Microbial Electrosynthesis: The Future of Next-Generation Biofuel Production—A Review
Abstract
1. Introduction
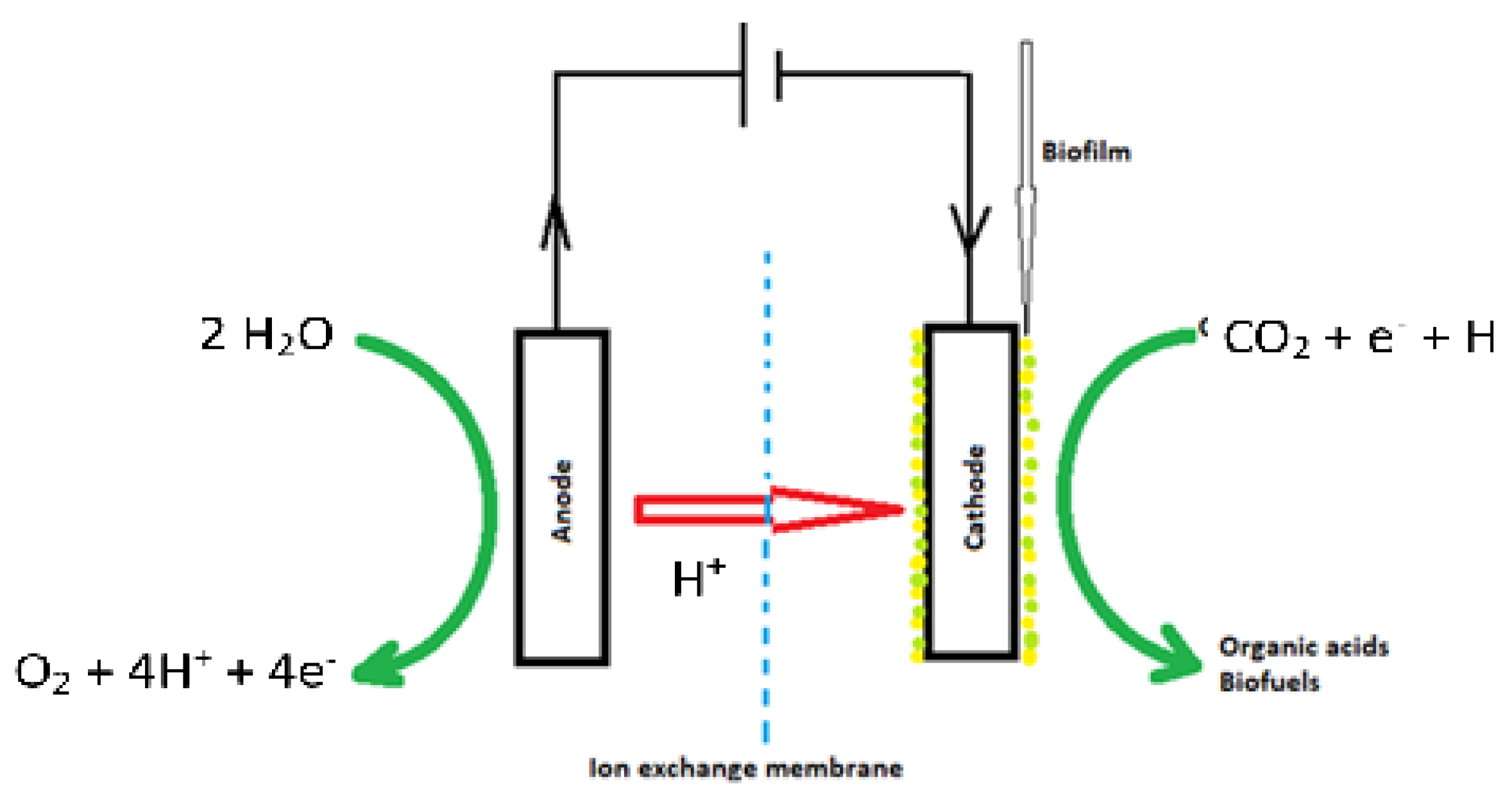
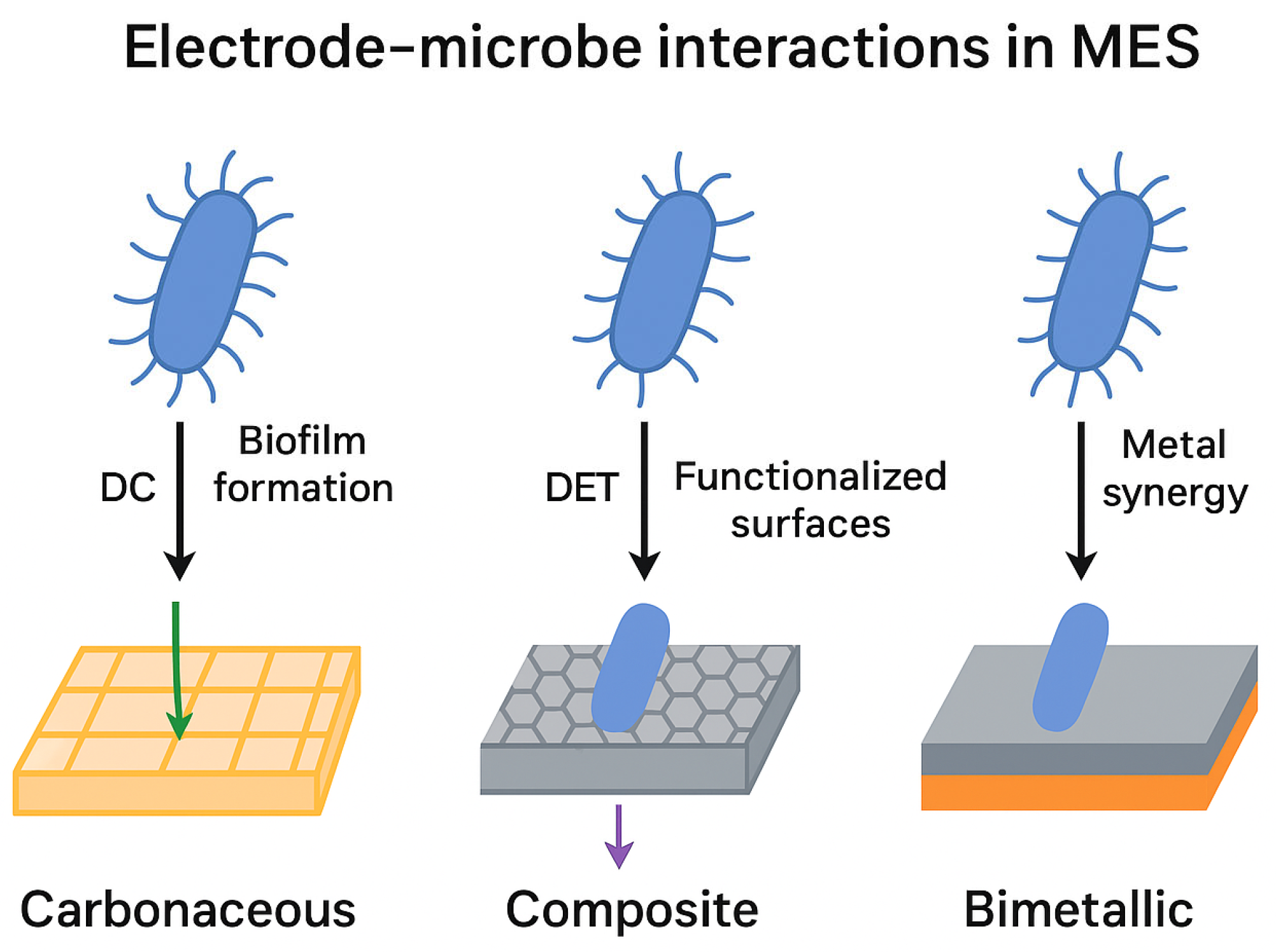
- Electrode–microbe electron transfer. Electrons originate at a cathode (driven by an external power source, preferably renewable). Electron transfer mechanisms include (a) direct extracellular electron transfer (EET) via conductive biofilms or redox proteins, and (b) indirect hydrogen-mediated transfer, wherein electrochemically produced H2 is consumed by hydrogenotrophic acetogens. Distinguishing between these pathways remains a major experimental and conceptual theme across the 2010–2025 studies [1,4].
- Electro-trophic metabolism. Many acetogens (e.g., Sporomusa, Acetobacterium, Clostridium) deploy the Wood–Ljungdahl pathway to fix CO2 to acetyl-CoA and then excrete acetate or reduce further to ethanol/butanol under specified conditions. Advances in metabolic understanding and synthetic biology approaches to expand the product scope intensified after 2020 [5,6].
- System design. The reactor architecture, cathode material and geometry, operational potential, pH, mass transfer and anode chemistry jointly determine the rates, titers and selectivity. From the initial graphite electrodes (2010) to engineered 3D-printed cathodes, materials and reactor choices have been central to MES progress [1,7].
2. Evolution of Electrode Materials for Microbial Electrosynthesis
2.1. Carbonaceous Materials and Graphene-Based Electrodes
2.1.1. Advantages and Disadvantages on Using Carbonaceous and Graphene-Based Electrodes in MES
- Advantages
- Carbonaceous electrodes (graphite, felt, RVC, paper): Low cost, scalable, chemically stable, and relatively easy to modify.
- Graphene-based electrodes: Outstanding conductivity, hierarchical porosity, tunable surface chemistry, and superior performance in terms of electron transfer and product selectivity.
- Circular-carbon electrodes: Sustainable, low-cost, derived from biomass or waste, high porosity and surface functionalization enable strong microbial adhesion and long-term durability.
- Disadvantages
- Plain carbon electrodes: Limited conductivity compared to advanced materials, poor catalytic activity toward hydrogen evolution requires high applied potentials.
- Graphene-based: High cost of synthesis, difficulties of large-scale and reproducible fabrication, potential instability under prolonged operation.
- Circular carbons: High variability in structure and performance depending on feedstock and processing conditions, often lower conductivity than engineered graphene-based.
2.1.2. Gaps and Challenges in Using Carbonaceous and Graphene-Based Electrodes in MES
2.1.3. Future Perspectives on Using Carbonaceous and Graphene-Based Electrodes in MES
- Hybrid electrodes: Combining biochar/circular carbons with thin graphene or catalytic skins may achieve both cost-effectiveness and high performance.
- Scalable manufacturing: Techniques such as 3D printing and roll-to-roll coating can bridge lab innovation with industrial feasibility.
- Functionalized surfaces: Rational tuning of oxygen groups, nitrogen doping, or heteroatom functionalization could steer microbial adhesion and selectivity.
- Integration with techno-economics: Future studies should assess not only performance metrics (current density, coulombic efficiency) but also life-cycle impacts and cost per unit product. Table 2 is summarizing the advantages, disadvantages, gaps and future perspectives for carbonaceous electrode materials.
2.2. Composite Electrodes (Carbon + Polymers/Metals/Catalysts)
2.2.1. Advantages and Disadvantages of Using Composite Electrodes
- Advantages
- Three-dimensional-printed carbon lattices (Ni/Mo-modified): Their engineered porosity and topology provide enhanced mass transfer and fine-tuned H2 delivery to microbial biofilms. Studies show significantly increased volumetric productivities, particularly for acetate, due to the localized control of the hydrogen flux near acetogens [12,27].
- Conductive polymer composites (poly-pyrrole, polyaniline, conductive PLA/ABS): These materials are low-cost, easily manufacturable, and mechanically flexible, enabling scalable electrode designs. Polymers also offer a tunable surface chemistry that supports microbial adhesion. Hybrid polymer–metal composites have shown stable long-term operation for acetate and methane production [19,21,25].
- Catalyst-assisted cathodes (Ni, Cu, Fe, Co, Mo, perovskites): The incorporation of catalytic coatings lowers the HER (hydrogen evolution reaction) overpotentials and provides a controlled, microbe-friendly H2 flux, avoiding damaging the local alkalinization. This leads to higher coulombic efficiencies and improved selectivity toward acetate and other reduced compounds [14,19,20,21].
- Disadvantages
- Three-dimensional-printed lattices require specialized manufacturing and may suffer from structural brittleness after prolonged use.
- Conductive polymers often face trade-offs between electrical conductivity and mechanical stability; their long-term bio-compatibility in harsh electrochemical environments is still under debate.
- Metal catalysts risk ion leaching, which can be toxic to microbial communities, and can increase system costs.
2.2.2. Gaps and Challenges in Using Composite Electrodes
2.2.3. Future Perspectives on Using Composite Electrodes
- Hybrid electrodes: Combining biochar or other circular carbons with thin catalytic coatings (Ni, Fe, Co) to balance performance and cost.
- Advanced manufacturing: Adoption of 3D printing, laser etching, and roll-to-roll coating for scalable, reproducible electrodes.
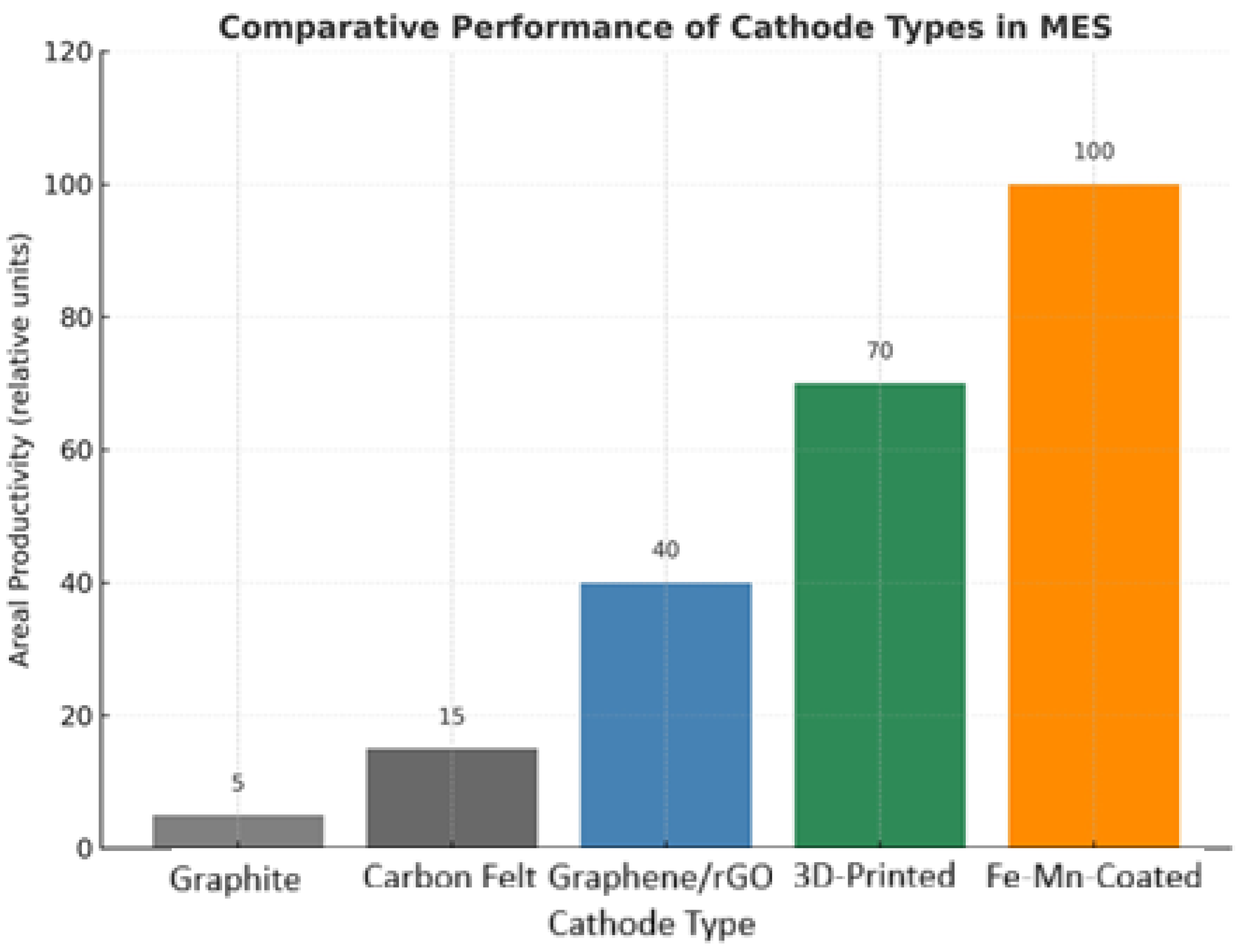
- Eco-friendly catalysts: Replacement of noble or heavy metals with earth-abundant, biodegradable alternatives (e.g., Fe- or Mn-based composites).
- Integrated techno-economic analysis: Studies should pair electrode innovations with LCA (life-cycle assessment) and cost modeling to assess the true scalability.
2.3. Bimetallic Oxide Cathodes
2.3.1. Advantages and Disadvantages of Using Bimetallic Oxide Cathodes
- Advantages
- Synergistic catalysis: Combining two or more metals enhances electron transfer kinetics and lowers overpotentials compared to monometallic oxides.
- Abundance and sustainability: Many BMOs (Fe-, Mn-, Ni-based) are earth-abundant, low-cost, and environmentally benign compared to noble metals.
- Enhanced biofilm performance: Oxide surfaces offer hydrophilicity, tunable porosity, and surface oxygen groups that promote microbial adhesion and stable electro-trophic growth.
- Selectivity: BMOs can regulate H2 evolution to match microbial uptake rates, avoiding accumulation and energy losses.
- Disadvantages
- Conductivity issues: Many oxides have lower intrinsic conductivity compared to carbon or graphene, requiring support materials or dopants.
- Metal leaching: Prolonged use may release ions (e.g., Ni2+, Co2+) potentially toxic to microbial communities.
- Complex synthesis: Hydrothermal, sol–gel, or electrodeposition methods can be costly and difficult to scale.
- Variability: Performance is highly dependent on metal ratios, synthesis conditions, and electrode architecture.
2.3.2. Gaps and Challenges in Using Bimetallic Oxide Cathodes
2.3.3. Future Perspectives on Using Bimetallic Oxide Cathodes
- Low-cost synthesis: Emphasis on scalable, eco-friendly methods (e.g., electrodeposition on felts, waste-derived metal oxides).
- Hybrid electrodes: Combination of BMOs with graphene or biochar supports to overcome conductivity limitations.
- Mechanistic studies: In situ spectroscopy and omics approaches could resolve how BMOs interact with electro-trophic consortia.
- Circular economy approaches: Utilizing waste streams (steel slag, mine tailings) as oxide precursors to reduce costs.
2.4. Cross-Cutting Insights on the Development of MES Electrodes Resulting from the 2010–2025 Literature
2.5. Anode and Anodic Reactions
3. Evolution of Microbial Communities and Biofilm Engineering
3.1. Carbonaceous Materials and Graphene
3.2. Composite Electrodes
3.3. Bi-Metallic Oxides (BMOs)
3.4. Gaps and Challenges in Co-Shape Communities
3.5. Future Perspectives on Biofilm Engineering
4. Evolution of Reactor Configurations and Process Engineering
4.1. Carbonaceous and Graphene Cathodes: Surface Area, Gas Handling, and GDEs
4.2. Composite Electrodes: Engineering the H2 Micro-Niche
4.3. Bimetallic Oxides: Low-Overpotential HER and Biofilm Compatibility
4.4. Scale-Up Lessons: Hydrodynamics, Compartmentalization, and Control
4.5. Gaps and Limitations in Reactor Configurations and Process Engineering
4.6. Future Perspectives on Reactor Configurations and Process Engineering
5. Performance Trends and Techno-Economic Signals
5.1. Performance Trends
5.2. Techno-Economic Signals
- Electrode Manufacturing and Carbon Footprint
- 2.
- Reactor Operation: Energy and Water Consumption
- 3.
- Product Separation and Resource Recovery
6. Conclusions
- Microbial electrosynthesis (MES) has progressed remarkably since its inception, moving from proof-of-concept studies in H-type reactors toward increasingly sophisticated systems that integrate novel electrode materials, microbial community engineering, and process optimization. The literature consistently highlights that electrode design and material innovation—ranging from carbonaceous substrates and graphene-based coatings to composite cathodes and bio-metallic oxides—are central drivers of improved performance, as they directly impact the electron transfer efficiency, biofilm formation, and hydrogen evolution dynamics.
- Parallel to these advances, microbial community engineering has transitioned from reliance on mixed consortia to more controlled and, in some cases, genetically optimized strains, offering opportunities to fine-tune product selectivity and stability. Similarly, reactor configurations have evolved from simple two-chamber setups to plate, tubular, and zero-gap flow designs, significantly reducing ohmic losses and improving mass transfer.
- Despite these advances, several challenges remain. MES still faces critical barriers to scale-up, including insufficient long-term stability, hydrogen management inefficiencies, incomplete understanding of microbe–electrode interactions, and economic constraints tied to the electricity demand and reactor capital costs. Current techno-economic assessments emphasize the need for higher current densities, improved Faradaic efficiencies, and durable low-cost materials to render MES competitive with conventional biotechnologies and power-to-X alternatives.
- Looking forward, the field is poised to benefit from integrative approaches—combining advanced material science, systems biology, and process engineering with data-driven modeling and techno-economic analysis. In particular, hybrid electrode architectures (graphene–oxide composites, catalytic coatings), biofilm engineering strategies, and intensified flow-through reactor designs hold promise for achieving the productivity thresholds required for industrial relevance. Furthermore, the alignment of MES research with renewable electricity integration and CO2 circular economy initiatives underscores its potential role in decarbonization strategies.
- Taken together, MES stands at the convergence of bioelectrochemistry, renewable energy integration, and carbon capture utilization (CCU). Its potential lies not in competing head-to-head with ethanol or biodiesel at current scales but in providing a new route to carbon-neutral liquid fuels that leverages cheap renewable electricity, reduces pressure on agricultural land, and transforms waste CO2 into valuable products. This positions MES as a critical component of the next generation of biofuel technologies, aligning with global priorities concerning carbon neutrality, energy security, and industrial decarbonization.
- In conclusion, while MES is not yet a mature industrial technology, the trajectory of scientific progress between 2010 and 2025 signals growing momentum toward practical application. Success will depend on bridging laboratory innovations with scalable, robust, and economically viable process configurations.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
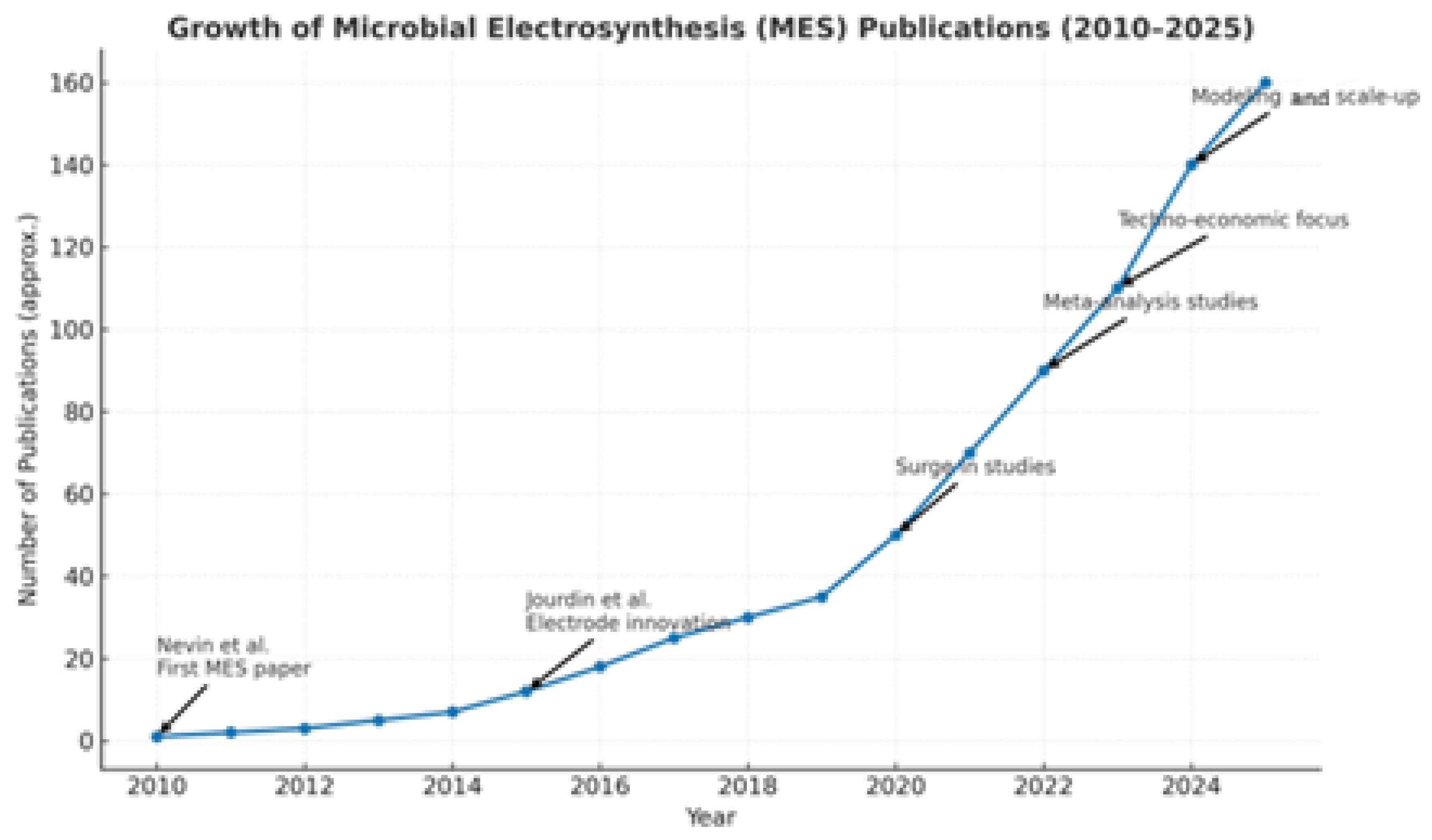
- Nevin, K.P.; Woodward, T.L.; Franks, A.E.; Summers, Z.M.; Lovley, D.R. Microbial Electrosynthesis: Feeding Microbes Electricity to Convert Carbon Dioxide and Water to Multicarbon Extracellular Organic Compounds. ASM J. 2010, 1, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Jourdin, L.; Grieger, T.; Monetti, J.; Flexer, V.; Freguia, S.; Lu, Y.; Chen, J.; Romano, M.; Wallace, G.G.; Keller, J. High Acetic Acid Production Rate Obtained by Microbial Electrosynthesis from Carbon Dioxide. Environ. Sci. Technol. 2015, 49, 13566–13574. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.; Slaehmin, M.; Balachandran, K.; Me, M.H.; Loh, K.; Bakar, M.; Jong, B.; Lim, S. Role of microbial electrosynthesis system in CO2 capture and conversion: A recent advancement toward cathode development. Front. Microbiol. 2023, 14, 1192187. [Google Scholar] [CrossRef] [PubMed]
- Zang, C.; Cao, Q.; Zhang, J.; Fu, B.; Zhang, Y.; Zhang, J.; Liu, F. Regulatory Mechanisms of Electron Supply Modes for Acetate Production in Microbial Electrosynthesis System. ACS Sustain. Chem. Eng. 2025, 13, 4406–4417. [Google Scholar] [CrossRef]
- Harnish, F.; Deutzmann, J.S.; Boto, S.T.; Rosenbaum, M.A. Microbial electrosynthesis: Opportunities for microbial pure cultures. Trends Biotechnol. 2024, 42, 1035–1047. [Google Scholar] [CrossRef]
- Zhang, D.; Foo, J.L.; Chang, M.W. Microbial electrosynthesis meets synthetic biology: Bioproduction from waste feedstocks. Biotechnol. Notes 2025, 6, 157–163. [Google Scholar] [CrossRef]
- Lekshmi, G.S.; Bazaka, K.; Ramakrishna, S.; Kumaravel, V. Microbial electrosynthesis: Carbonaceous electrode materials for CO2 conversion. Mater. Horiz. 2023, 10, 292–312. [Google Scholar] [CrossRef]
- Jourdin, L.; Freguia, S.; Flexer, V. Bringing High-Rate, CO2-Based Microbial Electrosynthesis Closer to Practical Implementation through Improved Electrode Design and Operating Conditions. Environ. Sci. Technol. 2016, 50, 1982–1989. [Google Scholar] [CrossRef]
- Xu, M.; Cobo, M.F.A.; Zeng, D. Leveraging 3D printing in microbial electrochemistry research: Current progress and future opportunities. Front. Environ. Sci. Eng. 2025, 19, 1. [Google Scholar] [CrossRef]
- Al-Mamun, A.; Ahmed, W.; Jafary, T.; Nayak, J.K.; Al-Nuaimi, A.; Sana, A. Recent advances in microbial electrosynthesis system: Metabolic investigation and process optimization. Biomed. Eng. J. 2023, 196, 108928. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, Y.; Chen, W.; Liu, C. Effect of different hydrogen evolution rates at cathode on bioelectrochemical reduction of CO2 to acetate. Sci. Total Environ. 2024, 913, 169744. [Google Scholar] [CrossRef]
- Lin, R.; Zheng, X.; Zhang, H.; Zhang, H.; He, Y.; Liu, M.; Xie, L. Cathode catalyst-assisted microbial electrosynthesis of acetate from carbon dioxide: Promising material selection. J. Environ. Sci. 2025, 160, 394–404. [Google Scholar] [CrossRef]
- Mills, S.; Dessi, P.; Pant, D.; Ferras, P.; Sloan, W.T.; Collins, G.; Ijaz, U.Z. A meta-analysis of acetogenic and methanogenic microbiomes in microbial electrosynthesis. Biofilms Microbiomes 2022, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.H.; Lee, Y.R.; Kim, H.S.; Lee, J.; Moon, M.; Park, G.W.; Lee, S.; Lee, S.Y. Evaluation of economic feasibility for the microbial electrosynthesis-based β-farnesene production technology: Process modeling and techno-economic assessment. J. CO2 Util. 2025, 97, 103110. [Google Scholar] [CrossRef]
- Adams, J.D.; Clark, D.S. Techno-Economic Assessment of Electromicrobial Production of n-Butanol from Air-Captured CO2. Environ. Sci. Technol. 2024, 58, 7302–7313. [Google Scholar] [CrossRef] [PubMed]
- Hengsbach, J.; Sabel-Becker, B.; Ulber, R.; Holtmann, D. Microbial electrosynthesis of methane and acetate—Comparison of pure and mixed cultures. Appl. Microbiol. Biotechnol. 2022, 106, 4427–4443. [Google Scholar] [CrossRef]
- Chu, N.; Wang, D.; Wang, H.; Liang, Q.; Chang, J.; Gao, Y.; Jiang, Y.; Zeng, R.J. Flow-Electrode Microbial Electrosynthesis for Increasing Production Rates and Lowering Energy Consumption. Engineering 2023, 25, 157–167. [Google Scholar] [CrossRef]
- Gharbi, R.; Vidales, A.G.; Omanovic, S.; Tartakovsky, B. Mathematical model of a microbial electrosynthesis cell for the conversion of carbon dioxide into methane and acetate. J. CO2 Util. 2022, 59, 101956. [Google Scholar] [CrossRef]
- Kargbo, H.; Harris, J.S.; Phan, N. “Drop-in” fuel production from biomass: Critical review on techno-economic feasibility and sustainability. Renew. Sustaianble Energy Rev. 2021, 135, 110168. [Google Scholar] [CrossRef]
- Marshall, C.; Ross, D.; Fichot, E.; Norman, R.; May, H. Long-term Operation of Microbial Electrosynthesis Systems Improves Acetate Production by Autotrophic Microbiomes. Environ. Sci. Technol. 2013, 47, 6023–6029. [Google Scholar] [CrossRef]
- Chung, T.H.; Rahman, A.; Chakrabarty, A.A.; Zakaria, B.S.; Khondoker, M.; Dhar, B. 3D printed cathodes for microbial electrolysis cell-assisted anaerobic digester: Evaluation of performance, resilience, and fluid dynamics. Journal of power sources 2024, 623, 235461. [Google Scholar] [CrossRef]
- Chen, L.F.; Yu, H.; Zhang, J.; Qin, H.Y. A short review of graphene in the microbial electrosynthesis of biochemicals from carbon dioxide. RSC Adv. 2022, 12, 22770–22782. [Google Scholar] [CrossRef]
- Cao, Q.; Zhang, C.; Zhang, J.; Zhang, J.; Zheng, Z.; Liu, H. Enhanced microbial electrosynthesis performance with 3-D algal electrodes under high CO2 sparging: Superior biofilm stability and biocathode-plankton interactions. Bioresour. Technol. 2024, 412, 131381. [Google Scholar] [CrossRef] [PubMed]
- Kracke, F.; Deutzmann, J.; Jayathilake, B.; Pang, S.; Chandrasekaran, S.; Baker, S.; Spormann, A. Efficient Hydrogen Delivery for Microbial Electrosynthesis via 3D-Printed Cathodes. Front. Microbiol. 2021, 12, 696473. [Google Scholar] [CrossRef] [PubMed]
- Nwanebu, E.; Omanovic, S.; Hrapovic, S.; Vidales, A.G.; Tartakovsky, B. Carbon dioxide conversion to acetate and methane in a microbial electrosynthesis cell employing an electrically-conductive polymer cathode modified by nickel-based coatings. Int. J. Hydrog. Energy 2022, 47, 203215. [Google Scholar] [CrossRef]
- Tian, S.; He, J.; Huang, H.; Song, T.-S.; Wu, X.; Xie, J.; Zhou, W. Perovskite-Based Multifunctional Cathode with Simultaneous Supplementation of Substrates and Electrons for Enhanced Microbial Electrosynthesis of Organics. ACS Appl. Mater. Interfaces 2020, 12, 30449–30456. [Google Scholar] [CrossRef]
- Izadi, P.; Fontmorin, J.-M.; Godain, A.; Yu, E.H.; Head, I.M. Parameters influencing the development of highly conductive and efficient biofilm during microbial electrosynthesis: The importance of applied potential and inorganic carbon source. npj Biofilms Microbiomes 2020, 6, 40. [Google Scholar] [CrossRef]
- Carrillo-Peña, D.; Mateos, R.; Morán, A.; Escapa, A. Reduced graphene oxide improves the performance of a methanogenic biocathode. Fuel 2022, 321, 123957. [Google Scholar] [CrossRef]
- Hui, S.; Jiang, Y.; Jiang, Y.; Lyu, Z.; Ding, S.; Song, B.; Zhu, W.; Zhu, J.-J. Cathode materials in microbial electrosynthesis systems for carbon dioxide reduction: Recent progress and perspectives. Energy Mater. 2023, 3, 3000055. [Google Scholar] [CrossRef]
- Zhuab, H.; Dongab, Z.; Huangc, Q.; Song, T.-S.; Xie, J. Fe3O4/granular activated carbon as an efficient three-dimensional electrode to enhance the microbial electrosynthesis of acetate from CO2. RSC Adv. 2019, 9, 34095–34101. [Google Scholar] [CrossRef]
- Liu, L.; Lin, R.N.; O’Shea, R.; Deng, C.; Xuan, X.; Xia, R.; Wall, D.M.; Murphy, J.D. Microbe-electrode interactions on biocathodes are facilitated through tip-enhanced electric fields during CO2-fed microbial electrosynthesis. Cell Rep. Phys. Sci. 2024, 5, 102262. [Google Scholar] [CrossRef]
- Bian, B.; Yu, N.; Yun, N.; Li, S.; Zhou, X.; Xie, C.; Rossi, R.; Logan, B.E. Substantially Improved Microbial Electrosynthesis of Methane Achieved by Improving Hydrogen Retention and Flow Distribution through Porous Electrodes. Environ. Sci. Technol. 2025, 59, 13719–13729. [Google Scholar] [CrossRef] [PubMed]
- Giddings, C.G.S.; Nevin, K.P.; Woodward, T.; Lovley, D.R.; Butler, C.S. Simplifying microbial electrosynthesis reactor design. Front. Microbiol. 2015, 6, 468. [Google Scholar] [CrossRef]
- Liu, Z.; Xue, X.; Cai, W.; Cui, K.; Patil, S.A.; Guo, K. Recent progress on microbial electrosynthesis reactor designs and strategies to enhance the reactor performance. Biochem. Eng. J. 2023, 190, 108745. [Google Scholar] [CrossRef]
- Deutzmann, J.S.; Callander, G.; Spormann, A.M. Improved reactor design enables productivity of microbial electrosynthesis on par with classical biotechnology. Bioresour. Technol. 2025, 416, 131733. [Google Scholar] [CrossRef]
- Bajracharya, S.; Krige, A.; Matsakas, L.; Rova, U.; Christakopoulos, P. Advances in cathode designs and reactor configurations of microbial electrosynthesis systems to facilitate gas electro-fermentation. Bioresour. Technol. 2022, 354, 127178. [Google Scholar] [CrossRef]
- Stöckl, M.; Lange, T.; Izadi, P.; Bolat, S.; Teetz, N.; Harnisch, F.; Holtmann, D. Application of gas diffusion electrodes in bioeconomy: An update. Biotechnol. Bioeng. 2023, 120, 1465–1477. [Google Scholar] [CrossRef]
- Corona-Martínez, D.A.; Martínez-Amador, S.Y.; la Garza, J.A.R.-D.; Laredo-Alcalá, E.I.; Pérez-Rodríguez, P. Recent Advances in Scaling up Bioelectrochemical Systems: A Review. BioTech 2025, 14, 8. [Google Scholar] [CrossRef]
- Fathima, A.; Ilankoon, I.M.S.K.; Zhang, Y.; Chong, M.N. Scaling up of dual-chamber microbial electrochemical systems—An appraisal using systems design approach. Sci. Total Environ. 2024, 912, 169186. [Google Scholar] [CrossRef]
- Nwanebua, E.; Jezernikb, M.; Lawsonb, C.; Bruanta, G.; Tartakovsky, B. Impact of cathodic pH and bioaugmentation on acetate and CH4 production in a microbial electrosynthesis cell. RSC Adv. 2024, 14, 22962–22973. [Google Scholar] [CrossRef]
- Rohbohm, N.; Angenent, L.T. A development study for liquid- and vapor-fed anode zero-gap bioelectrolysis cells. iScience 2025, 28, 112959. [Google Scholar] [CrossRef] [PubMed]
- Cabau-Peinado, O.; Winkelhorst, M.; Stroek, R.; de Kat Angelino, R.; Straathof, A.J.; Masania, K.; Daran, J.M.; Jourdin, L. Microbial electrosynthesis from CO2 reaches productivity of syngas and chain elongation fermentations. Trends Biotechnol. 2024, 42, 1503–1522. [Google Scholar] [CrossRef] [PubMed]
- Jourdin, L.; Sousa, J.; van Stralen, N.; Strik, D. Techno-economic assessment of microbial electrosynthesis from CO2 and/or organics: An interdisciplinary roadmap towards future research and application. Appl. Energy 2020, 279, 115775. [Google Scholar] [CrossRef]
- Bian, Y.; Leininger, A.; May, H.D.; Ren, Z.J. H2 mediated mixed culture microbial electrosynthesis for high titer acetate production from CO2. Environ. Sci. Ecotechnol. 2023, 19, 100324. [Google Scholar] [CrossRef]
- Bian, B.; Yu, N.; Akbari, A.; Shi, L.; Zhou, X.; Xie, C.; Saikaly, P.E.; Logan, B.E. Using a non-precious metal catalyst for long-term enhancement of methane production in a zero-gap microbial electrosynthesis cell. Water Res. 2024, 259, 121815. [Google Scholar] [CrossRef]
- Merve, B.A.; Schultz, T.; Sharma, N.; Browe, M.P. Optimizing Bimetallic NiRu@Ti3C2Tx Catalysts for Oxygen Evolution: The Impact of MXene Content on Ru Stability. Electrochim. Acta 2024, 513, 145529. [Google Scholar] [CrossRef]
- Barua, S.; Balciunaite, A.; Upskuviene, D.; Vaicuniene, J.; Tamašauskaitė-Tamašiūnaitė, L.; Norkus, E. Bimetallic Ni–Mn Electrocatalysts for Stable Oxygen Evolution Reaction in Simulated/Alkaline Seawater and Overall Performance in the Splitting of Alkaline Seawater. Coatings 2024, 14, 1074. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Yi, J.; Chen, Z.; Ge, L.; Liu, C.; Geng, H.; Chen, X.; Li, T.; Deng, D.; et al. Electromagnetic techniques in carbon fibre and carbon fibre composites manufacturing: A review. Compos. Part B 2025, 296, 112227. [Google Scholar] [CrossRef]
- Hu, H.; Guo, Y.; Zhao, J. Manufacturing Shape-Controllable Flexible PEDOT/rGO Composite Electrodes for Planar Micro-Supercapacitors. Materials 2024, 17, 2144. [Google Scholar] [CrossRef]
| System | CO2 Conversion Mechanism | Reaction Rate | Product Specificity | Energy Requirement | Advantages | Limitations |
|---|---|---|---|---|---|---|
| MES | Microbial catalysis at electrodes | Moderate | High for targeted compounds (e.g., acetate, methane) | Low to moderate (ambient conditions, requires applied voltage) | High selectivity, integrates with waste streams | Limited by microbial kinetics and mass transfer |
| AER | Inorganic electrocatalysis | High | Variable, depends on catalyst (CO, formate, hydrocarbons) | High (overpotentials, electricity input) | Fast conversion, tunable product selectivity | Competing side reactions, high energy input |
| PBS | Photosynthetic CO2 fixation | Low to moderate | Low to moderate (biomass, lipids) | Low (light-driven) | Uses solar energy directly, sustainable | Low product specificity, light and nutrient limitations |
| Electrode Type | Advantages | Disadvantages | Gaps/Challenges | Future Perspectives |
|---|---|---|---|---|
| Carbonaceous (graphite, felt, paper, RVC) | - Low cost, abundant, scalable - Chemically stable - High surface roughness → good biofilm attachment - Easy to modify with catalysts | - Limited conductivity - Low intrinsic catalytic activity (HER) - Requires high applied potentials | - Needs improved catalytic function - Limited long-term durability data - Scaling issues for high productivity | - Surface functionalization (doping, coatings) - Integration with conductive polymers - Engineering 3D-structured carbons |
| Graphene-based (rGO, aerogels, composites) | - Exceptional conductivity - Hierarchical porosity improves mass transfer - Tunable chemistry (functional groups) - Enhanced electron transfer and product selectivity | - High synthesis cost - Scale-up remains difficult - Potential instability during long-term runs | - Lack of standardized fabrication methods - Limited understanding of graphene–microbe interactions - Reproducibility concerns across labs | - Hybrid electrodes (graphene + biochar) - Roll-to-roll or scalable coating methods - Use in advanced 3D-printed cathodes |
| Circular carbons (biochar, waste-derived) | - Sustainable and low-cost - Derived from biomass or waste (circular economy) - High porosity and wettability - Comparable biofilm colonization to graphene | - Variability in properties (feedstock dependent) - Often lower conductivity than graphene - Inconsistent performance across studies | - Lack of standardization in feedstock processing - Limited comparative benchmarks with engineered electrodes | - Standardized production methods - Hybridization with conductive nanomaterials - Scalable sustainable electrodes for industrial MES |
| Composite Electrode Type | Advantages | Disadvantages | Gaps/Challenges | Future Perspectives |
|---|---|---|---|---|
| Three-dimensional-Printed Carbon Lattices (Ni/Mo, doped carbons) | - Tailored 3D geometry for high surface area and mass transfer - Enhanced localized H2 delivery to biofilms - High productivities for acetate and other products | - Requires specialized equipment - Mechanical brittleness under long-term use - Fabrication costs still high | - Limited durability testing (>1000 h) - Lack of standard print protocols - Scale-up feasibility unproven | - Scale-up via industrial 3D printing - Hybridization with low-cost biochars - Integration into modular reactor stacks |
| Conductive Polymers (poly-pyrrole, polyaniline, PLA/ABS blends) | - Low-cost and lightweight - Flexible, scalable manufacturing - Tunable chemistry enhances microbial adhesion - Good long-term stability in some studies | - Moderate conductivity compared to metals - Mechanical degradation in harsh electrochemical environments - Potential bio-compatibility concerns | - Limited reproducibility between labs - Unknown long-term chemical resistance - Need more data on polymer–biofilm interactions | - Polymer–metal or polymer–carbon hybrids - Development of biodegradable conductive polymers - Use in flexible/portable MES reactors |
| Catalyst-Coated Cathodes (Ni, Fe, Cu, Co, Mo, perovskites) | - Lower HER overpotentials - Controlled H2 flux prevents pH shocks - Increased coulombic efficiency and selectivity | - Risk of metal ion leaching (microbial toxicity) - Added costs (especially noble/perovskite catalysts) - Complex synthesis routes | - Few studies on biofilm community shifts under catalysts - Insufficient life-cycle and cost analyses - Variability in coating adhesion and durability | - Earth-abundant catalysts (Fe, Mn) - Thin catalytic coatings over low-cost supports - Techno-economic integration with renewable energy systems |
| Electrode Type | Advantages | Disadvantages | Gaps/Challenges | Future Perspectives |
|---|---|---|---|---|
| Bio-metallic Oxides (Fe-Mn, Ni-Co, Cu-Fe, perovskites) | - Synergistic catalytic activity - Earth-abundant, low-cost metals - Hydrophilic, biofilm-friendly surfaces - Tunable HER selectivity | - Low intrinsic conductivity - Risk of metal ion leaching (toxicity) - Complex and costly synthesis - Performance highly condition-dependent | - Limited long-term stability data - Lack of standardized testing - Poor understanding of electron transfer mechanisms - Few techno-economic studies | - Hybrid electrodes with carbon/graphene supports - Scalable electrodeposition or waste-derived oxides - Mechanistic in situ studies - Integration with circular economy feedstocks |
| Anode Reaction Type | Advantages | Disadvantages | Gaps/Challenges | Future Perspectives |
|---|---|---|---|---|
| Oxygen evolution reaction (OER, water oxidation) | Simple, well-studied reaction - Produces protons to balance cathodic CO2 reduction - No external feed required | - High overpotential → high energy demand - Generates O2, which can inhibit anaerobic microbes if it crosses over - No value-added product | - Need for robust, O2-tight separators - Lack of efficient low-cost OER catalysts compatible with MES | - Development of selective membranes preventing O2 crossover - Design of durable, low-overpotential OER catalysts (e.g., non-precious metal oxides) |
| Organic oxidation (e.g., acetate, glycerol, wastewater organics) | - Lower overpotential than the OER - Potential to co-produce value-added chemicals - Enables wastewater valorization | - Requires continuous organic feed - Possible fouling or toxicity from complex waste streams - Added complexity in feedstock logistics | - Limited studies on long-term operation - Incomplete understanding of anodic microbiome dynamics | - Integration of MES with wastewater treatment - Tailoring anodic catalysts for selective oxidation of targeted substrates |
| Sulfide oxidation | - Lower thermodynamic potential than the OER - Useful for treating sulfide-rich effluents - Produces elemental sulfur or sulfate as by-products | - Limited substrate availability (industrial niche) - Possible corrosion issues from sulfur species | - Few pilot-scale demonstrations - Need to control sulfur deposition on electrodes | - Coupling with biogas desulfurization - Development of sulfur-tolerant electrode materials |
| Ammonia oxidation | - Potential to co-produce nitrate/nitrite as fertilizers - Lower potential than OER - Useful in nitrogen-rich waste valorization | - Formation of undesirable intermediates (e.g., N2O, a greenhouse gas) - Limited microbial compatibility data | - Stability and selectivity of anodic catalysts not well explored - Environmental risks of incomplete oxidation | - Design of selective catalysts to minimize N2O - Integration with agricultural wastewater treatment for circular nutrient recovery |
| Microorganism | Type/Classification | Electron Transfer Mechanism | Typical Substrates | Main Products | Key Features in MES |
|---|---|---|---|---|---|
| Geobacter sulfurreducens | Gram-negative δ-proteobacteria | Direct electron transfer via pili (“nanowires”) | Acetate, lactate | Current, CO2 reduction products | Highly efficient anode-respiring bacteria, strong biofilm formation |
| Shewanella oneidensis | Gram-negative γ-proteobacteria | Direct electron transfer + soluble mediators (flavins) | Lactate, pyruvate | Current, H2 | Versatile metabolism, can transfer electrons to electrodes and metals |
| Clostridium spp. | Gram-positive anaerobes | Indirect electron transfer (mediators) | Sugars, organic acids | Acetate, butyrate, ethanol | Strong fermentative activity, useful in cathodic CO2 reduction |
| Methanogens (e.g., Methanococcus maripaludis) | Archaea | Direct or mediated electron uptake | H2, CO2 | Methane | Essential for microbial electrosynthesis of methane from CO2 |
| Acetobacterium woodii | Gram-positive anaerobe | Indirect via electron carriers | H2, CO2 | Acetate | Efficient CO2-to-acetate conversion in microbial electrosynthesis |
| Desulfuromonas acetexigens | Gram-negative δ-proteobacteria | Direct electron transfer | Acetate, lactate | Current | Strong anode respiration, used in high-current MES |
| Anaerobium acetethylicum | Gram-positive anaerobe | Mediated electron transfer | Sugars, pyruvate | Hydrogen, ethanol | Useful in biohydrogen production and MES cathodes |
| MES Stage | Key Activities/Processes | Carbon Footprint (CO2 eq) | Energy Consumption | Water Consumption | Notes/Mitigation Strategies |
|---|---|---|---|---|---|
| Electrode Manufacturing | Production of graphite, carbon cloth, nanomaterials; chemical/thermal treatments | High (due to raw material extraction and high-temp processes) | Moderate to high (pyrolysis, synthesis) | Low to moderate | Use low-carbon materials, recycle electrodes, extend electrode lifespan |
| Reactor Operation | Pumping, aeration, mixing, electrical input; maintaining microbial environment | Moderate (depends on electricity source) | High (continuous operation, electrical input) | Moderate to high (reaction medium, flow maintenance) | Optimize reactor design, reduce ohmic losses, use renewable electricity |
| Product Separation | Filtration, distillation, electrochemical extraction | Moderate (energy-intensive processes contribute indirectly) | High (heating, separation processes) | High (cooling, washing, extraction) | Implement in situ recovery, integrate energy-efficient separation techniques |
| Overall MES System | Combined impact of all stages | High to moderate (dominated by electrode and energy use) | High (operation + separation) | Moderate to high | Holistic optimization of materials, reactor, and separation reduces environmental footprint |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirea, R.; Popescu, E.; Zaharescu, T. Microbial Electrosynthesis: The Future of Next-Generation Biofuel Production—A Review. Energies 2025, 18, 5187. https://doi.org/10.3390/en18195187
Mirea R, Popescu E, Zaharescu T. Microbial Electrosynthesis: The Future of Next-Generation Biofuel Production—A Review. Energies. 2025; 18(19):5187. https://doi.org/10.3390/en18195187
Chicago/Turabian StyleMirea, Radu, Elisa Popescu, and Traian Zaharescu. 2025. "Microbial Electrosynthesis: The Future of Next-Generation Biofuel Production—A Review" Energies 18, no. 19: 5187. https://doi.org/10.3390/en18195187
APA StyleMirea, R., Popescu, E., & Zaharescu, T. (2025). Microbial Electrosynthesis: The Future of Next-Generation Biofuel Production—A Review. Energies, 18(19), 5187. https://doi.org/10.3390/en18195187







