Selective Adsorption of Aqueous Diclofenac Sodium, Naproxen Sodium, and Ibuprofen Using a Stable Fe3O4–FeBTC Metal–Organic Framework
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. FeBTC Synthesis
2.3. Fe3O4 (Magnetite) Synthesis
2.4. Synthesis of the Fe3O4–FeBTC Composite
2.5. Activation of the Materials
2.6. Characterization of the Materials
2.7. Evaluation of the Materials
3. Results
3.1. Material Characterization
3.2. Adsorption of Pollutants on Materials
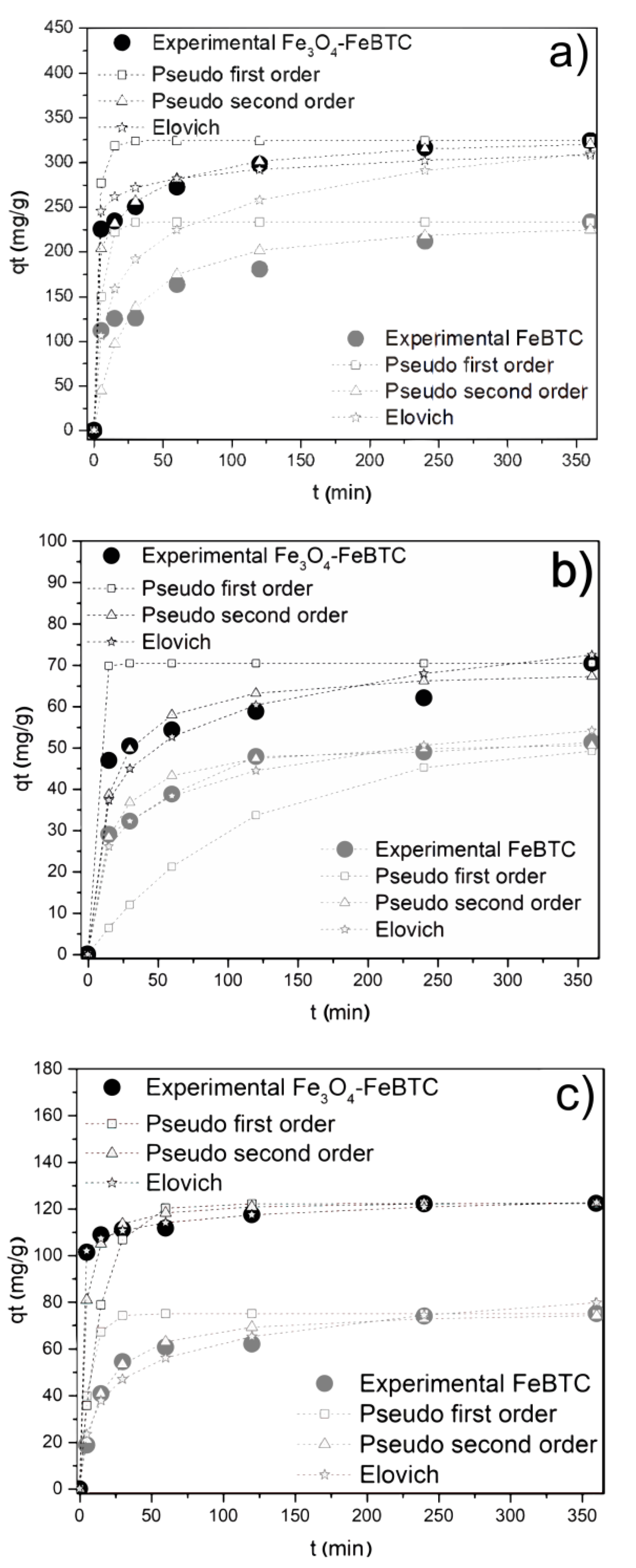
3.2.1. Adsorption Kinetics
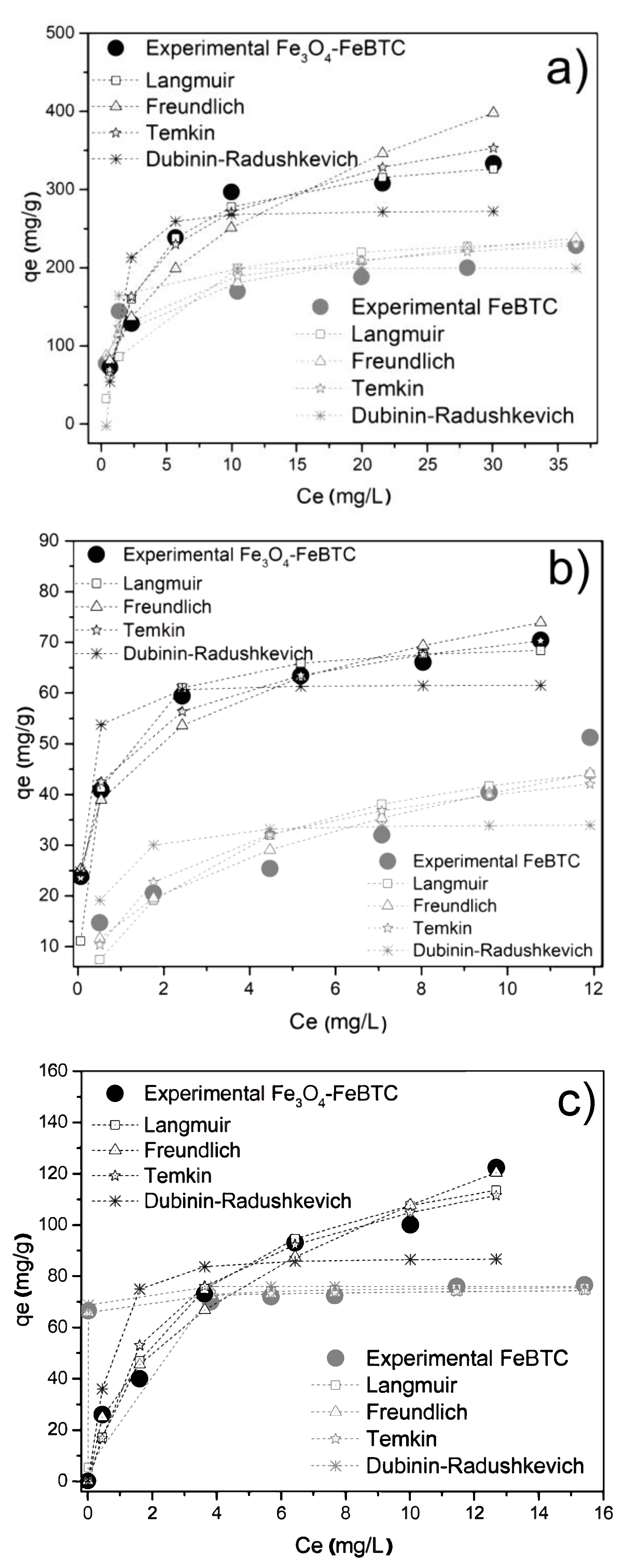
3.2.2. Adsorption Isotherms
3.2.3. Adsorption Thermodynamic Parameters
3.2.4. Multicomponent Mixture Adsorption
3.2.5. Effects of Adsorbent Mass, Temperature, and pH on the Adsorption Process
3.2.6. Characterization of Materials after Adsorption
3.2.7. Reutilization and Economy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Wang, S. Removal of Pharmaceuticals and Personal Care Products (PPCPs) from Wastewater: A Review. J. Environ. Manag. 2016, 1, 620–640. [Google Scholar] [CrossRef] [PubMed]
- Shailendra Mudgal, A.; De Toni, A.; Shailendra Mudgal, O.; Wiberg, O. Study on the Environmental Risks of Medicinal Products, Final Report Prepared for Executive Agency for Health and Consumers Photo Credit; Villa Deshayes: Paris, France, 2013. [Google Scholar]
- Wang, Q.; Shaheen, S.M.; Jiang, Y.; Li, R.; Slaný, M.; Abdelrahman, H.; Kwon, E.; Bolan, N.; Rinklebe, J.; Zhang, Z. Fe/Mn- and P-Modified Drinking Water Treatment Residuals Reduced Cu and Pb Phytoavailability and Uptake in a Mining Soil. J. Hazard. Mater. 2021, 403, 123628. [Google Scholar] [CrossRef]
- Sharma, S.; Bhattacharya, A. Drinking Water Contamination and Treatment Techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Paik Suh, M.; Reedijk, J. Terminology of Metal-Organic Frameworks and Coordination Polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Rojas, E.; Barrera, A.; Albiter, E.; Maubert, A.; Valenzuela, M. Application of MOFs and Their Composites Materials in Light-Driven Redox Reactions, Applications of Metal-Organic Frameworks and Their Derived Materials; Wiley-Scrivener Publisher: Beverly, MA, USA, 2019. [Google Scholar]
- Khan, N.A.; Jhung, S.H. Synthesis of Metal-Organic Frameworks (MOFs) with Microwave or Ultrasound: Rapid Reaction, Phase-Selectivity, and Size Reduction. Coord. Chem. Rev. 2015, 285, 11–23. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Horcajada, P.; Gibson, E.; Vishnuvarthan, M.; Vimont, A.; Grenèche, J.-M.; Serre, C.; Daturi, M.; Garcia, H. Comparison of Porous Iron Trimesates Basolite F300 and MIL-100(Fe) As Heterogeneous Catalysts for Lewis Acid and Oxidation Reactions: Roles of Structural Defects and Stability. ACS Catal. 2012, 2, 2060–2065. [Google Scholar] [CrossRef]
- Rojas, E.; Maubert, A.; Barrera, A.; Albiter, E.; Valenzuela, M. Synthesis of Carbon Nanotubes by Acetylene Decomposition in MCM-41 and SBA-15 Materials Modified with Ni, Fe & Co for H2 Adsorption; BOLETÍN del Grupo Español del Carbón: Zaragoza, Spain, 2019; pp. 23–27. [Google Scholar]
- Shahid, S.; Nijmeijer, K. High-Pressure Gas Separation Performance of Mixed-Matrix Polymer Membranes Containing Mesoporous Fe (BTC). J. Memb. Sci. 2014, 459, 33–44. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Ahmed, I.; Kim, S.; Jhung, S.H. Adsorptive Removal of Ibuprofen and Diclofenac from Water Using Metal-Organic Framework-Derived Porous Carbon. Chem. Eng. J. 2017, 314, 50–58. [Google Scholar] [CrossRef]
- Hasan, Z.; Choi, E.-J.; Jhung, S.H. Adsorption of Naproxen and Clofibric Acid over a Metal-Organic Framework MIL-101 Functionalized with Acidic and Basic Groups. Chem. Eng. J. 2013, 219, 537–544. [Google Scholar] [CrossRef]
- Petrova, T.M.; Fachikov, L.; Hristov, J. The Magnetite as Adsorbent for Some Hazardous Species from Aqueous Solutions: A Review. Int. Rev. Chem. Eng. 2011, 3, 134–152. [Google Scholar]
- Markeb, A.A.; Ordosgoitia, L.A.; Alonso, A.; Sánchez, A.; Font, X.; Kim, J.-Y.; Luo, T.; Meng, F.; Jin, Z.; Lin, D.; et al. Novel Magnetic Core-Shell Ce–Ti@Fe 3 O 4 Nanoparticles as an Adsorbent for Water Contaminants Removal. RSC Adv. 2016, 6, 56913–56917. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, X.; Yan, X.; Kong, L.; Zhang, G.; Liu, H.; Qiu, J.; Yeung, K.L. Synthesis of Fe3O4@ZIF-8 Magnetic Core-Shell Microspheres and Their Potential Application in a Capillary Microreactor. Chem. Eng. J. 2013, 228, 398–404. [Google Scholar] [CrossRef]
- Chen, G.; Yu, B.; Lu, C.; Zhang, H.; Shen, Y.; Cong, H. Controlled Synthesis of Fe3O4@ZIF-8 Nanoparticles for Drug Delivery. CrystEngComm 2018, 20, 7486–7491. [Google Scholar] [CrossRef]
- Pang, F.; He, M.; Ge, J. Controlled Synthesis of Fe3O4 /ZIF-8 Nanoparticles for Magnetically Separable Nanocatalysts. Chem. A Eur. J. 2015, 21, 6879–6887. [Google Scholar] [CrossRef] [PubMed]
- Min, X.; Yang, W.; Hui, Y.F.; Gao, C.Y.; Dang, S.; Sun, Z.M. Fe3O4@ZIF-8: A Magnetic Nanocomposite for Highly Efficient UO22+ Adsorption and Selective UO22+/Ln3+ Separation. Chem. Commun. 2017, 53, 4199–4202. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, X.L.; Geng, H.Y.; Hu, B.; Song, G.W.; Xu, Z.S. A MOF/Graphite Oxide Hybrid (MOF: HKUST-1) Material for the Adsorption of Methylene Blue from Aqueous Solution. J. Mater. Chem. A. 2013, 1, 10292–10299. [Google Scholar] [CrossRef]
- Liu, H.; Ren, X.; Chen, L. Synthesis and Characterization of Magnetic Metal-Organic Framework for the Adsorptive Removal of Rhodamine B from Aqueous Solution. J. Ind. Eng. Chem. 2016, 34, 278–285. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Y.; Wang, T.; Liang, N.; Hou, X. Core-Shell Fe3O4@MIL-100(Fe) Magnetic Nanoparticle for Effective Removal of Meloxicam and Naproxen in Aqueous Solution. J. Chem. Eng. Data 2019, 64, 2997–3007. [Google Scholar] [CrossRef]
- Li, S.; Cui, J.; Wu, X.; Zhang, X.; Hu, Q.; Hou, X. Rapid in Situ Microwave Synthesis of Fe3O4@MIL-100(Fe) for Aqueous Diclofenac Sodium Removal through Integrated Adsorption and Photodegradation. J. Hazard. Mater. 2019, 373, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Pérez, G.; Maubert, A. Materiales Compositos MWCNT@MOF FeBTC para la Reducción Fotocatalítica de CO2 a Combustibles Limpios: Fotosíntesis Artificial; BOLETÍN del Grupo Español del Carbón: Zaragoza, Spain, 2019; pp. 3–7. [Google Scholar]
- Cai, W.; Guo, M.; Weng, X.; Zhang, W.; Chen, Z. Adsorption of Doxorubicin Hydrochloride on Glutaric Anhydride Functionalized Fe3O4@SiO2 Magnetic Nanoparticles. Mater. Sci. Eng. C 2019, 98, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-F.; Meng, F.-Y.; Li, X.-H.; Wu, N.-N.; Deng, Y.-H.; Wei, L.-Y.; Zeng, X.-P. Magnetic Graphene Oxide-Fe 3 O 4 -PANI Nanoparticle Adsorbed Platinum Drugs as Drug Delivery Systems for Cancer Therapy. J. Nanosci. Nanotechnol. 2019, 19, 7517–7525. [Google Scholar] [CrossRef]
- Rojas-García, E.; López Medina, R.; May-Lozano, M.; Hernández-Pérez, I.; Hernández Pérez, I.; Valero, M.; Maubert-Franco, A. Adsorption of Azo-Dye Orange II from Aqueous Solutions Using a Metal-Organic Framework Material: Iron- Benzenetricarboxylate. Materials 2014, 7, 8037–8057. [Google Scholar] [CrossRef]
- Castañeda, A. Incorporación de Nanotubos de Carbono en Estructuras Metal-Orgánicas para el Almacenamiento de Hidrógeno. Master’s Thesis, Universidad Autónoma Metropolitana-A, Mexico City, Mexico, 2017. [Google Scholar]
- Jiang, X.; Chen, H.-Y.; Liu, L.-L.; Qiu, L.-G.; Jiang, X. Fe3O-1004 Embedded ZIF-8 Nanocrystals with Ultra-High Adsorption Capacity towards Hydroquinone. J. Alloys Compd. 2015, 646, 1075–1082. [Google Scholar] [CrossRef]
- Meteku, B.E.; Huang, J.; Zeng, J.; Subhan, F.; Feng, F.; Zhang, Y.; Qiu, Z.; Aslam, S.; Li, G.; Yan, Z. Magnetic Metal–Organic Framework Composites for Environmental Monitoring and Remediation. Coord. Chem. Rev. Elsevier BV 2020, 213261. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, J.; Li, X.; Song, C.; Meng, H.; Zhang, X. Adsorption Behavior of Methylene Blue on Fe3O4-Embedded Hybrid Magnetic Metal–Organic Framework. Desalin. Water Treat. 2016, 57, 25216–25225. [Google Scholar] [CrossRef]
- Jiménez-Cedillo, M.J.; Olguín, M.T.; Fall, C.; Colin-Cruz, A. As (III) and As (V) sorption on iron-modified non-pyrolyzed and pyrolyzed biomass from Petroselinum crispum (parsley). J. Environ. Manag. 2013, 117, 242–252. [Google Scholar] [CrossRef]
- Zijlstra, W.G.; Willem, G.; Buursma, A.; Assendelft, O.W.V. Visible and near Infrared Absorption Spectra of Human and Animal Haemoglobin: Determination and Application; VSP: Zeist, The Netherlands, 2000. [Google Scholar]
- Opanasenko, M.; Dhakshinamoorthy, A.; Čejka, J.; Garcia, H. Deactivation Pathways of the Catalytic Activity of Metal-Organic Frameworks in Condensation Reactions. ChemCatChem 2013, 5, 1553–1561. [Google Scholar] [CrossRef]
- Ding, J.; Tao, K.; Li, J.; Song, S.; Sun, K. Cell-Specific Cytotoxicity of Dextran-Stabilized Magnetite Nanoparticles. Colloids Surf. B Biointerfaces 2010, 79, 184–190. [Google Scholar] [CrossRef]
- Songvorawit, N.; Tuitemwong, K.; Tuitemwong, P. Single Step Synthesis of Amino-Functionalized Magnetic Nanoparticles with Polyol Technique at Low Temperature. Int. Sch. Res. Not. 2011, 1–6. [Google Scholar] [CrossRef]
- Khalil, M.I. Co-Precipitation in Aqueous Solution Synthesis of Magnetite Nanoparticles Using Iron (III) Salts as Precursors. Arab. J. Chem. 2015, 8, 279–284. [Google Scholar] [CrossRef]
- Slaný, M.; Jankovič, Ľ.; Madejová, J. Structural Characterization of Organo-Montmorillonites Prepared from a Series of Primary Alkylamines Salts: Mid-IR and near-IR Study. Appl. Clay Sci. 2019, 176, 11–20. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Tang, Z.; Niu, H.; Cai, Y.; Meng, W.; Wu, F.; Giesy, J.P. Synthesis of Magnetic Metal-Organic Framework (MOF) for Efficient Removal of Organic Dyes from Water. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Skumiel, A.; Józefczak, A.; Timko, M.; Kopčanský, P.; Herchl, F.; Koneracká, M.; Tomašovičová, N. Heating Effect in Biocompatible Magnetic Fluid. Int. J. Thermophys. 2007, 28, 1461–1469. [Google Scholar] [CrossRef]
- Sundman, A.; Byrne, J.M.; Bauer, I.; Menguy, N.; Kappler, A. Interactions between Magnetite and Humic Substances: Redox Reactions and Dissolution Processes. Geochem. Trans. 2017, 18, 1–12. [Google Scholar] [CrossRef]
- Huo, J.B.; Xu, L.; Yang, J.C.E.; Cui, H.J.; Yuan, B.; Fu, M.L. Magnetic Responsive Fe3O4-ZIF-8 Core-Shell Composites for Efficient Removal of As (III) from Water. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 539, 59–68. [Google Scholar] [CrossRef]
- Prestipino, C.; Regli, L.; Vitillo, J.G.; Bonino, F.; Damin, A.; Lamberti, C.; Zecchina, A.; Solari, P.L.; Kongshaug, K.O.; Bordiga, S. Local Structure of Framework Cu (II) in HKUST-1 Metallorganic Framework: Spectroscopic Characterization upon Activation and Interaction with Adsorbates. Chem. Mater. 2006, 18, 1337–1346. [Google Scholar] [CrossRef]
- Kim, H.K.; Yun, W.S.; Kim, M.; Kim, J.Y.; Bae, Y.; Lee, J.; Jeong, N.C. A Chemical Route to Activation of Open Metal Sites in the Copper-Based Metal−Organic Framework Materials HKUST-1 and Cu-MOF-2. J. Am. Chem. Soc. 2015, 222, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Moradi, S.E.; Haji Shabani, A.M.; Dadfarnia, S.; Emami, S. Effective Removal of Ciprofloxacin from Aqueous Solutions Using Magnetic Metal–Organic Framework Sorbents: Mechanisms, Isotherms and Kinetics. J. Iran. Chem. Soc. 2016, 13, 1617–1627. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Xiao, A.; Qiu, H.; Liu, L. Preparation of Magnetic Fe3O4/MIL-88A Nanocomposite and Its Adsorption Properties for Bromophenol Blue Dye in Aqueous Solution. Nanomaterials 2019, 9, 51. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, J.; Xue, X.; Liu, W.; Kong, Y.; Cheng, R.; Yua, D. Facile synthesis of Fe3O4@MOF-100(Fe) magnetic microspheres for the adsorption of diclofenac sodium in aqueous solution. Environ. Sci. Pollut. Res. 2018, 25, 31705–31717. [Google Scholar] [CrossRef]
- Gasparov, L.V.; Tanner, D.B.; Romero, D.B.; Berger, H.; Margaritondo, G.; Forró, L. Infrared and Raman Studies of the Verwey Transition in Magnetite. Phys. Rev. B-Condens. Matter Mater. Phys. 2000, 62, 7939–7944. [Google Scholar] [CrossRef]
- Castañeda, R.A.; Maubert, F.A. Adsorptive Removal of Diclofenac and Naproxen from Aquatic Environments by a New Metal-Organic Framework (MOF) Fe3O4-FeBTC; IMRC, MRS: Cancún, México, 2019; Volume 314, pp. 50–58. [Google Scholar]
- García, M.D.; Castañeda, R.A.; Maubert, F.A. Estructura Metal-orgánica híbrida Fe@CuBTC estable en medio acuoso para una elevada remoción de diclofenaco y naproxeno de agua. Rev. Tend. Docencia Investig. Química 2018, 4, 279–288. [Google Scholar]
- Hasan, Z.; Khan, N.A.; Jhung, S.H. Adsorptive Removal of Diclofenac Sodium from Water with Zr-Based Metal–Organic Frameworks. Chem. Eng. J. 2016, 284, 1406–1413. [Google Scholar] [CrossRef]
- Zhuang, S.; Cheng, R.; Wang, J. Adsorption of Diclofenac from Aqueous Solution Using UiO-66-Type Metal-Organic Frameworks. Chem. Eng. J. 2019, 359, 354–362. [Google Scholar] [CrossRef]
- Sun, W.; Li, H.; Li, H.; Li, S.; Cao, X. Adsorption Mechanisms of Ibuprofen and Naproxen to UiO-66 and UiO-66-NH2: Batch Experiment and DFT Calculation. Chem. Eng. J. 2019, 360, 645–653. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Wang, X.; Zhao, G.; Ai, Y.; Han, B.; Wen, T.; Hayat, T.; Alsaedi, A.; Wang, X. Experimental and Theoretical Study on Selenate Uptake to Zirconium Metal–Organic Frameworks: Effect of Defects and Ligands. Chem. Eng. J. 2017, 330, 1012–1021. [Google Scholar] [CrossRef]
- Zhang, C.; Han, C.; Sholl, D.S.; Schmidt, J.R. Computational Characterization of Defects in Metal-Organic Frameworks: Spontaneous and Water-Induced Point Defects in ZIF-8. J. Phys. Chem. Lett. 2016, 7, 459–464. [Google Scholar] [CrossRef]
- De Franco, M.A.E.; de Carvalho, C.B.; Bonetto, M.M.; de Pelegrini Soares, R.; Féris, L.A. Diclofenac Removal from Water by Adsorption Using Activated Carbon in Batch Mode and Fixed-Bed Column: Isotherms, Thermodynamic Study and Breakthrough Curves Modeling. J. Clean. Prod. 2018, 181, 145–154. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Zorpas, A.A. Heat of Adsorption, Adsorption Energy and Activation Energy in Adsorption and Ion Exchange Systems. Desalin. Water Treat. 2012, 39, 149–157. [Google Scholar] [CrossRef]
- Huang, M.; Xiang, W.; Zhou, T.; Mao, J.; Wu, X.; Guo, X. The Critical Role of the Surface Iron-Oxalate Complexing Species in Determining Photochemical Degradation of Norfloxacin Using Different Iron Oxides. Sci. Total Environ. 2019, 697, 134220. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, A.; Jurado, M.; Matz, O.; Calatayud, M.; Rojas, E.; Maubert, A. Hydrogen Adsorption in Metal-Organic Frameworks Cu-BTC and Fe-BTC: A Comparative Theoretical Study. J. Phys. Conf. Ser. 2019, 12016. [Google Scholar] [CrossRef]
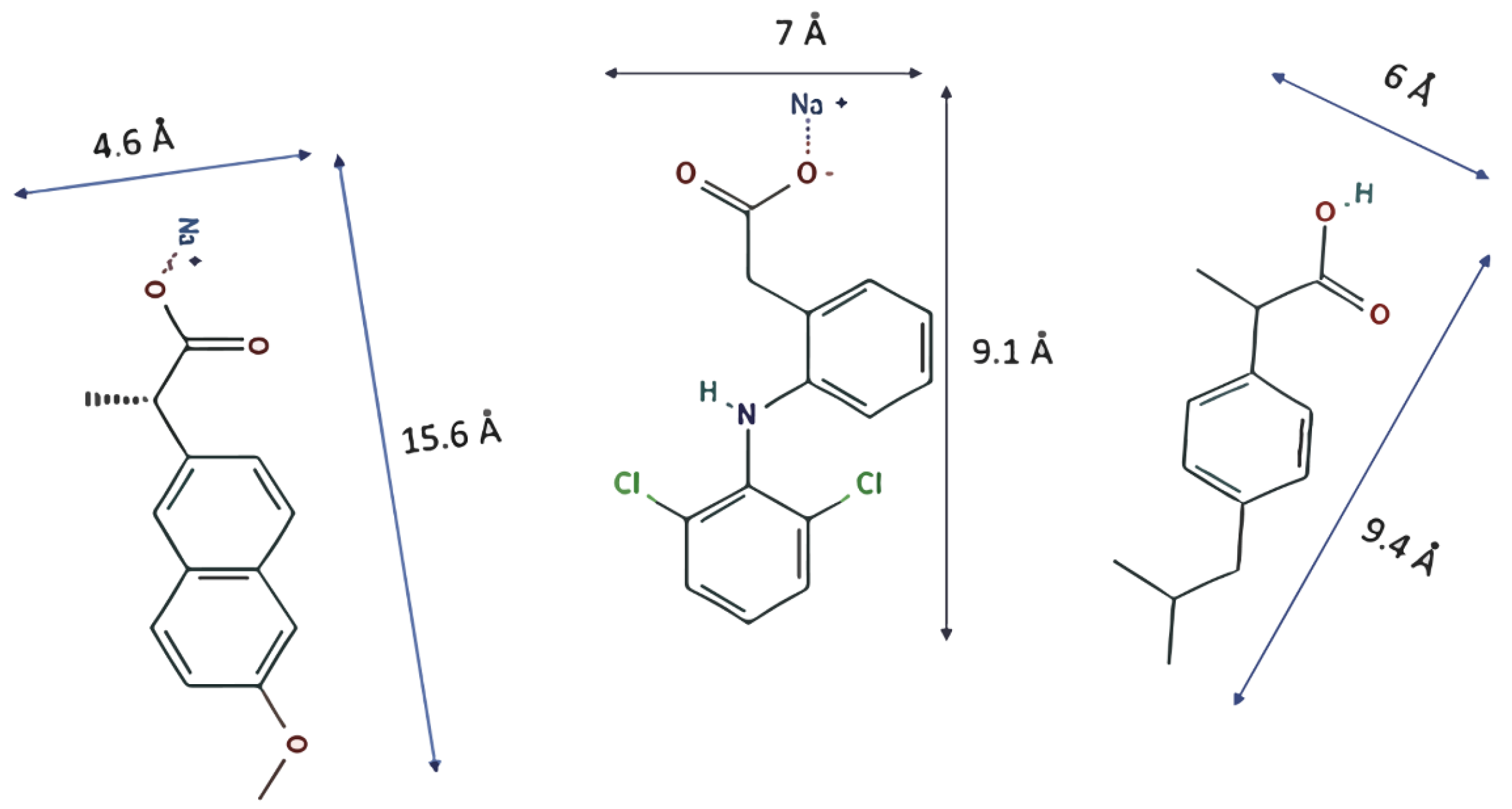






| Material | Surface Area BET (m2/g) | Total Pore Volume (m3/g) | Pore Diameter BJH (nm) |
|---|---|---|---|
| FeBTC | 815.84 | 0.92 | 3.26 |
| Fe3O4–FeBTC | 217.04 | 0.86 | 3.71 |
| Model | Parameter | Diclofenac Sodium | Naproxen Sodium | Ibuprofen | |||
|---|---|---|---|---|---|---|---|
| FeBTC | Fe3O4–FeBTC | FeBTC | Fe3O4–FeBTC | FeBTC | Fe3O4–FeBTC | ||
| Pseudo First-Order | qt (mg/g) | 139 | 233.1 | 51.2 | 70.4 | 75.1 | 102.6 |
| K1 (min−1) | 0.22 | 0.2 | 0.01 | 0.31 | 0.15 | 0.23 | |
| R2 | 0.994 | 0.981 | 0.920 | 0.962 | 0.950 | 0.920 | |
| Pseudo Second-Order | qt (mg/g) | 149.3 | 238.1 | 52.4 | 69.4 | 76.9 | 114.9 |
| K2 (g/mg min) | 9.3 × 10−6 | 1.6 × 10−6 | 8.8 × 10−7 | 3.5 × 10−7 | 2.9 × 10−5 | 2.3 × 10−5 | |
| R2 | 0.995 | 0.991 | 0.997 | 0.990 | 0.990 | 0.940 | |
| Elovich | β (mg/g) | 85.53 | 87.96 | 11.35 | 21.43 | 15.54 | 14.47 |
| α (g/mg min) | 0.068 | 0.021 | 0.113 | 0.090 | 0.076 | 0.052 | |
| R2 | 0.971 | 0.924 | 0.981 | 0.930 | 0.970 | 0.930 | |
| Model | Parameter | Diclofenac Sodium | Naproxen Sodium | Ibuprofen | |||
|---|---|---|---|---|---|---|---|
| FeBTC | Fe3O4–FeBTC | FeBTC | Fe3O4–FeBTC | FeBTC | Fe3O4–FeBTC | ||
| Langmuir | qm (mg/g) | 247.5 | 347.1 | 56.8 | 70.9 | 76.3 | 142.9 |
| KL (L/min) | 0.40 | 0.35 | 0.29 | 0.25 | 0.54 | 0.30 | |
| R2 | 0.987 | 0.989 | 0.854 | 0.98 | 0.990 | 0.961 | |
| Freundlich | n | 4.6 | 2.4 | 4.6 | 2.7 | 5.8 | 2.1 |
| KF ((g/mg) (L/mg)) | 108.3 | 96.1 | 44.2 | 17.1 | 70.8 | 36.4 | |
| R2 | 0.904 | 0.911 | 0.956 | 0.970 | 0.750 | 0.979 | |
| Temkin | KF (J/mol) | 79.4 | 36.9 | 268.9 | 248.6 | 207.1 | 88.9 |
| A (L/mg) | 34.0 | 3.9 | 16.7 | 5.6 | 21.5 | 4.0 | |
| R2 | 0.910 | 0.903 | 0.804 | 0.990 | 0.730 | 0.941 | |
| Dubinin–Radushkevich | qs (mg/g) | 199.5 | 272.3 | 34.0 | 61.6 | 75.9 | 86.9 |
| KDR ((mol/J)2) | 1 × 10−7 | 3 × 10−7 | 1 × 10−7 | 2 × 10−7 | 3 × 10−10 | 1 × 10−7 | |
| R2 | 0.876 | 0.791 | 0.617 | 0.890 | 0.821 | 0.731 | |
| Drug | Material | ΔH° (kJ/mol) | ΔS° (kJ/mol K) | ΔG° (kJ/mol) | R2 |
|---|---|---|---|---|---|
| DCF | FeBTC | −10.26 | −0.007 | −6.7 | 0.995 |
| Fe3O4–FeBTC | −34.09 | −0.083 | −7.4 | 0.990 | |
| NS | FeBTC | −28.87 | −0.079 | −4.2 | 0.988 |
| Fe3O4–FeBTC | −45.89 | −0.138 | −2.8 | 0.991 | |
| IB | FeBTC | −75.04 | −0.226 | −8.2 | 0.996 |
| Fe3O4–FeBTC | −80.03 | −0.240 | −9.0 | 0.994 |
| Material | Multicomponent Drug Adsorption (mg/g) | Multicomponent Drug Recovery (mg/g) | ||||
|---|---|---|---|---|---|---|
| DCF | NS | IB | DCF | NS | IB | |
| FeBTC | 162.5 | 18.7 | 102.7 | 146.3 (90%) | 16.4 (87%) | 13.3 (13%) |
| Fe3O4–FeBTC | 204.1 | 18.2 | 117.9 | 124.7 (61%) | 15.6 (85%) | 12.6 (11%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañeda Ramírez, A.A.; Rojas García, E.; López Medina, R.; Contreras Larios, J.L.; Suárez Parra, R.; Maubert Franco, A.M. Selective Adsorption of Aqueous Diclofenac Sodium, Naproxen Sodium, and Ibuprofen Using a Stable Fe3O4–FeBTC Metal–Organic Framework. Materials 2021, 14, 2293. https://doi.org/10.3390/ma14092293
Castañeda Ramírez AA, Rojas García E, López Medina R, Contreras Larios JL, Suárez Parra R, Maubert Franco AM. Selective Adsorption of Aqueous Diclofenac Sodium, Naproxen Sodium, and Ibuprofen Using a Stable Fe3O4–FeBTC Metal–Organic Framework. Materials. 2021; 14(9):2293. https://doi.org/10.3390/ma14092293
Chicago/Turabian StyleCastañeda Ramírez, Aldo Arturo, Elizabeth Rojas García, Ricardo López Medina, José L. Contreras Larios, Raúl Suárez Parra, and Ana Marisela Maubert Franco. 2021. "Selective Adsorption of Aqueous Diclofenac Sodium, Naproxen Sodium, and Ibuprofen Using a Stable Fe3O4–FeBTC Metal–Organic Framework" Materials 14, no. 9: 2293. https://doi.org/10.3390/ma14092293
APA StyleCastañeda Ramírez, A. A., Rojas García, E., López Medina, R., Contreras Larios, J. L., Suárez Parra, R., & Maubert Franco, A. M. (2021). Selective Adsorption of Aqueous Diclofenac Sodium, Naproxen Sodium, and Ibuprofen Using a Stable Fe3O4–FeBTC Metal–Organic Framework. Materials, 14(9), 2293. https://doi.org/10.3390/ma14092293





