Effect of the Preparation Method (Sol-Gel or Hydrothermal) and Conditions on the TiO2 Properties and Activity for Propene Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TiO2 Materials
2.3. Characterization
2.4. Photocatalytic Oxidation of Propene
3. Results and Discussion
3.1. Characterization of TiO2 Samples
3.1.1. XRD Analysis
- -
- Anatase: 25.3° (101), 37.8° (004), 48.0° (200), 54.5° (105), 55.0° (211), 62.7° (204), 70.4° (116) and 74.5° (220).
- -
- Brookite: 25.3° (120), 25.7° (111) and 30.8° (121).
- -
- Rutile: 27.5° (110), 36.1° (101) and 54.4° (211).
3.1.2. Textural Properties
3.1.3. Determination of the Band Gap Energy
3.2. Photocatalytic Activity
3.3. Effect of Avoiding Post-Synthesis Heat Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goldstein, B.D. Toxic substances in the atmospheric environement. A critical review. J. Air Pollut. Control Assoc. 1983, 33, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.J.; Sing, H.B. Distribution of volatile organic chemicals in outdoor and indoor air. Environ. Sci. Technol. 1988, 22, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Bello, L.; Rosal, R.; Satre, H.; Díez, F.V. Compuestos orgánicos volátiles y el medio ambiente. Ing. Química 1995, 5, 183–187. [Google Scholar]
- Blesa, M.A.; Sánchez, B. Eliminación de Contaminantes por Fotocatálisis Heterógenea; CIEMAT: Madrid, Spain, 2004; ISBN 987-43-3809-1. [Google Scholar]
- National Research Council. Rethinking the Ozone Problem in Urban and Regional Aire Pollution; National Academy Press: Washington, DC, USA, 1991; ISBN 0-309-56037-3. [Google Scholar]
- Bacsik, Z.; McGregor, J.; Mink, J. FTIR analysis of gaseous compounds in the mainstream smoke of regular and light cigarettes. Food Chem. Toxicol. 2007, 45, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Mühlberger, F.; Streibel, T.; Wieser, J.; Ulrich, A.; Zimmermann, R. Single photon ionization time-of-flight mass spectrometry with a pulsed electron beam pumped excimer VUV lamp for on-line gas analysis: Setup and first results on cigarette smoke and human breath. Anal. Chem. 2005, 77, 7408–7414. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, A.K.; Manjare, S.D. Selection of appropriate adsorption technique for recovery of VOCs: An analysis. J. Loss Prev. Process Ind. 2002, 15, 413–421. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Bouazza, N.; Berenguer Murcia, A.; Linares Salinas, J.; Soto, P.; Linares Solano, A. Photocatalytic oxidation of propene at low concentration. Appl. Catal. B Environ. 2007, 71, 298–309. [Google Scholar] [CrossRef]
- Bonjour, J.; Clausse, M. Psychrometric-like charts for the energy analysis of VOC recovery processes. Int. J. Therm. Sci. 2006, 45, 520–527. [Google Scholar] [CrossRef]
- Ozturk, B.; Yilmaz, D. Absorptive Removal of Volatile Organic Compounds from flue gas streams. Process Saf. Environ. Prot. 2006, 84, 391–398. [Google Scholar] [CrossRef]
- Heymes, F.; Manno-Demoustier, P.; Charbit, F.; Fanlo, J.L.; Moulin, P. A new efficient absorption liquid to treat exhaust air loaded with toluene. Chem. Eng. J. 2006, 115, 225–231. [Google Scholar] [CrossRef]
- Ruddy, E.N.; Carroll, L.A. Select the Best VOC Control strategy. Chem. Eng. Prog. 1993, 89, 28–35. [Google Scholar]
- Moretti, E.C.; Mukhopadhyay, N. VOC Control: Current practices and future trends. Chem. Eng. Prog. 1993, 89, 20–26. [Google Scholar]
- Tada, H.; Kiyonaga, T.; Naya, S. Rational design and applications of highly efficient reaction systems photocatalyzed by noble metal nanoparticle-loaded titanium(IV) dioxide. Chem. Soc. Rev. 2009, 38, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Shayegan, Z.; Lee, C.S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Zhou, R.; Eugene, A.J.; Guzman, M.I.; Grela, M.A. Cu2O/TiO2 heterostructures for CO2 reduction through a direct Z-scheme: Protecting Cu2O from photocorrosion. Appl. Catal. B Environ. 2017, 217, 485–493. [Google Scholar] [CrossRef]
- Reddy, K.R.; Hassan, M.; Gomes, V.G. Hybrid nanostructures based on titanium dioxide for enhanced photocatalysis. Appl. Catal. A Gen. 2015, 489, 1–16. [Google Scholar] [CrossRef]
- Wang, L.; Cai, Y.; Song, L.; Nie, W.; Zhou, Y.; Chen, P. High efficient photocatalyst of spherical TiO2 particles synthesized by a sol–gel method modified with glycol. Colloids Surf. A Physicochem. Eng. Asp. 2014, 461, 195–201. [Google Scholar] [CrossRef]
- Danish, M.; Ambreen, S.; Chauhan, A.; Pandey, A. Optimization and comparative evaluation of optical and photocatalytic properties of TiO2 thin films prepared via sol-gel method. J. Saudi Chem. Soc. 2015, 557–562. [Google Scholar] [CrossRef]
- Gupta, S.M.; Tripathi, M. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar] [CrossRef]
- Palmisano, L.; Augugliaro, V.; Bellardita, M.; Di Paola, A.; García López, E.; Loddo, V.; Marcì, G.; Palmisano, G.; Yurdakal, S. Titania photocatalysts for selective oxidations in water. ChemSusChem 2011, 4, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile? Model studies on epitaxial TiO2 films. Sci. Rep. 2015, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhu, G.; Fang, J.; Chen, J. Synthesis, Characterization, and Photocatalysis of Well-Dispersible Phase-Pure Anatase TiO2 Nanoparticles. Int. J. Photoenergy 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Mutuma, B.K.; Shao, G.N.; Kim, W.D.; Kim, H.T. Sol-gel synthesis of mesoporous anatase-brookite and anatase-brookite-rutile TiO2 nanoparticles and their photocatalytic properties. J. Colloid Interface Sci. 2015, 442, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, J.T.; Savenije, T.J.; Moulijn, J.A.; Mul, G. How Phase Composition Influences Optoelectronic and Photocatalytic Properties of TiO2. J. Phys. Chem. C 2010, 2211–2217. [Google Scholar] [CrossRef]
- Sahni, S.; Reddy, S.B.; Murty, B.S. Influence of process parameters on the synthesis of nano-titania by sol–gel route. Mater. Sci. Eng. A 2007, 452–453, 758–762. [Google Scholar] [CrossRef]
- Pulido Melián, E.; González Díaz, O.; Doña Rodríguez, J.M.; Colón, G.; Navío, J.A.; Pérez Peña, J. Effect of hydrothermal treatment on structural and photocatalytic properties of TiO2 synthesized by sol–gel method. Appl. Catal. A Gen. 2012, 411–412, 153–159. [Google Scholar] [CrossRef]
- Wen, B.; Liu, C.; Liu, Y. Optimization of the preparation methods. Synthesis of mesostructured TiO2 with high photocatalytic activities. J. Photochem. Photobiol. A Chem. 2005, 173, 7–12. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.; Zhang, L.; Ho, W. Enhancing effects of water content and ultrasonic irradiation on the photocatalytic activity of nano-sized TiO2 powders. J. Photochem. Photobiol. A 2002, 148, 263–271. [Google Scholar] [CrossRef]
- Colón, G.; Hidalgo, M.C.; Munuera, G.; Ferino, I.; Cutrufello, M.G.; Navío, J.A. Structural and surface approach to the enhanced photocatalytic activity of sulfated TiO2 photocatalyst. Appl. Catal. B Environ. 2006, 63, 45–59. [Google Scholar] [CrossRef]
- Liu, H.; Yang, W.; Ma, Y.; Cao, Y.; Yao, J. Promoted phase transition of titania nanoparticles prepared by a photo-assisted sol-gel method. New J. Chem. 2002, 26, 975–977. [Google Scholar] [CrossRef]
- Arconada, N.; Castro, Y.; Durán, A. Photocatalytic properties in aqueous solution of porous TiO2-anatase films prepared by sol-gel process. Appl. Catal. A Gen. 2010, 385, 101–107. [Google Scholar] [CrossRef]
- Yu, J.G.; Yu, J.C. Low Temperature Solvent Evaporation-induced Crystallization Synthesis of Nanocrystalline TiO2 Photocatalyst. Chin. J. Chem. 2003, 21, 994–997. [Google Scholar]
- Yu, J.G.; Yu, J.C.; Ho, W.K.; Jiang, Z.T. Effects of calcination temperature on the photocatalytic activity and photo-induced super-hydrophilicity of mesoporous TiO2 thin films. New J. Chem. 2002, 26, 607–613. [Google Scholar] [CrossRef]
- Li, S.; Ye, G.; Chen, G. Low-Temperature Preparation and Characterization of Nanocrystalline Anatase TiO2. J. Phys. Chem. C 2009, 113, 4031–4037. [Google Scholar] [CrossRef]
- Liu, A.R.; Wang, S.M.; Zhao, Y.R.; Zheng, Z. Low-temperature preparation of nanocrystalline TiO2 photocatalyst with a very large specific surface area. Mater. Chem. Phys. 2006, 99, 131–134. [Google Scholar] [CrossRef]
- Chuan, X. Preparation and photocatalytic performance of anatase-mounted natural porous silica, pumice, by hydrolysis under hydrothermal conditions. Appl. Catal. B Environ. 2004, 51, 255–260. [Google Scholar] [CrossRef]
- Kolenko, Y.V.; Maximov, V.D.; Burukhin, A.A.; Muhanov, V.A.; Churagulov, B.R. Synthesis of ZrO2 and TiO2 nanocrystalline powders by hydrothermal process. Mater. Sci. Eng. C 2003, 23, 1033–1038. [Google Scholar] [CrossRef]
- Dai, S.; Wu, Y.; Sakai, T.; Du, Z.; Sakai, H.; Abe, M. Preparation of highly crystalline TiO2 nanostructures by acid-assisted hydrothermal treatment of hexagonal-structured nanocrystalline titania/cetyltrimethyammonium bromide nanoskeleton. Nanoscale Res. Lett. 2010, 5, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Osterlund, L.; Ljungstrom, S.; Palmqvist, A. Preparation of Nanosize Anatase and Rutile TiO2 by Hydrothermal Treatment of Microemulsions and Their Activity for Photocatalytic Wet Oxidation of Phenol. J. Phys. Chem. B 2002, 106, 10674–10679. [Google Scholar] [CrossRef]
- Zhou, J.; Song, B.; Zhao, G.; Han, G. Effects of acid on the microstructures and properties of three-dimensional TiO2 hierarchical structures by solvothermal method. Nanoscale Res. Lett. 2012, 7, 217. [Google Scholar] [CrossRef] [PubMed]
- Ao, Y.; Xu, J.; Fu, D. Study on the effect of different acids on the structure and photocatalytic activity of mesoporous titania. Appl. Surf. Sci. 2009, 256, 239–245. [Google Scholar] [CrossRef]
- Serrano, D.P.; Calleja, G.; Sanz, R.; Pizarro, P. Preparation of bimodal micro-mesoporous TiO2 with tailored crystalline properties. Chem. Commun. 2004, 10, 1000–1001. [Google Scholar] [CrossRef] [PubMed]
- Cano-Casanova, L.; Amorós-Pérez, A.; Ouzzine, M.; Lillo-Ródenas, M.A.; Román-Martínez, M.C. One step hydrothermal synthesis of TiO2 with variable HCl concentration: Detailed characterization and photocatalytic activity in propene oxidation. Appl. Catal. B Environ. 2018, 220, 645–653. [Google Scholar] [CrossRef]
- Wu, M.; Long, J.; Huang, A.; Luo, Y.; Feng, S.; Xu, R. Microemulsion-mediated hydrothermal synthesis and characterization of nanosize rutile and anatase particles. Langmuir 1999, 15, 8822–8825. [Google Scholar] [CrossRef]
- Hoque, M.; Guzman, M.; Hoque, M.A.; Guzman, M.I. Photocatalytic Activity: Experimental Features to Report in Heterogeneous Photocatalysis. Materials 2018, 11, 1990. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ji, L.; Wu, B.; Gong, Q.; Zhu, Y.; Liang, J. Influence of surface treatment on preparing nanosized TiO2 supported on carbon nanotubes. Appl. Surf. Sci. 2008, 255, 3263–3266. [Google Scholar] [CrossRef]
- Rodriguez-Reinoso, F.; Linares-Solano, A. Microporous Structure of Activated Carbons as Revealed by Adsorption Methods; Thrower, P.A., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1989. [Google Scholar]
- Gandhe, A.R.; Fernandes, J.B. A simple method to synthesize N-doped rutile titania with enhanced photocatalytic activity in sunlight. J. Solid State Chem. 2005, 178, 2953–2957. [Google Scholar] [CrossRef]
- Aguilar, T.; Navas, J.; Alcántara, R.; Fernández-Lorenzo, C.; Gallardo, J.J.; Blanco, G.; Martín-Calleja, J. A route for the synthesis of Cu-doped TiO2 nanoparticles with a very low band gap. Chem. Phys. Lett. 2013, 571, 49–53. [Google Scholar] [CrossRef]
- ICDD: The International Centre for Diffraction Data. Available online: http://www.icdd.com/ (accessed on 10 October 2018).
- Zhang, H.; Banfield, J.F. Understanding Polymorphic Phase Transformation Behavior during Growth of Nanocrystalline Aggregates: Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Jensen, H.; Joensen, K.D.; Jørgensen, J.E.; Pedersen, J.S.; Søgaard, E.G. Characterization of nanosized partly crystalline photocatalysts. J. Nanopart. Res. 2004, 6, 519–526. [Google Scholar] [CrossRef]
- Bellardita, M.; Di Paola, A.; Megna, B.; Palmisano, L. Absolute crystallinity and photocatalytic activity of brookite TiO2 samples. Appl. Catal. B Environ. 2017, 201, 150–158. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Bellardita, M.; Di Paola, A.; Megna, B.; Palmisano, L. Determination of the crystallinity of TiO2 photocatalysts. J. Photochem. Photobiol. A Chem. 2018, 367, 312–320. [Google Scholar] [CrossRef]
- Wang, X.; Sø, L.; Su, R.; Wendt, S.; Hald, P.; Mamakhel, A.; Yang, C.; Huang, Y.; Iversen, B.B.; Besenbacher, F. The influence of crystallite size and crystallinity of anatase nanoparticles on the photo-degradation of phenol. J. Catal. 2014, 310, 100–108. [Google Scholar] [CrossRef]
- Jensen, H.; Soloviev, A.; Li, Z.; Søgaard, E.G. XPS and FTIR investigation of the surface properties of different prepared titania nano-powders. Appl. Surf. Sci. 2005, 246, 239–249. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L.; Barbieriková, Z.; Brezová, V. Influence of crystallinity and OH surface density on the photocatalytic activity of TiO2 powders. J. Photochem. Photobiol. A Chem. 2014, 273, 59–67. [Google Scholar] [CrossRef]
- Marcì, G.; García-López, E.; Bellardita, M.; Parisi, F.; Colbeau-Justin, C.; Sorgues, S.; Liotta, L.F.; Palmisano, L. Keggin heteropolyacid H3PW12O40 supported on different oxides for catalytic and catalytic photo-assisted propene hydration. Phys. Chem. Chem. Phys. 2013, 15, 13329–13342. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Phisisorption data for Gas/Solid systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1982, 54, 2201–2218. [Google Scholar] [CrossRef]
- Sayilkan, F.; Asilturk, M. Characterization of TiO2 Synthesized in Alcohol by a Sol-Gel Process: The Effects of Annealing Temperature and Acid Catalyst. Turkish J. Chem 2005, 29, 697–706. [Google Scholar]
- Coronado, J.; Portela, R.; Fresno, F.; Hernández-Alonso, M.D. Design of Advanced Photocatalytic Materials for Energy and Environmental; Springer: London, UK, 2013; pp. 85–102. ISBN 978-1-4471-5061-9. [Google Scholar]
- Di Paola, A.; Bellardita, M.; Palmisano, L. Brookite, the Least Known TiO2 Photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.-C.; Zakaria, R.; Ying, J.Y. Role of Particle Size in Nanocrystalline TiO2-Based Photocatalysts. J. Phys. Chem. B 1998, 102, 10871–10878. [Google Scholar] [CrossRef]
- Naik, V.M.; Haddad, D.; Naik, R.; Benci, J.; Auner, G.W. Optical Properties of Anatase, Rutile and Amorphous Phases of TiO2 Thin Films Grown at Room Temperature by RF Magnetron Sputtering. Mater. Res. 2003, 775, 1–9. [Google Scholar] [CrossRef]
- Valencia, S.; Marín, J.M.; Restrepo, G. Study of the Bandgap of Synthesized Titanium Dioxide Nanoparticules Using the Sol-Gel Method and a Hydrothermal Treatment. Open Mater. Sci. J. 2010, 4, 9–14. [Google Scholar] [CrossRef]
- Ouzzine, M.; Lillo-Ródenas, M.A.; Linares-Solano, A. Photocatalytic oxidation of propene in gas phase at low concentration by optimized TiO2 nanoparticles. Appl. Catal. B Environ. 2013, 134–135, 333–343. [Google Scholar] [CrossRef]
- Bacsa, R.R.; Kiwi, J. Effect of rutile phase on the photocatalytic properties of nanocrystalline titania during the degradation of p-coumaric acid. Appl. Catal. B Environ. 1998, 16, 19–29. [Google Scholar] [CrossRef]
- Warson, J.M.; Cooper, A.T.; Flora, J.R.V. Nanoglued Titanium Dioxide Aerogels for Photocatalysis. Environ. Eng. Sci. 2005, 22, 666–675. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Crist, S.E.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Probing reaction mechanisms in mixed phase TiO2 by EPR. J. Electron Spectros. Relat. Phenomena 2006, 150, 155–163. [Google Scholar] [CrossRef]
- Jaramillo-Páez, C.; Navío, J.A.; Hidalgo, M.C. Silver-modified ZnO highly UV-photoactive. J. Photochem. Photobiol. A Chem. 2018, 356, 112–122. [Google Scholar] [CrossRef]
- Fernández-Catalá, J.; Cano-Casanova, L.; Lillo-Ródenas, M.Á.; Berenguer-Murcia, Á.; Cazorla-Amorós, D. Synthesis of TiO2 with hierarchical porosity for the photooxidation of propene. Molecules 2017, 22, 2243. [Google Scholar] [CrossRef] [PubMed]

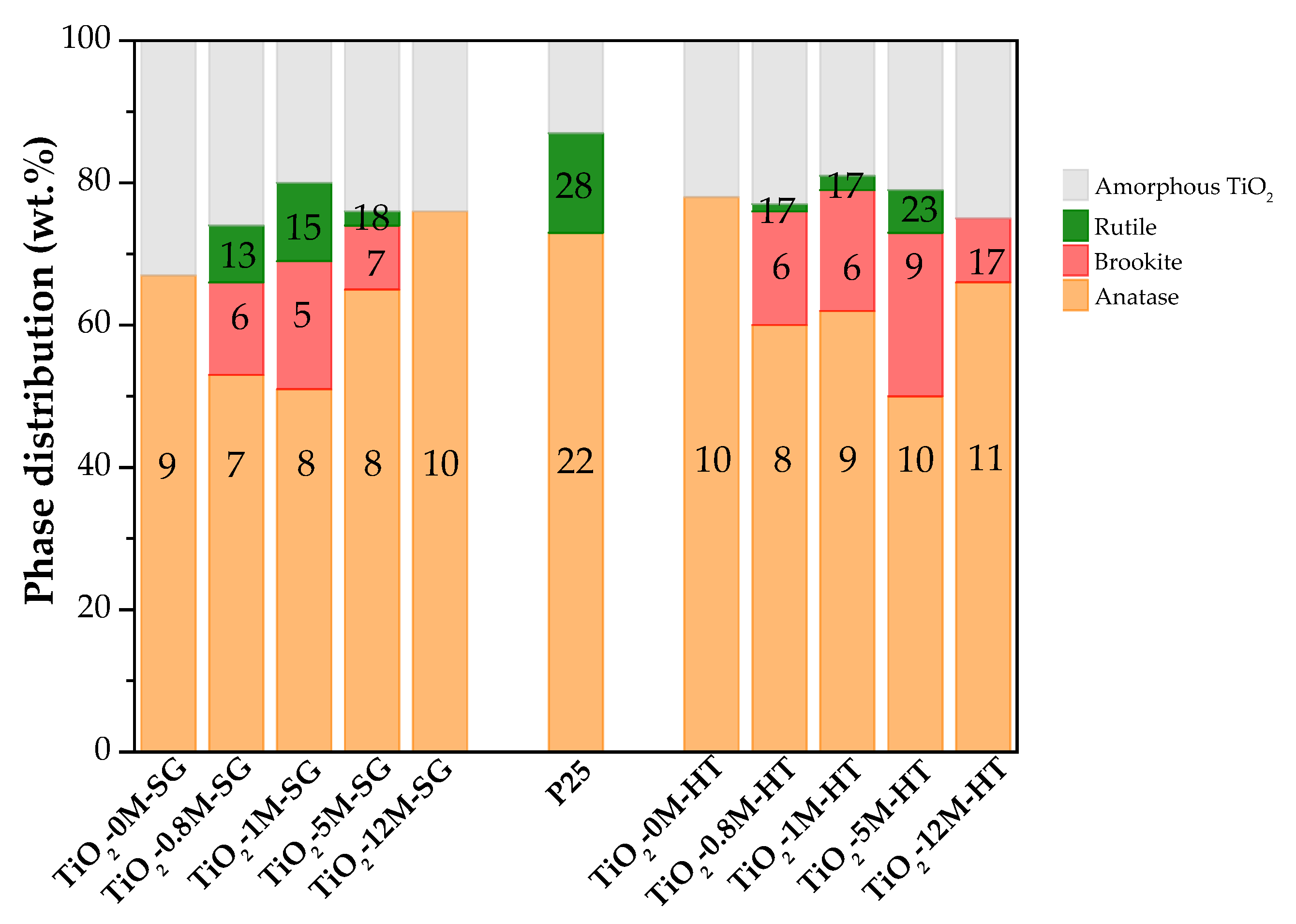
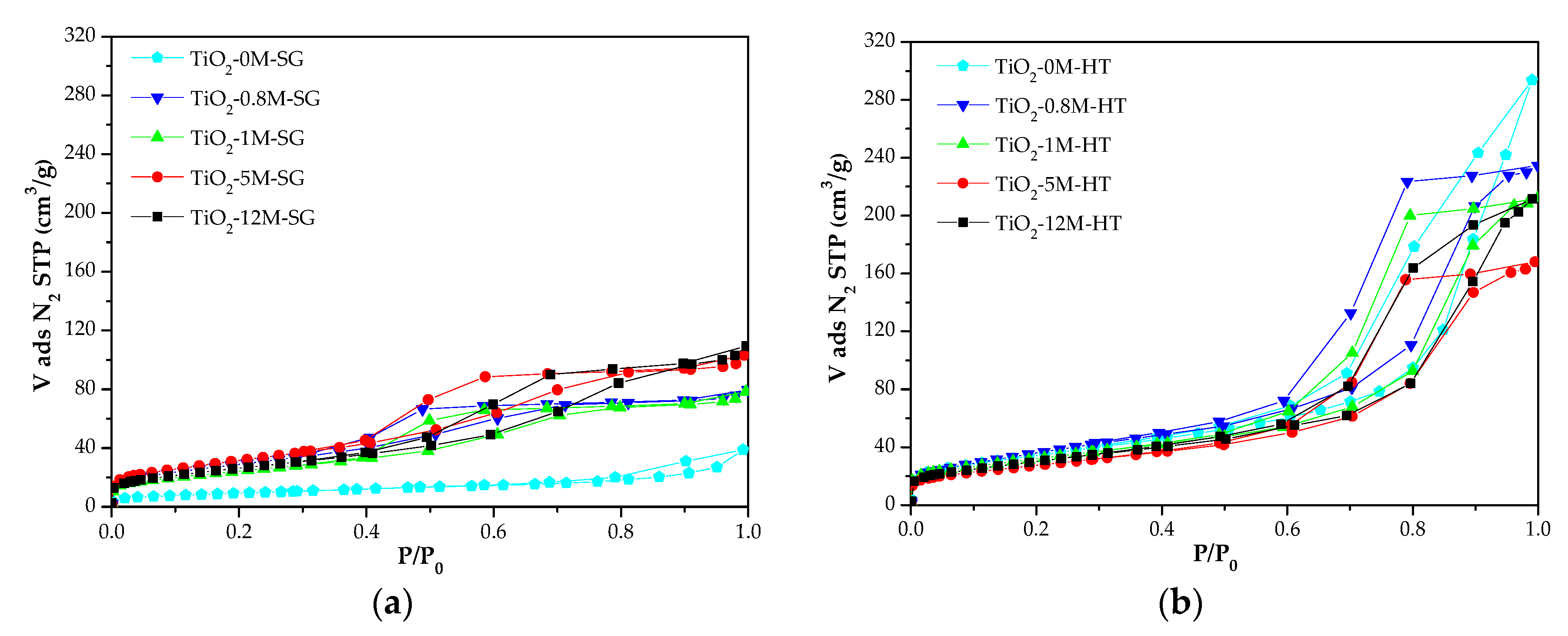

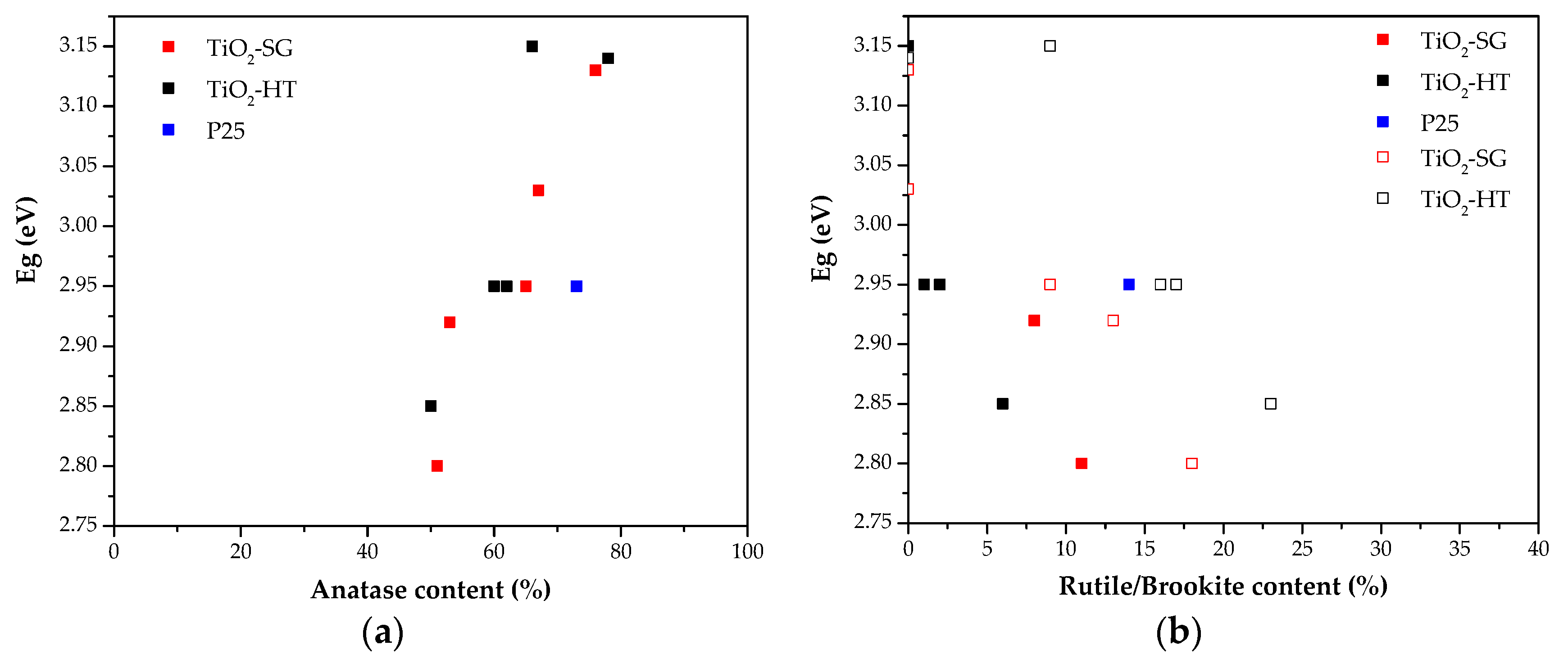
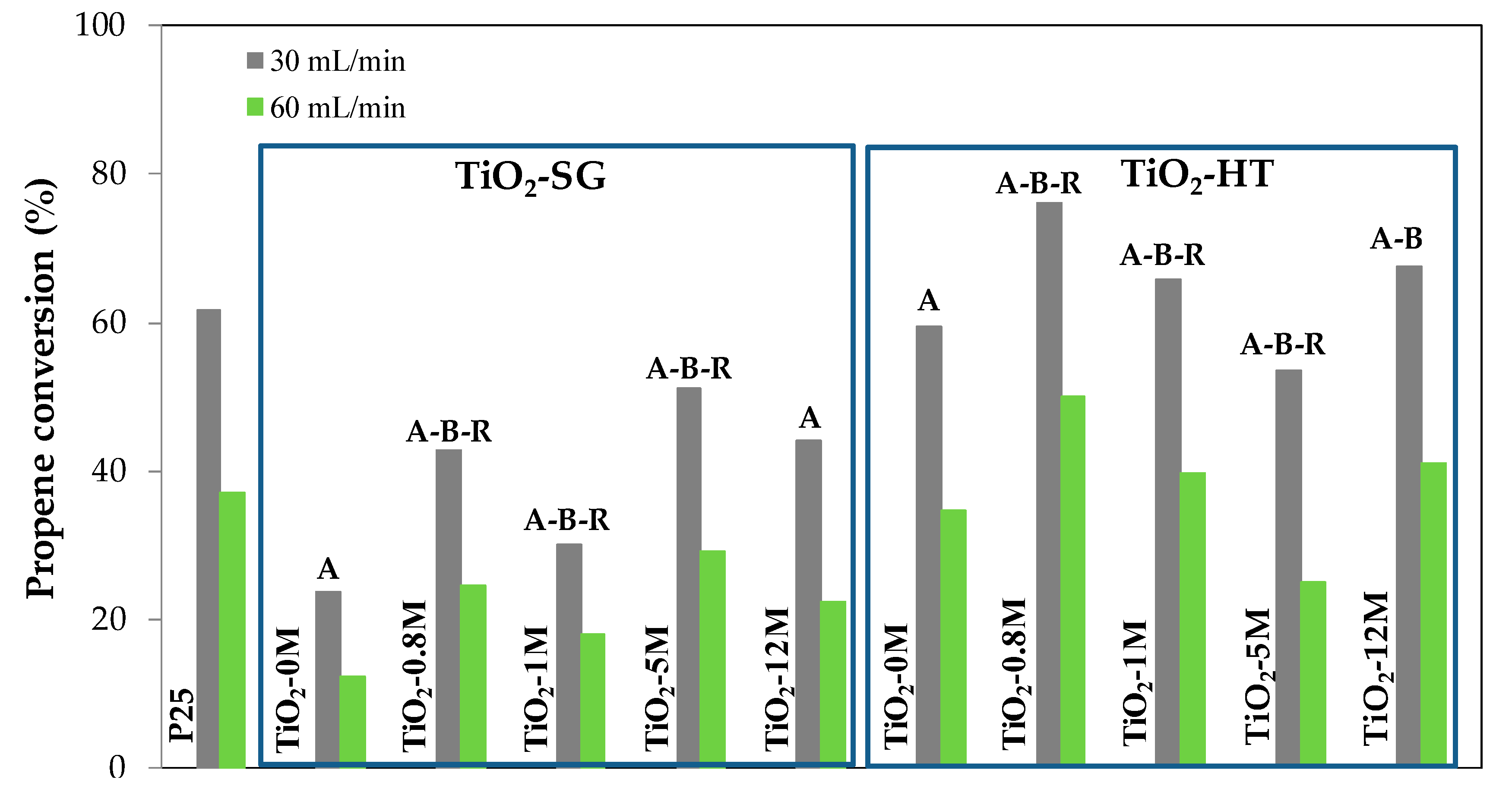
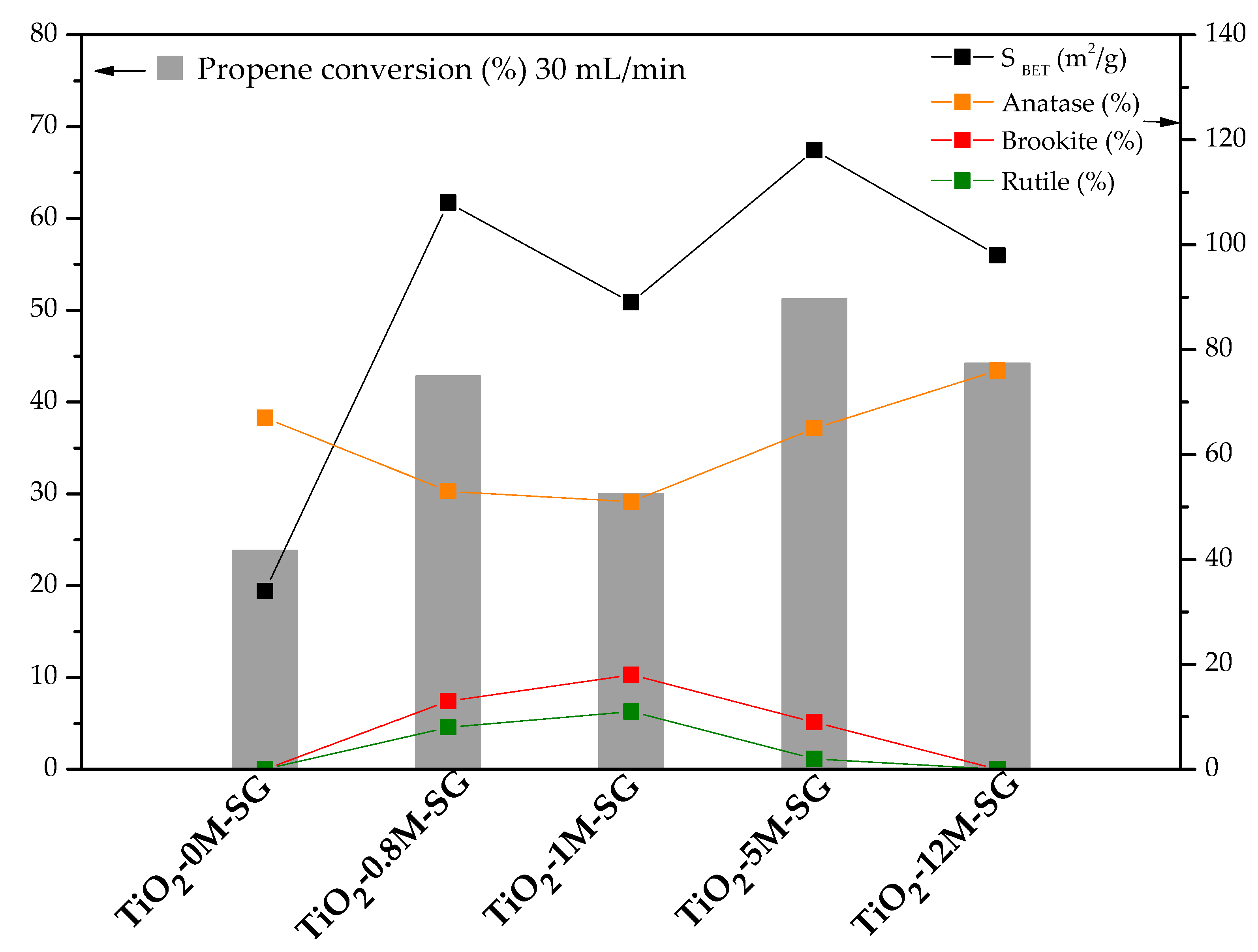
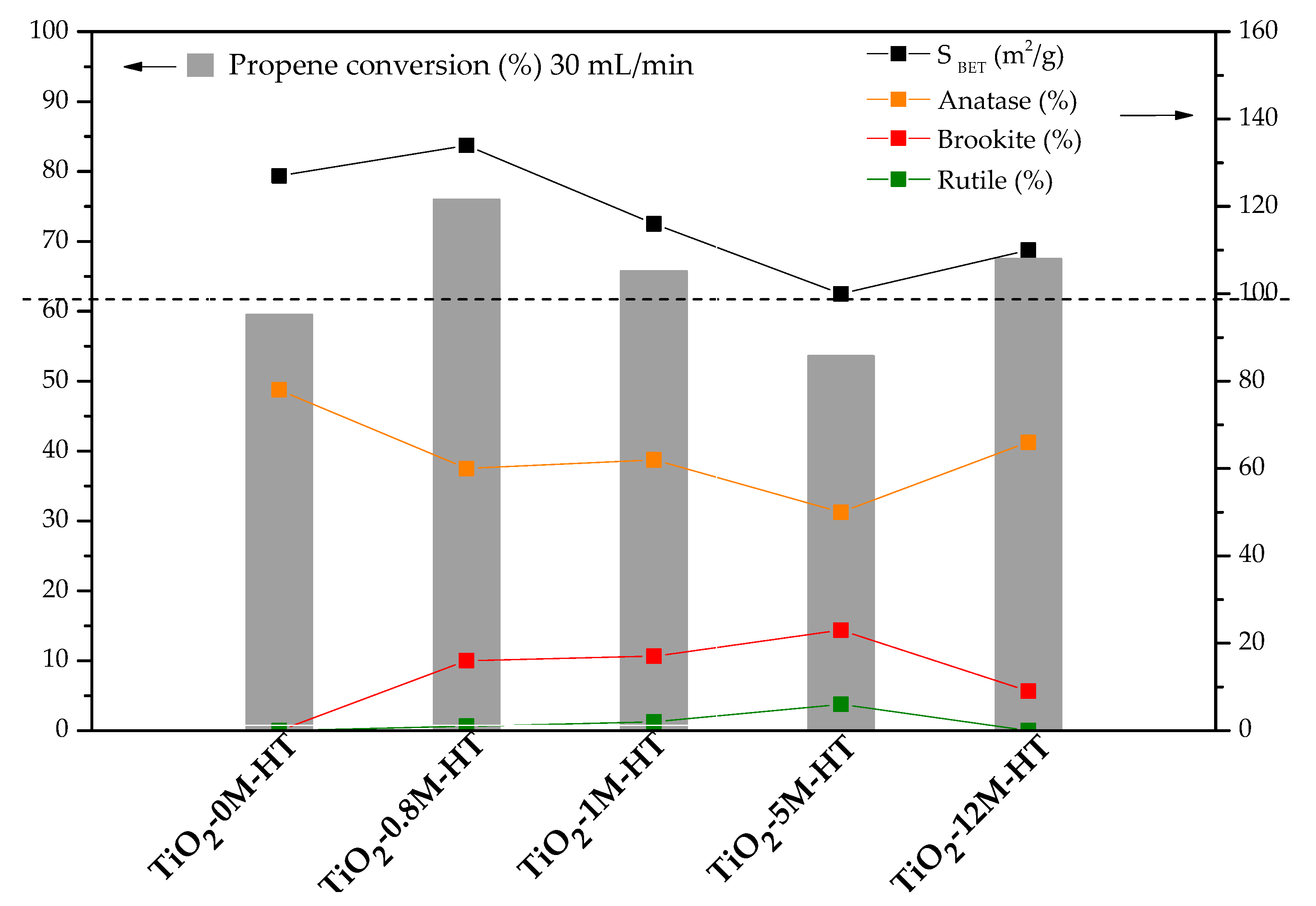
| Sample | SBET (m2/g) | VDR N2 (cm3/g) | Vmeso (cm3/g) | VT (cm3/g) |
|---|---|---|---|---|
| TiO2-0M-SG | 34 | 0.01 | 0.04 | 0.06 |
| TiO2-0.8M-SG | 108 | 0.04 | 0.07 | 0.12 |
| TiO2-1M-SG | 89 | 0.03 | 0.08 | 0.11 |
| TiO2-5M-SG | 118 | 0.04 | 0.10 | 0.16 |
| TiO2-12M-SG | 98 | 0.04 | 0.10 | 0.17 |
| TiO2-0M-HT | 127 | 0.05 | 0.38 | 0.45 |
| TiO2-0.8M-HT | 134 | 0.05 | 0.29 | 0.36 |
| TiO2-1M-HT | 116 | 0.04 | 0.25 | 0.33 |
| TiO2-5M-HT | 100 | 0.04 | 0.20 | 0.26 |
| TiO2-12M-HT | 110 | 0.04 | 0.26 | 0.32 |
| P25 | 55 | 0.02 | 0.07 | 0.18 |
| Sample | Eg (eV) |
|---|---|
| TiO2-0M-SG | 3.03 |
| TiO2-0.8M SG | 2.92 |
| TiO2-1M SG | 2.80 |
| TiO2-5M SG | 2.95 |
| TiO2-12M SG | 3.13 |
| TiO2-0M-HT | 3.14 |
| TiO2-0.8M HT | 2.95 |
| TiO2-1M HT | 2.95 |
| TiO2-5M HT | 2.85 |
| TiO2-12M HT | 3.15 |
| P25 | 2.95 |
| Sample | SBET (m2/g) | Crystalline TiO2 (wt.%) | Average Anatase Crystallite Size (nm) | Propene Conversion (%) |
|---|---|---|---|---|
| TiO2-0M-SG-WT | 450 | - | - | 6.0 |
| TiO2-0M-SG-350 | 34 | A (67) | 9.0 | 24.0 |
| TiO2-0M-HT-WT | 131 | A (75) | 9.0 | 70.0 |
| TiO2-0M-HT-350 | 127 | A (78) | 10 | 60.0 |
| TiO2-0.8M-HT-WT | 147 | A (53)-B (16)-R (1) | 7.0 | 78.0 |
| TiO2-0.8M-HT-350 | 134 | A (60)-B (16)-R (1) | 8.0 | 76.0 |
| TiO2-12M-SG-WT | 160 | A (51) | 5.0 | 68.0 |
| TiO2-12M-SG-350 | 98 | A (76) | 10.0 | 44.0 |
| P25 | 55 | A (73) | 22 | 61.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano-Casanova, L.; Amorós-Pérez, A.; Lillo-Ródenas, M.Á.; Román-Martínez, M.d.C. Effect of the Preparation Method (Sol-Gel or Hydrothermal) and Conditions on the TiO2 Properties and Activity for Propene Oxidation. Materials 2018, 11, 2227. https://doi.org/10.3390/ma11112227
Cano-Casanova L, Amorós-Pérez A, Lillo-Ródenas MÁ, Román-Martínez MdC. Effect of the Preparation Method (Sol-Gel or Hydrothermal) and Conditions on the TiO2 Properties and Activity for Propene Oxidation. Materials. 2018; 11(11):2227. https://doi.org/10.3390/ma11112227
Chicago/Turabian StyleCano-Casanova, Laura, Ana Amorós-Pérez, María Ángeles Lillo-Ródenas, and María del Carmen Román-Martínez. 2018. "Effect of the Preparation Method (Sol-Gel or Hydrothermal) and Conditions on the TiO2 Properties and Activity for Propene Oxidation" Materials 11, no. 11: 2227. https://doi.org/10.3390/ma11112227
APA StyleCano-Casanova, L., Amorós-Pérez, A., Lillo-Ródenas, M. Á., & Román-Martínez, M. d. C. (2018). Effect of the Preparation Method (Sol-Gel or Hydrothermal) and Conditions on the TiO2 Properties and Activity for Propene Oxidation. Materials, 11(11), 2227. https://doi.org/10.3390/ma11112227







