Preparation and Characterization of Phenolic Foam Modified with Bio-Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.2.1. Synthesis and Characterization of BPRs
2.2.2. Preparation and Characterization of BPFs
2.3. Analysis
3. Results and Discussion
3.1. Components of the Whole Bio-Oil
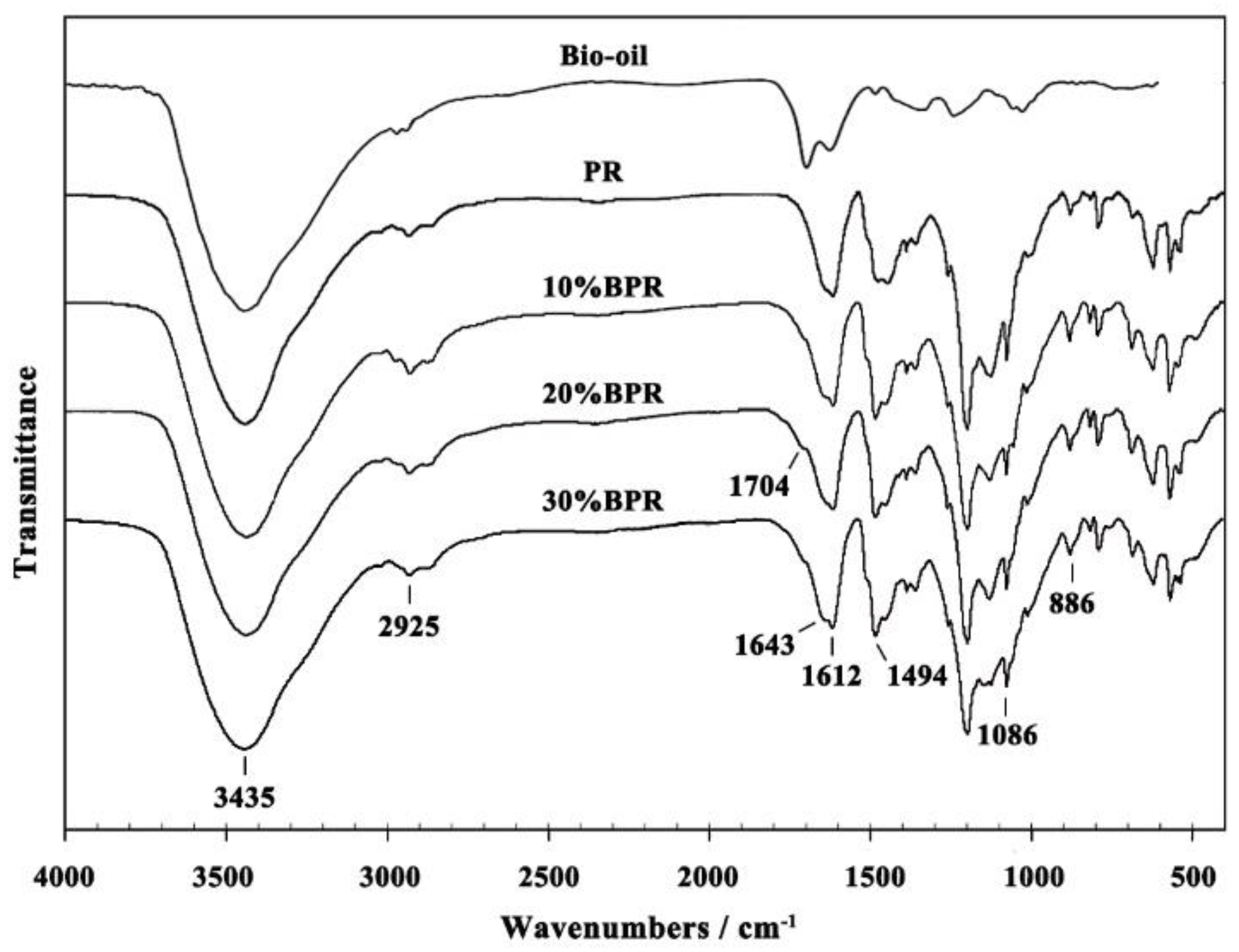
3.2. FT-IR Analysis of BPRs
3.3. Characteristics of BPFs
3.3.1. Microstructure of BPFs
3.3.2. Basic Characteristics of BPFs
3.3.3. Thermal Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liang, B.; Li, X.; Hua, L.; Bo, C.; Zhou, J.; Zhou, Y. Foaming resol resin modified with polyhydroxylated cardanol and its application to phenolic foams. Ind. Crop. Prod. 2016, 80, 194–196. [Google Scholar] [CrossRef]
- Bo, C.; Wei, S.; Hu, L.; Jia, P.; Liang, B.; Zhou, J.; Zhou, Y. Synthesis of a cardanol-based phosphorus-containing polyurethane prepolymer and its application in phenolic foams. RSC Adv. 2016, 6, 62999–63005. [Google Scholar] [CrossRef]
- Saz-Orozco, B.D.; Alonso, M.V.; Oliet, M.; Dominguez, J.C.; Rodriguez, F. Mechanical, thermal and morphological characterization of cellulose fiber-reinforced phenolic foams. Compos. Part B Eng. 2015, 75, 367–372. [Google Scholar] [CrossRef]
- Hu, X.; Wang, D.; Cheng, W.; Zhou, G. Effect of polyethylene glycol on the mechanical property, microstructure, thermal stability, and flame resistance of phenol-urea-formaldehyde foams. J. Mater. Sci. 2014, 49, 1556–1565. [Google Scholar] [CrossRef]
- Yang, Z.; Yuan, L.; Gu, Y.; Li, M.; Sun, Z.; Zhang, Z. Improvement in Mechanical and thermal properties of phenolic foam reinforced with multiwalled carbon nanotubes. J. Appl. Polym. Sci. 2013, 130, 1479–1488. [Google Scholar] [CrossRef]
- Saz-Orozco, B.D.; Oliet, M.; Alonso, M.V.; Rojo, E.; Rodríguez, F. Formulation optimization of unreinforced and lignin nanoparticle-reinforced phenolic foams using an analysis of variance approach. Compos. Sci. Technol. 2012, 72, 667–674. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Z. Phenolic foams, modified by nano-metallic oxides, improved in mechanical strengths and friability. Iran. Polym. J. 2016, 25, 579–587. [Google Scholar] [CrossRef]
- Song, S.A.; Chung, Y.S.; Kim, S.S. The mechanical and thermal characteristics of phenolic foams reinforced with carbon nanoparticles. Compos. Sci. Technol. 2014, 103, 85–93. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, Z.; Chen, Y.; Wei, D.; Xu, T. Fabrication and mechanical properties of phenolic foam reinforced with graphene oxid. Polym. Compos. 2014, 35, 581–586. [Google Scholar] [CrossRef]
- Choe, J.; Kim, M.; Kim, J.; Lee, D.G. A microwave foaming method for fabricating glass fiber reinforced phenolic foam. Compos. Struct. 2016, 152, 239–246. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, Z.; Chen, Y.; Wei, D.; Wu, Y. Thermomechanical analyses of phenolic foam reinforced with glass fiber mat. Mater. Des. 2013, 51, 131–135. [Google Scholar] [CrossRef]
- Liu, L.; Fu, M.; Wang, Z. Synthesis of boron-containing toughening agents and their application in phenolic foams. Ind. Eng. Chem. Res. 2015, 54, 1962–1970. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Yuan, H.; Song, L.; Hu, Y.; Yuen, R.K. Fire performance and mechanical properties of phenolic foams modified by phosphorus-containing polyethers. J. Polym. Res. 2012, 19, 1–10. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Yu, B.; Yuan, H.; Song, L.; Hu, Y.; Yuen, R.K.; Yeoh, G.H. A novel polyurethane prepolymer as toughening agent: Preparation, characterization, and its influence on mechanical and flame retardant properties of phenolic foam. J. Appl. Polym. Sci. 2013, 128, 2720–2728. [Google Scholar] [CrossRef]
- Saz-Orozco, B.D.; Alonso, M.V.; Oliet, M.; Domínguez, J.C.; Rojo, E.; Rodriguez, F. Lignin particle- and wood flour-reinforced phenolic foams: Friability, thermal stability and effect of hygrothermal aging on mechanical properties and morphology. Compos. Part B 2015, 80, 154–161. [Google Scholar] [CrossRef]
- Hu, L.; Zhou, Y.; Liu, R.; Zhang, M.; Yang, X. Synthesis of foaming resol resin modified with oxidatively degraded lignosulfonate. Ind. Crop. Prod. 2013, 44, 364–366. [Google Scholar] [CrossRef]
- Meikleham, J.; Pizzi, A. Acid- and alkali-catalyzed tannin-based rigid foams. J. Appl. Polym. Sci. 1994, 53, 1547–1556. [Google Scholar] [CrossRef]
- Lagel, M.C.; Pizzi1, A.; Giovando, S.; Celzard, A. Development and characterisation of phenolic foams with phenol-formaldehyde-chestnut tannins resin. J. Renew. Mater. 2014, 2, 220–229. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Ye, D.; Cai, L.; Shi, S.Q. Catalytic pyrolysis of larch sawdust for phenol-rich bio-oil using different catalysts. Renew. Energy 2018, 121, 146–152. [Google Scholar] [CrossRef]
- Kim, J.S. Prodution, separation and applications of phenolic-rich bio-oil—A review. Bioresour. Technol. 2015, 178, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Asadullah, M.; Rasid, N.S.; Kadir, S.A.; Azdarpour, A. Production and detailed characterization of bio-oil from fast pyrolysis of palm kernel shell. Biomass Bioenergy 2013, 59, 316–324. [Google Scholar] [CrossRef]
- Sukhbaatar, B.; Steele, P.H.; Kima, M.G. Use of lignin separated form bio-oil in oriented strand board binder phenol-formaldehyde resin. BioResources 2009, 4, 789–804. [Google Scholar]
- Choi, G.G.; Oh, S.J.; Lee, S.J. Production of bio-based phenolic resin and activated carbon from bio-oil and biochar derived from fast pyrolysis of palm kernel shells. Bioresour. Technol. 2014, 178, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Chang, J.; Gou, J.; Xia, B.; Ren, X. On the cure acceleration of oil-phenol-formaldehyde resins with different catalysts. J. Adhes. 2010, 86, 834–843. [Google Scholar] [CrossRef]
- Choi, M.H.; Byun, H.Y.; Chung, I.J. The effect of chain length of flexible diacid on morphology and mechanical property of modified phenolic resin. Polymer 2002, 43, 4437–4444. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, P.; Chen, C.; Chang, J.; Li, L. Formaldehyde emission behavior of plywood with phenol-formaldehyde resin modified by bio-oil under radiant floor heating condition. Build. Environ. 2018, 144, 565–572. [Google Scholar] [CrossRef]
- Aslan, M.; Özbay, G.; Ayrilmis, N. Adhesive characteristics and bonding performance of phenol formaldehyde modified with phenol-rich fraction of crude bio-oil. J. Adhes. Sci. Technol. 2015, 29, 2679–2691. [Google Scholar] [CrossRef]
- Özbay, G.; Ayrilmis, N. Bonding performance of wood bonded with adhesive mixtures composed of phenol-formaldehyde and bio-oil. Ind. Crop. Prod. 2015, 66, 68–72. [Google Scholar]
- Chaouch, M.; Diouf, P.N.; Laghdir, A.; Yin, S. Bio-oil from whole-tree feedstock in resol-type phenolic resins. J. Appl. Polym. Sci. 2014, 131, 596–602. [Google Scholar] [CrossRef]



| Resins | Viscosity (25 °C, mPa·s) | Solids Content (%) | Curing Time (75 °C, s) |
|---|---|---|---|
| PR | 1743 ± 67 | 79.8 ± 0.4 | 698 ± 29 |
| 10%BPR | 2852 ± 35 | 80.3 ± 0.3 | 823 ± 32 |
| 20%BPR | 3889 ± 48 | 78.6 ± 0.3 | 1062 ± 43 |
| 30%BPR | 3205 ± 81 | 74.7 ± 0.5 | 1605 ± 35 |
| Compounds | Molecular Formula | Peak Area (%) |
|---|---|---|
| Phenols | 33.08 | |
| Phenol | C6H6O | 4.23 |
| Cresols | C7H8O | 3.59 |
| Catechol | C6H6O2 | 1.11 |
| Guaiacol | C7H8O2 | 2.67 |
| 4-methylcatechol | C7H8O2 | 2.50 |
| 2-methoxy-4-methylphenol | C8H10O2 | 3.14 |
| 4-ethylresorcinol | C8H10O2 | 1.84 |
| 4-ethylguaiacol | C9H12O2 | 1.14 |
| 3,4-dimethoxyphenol | C8H10O3 | 2.10 |
| Eugenol | C10H12O2 | 1.27 |
| 4-allyl-2,6-dimethoxyphenol | C11H14O3 | 1.80 |
| Other phenols | 7.68 | |
| Ketones | 17.68 | |
| Hydroxyacetone | C3H6O2 | 4.08 |
| 2-butanone | C4H8O | 1.94 |
| 4-hydroxyacetophenone | C8H8O2 | 1.46 |
| Acetovanillone | C9H10O3 | 1.22 |
| 2,4-dimethoxyacetophenone | C10H12O3 | 1.62 |
| Other ketones | 7.36 | |
| Aldehydes | 11.18 | |
| Acetaldehyde | C2H4O2 | 4.91 |
| 2-Furaldehyde | C5H4O2 | 2.08 |
| Vanillin | C8H8O3 | 1.27 |
| Syringaldehyde | C9H10O4 | 0.86 |
| Other aldehydes | 2.07 | |
| Sugars | 10.35 | |
| D-Mannose | C6H12O6 | 6.53 |
| β-D-lactose | C6H12O6 | 1.22 |
| Other sugers | 2.60 | |
| Acids | 9.24 | |
| Acetic acid | C2H4O2 | 2.97 |
| 4-hydroxybenzoic acid | C7H6O3 | 1.84 |
| Homovanillic acid | C9H10O4 | 1.22 |
| 4-methylnonanoic acid | C10H20O2 | 1.17 |
| Nonadecanoic acid | C19H38O2 | 0.94 |
| Other acids | 1.10 | |
| Esters | 6.75 | |
| Methyl acetate | C3H6O2 | 1.94 |
| Ethyl methacrylate | C6H10O2 | 1.03 |
| Ethylene glycol diacetate | C6H10O4 | 1.59 |
| Octyl acetate | C10H20O2 | 1.20 |
| Other esters | 1.00 | |
| Alcohols | 5.89 | |
| Ethylene glycol | C2H6O2 | 1.03 |
| Furfuryl alcohol | C5H6O2 | 1.60 |
| 4-hydroxychroman | C9H10O2 | 1.17 |
| Heneicosyl alcohol | C21H44O | 0.94 |
| Other alcohols | 1.15 | |
| Others | 5.81 | |
| Total | 100.00 |
| Wave Number (cm−1) | Vibration | Assignment |
|---|---|---|
| 3435 | ν (OH) | Phenolic OH and aliphatic OH stretching vibration |
| 2925 | ν (CH2) | Aliphatic CH2 asymmetric stretching vibration |
| 1704, 1643 | ν (C=O) | (Phenolic) C=O stretching vibration |
| 1612, 1494 | ν (C=C) | C=C aromatic ring stretching vibration |
| 1086 | ν (C–O–C) | Phenolic C–O–C stretching vibration |
| Foams | Apparent Density (kg·m−3) | Pulverization Ratio (%) | Compressive Strength (MPa) | Flexural Strength (MPa) |
|---|---|---|---|---|
| PF | 49.2 ± 1.9 | 14.5 ± 0.4 | 0.19 ± 0.03 | 0.24 ± 0.03 |
| 10%BPF | 53.2 ± 1.3 | 12.4 ± 0.2 | 0.21 ± 0.04 | 0.30 ± 0.04 |
| 20%BPF | 58.1 ± 1.6 | 8.9 ± 0.2 | 0.28 ± 0.05 | 0.36 ± 0.02 |
| 30%BPF | 45.6 ± 0.9 | 13.6 ± 0.3 | 0.16 ± 0.02 | 0.19 ± 0.02 |
| Foams | T−5% (°C) | Tmas (°C) | Residue at 600 °C (%) |
|---|---|---|---|
| PF | 126 | 505 | 70.27 |
| 10%BPF | 169 | 495 | 70.10 |
| 20%BPF | 156 | 489 | 65.22 |
| 30%BPF | 103 | 482 | 59.79 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Wang, Y.; Xu, P.; Chang, J. Preparation and Characterization of Phenolic Foam Modified with Bio-Oil. Materials 2018, 11, 2228. https://doi.org/10.3390/ma11112228
Yu Y, Wang Y, Xu P, Chang J. Preparation and Characterization of Phenolic Foam Modified with Bio-Oil. Materials. 2018; 11(11):2228. https://doi.org/10.3390/ma11112228
Chicago/Turabian StyleYu, Yuxiang, Yufei Wang, Pingping Xu, and Jianmin Chang. 2018. "Preparation and Characterization of Phenolic Foam Modified with Bio-Oil" Materials 11, no. 11: 2228. https://doi.org/10.3390/ma11112228
APA StyleYu, Y., Wang, Y., Xu, P., & Chang, J. (2018). Preparation and Characterization of Phenolic Foam Modified with Bio-Oil. Materials, 11(11), 2228. https://doi.org/10.3390/ma11112228





