Cationic Substitutions in Hydroxyapatite: Current Status of the Derived Biofunctional Effects and Their In Vitro Interrogation Methods
Abstract
:1. Introduction
2. Preparation Methods and Synthesis Routes of Hydroxyapatite Materials
2.1. Preparation of Bulk Hydroxyapatite (HA) from Natural Resources
2.2. Synthesis of Bulk Synthetic Substituted HA
2.3. Fabrication of Substituted HA Coatings
3. Cation-Substituted Hydroxyapatites
3.1. s-Block Cation-Substituted Hydroxyapatites
3.2. p-Block Cation-Substituted Hydroxyapatites
3.3. d-Block Cation-Substituted Hydroxyapatites
3.4. f-Block Cation-Substituted Hydroxyapatites
3.5. Cytotoxic Concentration of Cationic Species
4. Rigorous In Vitro Testing of Bioactive Materials
- ISO 10993-14:2001—Biological Evaluation of Medical Devices—Part 14: Identification and Quantification of Degradation Products from Ceramics.Medium for extreme tests: buffered citric acid solution, pH = 3.0 ± 0.2 at a temperature of 37 ± 1 °C, in normal atmosphere;Solution for simulated tests: buffered tris(hydroxymethyl)aminomethane (Tris)-HCl solution, pH = 7.4 ± 0.1 at a temperature of 37 ± 1 °C, in normal atmosphere.
- ISO 16428:2005—Implants for Surgery—Test Solutions and Environmental Conditions for Static and Dynamic Corrosion Tests on Implantable Materials and Medical Devices.Medium: aqueous solution of sodium chloride (0.9% NaCl mass fraction) or Ringer’s solution isotonic aqueous solution of NaCl, pH = 7.0 at a temperature of 37 ± 1 °C, in normal atmosphere.
- ISO 16429:2004—Implants for Surgery—Measurements of Open-Circuit Potential to Assess Corrosion Behaviour of Metallic Implantable Materials and Medical Devices over Extended Time Periods.Medium: aqueous solution of sodium chloride (0.9% NaCl mass fraction), pH = 7.0 at a temperature of 37 ± 1 °C, in normal atmosphere. For more stringent test conditions, more acidic test solutions are recommended.
- ISO 23317:2014—Implants for surgery—In vitro Evaluation for Apatite-Forming Ability of Implant Materials. (i.e., Bioactivity/Biomineralization Capacity Testing).Medium: Tris-buffered simulated body fluid (ionic concentration in mM: 142.0 Na+, 5.0 K+, 1.5 Mg2+, 2.5 Ca2+, 147.8 Cl−, 4.2 HCO3−, 1.0 HPO42−, and 0.5 SO42−), pH = 7.4 at a temperature of 36.5 ± 0.2 °C, in normal atmosphere.
- ISO 10993-5:2009—Biological Evaluation of Medical Devices—Part 5: Tests for in vitro Cytotoxicity.Medium: culture medium (e.g., Dulbecco’s Modified Eagle Medium) with or without serum such as to meet the growth requirements of the selected cell line, pH = 7.4 at a temperature of (37 ± 1) °C, in a humidified atmosphere of 5% CO2.
- ISO 22196:2011—Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces.Medium for suspension assays: nutrient broth (containing meat extract, peptone, NaCl), at a temperature of (35 ± 1) °C and a relative humidity of not less than 90% for 24 ± 1 h, in normal atmosphere.
4.1. Biomineralization Capability (Bioactivity Testing)
4.2. Degradation and Corrosion Tests
- Using pure inorganic fluids for testing (i.e., citric acid, (Tris)-HCl, 0.9% NaCl, Ringer’s solutions) is not a viable choice because, as presented before, the organic component of the intercellular fluid interacts with the implant surface and greatly modifies the interactions with the biomaterial. The use of a suitable testing environment is of foremost importance since these specific material features (degradation rate and corrosion resistance) are dependent on the material surface properties and its ability to adsorb organic moieties, partial dissolution and the consequent ionic exchanges.
- In the attempt to compress the time needed for a degradation test and peek into the future, the ISO 10993-14:2001 standard uses buffered citric acid solution (at a pH = 3.0 ± 0.2) to force degradation. However, since this solution is only inorganic and with a pH value never to be encountered at the implantation site, results can significantly vary from the actual events that will occur in vivo for the tested material over the long-term.
- Such standards are designed mainly for testing bulk materials, and are focused on the weight of the specimen, not taking into account one of the most important parameters: the contact area with the fluid. The focus is on the ratio between the mass of specimen and volume of fluid, but systems to be studied differ a lot with respect to the interaction area per gram of substance. Pellets, scaffolds (with macro- and micro-porosity), powders with different particle size, and thin (or thick) smooth (or rough) films induce huge differences in the ratios between the mass of substance and the area of interaction with the testing medium. An overview of this particular matter along with a several proposals can be found in [492].
4.3. Biocompatibility Assays
- Classic, MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide), MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)), XTT (2,3-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanilide) assays, that returns a value linked to general mitochondrial activity of the cells. Errors are given by different factors (e.g., differentiation of stem cells induces growth of mitochondria number per cell and increased activity). Advantages: simple and fast procedure, reliable results when working with homogenous terminally differentiated cells, cheap equipment and kits; Disadvantages: low reliability when working with heterogeneous cell cultures for differentiating experiments, indirect measure of proliferation;
- Quantifying double-stranded (ds) DNA by fluorescence (more ds-DNA, means more cells, ergo higher proliferation). Commercial kits are available. Advantages: direct measure of proliferation, very good and reliable results when working with heterogeneous cell cultures with many cell types (differentiation experiments), affordable equipment (98 well fluorescence reader), commercial kits are available; Disadvantage: complicated procedure;
- Cell counting when possible. Advantage: can be somewhat automated with a flow cytometer; Disadvantages: the classic counting technique uses microscopy, which is very laborious, time consuming, and impossible when dealing with a large number of situations (i.e., at least 10 microscopy fields per situation are required, with minimum 500 cells, numbered by three different examiners).
- Studying their morphology, when possible (as presented in ISO 10993-5:2009). This is a laborious method as it requires examination of a minimum 500 cells per situation acquired from a minimum 10 different randomly-chosen microscopy fields by three separate individuals. This renders the method almost impossible, when the experiment would involve a large number of materials;
- Measuring the LDH (lactate dehydrogenase) activity in the medium in which the cells were cultivated. LDH is an active intracellular enzyme found in all cells. Upon death, the cell releases this LDH into the medium and, therefore, this enzyme activity is proportional to the number of dead cells [501]. The method is easy to perform, fast, and returns reliable results on the same samples investigated for cell proliferation by mitochondrial activity tests;
- Measuring mitochondrial activity (MTT, MTS, XTT), as presented in the ISO 10993-5:2009 standard. It is a surrogate test for cytotoxicity: lower values with respect to control, due to lower general mitochondrial activity, are interpreted as results of cellular death, but this can also be an effect of slower proliferation values induced by the material. Thereby, it should not be used as stand-alone assay for cytotoxicity;
- Fluorescence apoptosis and cell viability kits (e.g., DAPI, annexinV, propidium iodide kit and Calcein AM/EthD-1 kit) are simple and widely used assays that provide good results, especially for flat substrates and examination with a confocal microscope. Calcein AM enters live cells and is converted in the cytoplasm in a green fluorescent compound, which does not exit from the cytoplasm. The dead cell nuclei have a red fluorescence due to EthD-1 that can penetrate only through the membrane of dead cells. As such, by fluorescence confocal microscopy the ratio of dead cells can be assessed. For 3D scaffolds it provides good results when the reading is done by a flow cytometer only, if the protocol recuperates and counts also the prior detached cells (which makes it a more difficult variant);
- Measuring the intracellular colorant uptake, as presented in the ISO 10993-5:2009 standard. The procedure is time consuming, but offers reliable results.
4.4. Osteoinduction Ability
4.5. Cell Differentiation Capacity
4.6. Pro-Angiogenic Properties
4.7. Antimicrobial Activity
- The tested material should be flat and compact with a surface of minimum 6.25 cm2, of which 4 cm2 should be reserved for bacterial interaction;
- Various types of nutrient broth have been observed to interact differently with the biomaterials, causing a variety of degradation rates, and therefore dissimilar antibacterial activities;
- Because of their nature and geometry, powders and 3D scaffolds with macro- and micro-porosity, cannot be tested according to this ISO standard protocol. Therefore, adaptive measures should be devised.
- ○
- A nutrient media powder suspension is inoculated with a known number of colony-forming units (CFU) to a final concentration of around 105−106 CFU mL−1, under continuous agitation in an incubator at 37 °C for a desired period of time. The number of bacterial cells that remained viable (viable cell count, VCC) is to be investigated by serial dilutions from each situation and seeding on simple agar plates (in an analogue manner to the ISO standard protocol);
- ○
- Colorimetric or fluorescence tests can be performed on samples, and rapid results are obtained based on previous control measuring curves established for each type of bacteria (e.g., MTS/XTT, cresyl violet, fluorescein diacetate). The fluorescence techniques use more expensive reagents and readers, but their measurement is more reliable since turbidity of the sample generated by powder material dissolution does not affect the reading. Fluorescein diacetate is used in a standard method for the assessment of water contaminated with microorganisms and could be considered very reliable.
- ○
- The scaffold would require an incubation in a given volume of nutrient media inoculated with a known number of CFU;
- ○
- Antimicrobial activity of a 3D structure is very hard to investigate because not all the bacterial cells can be harvested, since some of them could be very strongly adhered inside the scaffold, and therefore hard to detach;
- ○
- After the desired testing period, since the bacterial cells could be adhered inside the scaffold and cannot be reached, only a reading of a soluble coloured/fluorescent product of bacterial metabolism can provide insights. Some materials absorb coloured substances and make such tests impossible to carry out.
5. Future Perspectives: Co-Substituted Hydroxyapatite Bioceramics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Antoniac, I.V. Handbook of Bioceramics and Biocomposites; Springer: Berlin, Germany, 2016; ISBN 978-3-319-12461-2. [Google Scholar]
- Mucalo, M. Hydroxyapatite (HAp) for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 978-1-782-42033-0. [Google Scholar]
- Sassoni, E. Hydroxyapatite and other calcium phosphates for the conservation of cultural heritage: A review. Materials 2018, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Šupová, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Fihri, A.; Len, C.; Varma, R.S.; Solhy, A. Hydroxyapatite: A review of syntheses, structure and applications in heterogeneous catalysis. Coord. Chem. Rev. 2017, 347, 48–76. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate deposits: Preparation, properties and biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 272–326. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Thompson, I. Twenty-first century challenges for biomaterials. J. R. Soc. Interface 2010, 7, S379–S391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Toscano, M.; Bottagisio, M. Recent evidence on bioactive glass antimicrobial and antibiofilm activity: A mini-review. Materials 2018, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Hamzehlou, S.; Baino, F. Potential of bioactive glasses for cardiac and pulmonary tissue engineering. Materials 2017, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.W.; Gimeno-Fabra, M.; Segal, J.; Ahmed, I.; Grant, D.M. Degradation and characterization of resorbable phosphate-based glass thin-film coatings applied by radio-frequency magnetron sputtering. ACS Appl. Mater. Interfaces 2015, 7, 27362–27372. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.W.; Gimeno-Fabra, M.; Segal, J.; Ahmed, I.; Grant, D.M. Mechanical, structural and dissolution properties of heat treated thin-film phosphate based glasses. Appl. Surf. Sci. 2017, 416, 605–617. [Google Scholar] [CrossRef]
- Vichery, C.; Nedelec, J.-M. Bioactive glass nanoparticles: From synthesis to materials design for biomedical applications. Materials 2016, 9, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popa, A.C.; Stan, G.E.; Enculescu, M.; Tanase, C.; Tulyaganov, D.U.; Ferreira, J.M.F. Superior biofunctionality of dental implant fixtures uniformly coated with durable bioglass films by magnetron sputtering. J. Mech. Behav. Biomed. Mater. 2015, 51, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.W.; Grant, C.A.; Stan, G.E.; Popa, A.C.; Titman, J.J.; Grant, D.M. Gallium incorporation into phosphate based glasses: Bulk and thin film properties. J. Mech. Behav. Biomed. Mater. 2018, 82, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Popa, A.C.; Marques, V.; Stan, G.E.; Husanu, M.A.; Galca, A.C.; Ghica, C.; Tulyaganov, D.U.; Lemos, A.F.; Ferreira, J.M.F. Nanomechanical characterization of bioglass films synthesized by magnetron sputtering. Thin Solid Films 2014, 553, 166–172. [Google Scholar] [CrossRef]
- Stan, G.E.; Popa, A.C.; Bojin, D. Bioreactivity evaluation in simulated body fluid of magnetron sputtered glass and glass-ceramic coatings: A FTIR spectroscopy study. Dig. J. Nanomater. Biostruct. 2010, 5, 557–566. [Google Scholar]
- Gerhardt, L.-C.; Boccaccini, A.R. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef] [PubMed]
- Szczes, A.; Holysz, L.; Chibowski, E. Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Interface Sci. 2017, 249, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Rahavi, S.S.; Ghaderi, O.; Monshi, A.; Fathi, M.H. A comparative study on physicochemical properties of hydroxyapatite powders derived from natural and synthetic sources. Russ. J. Non-Ferrous Met. 2017, 58, 276–286. [Google Scholar] [CrossRef]
- Oladele, I.; Agbabiaka, O.; Olasunkanmi, O. Non-synthetic sources for the development of hydroxyapatite. J. Appl. Biotechnol. Bioeng. 2018, 5, 92–99. [Google Scholar] [CrossRef]
- Sima, L.E.; Stan, G.E.; Morosanu, C.O.; Melinescu, A.; Ianculescu, A.; Melinte, R.; Neamtu, J.; Petrescu, S.M. Differentiation of mesenchymal stem cells onto highly adherent radio frequency-sputtered carbonated hydroxylapatite thin films. J. Biomed. Mater. Res. Part A 2010, 95, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhao, L.; Liu, J.; Weir, M.D.; Zhou, X.; Xu, H.H. Bone tissue engineering via nanostructured calcium phosphate biomaterials and stem cells. Bone Res. 2014, 2, 14017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sima, L.E.; Petrescu, S.M. Adhesion and osteogenic differentiation of human mesenchymal stem cells: Supported by b-type carbonated hydroxylapatite. In Stem Cells and Cancer Stem Cells; Hayat, M.A., Ed.; Springer: Berlin, Germany, 2012; Volume 6, pp. 247–259. ISBN 978-94-007-2992-6. [Google Scholar]
- Willerth, S.M.; Sakiyama-Elbert, S.E. Combining stem cells and biomaterial scaffolds for constructing tissues and cell delivery. In StemBook; Harvard Stem Cell Institute: Cambridge, UK, 2008. [Google Scholar]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current concepts in scaffolding for bone tissue engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar] [PubMed]
- Krishnamurithy, G. A review on hydroxyapatite-based scaffolds as a potential bone graft substitute for bone tissue engineering applications. J. Health Transl. Med. 2013, 16, 22–27. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2017, 3, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.F.; Perera, F.H.; Marote, A.; Ferreira, S.; Vieira, S.I.; Olhero, S.; Miranda, P.; Ferreira, J.M.F. Biphasic calcium phosphate scaffolds fabricated by direct write assembly: Mechanical, anti-microbial and osteoblastic properties. J. Eur. Ceram. Soc. 2017, 37, 359–368. [Google Scholar] [CrossRef]
- Kundu, B.; Lemos, A.; Soundrapandian, C.; Sen, P.; Datta, S.; Ferreira, J.; Basu, D. Development of porous HAp and β-TCP scaffolds by starch consolidation with foaming method and drug-chitosan bilayered scaffold based drug delivery system. J. Mater. Sci. Mater. Med. 2010, 21, 2955–2969. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nissan, B. Advances in Calcium Phosphate Biomaterials; Springer: Berlin, Germany, 2014; ISBN 978-3-642-53980-0. [Google Scholar]
- Avila, I.; Pantchev, K.; Holopainen, J.; Ritala, M.; Tuukkanen, J. Adhesion and mechanical properties of nanocrystalline hydroxyapatite coating obtained by conversion of atomic layer-deposited calcium carbonate on titanium substrate. J. Mater. Sci. Mater. Med 2018, 29, 111. [Google Scholar] [CrossRef] [PubMed]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis—A review. Acta Biomater. 2014, 10, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Boi, M.; Bianchi, M. A Review on ionic substitutions in hydroxyapatite thin films: Towards complete biomimetism. Coatings 2018, 8, 269. [Google Scholar] [CrossRef]
- El Hadad, A.A.; Peón, E.; García-Galván, F.R.; Barranco, V.; Parra, J.; Jiménez-Morales, A.; Galván, J.C. Biocompatibility and corrosion protection behaviour of hydroxyapatite sol-gel-derived coatings on Ti6Al4V alloy. Materials 2017, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Oskouei, R.H.; Fallahnezhad, K.; Kuppusami, S. An investigation on the wear resistance and fatigue behaviour of Ti-6Al-4V notched members coated with hydroxyapatite coatings. Materials 2016, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-K.; Byun, S.-H.; Woo, J.-M.; Kim, S.-M.; Lee, S.-M.; Kim, B.-J.; Kim, H.-E.; Lee, J.-W.; Kim, S.-M.; Lee, J.-H. Biocompatibility and biocorrosion of hydroxyapatite-coated magnesium plate: Animal experiment. Materials 2017, 10, 1149. [Google Scholar] [CrossRef] [PubMed]
- Berndt, C.; Hasan, F.; Tietz, U.; Schmitz, K.-P. A review of hydroxyapatite coatings manufactured by thermal spray. In Advances in Calcium Phosphate Biomaterials; Ben-Nissan, B., Ed.; Springer: Berlin, Germany, 2014; pp. 267–329. ISBN 978-3-642-53980-0. [Google Scholar]
- Fukada, E.; Yasuda, I. On the piezoelectric effect of bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Ulstrup, A.K. Biomechanical concepts of fracture healing in weight-bearing long bones. Acta Orthop. Belg. 2008, 74, 291–302. [Google Scholar] [PubMed]
- Uto, Y.; Kuroshima, S.; Nakano, T.; Ishimoto, T.; Inaba, N.; Uchida, Y.; Sawase, T. Effects of mechanical repetitive load on bone quality around implants in rat maxillae. PLoS ONE 2017, 12, e0189893. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Fujiwara, S.; Mizuno, K. Roles of the cytoskeleton, cell adhesion and rho signalling in mechanosensing and mechanotransduction. J. Biochem. 2017, 161, 245–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gittings, J.; Bowen, C.R.; Dent, A.C.; Turner, I.G.; Baxter, F.R.; Chaudhuri, J.B. Electrical characterization of hydroxyapatite-based bioceramics. Acta Biomater. 2009, 5, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Kitagaki, K.; Umegaki, T. Thermal instability and proton conductivity of ceramic hydroxyapatite at high temperatures. J. Am. Ceram. Soc. 1995, 78, 1191–1197. [Google Scholar] [CrossRef]
- Yamashita, K.; Owada, H.; Umegaki, T.; Kanazawa, T.; Futagami, T. Ionic conduction in apatite solid solutions. Solid State Ion. 1988, 28, 660–663. [Google Scholar] [CrossRef]
- Gandhi, A.A.; Wojtas, M.; Lang, S.B.; Kholkin, A.L.; Tofail, S.A. Piezoelectricity in poled hydroxyapatite ceramics. J. Am. Ceram. Soc. 2014, 97, 2867–2872. [Google Scholar] [CrossRef]
- Lang, S.; Tofail, S.; Gandhi, A.; Gregor, M.; Wolf-Brandstetter, C.; Kost, J.; Bauer, S.; Krause, M. Pyroelectric, piezoelectric, and photoeffects in hydroxyapatite thin films on silicon. Appl. Phys. Lett. 2011, 98, 123703. [Google Scholar] [CrossRef]
- Lang, S.; Tofail, S.; Kholkin, A.; Wojtaś, M.; Gregor, M.; Gandhi, A.; Wang, Y.; Bauer, S.; Krause, M.; Plecenik, A. Ferroelectric polarization in nanocrystalline hydroxyapatite thin films on silicon. Sci. Rep. 2013, 3, 2215. [Google Scholar] [CrossRef] [PubMed]
- Basirun, W.J.; Nasiri-Tabrizi, B.; Baradaran, S. Overview of hydroxyapatite–graphene nanoplatelets composite as bone graft substitute: Mechanical behavior and in-vitro biofunctionality. Crit. Rev. Solid State Mat. Sci. 2017, 43, 177–212. [Google Scholar] [CrossRef]
- Combes, C.; Cazalbou, S.; Rey, C. Apatite biominerals. Minerals 2016, 6, 34. [Google Scholar] [CrossRef]
- Fleet, M.E.; Liu, X. Coupled substitution of type A and B carbonate in sodium-bearing apatite. Biomaterials 2007, 28, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Markovic, M.; Fowler, B.O.; Tung, M.S. Preparation and comprehensive characterization of a calcium hydroxyapatite reference material. J. Res. Natl. Inst. Stand. Technol. 2004, 109, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Fleet, M.E.; Pan, Y. Site preference of rare earth elements in fluorapatite: Binary (LREE + HREE)-substituted crystals. Am. Miner. 1997, 82, 870–877. [Google Scholar] [CrossRef]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2013, 49, 1461–1475. [Google Scholar] [CrossRef]
- Oktar, F.; Yetmez, M.; Gunduz, O. Novel hydroxyapatite (HA) production from synthetic and natural sources. Bioceram. Dev. Appl. 2017, 7, e109. [Google Scholar] [CrossRef]
- Miculescu, F.; Mocanu, A.C.; Stan, G.E.; Miculescu, M.; Maidaniuc, A.; Cîmpean, A.; Mitran, V.; Voicu, S.I.; Machedon-Pisu, T.; Ciocan, L.T. Influence of the modulated two-step synthesis of biogenic hydroxyapatite on biomimetic products’ surface. Appl. Surf. Sci. 2018, 438, 147–157. [Google Scholar] [CrossRef]
- Maidaniuc, A.; Miculescu, F.; Voicu, S.I.; Andronescu, C.; Miculescu, M.; Matei, E.; Mocanu, A.C.; Pencea, I.; Csaki, I.; Machedon-Pisu, T. Induced wettability and surface-volume correlation of composition for bovine bone derived hydroxyapatite particles. Appl. Surf. Sci. 2018, 438, 158–166. [Google Scholar] [CrossRef]
- Miculescu, F.; Maidaniuc, A.; Voicu, S.I.; Thakur, V.K.; Stan, G.E.; Ciocan, L. Progress in hydroxyapatite–starch based sustainable biomaterials for biomedical bone substitution applications. ACS Sustain. Chem. Eng. 2017, 5, 8491–8512. [Google Scholar] [CrossRef]
- Rocha, J.; Lemos, A.; Agathopoulos, S.; Kannan, S.; Valerio, P.; Ferreira, J. Hydrothermal growth of hydroxyapatite scaffolds from aragonitic cuttlefish bones. J. Biomed. Mater. Res. Part A 2006, 77, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Lemos, A.; Agathopoulos, S.; Valério, P.; Kannan, S.; Oktar, F.; Ferreira, J. Scaffolds for bone restoration from cuttlefish. Bone 2005, 37, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Lemos, A.; Kannan, S.; Agathopoulos, S.; Ferreira, J.M.F. Hydroxyapatite scaffolds hydrothermally grown from aragonitic cuttlefish bones. J. Mater. Chem. 2005, 15, 5007–5011. [Google Scholar] [CrossRef]
- Gunduz, O.; Sahin, Y.; Agathopoulos, S.; Ben-Nissan, B.; Oktar, F.N. A new method for fabrication of nanohydroxyapatite and TCP from the sea snail Cerithium vulgatum. J. Nanomater. 2014, 2014, 382861. [Google Scholar] [CrossRef]
- Kannan, S.; Rocha, J.H.; Agathopoulos, S.; Ferreira, J.M.F. Fluorine-substituted hydroxyapatite scaffolds hydrothermally grown from aragonitic cuttlefish bones. Acta Biomater. 2007, 3, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Deniaud, A.; Chevallet, M.; Michaud-Soret, I.; Buton, N.; Prodan, A.M. Textural, structural and biological evaluation of hydroxyapatite doped with zinc at low concentrations. Materials 2017, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Popa, C.L.; Chapon, P.; Groza, A.; Iconaru, S.L. Evaluation of the antimicrobial activity of different antibiotics enhanced with silver-doped hydroxyapatite thin films. Materials 2016, 9, 778. [Google Scholar] [CrossRef] [PubMed]
- Vladescu, A.; Cotrut, C.M.; Azem, F.A.; Bramowicz, M.; Pana, I.; Braic, V.; Birlik, I.; Kiss, A.; Braic, M.; Abdulgader, R. Sputtered Si and Mg doped hydroxyapatite for biomedical applications. Biomed. Mater. 2018, 13, 025011. [Google Scholar] [CrossRef] [PubMed]
- Vladescu, A.; Padmanabhan, S.; Azem, F.A.; Braic, M.; Titorencu, I.; Birlik, I.; Morris, M.; Braic, V. Mechanical properties and biocompatibility of the sputtered ti doped hydroxyapatite. J. Mech. Behav. Biomed. Mater. 2016, 63, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, J.T.B.; Mucalo, M.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B 2017, 105, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.; Salma-Ancane, K.; Stipniece, L.; Meenan, B.J.; Boyd, A.R. The deposition of strontium and zinc co-substituted hydroxyapatite coatings. J. Mater. Sci. Mater. Med. 2017, 28, 51. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Sallam, S.M.; Tohami, K.M.; Sallam, A.M.; Salem, L.I.A.; Mohamed, F.A. Synthesis and characterization of hydroxyapatite contain chromium. J. Biophys. Chem. 2012, 3, 278–282. [Google Scholar] [CrossRef]
- Golovanova, O.A.; Shlyapov, R.M.; Amerkhanova, S.K.; Uali, A.S.; Vlasov, V.A. Effect of cations (Mg2+, Zn2+, Cd2+) on formation of the mineral phase in Ca(NO3)2-Mg(NO3)2-Na2HPO4-H2O system. IOP Conf. Ser. Mater. Sci. Eng. 2015, 81, 012065. [Google Scholar] [CrossRef]
- Mishra, U. Development of Cobalt-Magnesium Doped Hydroxyapatite for Bone Tissue Engineering Application. Bachelor’s Thesis, Department of Biotechnology and Medical Engineering, National Institute of Technology, Odisha, India, 2013. [Google Scholar]
- Nandyala, S.H.; Santos, J.D. Current Trends on Glass and Ceramic Materials; Bentham Science Publishers: Sharjah, UAE, 2013; ISBN 978-1-60805-514-2. [Google Scholar]
- Renaudin, G.; Gomes, S.; Nedelec, J.M. First-row transition metal doping in calcium phosphate bioceramics: A detailed crystallographic study. Materials 2017, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.P.; Mahfuz, H.; Rondinone, A.J.; Leventouri, T. Improvement of the fracture toughness of hydroxyapatite (HAp) by incorporation of carboxyl functionalized single walled carbon nanotubes (CfSWCNTs) and nylon. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Uysal, I.; Severcan, F.; Tezcaner, A.; Evis, Z. Co-doping of hydroxyapatite with zinc and fluoride improves mechanical and biological properties of hydroxyapatite. Prog. Nat. Sci. 2014, 24, 340–349. [Google Scholar] [CrossRef]
- Zyman, Z.; Tkachenko, M.; Epple, M.; Polyakov, M.; Naboka, M. Magnesium-substituted hydroxyapatite ceramics. Materialwiss. Werkstofftech. 2006, 37, 474–477. [Google Scholar] [CrossRef]
- Lala, S.; Maity, T.; Singha, M.; Biswas, K.; Pradhan, S. Effect of doping (Mg, Mn, Zn) on the microstructure and mechanical properties of spark plasma sintered hydroxyapatites synthesized by mechanical alloying. Ceram. Int. 2017, 43, 2389–2397. [Google Scholar] [CrossRef]
- Laskus, A.; Kolmas, J. Ionic substitutions in non-apatitic calcium phosphates. Int. J. Mol. Sci. 2017, 18, 2542. [Google Scholar] [CrossRef] [PubMed]
- WHO Warns against ‘Post-Antibiotic’ Era. Nature. 30 April 2014. Available online: https://www.nature.com/news/who-warns-against-post-antibiotic-era-1.15135 (accessed on 12 October 2018).
- World Health Organization. WHO’s First Global Report on Antibiotic Resistance Reveals Serious, Worldwide Threat to Public Health. 2014. Available online: http://www.who.int/mediacentre/news/releases/2014/amr-report/en/ (accessed on 12 October 2018).
- 14th Annual Report 2017. National Joint Registry. 2017. Available online: http://www.njrreports.org.uk/ (accessed on 12 October 2018).
- Miculescu, F.; Bojin, D.; Ciocan, L.; Antoniac, I.; Miculescu, M.; Miculescu, N. Experimental researches on biomaterial-tissue interface interactions. J. Optoelectron. Adv. Mater. 2007, 9, 3303–3306. [Google Scholar]
- Miculescu, F.; Ciocan, L.; Miculescu, M.; Ernuteanu, A. Effect of heating process on micro structure level of cortical bone prepared for compositional analysis. Dig. J. Nanomater. Biostruct. 2011, 6, 225–233. [Google Scholar]
- Miculescu, F.; Jepu, I.; Lungu, C.; Miculescu, M.; Bane, M. Researches regarding the microanalysis results optimisation on multilayer nanostructures investigations. Dig. J. Nanomater. Biostruct. 2011, 6, 769–778. [Google Scholar]
- Miculescu, F.; Mocanu, A.-C.; Dascalu, C.A.; Maidaniuc, A.; Batalu, D.; Berbecaru, A.; Voicu, S.I.; Miculescu, M.; Thakur, V.K.; Ciocan, L.T. Facile synthesis and characterization of hydroxyapatite particles for high value nanocomposites and biomaterials. Vacuum 2017, 146, 614–622. [Google Scholar] [CrossRef]
- Duta, L.; Oktar, F.; Stan, G.; Popescu-Pelin, G.; Serban, N.; Luculescu, C.; Mihailescu, I. Novel doped hydroxyapatite thin films obtained by pulsed laser deposition. Appl. Surf. Sci. 2013, 265, 41–49. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 722/2012. Off. J. Eur. Union 2012. Available online: https://publications.europa.eu/en/publication-detail/-/publication/7fdb9dff-e222-11e1-905c-01aa75ed71a1 (accessed on 23 October 2018).
- ISO 22442:2007—Medical Devices Utilizing Animal Tissues and Their Derivatives: Part 3. Validation of the Elimination and/or Inactivation of Viruses and Transmissible Spongiform Encephalopathy (TSE) Agents; ISO: Geneva, Switzerland, 2007.
- Rincón-López, J.A.; Hermann-Muñoz, J.A.; Giraldo-Betancur, A.L.; De Vizcaya-Ruiz, A.; Alvarado-Orozco, J.M.; Muñoz-Saldaña, J. Synthesis, characterization and in vitro study of synthetic and bovine-derived hydroxyapatite ceramics: A comparison. Materials 2018, 11, 333. [Google Scholar] [CrossRef] [PubMed]
- Bano, N.; Jikan, S.S.; Basri, H.; Bakar, S.A.A.; Nuhu, A.H. Natural hydroxyapatite extracted from bovine bone. J. Sci. Technol. 2017, 9, 22–28. [Google Scholar]
- Ramesh, S.; Loo, Z.Z.; Tan, C.Y.; Chew, W.J.K.; Ching, Y.C.; Tarlochan, F.; Chandran, H.; Krishnasamy, S.; Bang, L.T.; Sarhan, A.A.D. Characterization of biogenic hydroxyapatite derived from animal bones for biomedical applications. Ceram. Int. 2018, 44, 10525–10530. [Google Scholar] [CrossRef]
- Rana, M.; Akhtar, N.; Rahman, S.; Jamil, H.; Asaduzzaman, S. Extraction of hydroxyapatite from bovine and human cortical bone by thermal decomposition and effect of gamma radiation: A comparative study. Int. J. Complement. Altern. Med. 2017, 8, 00263. [Google Scholar]
- Goller, G.; Oktar, F.; Agathopoulos, S.; Tulyaganov, D.; Ferreira, J.; Kayali, E.; Peker, I. Effect of sintering temperature on mechanical and microstructural properties of bovine hydroxyapatite (BHA). J. Sol-Gel Sci. Technol. 2006, 37, 111–115. [Google Scholar] [CrossRef]
- Mihailescu, N.; Stan, G.; Duta, L.; Chifiriuc, M.C.; Bleotu, C.; Sopronyi, M.; Luculescu, C.; Oktar, F.; Mihailescu, I. Structural, compositional, mechanical characterization and biological assessment of bovine-derived hydroxyapatite coatings reinforced with MgF2 or MgO for implants functionalization. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Popescu, A.; Florian, P.; Stan, G.; Popescu-Pelin, G.; Zgura, I.; Enculescu, M.; Oktar, F.; Trusca, R.; Sima, L.; Roseanu, A. Physical-chemical characterization and biological assessment of simple and lithium-doped biological-derived hydroxyapatite thin films for a new generation of metallic implants. Appl. Surf. Sci. 2018, 439, 724–735. [Google Scholar] [CrossRef]
- Ofudje, E.A.; Rajendran, A.; Adeogun, A.I.; Idowu, M.A.; Kareem, S.O.; Pattanayak, D.K. Synthesis of organic derived hydroxyapatite scaffold from pig bone waste for tissue engineering applications. Adv. Powder Technol. 2018, 29, 1–8. [Google Scholar] [CrossRef]
- Ramirez-Gutierrez, C.F.; Londoño-Restrepo, S.M.; del Real, A.; Mondragón, M.A.; Rodriguez-García, M.E. Effect of the temperature and sintering time on the thermal, structural, morphological, and vibrational properties of hydroxyapatite derived from pig bone. Ceram. Int. 2017, 43, 7552–7559. [Google Scholar] [CrossRef]
- Sobczak-Kupiec, A.; Pluta, K.; Drabczyk, A.; Włoś, M.; Tyliszczak, B. Synthesis and characterization of ceramic–polymer composites containing bioactive synthetic hydroxyapatite for biomedical applications. Ceram. Int. 2018, 44, 13630–13638. [Google Scholar] [CrossRef]
- Jaber, H.L.; Hammood, A.S.; Parvin, N. Synthesis and characterization of hydroxyapatite powder from natural Camelus bone. J. Aust. Ceram. Soc. 2017, 54, 1–10. [Google Scholar] [CrossRef]
- Ekren, N. Reinforcement of sheep-bone derived hydroxyapatite with bioactive glass. J. Ceram. Process. Res. 2017, 18, 64–68. [Google Scholar]
- Karacayli, U.; Gunduz, O.; Salman, S.; Ozyegin, L.; Agathopoulos, S.; Oktar, F. Effect of sintering temperature on mechanical properties and microstructure of sheep-bone derived hydroxyapatite (SHA). In Proceedings of the 13th International Conference on Biomedical Engineering, Munich, Germany, 7–12 September 2009; pp. 1271–1274. [Google Scholar] [CrossRef]
- Duta, L.; Mihailescu, N.; Popescu, A.; Luculescu, C.; Mihailescu, I.; Çetin, G.; Gunduz, O.; Oktar, F.; Popa, A.; Kuncser, A. Comparative physical, chemical and biological assessment of simple and titanium-doped ovine dentine-derived hydroxyapatite coatings fabricated by pulsed laser deposition. Appl. Surf. Sci. 2017, 413, 129–139. [Google Scholar] [CrossRef]
- Alshemary, A.Z.; Akram, M.; Taha, A.; Tezcaner, A.; Evis, Z.; Hussain, R. Physico-chemical and biological properties of hydroxyapatite extracted from chicken beaks. Mater. Lett. 2018, 215, 169–172. [Google Scholar] [CrossRef]
- Zhu, H.; Song, W.; Deng, Y. Hydroxyapatite extracted by animal bone image analysis in ionic liquid choline chloride-glycerol. EURASIP J. Image Video Process. 2018, 2018, 56. [Google Scholar] [CrossRef]
- Elkayar, A.; Elshazly, Y.; Assaad, M. Properties of hydroxyapatite from bovine teeth. Bone Tissue Regen. Insights 2009, 2, 31–36. [Google Scholar] [CrossRef]
- Akyurt, N.; Yetmez, M.; Karacayli, U.; Gunduz, O.; Agathopoulos, S.; Gökçe, H.; Öveçoğlu, M.; Oktar, F. A new natural biomaterial: Sheep dentine derived hydroxyapatite. Key Eng. Mater. 2012, 493–494, 281–286. [Google Scholar] [CrossRef]
- Roudan, M.A.; Ramesh, S.; Niakan, A.; Wong, Y.; Zavareh, M.A.; Chandran, H.; Teng, W.; Lwin, N.; Sutharsini, U. Thermal phase stability and properties of hydroxyapatite derived from bio-waste eggshells. J. Ceram. Process. Res. 2017, 18, 69–72. [Google Scholar]
- Cahyanto, A.; Kosasih, E.; Aripin, D.; Hasratiningsih, Z. Fabrication of hydroxyapatite from fish bones waste using reflux method. IOP Conf. Ser. Mater. Sci. Eng. 2017, 172, 012006. [Google Scholar] [CrossRef] [Green Version]
- Sunil, B.R.; Jagannatham, M. Producing hydroxyapatite from fish bones by heat treatment. Mater. Lett. 2016, 185, 411–414. [Google Scholar] [CrossRef]
- Walsh, P.J.; Buchanan, F.J.; Dring, M.; Maggs, C.; Bell, S.; Walker, G.M. Low-pressure synthesis and characterisation of hydroxyapatite derived from mineralise red algae. Chem. Eng. J. 2008, 137, 173–179. [Google Scholar] [CrossRef]
- Yelten, A.; Yilmaz, S. Comparison of naturally and synthetically derived hydroxyapatite powders. Acta Phys. Pol. A 2017, 131, 55–58. [Google Scholar] [CrossRef]
- Balaz, M. Ball milling of eggshell waste as a green and sustainable approach: A review. Adv. Colloid Interface Sci. 2018, 256, 256–275. [Google Scholar] [CrossRef] [PubMed]
- Antoniac, I.V.; Lesci, I.G.; Blajan, A.I.; Vitioanu, G.; Antoniac, A. Bioceramics and biocomposites from marine sources. Key Eng. Mater. 2016, 672, 276–292. [Google Scholar] [CrossRef]
- Granito, R.N.; Renno, A.C.M.; Yamamura, H.; de Almeida, M.C.; Ruiz, P.L.M.; Ribeiro, D.A. Hydroxyapatite from fish for bone tissue engineering: A promising approach. Int. J. Mol. Cell. Med. 2018, 7, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Inan, A.; Komur, B.; Ekren, N.; Aydogdu, M.; Gokce, H.; Ficai, A.; Salman, S.; Oktar, F.; Gunduz, O. Physical characterization of Turbot (Psetta Maxima) originated natural hydroxyapatite. Acta Phys. Pol. A 2017, 131, 397–399. [Google Scholar] [CrossRef]
- Abdulrahman, I.; Tijani, H.I.; Mohammed, B.A.; Saidu, H.; Yusuf, H.; Ndejiko Jibrin, M.; Mohammed, S. From garbage to biomaterials: An overview on egg shell based hydroxyapatite. J. Mater. 2014, 2014, 802467. [Google Scholar] [CrossRef]
- Ronan, K.; Kannan, M.B. Novel sustainable route for synthesis of hydroxyapatite biomaterial from biowastes. ACS Sustain. Chem. Eng. 2017, 5, 2237–2245. [Google Scholar] [CrossRef]
- Wu, S.-C.; Hsu, H.-C.; Hsu, S.-K.; Chang, Y.-C.; Ho, W.-F. Synthesis of hydroxyapatite from eggshell powders through ball milling and heat treatment. J. Asian Ceram. Soc. 2016, 4, 85–90. [Google Scholar] [CrossRef]
- Büdinger, L.; Hertl, M.; Büdinger, L. Immunologic mechanisms in hypersensitivity reactions to metal ions: An overview. Allergy 2000, 55, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Kaygili, O.; Dorozhkin, S.V.; Keser, S. Synthesis and characterization of Ce-substituted hydroxyapatite by sol-gel method. Mater. Sci. Eng. C Biol. Appl. 2014, 42, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Kaygili, O.; Keser, S.; Bulut, N.; Ates, T. Characterization of Mg-containing hydroxyapatites synthesized by combustion method. Physica B 2018, 537, 63–67. [Google Scholar] [CrossRef]
- Kolmas, J.; Groszyk, E.; Kwiatkowska-Rozycka, D. Substituted hydroxyapatites with antibacterial properties. BioMed Res. Int. 2014, 2014, 178123. [Google Scholar] [CrossRef] [PubMed]
- Perera, T.S.H.; Han, Y.; Lu, X.; Wang, X.; Dai, H.; Li, S. Rare earth doped apatite nanomaterials for biological application. J. Nanomater. 2015, 2015, 705390. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ooi, C.P.; Philip Hong Ning, C.; Aik Khor, K. Synthesis and characterization of Neodymium(III) and Gadolinium(III)-substituted hydroxyapatite as biomaterials. Int. J. Appl. Ceram. Technol. 2009, 6, 501–512. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.; Gong, H.; Jiang, X.; Wang, H.; Li, K. Effects of synthesis conditions on the morphology of hydroxyapatite nanoparticles produced by wet chemical process. Powder Technol. 2010, 203, 315–321. [Google Scholar] [CrossRef]
- Rh Owen, G.; Dard, M.; Larjava, H. Hydoxyapatite/beta-tricalcium phosphate biphasic ceramics as regenerative material for the repair of complex bone defects. J. Biomed. Mater. Res. Part B 2018, 106, 2493–2512. [Google Scholar] [CrossRef] [PubMed]
- Surmenev, R.A. A review of plasma-assisted methods for calcium phosphate-based coatings fabrication. Surf. Coat. Technol. 2012, 206, 2035–2056. [Google Scholar] [CrossRef]
- Durham, J.W.; Montelongo, S.A.; Ong, J.L.; Guda, T.; Allen, M.J.; Rabiei, A. Hydroxyapatite coating on PEEK implants: Biomechanical and histological study in a rabbit model. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 723–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bono, J.V.; McCarthy, J.C.; Thornhill, T.S.; Bierbaum, B.E.; Turner, R.H. Revision Total Hip Arthroplasty; Springer: Berlin, Germany, 1999; ISBN 978-1-4612-1406-9. [Google Scholar]
- Epinette, J.-A.; Manley, M.T. Fifteen Years of Clinical Experience with Hydroxyapatite Coatings in Joint Arthroplasty; Springer: Berlin, Germany, 2013; ISBN 978-2-8178-0851-2. [Google Scholar]
- Graziani, G.; Bianchi, M.; Sassoni, E.; Russo, A.; Marcacci, M. Ion-substituted calcium phosphate coatings deposited by plasma-assisted techniques: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Vilardell, A.M.; Cinca, N.; Jokinen, A.; Garcia-Giralt, N.; Dosta, S.; Cano, I.G.; Guilemany, J.M. Real-time protein and cell binding measurements on hydroxyapatite coatings. J. Funct. Biomater. 2016, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Trujillo, C.; Peón, E.; Chicardi, E.; Pérez, H.; Rodríguez-Ortiz, J.A.; Pavón, J.J.; García-Couce, J.; Galván, J.; García-Moreno, F.; Torres, Y. Sol-gel deposition of hydroxyapatite coatings on porous titanium for biomedical applications. Surf. Coat. Technol. 2018, 333, 158–162. [Google Scholar] [CrossRef]
- Stoica, T.; Morosanu, C.; Slav, A.; Stoica, T.; Osiceanu, P.; Anastasescu, C.; Gartner, M.; Zaharescu, M. Hydroxyapatite films obtained by sol–gel and sputtering. Thin Solid Films 2008, 516, 8112–8116. [Google Scholar] [CrossRef]
- Đošić, M.; Eraković, S.; Janković, A.; Vukašinović-Sekulić, M.; Matić, I.Z.; Stojanović, J.; Rhee, K.Y.; Mišković-Stanković, V.; Park, S.-J. In vitro investigation of electrophoretically deposited bioactive hydroxyapatite/chitosan coatings reinforced by graphene. J. Ind. Eng. Chem. 2017, 47, 336–347. [Google Scholar] [CrossRef]
- Dudek, K.; Dulski, M.; Goryczka, T.; Gerle, A. Structural changes of hydroxyapatite coating electrophoretically deposited on NiTi shape memory alloy. Ceram. Int. 2018, 44, 11292–11300. [Google Scholar] [CrossRef]
- Chakraborty, R.; Saha, P. A comparative study on surface morphology and electrochemical behaviour of hydroxyapatite-calcium hydrogen phosphate composite coating synthesized in-situ through electro chemical process under various deposition conditions. Surf. Interfaces 2018, 12, 160–167. [Google Scholar] [CrossRef]
- El-Wassefy, N.; Reicha, F.; Aref, N. Electro-chemical deposition of nano hydroxyapatite-zinc coating on titanium metal substrate. Int. J. Implant Dent. 2017, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Sandukas, S.; Yamamoto, A.; Rabiei, A. Osteoblast adhesion to functionally graded hydroxyapatite coatings doped with silver. J. Biomed. Mater. Res. Part A 2011, 97, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Aktug, S.L.; Kutbay, I.; Usta, M. Characterization and formation of bioactive hydroxyapatite coating on commercially pure zirconium by micro arc oxidation. J. Alloys Compd. 2017, 695, 998–1004. [Google Scholar] [CrossRef]
- Tang, H.; Gao, Y. Preparation and characterization of hydroxyapatite containing coating on AZ31 magnesium alloy by micro-arc oxidation. J. Alloys Compd. 2016, 688, 699–708. [Google Scholar] [CrossRef]
- Fatehi, K.; Moztarzadeh, F.; Solati-Hashjin, M.; Tahriri, M.; Rezvannia, M.; Ravarian, R. In vitro biomimetic deposition of apatite on alkaline and heat treated Ti6A14V alloy surface. Bull. Mat. Sci. 2008, 31, 101. [Google Scholar] [CrossRef]
- Iijima, K.; Sakai, A.; Komori, A.; Sakamoto, Y.; Matsuno, H.; Serizawa, T.; Hashizume, M. Control of biomimetic hydroxyapatite deposition on polymer substrates using different protein adsorption abilities. Colloid Surf. B Biointerfaces 2015, 130, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Pylypchuk, I.V.; Petranovskaya, A.; Gorbyk, P.; Korduban, A.; Markovsky, P.; Ivasishin, O. Biomimetic hydroxyapatite growth on functionalized surfaces of Ti-6Al-4V and Ti-Zr-Nb Alloys. Nanoscale Res. Lett. 2015, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Surmeneva, M.; Tyurin, A.; Surmenev, R. Correlation between structural and mechanical properties of RF magnetron sputter deposited hydroxyapatite coating. Mater. Charact. 2018, 142, 261–269. [Google Scholar] [CrossRef]
- Monsees, T.; Ak Azem, F.; Cotrut, C.; Braic, M.; Abdulgader, R.; Pana, I.; Birlik, I.; Kiss, A.; Booysen, R.; Vladescu, A. Biodegradable ceramics consisting of hydroxyapatite for orthopaedic implants. Coatings 2017, 7, 184. [Google Scholar] [CrossRef]
- Stan, G.E. Adherent functional graded hydroxylapatite coatings produced by sputtering deposition techniques. J. Optoelectron. Adv. Mater. 2009, 11, 1132–1138. [Google Scholar]
- Bianchi, M.; Degli Esposti, L.; Ballardini, A.; Liscio, F.; Berni, M.; Gambardella, A.; Leeuwenburgh, S.C.; Sprio, S.; Tampieri, A.; Iafisco, M. Strontium doped calcium phosphate coatings on poly (etheretherketone) (PEEK) by pulsed electron deposition. Surf. Coat. Technol. 2017, 319, 191–199. [Google Scholar] [CrossRef]
- Bianchi, M.; Gambardella, A.; Graziani, G.; Liscio, F.; Maltarello, M.C.; Boi, M.; Berni, M.; Bellucci, D.; Marchiori, G.; Valle, F. Plasma-assisted deposition of bone apatite-like thin films from natural apatite. Mater. Lett. 2017, 199, 32–36. [Google Scholar] [CrossRef]
- Bianchi, M.; Pisciotta, A.; Bertoni, L.; Berni, M.; Gambardella, A.; Visani, A.; Russo, A.; De Pol, A.; Carnevale, G. Osteogenic differentiation of hDPSCs on biogenic bone apatite thin films. Stem Cells Int. 2017, 2017, 3579283. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Carnevale, G.; Pisciotta, A.; Bertoni, L.; Boi, M.; Gambardella, A.; Berni, M.; Marchiori, G.; Russo, A.; De Pol, A. Pulsed electron deposition of bone-like apatite thin films from a biogenic source: From material characterization to in vitro stem cell differentiation. Orthop. Proc. 2018, 100, 1. [Google Scholar]
- Boanini, E.; Torricelli, P.; Sima, F.; Axente, E.; Fini, M.; Mihailescu, I.N.; Bigi, A. Gradient coatings of strontium hydroxyapatite/zinc β-tricalcium phosphate as a tool to modulate osteoblast/osteoclast response. J. Inorg. Biochem. 2018, 183, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Janković, A.; Eraković, S.; Ristoscu, C.; Mihailescu, N.; Duta, L.; Visan, A.; Stan, G.; Popa, A.; Husanu, M.; Luculescu, C. Structural and biological evaluation of lignin addition to simple and silver-doped hydroxyapatite thin films synthesized by matrix-assisted pulsed laser evaporation. J. Mater. Sci. Mater. Med. 2015, 26, 17. [Google Scholar] [CrossRef] [PubMed]
- Popescu-Pelin, G.; Sima, F.; Sima, L.; Mihailescu, C.; Luculescu, C.; Iordache, I.; Socol, M.; Socol, G.; Mihailescu, I. Hydroxyapatite thin films grown by pulsed laser deposition and matrix assisted pulsed laser evaporation: Comparative study. Appl. Surf. Sci. 2017, 418, 580–588. [Google Scholar] [CrossRef]
- Visan, A.; Stan, G.E.; Ristoscu, C.; Popescu-Pelin, G.; Sopronyi, M.; Besleaga, C.; Luculescu, C.; Chifiriuc, M.C.; Hussien, M.; Marsan, O. Combinatorial MAPLE deposition of antimicrobial orthopedic maps fabricated from chitosan and biomimetic apatite powders. Int. J. Pharm. 2016, 511, 505–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosco, R.; Van Den Beucken, J.; Leeuwenburgh, S.; Jansen, J. Surface engineering for bone implants: A trend from passive to active surfaces. Coatings 2012, 2, 95–119. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, R.; Seshadri, S.K.; Kwon, T.Y.; Kim, K.H. Calcium phosphate-based coatings on titanium and its alloys. J. Biomed. Mater. Res. Part B 2008, 85, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Hontsu, S.; Nakamoru, M.; Nishikawa, H.; Kusunoki, M. Characteristics of a humidity sensor using a Na-doped hydroxyapatite thin film. Mem. Fac. Biol.-Oriented Sci. Technol. Kinki Univ. 2010, 26, 87–91. [Google Scholar]
- Essamlali, Y.; Amadine, O.; Larzek, M.; Len, C.; Zahouily, M. Sodium modified hydroxyapatite: Highly efficient and stable solid-base catalyst for biodiesel production. Energy Conv. Manag. 2017, 149, 355–367. [Google Scholar] [CrossRef]
- Sugiyama, S.; Iguchi, Y.; Nishioka, H.; Minami, T.; Moriga, T.; Hayashi, H.; Moffat, J.B. Effects of the thermal stability and the fine structure changes of strontium hydroxyapatites ion-exchanged with lead on methane oxidation in the presence and absence of tetrachloromethane. J. Catal. 1998, 176, 25–34. [Google Scholar] [CrossRef]
- Fierascu, I.; Avramescu, S.M.; Petreanu, I.; Marinoiu, A.; Soare, A.; Nica, A.; Fierascu, R.C. Efficient removal of phenol from aqueous solutions using hydroxyapatite and substituted hydroxyapatites. React. Kinet. Mech. Catal. 2017, 122, 155–175. [Google Scholar] [CrossRef]
- Sugiyama, S.; Shono, T.; Nitta, E.; Hayashi, H. Effects of gas- and solid-phase additives on oxidative dehydrogenation of propane on strontium and barium hydroxyapatites. Appl. Catal. A Gen. 2001, 211, 123–130. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Mao, L.; Gong, L.; Sun, W.; Feng, L. Effect of cation doping on the structure of hydroxyapatite and the mechanism of defluoridation. Ceram. Int. 2018, 44, 6002–6009. [Google Scholar] [CrossRef]
- Nie, Y.; Hu, C.; Kong, C. Enhanced fluoride adsorption using Al(III) modified calcium hydroxyapatite. J. Hazard. Mater. 2012, 233–234, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Thom, N.T.; Thanh, D.T.M.; Nam, P.T.; Phuong, N.T.; Hong, C.T.; Xuyen, N.T.; Van Trang, N.; Buess-Herman, C. Treatment of Cd2+ ions using aluminum doped hydroxyapatite (AlHAp) powder. Viet. J. Chem. 2017, 55, 393–399. [Google Scholar] [CrossRef]
- Rahmanian, A.; Ghaziaskar, H.S. Continuous dehydration of ethanol to diethyl ether over aluminum phosphate–hydroxyapatite catalyst under sub and supercritical condition. J. Supercrit. Fluids 2013, 78, 34–41. [Google Scholar] [CrossRef]
- Neidel, L.L.; Moore, R.C.; Salas, F.; Grouios, F.; Holt, K.C.; Helean, K.B. Sequestration of radionuclides and heavy metals by hydroxyapatite doped with Fe, Cu and Sn. Geochim. Cosmochim. Acta 2005, 69, A70. [Google Scholar] [CrossRef]
- Matsumura, Y.; Moffat, J.B.; Sugiyama, S.; Hayashi, H.; Shigemoto, N.; Saitoh, K. Selective oxidative coupling of methane catalysed over hydroxyapatite ion-exchanged with lead. J. Chem. Soc. Faraday Trans. 1994, 90, 2133–2140. [Google Scholar] [CrossRef]
- Oh, S.C.; Xu, J.; Tran, D.T.; Liu, B.; Liu, D. Effects of controlled crystalline surface of hydroxyapatite on methane oxidation reactions. ACS Catal. 2018, 8, 4493–4507. [Google Scholar] [CrossRef]
- Wei, X.; Yates, M.Z. Yttrium-doped hydroxyapatite membranes with high proton conductivity. Chem. Mater. 2012, 24, 1738–1743. [Google Scholar] [CrossRef]
- Hu, A.; Li, M.; Chang, C.; Mao, D. Preparation and characterization of a titanium-substituted hydroxyapatite photocatalyst. J. Mol. Catal. A Chem. 2007, 267, 79–85. [Google Scholar] [CrossRef]
- Salhi, A.; Aarfane, A.; Tahiri, S.; Khamliche, L.; Bensitel, M.; Bentiss, F.; El Krati, M. Study of the photocatalytic degradation of methylene blue dye using titanium-doped hydroxyapatite. Mediterr. J. Chem. 2015, 4, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Wakamura, M.; Hashimoto, K.; Watanabe, T. Photocatalysis by calcium hydroxyapatite modified with Ti(IV): Albumin decomposition and bactericidal effect. Langmuir 2003, 19, 3428–3431. [Google Scholar] [CrossRef]
- Nishikawa, M.; Tan, L.H.; Nakabayashi, Y.; Hasegawa, T.; Shiroishi, W.; Kawahara, S.; Saito, N.; Nosaka, A.; Nosaka, Y. Visible light responsive vanadium-substituted hydroxyapatite photocatalysts. J. Photochem. Photobiol. A Chem. 2015, 311, 30–34. [Google Scholar] [CrossRef]
- Chlala, D.; Griboval-Constant, A.; Nuns, N.; Giraudon, J.M.; Labaki, M.; Lamonier, J.F. Effect of Mn loading onto hydroxyapatite supported Mn catalysts for toluene removal: Contribution of PCA assisted ToF-SIMS. Catal. Today 2018, 307, 41–47. [Google Scholar] [CrossRef]
- Ravindranadh, K.; Babu, B.; Pushpa Manjari, V.; Thirumala Rao, G.; Rao, M.C.; Ravikumar, R.V.S.S.N. Optical and structural properties of undoped and Mn2+ doped Ca–Li hydroxyapatite nanopowders using mechanochemical synthesis. J. Lumines. 2015, 159, 119–127. [Google Scholar] [CrossRef]
- Kanchana, P.; Radhakrishnan, S.; Navaneethan, M.; Arivanandhan, M.; Hayakawa, Y.; Sekar, C. Electrochemical sensor based on fe doped hydroxyapatite-carbon nanotubes composite for l-dopa detection in the presence of uric acid. J. Nanosci. Nanotechnol. 2016, 16, 6185–6192. [Google Scholar] [CrossRef] [PubMed]
- Khachani, M.; Kacimi, M.; Ensuque, A.; Piquemal, J.-Y.; Connan, C.; Bozon-Verduraz, F.; Ziyad, M. Iron–calcium–hydroxyapatite catalysts: Iron speciation and comparative performances in butan-2-ol conversion and propane oxidative dehydrogenation. Appl. Catal. A Gen. 2010, 388, 113–123. [Google Scholar] [CrossRef]
- Padayachee, D.; Dasireddy, V.D.B.C.; Bharuth-Ram, K.; Singh, S.; Friedrich, H.B. Phase transformation of iron in hydroxyapatite in the activation of n-octane. Hyperfine Interact. 2014, 231, 131–136. [Google Scholar] [CrossRef]
- Mene, R.U.; Mahabole, M.P.; Khairnar, R. Surface modification of cobalt doped hydroxyapatite thick films via swift heavy ion irradiations for CO and CO2 gas sensing application. In Proceedings of the 14th International Meeting on Chemical Sensors (IMCS 2012), Nuremberg, Germany, 20–23 May 2012; pp. 1180–1183. [Google Scholar] [CrossRef]
- Emayavaramban, P.; Babu, S.G.; Karvembu, R.; Dharmaraj, N. Nickel oxide doped hydroxyapatite for catalytic oxidation of alcohols to carbonyl compounds at room temperature. Adv. Sci. Eng. Med. 2014, 6, 659–666. [Google Scholar] [CrossRef]
- Miniach, E.; Śliwak, A.; Moyseowicz, A.; Gryglewicz, G. Growth of carbon nanofibers from methane on a hydroxyapatite-supported nickel catalyst. J. Mater. Sci. 2016, 51, 5367–5376. [Google Scholar] [CrossRef] [Green Version]
- Neelakandeswari, N.; Sangami, G.; Emayavaramban, P.; Karvembu, R.; Dharmaraj, N.; Kim, H.Y. Mesoporous nickel hydroxyapatite nanocomposite for microwave-assisted Henry reaction. Tetrahedron Lett. 2012, 53, 2980–2984. [Google Scholar] [CrossRef]
- Rego de Vasconcelos, B.; Pham Minh, D.; Sharrock, P.; Nzihou, A. Regeneration study of Ni/hydroxyapatite spent catalyst from dry reforming. Catal. Today 2018, 310, 107–115. [Google Scholar] [CrossRef]
- Kamieniak, J.; Bernalte, E.; Foster, C.; Doyle, A.; Kelly, P.; Banks, C. High yield synthesis of hydroxyapatite (HAP) and palladium doped HAP via a wet chemical synthetic route. Catalysts 2016, 6, 119. [Google Scholar] [CrossRef]
- Kamieniak, J.; Kelly, P.J.; Doyle, A.M.; Banks, C.E. Influence of the metal/metal oxide redox cycle on the catalytic activity of methane oxidation over Pd and Ni doped hydroxyapatite. Catal. Commun. 2018, 107, 82–86. [Google Scholar] [CrossRef]
- Takarroumt, N.; Kacimi, M.; Bozon-Verduraz, F.; Liotta, L.F.; Ziyad, M. Characterization and performance of the bifunctional platinum-loaded calcium-hydroxyapatite in the one-step synthesis of methyl isobutyl ketone. J. Mol. Catal. A Chem. 2013, 377, 42–50. [Google Scholar] [CrossRef]
- Vukomanović, M.; Žunič, V.; Otoničar, M.; Repnik, U.; Turk, B.; Škapin, S.D.; Suvorov, D. Hydroxyapatite/platinum bio-photocatalyst: A biomaterial approach to self-cleaning. J. Mater. Chem. 2012, 22, 10571–10580. [Google Scholar] [CrossRef]
- Chlala, D.; Giraudon, J.M.; Nuns, N.; Labaki, M.; Lamonier, J.F. Highly active noble-metal-free copper hydroxyapatite catalysts for the total oxidation of toluene. ChemCatChem 2017, 9, 2275–2283. [Google Scholar] [CrossRef]
- Guo, J.; Yu, H.; Dong, F.; Zhu, B.; Huang, W.; Zhang, S. High efficiency and stability of Au–Cu/hydroxyapatite catalyst for the oxidation of carbon monoxide. RSC Adv. 2017, 7, 45420–45431. [Google Scholar] [CrossRef] [Green Version]
- Othmani, M.; Bachoua, H.; Ghandour, Y.; Aissa, A.; Debbabi, M. Synthesis, characterization and catalytic properties of copper-substituted hydroxyapatite nanocrystals. Mater. Res. Bull. 2018, 97, 560–566. [Google Scholar] [CrossRef]
- Tounsi, H.; Djemal, S.; Petitto, C.; Delahay, G. Copper loaded hydroxyapatite catalyst for selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B Environ. 2011, 107, 158–163. [Google Scholar] [CrossRef]
- Jahan, S.A.; Mollah, M.Y.A.; Ahmed, S.; Susan, M.A.B.H. Copper-doped hydroxyapatite for removal of Arsenic(V) from aqueous system. J. Sci. Res. 2017, 9, 383–402. [Google Scholar] [CrossRef]
- Kumar, P.A.; Reddy, M.P.; Ju, L.K.; Phil, H.H. Novel silver loaded hydroxyapatite catalyst for the selective catalytic reduction of NOx by propene. Catal. Lett. 2008, 126, 78–83. [Google Scholar] [CrossRef]
- Mitsudome, T.; Mikami, Y.; Mori, H.; Arita, S.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Supported silver nanoparticle catalyst for selective hydration of nitriles to amides in water. Chem. Commun. 2009, 0, 3258–3260. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, X.-C.; Yang, X.-J.; Han, Y.-F. Silver/hydroxyapatite foam as a highly selective catalyst for acetaldehyde production via ethanol oxidation. Catal. Today 2016, 276, 19–27. [Google Scholar] [CrossRef]
- Domínguez, M.I.; Romero-Sarria, F.; Centeno, M.A.; Odriozola, J.A. Gold/hydroxyapatite catalysts: Synthesis, characterization and catalytic activity to CO oxidation. Appl. Catal. B Environ. 2009, 87, 245–251. [Google Scholar] [CrossRef]
- Phonthammachai, N.; Ziyi, Z.; Jun, G.; Fan, H.Y.; Whitea, T. Synthesis of high performance hydroxyapatite-gold catalysts for CO oxidation. Gold Bull. 2008, 41, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Lakshmi Kantam, M.; Balasubrahmanyam, V.; Kumar, K.S. Zinc hydroxyapatite–catalyzed efficient synthesis of 5-substituted 1 H-tetrazoles. Synth. Commun. 2006, 36, 1809–1814. [Google Scholar] [CrossRef]
- Low, H.R.; Avdeev, M.; Ramesh, K.; White, T.J. Zinc hydroxyapatite catalyst for decomposition of 2-propanol. Adv. Mater. 2012, 24, 4175–4179. [Google Scholar] [CrossRef] [PubMed]
- Riad, M.; Mikhail, S. Zinc incorporated hydroxyapatite catalysts: Preparation and characterization. Energy Sources Part A Recover. Util. Environ. Eff. 2013, 35, 445–454. [Google Scholar] [CrossRef]
- Latshaw, A.M.; Hughey, K.D.; Smith, M.D.; Yeon, J.; Zur Loye, H.C. Photoluminescent and magnetic properties of lanthanide containing apatites: NaxLn10-x(SiO4)6O2-yFy, CaxLn10-x(SiO4)6O2-yFy (Ln = Eu, Gd, and Sm), Gd9.34(SiO4)6O2, and K1.32Pr8.68(SiO4)6O1.36F0.64. Inorg. Chem. 2015, 54, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Kottaisamy, M.; Jagannathan, R.; Jeyagopal, P.; Rao, R.; Narayanan, R. Eu2+ luminescence in M5(PO4)3X apatites, where M is Ca2+, Sr2+ and Ba2+, and X is F−, Cl−, Br− and OH−. J. Phys. D Appl. Phys. 1994, 27, 2210. [Google Scholar] [CrossRef]
- Constantin, L.; Iconaru, S.; Ciobanu, C. Europium doped hydroxyapatite for applications in environmental field. Rom. Rep. Phys. 2012, 64, 788–794. [Google Scholar]
- Jiménez-Flores, Y.; Suárez-Quezada, M.; Rojas-Trigos, J.B.; Lartundo-Rojas, L.; Suárez, V.; Mantilla, A. Characterization of Tb-doped hydroxyapatite for biomedical applications: Optical properties and energy band gap determination. J. Mater. Sci. 2017, 52, 9990–10000. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wang, H.; Chen, L.; Guo, D.X.; Cai, K.Q.; Liu, X.J.; Huang, Z.L. Synthesis of Tb3+-doped Ca-deficient hydroxyapatite and its photoluminescence for white light-emitting diode application. Adv. Mater. Res. 2012, 560–561, 825–829. [Google Scholar] [CrossRef]
- Zhang, Y.; Mei, L.; Liu, H.; Yang, D.; Liao, L.; Huang, Z. Dysprosium doped novel apatite-type white-emitting phosphor Ca9La(PO4)5(GeO4)F2 with satisfactory thermal properties for n-UV w-LEDs. Dyes Pigments 2017, 139, 180–186. [Google Scholar] [CrossRef]
- Hager, E.; Dziambor, H.; Höhmann, D.; Winkler, P.; Strama, H. Effects of lithium on thrombopoiesis in patients with low platelet cell counts following chemotherapy or radiotherapy. Biol. Trace Elem. Res. 2001, 83, 139–148. [Google Scholar] [CrossRef]
- Hager, E.D.; Dziambor, H.; Winkler, P.; Hohmann, D.; Macholdt, K. Effects of lithium carbonate on hematopoietic cells in patients with persistent neutropenia following chemotherapy or radiotherapy. J. Trace Elem. Med. Biol. 2002, 16, 91–97. [Google Scholar] [CrossRef]
- Levitt, L.J.; Quesenberry, P.J. The effect of lithium on murine hematopoiesis in a liquid culture system. N. Engl. J. Med. 1980, 302, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Klontzas, M.E.; Kenanidis, E.I.; MacFarlane, R.J.; Michail, T.; Potoupnis, M.E.; Heliotis, M.; Mantalaris, A.; Tsiridis, E. Investigational drugs for fracture healing: Preclinical & clinical data. Expert Opin. Investig. Drugs 2016, 25, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Bernick, J.; Wang, Y.; Sigal, I.A.; Alman, B.A.; Whyne, C.M.; Nam, D. Parameters for lithium treatment are critical in its enhancement of fracture-healing in rodents. J. Bone Jt. Surg. Am. Vol. 2014, 96, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Whetstone, H.C.; Lin, A.C.; Nadesan, P.; Wei, Q.; Poon, R.; Alman, B.A. Beta-catenin signaling plays a disparate role in different phases of fracture repair: Implications for therapy to improve bone healing. PLoS Med. 2007, 4, e249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Peng, X.; Qin, Y.; Wang, R.; Tang, J.; Cui, X.; Wang, T.; Liu, W.; Pan, H.; Li, B. Acceleration of bone regeneration by activating wnt/β-catenin signalling pathway via lithium released from lithium chloride/calcium phosphate cement in osteoporosis. Sci. Rep. 2017, 7, 45204. [Google Scholar] [CrossRef] [PubMed]
- Satija, N.K.; Sharma, D.; Afrin, F.; Tripathi, R.P.; Gangenahalli, G. High throughput transcriptome profiling of lithium stimulated human mesenchymal stem cells reveals priming towards osteoblastic lineage. PLoS ONE 2013, 8, e55769. [Google Scholar] [CrossRef] [PubMed]
- Vachhani, K.; Whyne, C.; Wang, Y.; Burns, D.M.; Nam, D. Low-dose lithium regimen enhances endochondral fracture healing in osteoporotic rodent bone. J. Orthop. Res. 2018, 36, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, X.; Gu, Z.; Qin, H.; Li, L.; Liu, J.; Yu, X. In vitro study on the degradation of lithium-doped hydroxyapatite for bone tissue engineering scaffold. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 66, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Huifang, L.; Zhao, J.; Yang, Z.; Xie, X.; Wei, Z.; Li, D.; Kang, P. Porous lithium-doped hydroxyapatite scaffold seeded with hypoxia-preconditioned bone-marrow mesenchymal stem cells for bone-tissue regeneration. Biomed. Mater. 2018, 13, 055002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shainberg, A.P.M.; Valério, P.; Zonari, A.; Oktar, F.N.; Ozyegin, L.S.; Graça, M.P.F.; Leite, M.F.; Goes, A.M. Attachment and proliferation of osteoblasts on lithium-hydroxyapatite composites. Adv. Mater. Sci. Eng. 2012, 2012, 650574. [Google Scholar] [CrossRef]
- Luo, Y.; Li, D.; Zhao, J.; Yang, Z.; Kang, P. In vivo evaluation of porous lithium-doped hydroxyapatite scaffolds for the treatment of bone defect. Bio-Med. Mater. Eng. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Ginty, F.; Flynn, A.; Cashman, K.D. The effect of dietary sodium intake on biochemical markers of bone metabolism in young women. Br. J. Nutr. 1998, 79, 343–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohl, H.R.; Wheeler, J.S.; Murray, H.E. Sodium and potassium in health and disease. In Interrelations Between Essential Metal Ions and Human Diseases; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer: Berlin, Germany, 2013; pp. 29–47. ISBN 978-94-007-7500-8. [Google Scholar]
- Sang Cho, J.; Um, S.H.; Su Yoo, D.; Chung, Y.C.; Hye Chung, S.; Lee, J.C.; Rhee, S.H. Enhanced osteoconductivity of sodium-substituted hydroxyapatite by system instability. J. Biomed. Mater. Res. Part B 2014, 102, 1046–1062. [Google Scholar] [CrossRef] [PubMed]
- Sigel, A.; Sigel, H.; Sigel, R.K. Interrelations between Essential Metal Ions and Human Diseases; Springer: Berlin, Germany, 2013; ISBN 978-94-007-7500-8. [Google Scholar]
- Li, H.; Zhao, X.; Cao, S.; Li, K.; Chen, M.; Xu, Z.; Lu, J.; Zhang, L. Na-doped hydroxyapatite coating on carbon/carbon composites: Preparation, in vitro bioactivity and biocompatibility. Appl. Surf. Sci. 2012, 263, 163–173. [Google Scholar] [CrossRef]
- Wiesmann, H.-P.; Plate, U.; Zierold, K.; Höhling, H. Potassium is involved in apatite biomineralization. J. Dent. Res. 1998, 77, 1654–1657. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Ventura, J.M.G.; Ferreira, J.M.F. Synthesis and thermal stability of potassium substituted hydroxyapatites and hydroxyapatite/β-tricalciumphosphate mixtures. Ceram. Int. 2007, 33, 1489–1494. [Google Scholar] [CrossRef]
- Weissmueller, N.T.; Schiffter, H.A.; Carlisle, R.C.; Rollier, C.S.; Pollard, A.J. Needle-free dermal delivery of a diphtheria toxin CRM197 Mutant on potassium-doped hydroxyapatite microparticles. Clin. Vaccine Immunol. 2015, 22, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Weissmueller, N.T.; Schiffter, H.A.; Pollard, A.J.; Cuneyt Tas, A. Molten salt synthesis of potassium-containing hydroxyapatite microparticles used as protein substrate. Mater. Lett. 2014, 128, 421–424. [Google Scholar] [CrossRef]
- Stipniece, L.; Salma-Ancane, K.; Borodajenko, N.; Sokolova, M.; Jakovlevs, D.; Berzina-Cimdina, L. Characterization of Mg-substituted hydroxyapatite synthesized by wet chemical method. Ceram. Int. 2014, 40, 3261–3267. [Google Scholar] [CrossRef]
- Landi, E.; Logroscino, G.; Proietti, L.; Tampieri, A.; Sandri, M.; Sprio, S. Biomimetic Mg-substituted hydroxyapatite: From synthesis to in vivo behaviour. J. Mater. Sci. Mater. Med. 2008, 19, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Andres, N.C.; D’Elia, N.L.; Ruso, J.M.; Campelo, A.E.; Massheimer, V.L.; Messina, P.V. Manipulation of Mg(2+)-Ca(2+) switch on the development of bone mimetic hydroxyapatite. ACS Appl. Mater. Interfaces 2017, 9, 15698–15710. [Google Scholar] [CrossRef] [PubMed]
- Andres, N.C.; Sieben, J.M.; Baldini, M.; Rodriguez, C.H.; Famiglietti, A.; Messina, P.V. Electroactive Mg(2+)-hydroxyapatite nanostructured networks against drug-resistant bone infection strains. ACS Appl. Mater. Interfaces 2018, 10, 19534–19544. [Google Scholar] [CrossRef] [PubMed]
- de Lima, I.R.; Alves, G.G.; Soriano, C.A.; Campaneli, A.P.; Gasparoto, T.H.; Ramos, E.S., Jr.; de Sena, L.A.; Rossi, A.M.; Granjeiro, J.M. Understanding the impact of divalent cation substitution on hydroxyapatite: An in vitro multiparametric study on biocompatibility. J. Biomed. Mater. Res. Part A 2011, 98, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Zhang, S.; Khor, K.A.; Lye, S.W.; Zeng, X.; Weng, W.; Liu, C.; Venkatraman, S.S.; Ma, L.L. Osteoblastic cell response on magnesium-incorporated apatite coatings. Appl. Surf. Sci. 2008, 255, 304–307. [Google Scholar] [CrossRef]
- Ran, J.; Jiang, P.; Sun, G.; Ma, Z.; Hu, J.; Shen, X.; Tong, H. Comparisons among Mg, Zn, Sr, and Si doped nano-hydroxyapatite/chitosan composites for load-bearing bone tissue engineering applications. Mater. Chem. Front. 2017, 1, 900–910. [Google Scholar] [CrossRef]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable materials and metallic implants—A review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Sutha, S.; Dhineshbabu, N.; Prabhu, M.; Rajendran, V. Mg-doped hydroxyapatite/chitosan composite coated 316l stainless steel implants for biomedical applications. J. Nanosci. Nanotechnol. 2015, 15, 4178–4187. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.C.; Jamshidi, P.; Grover, L.M.; Mallick, K.K. Preparation and characterisation of nanophase Sr, Mg, and Zn substituted hydroxyapatite by aqueous precipitation. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 35, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Aina, V.; Bergandi, L.; Lusvardi, G.; Malavasi, G.; Imrie, F.E.; Gibson, I.R.; Cerrato, G.; Ghigo, D. Sr-containing hydroxyapatite: Morphologies of HA crystals and bioactivity on osteoblast cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Capuccini, C.; Torricelli, P.; Sima, F.; Boanini, E.; Ristoscu, C.; Bracci, B.; Socol, G.; Fini, M.; Mihailescu, I.N.; Bigi, A. Strontium-substituted hydroxyapatite coatings synthesized by pulsed-laser deposition: In vitro osteoblast and osteoclast response. Acta Biomater. 2008, 4, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Hosick, H.L.; Bandyopadhyay, A.; Bose, S.; Ding, C.; Luk, K.D.K.; Cheung, K.M.C.; Lu, W.W. Preparation and cell–materials interactions of plasma sprayed strontium-containing hydroxyapatite coating. Surf. Coat. Technol. 2007, 201, 4685–4693. [Google Scholar] [CrossRef]
- Caverzasio, J. Strontium ranelate promotes osteoblastic cell replication through at least two different mechanisms. Bone 2008, 42, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wei, S.; Lu, M.; Shao, Z.; Lu, J.; Xia, L.; Lin, K.; Zou, D. Dose-dependent effects of strontium ranelate on ovariectomy rat bone marrow mesenchymal stem cells and human umbilical vein endothelial cells. Int. J. Biol. Sci. 2016, 12, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, D.; Tu, J.; Zheng, Q.; Cai, L.; Wang, L. Strontium enhances osteogenic differentiation of mesenchymal stem cells and in vivo bone formation by activating Wnt/catenin signaling. Stem Cells 2011, 29, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Fielding, G.A.; Roy, M.; Bandyopadhyay, A.; Bose, S. Antibacterial and biological characteristics of silver containing and strontium doped plasma sprayed hydroxyapatite coatings. Acta Biomater. 2012, 8, 3144–3152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suganthi, R.; Elayaraja, K.; Joshy, M.A.; Chandra, V.S.; Girija, E.; Kalkura, S.N. Fibrous growth of strontium substituted hydroxyapatite and its drug release. Mater. Sci. Eng. C Mater. Biol. Appl. 2011, 31, 593–599. [Google Scholar] [CrossRef]
- Yu, N.; Cai, S.; Wang, F.; Zhang, F.; Ling, R.; Li, Y.; Jiang, Y.; Xu, G. Microwave assisted deposition of strontium doped hydroxyapatite coating on AZ31 magnesium alloy with enhanced mineralization ability and corrosion resistance. Ceram. Int. 2017, 43, 2495–2503. [Google Scholar] [CrossRef]
- Montesi, M.; Panseri, S.; Dapporto, M.; Tampieri, A.; Sprio, S. Sr-substituted bone cements direct mesenchymal stem cells, osteoblasts and osteoclasts fate. PLoS ONE 2017, 12, e0172100. [Google Scholar] [CrossRef] [PubMed]
- Landi, E.; Tampieri, A.; Celotti, G.; Sprio, S.; Sandri, M.; Logroscino, G. Sr-substituted hydroxyapatites for osteoporotic bone replacement. Acta Biomater. 2007, 3, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.X.; Chiu, K.Y.; Lu, W.W.; Wang, Y.; Zhang, Y.G.; Hao, L.B.; Li, Z.Y.; Lam, W.M.; Lu, S.B.; Luk, K.D. Strontium-containing hydroxyapatite bioactive bone cement in revision hip arthroplasty. Biomaterials 2006, 27, 4348–4355. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.H.; Shepherd, D.V.; Best, S.M. Substituted hydroxyapatites for bone repair. J. Mater. Sci. Mater. Med. 2012, 23, 2335–2347. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Ge, K.; Gao, F.; Yan, W.; Liu, H.; Xue, L.; Jin, Y.; Ma, H.; Zhang, J. Biomimetic mineralized strontium-doped hydroxyapatite on porous poly (l-lactic acid) scaffolds for bone defect repair. Int. J. Nanomed. 2018, 13, 1707–1721. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, A.; Che Ab Aziz, Z.A. Bioceramic-based root canal sealers: A review. Int. J. Biomater. 2016, 2016, 9753210. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M.; Yamazaki, A.; Akao, M.; Aoki, H. Cytotoxicity of synthetic barium hydroxyapatite. Bio-Med. Mater. Eng. 1996, 6, 405–413. [Google Scholar]
- Shibata, S.; Doi, Y.; Takezawa, Y.; Wakamatsu, N.; Horiguchi, T.; Kamemizu, H.; Moriwaki, Y.; Kubo, F.; Haeuchi, Y. Self-setting apatite cement. VII. Barium-apatite as radio-opaque medium. Shika Zairyo Kikai/J. Jpn. Soc. Dent. Mater. Devices 1989, 8, 77–82. [Google Scholar]
- Kaygili, O.; Keser, S.; Dorozhkin, S.V.; Yakuphanoglu, F.; Al-Ghamdi, A.A.; Kirbag, S.; Sertkaya, D.; Ates, T.; Gursoy, N.C. Structural and dielectrical properties of Ag- and Ba-substituted hydroxyapatites. J. Inorg. Organomet. Polym. Mater. 2014, 24, 1001–1008. [Google Scholar] [CrossRef]
- Alshemary, A.Z.; Akram, M.; Goh, Y.-F.; Butt, F.K.; Hussian, R. In vitro bioactivity of barium doped biphasic calcium phosphate. J. Appl. Sci. Agric. 2014, 9, 102–106. [Google Scholar]
- Kolekar, T.V.; Thorat, N.D.; Yadav, H.M.; Magalad, V.T.; Shinde, M.A.; Bandgar, S.S.; Kim, J.H.; Agawane, G.L. Nanocrystalline hydroxyapatite doped with aluminium: A potential carrier for biomedical applications. Ceram. Int. 2016, 42, 5304–5311. [Google Scholar] [CrossRef]
- Mellier, C.; Fayon, F.; Boukhechba, F.; Verron, E.; LeFerrec, M.; Montavon, G.; Lesoeur, J.; Schnitzler, V.; Massiot, D.; Janvier, P.; et al. Design and properties of novel gallium-doped injectable apatitic cements. Acta Biomater. 2015, 24, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, P.; Teixeira, A.R.; Malzac, A.; Coelho, M.D.B. Gallium-containing hydroxyapatite for potential use in orthopedics. Mater. Chem. Phys. 2009, 117, 86–90. [Google Scholar] [CrossRef]
- Kurtjak, M.; Vukomanović, M.; Suvorov, D. Antibacterial nanocomposite of functionalized nanogold and gallium-doped hydroxyapatite. Mater. Lett. 2017, 193, 126–129. [Google Scholar] [CrossRef]
- Webster, T.J.; Massa-Schlueter, E.A.; Smith, J.L.; Slamovich, E.B. Osteoblast response to hydroxyapatite doped with divalent and trivalent cations. Biomaterials 2004, 25, 2111–2121. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, M.; Srivastava, P.; Pawar, H.S.; Francis, N.K.; Das, B.; Sathishkumar, G.; Subramanian, B.; Jaganathan, S.K.; George, G.; Anandhan, S. On-demand guided bone regeneration with microbial protection of ornamented SPU scaffold with bismuth-doped single crystalline hydroxyapatite: Augmentation and cartilage formation. ACS Appl. Mater. Interfaces 2016, 8, 4086–4100. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, G.; Bargan, A.M.; Luca, C. New bismuth-substituted hydroxyapatite nanoparticles for bone tissue engineering. JOM 2015, 67, 2534–2542. [Google Scholar] [CrossRef]
- Zare, B.; Faramarzi, M.A.; Sepehrizadeh, Z.; Shakibaie, M.; Rezaie, S.; Shahverdi, A.R. Biosynthesis and recovery of rod-shaped tellurium nanoparticles and their bactericidal activities. Mater. Res. Bull. 2012, 47, 3719–3725. [Google Scholar] [CrossRef]
- Zhong, C.L.; Qin, B.Y.; Xie, X.Y.; Bai, Y. Antioxidant and antimicrobial activity of tellurium dioxide nanoparticles sols. J. Nano Res. 2013, 25, 8–15. [Google Scholar] [CrossRef]
- Yahia, I.S.; Shkir, M.; AlFaify, S.; Ganesh, V.; Zahran, H.Y.; Kilany, M. Facile microwave-assisted synthesis of Te-doped hydroxyapatite nanorods and nanosheets and their characterizations for bone cement applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Y.; Courtney, H.S.; Bettenga, M.; Agrawal, C.M.; Bumgardner, J.D.; Ong, J.L. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 2006, 27, 5512–5517. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.-J.; Hsieh, M.-F.; Huang, K.-C.; Perng, L.-H.; Chou, F.-I.; Chin, T.-S. Anti-microbial hydroxyapatite particles synthesized by a sol–gel route. J. Sol-Gel Sci. Technol. 2005, 33, 229–239. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Chifiriuc, M.C.; Costescu, A.; Le Coustumer, P.; Predoi, D. Synthesis and antimicrobial activity of silver-doped hydroxyapatite nanoparticles. BioMed Res. Int. 2013, 2013, 916218. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Iconaru, S.L.; Le Coustumer, P.; Constantin, L.V.; Predoi, D. Antibacterial activity of silver-doped hydroxyapatite nanoparticles against gram-positive and gram-negative bacteria. Nanoscale Res. Lett. 2012, 7, 324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groza, A.; Ciobanu, C.; Popa, C.; Iconaru, S.; Chapon, P.; Luculescu, C.; Ganciu, M.; Predoi, D. Structural properties and antifungal activity against candida albicans biofilm of different composite layers based on Ag/Zn doped hydroxyapatite-polydimethylsiloxanes. Polymers 2016, 8, 131. [Google Scholar] [CrossRef]
- Kim, Y.S.; Min, B.G. Preparation of bio-polyurethane using castor oil and antibacterial hybrid films thereof with silver-doped hydroxyapatite. Fibers Polym. 2017, 18, 1841–1847. [Google Scholar] [CrossRef]
- Mirzaee, M.; Vaezi, M.; Palizdar, Y. Synthesis and characterization of silver doped hydroxyapatite nanocomposite coatings and evaluation of their antibacterial and corrosion resistance properties in simulated body fluid. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Rameshbabu, N.; Sampath Kumar, T.S.; Prabhakar, T.G.; Sastry, V.S.; Murty, K.V.; Prasad Rao, K. Antibacterial nanosized silver substituted hydroxyapatite: Synthesis and characterization. J. Biomed. Mater. Res. Part A 2007, 80, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Samani, S.; Hossainalipour, S.M.; Tamizifar, M.; Rezaie, H.R. In vitro antibacterial evaluation of sol-gel-derived Zn-, Ag-, and (Zn+Ag)-doped hydroxyapatite coatings against methicillin-resistant Staphylococcus aureus. J. Biomed. Mater. Res. Part A 2013, 101, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Dubey, A.K.; Kumar, S.; Saha, N.; Basu, B.; Gupta, R. In vitro biocompatibility and antimicrobial activity of wet chemically prepared Ca10−xAgx(PO4)6(OH)2 (0.0 ≤ x ≤ 0.5) hydroxyapatites. Mater. Sci. Eng. C Mater. Biol. Appl. 2011, 31, 1320–1329. [Google Scholar] [CrossRef]
- Ueno, M.; Miyamoto, H.; Tsukamoto, M.; Eto, S.; Noda, I.; Shobuike, T.; Kobatake, T.; Sonohata, M.; Mawatari, M. Silver-containing hydroxyapatite coating reduces biofilm formation by Methicillin-Resistant Staphylococcus aureus in vitro and in vivo. BioMed Res. Int. 2016, 2016, 8070597. [Google Scholar] [CrossRef] [PubMed]
- Dubnika, A.; Loca, D.; Rudovica, V.; Parekh, M.B.; Berzina-Cimdina, L. Functionalized silver doped hydroxyapatite scaffolds for controlled simultaneous silver ion and drug delivery. Ceram. Int. 2017, 43, 3698–3705. [Google Scholar] [CrossRef]
- Zhuk, I.; Rasskazova, L.; Korotchenko, N.; Kozik, V.; Kurzina, I. Synthesis and investigation of physico-chemical, antibacterial, biomymetic properties of silver and zinc containing hydroxyapatite. AIP Conf. Proc. 2017, 1899, 020017. [Google Scholar] [CrossRef]
- Fonseca, F.; Costa, A.; Campos, J.; Marçal, R.; Rocha, D. Bioactivity assessment of Ag-HA. Biomater. Med. Appl. 2017, 1, 1000106. [Google Scholar] [CrossRef]
- Begam, H.; Kundu, B.; Chanda, A.; Nandi, S.K. MG63 osteoblast cell response on Zn doped hydroxyapatite (HAp) with various surface features. Ceram. Int. 2017, 43, 3752–3760. [Google Scholar] [CrossRef]
- Hidalgo-Robatto, B.M.; López-Álvarez, M.; Azevedo, A.S.; Dorado, J.; Serra, J.; Azevedo, N.F.; González, P. Pulsed laser deposition of copper and zinc doped hydroxyapatite coatings for biomedical applications. Surf. Coat. Technol. 2018, 333, 168–177. [Google Scholar] [CrossRef]
- Li, Y.; Shi, X.; Li, W. Zinc-containing hydroxyapatite enhances cold-light-activated tooth bleaching treatment in vitro. BioMed Res. Int. 2017, 2017, 6261248. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, Y.; Huang, T.; Yu, Z.; Ma, K.; Yang, M.; Liu, Q.; Pan, H.; Wang, H.; Wang, J. Runx2/osterix and zinc uptake synergize to orchestrate osteogenic differentiation and citrate containing bone apatite formation. Adv. Sci. 2018, 5, 1700755. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.L.; Deniaud, A.; Michaud-Soret, I.; Guégan, R.; Motelica-Heino, M.; Predoi, D. Structural and biological assessment of zinc doped hydroxyapatite nanoparticles. J. Nanomater. 2016, 2016, 1062878. [Google Scholar] [CrossRef]
- Zhong, Z.; Ma, J. Fabrication, characterization, and in vitro study of zinc substituted hydroxyapatite/silk fibroin composite coatings on titanium for biomedical applications. J. Biomater. Appl. 2017, 32, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Akbar, S.; Sadiqa, A.; Kazmi, M. Novel continuous flow synthesis, characterization and antibacterial studies of nanoscale zinc substituted hydroxyapatite bioceramics. Inorg. Chim. Acta 2016, 453, 16–22. [Google Scholar] [CrossRef]
- Ohtsu, N.; Kakuchi, Y.; Ohtsuki, T. Antibacterial effect of zinc oxide/hydroxyapatite coatings prepared by chemical solution deposition. Appl. Surf. Sci. 2018, 445, 596–600. [Google Scholar] [CrossRef]
- Sathiskumar, S.; Vanaraj, S.; Sabarinathan, D.; Preethi, K. Evaluation of antibacterial and antibiofilm activity of synthesized zinc-hydroxyapatite biocomposites from Labeo rohita fish scale waste. Mater. Res. Express 2018, 5, 025407. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Prodan, A.M.; Buton, N.; Predoi, D. Structural characterization and antifungal studies of zinc-doped hydroxyapatite coatings. Molecules 2017, 22, 604. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Qu, X.; Lin, W.; Wang, C.; Zhu, D.; Dai, K.; Zheng, Y. In vitro and in vivo studies on zinc-hydroxyapatite composites as novel biodegradable metal matrix composite for orthopedic applications. Acta Biomater. 2018, 71, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Begam, H.; Nandi, S.K.; Chanda, A.; Kundu, B. Effect of bone morphogenetic protein on zn-hap and zn-hap/collagen composite: A systematic in vivo study. Res. Vet. Sci. 2017, 115, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Gopal, B. Copper substituted hydroxyapatite and fluorapatite: Synthesis, characterization and antimicrobial properties. Ceram. Int. 2014, 40, 15655–15662. [Google Scholar] [CrossRef]
- Stanić, V.; Dimitrijević, S.; Antić-Stanković, J.; Mitrić, M.; Jokić, B.; Plećaš, I.B.; Raičević, S. Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders. Appl. Surf. Sci. 2010, 256, 6083–6089. [Google Scholar] [CrossRef]
- Li, Y.; Ho, J.; Ooi, C.P. Antibacterial efficacy and cytotoxicity studies of copper (II) and titanium (IV) substituted hydroxyapatite nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2010, 30, 1137–1144. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Zhao, R.; Mao, H.; Yan, Y.; Pang, X. Antibacterial efficacy, corrosion resistance, and cytotoxicity studies of copper-substituted carbonated hydroxyapatite coating on titanium substrate. J. Mater. Sci. 2015, 50, 1688–1700. [Google Scholar] [CrossRef]
- Shi, F.; Liu, Y.; Zhi, W.; Xiao, D.; Li, H.; Duan, K.; Qu, S.; Weng, J. The synergistic effect of micro/nano-structured and Cu(2+)-doped hydroxyapatite particles to promote osteoblast viability and antibacterial activity. Biomed. Mater. 2017, 12, 035006. [Google Scholar] [CrossRef] [PubMed]
- Ananth, K.; Sun, J.; Bai, J. An innovative approach to manganese-substituted hydroxyapatite coating on zinc oxide–coated 316L SS for implant application. Int. J. Mol. Sci. 2018, 19, 2340. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ding, Q.; Han, S.; Yan, Y.; Pang, X. Characterisation, corrosion resistance and in vitro bioactivity of manganese-doped hydroxyapatite films electrodeposited on titanium. J. Mater. Sci. Mater. Med. 2013, 24, 1853–1864. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-I.; Son, M.-K.; Choe, H.-C.; Brantley, W.A. Bone-like apatite formation on manganese-hydroxyapatite coating formed on Ti-6Al-4V alloy by plasma electrolytic oxidation. Thin Solid Films 2016, 620, 126–131. [Google Scholar] [CrossRef]
- Kandori, K.; Murata, R.; Yamaguchi, Y.; Yoshioka, A. Protein adsorption behaviors onto Mn(II)-doped calcium hydroxyapatite particles with different morphologies. Colloid Surf. B Biointerfaces 2018, 167, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Widodo, J.; Lim, S.; Ooi, C.P. Synthesis and cytocompatibility of manganese (II) and iron (III) substituted hydroxyapatite nanoparticles. J. Mater. Sci. 2012, 47, 754–763. [Google Scholar] [CrossRef]
- Zilm, M.E.; Yu, L.; Hines, W.A.; Wei, M. Magnetic properties and cytocompatibility of transition-metal-incorporated hydroxyapatite. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 87, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Sarath Chandra, V.; Baskar, G.; Suganthi, R.; Elayaraja, K.; Ahymah Joshy, M.; Sofi Beaula, W.; Mythili, R.; Venkatraman, G.; Narayana Kalkura, S. Blood compatibility of iron-doped nanosize hydroxyapatite and its drug release. ACS Appl. Mater. Interfaces 2012, 4, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Ulum, M.; Arafat, A.; Noviana, D.; Yusop, A.; Nasution, A.; Kadir, M.A.; Hermawan, H. In vitro and in vivo degradation evaluation of novel iron-bioceramic composites for bone implant applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 36, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, L.; Sinha, S.; Singhababu, Y.N.; Verma, V.; Tripathy, S.; Nayar, S. Traversing the profile of biomimetically nanoengineered iron substituted hydroxyapatite: Synthesis, characterization, property evaluation, and drug release modeling. RSC Adv. 2018, 8, 19389–19401. [Google Scholar] [CrossRef]
- Hou, C.H.; Hou, S.M.; Hsueh, Y.S.; Lin, J.; Wu, H.C.; Lin, F.H. The in vivo performance of biomagnetic hydroxyapatite nanoparticles in cancer hyperthermia therapy. Biomaterials 2009, 30, 3956–3960. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.T.; Li, K.Y.; Meng, F.Q.; Lin, J.F.; Young, I.C.; Ivkov, R.; Lin, F.H. ROS-induced HepG2 cell death from hyperthermia using magnetic hydroxyapatite nanoparticles. Nanotechnology 2018, 29, 375101. [Google Scholar] [CrossRef] [PubMed]
- Sidambe, A.T. Biocompatibility of advanced manufactured titanium implants—A review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, M.; Yamada, M.; Wakamura, M.; Egusa, H.; Sakurai, K. Activation of osteoblastic function on titanium surface with titanium-doped hydroxyapatite nanoparticle coating: An in vitro study. Int. J. Oral Maxillofac. Implants 2017, 32, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Siek, D.; Czechowska, J.; Mróz, W.; Zima, A.; Burdyńska, S.; Załęczny, R.; Ślósarczyk, A. Bioactivity of cement type bone substitutes. Bull. Pol. Acad. Sci.-Tech. Sci. 2013, 61, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Surmeneva, M.A.; Vladescu, A.; Surmenev, R.A.; Pantilimon, C.M.; Braic, M.; Cotrut, C.M. Study on a hydrophobic ti-doped hydroxyapatite coating for corrosion protection of a titanium based alloy. RSC Adv. 2016, 6, 87665–87674. [Google Scholar] [CrossRef]
- Bandgar, S.S.; Yadav, H.M.; Shirguppikar, S.S.; Shinde, M.A.; Shejawal, R.V.; Kolekar, T.V.; Bamane, S.R. Enhanced hemolytic biocompatibility of hydroxyapatite by chromium (Cr3+) doping in hydroxyapatite nanoparticles synthesized by solution combustion method. J. Korean Ceram. Soc. 2017, 54, 158–166. [Google Scholar] [CrossRef]
- Pantaleão, S.D.M.; Santos, M.D.; Anjos, H.A.; Lima, A.D.; Valadares, B.L.B.; Nunes, R.; Feitosa, M.B.D.J. Evaluation of mutagenic effects of pure hydroxyapatite doped with chromium (III) through the SMART Test in Drosophila melanogaster Meigen, 1830 (Diptera: Drosophilidae). Braz. J. Biol. Sci. 2017, 4, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Tank, K.P.; Chudasama, K.S.; Thaker, V.S.; Joshi, M.J. Cobalt-doped nanohydroxyapatite: Synthesis, characterization, antimicrobial and hemolytic studies. J. Nanopart. Res. 2013, 15, 1644. [Google Scholar] [CrossRef]
- Kulanthaivel, S.; Roy, B.; Agarwal, T.; Giri, S.; Pramanik, K.; Pal, K.; Ray, S.S.; Maiti, T.K.; Banerjee, I. Cobalt doped proangiogenic hydroxyapatite for bone tissue engineering application. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Ignjatović, N.; Ajduković, Z.; Rajković, J.; Najman, S.; Mihailović, D.; Uskoković, D. Enhanced osteogenesis of nanosized cobalt-substituted hydroxyapatite. J. Bionic Eng. 2015, 12, 604–612. [Google Scholar] [CrossRef]
- Dhal, J.; Bose, S.; Bandyopadhyay, A. Influence of pentavalent dopant addition to polarization and bioactivity of hydroxyapatite. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3061–3068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ligot, S.; Godfroid, T.; Music, D.; Bousser, E.; Schneider, J.M.; Snyders, R. Tantalum-doped hydroxyapatite thin films: Synthesis and characterization. Acta Mater. 2012, 60, 3435–3443. [Google Scholar] [CrossRef]
- Civjan, S.; Huget, E.F.; DeSimon, L.B. Potential applications of certain nickel-titanium (nitinol) alloys. J. Dent. Res. 1975, 54, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.; Aldini, N.N.; Torricelli, P.; Giavaresi, G.; Borsari, V.; Lenger, H.; Bernauer, J.; Giardino, R.; Chiesa, R.; Cigada, A. A new austenitic stainless steel with negligible nickel content: An in vitro and in vivo comparative investigation. Biomaterials 2003, 24, 4929–4939. [Google Scholar] [CrossRef]
- Priya, B.A.; Senthilguru, K.; Agarwal, T.; Narayana, S.G.H.; Giri, S.; Pramanik, K.; Pal, K.; Banerjee, I. Nickel doped nanohydroxyapatite: Vascular endothelial growth factor inducing biomaterial for bone tissue engineering. RSC Adv. 2015, 5, 72515–72528. [Google Scholar] [CrossRef]
- Alshemary, A.Z.; Akram, M.; Goh, Y.-F.; Tariq, U.; Butt, F.K.; Abdolahi, A.; Hussain, R. Synthesis, characterization, in vitro bioactivity and antimicrobial activity of magnesium and nickel doped silicate hydroxyapatite. Ceram. Int. 2015, 41, 11886–11898. [Google Scholar] [CrossRef]
- Abutalib, M.M.; Yahia, I.S. Novel and facile microwave-assisted synthesis of Mo-doped hydroxyapatite nanorods: Characterization, gamma absorption coefficient, and bioactivity. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Sambito, M.A.; Aslani, A.; Kalkhoran, N.M.; Slamovich, E.B.; Webster, T.J. Increased osteoblast functions on undoped and yttrium-doped nanocrystalline hydroxyapatite coatings on titanium. Biomaterials 2006, 27, 2358–2369. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Bizios, R. Hydroxylapatite with substituted magnesium, zinc, cadmium, and yttrium. II. Mechanisms of osteoblast adhesion. J. Biomed. Mater. Res. 2002, 59, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Gabriel, M.C.; de Souza, S.A.; Gomes, S.C.; Assi, P.E.; Pinheiro Perri, M.L.; Liberato, W.; Matushita, C.S.; Gutfilen, B.; da Fonseca, L.M. 90 Yttrium-hydroxyapatite: A new therapeutic option for radioactive synovectomy in haemophilic synovitis. Haemophilia 2011, 17, e985–e989. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yang, Y.; Lv, M.; Song, W.; Song, Z. Oxidative stress and DNA damage in zebrafish liver due to hydroxyapatite nanoparticles-loaded cadmium. Chemosphere 2018, 202, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Qiu, W.; Yu, Z.; Song, Z. Toxic effect of cadmium adsorbed by different sizes of nano-hydroxyapatite on the growth of rice seedlings. Environ. Toxicol. Pharmacol. 2017, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shkir, M.; Kilany, M.; Yahia, I.S. Facile microwave-assisted synthesis of tungsten-doped hydroxyapatite nanorods: A systematic structural, morphological, dielectric, radiation and microbial activity studies. Ceram. Int. 2017, 43, 14923–14931. [Google Scholar] [CrossRef]
- Maggiorella, L.; Barouch, G.; Devaux, C.; Pottier, A.; Deutsch, E.; Bourhis, J.; Borghi, E.; Levy, L. Nanoscale radiotherapy with hafnium oxide nanoparticles. Future Oncol. 2012, 8, 1167–1181. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.G.; Frutis, M.A.; Flores, G.A.; Hipólito, M.G.; Cerda, A.M.; Nieto, J.A.; Montalvo, T.R.; Falcony, C. Synthesis and characterization of hafnium oxide films for thermo and photoluminescence applications. Appl. Radiat. Isot. 2010, 68, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Hanagata, N.; Ikoma, T.; Huang, J.Y.; Li, K.Y.; Lin, C.P.; Lin, F.H. Hafnium-doped hydroxyapatite nanoparticles with ionizing radiation for lung cancer treatment. Acta Biomater. 2016, 37, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Lou, W.; Dong, Y.; Zhang, H.; Jin, Y.; Hu, X.; Ma, J.; Liu, J.; Wu, G. Preparation and characterization of lanthanum-incorporated hydroxyapatite coatings on titanium substrates. Int. J. Mol. Sci. 2015, 16, 21070–21086. [Google Scholar] [CrossRef] [PubMed]
- Bulina, N.V.; Chaikina, M.V.; Prosanov, I.Y.; Dudina, D.V.; Solovyov, L.A. Fast synthesis of La-substituted apatite by the dry mechanochemical method and analysis of its structure. J. Solid State Chem. 2017, 252, 93–99. [Google Scholar] [CrossRef]
- Guo, D.G.; Wang, A.H.; Han, Y.; Xu, K.W. Characterization, physicochemical properties and biocompatibility of La-incorporated apatites. Acta Biomater. 2009, 5, 3512–3523. [Google Scholar] [CrossRef] [PubMed]
- Hendi, A.A. Hydroxyapatite based nanocomposite ceramics. J. Alloys Compd. 2017, 712, 147–151. [Google Scholar] [CrossRef]
- Sundarabharathi, L.; Chinnaswamy, M.; Ponnamma, D.; Parangusan, H.; Al-Maadeed, M.A.A. Investigation of antimicrobial properties and in-vitro bioactivity of Ce3+-Sr2+ dual-substituted nano hydroxyapatites. J. Am. Ceram. Soc. 2018. [Google Scholar] [CrossRef]
- Kaur, K.; Singh, K.J.; Anand, V.; Islam, N.; Bhatia, G.; Kalia, N.; Singh, J. Lanthanide (=Ce, Pr, Nd and Tb) ions substitution at calcium sites of hydroxyl apatite nanoparticles as fluorescent bio probes: Experimental and density functional theory study. Ceram. Int. 2017, 43, 10097–10108. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Ning, C.; Nan, K.; Han, Y. Preparation and properties of a cerium-containing hydroxyapatite coating on commercially pure titanium by micro-arc oxidation. Rare Met. 2008, 27, 257–260. [Google Scholar] [CrossRef]
- Khusayfan, N.M. Ferroelectric properties of Ce doped hydroxyapatite nanoceramics. J. Alloys Compd. 2016, 685, 350–354. [Google Scholar] [CrossRef]
- Priyadarshini, B.; Anjaneyulu, U.; Vijayalakshmi, U. Preparation and characterization of sol-gel derived Ce4+ doped hydroxyapatite and its in vitro biological evaluations for orthopedic applications. Mater. Des. 2017, 119, 446–455. [Google Scholar] [CrossRef]
- Yuan, Q.; Qin, C.; Wu, J.; Xu, A.; Zhang, Z.; Liao, J.; Lin, S.; Ren, X.; Zhang, P. Synthesis and characterization of cerium-doped hydroxyapatite/polylactic acid composite coatings on metal substrates. Mater. Chem. Phys. 2016, 182, 365–371. [Google Scholar] [CrossRef]
- Setiawan, D.; Nurhasan, D. Sintesis dan karakterisasi praseodymium-142 hidroksiapatit (142pr-ha). In Proceedings of the Penelitian Dasar Ilmu Pengetahuan dan Teknologi Nuklir 2015 Pusat Sains dan Teknologi Akselerator, Yogyakarta, Indonesia, 9–10 June 2015; pp. 27–32. [Google Scholar]
- Starostenko, N.V.; Get’man, E.I.; Loboda, S.N.; Pasechnik, L.V.; Marchenko, V.I. Substitutions of praseodymium and silicon for calcium and phosphorus in hydroxyapatite structure. Russ. J. Inorg. Chem. 2012, 57, 1192–1195. [Google Scholar] [CrossRef]
- Victor, S.P.; Paul, W.; Vineeth, V.M.; Komeri, R.; Jayabalan, M.; Sharma, C.P. Neodymium doped hydroxyapatite theranostic nanoplatforms for colon specific drug delivery applications. Colloid Surf. B Biointerfaces 2016, 145, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Drouet, C.; Al-Kattan, A.; Navrotsky, A. Energetics of lanthanide-doped calcium phosphate apatite. Am. Miner. 2014, 99, 2320–2327. [Google Scholar] [CrossRef] [Green Version]
- Sathishkumar, S.; Louis, K.; Shinyjoy, E.; Gopi, D. Tailoring the Sm/Gd-substituted hydroxyapatite coating on biomedical AISI 316L SS: Exploration of corrosion resistance, protein profiling, osteocompatibility, and osteogenic differentiation for orthopedic implant applications. Ind. Eng. Chem. Res. 2016, 55, 6331–6344. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Popa, C.L.; Motelica-Heino, M.; Predoi, D. Evaluation of samarium doped hydroxyapatite, ceramics for medical application: Antimicrobial activity. J. Nanomater. 2015, 2015, 849216. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Maddila, S.N.; Jonnalagadda, S.B. Nanostructured samarium doped fluorapatites and their catalytic activity towards synthesis of 1,2,4-triazoles. Molecules 2016, 21, 1281. [Google Scholar] [CrossRef] [PubMed]
- Iconaru, S.; Stanciu, G.; Hristu, R.; Ghita, R. Properties of samarium doped hydroxyapatite thin films deposited by evaporation. Rom. Rep. Phys. 2017, 69, 508. [Google Scholar]
- Lv, Y.; Shi, Q.; Jin, Y.; Ren, H.; Qin, Y.; Wang, B.; Song, S. Preparation and luminescent properties of the antibacterial materials of the La3+ doped Sm3+-hydroxyapatite. J. Phys. Conf. Ser. 2018, 986, 012010. [Google Scholar] [CrossRef]
- Morais, D.S.; Coelho, J.; Ferraz, M.P.; Gomes, P.S.; Fernandes, M.H.; Hussain, N.S.; Santos, J.D.; Lopes, M.A. Samarium doped glass-reinforced hydroxyapatite with enhanced osteoblastic performance and antibacterial properties for bone tissue regeneration. J. Mater. Chem. B 2014, 2, 5872–5881. [Google Scholar] [CrossRef]
- Prichodko, A.; Enrichi, F.; Stankeviciute, Z.; Benedetti, A.; Grigoraviciute-Puroniene, I.; Kareiva, A. Study of Eu3+ and Tm3+ substitution effects in sol–gel fabricated calcium hydroxyapatite. J. Sol-Gel Sci. Technol. 2016, 81, 261–267. [Google Scholar] [CrossRef]
- Mondéjar, S.P.; Kovtun, A.; Epple, M. Lanthanide-doped calcium phosphate nanoparticles with high internal crystallinity and with a shell of DNA as fluorescent probes in cell experiments. J. Mater. Chem. 2007, 17, 4153–4159. [Google Scholar] [CrossRef]
- Andronescu, E.; Surugiu, A.; Badea, M.L.; Ciobanu, C.S.; Iosif, A. Antimicrobial activity of europium doped hydroxyapatite powders after immersion in SBF solution. Univ. Politeh. Buchar. Sci. Bull. Ser. B Chem. Mater. Sci. 2016, 78, 147–154. [Google Scholar]
- Frumosu, F.; Iconaru, S.; Predoi, D. Europium concentration effect of europium doped hydroxyapatite on proliferation of osteoblast cells. Dig. J. Nanomater. Biostruct. 2011, 6, 1859–1865. [Google Scholar]
- Miranda-Melendez, P.G.; Martinez-Castanon, G.A.; Nino-Martinez, N.; Patino-Marin, N.; Casillas-Santana, M.A.; Castillo-Silva, B.E.; Ruiz, F. Facile synthesis, characterization, and cytotoxic activity of europium-doped nanohydroxyapatite. Bioinorg. Chem. Appl. 2016, 2016, 1057260. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.L.; Ciobanu, C.S.; Iconaru, S.L.; Stan, M.; Dinischiotu, A.; Negrila, C.C.; Motelica-Heino, M.; Guegan, R.; Predoi, D. Systematic investigation and in vitro biocompatibility studies on mesoporous europium doped hydroxyapatite. Cent. Eur. J. Chem. 2014, 12, 1032–1046. [Google Scholar] [CrossRef] [Green Version]
- Victor, S.P.; Gayathri Devi, M.G.; Paul, W.; Vijayan, V.M.; Muthu, J.; Sharma, C.P. Europium doped calcium deficient hydroxyapatite as theranostic nanoplatforms: Effect of structure and aspect ratio. ACS Biomater. Sci. Eng. 2017, 3, 3588–3595. [Google Scholar] [CrossRef]
- Xu, L.; Zheng, T.; Yang, S.; Zhang, L.; Wang, J.; Liu, W.; Chen, L.; Diwu, J.; Chai, Z.; Wang, S. Uptake mechanisms of Eu(III) on hydroxyapatite: A potential permeable reactive barrier backfill material for trapping trivalent minor actinides. Environ. Sci. Technol. 2016, 50, 3852–3859. [Google Scholar] [CrossRef] [PubMed]
- Cipreste, M.F.; Peres, A.M.; Cotta, A.A.C.; Aragón, F.H.; Antunes, A.D.M.; Leal, A.S.; Macedo, W.A.A.; de Sousa, E.M.B. Synthesis and characterization of 159 Gd-doped hydroxyapatite nanorods for bioapplications as theranostic systems. Mater. Chem. Phys. 2016, 181, 301–311. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Lin, C.; Zhong, W. Sol-gel derived terbium-containing mesoporous bioactive glasses nanospheres: In vitro hydroxyapatite formation and drug delivery. Colloid Surf. B Biointerfaces 2017, 160, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-Z.; Yang, M.; Zhang, H.-B.; Zhu, J.; Zhou, K.-C. Characterization of terbium-doped nano-hydroxyapatite and surface modification. J. Cent. South Univ. 2016, 23, 1548–1555. [Google Scholar] [CrossRef]
- Unni, P.; Chaudhari, P.; Venkatesh, M.; Ramamoorthy, N.; Pillai, M. Preparation and bioevaluation of 166Ho labelled hydroxyapatite (HA) particles for radiosynovectomy. Nucl. Med. Biol. 2002, 29, 199–209. [Google Scholar] [CrossRef]
- Alshemary, A.Z.; Akram, M.; Goh, Y.-F.; Abdul Kadir, M.R.; Abdolahi, A.; Hussain, R. Structural characterization, optical properties and in vitro bioactivity of mesoporous erbium-doped hydroxyapatite. J. Alloys Compd. 2015, 645, 478–486. [Google Scholar] [CrossRef] [Green Version]
- Zavala, L.A.; Fernández, P.; Novitskaya, E.; Díaz, J.N.; Herrera, M.; Graeve, O.A. Interconfigurational and intraconfigurational transitions of Yb2+ and Yb3+ ions in hydroxyapatite: A cathodoluminescence study. Acta Mater. 2017, 135, 35–43. [Google Scholar] [CrossRef]
- Chatelain, G.; Bourgeois, D.; Ravaux, J.; Averseng, O.; Vidaud, C.; Meyer, D. Alternate dipping preparation of biomimetic apatite layers in the presence of carbonate ions. Biomed. Mater. 2014, 9, 015003. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, G.; Bourgeois, D.; Ravaux, J.; Averseng, O.; Vidaud, C.; Meyer, D. Incorporation of uranium into a biomimetic apatite: Physicochemical and biological aspects. J. Biol. Inorg. Chem. 2015, 20, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Cawthray, J.F.; Creagh, A.L.; Haynes, C.A.; Orvig, C. Ion exchange in hydroxyapatite with lanthanides. Inorg. Chem. 2015, 54, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, Z.; Cheng, J. Preparation, Characterization and Antibacterial Property of Cerium Substituted Hydroxyapatite Nanoparticles. J. Rare Earths 2007, 25, 452–456. [Google Scholar] [CrossRef]
- El-Meliegy, E.; Farag, M.M.; El-Kady, A.M.; Mohamed, M.S.; Abdelhakim, H.K.; Moaness, M. Evaluation of solubility and cytotoxicity of lanthanum-doped phosphate glasses nanoparticles for drug delivery applications. J. Non-Cryst. Solids 2017, 475, 59–70. [Google Scholar] [CrossRef]
- Ahymah Joshy, M.I.; Elayaraja, K.; Suganthi, R.V.; Chandra Veerla, S.; Kalkura, S.N. In vitro sustained release of amoxicillin from lanthanum hydroxyapatite nano rods. Curr. Appl. Phys. 2011, 11, 1100–1106. [Google Scholar] [CrossRef]
- Jadalannagari, S.; Deshmukh, K.; Verma, A.K.; Kowshik, R.V.; Meenal Ramanan, S.R. Lanthanum-doped hydroxyapatite nanoparticles as biocompatible fluorescent probes for cellular internalization and biolabeling. Sci. Adv. Mater. 2014, 6, 312–319. [Google Scholar] [CrossRef]
- Baskaran, P.; Udduttula, A.; Uthirapathy, V. Development and characterisation of novel Ce-doped hydroxyapatite–Fe3O4 nanocomposites and their in vitro biological evaluations for biomedical applications. IET Nanobiotechnol. 2018, 12, 138–146. [Google Scholar] [CrossRef]
- Ciobanu, C.; Popa, C.; Predoi, D. Cerium doped hydroxyapatite nanoparticles synthesized by coprecipitation method. J. Serb. Chem. Soc. 2016, 81, 433–446. [Google Scholar] [CrossRef]
- Heshmatpour, F.; Lashteneshaee, S.H.; Samadipour, M. Study of in vitro bioactivity of nano hydroxyapatite composites doped by various cations. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2063–2068. [Google Scholar] [CrossRef]
- Rajeswari, D.; Gopi, D.; Ramya, S.; Kavitha, L. Investigation of anticorrosive, antibacterial and in vitro biological properties of a sulphonated poly (etheretherketone)/strontium, cerium co-substituted hydroxyapatite composite coating developed on surface treated surgical grade stainless steel for orthopedic applications. RSC Adv. 2014, 4, 61525–61536. [Google Scholar] [CrossRef]
- Ciobanu, G.; Maria Bargan, A.; Luca, C. New cerium(IV)-substituted hydroxyapatite nanoparticles: Preparation and characterization. Ceram. Int. 2015, 41, 12192–12201. [Google Scholar] [CrossRef]
- Huang, W.; Mao, Z.; Chen, L.; Chi, Y.; Jiang, H.; Zimba, B.L.; Xiong, G.; Wu, Q. Synthesis and characterisation of fluorescent and biocompatible hydroxyapatite nanoparticles with cerium doping. Micro Nano Lett. 2018, 13, 699–703. [Google Scholar] [CrossRef]
- Sanyal, V.; Raja, C.R. Influence of sol–gel derived strontium–cerium co-substitution in fluorohydroxyapatite and its in-vitro bioactivity. J. Sol-Gel Sci. Technol. 2017, 83, 596–608. [Google Scholar] [CrossRef]
- Andronescu, E.; Iordache, F.; Ciobanu, C.; Badea, M.; Costescu, A.; Prodan, A. Optical properties of bioactive europium doped hydroxyapatite (HAp: Eu3+). Optoelectron. Adv. Mater. Rapid Commun. 2015, 9, 1155–1159. [Google Scholar]
- Iconaru, S.-L.; Motelica-Heino, M.; Predoi, D. Study on europium-doped hydroxyapatite nanoparticles by fourier transform infrared spectroscopy and their antimicrobial properties. J. Spectrosc. 2013, 2013, 284285. [Google Scholar] [CrossRef] [Green Version]
- Ciobanu, C.; Massuyeau, F.; Andronescu, E.; Stan, M.; Dinischiotu, A.; Predoi, D. Biocompatibility study of europium doped crystalline hydroxyapatite bioceramics. Dig. J. Nanomater. Biostruct. 2011, 6, 1639–1647. [Google Scholar]
- Ma, X.; Liu, Y.; Wu, X.M.; Wang, C.; Li, Q.; Fu, T. Synthesis of europium-doped nanohydroxyapatite and its cytocompatibility with endothelial cells in vitro. Mater. Technol. 2016, 31, 23–27. [Google Scholar] [CrossRef]
- Tesch, A.; Wenisch, C.; Herrmann, K.H.; Reichenbach, J.R.; Warncke, P.; Fischer, D.; Muller, F.A. Luminomagnetic Eu(3+)- and Dy(3+)-doped hydroxyapatite for multimodal imaging. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, M.; Hui, J.; Fan, D.; Ma, H.; Zhang, X.; Wang, Y.; Wei, Y. Ln3+-doped hydroxyapatite nanocrystals: Controllable synthesis and cell imaging. Phys. Chem. Chem. Phys. 2015, 17, 20301–20307. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Flores, Y.; Suárez-Quezada, M.; Rojas-Trigos, J.B.; Suárez, V.; Mantilla, A. Sol-gel synthesis of Tb-doped hydroxyapatite with high performance as photocatalyst for 2, 4 dichlorophenoxyacetic acid mineralization. J. Chem. Technol. Biotechnol. 2017, 92, 1521–1530. [Google Scholar] [CrossRef]
- Lu, Y.H.; Xiao, X.F.; Zheng, X.; Wu, S.S.; Liu, R.F. Hydrothermal synthesis and characterization of Tb3+ doped hydroxyapatite. Adv. Mater. Res. 2011, 391–392, 709–713. [Google Scholar] [CrossRef]
- Luque, P.; Cervantes, D.; Gomez-Gutierrez, C.; Carrillo-Castillo, A.; Mota-Gonzalez, M.; Vilchis-Nestor, A.; Olivas, A. Synthesis and characterization of terbium doped hydroxyapatite at different percentages by weight. Dig. J. Nanomater. Biostruct. 2017, 12, 135–139. [Google Scholar]
- Yin, H.; Li, Y.; Bai, J.; Ma, M.; Liu, J. Effect of calcinations temperature on the luminescence intensity and fluorescent lifetime of Tb3+-doped hydroxyapatite (Tb-HA) nanocrystallines. J. Materiomics 2017, 3, 144–149. [Google Scholar] [CrossRef]
- Sun, L.J.; Ni, P.F.; Guo, D.G.; Fang, C.Q.; Wang, J.; Yang, F.; Huang, X.F.; Hao, Y.Z.; Zhu, H.; Xu, K.W. Synthesis and characterization of Tb-incorporated apatite nano-scale powders. J. Mater. Sci. Technol. 2012, 28, 773–778. [Google Scholar] [CrossRef]
- Wei, Y.; He, Y.; Li, X.; Chen, H.; Deng, X. Cellular uptake and delivery-dependent effects of Tb(3+)-doped hydroxyapatite nanorods. Molecules 2017, 22, 1043. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Manivasagan, P.; Bharathiraja, S.; Santha Moorthy, M.; Kim, H.H.; Seo, H.; Lee, K.D.; Oh, J. Magnetic hydroxyapatite: A promising multifunctional platform for nanomedicine application. Int. J. Nanomed. 2017, 12, 8389–8410. [Google Scholar] [CrossRef] [PubMed]
- Zarinfar, A.; Shafaei, M.; Ziaie, F. Synthesis, characterization and thermoluminescence properties of nano-structure gadolinium doped hydroxyapatite (HAP:Gd). Procedia Mater. Sci. 2015, 11, 293–298. [Google Scholar] [CrossRef]
- Laranjeira, M.S.; Moco, A.; Ferreira, J.; Coimbra, S.; Costa, E.; Santos-Silva, A.; Ferreira, P.J.; Monteiro, F.J. Different hydroxyapatite magnetic nanoparticles for medical imaging: Its effects on hemostatic, hemolytic activity and cellular cytotoxicity. Colloid Surf. B Biointerfaces 2016, 146, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Lafarga, A.K.; Pacheco Moises, F.P.; Gurinov, A.; Ortiz, G.G.; Carbajal Arizaga, G.G. Dual responsive dysprosium-doped hydroxyapatite particles and toxicity reduction after functionalization with folic and glucuronic acids. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.-H.; Van, H.N.; Tam, P.D.; Ha, H.N.T. A novel 1540 nm light emission from erbium doped hydroxyapatite/β-tricalcium phosphate through co-precipitation method. Mater. Lett. 2016, 167, 145–147. [Google Scholar] [CrossRef]
- Yang, H.; Yang, G.; Wang, C.; Xu, K.W. Preparation and biocompatibility of nanoscaled La/Ag/HAP powder. Adv. Mater. Res. 2011, 308–310, 2173–2179. [Google Scholar] [CrossRef]
- Lanone, S.; Rogerieux, F.; Geys, J.; Dupont, A.; Maillot-Marechal, E.; Boczkowski, J.; Lacroix, G.; Hoet, P. Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines. Part. Fibre Toxicol. 2009, 6, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wataha, J.C.; Hanks, C.; Craig, R.G. In vitro effect of metal ions on cellular metabolism and the correlation between these effects and the uptake of the ions. J. Biomed. Mater. Res. 1994, 28, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Wataha, J.C.; Hanks, C.T.; Sun, Z. Effect of cell line on in vitro metal ion cytotoxicity. Dent. Mater. 1994, 10, 156–161. [Google Scholar] [CrossRef]
- Bhugra, C.; Pikal, M.J. Role of thermodynamic, molecular, and kinetic factors in crystallization from the amorphous state. J. Pharm. Sci. 2008, 97, 1329–1349. [Google Scholar] [CrossRef] [PubMed]
- Karavelidis, V.; Karavas, E.; Giliopoulos, D.; Papadimitriou, S.; Bikiaris, D. Evaluating the effects of crystallinity in new biocompatible polyester nanocarriers on drug release behavior. Int. J. Nanomed. 2011, 6, 3021–3032. [Google Scholar] [CrossRef]
- Hsiao, I.-L.; Huang, Y.-J. Effects of serum on cytotoxicity of nano-and micro-sized ZnO particles. J. Nanopart. Res. 2013, 15, 1829. [Google Scholar] [CrossRef] [PubMed]
- Park, M.V.; Neigh, A.M.; Vermeulen, J.P.; de la Fonteyne, L.J.; Verharen, H.W.; Briedé, J.J.; van Loveren, H.; de Jong, W.H. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 2011, 32, 9810–9817. [Google Scholar] [CrossRef] [PubMed]
- Sohaebuddin, S.K.; Thevenot, P.T.; Baker, D.; Eaton, J.W.; Tang, L. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part. Fibre Toxicol. 2010, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Honma, R.; Sumita, M.; Hanawa, T. Cytotoxicity evaluation of ceramic particles of different sizes and shapes. J. Biomed. Mater. Res. Part A 2004, 68, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Zapór, L. Effects of silver nanoparticles of different sizes on cytotoxicity and oxygen metabolism disorders in both reproductive and respiratory system cells. Arch. Environ. Prot. 2016, 42, 32–47. [Google Scholar] [CrossRef] [Green Version]
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Particle shape: A new design parameter for micro-and nanoscale drug delivery carriers. J. Control. Release 2007, 121, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Gratton, S.E.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Liao, J.; Shao, X.; Li, Q.; Lin, Y. The effect of shape on cellular uptake of gold nanoparticles in the forms of stars, rods, and triangles. Sci. Rep. 2017, 7, 3827. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ng, S.; Heng, B.C.; Guo, J.; Ma, L.; Tan, T.T.Y.; Ng, K.W.; Loo, S.C.J. Cytotoxicity of hydroxyapatite nanoparticles is shape and cell dependent. Arch. Toxicol. 2013, 87, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, K.A.; Landolph, J.R. Role of valence state and solubility of chromium compounds on induction of cytotoxicity, mutagenesis, and anchorage independence in diploid human fibroblasts. Cancer Res. 1990, 50, 7835–7842. [Google Scholar] [PubMed]
- Collins, B.J.; Stout, M.D.; Levine, K.E.; Kissling, G.E.; Melnick, R.L.; Fennell, T.R.; Walden, R.; Abdo, K.; Pritchard, J.B.; Fernando, R.A. Exposure to hexavalent chromium resulted in significantly higher tissue chromium burden compared with trivalent chromium following similar oral doses to male F344/N rats and female B6C3F1 mice. Toxicol. Sci. 2010, 118, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Dasari, T.S.; Zhang, Y.; Yu, H. Antibacterial activity and cytotoxicity of gold (I) and (III) ions and gold nanoparticles. Biochem. Pharmacol. 2015, 4, 199. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, T.; Traversa, E. The effect of cerium valence states at cerium oxide nanoparticle surfaces on cell proliferation. Biomaterials 2014, 35, 4441–4453. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wu, C.; Lei, X.; Zhang, K.; Liu, C.; Ding, J.; Shi, X. Effect of oxidation time on cytocompatibility of ultrafine-grained pure Ti in micro-arc oxidation treatment. Surf. Coat. Technol. 2018, 342, 12–22. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, L.; Li, M.; Zhao, R.; Yang, X.; Ji, T.; Gu, Z.; Yin, J.-J.; Gao, X.; Nie, G. Deciphering the underlying mechanisms of oxidation-state dependent cytotoxicity of graphene oxide on mammalian cells. Toxicol. Lett. 2015, 237, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Namvar, F.; Rahman, H.S.; Mohamad, R.; Baharara, J.; Mahdavi, M.; Amini, E.; Chartrand, M.S.; Yeap, S.K. Cytotoxic effect of magnetic iron oxide nanoparticles synthesized via seaweed aqueous extract. Int. J. Nanomed. 2014, 9, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Foxx, D.N.; Ishaque, A.B.; Shen, E. Lead-induced cytotoxicity and transcriptional activation of stress genes in human liver carcinoma (HepG2) cells. Mol. Cell. Biochem. 2004, 255, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Allagui, M.; Vincent, C.; Gaubin, Y.; Croute, F. Lithium toxicity and expression of stress-related genes or proteins in A549 cells. Biochim. Biophys. Acta Mol. Cell 2007, 1773, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Madiehe, A.M.; Mampuru, L.J.; Tyobeka, E.M. Induction of apoptosis in HL-60 cells by lithium. Biochem. Biophys. Res. Commun. 1995, 209, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Repetto, G.; Del Peso, A.; Sanz, P.; Repetto, M. In vitro effects of lithium and nickel at different levels on Neuro-2a mouse Neuroblastoma cells. Toxicol. In Vitro 2001, 15, 363–368. [Google Scholar] [CrossRef]
- Garland, E.; Parr, J.; Williamson, D.; Cohen, S. In vitro cytotoxicity of the sodium, potassium and calcium salts of saccharin, sodium ascorbate, sodium citrate and sodium chloride. Toxicol. In Vitro 1989, 3, 201–205. [Google Scholar] [CrossRef]
- Hasan, M.S.; Werner-Zwanziger, U.; Boyd, D. Composition-structure-properties relationship of strontium borate glasses for medical applications. J. Biomed. Mater. Res. Part A 2015, 103, 2344–2354. [Google Scholar] [CrossRef] [PubMed]
- Jeng, J.-H.; Hsieh, C.; Lan, W.; Chang, M.; Lin, S.; Hahn, L.; Kuo, M. Cytotoxicity of sodium fluoride on human oral mucosal fibroblasts and its mechanisms. Cell Biol. Toxicol. 1998, 14, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Slameňová, D.; Gabelova, A.; Ruppova, K. Cytotoxicity and genotoxicity testing of sodium fluoride on Chinese hamster V79 cells and human EUE cells. Mutat. Res. 1992, 279, 109–115. [Google Scholar] [CrossRef]
- Gao, J.-C.; Qiao, L.-Y.; Xin, R.-L. Effect of Mg2+ concentration on biocompatibility of pure magnesium. Front. Mater. Sci. China 2010, 4, 126–131. [Google Scholar] [CrossRef]
- Liu, X.; Xi, T.; Zheng, Y. Influence of the extraction parameters on the cytotoxicity test results of Mg materials. Prog. Nat. Sci. 2014, 24, 507–515. [Google Scholar] [CrossRef]
- Mirmalek, S.; Jangholi, E.; Jafari, M.; Yadollah-Damavandi, S.; Javidi, M.; Parsa, Y.; Parsa, T.; Salimi-Tabatabaee, S.A.; Kolagar, H.G.; Jalil, S.K. Comparison of in vitro cytotoxicity and apoptogenic activity of magnesium chloride and cisplatin as conventional chemotherapeutic agents in the MCF-7 cell line. Asian Pac. J. Cancer Prev. 2016, 17, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.M.; Yeung, K.W.; Lam, K.O.; Tam, V.; Chu, P.K.; Luk, K.D.; Cheung, K.M. A biodegradable polymer-based coating to control the performance of magnesium alloy orthopaedic implants. Biomaterials 2010, 31, 2084–2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aimaiti, A.; Maimaitiyiming, A.; Boyong, X.; Aji, K.; Li, C.; Cui, L. Low-dose strontium stimulates osteogenesis but high-dose doses cause apoptosis in human adipose-derived stem cells via regulation of the ERK1/2 signaling pathway. Stem Cell Res. Ther. 2017, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Bonnelye, E.; Chabadel, A.; Saltel, F.; Jurdic, P. Dual effect of strontium ranelate: Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 2008, 42, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Er, K.; Polat, Z.A.; Özan, F.; Taşdemir, T.; Sezer, U.; Siso, Ş.H. Cytotoxicity analysis of strontium ranelate on cultured human periodontal ligament fibroblasts: A preliminary report. J. Formos. Med. Assoc. 2008, 107, 609–615. [Google Scholar] [CrossRef]
- Hallab, N.J.; Anderson, S.; Caicedo, M.; Jacobs, J.J. Zirconium and niobium affect human osteoblasts, fibroblasts, and lymphocytes in a similar manner to more traditional implant alloy metals. J. ASTM Int. 2006, 3, 1–12. [Google Scholar] [CrossRef]
- Puleo, D.A.; Huh, W.W. Acute toxicity of metal ions in cultures of osteogenic cells derived from bone marrow stromal cells. J. Appl. Biomater. 1995, 6, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.L.; Wataha, J.C.; Hanks, C.T. Effects of metal ions on osteoblast-like cell metabolism and differentiation. J. Biomed. Mater. Res. 1997, 34, 29–37. [Google Scholar] [CrossRef]
- Milheiro, A.; Nozaki, K.; Kleverlaan, C.J.; Muris, J.; Miura, H.; Feilzer, A.J. In vitro cytotoxicity of metallic ions released from dental alloys. Odontology 2016, 104, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Damati, A.; Vlastos, D.; Philippopoulos, A.I.; Matthopoulos, D.P. Inorganic tin compounds do not induce micronuclei in human lymphocytes in the absence of metabolic activation. Drug Chem. Toxicol. 2014, 37, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.S.; Pierre, T.H.; McCall, R.; Wu, J.; Gato, W.E. Evaluating the cytotoxicity of tin dioxide nanofibers. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2018, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Aydin, N.; Arslan, M.E.; Sonmez, E.S.; Turkez, H. Cytotoxicity analysis of tellurium dioxide nanoparticles on cultured human pulmonary alveolar epithelial and peripheral blood cell cultures. Biomed. Res. 2017, 28, 3300–3304. [Google Scholar]
- Mahto, S.K.; Vinod, T.; Kim, J.-K.; Rhee, S.-W. Cytotoxic potentials of tellurium nanowires in BALB/3T3 fibroblast cells. Bull. Korean Chem. Soc. 2011, 32, 3405–3410. [Google Scholar] [CrossRef]
- Wen, H.; Zhong, J.; Shen, B.; Gan, T.; Fu, C.; Zhu, Z.; Li, R.; Yang, X. Comparative study of the cytotoxicity of the nanosized and microsized tellurium powders on HeLa cells. Front. Biol. 2013, 8, 444–450. [Google Scholar] [CrossRef]
- Aykin-Burns, N.; Laegeler, A.; Kellogg, G.; Ercal, N. Oxidative effects of lead in young and adult Fisher 344 rats. Arch. Environ. Contam. Toxicol. 2003, 44, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Bruinink, A.; Reiser, P.; Müller, M.; Gähwiler, B.; Zbinden, G. Neurotoxic effects of bismuth in vitro. Toxicol. In Vitro 1992, 6, 285–293. [Google Scholar] [CrossRef]
- Hernandez-Delgadillo, R.; Badireddy, A.R.; Zaragoza-Magaña, V.; Sánchez-Nájera, R.I.; Chellam, S.; Cabral-Romero, C. Effect of lipophilic bismuth nanoparticles on erythrocytes. J. Nanomater. 2015, 2015, 264024. [Google Scholar] [CrossRef]
- Liman, R. Genotoxic effects of Bismuth(III) oxide nanoparticles by Allium and Comet assay. Chemosphere 2013, 93, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, C.; Qiao, Y.; Hossain, M.; Ma, L.; Su, M. In vitro cytotoxicity of surface modified bismuth nanoparticles. J. Mater. Sci. Mater. Med. 2012, 23, 2563–2573. [Google Scholar] [CrossRef] [PubMed]
- Apaza-Bedoya, K.; Bijukumar, D.; Benfatti, C.; Mathew, M.; da Silva, J.; Souza, J. Adverse local and systemic effect of nanoparticles released from oral and cranio-maxillofacial implants. In Nanostructured Biomaterials for Cranio-Maxillofacial and Oral Applications; Souza, J., Hotza, D., Henrique, B., Boccaccini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 63–79. ISBN 9-780-128-14621-7. [Google Scholar]
- Khan, M.; Naqvi, A.H.; Ahmad, M. Comparative study of the cytotoxic and genotoxic potentials of zinc oxide and titanium dioxide nanoparticles. Toxicol. Rep. 2015, 2, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Bellusci, M.; La Barbera, A.; Padella, F.; Mancuso, M.; Pasquo, A.; Grollino, M.G.; Leter, G.; Nardi, E.; Cremisini, C.; Giardullo, P. Biodistribution and acute toxicity of a nanofluid containing manganese iron oxide nanoparticles produced by a mechanochemical process. Int. J. Nanomed. 2014, 9, 1919–1929. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, A.M.; Santos, D.; Au, C.; Milatovic, D.; Aschner, M.; Batoréu, M.C.C. Antioxidants prevent the cytotoxicity of manganese in RBE4 cells. Brain Res. 2008, 1236, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Hernroth, B.; Holm, I.; Gondikas, A.; Tassidis, H. Manganese inhibits viability of prostate cancer cells. Anticancer Res. 2018, 38, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Samim, M.; Abdin, M.; Ahmed, F.J.; Maitra, A.; Prashant, C.; Dinda, A.K. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int. J. Nanomed. 2010, 5, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Garza-Cervantes, J.A.; Chávez-Reyes, A.; Castillo, E.C.; García-Rivas, G.; Ortega-Rivera, O.A.; Salinas, E.; Ortiz-Martínez, M.; Gómez-Flores, S.L.; Peña-Martínez, J.A.; Pepi-Molina, A. Synergistic antimicrobial effects of silver/transition-metal combinatorial treatments. Sci. Rep. 2017, 7, 903. [Google Scholar] [CrossRef] [PubMed]
- Kanaji, A.; Orhue, V.; Caicedo, M.S.; Virdi, A.S.; Sumner, D.R.; Hallab, N.J.; Yoshiaki, T.; Sena, K. Cytotoxic effects of cobalt and nickel ions on osteocytes in vitro. J. Orthop. Surg. Res. 2014, 9, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ada, K.; Turk, M.; Oguztuzun, S.; Kilic, M.; Demirel, M.; Tandogan, N.; Ersayar, E.; Latif, O. Cytotoxicity and apoptotic effects of nickel oxide nanoparticles in cultured HeLa cells. Folia Histochem. Cytobiol. 2010, 48, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M. Toxic response of nickel nanoparticles in human lung epithelial A549 cells. Toxicol. In Vitro 2011, 25, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Anchordoquy, J.M.; Anchordoquy, J.P.; Nikoloff, N.; Pascua, A.M.; Furnus, C.C. High copper concentrations produce genotoxicity and cytotoxicity in bovine cumulus cells. Environ. Sci. Pollut. Res. 2017, 24, 20041–20049. [Google Scholar] [CrossRef] [PubMed]
- Sahu, D.; Kannan, G.; Tailang, M.; Vijayaraghavan, R. In vitro cytotoxicity of nanoparticles: A comparison between particle size and cell type. J. Nanosci. 2016, 2016, 4023852. [Google Scholar] [CrossRef]
- Andelman, T.; Gordonov, S.; Busto, G.; Moghe, P.V.; Riman, R.E. Synthesis and cytotoxicity of Y2O3 nanoparticles of various morphologies. Nanoscale Res. Lett. 2010, 5, 263. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kasuga, T.; Kato, K. Effects of Y2O3 particle size on cytotoxicity and cell morphology. J. Ceram. Soc. Jpn. 2010, 118, 428–433. [Google Scholar] [CrossRef]
- Selvaraj, V.; Bodapati, S.; Murray, E.; Rice, K.M.; Winston, N.; Shokuhfar, T.; Zhao, Y.; Blough, E. Cytotoxicity and genotoxicity caused by yttrium oxide nanoparticles in HEK293 cells. Int. J. Nanomed. 2014, 9, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Braydich-Stolle, L.; Hussain, S.; Schlager, J.J.; Hofmann, M.-C. In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol. Sci. 2005, 88, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.A.; Saquib, Q.; Ahamed, M.; Farshori, N.N.; Ahmad, J.; Wahab, R.; Khan, S.T.; Alhadlaq, H.A.; Musarrat, J.; Al-Khedhairy, A.A. Molybdenum nanoparticles-induced cytotoxicity, oxidative stress, G2/M arrest, and DNA damage in mouse skin fibroblast cells (L929). Colloid Surf. B Biointerfaces 2015, 125, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Ostad, S.N.; Dehnad, S.; Nazari, Z.E.; Fini, S.T.; Mokhtari, N.; Shakibaie, M.; Shahverdi, A.R. Cytotoxic activities of silver nanoparticles and silver ions in parent and tamoxifen-resistant T47D human breast cancer cells and their combination effects with tamoxifen against resistant cells. Avicenna J. Med. Biotechnol. 2010, 2, 187. [Google Scholar] [PubMed]
- Piao, M.J.; Kang, K.A.; Lee, I.K.; Kim, H.S.; Kim, S.; Choi, J.Y.; Choi, J.; Hyun, J.W. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol. Lett. 2011, 201, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Składanowski, M.; Golinska, P.; Rudnicka, K.; Dahm, H.; Rai, M. Evaluation of cytotoxicity, immune compatibility and antibacterial activity of biogenic silver nanoparticles. Med. Microbiol. Immunol. 2016, 205, 603–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, T.A.; Franchi, L.P.; Rosa, L.R.; da Veiga, M.A.; Takahashi, C.S. Cytotoxicity and genotoxicity of silver nanoparticles of different sizes in CHO-K1 and CHO-XRS5 cell lines. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 795, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, R.; Wang, F.; Liu, X.; Zhang, J.; Hu, L.; Shi, J.; He, B.; Zhou, Q.; Song, M. Determining the cytotoxicity of rare earth element nanoparticles in macrophages and the involvement of membrane damage. Environ. Sci. Technol. 2017, 51, 13938–13948. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I.; Momekov, G.; Tzanova, T.; Karaivanova, M. Synthesis, characterization, and cytotoxic activity of new lanthanum(III) complexes of bis-coumarins. Bioinorg. Chem. Appl. 2006, 2006, 25651. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.-H. Toxicity of two different sized lanthanum oxides in cultured cells and Sprague-Dawley rats. Toxicol. Res. 2015, 31, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, W.; Perone, P.; Walker, K.; Bhagavathula, N.; Aslam, M.N.; DaSilva, M.; Dame, M.K.; Varani, J. Fibroblast response to lanthanoid metal ion stimulation: Potential contribution to fibrotic tissue injury. Biol. Trace Elem. Res. 2011, 144, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ansari, A.A.; Rolfo, C.; Coelho, A.; Abdulla, M.; Al-Khayal, K.; Ahmad, R. Evaluation of in vitro cytotoxicity, biocompatibility, and changes in the expression of apoptosis regulatory proteins induced by cerium oxide nanocrystals. Sci. Technol. Adv. Mater. 2017, 18, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Huang, Y.-W.; Zhou, X.-D.; Ma, Y. Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int. J. Toxicol. 2006, 25, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Andiappan, K.; Sanmugam, A.; Deivanayagam, E.; Karuppasamy, K.; Kim, H.-S.; Vikraman, D. In vitro cytotoxicity activity of novel Schiff base ligand–lanthanide complexes. Sci. Rep. 2018, 8, 3054. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I.; Momekov, G. Synthesis, characterization and cytotoxicity evaluation of new cerium(III), lanthanum(III) and neodymium(III) complexes. Appl. Organomet. Chem. 2007, 21, 226–233. [Google Scholar] [CrossRef]
- Harper, S.; Usenko, C.; Tanguay, R. Differential distribution and toxicity of nanomaterials in vivo. In Proceedings of the 2006 AIChE Annual Meeting, San Francisco, CA, USA, 12–17 November 2006; pp. 12–17. [Google Scholar]
- Cho, S.; Lee, Y.; Lee, S.; Choi, Y.J.; Chung, H.W. Enhanced cytotoxic and genotoxic effects of gadolinium following ELF-EMF irradiation in human lymphocytes. Drug Chem. Toxicol. 2014, 37, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.C.; Kuhlmann, M.K.; Kohlbacher, S.; Scheer, M.; Grgic, A.; Heckmann, M.B.; Uder, M. Cytotoxicity of iodinated and gadolinium-based contrast agents in renal tubular cells at angiographic concentrations: In vitro study. Radiology 2007, 242, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Paltoo, D. Effects of terbium on the cytotoxicity of cisplatin in FaDu human head and neck squamous cell carcinoma. Cancer Biochem. Biophys. 1998, 16, 213–227. [Google Scholar] [PubMed]
- Atabaev, T.S.; Shin, Y.C.; Song, S.-J.; Han, D.-W.; Hong, N.H. Toxicity and T2-weighted magnetic resonance imaging potentials of holmium oxide nanoparticles. Nanomaterials 2017, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Carmona, A.; Malard, V.; Avazeri, E.; Roudeau, S.; Porcaro, F.; Paredes, E.; Vidaud, C.; Bresson, C.; Ortega, R. Uranium exposure of human dopaminergic cells results in low cytotoxicity, accumulation within sub-cytoplasmic regions, and down regulation of MAO-B. Neurotoxicology 2018, 68, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Thiébault, C.; Carriere, M.; Milgram, S.; Simon, A.; Avoscan, L.; Gouget, B. Uranium induces apoptosis and is genotoxic to normal rat kidney (NRK-52E) proximal cells. Toxicol. Sci. 2007, 98, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Popa, A.C.; Stan, G.E.; Husanu, M.A.; Mercioniu, I.; Santos, L.; Fernandes, H.R.; Ferreira, J.M.F. Bioglass implant-coating interactions in synthetic physiological fluids with varying degrees of biomimicry. Int. J. Nanomed. 2017, 12, 683–707. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Sueoka, M.; Shirosaki, Y.; Shinozaki, N.; Shiraishi, T. Development of hafnium metal and titanium-hafnium alloys having apatite-forming ability by chemical surface modification. J. Biomed. Mater. Res. Part B 2017. [Google Scholar] [CrossRef] [PubMed]
- Renke-Gluszko, M.; El Fray, M. The effect of simulated body fluid on the mechanical properties of multiblock poly (aliphatic/aromatic-ester) copolymers. Biomaterials 2004, 25, 5191–5198. [Google Scholar] [CrossRef] [PubMed]
- Thaveedeetrakul, A.; Witit-anun, N.; Boonamnuayvitaya, V. The role of target-to-substrate distance on the DC magnetron sputtered zirconia thin films’ bioactivity. Appl. Surf. Sci. 2012, 258, 2612–2619. [Google Scholar] [CrossRef]
- Bohner, M.; Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.T.; Leng, Y.; Chow, K.L.; Ren, F.; Ge, X.; Wang, K.; Lu, X. Cell culture medium as an alternative to conventional simulated body fluid. Acta Biomater. 2011, 7, 2615–2622. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Tao, J.; Cai, Y.; Pan, H.; Xu, X.; Tang, R. Role of fetal bovine serum in the prevention of calcification in biological fluids. J. Cryst. Growth 2008, 310, 4672–4675. [Google Scholar] [CrossRef]
- Mahmood, T.; Davies, J. Incorporation of amino acids within the surface reactive layers of bioactive glass in vitro: An XPS study. J. Mater. Sci. Mater. Med. 2000, 11, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Tas, A. The use of physiological solutions or media in calcium phosphate synthesis and processing. Acta Biomater. 2014, 10, 1771–1792. [Google Scholar] [CrossRef] [PubMed]
- Popa, A.C.; Stan, G.E.; Besleaga, C.; Ion, L.; Maraloiu, V.A.; Tulyaganov, D.U.; Ferreira, J.M.F. Submicrometer hollow bioglass cones deposited by radio frequency magnetron sputtering: Formation mechanism, properties, and prospective biomedical applications. ACS Appl. Mater. Interfaces 2016, 8, 4357–4367. [Google Scholar] [CrossRef] [PubMed]
- Almubarak, S.; Nethercott, H.; Freeberg, M.; Beaudon, C.; Jha, A.; Jackson, W.; Marcucio, R.; Miclau, T.; Healy, K.; Bahney, C. Tissue engineering strategies for promoting vascularized bone regeneration. Bone 2016, 83, 197–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalczewski, C.J.; Saul, J.M. Biomaterials for the delivery of growth factors and other therapeutic agents in tissue engineering approaches to bone regeneration. Front. Pharmacol. 2018, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.K.; Kundu, B.; Mukherjee, J.; Mahato, A.; Datta, S.; Balla, V.K. Converted marine coral hydroxyapatite implants with growth factors: In vivo bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Kalajzic, I.; Staal, A.; Yang, W.-P.; Wu, Y.; Johnson, S.E.; Feyen, J.H.; Krueger, W.; Maye, P.; Yu, F.; Zhao, Y.; et al. Expression profile of osteoblast lineage at defined stages of diferentiation. J. Biol. Chem. 2005, 280, 24618–24626. [Google Scholar] [CrossRef] [PubMed]
- Klontzas, M.E.; Vernardis, S.I.; Heliotis, M.; Tsiridis, E.; Mantalaris, A. Metabolomics analysis of the osteogenic differentiation of umbilical cord blood mesenchymal stem cells reveals differential sensitivity to osteogenic agents. Stem Cells Dev. 2017, 26, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lee, S.G.; Lee, S.W.; Oh, B.J.; Kim, J.H.; Kim, J.A.; Lee, G.; Jang, J.D.; Joe, Y.A. A subset of paracrine factors as efficient biomarkers for predicting vascular regenerative efficacy of mesenchymal stromal/stem cells. Stem Cells 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, E.; Castelli, G.; Testa, U. Endothelial progenitors. Blood Cells Mol. Dis. 2014, 52, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Liu, W.; Deng, Y.; Li, S.; Liu, X.; Gao, Y.; Zhou, L. Angiogenesis and bone regeneration of porous nano-hydroxyapatite/coralline blocks coated with rhVEGF165 in critical-size alveolar bone defects in vivo. Int. J. Nanomed. 2015, 10, 2555–2565. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.; Fielding, G.; Tarafder, S.; Bandyopadhyay, A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013, 31, 594–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulanthaivel, S.; Mishra, U.; Agarwal, T.; Giri, S.; Pal, K.; Pramanik, K.; Banerjee, I. Improving the osteogenic and angiogenic properties of synthetic hydroxyapatite by dual doping of bivalent cobalt and magnesium ion. Ceram. Int. 2015, 41, 11323–11333. [Google Scholar] [CrossRef]
- Birgani, Z.T.; Fennema, E.; Gijbels, M.J.; de Boer, J.; van Blitterswijk, C.A.; Habibovic, P. Stimulatory effect of cobalt ions incorporated into calcium phosphate coatings on neovascularization in an in vivo intramuscular model in goats. Acta Biomater. 2016, 36, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Giuffrida, R.; Forte, S.; Salvatorelli, L.; Fabbi, C.; Figallo, E.; Gulisano, M.; Parenti, R.; Magro, G.; Colarossi, C. Bone augmentation after ectopic implantation of a cell-free collagen-hydroxyapatite scaffold in the mouse. Sci. Rep. 2016, 6, 36399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L.; Li, D.; Yang, Z.; Xie, X.; Kang, P. Repair of the calvarial defect in goat model using magnesium-doped porous hydroxyapatite combined with recombinant human bone morphogenetic protein-2. Bio-Med. Mater. Eng. 2017, 28, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Ehret, C.; Aid-Launais, R.; Sagardoy, T.; Siadous, R.; Bareille, R.; Rey, S.; Pechev, S.; Etienne, L.; Kalisky, J.; De Mones, E. Strontium-doped hydroxyapatite polysaccharide materials effect on ectopic bone formation. PLoS ONE 2017, 12, e0184663. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xie, X.; Yang, Z.; Wang, C.; Wei, Z.; Kang, P. Enhanced bone defect repairing effects in glucocorticoid-induced osteonecrosis of the femoral head using a porous nano-lithium-hydroxyapatite/gelatin microsphere/erythropoietin composite scaffold. Biomater. Sci. 2018, 6, 519–537. [Google Scholar] [CrossRef] [PubMed]
- Madden, L.R.; Mortisen, D.J.; Sussman, E.M.; Dupras, S.K.; Fugate, J.A.; Cuy, J.L.; Hauch, K.D.; Laflamme, M.A.; Murry, C.E.; Ratner, B.D. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 15211–15216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliviero, O.; Ventre, M.; Netti, P. Functional porous hydrogels to study angiogenesis under the effect of controlled release of vascular endothelial growth factor. Acta Biomater. 2012, 8, 3294–3301. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Wang, W.; Liu, D.; Zhang, H.; Gao, P.; Geng, L.; Yuan, Y.; Lu, J.; Wang, Z. The promotion of angiogenesis induced by three-dimensional porous beta-tricalcium phosphate scaffold with different interconnection sizes via activation of Pi3K/Akt pathways. Sci. Rep. 2015, 5, 9409. [Google Scholar] [CrossRef] [PubMed]
- Artel, A.; Mehdizadeh, H.; Chiu, Y.-C.; Brey, E.M.; Cinar, A. An agent-based model for the investigation of neovascularization within porous scaffolds. Tissue Eng. A 2011, 17, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, Y.; Jin, Q.; Takita, H. Geometry of carriers controlling phenotypic expression in bmp-induced osteogenesis and chondrogenesis. J. Bone Jt. Surg. Am. Vol. 2001, 83, S1–S105. [Google Scholar] [CrossRef]
- Wang, Z.; Chang, J.; Bai, F.; Sun, X.; Lu, J.; Lin, L.; Chen, L.; Rong, D. Role of the porous structure of the bioceramic scaffolds in bone tissue engineering. Nat. Preced. 2010. Available online: http://precedings.nature.com/documents/4148/version/1 (accessed on 12 October 2018).
- Shanmugam, S.; Gopal, B. Antimicrobial and cytotoxicity evaluation of aliovalent substituted hydroxyapatite. Appl. Surf. Sci. 2014, 303, 277–281. [Google Scholar] [CrossRef]
- Turkoz, M.; Atilla, A.O.; Evis, Z. Silver and fluoride doped hydroxyapatites: Investigation by microstructure, mechanical and antibacterial properties. Ceram. Int. 2013, 39, 8925–8931. [Google Scholar] [CrossRef]
- Gopi, D.; Shinyjoy, E.; Kavitha, L. Synthesis and spectral characterization of silver/magnesium co-substituted hydroxyapatite for biomedical applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 127, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.; El-Dek, S.; Dorozhkin, S.; Ahmed, M. Physico-mechanical properties of Mg and Ag doped hydroxyapatite/chitosan biocomposites. New J. Chem. 2017, 41, 13773–13783. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, Z.; Zhang, X.; Chang, X.; Zhang, X.; Li, Y.; Ye, T.; Han, R.; Han, S.; Gao, Y. Nanotube-formed Ti substrates coated with silicate/silver co-doped hydroxyapatite as prospective materials for bone implants. J. Alloys Compd. 2017, 697, 182–199. [Google Scholar] [CrossRef]
- Geng, Z.; Cui, Z.; Li, Z.; Zhu, S.; Liang, Y.; Liu, Y.; Li, X.; He, X.; Yu, X.; Wang, R. Strontium incorporation to optimize the antibacterial and biological characteristics of silver-substituted hydroxyapatite coating. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Wang, R.; Zhuo, X.; Li, Z.; Huang, Y.; Ma, L.; Cui, Z.; Zhu, S.; Liang, Y.; Liu, Y. Incorporation of silver and strontium in hydroxyapatite coating on titanium surface for enhanced antibacterial and biological properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Kadir, M.R.A.; Mahmood, N.H.; Salim, N.; Froemming, G.R.; Balaji, H.; Kamarul, T. Characterization, antibacterial and in vitro compatibility of zinc–silver doped hydroxyapatite nanoparticles prepared through microwave synthesis. Ceram. Int. 2014, 40, 4507–4513. [Google Scholar] [CrossRef]
- Gopi, D.; Sathishkumar, S.; Karthika, A.; Kavitha, L. Development of Ce3+/Eu3+ dual-substituted hydroxyapatite coating on surgical grade stainless steel for improved antimicrobial and bioactive properties. Ind. Eng. Chem. Res. 2014, 53, 20145–20153. [Google Scholar] [CrossRef]
- Gopi, D.; Ramya, S.; Rajeswari, D.; Karthikeyan, P.; Kavitha, L. Strontium, cerium co-substituted hydroxyapatite nanoparticles: Synthesis, characterization, antibacterial activity towards prokaryotic strains and in vitro studies. Colloid Surf. A Physicochem. Eng. Asp. 2014, 451, 172–180. [Google Scholar] [CrossRef]
- Huang, Y.; Hao, M.; Nian, X.; Qiao, H.; Zhang, X.; Zhang, X.; Song, G.; Guo, J.; Pang, X.; Zhang, H. Strontium and copper co-substituted hydroxyapatite-based coatings with improved antibacterial activity and cytocompatibility fabricated by electrodeposition. Ceram. Int. 2016, 42, 11876–11888. [Google Scholar] [CrossRef]
- Lowry, N.; Brolly, M.; Han, Y.; McKillop, S.; Meenan, B.; Boyd, A. Synthesis and characterisation of nanophase hydroxyapatite co-substituted with strontium and zinc. Ceram. Int. 2018, 44, 7761–7770. [Google Scholar] [CrossRef]
- Lowry, N.; Han, Y.; Meenan, B.; Boyd, A. Strontium and zinc co-substituted nanophase hydroxyapatite. Ceram. Int. 2017, 43, 12070–12078. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Mao, H.; Li, T.; Zhao, R.; Yan, Y.; Pang, X. Osteoblastic cell responses and antibacterial efficacy of Cu/Zn co-substituted hydroxyapatite coatings on pure titanium using electrodeposition method. RSC Adv. 2015, 5, 17076–17086. [Google Scholar] [CrossRef]
- Ramya, J.R.; Arul, K.T.; Elayaraja, K.; Kalkura, S.N. Physicochemical and biological properties of iron and zinc ions co-doped nanocrystalline hydroxyapatite, synthesized by ultrasonication. Ceram. Int. 2014, 40, 16707–16717. [Google Scholar] [CrossRef]
- Karthika, A. Aliovalent ions substituted hydroxyapatite coating on titanium for improved medical applications. Mater. Today Proc. 2018, 5, 8768–8774. [Google Scholar] [CrossRef]
- Sridevi, C.; Sathishkumar, S.; Elavarasan, S.; Rajavel, R.; Maheswaran, P. Investigation of anticorrosion, antibacterial and in vitro biological performance of terbium/gadolinium dual substituted hydroxyapatite coating on surgical grade stainless steel for biomedical applications. Chem. Sci. Trans. 2018, 7, 319–328. [Google Scholar] [CrossRef]
- Sanyal, V.; Raja, C.R. Structural and antibacterial activity of hydroxyapatite and fluorohydroxyapatite co-substituted with zirconium–cerium ions. Appl. Phys. A Mater. Sci. Process. 2016, 122, 132. [Google Scholar] [CrossRef]
- Sandhyarani, M.; Rameshbabu, N.; Venkateswarlu, K.; Ravisankar, K.; Ashok, M.; Anandan, S. Photocatalytic and antibacterial activity of titanium, fluorine and silver co-substituted hydroxyapatite. Int. J. Mod. Phys. Conf. Ser. 2013, 22, 268–277. [Google Scholar] [CrossRef]
- Rajendran, A.; Balakrishnan, S.; Kulandaivelu, R.; Nellaiappan, S.N.T. Multi-element substituted hydroxyapatites: Synthesis, structural characteristics and evaluation of their bioactivity, cell viability, and antibacterial activity. J. Sol-Gel Sci. Technol. 2018, 86, 441–458. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, L. Multifunction Sr, Co and F co-doped microporous coating on titanium of antibacterial, angiogenic and osteogenic activities. Sci. Rep. 2016, 6, 29069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bir, F.; Khireddine, H.; Touati, A.; Sidane, D.; Yala, S.; Oudadesse, H. Electrochemical depositions of fluorohydroxyapatite doped by Cu2+, Zn2+, Ag+ on stainless steel substrates. Appl. Surf. Sci. 2012, 258, 7021–7030. [Google Scholar] [CrossRef]
- Hu, C.; Guo, J.; Qu, J.; Hu, X. Efficient destruction of bacteria with Ti(IV) and antibacterial ions in co-substituted hydroxyapatite films. Appl. Catal. B Environ. 2007, 73, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Quarto, R.; Mastrogiacomo, M.; Cancedda, R.; Kutepov, S.M.; Mukhachev, V.; Lavroukov, A.; Kon, E.; Marcacci, M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N. Engl. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Marcacci, M.; Kon, E.; Moukhachev, V.; Lavroukov, A.; Kutepov, S.; Quarto, R.; Mastrogiacomo, M.; Cancedda, R. Stem cells associated with macroporous bioceramics for long bone repair: 6-to 7-year outcome of a pilot clinical study. Tissue Eng. 2007, 13, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Sankhla, S.; Gupta, A.; Changani, R.; Gagal, K. Percutaneous autologus bone marrow injection in the treatment of delayed or nonunion. Indian J. Orthop. 2007, 41, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Sangwan, S.; Siwach, R.; Ali, A.M. Percutaneous bone marrow grafting for the treatment of tibial non-union. Injury 2005, 36, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Poignard, A.; Beaujean, F.; Rouard, H. Percutaneous autologous bone-marrow grafting for nonunions: Influence of the number and concentration of progenitor cells. J. Bone Jt. Surg. Am. Vol. 2005, 87, 1430–1437. [Google Scholar] [CrossRef]
- Kettunen, J.; Mäkelä, E.A.; Turunen, V.; Suomalainen, O.; Partanen, K. Percutaneous bone grafting in the treatment of the delayed union and non-union of tibial fractures. Injury 2002, 33, 239–245. [Google Scholar] [CrossRef]
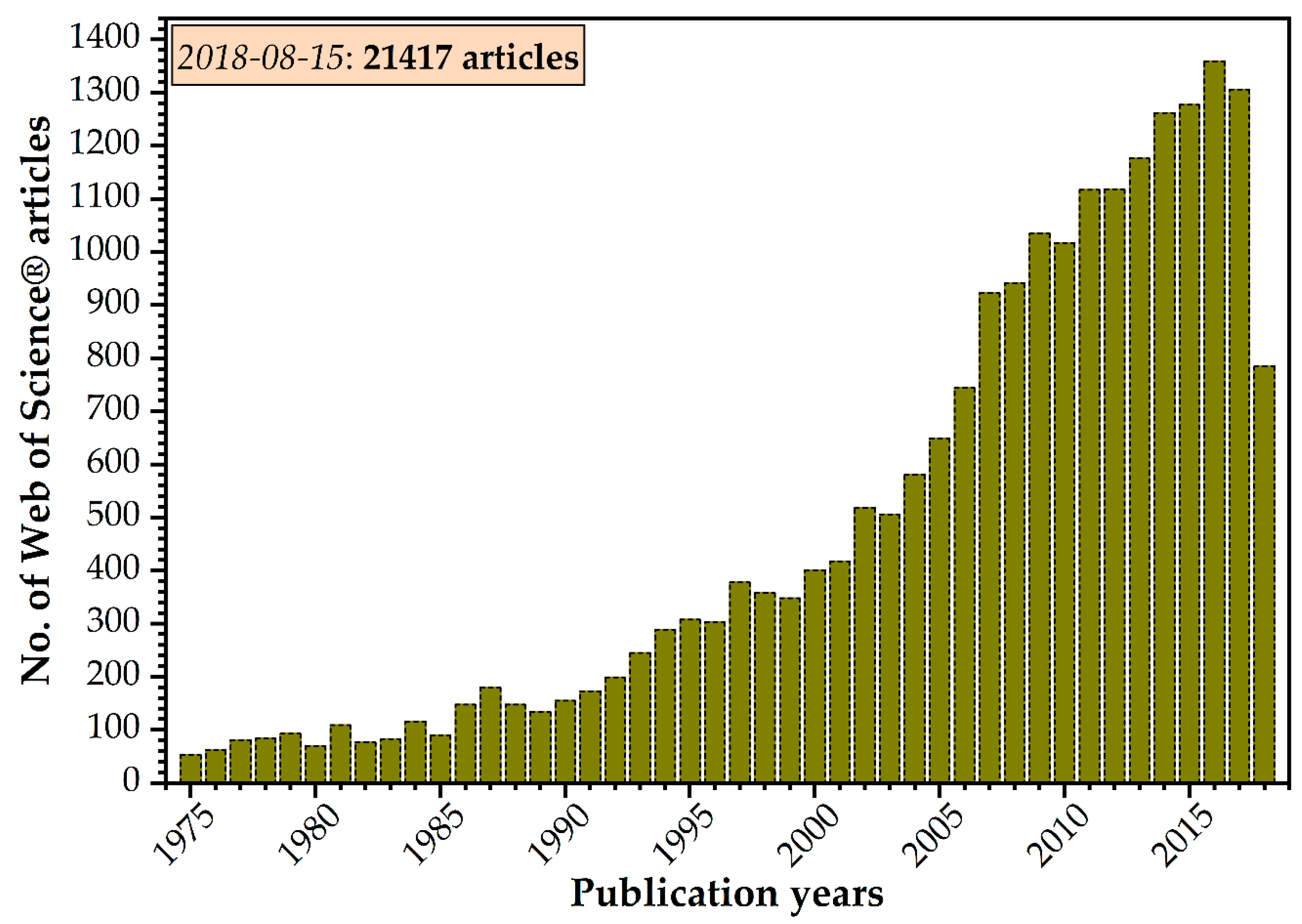
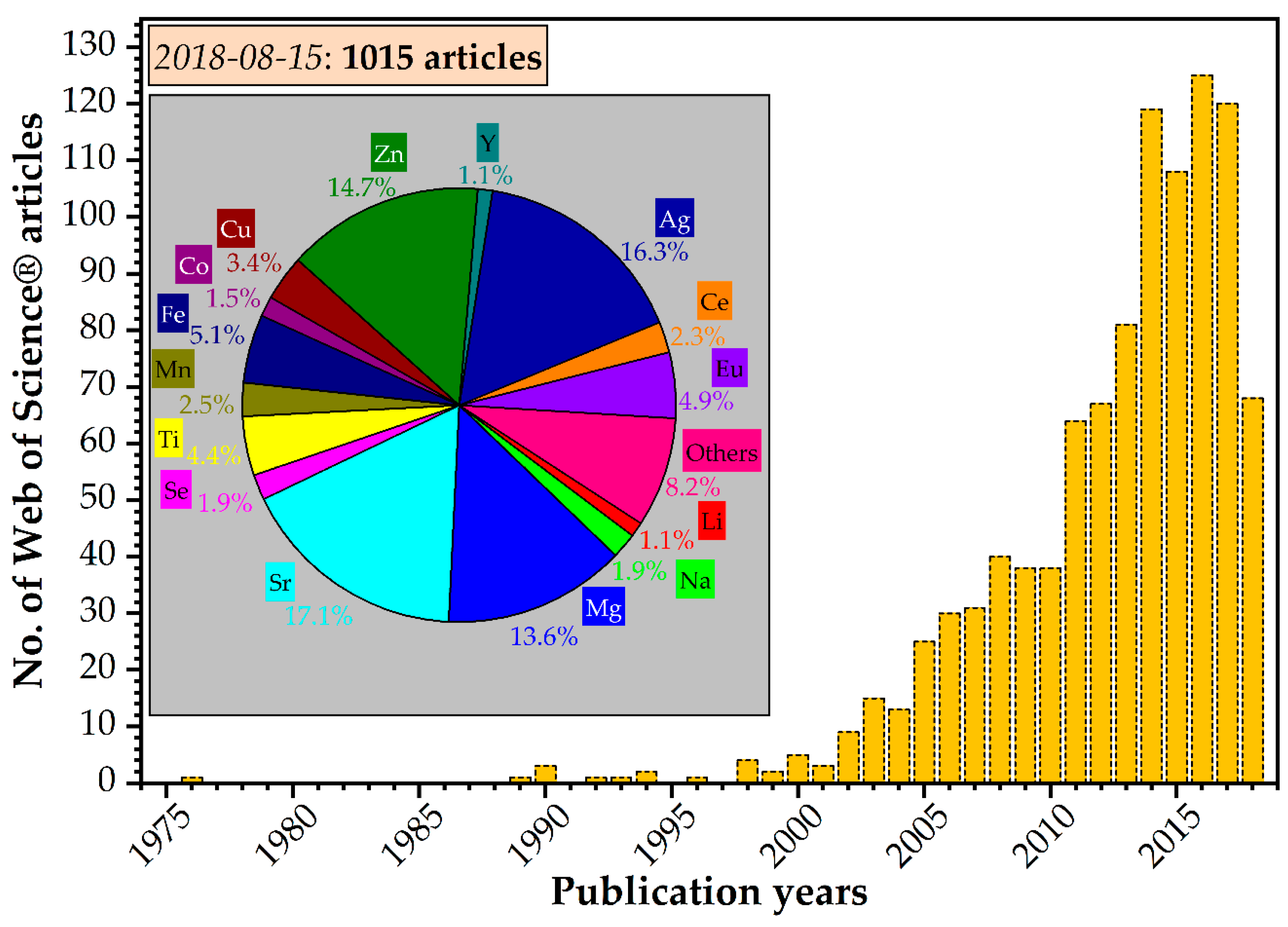
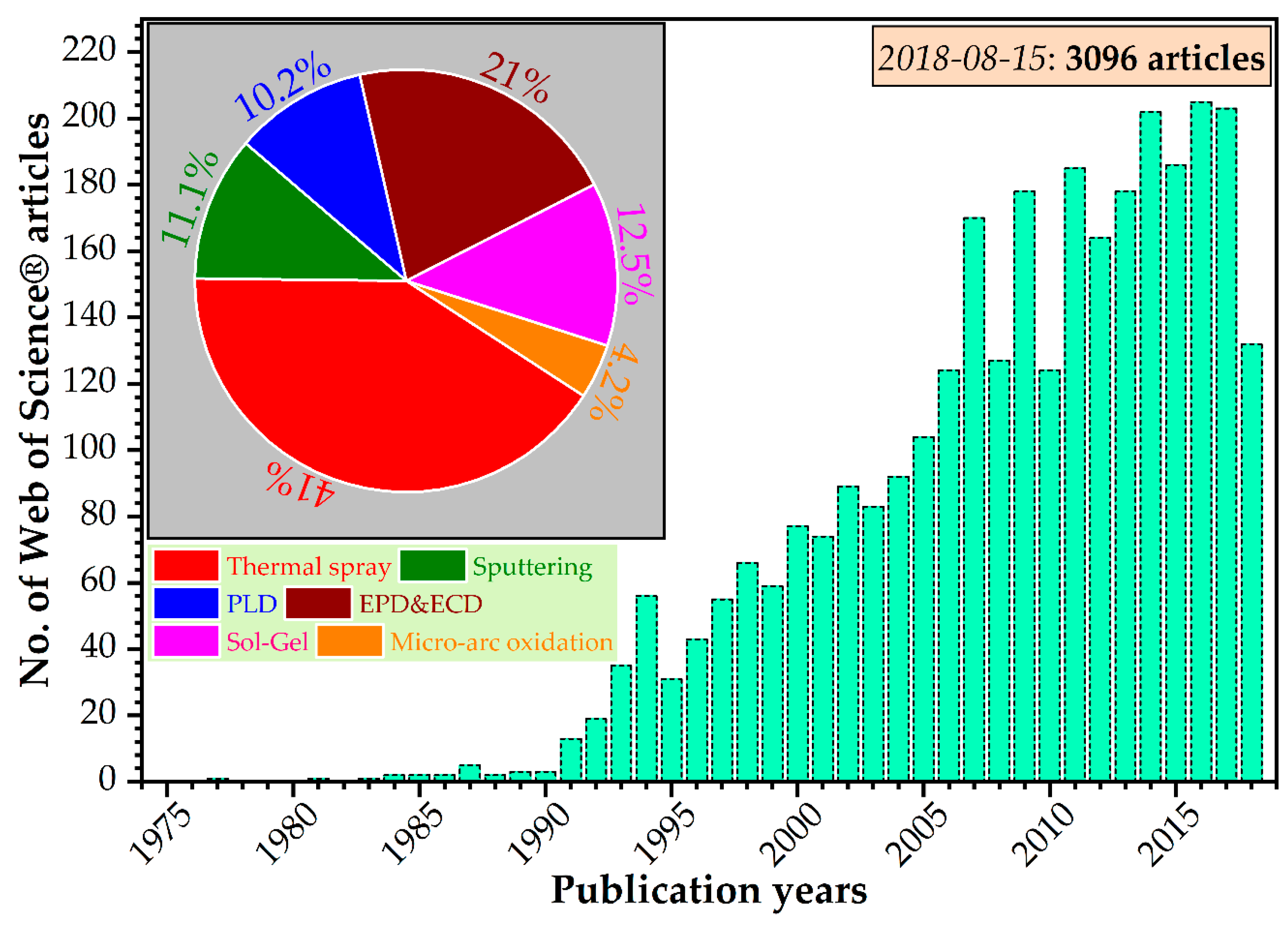
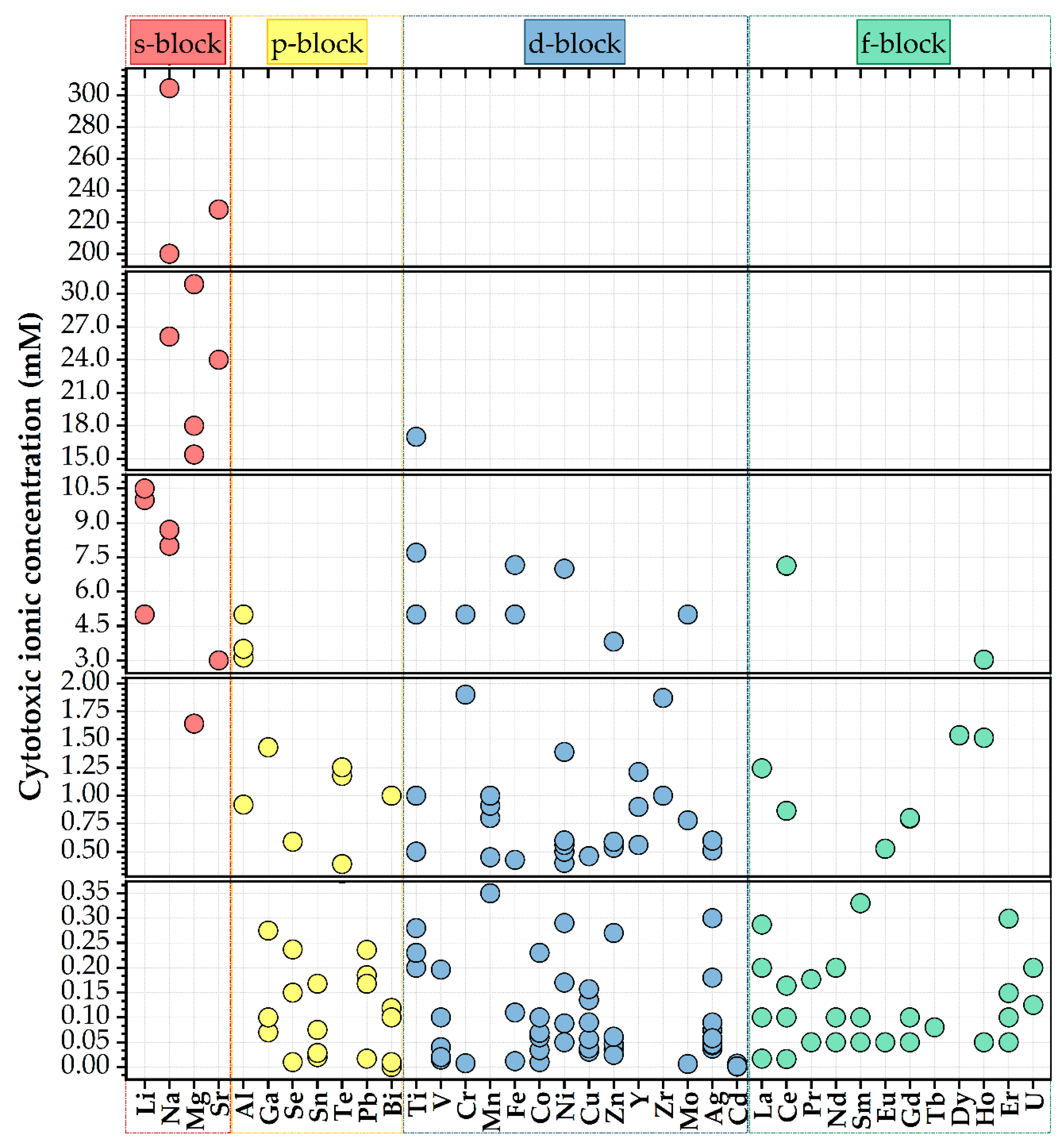
| Elements | Source | Synthesis Method | Refs. |
|---|---|---|---|
| Bovine | Cortical bone | Pre-cleaning: (i) removal of soft tissue; (ii) cut into small pieces and boiled in water for 2 to 3 h; (iii) dry in an oven at 80 °C for 72 h; (iv) crush and subsequently grind by ball milling for 24 h. Heating: calcination in a furnace at temperatures in the range of 600–1100 °C, for 3 h, with heating and cooling rates of 5 °C min−1. | [93] |
| Cortical bone | Pre-cleaning: (i) removal of soft tissue; (ii) crushing and milling process. Heating: sintering in a furnace at 1200 °C for 2 h or 4 h, with a heating and cooling rate of 5 °C min−1 and 10 °C min−1, respectively. | [92] | |
| Teeth | Pre-cleaning: (i) removal of soft tissue; (ii) removal of remnant impurities by mechanical scraping; (iii) boiling in distilled water for 30 min; (iv) repeated the aforementioned steps three times; (v) drying in the sun for 3 days. Heating: (i) calcination in humid atmosphere at 735 °C for 1 h with a heating rate of 7 °C min−1; (ii) sintering at 1150 °C for 1 h with a heating rate of 7 °C min−1. | [108] | |
| Pig | Cortical bone | Pre-cleaning: (i) hot water treatment; (ii) removal of organic compounds by scraping; (iii) de-proteinization in a boiled mixture of 1 M NaOH and 1 M HCl at 100 °C for 5–10 min; (iv) dried in an oven at 100 °C overnight; (v) crushing and grinding. Heating: calcination in air at 600 °C, 800 °C or 1000 °C at a heating rate of 5 °C min−1 followed by cooling to room temperature. | [99] |
| Cortical bone | Pre-cleaning: (i) removal of soft tissues and fluids; (ii) boiling bone slices at 154 °C, 4 atm; (iii) drying in vacuum; (iv) milling; (v) removal of remnant fat and protein moieties by hydrothermal process. Heating: (i) calcination at 5 °C min−1 at 600 °C (allows the decomposition of organic tissue); (ii) sintering at 1000 °C (induces physico-chemical changes) from 1 to 50 h; (iii) cooling in the furnace in air. | [100] | |
| Camel | Cortical bone | Pre-cleaning: (i) removal of organic compound; (ii) dry-heating at 100 °C for 1 h; (iii) cut in small pieces and immersion in acetone for 1 h. Heating: calcination at 1000 °C for 3 h at a heating rate of 10 °C min−1, and then slowly cooled down to room temperature. | [102] |
| Sheep | Cortical bone | Pre-cleaning: (i) removal of femoral heads; (ii) de-proteinization with NaOH; (iii) washing and drying. Heating: (i) calcination at 850 °C for 4 h in air; (ii) crushing and milling. | [103] |
| Dentine | Pre-cleaning: cleaning and washing the teeth. Heating: (i) calcination at 750 °C for 5–6 h; (ii) separation of dentine from enamel; (iii) ball grinding; (iv) sintering at 1000–1300 °C for 4 h. | [109] | |
| Chicken | Egg-shells | Pre-cleaning and synthesis: (i) crushing egg-shells; (ii) simultaneous removal of organics and transformation of CaCO3 into CaO by calcination at 900 °C for 1 h; (iii) addition of water and phosphoric acid; (iv) precipitation overnight, followed by filtration and washing; (v) drying the HA product at 60 °C for 24 h. Heating: sintering in air (after sieving and pressing) at 900–1300 °C for 1 h, with a heating rate of 10 °C. | [110] |
| Fish | Bones | Pre-cleaning: (i) removal of organic compounds by brushing and then boiling at 100 °C for 10 min; (ii) drying at 90 °C for 100 min and then crushing to powder; (iii) de-proteinization by reflux method using a 5% KOH solution. Heating: sintering at 600–1000 °C. | [111,112] |
| Mussel | Shells | Pre-cleaning and synthesis: (i) mechanical cleaning and calcination in air at 1300 °C for 6 h (ii) Rathje fabrication method: mixing seashells powder with water and H3PO4 with magnetic stirring during the synthesis for 2 h at 700 rpm; (iii) filtering, followed by drying at room temperature for 168 h, and then at 100 °C for 24 h. Heating: sintering at 1200 °C for 10 h. | [57] |
| Snail | Shells | Pre-cleaning and synthesis: (i) thoroughly cleaning of sand particles and other foreign materials; (ii) drying, crushing into small particles, ball-milling; (iii) sieving; (iv) mixing the as-obtained CaCO3 powder with water and H3PO4 solution, followed by continuous stirring at 80 °C for 8 h; (v) drying at 100 °C overnight in an incubator. Heating: calcination at 800 °C for 4 h in air. | [63] |
| Cuttlefish | Whole | Pre-cleaning and synthesis: (i) cutting into small pieces; (ii) heat-treatment at 110, 500, 1000 °C with a heating rate of 5 °C min−1; (iii) mixing the as-obtained CaCO3 powder with an aqueous NH4H2PO4 solution to a Ca/P molar ratio of 1.67; (v) drying at 200 °C for 1–72 h, using heating and cooling rates of 5 °C min−1. | [60] |
| Dolomic marble | Origin: Ruschiţa, Romania | Pre-cleaning and synthesis: (i) mechanical cleaning and calcination in air at 1300 °C for 6 h (ii) Rathje fabrication method: mixing seashells powder with water and H3PO4 with magnetic stirring during the synthesis for 2 h at 700 rpm; (iii) filtering, followed by drying at room temperature for 168 h, and then at 100 °C for 24 h. Heating: sintering at 1200 °C for 10 h. | [57] |
| Red algae | Whole | Pre-cleaning: (i) rinsing at high-pressure; (ii) drying at room temperature for 24 h; (iii) sieving; (iv) thermal treatment to burn-off the organic material, at 650–700 °C for 12 h, with a low heating rate of 0.5 °C min−1 to prevent decomposition of algae; (v) alkalinisation with ammonium hydroxide at ambient pressure and 100 °C for 12 h under continuous stirring at speed of 100 rpm; (vi) filtration and neutralisation by repeating washing and drying overnight at 90 °C. Heating: thermal treatment at 60 °C, 105 °C, 450 °C, 550 °C and 1000 °C for 1 h each. | [113] |
| Cation Dopant | Field of Application [Refs.] |
|---|---|
| Na | Sensors [162]; Catalysis [163] |
| Sr | Catalysis [164] |
| Ba | Water decontamination [165]; Catalysis [166] |
| Al | Environment decontamination [167,168,169]; Catalysis [170] |
| Sn | Radionuclides and heavy metals scavengers (decontamination) [171] |
| Pb | Catalysis [172,173] |
| Y | Electrochemical devices [174] |
| Ti | Catalysis [175,176,177] |
| V | Catalysis [178] |
| Mn | Catalysis [179]; Optoelectronics [180] |
| Fe | Sensors [181]; Catalysis [182,183] |
| Co | Sensors [184] |
| Ni | Catalysis [185,186,187,188] |
| Pd | Catalysis [189,190] |
| Pt | Catalysis [191,192] |
| Cu | Catalysis [193,194,195,196]; Water decontamination [197] |
| Ag | Catalysis [198,199,200] |
| Au | Catalysis [194,201,202] |
| Zn | Catalysis [203,204,205] |
| Sm | Optoelectronics [206] |
| Eu | Optoelectronics [206,207]; Environmental [208] |
| Gd | Optoelectronics [206] |
| Tb | Catalysis [209]; Optoelectronics [210] |
| Dy | Optoelectronics [211] |
| Cation (M) | Sample Form | Doping Range [M/(M + Ca)]∙100 (at.%) | Bio-Functionality/Effect of the Dopant | Refs. |
| Li | Powder Scaffold Coating | 0.5–2 |
| [98,221,222,223,224] |
| Na | Powder Coating | 5 |
| [227,229] |
| K | Powder | 2.5–47 |
| [232,233] |
| Mg | Powder Coating | 1–53 |
| [235,236,237,238,239,240,242,243] |
| Sr | Powder Coating | 1–40 |
| [240,243,244,245,246,249,250,251,252,255,257] |
| Ba | Powder | 0.5–2 |
| [261,262] |
| Al | Powder | 0.5–2.5 |
| [263] |
| Ga | Powder | n/a |
| [264,266] |
| In | Powder | 1; 3 |
| [238,267] |
| Bi | Powder | 5–25 |
| [267,268,269] |
| Te | Powder | 0.04–0.22 |
| [272] |
| Ag | Powder Scaffold Coating | 0.5–5 |
| [250,261,273,275,276,277,278,279,280,281,282,283,284,285,286] |
| Zn | Powder Coating | 0.1–50 |
| [66,238,243,274,277,281,282,287,288,289,291,292,293,294,295,296,297,298] |
| Cu | Powder Coating | 0.04–5 |
| [238,299,300,301,302,303] |
| Mn | Powder Coating | 0.4–20 |
| [304,305,306,307,308,309] |
| Fe | Powder | 1–50 |
| [308,309,310,311,312,313,314] |
| Ti | Powder Coating | 1–13 |
| [68,177,301,316,317,318] |
| Cr | Powder | 0.5–2.5 |
| [319,320] |
| Co | Powder | 0.2–27 |
| [238,321,322,323] |
| Ta | Powder | 0.13–0.27 |
| [324] |
| Ni | Powder | 0.8–8.3 (theoretical)0.2–2.4(determined by ICP-OES) |
| [328,329] |
| Mo | Powder | 0.05–5.2 |
| [330] |
| Y | Powder Coating | 1.3–7 |
| [267,331,332,333] |
| Cd | Powder | n/a |
| [334,335] |
| W | Powder | 0.7–32.3 |
| [336] |
| Hf | Powder | 0.5–15 |
| [339] |
| La | Powder Coating | 2–30 |
| [314,340,342,379,380,405] |
| Ce (3+) | Powder Coating | 4–20 |
| [344,346,377,382,383,384] |
| Ce (4+) | Powder | 0.1–0.5 |
| [348,381,385] |
| Sm | Powder Coating | 0.2–0.5 |
| [355,357] |
| Eu | Powder | 0.1–20 |
| [361,362,363,364,365,366,388,389,390,391,392,393] |
| Tb | Powder | 2–17 |
| [361,393,399] |
| Gd | Powder | 1–17 |
| [128] |
| Dy | Powder | 0.5–10 |
| [392,403] |
| Nd | Powder | 1–17 |
| [128,352] |
| Er | Powder | 2–10 |
| [372] |
| U | Solution | 0.1–10 |
| [375] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tite, T.; Popa, A.-C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.F.; Stan, G.E. Cationic Substitutions in Hydroxyapatite: Current Status of the Derived Biofunctional Effects and Their In Vitro Interrogation Methods. Materials 2018, 11, 2081. https://doi.org/10.3390/ma11112081
Tite T, Popa A-C, Balescu LM, Bogdan IM, Pasuk I, Ferreira JMF, Stan GE. Cationic Substitutions in Hydroxyapatite: Current Status of the Derived Biofunctional Effects and Their In Vitro Interrogation Methods. Materials. 2018; 11(11):2081. https://doi.org/10.3390/ma11112081
Chicago/Turabian StyleTite, Teddy, Adrian-Claudiu Popa, Liliana Marinela Balescu, Iuliana Maria Bogdan, Iuliana Pasuk, José M. F. Ferreira, and George E. Stan. 2018. "Cationic Substitutions in Hydroxyapatite: Current Status of the Derived Biofunctional Effects and Their In Vitro Interrogation Methods" Materials 11, no. 11: 2081. https://doi.org/10.3390/ma11112081
APA StyleTite, T., Popa, A.-C., Balescu, L. M., Bogdan, I. M., Pasuk, I., Ferreira, J. M. F., & Stan, G. E. (2018). Cationic Substitutions in Hydroxyapatite: Current Status of the Derived Biofunctional Effects and Their In Vitro Interrogation Methods. Materials, 11(11), 2081. https://doi.org/10.3390/ma11112081







