Photocatalytic Oxidation of VOCs in Gas Phase Using Capillary Microreactors with Commercial TiO2 (P25) Fillings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
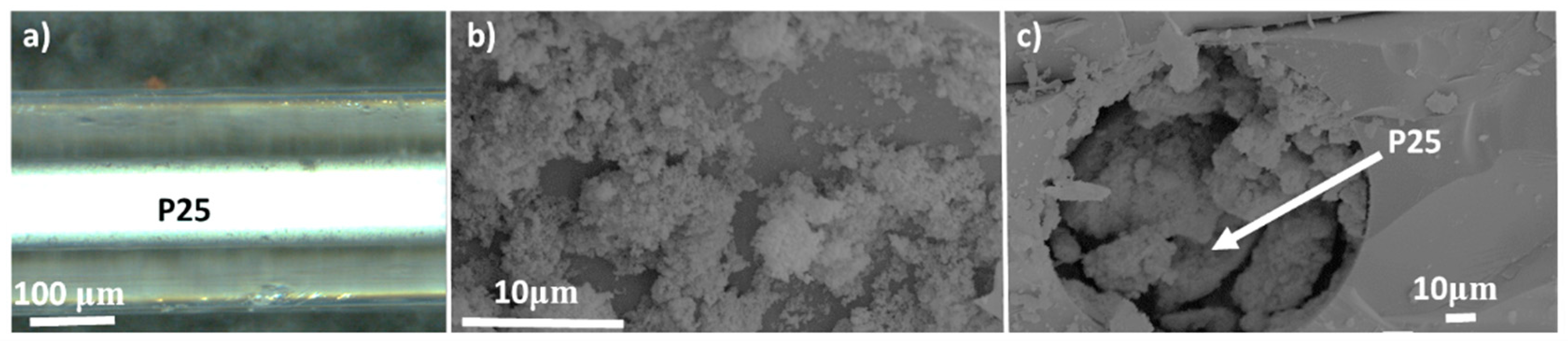
2.2. Incorporation of P25 Inside Silica Capillary
2.3. Sample Characterization
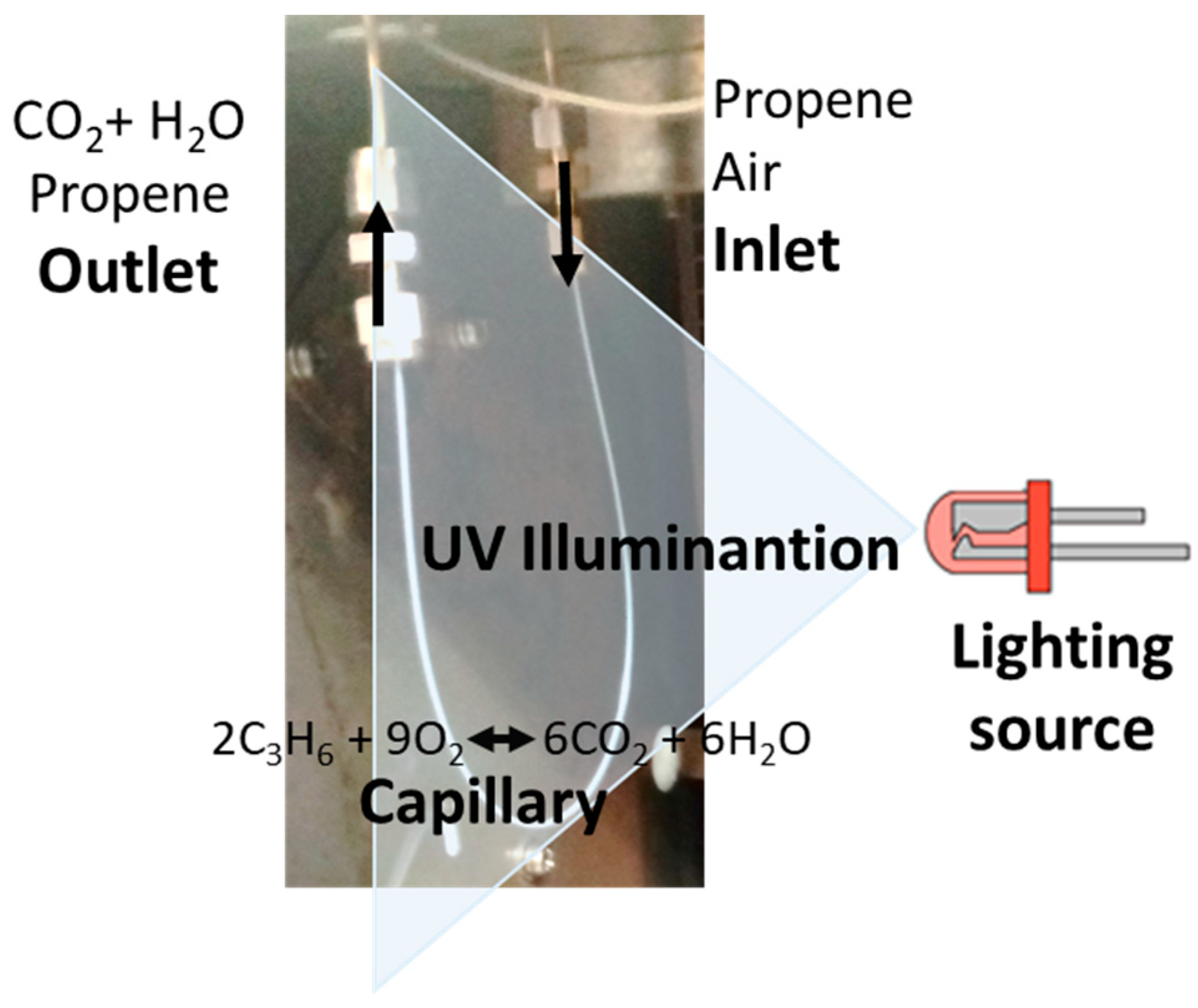
2.4. Catalytic Tests.
3. Results and Discussion
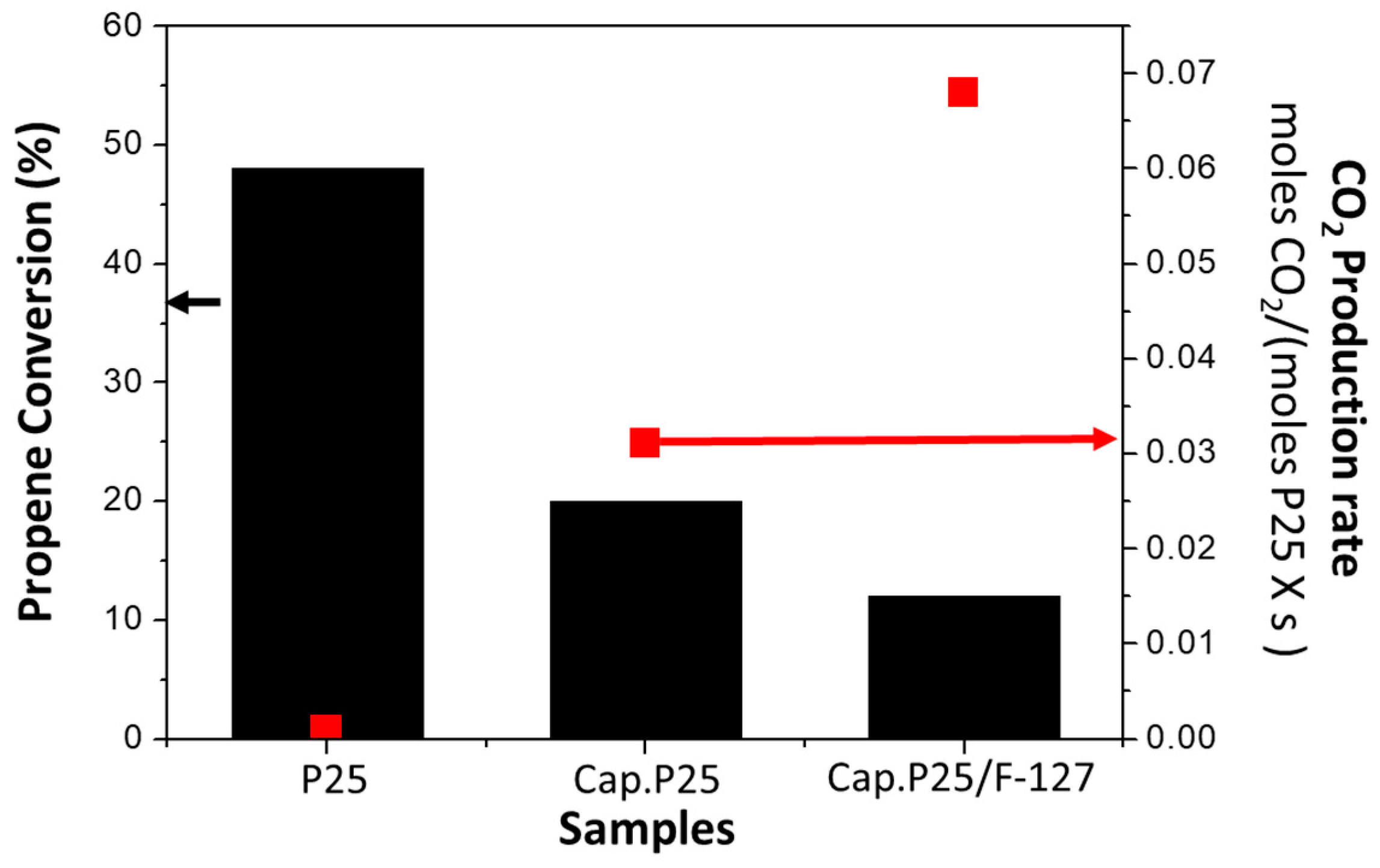
3.1. Study of the Capillary Filled with P25 (Cap. P25)
3.1.1. Characterization of the Capillary Filled with P25 (Cap. P25)
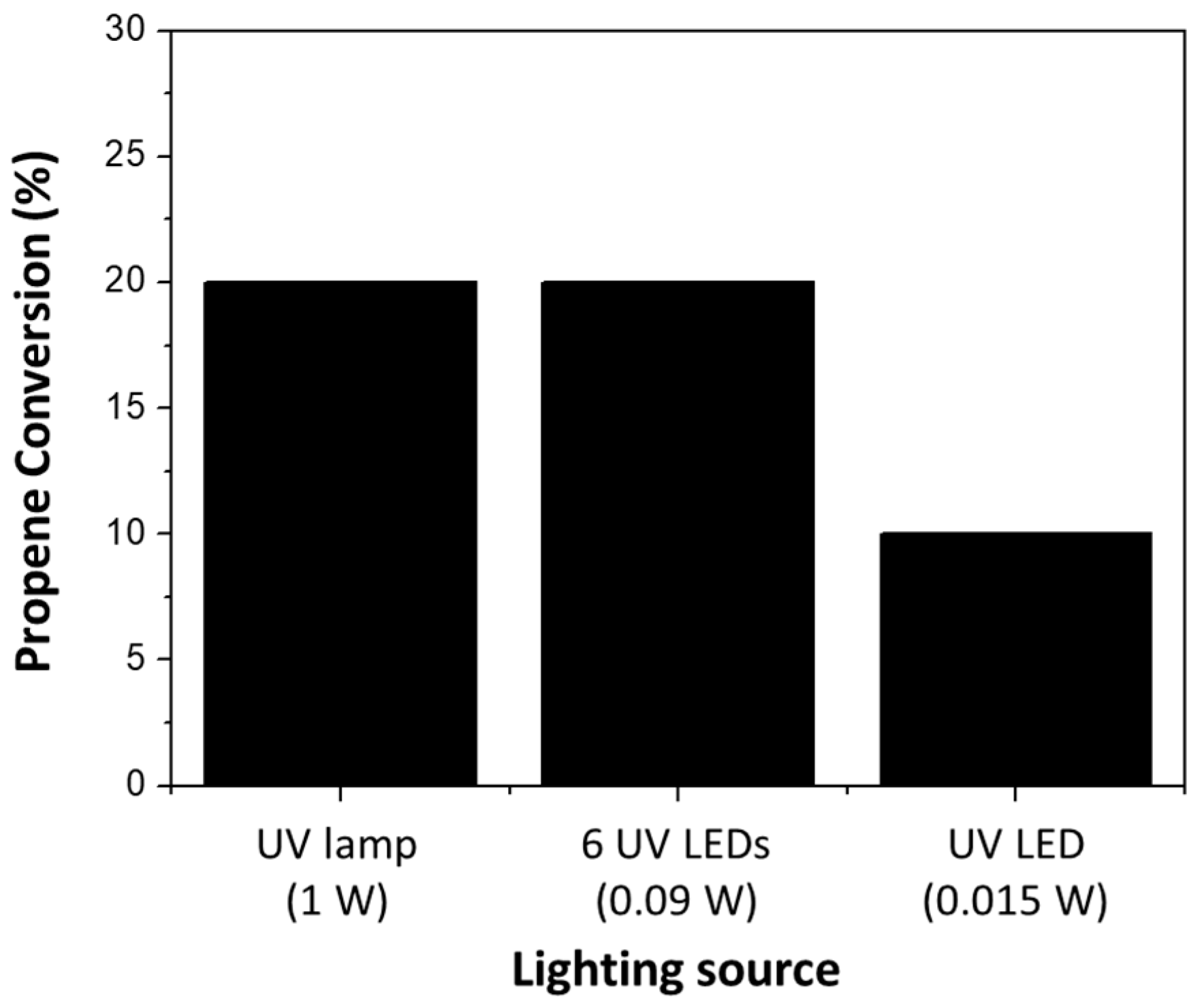
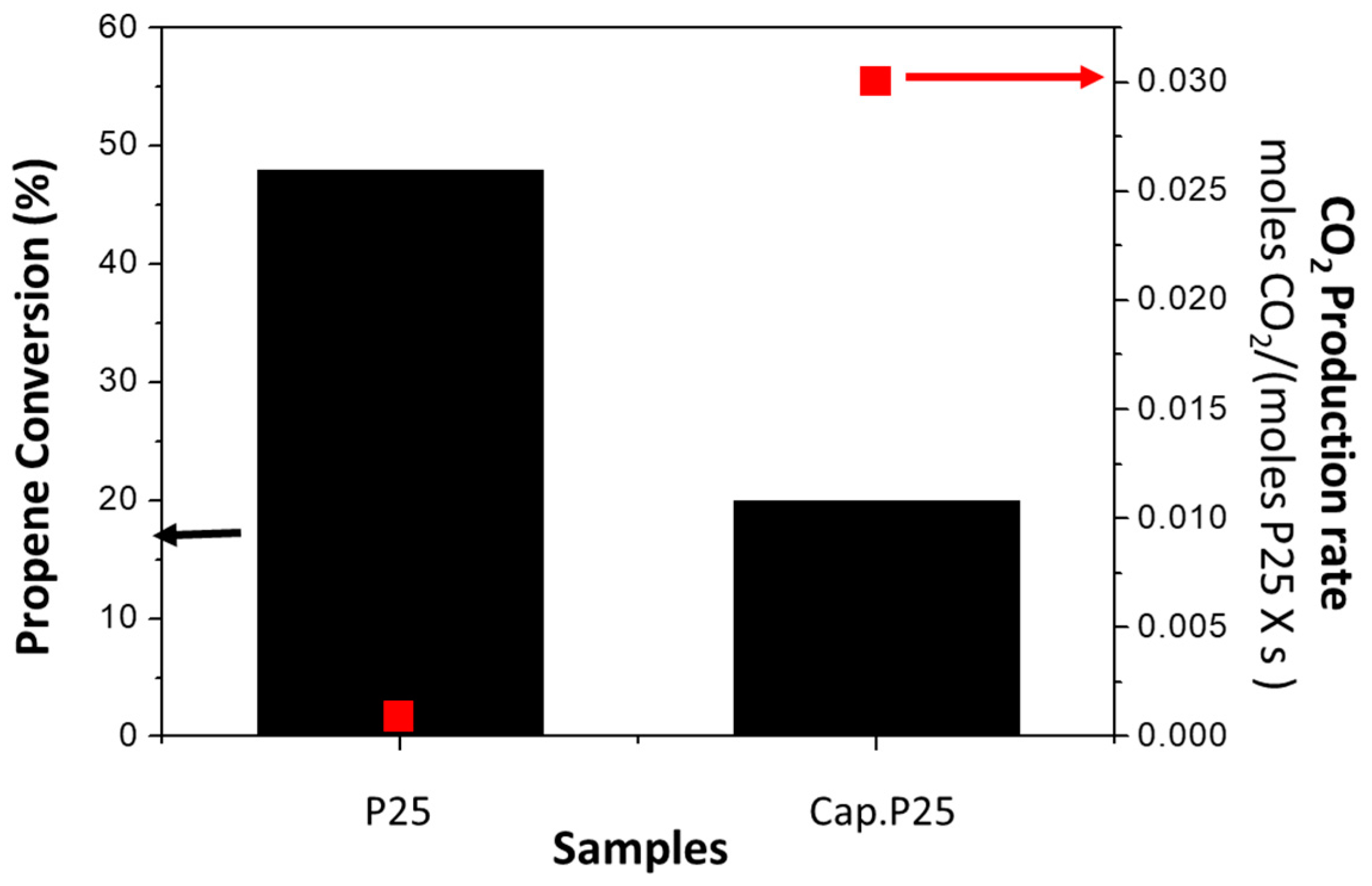
3.1.2. Photocatalytic Activity of the Capillary Filled with P25 (Cap. P25)
 6CO2 + 6H2O
6CO2 + 6H2O
3.2. Study of the Capillary Filled with P25 and F-127(Cap. P25/F-127)
3.2.1. Characterization of the Capillary Filled with P25 and F-127(Cap. P25/F-127)
3.2.2. Photocatalytic Activity of the Capillary Filled with P25 Using Surfactant (Cap. P25/F-127)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rafson Odor, H.J. VOC Control Handbook, 1st ed.; McGraw Hill: New York, NY, USA, 1998. [Google Scholar]
- Manahan, S.E. Environmental Chemistry, 8th ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Myers, I.; Maynard, R.L. Polluted air—Outdoors and indoors. Occup. Med. 2005, 55, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Lillo-Ródenas, M.A.; Bouazza, N.; Berenguer-Murcia, A.; Linares-Salinas, J.J.; Soto, P.; Linares-Solano, A. Photocatalytic oxidation of propene at low concentration. Appl. Catal. B Environ. 2007, 71, 298–309. [Google Scholar] [CrossRef]
- Fernánadez-Catalá, J.; Cano-Casanova, L.; Lillo-Ródenas, M.A.; Berenguer-Murcia, A.; Cazorla-Amorós, D. Synthesis of TiO2 with Hierarchical Porosity for the Photooxidation of Propene. Molecules 2017, 22, 2243. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Okura, I. Photocatalysis: Science and Technology, 1st ed.; Springer: Berlin, Germany, 2002. [Google Scholar]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Shayegan, Z.; Lee, C.S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Ryu, J.; Choi, W. Substrate-Specific photocatalytic activities of TiO2 and multiactivity test for water treatment application. Environ. Sci. Technol. 2008, 42, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Šalić, A.; Tušek, A.; Zelić, B. Application of microreactors in medicine and biomedicine. J. Appl. Biomed. 2012, 10, 137–153. [Google Scholar] [CrossRef]
- Rebrov, E.V.; Berenguer-Murcia, A.; Skelton, H.E.; Johnson, B.F.G.; Wheatley, A.E.H.; Schouten, J.C. Capillary microreactors wall-coated with mesoporous titania thin film catalyst supports. Lab Chip 2009, 9, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Elvira, K.S.; Solvas, X.C.; Wootton, R.C.R.; deMello, A.J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 2013, 5, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Watts, P.; Wiles, C. Recent advances in synthetic micro reaction technology. Chem. Commun. 2007, 5, 443–467. [Google Scholar] [CrossRef] [PubMed]
- Colmenares, J.C.; Varma, R.S.; Nair, V. Selective photocatalysis of lignin-inspired chemicals by integrating hybrid nanocatalysis in microfluidic reactors. Chem. Soc. Rev. 2017, 2, 6675–6686. [Google Scholar] [CrossRef] [PubMed]
- Tanimu, A.; Jaenicke, S.; Alhooshani, K. Heterogeneous catalysis in continuous flow microreactors: A review of methods and applications. Chem. Eng. J. 2017, 327, 792–821. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Y.; Du, L.; Liu, J.; Yao, J. Review of the applications of microreactors. Renew. Sustain. Energy Rev. 2015, 47, 519–539. [Google Scholar] [CrossRef]
- Oelgemöller, M.; Shvydkiv, O. Recent advances in microflow photochemistry. Molecules 2011, 16, 7522–7550. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, I.; Habba, Y.G.; Capochichi-Gnambodoe, M.; Marty, F.; Vial, J.; Leprince-Wang, Y.; Bourouina, T. Zinc oxide nano-enabled microfluidic reactor for water purification and its applicability to volatile organic compounds. Microsyst. Nanoeng. 2018, 4, 17093. [Google Scholar] [CrossRef]
- Kolb, G.; Hessel, V. Micro-structured reactors for gas phase reactions. Chem. Eng. J. 2004, 98, 1–38. [Google Scholar] [CrossRef]
- Munirathinam, R.; Huskens, J.; Verboom, W. Supported catalysis in continuous-flow microreactors. Adv. Synth. Catal. 2015, 357, 1093–1123. [Google Scholar] [CrossRef]
- Shen, C.; Wang, Y.J.; Xu, J.H.; Luo, G.S. Glass capillaries with TiO2 supported on inner wall as microchannel reactors. Chem. Eng. J. 2015, 277, 48–55. [Google Scholar] [CrossRef]
- Miguel-García, I.; Navlani-García, M.; García-Aguilar, J.; Berenguer-Murcia, Á.; Lozano-Castelló, D.; Cazorla-Amorós, D. Capillary microreactors based on hierarchical SiO2 monoliths incorporating noble metal nanoparticles for the Preferential Oxidation of CO. Chem. Eng. J. 2015, 275, 71–78. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, S.S.H.; Niu, R.; Xu, L.; Lu, Y.; Cao, J.; Lee, S. Removal of indoor volatile organic compounds via photocatalytic oxidation: A short review and prospect. Molecules 2016, 21, 56. [Google Scholar] [CrossRef] [PubMed]

| Samples | Mass of P25 (mg) | Propene Flux (mL/min) | Residence Time (mg·min/mL) | Pressure Drop (bar) |
|---|---|---|---|---|
| Cap. P25 | 0.116 | 0.35 | 0.33 | 4.5 |
| Cap. P25/F-127 | 0.103 | 1 | 0.103 | 0.5 |
| P25 in conventional reactor | 110 | 30 | 3.66 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Catalá, J.; Berenguer-Murcia, Á.; Cazorla-Amorós, D. Photocatalytic Oxidation of VOCs in Gas Phase Using Capillary Microreactors with Commercial TiO2 (P25) Fillings. Materials 2018, 11, 1149. https://doi.org/10.3390/ma11071149
Fernández-Catalá J, Berenguer-Murcia Á, Cazorla-Amorós D. Photocatalytic Oxidation of VOCs in Gas Phase Using Capillary Microreactors with Commercial TiO2 (P25) Fillings. Materials. 2018; 11(7):1149. https://doi.org/10.3390/ma11071149
Chicago/Turabian StyleFernández-Catalá, Javier, Ángel Berenguer-Murcia, and Diego Cazorla-Amorós. 2018. "Photocatalytic Oxidation of VOCs in Gas Phase Using Capillary Microreactors with Commercial TiO2 (P25) Fillings" Materials 11, no. 7: 1149. https://doi.org/10.3390/ma11071149
APA StyleFernández-Catalá, J., Berenguer-Murcia, Á., & Cazorla-Amorós, D. (2018). Photocatalytic Oxidation of VOCs in Gas Phase Using Capillary Microreactors with Commercial TiO2 (P25) Fillings. Materials, 11(7), 1149. https://doi.org/10.3390/ma11071149







