Influence of Absorbable Calcium Sulfate-Based Bone Substitute Materials on Human Haemostasis—In Vitro Biological Behavior of Antibiotic Loaded Implants
Abstract
:1. Introduction
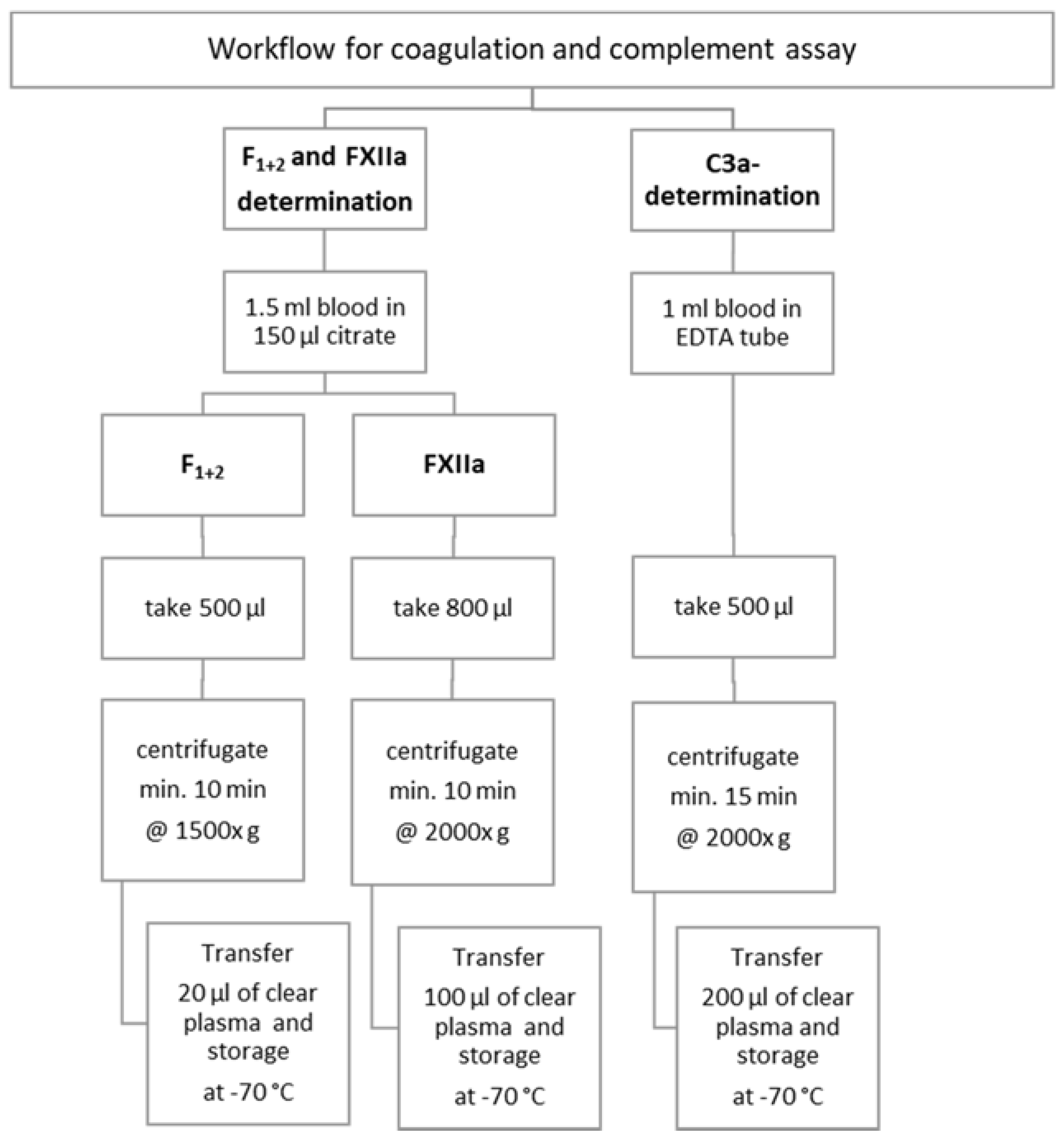
2. Materials and Methods
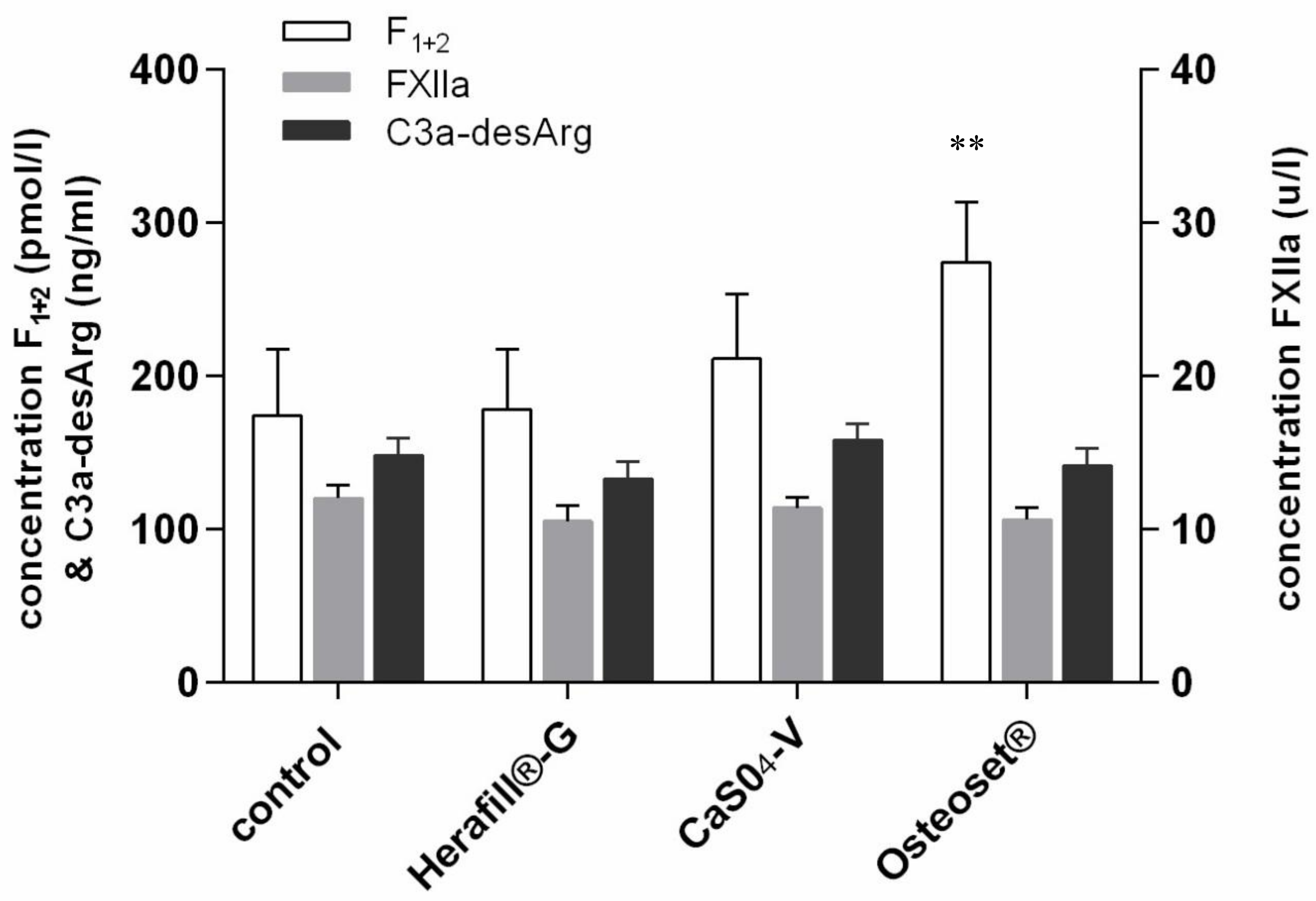
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pforringer, D.; Obermeier, A.; Kiokekli, M.; Buchner, H.; Vogt, S.; Stemberger, A.; Burgkart, R.; Lucke, M. Antimicrobial formulations of absorbable bone substitute materials as drug carriers based on calcium sulfate. Antimicrob. Agents Chemother. 2016, 60, 3897–3905. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, T. Basic concept for evaluating coagulation and fibrinolysis data. Rinsho Ketsueki 2017, 58, 2096–2103. [Google Scholar] [PubMed]
- Lalidou, F.; Kolios, G.; Drosos, G.I. Bone infections and bone graft substitutes for local antibiotic therapy. Surg. Technol. Int. 2014, 24, 353–362. [Google Scholar] [PubMed]
- Peltier, L.F. The use of plaster of Paris to fill large defects in bone. Am. J. Surg. 1959, 97, 311–315. [Google Scholar] [CrossRef]
- Helgeson, M.D.; Potter, B.K.; Tucker, C.J.; Frisch, H.M.; Shawen, S.B. Antibiotic-impregnated calcium sulfate use in combat-related open fractures. Orthopedics 2009, 32, 323. [Google Scholar] [CrossRef] [PubMed]
- Beuerlein, M.J.; McKee, M.D. Calcium sulfates: What is the evidence? Orthop. Trauma 2010, 24 (Suppl. S1), S46–S51. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, A.; Matl, F.D.; Schwabe, J.; Zimmermann, A.; Kuhn, K.D.; Lakemeier, S.; von Eisenhart-Rothe, R.; Stemberger, A.; Burgkart, R. Novel fatty acid gentamicin salts as slow-release drug carrier systems for anti-infective protection of vascular biomaterials. J. Mater. Sci. Mater. Med. 2012, 23, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.; Bader, A.; Kirschfink, M.; Rother, U.; Schrod, L.; Worner, I.; Zilow, G. Functional analysis and quantification of the complement c3 derived anaphylatoxin c3a with a monoclonal antibody. Clin. Exp. Immunol. 1987, 68, 703–711. [Google Scholar] [PubMed]
- Scarano, A.; Sinjari, B.; Murmura, G.; Mijiritsky, E.; Iaculli, F.; Mortellaro, C.; Tete, S. Hemostasis control in dental extractions in patients receiving oral anticoagulant therapy: An approach with calcium sulfate. J. Craniofac. Surg. 2014, 25, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Pforringer, D.; Harrasser, N.; Muhlhofer, H.; Kiokekli, M.; Stemberger, A.; van Griensven, M.; Lucke, M.; Burgkart, R.; Obermeier, A. Osteoinduction and -conduction through absorbable bone substitute materials based on calcium sulfate: In vivo biological behavior in a rabbit model. J. Mater. Sci. Mater Med. 2018, 29, 17. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, C.; Maazouz, Y.; Yang, D. Measuring wettability of biosurfaces at the microscale. Nanotechnol. Regen. Med. 2012, 811, 163–177. [Google Scholar] [CrossRef]
- Lu, Y.; Tang, N.; Lian, R.; Qi, J.; Wu, W. Understanding the relationship between wettability and dissolution of solid dispersion. Int. J. Pharm. 2014, 465, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Bhamla, M.S.; Nash, W.L.; Elliott, S.; Fuller, G.G. Influence of lipid coatings on surface wettability characteristics of silicone hydrogels. Langmuir 2015, 31, 3820–3828. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable materials for bone repair and tissue engineering applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Remedios, A. Bone and bone healing. Vet. Clin. N. Am. Small Anim. Pract. 1999, 29, 1029–1044. [Google Scholar] [CrossRef]
- Malhotra, A.; Pelletier, M.H.; Yu, Y.; Walsh, W.R. Can platelet-rich plasma (PRP) improve bone healing? A comparison between the theory and experimental outcomes. Arch. Orthop. Trauma Surg. 2013, 133, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, K.E.; Firth, E.C. Mechanisms of bone response to injury. J. Vet. Diagn. Investig. 2017, 29, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Shiu, H.T.; Leung, P.C.; Ko, C.H. The roles of cellular and molecular components of a hematoma at early stage of bone healing. J. Tissue Eng. Regen. Med. 2018, 12, e1911–e1925. [Google Scholar] [CrossRef] [PubMed]
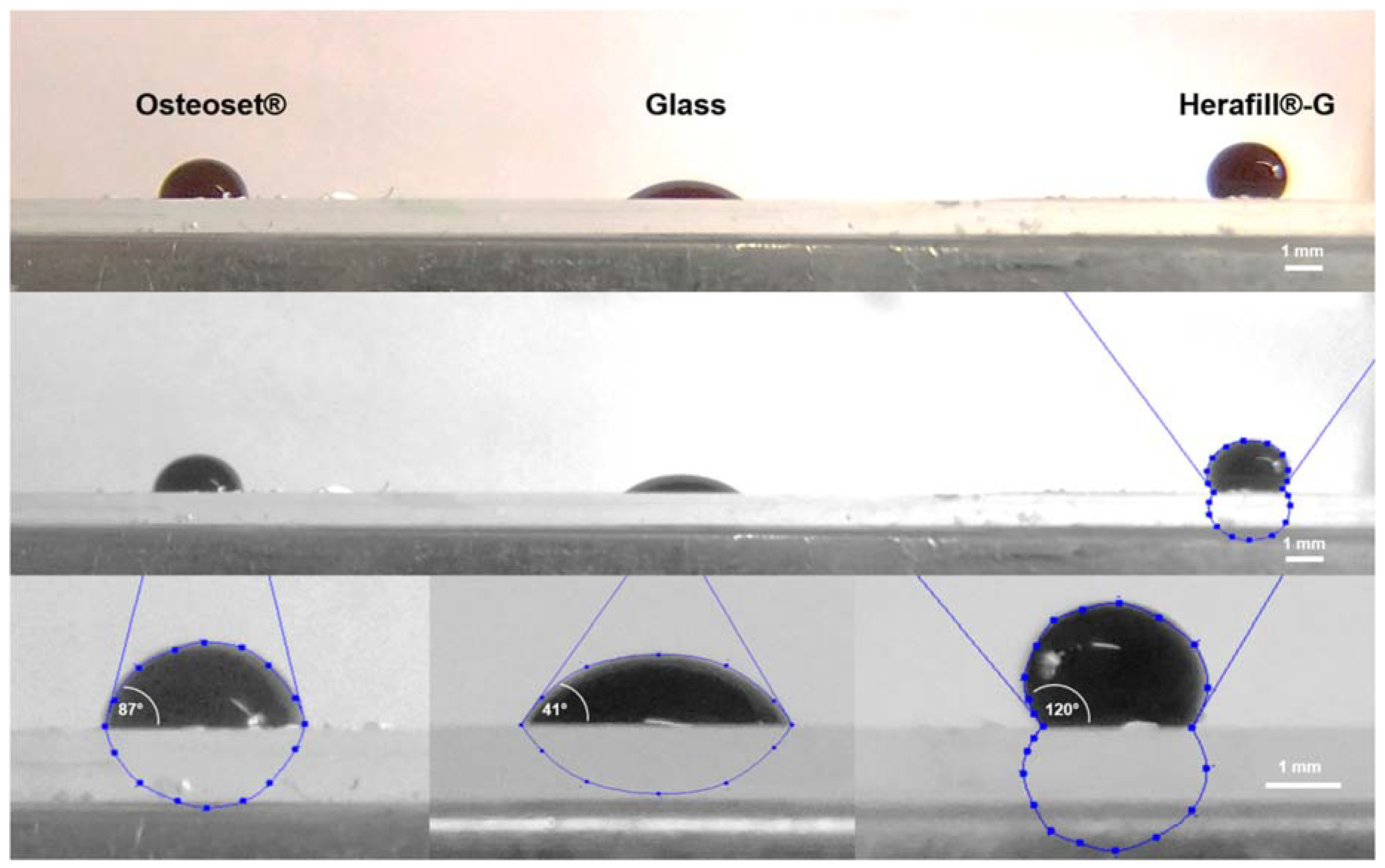
| Interfaces | Contact Angle (±SD) | Significance |
|---|---|---|
| Glass | 41.1 (±9.6)° | |
| Herafill®-G | 119.6 (±10.4)° | p < 0.001; *** |
| Osteoset® | 86.6 (±8.4)° | p < 0.001; *** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pförringer, D.; Harrasser, N.; Beirer, M.; Crönlein, M.; Stemberger, A.; Van Griensven, M.; Lucke, M.; Burgkart, R.; Obermeier, A. Influence of Absorbable Calcium Sulfate-Based Bone Substitute Materials on Human Haemostasis—In Vitro Biological Behavior of Antibiotic Loaded Implants. Materials 2018, 11, 935. https://doi.org/10.3390/ma11060935
Pförringer D, Harrasser N, Beirer M, Crönlein M, Stemberger A, Van Griensven M, Lucke M, Burgkart R, Obermeier A. Influence of Absorbable Calcium Sulfate-Based Bone Substitute Materials on Human Haemostasis—In Vitro Biological Behavior of Antibiotic Loaded Implants. Materials. 2018; 11(6):935. https://doi.org/10.3390/ma11060935
Chicago/Turabian StylePförringer, Dominik, Norbert Harrasser, Marc Beirer, Moritz Crönlein, Axel Stemberger, Martijn Van Griensven, Martin Lucke, Rainer Burgkart, and Andreas Obermeier. 2018. "Influence of Absorbable Calcium Sulfate-Based Bone Substitute Materials on Human Haemostasis—In Vitro Biological Behavior of Antibiotic Loaded Implants" Materials 11, no. 6: 935. https://doi.org/10.3390/ma11060935
APA StylePförringer, D., Harrasser, N., Beirer, M., Crönlein, M., Stemberger, A., Van Griensven, M., Lucke, M., Burgkart, R., & Obermeier, A. (2018). Influence of Absorbable Calcium Sulfate-Based Bone Substitute Materials on Human Haemostasis—In Vitro Biological Behavior of Antibiotic Loaded Implants. Materials, 11(6), 935. https://doi.org/10.3390/ma11060935






