Development and Characterization of Clean Fracturing Fluid Based on Gemini Surfactant for Coalbed Methane Extraction
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Instruments
2.1.1. Materials
2.1.2. Instruments
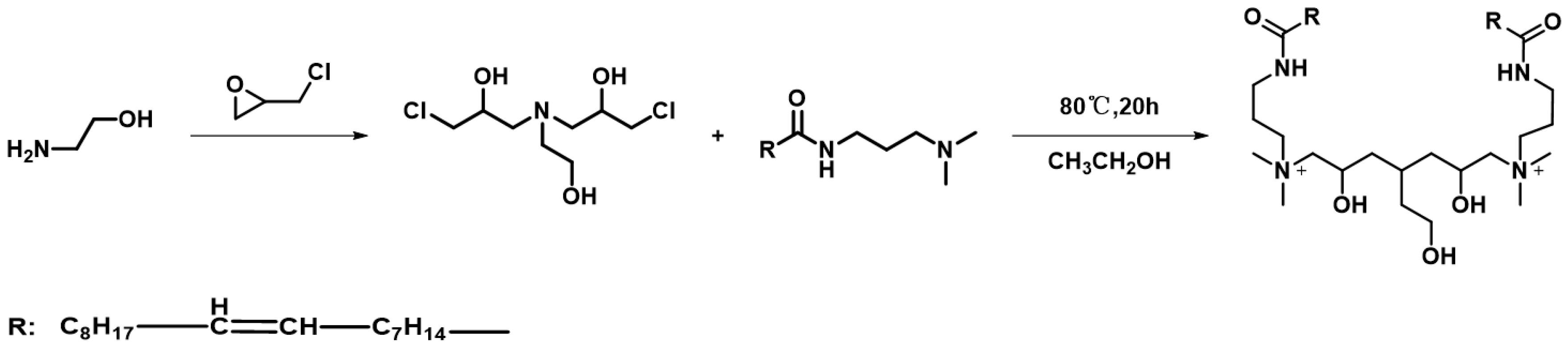
2.2. Synthesis Process
2.3. Structural Characterization
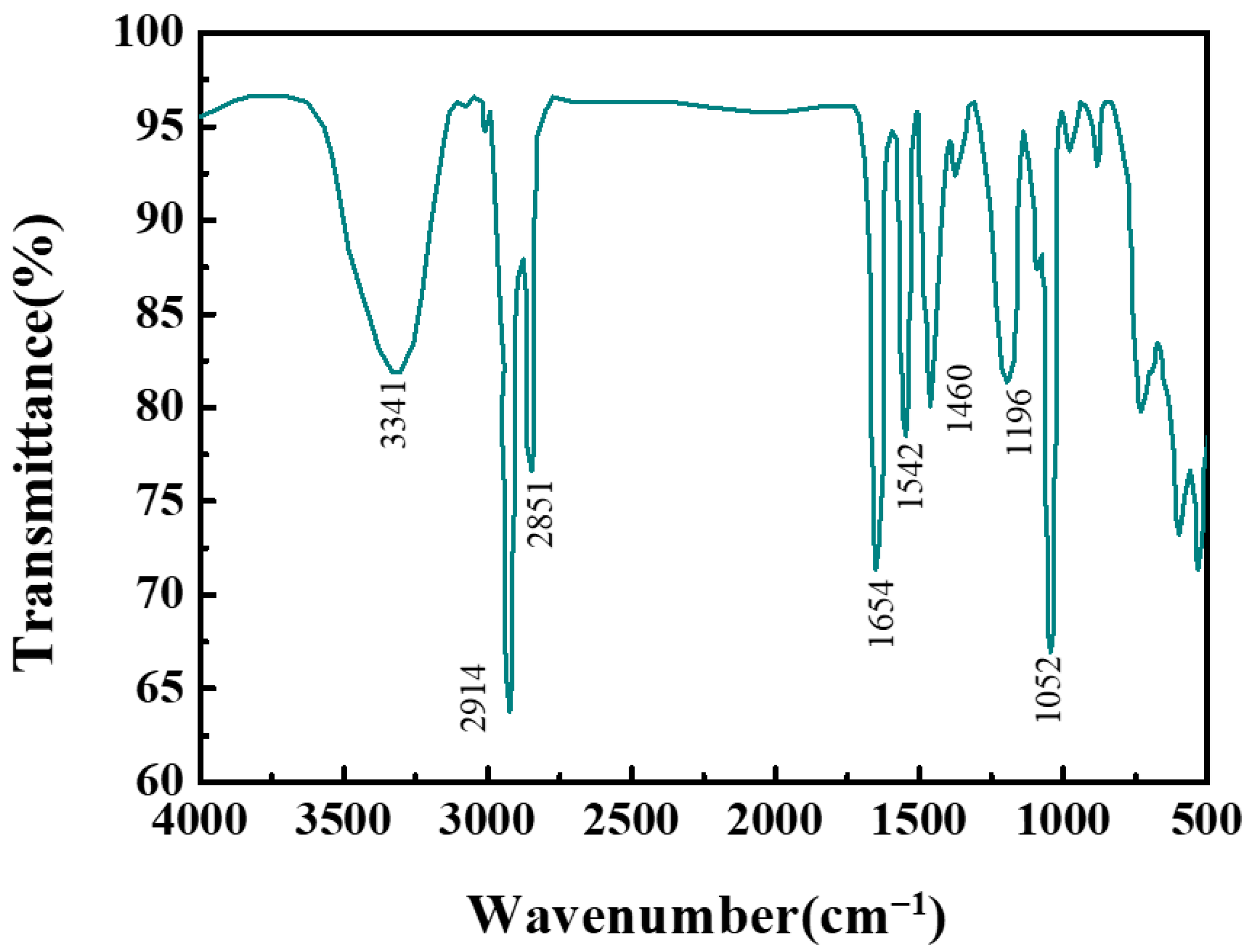
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.3.2. Elemental Analysis (EA)
2.4. Surface Activity Testing
2.5. Analysis of Coal Reservoir Physical Properties
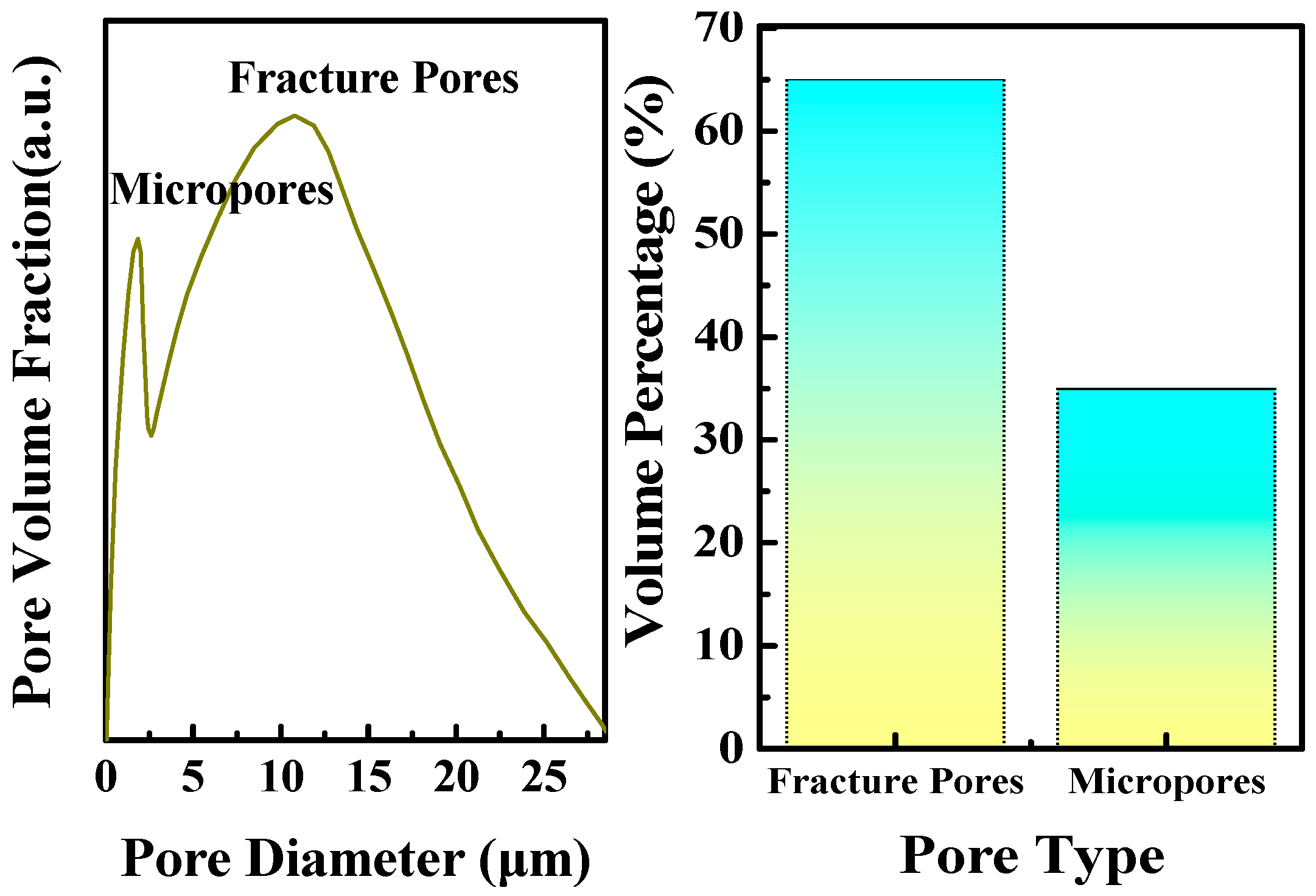
2.5.1. Pore Structure Analysis
2.5.2. Stress Sensitivity Testing
2.6. Performance Evaluation Methods of Clean Fracturing Fluid
2.6.1. Rheological Property Testing
2.6.2. Sand-Carrying Capacity Testing
2.6.3. Fluid Loss Property Testing
2.6.4. Reservoir Damage Testing
3. Results and Discussion
3.1. Correlation Between Structure and Surface Activity of Gemini Surfactant GEM-CBM
3.1.1. Molecular Structure Verification of GEM-CBM
3.1.2. Surface Activity Performance of GEM-CBM
3.2. Constraint Mechanism of Coal Reservoir Properties on Fracturing Fluid
3.2.1. Pore Structure Constraints of Coal Reservoirs
3.2.2. Stress Sensitivity Constraints of Coal Reservoirs
3.3. Performance Advantages and Reservoir Adaptability of Clean Fracturing Fluid
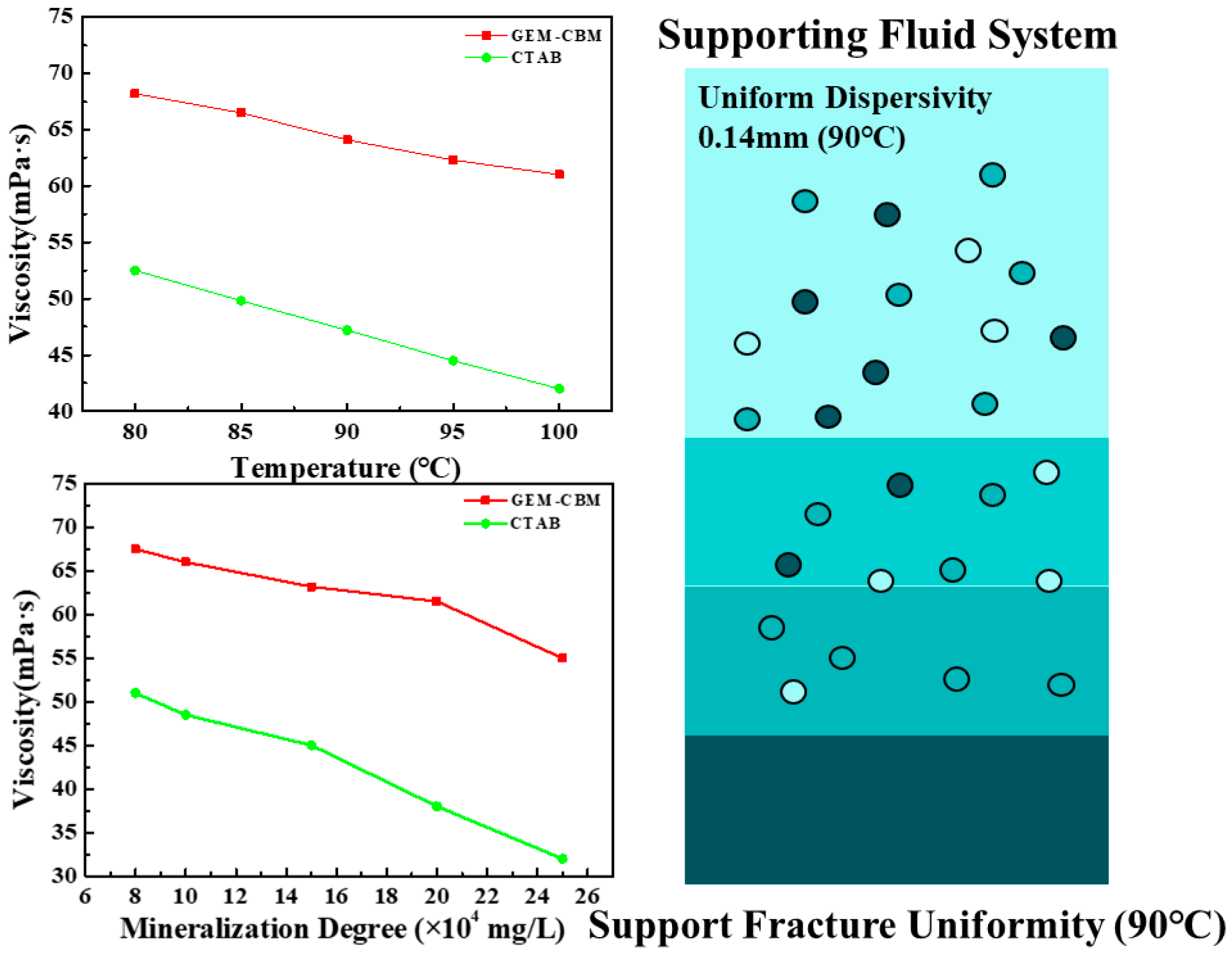
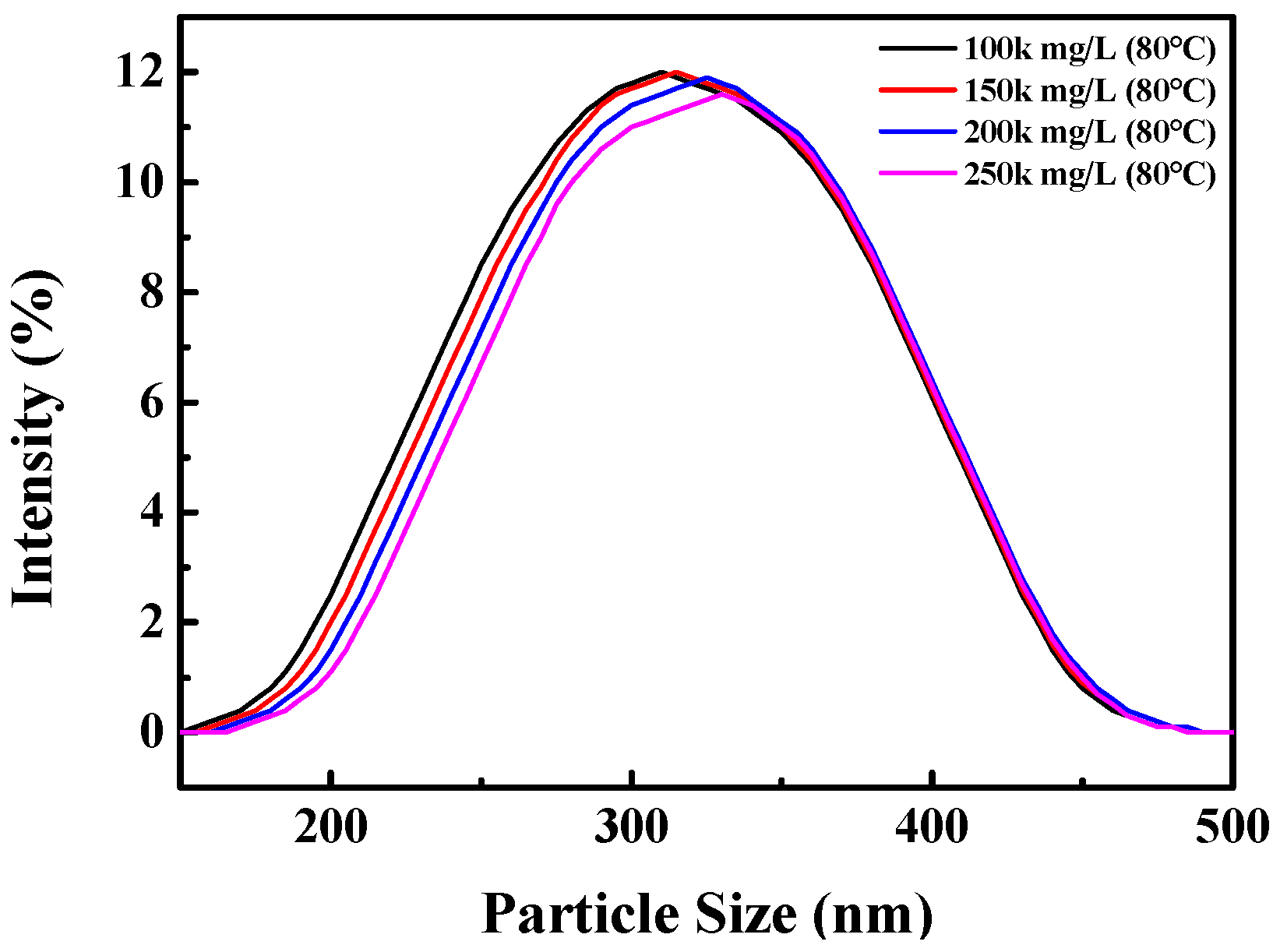
3.3.1. Temperature/Salt Resistance and Sand-Carrying Capacity
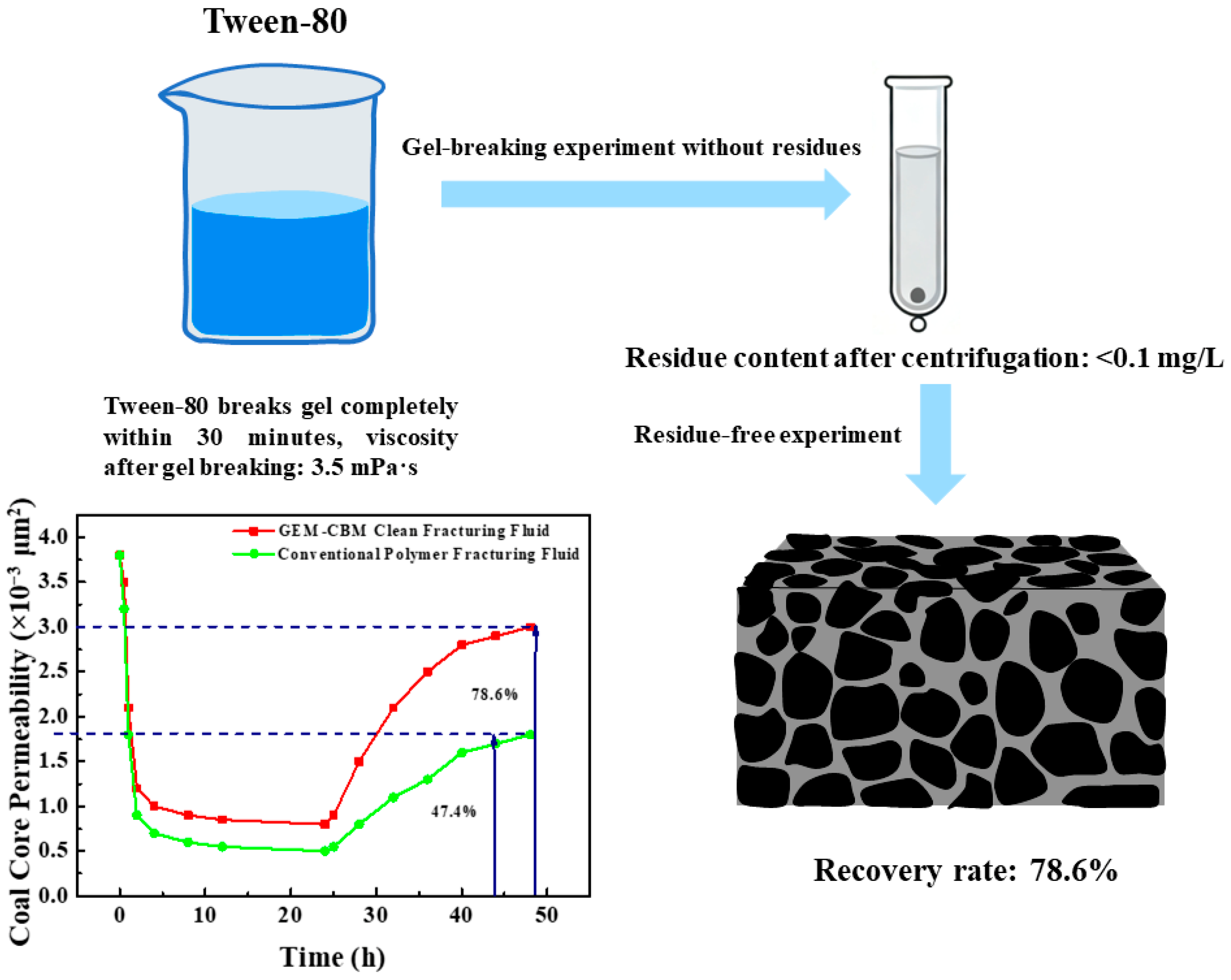
3.3.2. Gel-Breaking Performance and Reservoir Damage Control
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Abbreviation/Symbol | Full Name |
| Amin | Minimum molecular area at the interface |
| API | American Petroleum Institute |
| C | Fluid loss coefficient |
| CMC | Critical micelle concentration |
| EA | Elemental Analysis |
| EAPB | Alkylamidopropyl betaine |
| FTIR | Fourier Transform Infrared Spectroscopy |
| G′ | Storage modulus |
| G″ | Loss modulus |
| GEM-CBM | Gemini surfactant for coalbed methane extraction |
| Γmax | Maximum surface excess concentration |
| γCMC | Surface tension at critical micelle concentration |
| LVR | Linear Viscoelastic Region |
| PDI | Polydispersity Index |
| V0 | Initial fluid loss |
| VES | Viscoelastic Surfactant |
| CAPHS | Carboxybetaine surfactant |
| CTAB | Cetyltrimethylammonium bromide |
| SDBS | Sodium dodecyl benzene sulfonate |
| C12-APB | Dodecylamidopropyl betaine |
| C16-2-16 | Gemini surfactant with C16 alkyl chains and ethylene spacer |
| K0 | Initial permeability |
| Kr | Recovered permeability |
| Mineralization | Mineralization degree of formation water |
| Viscosity | Apparent viscosity of fracturing fluid |
| Proppant settlement velocity | Proppant settling velocity |
| Permeability recovery rate | Coal core permeability recovery rate |
| Surface tension | Gas–liquid interfacial tension |
| Particle size | Micelle particle size (dynamic light scattering) |
| Shear rate | Shear rate in rheological test |
| Temperature | Reservoir temperature/test temperature |
References
- Chen, S.D.; Zhang, Y.F.; Tang, D.Z.; Tao, S.; Pu, Y.F.; Chen, Z.H. Present-day stress regime, permeability, and fracture stimulations of coal reservoirs in the Qinshui Basin, northern China. AAPG Bull. 2024, 108, 1509–1536. [Google Scholar] [CrossRef]
- Guo, C.; Gou, J.; Ma, D. Adsorption and desorption behavior under coal-water-gas coupling conditions of high- and low-rank coal samples. Front. Earth Sci. 2023, 17, 145–157. [Google Scholar] [CrossRef]
- Lyu, C.; Meng, J.; Li, X.; Lyu, Y.; Wang, J.; Wang, H.; Deng, Y.; Li, X.; Nie, B. Effect of unconventional surfactants on coalbed methane migration and its regulation mechanisms. Fuel 2025, 405, 136661. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, L.; Wan, X.; Tang, Y.; Liu, Q.; Li, W.; Liao, J. Synthesis and Characterization of a Temperature-Sensitive Microcapsule Gelling Agent for High-Temperature Acid Release. ACS Omega 2024, 9, 20849–20858. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Lin, D.; Xiong, Z.; Spicer, R.; Farnsworth, A.; Valdes, P.; Wang, C.; Cai, F.; Wang, H.; Sun, Y.; et al. A distinctive Eocene Asian monsoon and modern biodiversity resulted from the rise of eastern Tibet. Sci. Bull. 2022, 21, 2245–2258. [Google Scholar] [CrossRef]
- Wang, S.; Kong, L.; Li, Z. Propagation and distribution of shear and tensile hydraulic fractures affected by frictional coal beddings. Sci. Rep. 2024, 14, 27880. [Google Scholar] [CrossRef]
- Meng, T.; Liu, R.; Cai, J.; Cheng, X.; He, Z.; Zhao, Z. Breaking structural symmetry of atomically dispersed Co sites for boosting oxygen reduction. Adv. Funct. Mater. 2025, e22046. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Li, D. Numerical Simulation of Hydraulic Fracture Propagation in Coal Seams with Discontinuous Natural Fracture Networks. Processes 2018, 6, 113. [Google Scholar] [CrossRef]
- Huang, F.; Pu, C.; Lu, L.; Pei, Z.; Gu, X.; Lin, S.; Wu, F.; Liu, J. Gemini Surfactant with Unsaturated Long Tails for Viscoelastic Surfactant (VES) Fracturing Fluid Used in Tight Reservoirs. ACS Omega 2021, 6, 1593–1602. [Google Scholar] [CrossRef]
- Cun, M.; Mao, J.; Sun, H.; Wei, G.; Tang, F.; Zhang, W.; Tian, J.; Yang, X.; Lin, C.; Huang, Z. Development of a novel temperature-resistant and salt-resistant double-cationic surfactant with “super thick hydration layer” for clean fracturing fluid. Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126306. [Google Scholar] [CrossRef]
- Tang, H.; Song, J.; Zhao, M.; Zhang, Z.; Liu, W.; Yan, Z. Performance Evaluation and Mechanism Study of Seawater-Based Circulatory Fracturing Fluid Based on pH-Regulated WormLike Micelles. Front. Chem. 2022, 10, 848269. [Google Scholar] [CrossRef]
- Yang, X.; Mao, J.; Chen, Z.; Chen, Y.; Zhao, J. Clean fracturing fluids for tight reservoirs: Opportunities with viscoelastic surfactant. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 41, 1446–1459. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, B.; Zhang, Y.; Wang, Q.; Yao, W.; Chen, K.; Zhou, Q.; Xie, C.; Yan, C. Preparation and performance evaluation of a low-damage thickener suitable for fracturing medium and deep coalbed methane. J. Dispers. Sci. Technol. 2025, 45, 1–11. [Google Scholar] [CrossRef]
- You, Q.; Wang, C.; Ding, Q.; Zhao, G.; Fang, J.; Liu, Y.; Zhao, M.; Dai, C.; Geng, M. Impact of surfactant in fracturing fluid on the adsorption–desorption processes of coalbed methane. J. Nat. Gas. Sci. Eng. 2015, 26, 35–41. [Google Scholar] [CrossRef]
- Yin, T.; Liu, H.; Wen, X. pH-responsive clean fracturing fluid based on pseudo-trimeric surfactants. Colloid. Polym. Sci. 2023, 301, 189–197. [Google Scholar] [CrossRef]
- Qi, Y.; Tian, L.; Cao, Y.; Shi, B.; Long, P. Effect of Surfactant Modification on Coalbed Methane Desorption and Its Behavioral Mechanism. Energy Fuels 2024, 38, 14235–14245. [Google Scholar] [CrossRef]
- Zhang, W.; Mao, J.; Yang, X.; Zhang, H.; Zhao, J.; Tian, J.; Lin, C.; Mao, J. Development of a sulfonic gemini zwitterionic viscoelastic surfactant with high salt tolerance for seawater-based clean fracturing fluid. Chem. Eng. Sci. 2019, 207, 688–701. [Google Scholar] [CrossRef]
- Li, J.; Wen, M.; Yang, J.; Liu, Y.; Jiang, Z.; Chen, J. Synthesis and analysis of magnetic nanoparticles within foam matrix for foam drainage gas production. Geoenergy Sci. Eng. 2024, 238, 212887. [Google Scholar] [CrossRef]
- Rong, L.; Zhang, B.; Qiu, H.; Zhang, H.; Yu, J.; Yuan, Q.; Wu, L.; Chen, H.; Mo, Y.; Zou, X.; et al. Significant generational effects of tetracyclines upon the promoting plasmid-mediated conjugative transfer between typical wastewater bacteria and its mechanisms. Water Res. 2025, 287, 124290. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Q.; Xia, T. Development and study of a salt tolerance Gemini cationic viscoelastic surfactant with spacer of neopentyl-glycol applied in seawater-based clean fracturing fluid. Colloid. Polym. Sci. 2025, 303, 547–561. [Google Scholar] [CrossRef]
- Yan, Z.; Wu, Y.; Zhao, M.; Yu, L.; Zhang, S. Study on synthesis, surface activity and quantum chemical properties of anionic-nonionic Gemini surfactant. J. Mol. Liq. 2023, 392, 123439. [Google Scholar] [CrossRef]
- Morishima, K.; Sugawara, S.; Yoshimura, T.; Shibayama, M. Structure and Rheology of Wormlike Micelles Formed by Fluorocarbon–Hydrocarbon-Type Hybrid Gemini Surfactant in Aqueous Solution. Langmuir 2017, 33, 6084–6091. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, L.; Zhang, Q.; Ni, C.; Deng, N.; Huang, X. Efficient Adsorptive Removal of Phosphonate Antiscalant HEDP by Mg-Al LDH. Separations 2025, 12, 259. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Zhuang, W. Spacer length effect on the aggregation behaviours of gemini surfactants in EAN. Colloid. Polym. Sci. 2021, 299, 685–692. [Google Scholar] [CrossRef]
- Dai, R.Y.; Shen, J.; Xu, H.J. Study on the synthesis and properties of Gemini surfactant with ester spacers. Tenside Surfactants Deterg. 2025, 62, 109–117. [Google Scholar] [CrossRef]
- Yang, S.J.; Liu, H.L.; Feng, J.; Ma, H.H.; Zhu, Y.; Zhang, L.; Zhang, L. Interfacial dilational properties of anionic gemini surfactants with polymethylene spacers. J. Dispers. Sci. Technol. 2017, 39, 531–538. [Google Scholar] [CrossRef]
- Fei, G.; Liu, B. Development and Characterization of a Novel Erucyl Ultra-Long-Chain Gemini Surfactant. Polymers 2025, 17, 2257. [Google Scholar] [CrossRef]
- Guo, C.; Xu, Y.; Deng, N.; Huang, X. Efficient degradation of organophosphorus pesticides and in situ phosphate recovery via NiFe-LDH activated peroxymonosulfate. Chem. Eng. J. 2025, 524, 169107. [Google Scholar] [CrossRef]
- Hussain, S.S.; Kamal, M.S.; Murtaza, M. Effect of aromatic spacer groups and counterions on aqueous micellar and thermal properties of the synthesized quaternary ammonium gemini surfactants. J. Mol. Liq. 2019, 296, 111837. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Qin, Y. Coal wettability in coalbed methane production: A critical review. Fuel 2021, 303, 121277. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Q.; Zhang, Y.; Zhang, Q.; Li, L.; Wang, Y.; Sun, B.; Liu, R. Effects of dual Gemini-based fracturing fluid on the physicochemical properties of coking coal: Material preparation and wetting mechanism. Energy 2024, 289, 130055. [Google Scholar] [CrossRef]
- Ni, C.; Zhang, Q.; Xu, B.; Deng, N.; Huang, X. The Role of Solid Electrolytes in Suppressing Joule Heating Effect for Scalable H2O2 Electrosynthesis. ACS Sustainable Chem. Eng. 2025, 13, 17958–17968. [Google Scholar] [CrossRef]
- Fu, L.; Liao, K.; Ge, J.; Huang, W.; Chen, L.; Sun, X.; Zhang, S. Study on the damage and control method of fracturing fluid to tight reservoir matrix. J. Nat. Gas. Sci. Eng. 2020, 82, 103465. [Google Scholar] [CrossRef]
- Wu, J.; Liu, A.; Zhang, F.; Liu, Y.; Yan, L.; Jie, Y.; Wang, C. Research on Damage Characteristics of Clean Fracturing Fluid in Deep Coal Seam. Processes 2025, 13, 2669. [Google Scholar] [CrossRef]
- Pan, X.; Huang, X.; Deng, N. Short-Chain Carboxylic Acids Influencing Mineralization Mechanisms of Ferrihydrite Transformation to Hematite and Goethite. Environ. Sci. Technol. 2025, 59, 12910–12919. [Google Scholar] [CrossRef]
- Xue, S.; Huang, Q.; Wang, G.; Bing, W.; Li, J. Experimental study of the influence of water-based fracturing fluids on the pore structure of coal. J. Nat. Gas. Sci. Eng. 2021, 88, 103863. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, J.; Zhang, L.; Gong, S. Physicochemical Effect on Coal Pores of Hydrocarbon Chain Length and Mixing of Viscoelastic Surfactants in Clean Fracturing Fluids. ACS Omega 2024, 9, 19418–19427. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Yuan, C.; Du, R.; Qu, Y. Development and Characterization of Clean Fracturing Fluid Based on Gemini Surfactant for Coalbed Methane Extraction. Energies 2025, 18, 6094. https://doi.org/10.3390/en18236094
Liu J, Yuan C, Du R, Qu Y. Development and Characterization of Clean Fracturing Fluid Based on Gemini Surfactant for Coalbed Methane Extraction. Energies. 2025; 18(23):6094. https://doi.org/10.3390/en18236094
Chicago/Turabian StyleLiu, Jun, Chao Yuan, Rongjie Du, and Yansi Qu. 2025. "Development and Characterization of Clean Fracturing Fluid Based on Gemini Surfactant for Coalbed Methane Extraction" Energies 18, no. 23: 6094. https://doi.org/10.3390/en18236094
APA StyleLiu, J., Yuan, C., Du, R., & Qu, Y. (2025). Development and Characterization of Clean Fracturing Fluid Based on Gemini Surfactant for Coalbed Methane Extraction. Energies, 18(23), 6094. https://doi.org/10.3390/en18236094





