL@NH2-MIL-101 (Al)) increased the LOI value of the epoxy to 29.8%, decreased pHRR by 51.2%, smoke production rate by 37.8%, and CO release rate by 44.8% with respect to those of blank epoxy. | [99] |
| MOFs as the Modifiers of Other Properties | |||
|---|---|---|---|
| No | MOFs | Main Findings | Reference |
| 1 | MOF-5 | Incorporation of 0.3% wt. MOF-5 led to 68% rise in the impact strength and 230% increase in the fracture energy of epoxy. | [104] |
| 2 | UiO-66 * UiO-66-NH2 | The values of tensile strength and elongation at break for UiO-66-NH2/EP were 40.4 MPa and 2.60%, respectively, which were higher than those of neat epoxy (35.2 MPa and 1.94%, respectively) and UiO-66/epoxy (37.0 MPa and 2.56%, respectively). | [103] |
| 3 | ZIF-8 | Addition of 25 vol.% ZIF-8 increased the Young’s modulus by 20% and decreased the dielectric constant of epoxy from 3.9 to 3.2 at 100 kHz. | [107] |
| 4 | ZIF-8 | The dielectric constant of the epoxy/ZIF-8 composite (0.3 wt.%) was decreased from 4.12 to 3.45. | [108] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seidi, F.; Jouyandeh, M.; Taghizadeh, M.; Taghizadeh, A.; Vahabi, H.; Habibzadeh, S.; Formela, K.; Saeb, M.R. Metal-Organic Framework (MOF)/Epoxy Coatings: A Review. Materials 2020, 13, 2881. https://doi.org/10.3390/ma13122881
Seidi F, Jouyandeh M, Taghizadeh M, Taghizadeh A, Vahabi H, Habibzadeh S, Formela K, Saeb MR. Metal-Organic Framework (MOF)/Epoxy Coatings: A Review. Materials. 2020; 13(12):2881. https://doi.org/10.3390/ma13122881
Chicago/Turabian StyleSeidi, Farzad, Maryam Jouyandeh, Mohsen Taghizadeh, Ali Taghizadeh, Henri Vahabi, Sajjad Habibzadeh, Krzysztof Formela, and Mohammad Reza Saeb. 2020. "Metal-Organic Framework (MOF)/Epoxy Coatings: A Review" Materials 13, no. 12: 2881. https://doi.org/10.3390/ma13122881
APA StyleSeidi, F., Jouyandeh, M., Taghizadeh, M., Taghizadeh, A., Vahabi, H., Habibzadeh, S., Formela, K., & Saeb, M. R. (2020). Metal-Organic Framework (MOF)/Epoxy Coatings: A Review. Materials, 13(12), 2881. https://doi.org/10.3390/ma13122881












