Comparative Evaluation of Performance Parameters of Conventional and Waste Fuels for Diesel Engines Towards Sustainable Transport
Abstract
1. Introduction
2. Materials and Methods
2.1. Tested Fuels
2.2. Experimental Methodology and Measurement Equipment
3. Results and Discussion
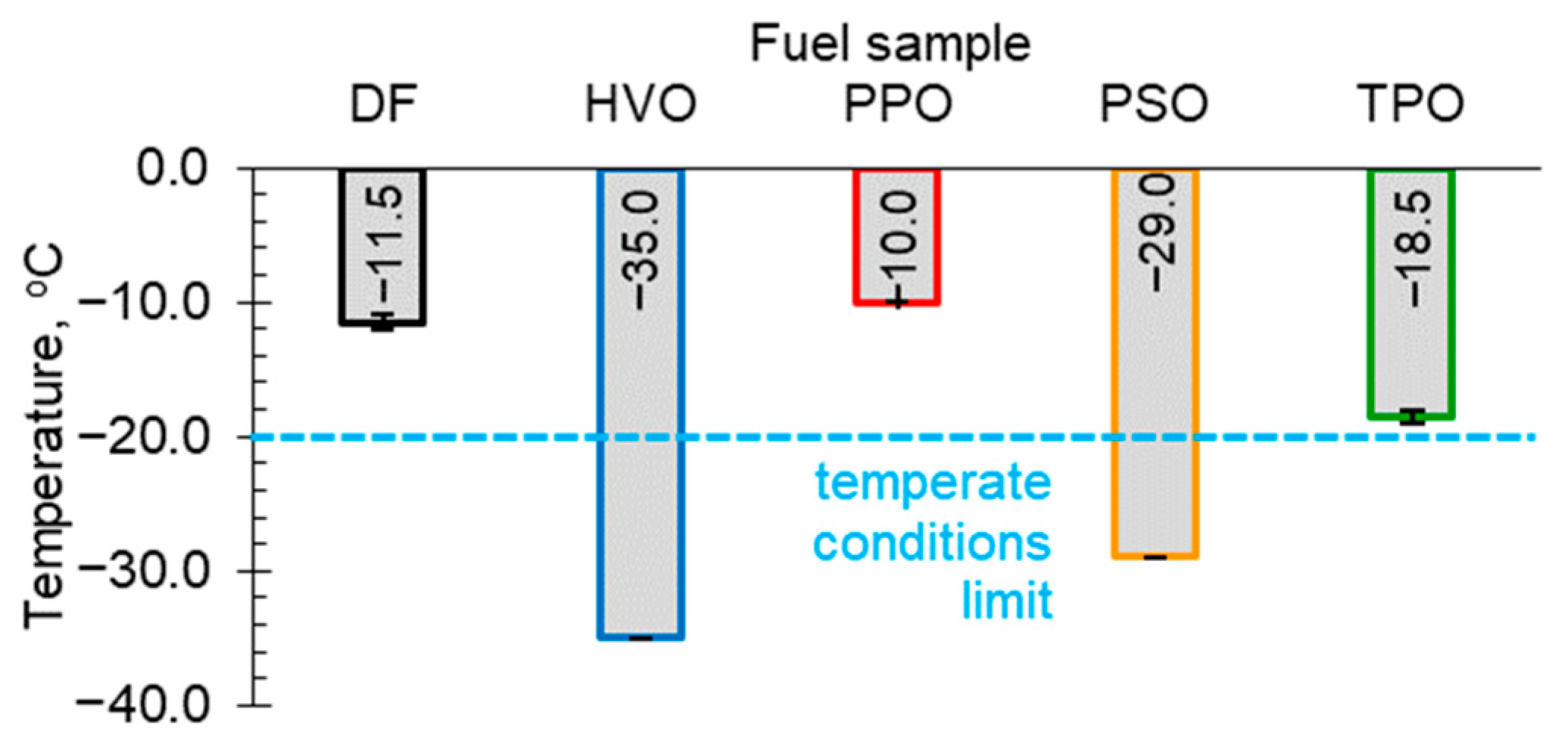
3.1. Cold Filter Plugging Point
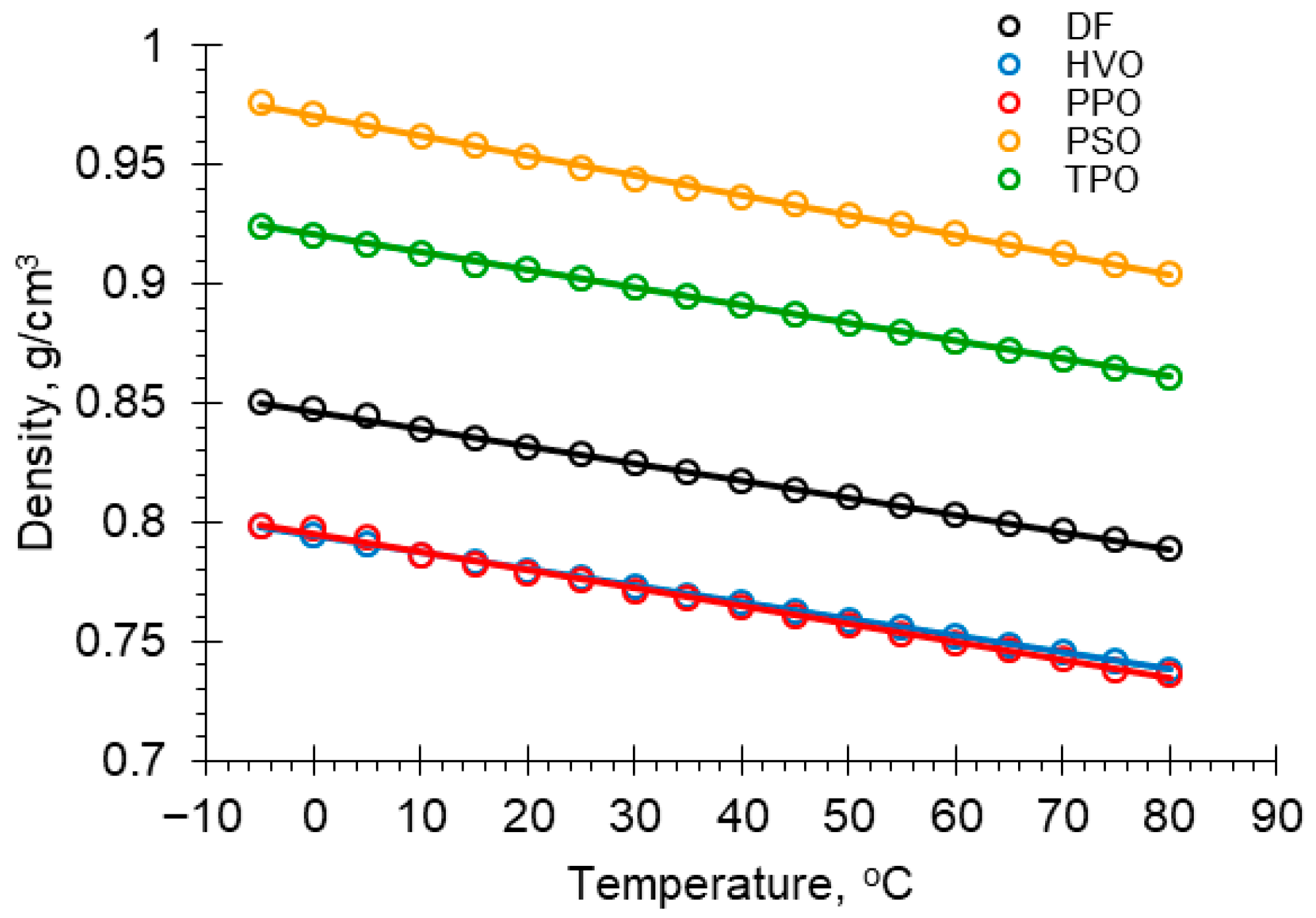
3.2. Density
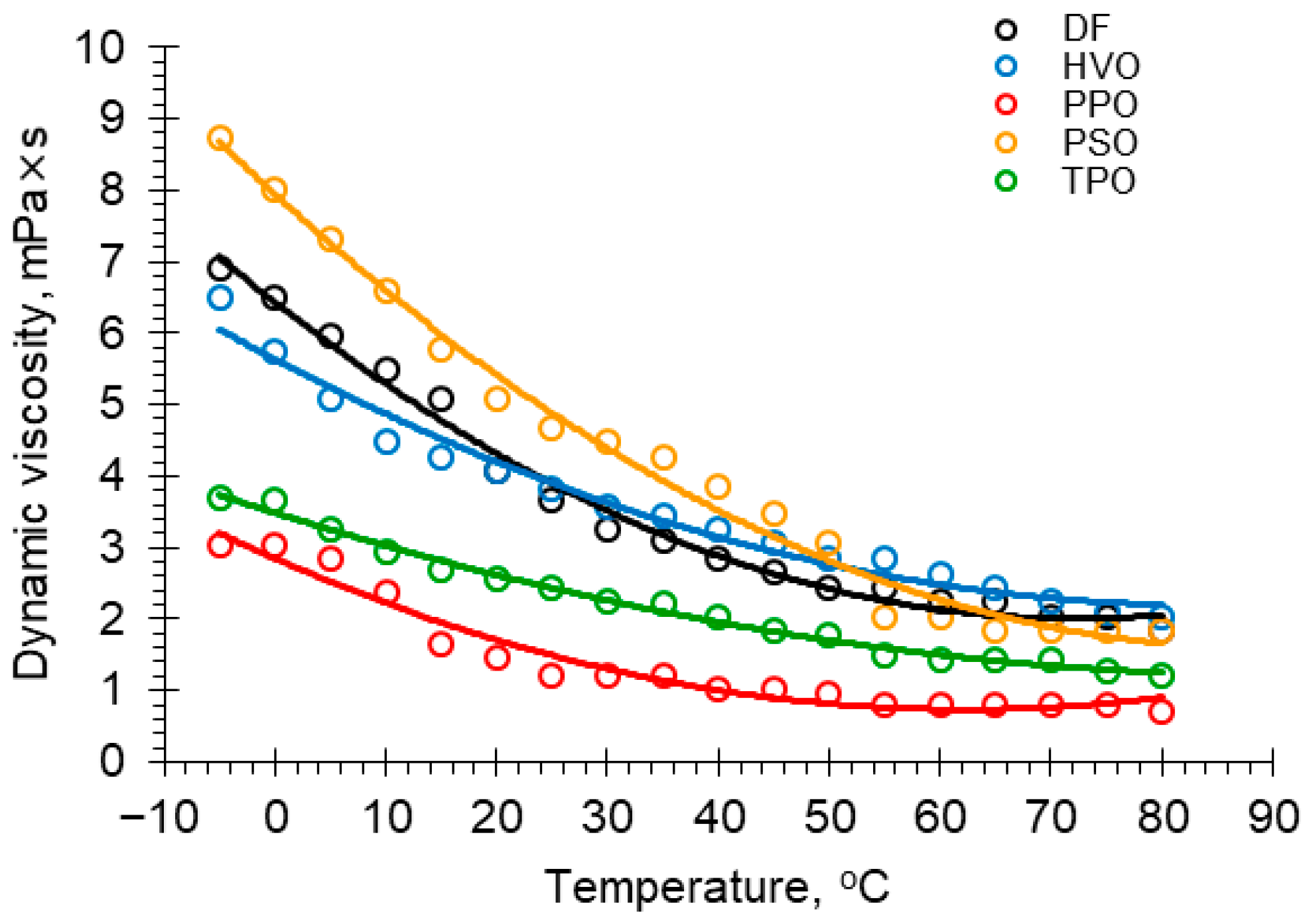
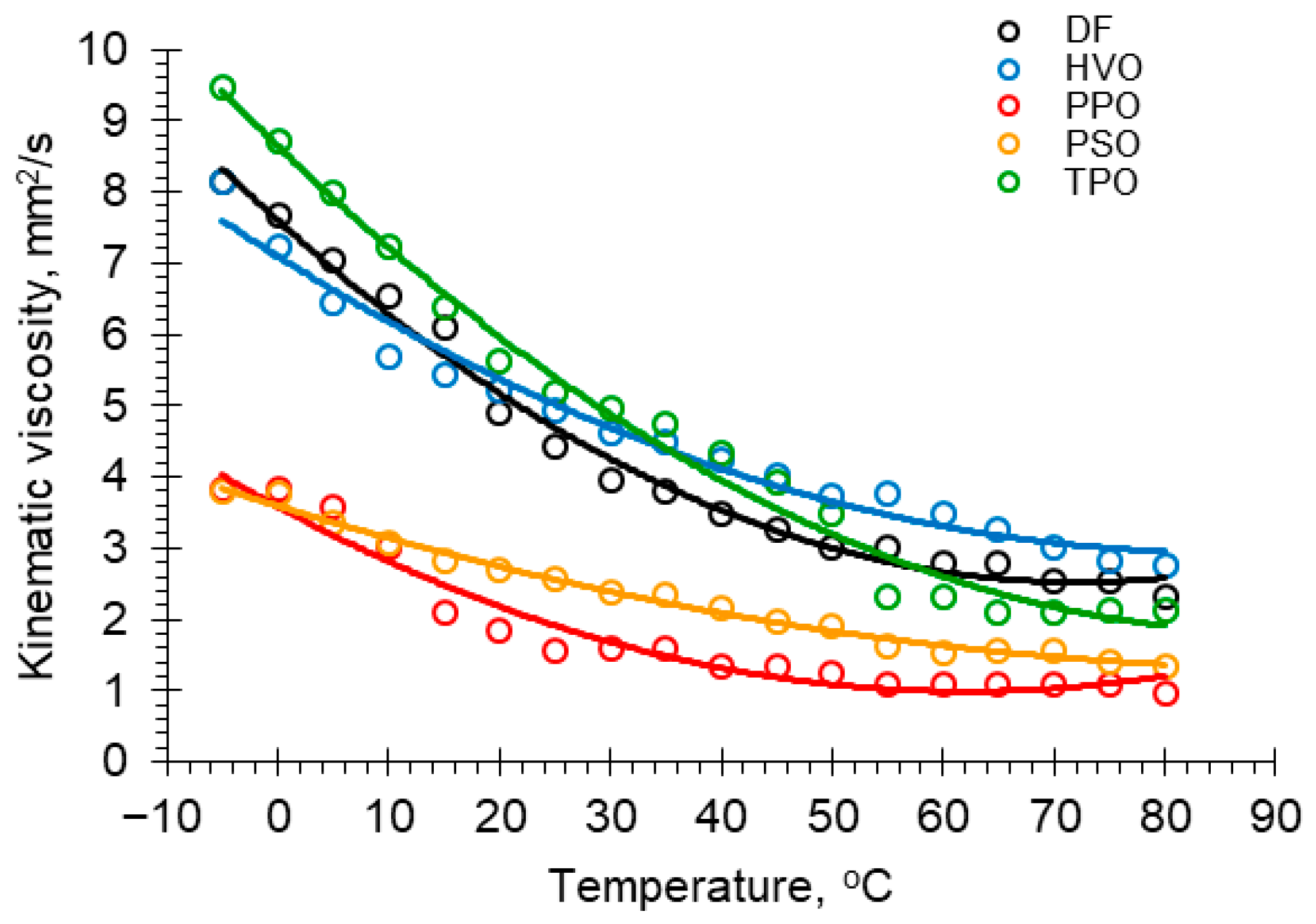
3.3. Viscosity
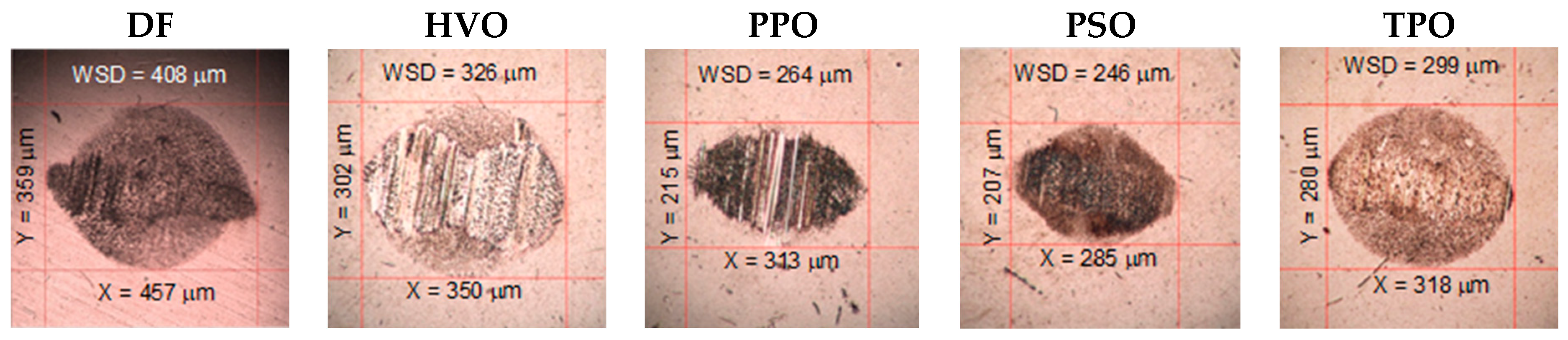
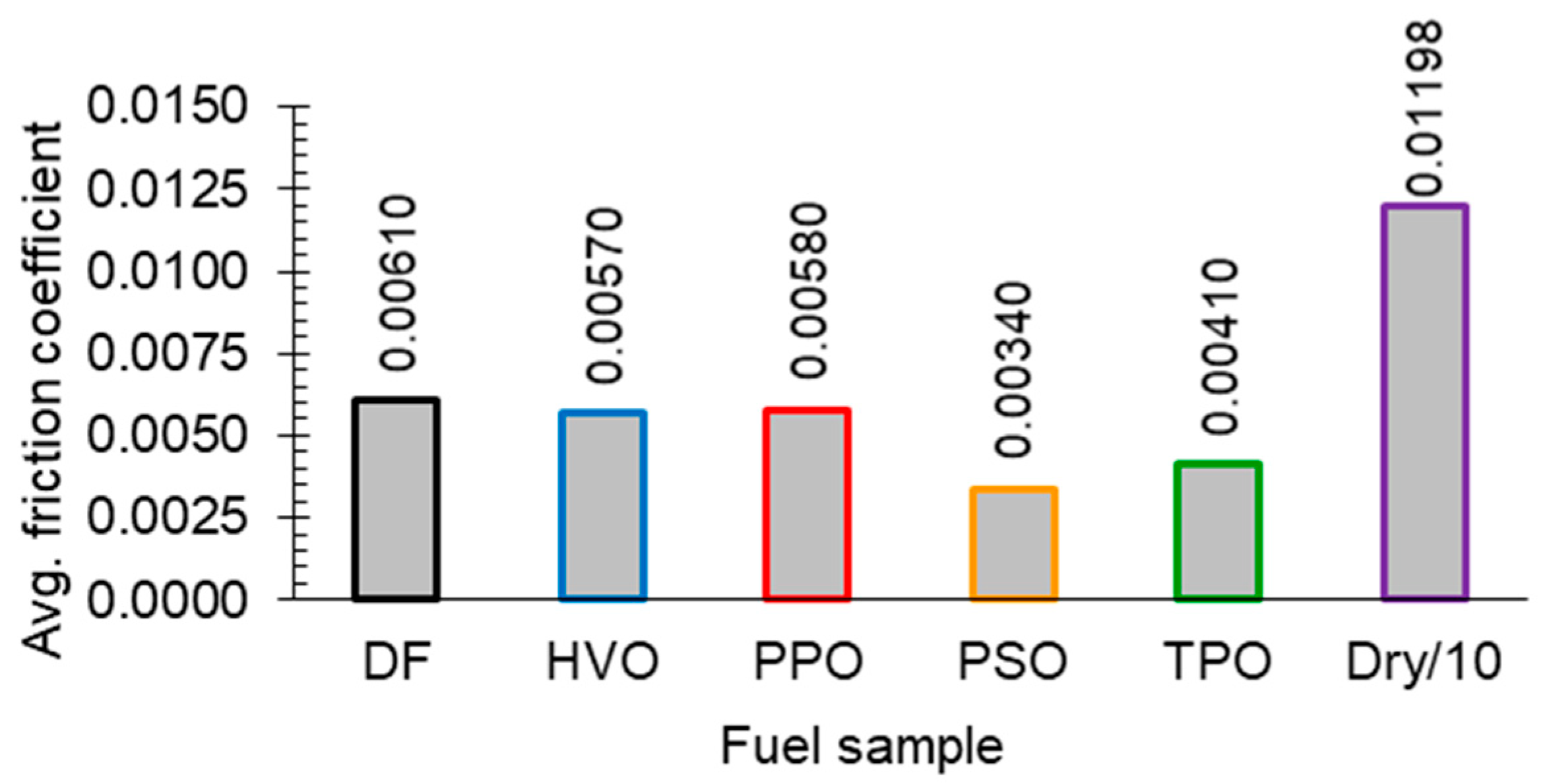
3.4. Lubricity and Coefficient of Friction
3.4.1. Four-Ball Method
3.4.2. HFRR Method
3.4.3. Ball-on-Disc Method
4. Conclusions
5. Future Research
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BEVs | battery electric vehicles |
| BOTD | ball-on-three discs |
| BOTS | ball-on-three seats |
| CFPP | cold filter plugging point |
| DF | diesel fuel |
| FAME | fatty acid methyl ester |
| FBR | four-ball rig |
| HFRR | high-frequency reciprocating rig |
| HPDE | high-density polyethylene |
| HVO | hydrotreated vegetable oil |
| LPDE | low-density polyethylene |
| PET | polyethylene terephthalate |
| PHEVs | plug-in hybrid electric vehicles |
| BOD | ball-on-disc |
| PP | polypropylene |
| PPO | polypropylene oil |
| PS | polystyrene |
| PSO | polystyrene oil |
| PVC | polyvinyl chloride |
| PVO | pure vegetable oil |
| SDGs | Sustainable Development Goals |
| SLBOCLE | scuffing load ball-on-cylinder lubricity evaluator |
| TPO | automobile tyre oil |
| TRLs | technology readiness levels |
| VAPs | value-added products |
| VO | vegetable oil |
Appendix A
| Parameter | Analyzer | Method | Unit | Accuracy |
|---|---|---|---|---|
| Nitrogen content | Kjeldahl FoodAlyt D5000, OMNILAB, Bremen, Germany | Kjeldahla method | mg/kg | 1% |
| Sulphur content | S2 PICOFOX, Bruker, Berlin, Germany | X-ray fluorescence | mg/kg | 0.3% |
| Carbon content | TOC multi N/C3100 TOC, Analytik Jena, Jena, Germany | NDIR detector | %wt. | 0.69% |
| Hydrogen content | UNICUBE analytic functional testing, Elementar Analysensysteme, Langenselbold, Germany | direct TPD technology | %wt. | 0.3% |
| Total aromatics content | ERASPEC, Eralytics GmbH, Vienna, Austria | mid-FTIR | %wt. | 0.3% |
| FAME content | ERASPEC, Eralytics GmbH, Vienna, Austria | EN 14078 [92] | %v | 0.2% |
| Water content | Titro Line 7500 KF TRACE, SI Analytics/Xylem Analytics, Weilheim, Germany | EN 61326-1 [93] | ppm | 0.3% |
| Derived cetane number | CID 510 Herzog (PAC Group), Paris, France | ASTM D7668 [94] | - | 0.6% |
| Lower heating value | PARR 6100, Parr Instrument Company, Moline, IL, USA | ASTM D240 [95], ASTM D4809 [96] | MJ/kg | 0.2% |
| Flash point | NPM 450, Normalab, Valliquerville, France | Pensky-Martens ASTM D 93 [97] | °C | 0.3% |
| Parameter | Unit | Value |
|---|---|---|
| Volume of fuel sample | cm3 | 2.0 ± 0.2 |
| Stroke length | mm | 1.00 ± 0.02 |
| Frequency | Hz | 50 ± 1 |
| Fuel sample temperature | °C | 60 ± 2 |
| Test mass | g | 200 ± 1 |
| Test duration | min | 75.0 ± 0.1 |
| Parameter | Analyzer | Method/Standard | Unit | Accuracy |
|---|---|---|---|---|
| Cold filter blocking tendency | FPP 5Gs, ISL (PAC Group), Paris, France | PN-EN 116 [74] | °C | 1 |
| Density | DMA 4100 M, Anton Paar, Graz, Austria | ASTM D4052 [75] | g/cm3 | 0.0001 |
| Dynamic viscosity | BROOKFIELD DV-II+ Pro, AMETEK Brookfield, Middleborough, MA, USA | ISO 2555 [76] | mPa × s | ±1% |
| Lubricity | T-02, Instytut Technologii Eksploatacji–PIB (ITeE), Radom, Poland | Four-ball method ISO 20623 [77], ASTM D 2783 [78] | kg μm | 0.1 to 1.0 0.1 |
| Lubricity and coefficient of friction | PSC, PCS Instruments, London, UK | HFRR method: ISO 12156 [79], ASTM D6079 [98] | kg μm | 0.1 to 1.0 0.5 |
| Coefficient of friction | T-20, Instytut Technologii Eksploatacji–PIB (ITeE), Radom, Poland | Ball-on-disc method Ball-cratering | N mm | 0.01 0.01 |
Appendix B
| T, °C | Density ρ, g/cm3 Fuel Sample | T, °C | Density ρ, g/cm3 Fuel Sample | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DF | HVO | PPO | PSO | TPO | DF | HVO | PPO | PSO | TPO | ||
| −5 | 0.8504 | 0.7985 | 0.7987 | 0.9758 | 0.9244 | 40 | 0.8174 | 0.7661 | 0.7643 | 0.9366 | 0.8911 |
| 0 | 0.8476 | 0.7945 | 0.7973 | 0.9711 | 0.9208 | 45 | 0.8140 | 0.7626 | 0.7605 | 0.9333 | 0.8873 |
| 5 | 0.8446 | 0.7905 | 0.7936 | 0.9668 | 0.9167 | 50 | 0.8104 | 0.7592 | 0.7565 | 0.9290 | 0.8836 |
| 10 | 0.8388 | 0.7867 | 0.7858 | 0.9624 | 0.9130 | 55 | 0.8069 | 0.7557 | 0.7531 | 0.9248 | 0.8798 |
| 15 | 0.8355 | 0.7834 | 0.7822 | 0.9583 | 0.9086 | 60 | 0.8034 | 0.7522 | 0.7498 | 0.9209 | 0.8760 |
| 20 | 0.8318 | 0.7797 | 0.7784 | 0.9532 | 0.9058 | 65 | 0.7998 | 0.7487 | 0.7461 | 0.9168 | 0.8722 |
| 25 | 0.8285 | 0.7768 | 0.7754 | 0.9491 | 0.9021 | 70 | 0.7963 | 0.7452 | 0.7426 | 0.9127 | 0.8685 |
| 30 | 0.8246 | 0.7730 | 0.7715 | 0.9443 | 0.8986 | 75 | 0.7928 | 0.7417 | 0.7378 | 0.9084 | 0.8646 |
| 35 | 0.8209 | 0.7695 | 0.7685 | 0.9404 | 0.8948 | 80 | 0.7892 | 0.7382 | 0.7364 | 0.9043 | 0.8608 |
| T, °C | Dynamic Viscosities η, mPa × s Fuel Sample | T, °C | Dynamic Viscosities η, mPa × s Fuel Sample | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DF | HVO | PPO | PSO | TPO | DF | HVO | PPO | PSO | TPO | ||
| −5 | 6.913 | 6.507 | 3.05 | 8.743 | 3.694 | 40 | 2.847 | 3.253 | 1.017 | 3.863 | 2.033 |
| 0 | 6.507 | 5.751 | 3.05 | 8.020 | 3.660 | 45 | 2.643 | 3.073 | 1.017 | 3.481 | 1.830 |
| 5 | 5.956 | 5.083 | 2.847 | 7.312 | 3.253 | 50 | 2.440 | 2.847 | 0.943 | 3.070 | 1.772 |
| 10 | 5.490 | 4.473 | 2.372 | 6.588 | 2.948 | 55 | 2.440 | 2.847 | 0.813 | 2.033 | 1.491 |
| 15 | 5.083 | 4.270 | 1.651 | 5.788 | 2.690 | 60 | 2.237 | 2.628 | 0.813 | 2.033 | 1.423 |
| 20 | 4.067 | 4.067 | 1.446 | 5.083 | 2.567 | 65 | 2.237 | 2.44 | 0.813 | 1.842 | 1.423 |
| 25 | 3.66 | 3.836 | 1.220 | 4.685 | 2.44 | 70 | 2.033 | 2.252 | 0.813 | 1.830 | 1.423 |
| 30 | 3.253 | 3.565 | 1.220 | 4.473 | 2.237 | 75 | 2.019 | 2.093 | 0.813 | 1.830 | 1.280 |
| 35 | 3.108 | 3.457 | 1.220 | 4.255 | 2.210 | 80 | 1.830 | 2.033 | 0.702 | 1.830 | 1.220 |
| T, °C | Kinematic Viscosities ν, mm2/s Fuel Sample | T, °C | Kinematic Viscosities ν, mm2/s Fuel Sample | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DF | HVO | PPO | PSO | TPO | DF | HVO | PPO | PSO | TPO | ||
| −5 | 8.130 | 8.149 | 3.819 | 3.785 | 9.458 | 40 | 3.483 | 4.247 | 1.330 | 2.171 | 4.335 |
| 0 | 7.677 | 7.239 | 3.825 | 3.769 | 8.710 | 45 | 3.247 | 4.029 | 1.337 | 1.961 | 3.923 |
| 5 | 7.052 | 6.431 | 3.587 | 3.365 | 7.977 | 50 | 3.011 | 3.750 | 1.246 | 1.907 | 3.475 |
| 10 | 6.545 | 5.686 | 3.019 | 3.064 | 7.216 | 55 | 3.024 | 3.767 | 1.080 | 1.612 | 2.311 |
| 15 | 6.084 | 5.451 | 2.11 | 2.807 | 6.37 | 60 | 2.784 | 3.493 | 1.085 | 1.546 | 2.321 |
| 20 | 4.889 | 5.216 | 1.858 | 2.693 | 5.612 | 65 | 2.797 | 3.259 | 1.09 | 1.553 | 2.112 |
| 25 | 4.418 | 4.938 | 1.573 | 2.571 | 5.194 | 70 | 2.553 | 3.022 | 1.095 | 1.559 | 2.107 |
| 30 | 3.945 | 4.612 | 1.581 | 2.369 | 4.978 | 75 | 2.546 | 2.822 | 1.102 | 1.409 | 2.117 |
| 35 | 3.786 | 4.492 | 1.588 | 2.350 | 4.756 | 80 | 2.319 | 2.754 | 0.954 | 1.349 | 2.126 |
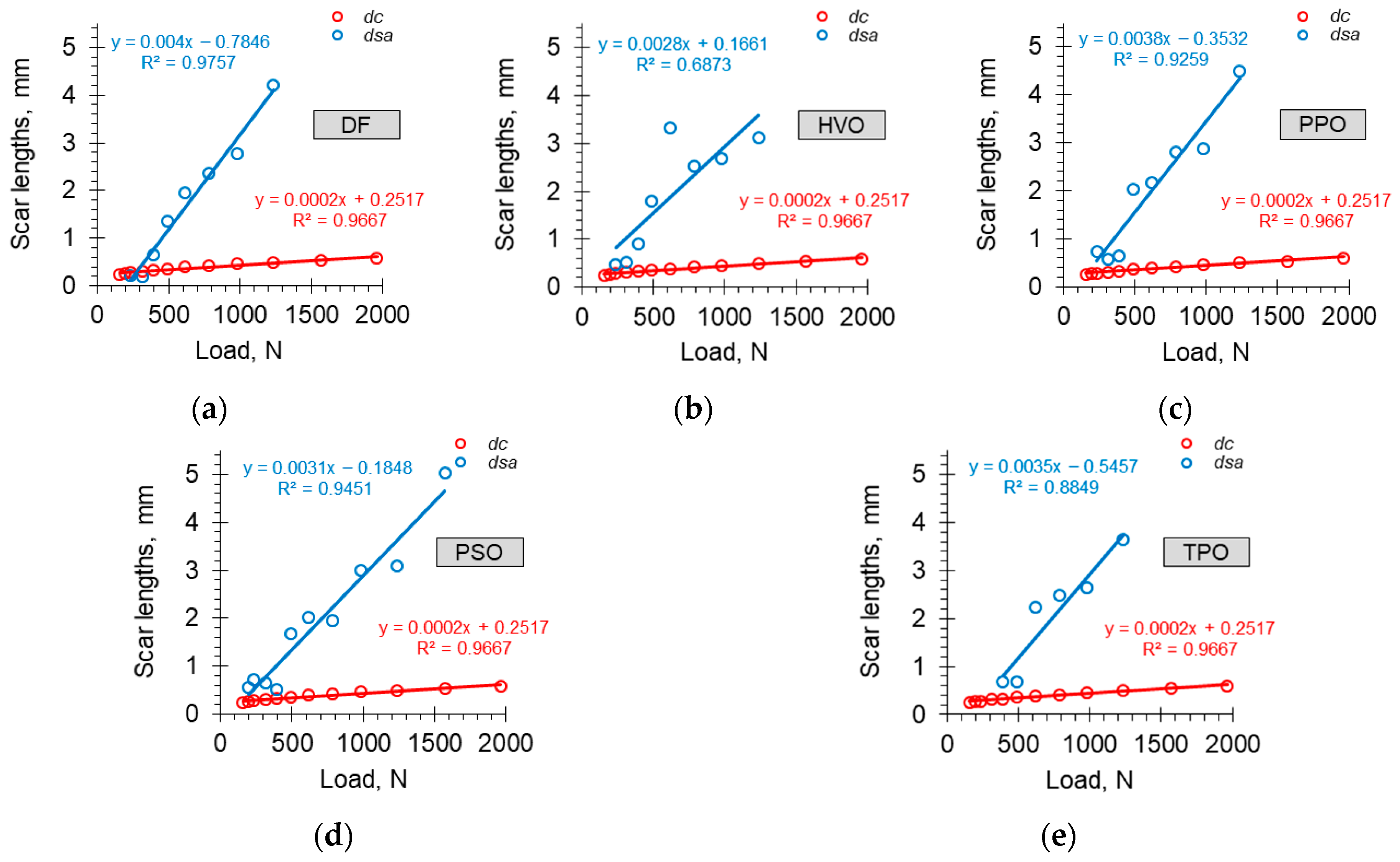
| F, N | Fuel Sample | |||||
|---|---|---|---|---|---|---|
| Stand. | DF | HVO | PPO | PSO | TPO | |
| dc, mm | dsa, mm | dsa, mm | dsa, mm | dsa, mm | dsa, mm | |
| 156.91 | 0.25 | - | - | - | no scars | - |
| 196.14 | 0.27 | no scars | no scars | no scars | 0.56 | - |
| 235.37 | 0.28 | 0.23 | 0.48 | 0.73 | 0.71 | - |
| 313.82 | 0.31 | 0.19 | 0.51 | 0.57 | 0.66 | no scars |
| 392.28 | 0.33 | 0.66 | 0.90 | 0.65 | 0.52 | 0.68 |
| 490.35 | 0.36 | 1.35 | 1.80 | 2.04 | 1.67 | 0.68 |
| 617.84 | 0.39 | 1.96 | 3.32 | 2.18 | 2.02 | 2.23 |
| 784.56 | 0.42 | 2.37 | 2.53 | 2.82 | 1.96 | 2.48 |
| 980.70 | 0.46 | 2.78 | 2.69 | 2.87 | 3.00 | 2.65 |
| 1235.68 | 0.50 | 4.21 | 3.12 | 4.50 | 3.09 | 3.64 |
| 1569.12 | 0.54 | welded | welded | welded | 5.02 | welded |
| 1961.40 | 0.59 | - | - | - | welded | - |
References
- Walsh, M. Global trends in motor vehicle use and emissions. Annu. Rev. Energy 1990, 15, 217–243. [Google Scholar] [CrossRef]
- Kalghatgi, G.; Agarwal, A.K.; Leach, L.; Senecal, L. Engines and Fuels for Future Transport; Springer: Berlin/Heidelberg, Germany, 2022; ISBN 9789811687167. [Google Scholar]
- Ritchie, H. A Global Breakdown of Greenhouse Gas Emissions by Sector. Available online: https://www.visualcapitalist.com/cp/a-global-breakdown-of-greenhouse-gas-emissions-by-sector/ (accessed on 8 March 2023).
- Robiou du Pont, Y.; Meinshausen, M. Warming assessment of the bottom-up Paris Agreement emissions pledges. Nat. Commun. 2018, 9, 4810. [Google Scholar] [CrossRef]
- Clairotte, M.; Suarez-Bertoa, R.; Zardini, A.A.; Giechaskiel, B.; Pavlovic, J.; Valverde, V.; Ciuffo, B.; Astorga, C. Exhaust emission factors of greenhouse gases (GHGs) from European road vehicles. Environ. Sci. Eur. 2020, 32, 125. [Google Scholar] [CrossRef]
- Schlacke, S.; Wentzien, H.; Thierjung, E.-M.; Köster, M. Implementing the EU Climate Law via the ‘Fit for 55’ package. Oxf. Open Energy 2022, 1, oiab002. [Google Scholar] [CrossRef]
- European Parliament. BRIEFING EU Legislation in Progress-Revising the Energy Efficiency Directive: Fit for 55 Package; BRIEFING EU Legislation in Progress; European Parliament: Strasbourg, France, 2021. [Google Scholar]
- Council of the European Union. Council Directive 2014/94/EU of 22 October 2014 on the deployment of alternative fuels infrastructure. Off. J. Eur. Union 2014, 307, 1–20. [Google Scholar]
- Szpica, D. Combustion Systems and Fuels Used in Engines—A Short Review. Appl. Sci. 2023, 13, 3126. [Google Scholar] [CrossRef]
- Kremer, M.; Hulshorst, T. In-Market Application of Start-Stop Systems in European Market; FEV Group Ltd.: Aachen, Germany, 2011. [Google Scholar]
- Mueller, N.; Strauss, S.; Tumback, S.; Goh, G.C.; Christ, A. Next Generation Engine Start/Stop Systems: “Free-Wheeling”. SAE Int. J. Engines 2011, 4, 874–887. [Google Scholar] [CrossRef]
- Bucsky, P.; Juhász, M. Is car ownership reduction impact of car sharing lower than expected? A Europe wide empirical evidence. Case Stud. Transp. Policy 2022, 10, 2208–2217. [Google Scholar] [CrossRef]
- Caban, J.; Vrábel, J.; Šarkan, B.; Ignaciuk, P. About eco-driving, genesis, challenges and benefits, application possibilities. Transp. Res. Procedia 2019, 40, 1281–1288. [Google Scholar] [CrossRef]
- Mustayen, A.G.M.B.; Rasul, M.G.; Wang, X.; Hazrat, M.A.; Negnevitsky, M.; Jahirul, M.I. Impact of waste-plastic-derived diesel on the performance and emission characteristics of a diesel engine under low load conditions. Energy Convers. Manag. 2023, 283, 116936. [Google Scholar] [CrossRef]
- Diouf, B. The electric vehicle transition. Environ. Sci. Adv. 2024, 3, 332–345. [Google Scholar] [CrossRef]
- ExxonMobil Energy Demand: Three Drivers|ExxonMobil. Available online: https://corporate.exxonmobil.com/what-we-do/energy-supply/global-outlook/energy-demand (accessed on 20 January 2023).
- British Petroleum. The Energy Outlook Explores the Forces Shaping the Global Energy Transition out to 2040 and the Key Uncertainties Surrounding That; BP Energy Outlook 2019 Edition; British Petroleum: London, UK, 2019; pp. 22–24, 72–76, 144–148. [Google Scholar]
- Koszalka, G.; Hunicz, J. Comparative study of energy losses related to the ring pack operation in homogeneous charge compression ignition and spark ignition combustion. Energy 2021, 235, 121388. [Google Scholar] [CrossRef]
- European Commission. CO2 emission performance standards for cars and vans. Eur. Comm. 2020, 2, 02019R0631-20201119. [Google Scholar]
- Vásquez, M.C.; Silva, E.E.; Castillo, E.F. Hydrotreatment of vegetable oils: A review of the technologies and its developments for jet biofuel production. Biomass Bioenergy 2017, 105, 197–206. [Google Scholar] [CrossRef]
- Aatola, H.; Larmi, M.; Sarjovaara, T.; Mikkonen, S. Hydrotreated vegetable Oil (HVO) as a renewable diesel fuel: Trade-off between NOx, particulate emission, and fuel consumption of a heavy duty engine. SAE Int. J. Engines 2009, 1, 1251–1262. [Google Scholar] [CrossRef]
- Hunicz, J.; Matijošius, J.; Rimkus, A.; Kilikevičius, A.; Kordos, P.; Mikulski, M. Efficient hydrotreated vegetable oil combustion under partially premixed conditions with heavy exhaust gas recirculation. Fuel 2020, 268, 117350. [Google Scholar] [CrossRef]
- Bezergianni, S.; Dimitriadis, A. Comparison between different types of renewable diesel. Renew. Sustain. Energy Rev. 2013, 21, 110–116. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Prussi, M. Pure vegetable oil for energy and transport. Int. J. Oil Gas Coal Technol. 2009, 2, 186–198. [Google Scholar] [CrossRef]
- Gil, L.; Pieniak, D.; Walczak, M.; Ignaciuk, P.; Sawa, J. Impact of acid number of fuels on the wear process of apparatus fuel injection in diesel engines. Adv. Sci. Technol. Res. J. 2014, 8, 54–57. [Google Scholar] [CrossRef]
- Kumar, J.S.; Bapu, B.R.R.; Sivasaravanan, S.; Prabhu, M.; Kumar, S.M.; Abubacker, M.A. Experimental studies on emission reduction in a DI diesel engine by using a nano catalyst coated catalytic converter. Int. J. Ambient. Energy 2019, 43, 1241–1247. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A review of pyrolysis technologies and feedstock: A blending approach for plastic and biomass towards optimum biochar yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Januszewicz, K.; Hunicz, J.; Kazimierski, P.; Rybak, A.; Suchocki, T.; Duda, K.; Mikulski, M. An experimental assessment on a diesel engine powered by blends of waste-plastic-derived pyrolysis oil with diesel. Energy 2023, 281, 128330. [Google Scholar] [CrossRef]
- Pumpuang, A.; Klinkaew, N.; Wathakit, K.; Sukhom, A.; Sukjit, E. The influence of plastic pyrolysis oil on fuel lubricity and diesel engine performance. RSC Adv. 2024, 14, 10070–10087. [Google Scholar] [CrossRef]
- Mikulski, M.; Ambrosewicz-Walacik, M.; Hunicz, J.; Nitkiewicz, S. Combustion engine applications of waste tyre pyrolytic oil. Prog. Energy Combust. Sci. 2021, 85, 100915. [Google Scholar] [CrossRef]
- Hamid, M.; Wesołowski, M. Waste-to-energy technologies as the future of internal combustion engines. Combust. Engines 2023, 193, 52–63. [Google Scholar] [CrossRef]
- Ferdoush, M.R.; Al Aziz, R.; Karmaker, C.L.; Debnath, B.; Limon, M.H.; Bari, A.B.M.M. Unraveling the challenges of waste-to-energy transition in emerging economies: Implications for sustainability. Innov. Green Dev. 2024, 3, 100121. [Google Scholar] [CrossRef]
- Muthukumar, K.; Kasiraman, G. Utilization of fuel energy from single-use Low-density polyethylene plastic waste on CI engine with hydrogen enrichment—An experimental study. Energy 2024, 289, 129926. [Google Scholar] [CrossRef]
- EN 590; Automotive Fuels—Diesel—Requirements and Test Methods. European Committee for Standardization: Brussels, Belgium, 2022.
- Zeman, P.; Hönig, V.; Kotek, M.; Táborský, J.; Obergruber, M.; Mařík, J.; Hartová, V.; Pechout, M. Hydrotreated vegetable oil as a fuel from waste materials. Catalysts 2019, 9, 337. [Google Scholar] [CrossRef]
- Murphy, F.; Devlin, G.; Mcdonnell, K. The evaluation of flash point and cold filter plugging point with blends of diesel and Cyn-diesel pyrolysis fuel for automotive engines. Open Fuels Energy Sci. J. 2013, 6, 1–8. [Google Scholar] [CrossRef][Green Version]
- Gala, A.; Guerrero, M.; Guirao, B.; Domine, M.E.; Serra, J.M. Characterization and Distillation of Pyrolysis Liquids Coming from Polyolefins Segregated of MSW for Their Use as Automotive Diesel Fuel. Energy Fuels 2020, 34, 5969–5982. [Google Scholar] [CrossRef]
- Ambrosewicz-Walacik, M.; Wierzbicki, S.; Mikulski, M.; Podciborski, T. Ternary fuel mixture of diesel, rapeseed oil and tyre pyrolytic oil suitable for modern CRDI engines. Transport 2018, 33, 727–740. [Google Scholar] [CrossRef]
- Acharya, N.; Nanda, P.; Panda, S.; Acharya, S. Analysis of properties and estimation of optimum blending ratio of blended mahua biodiesel. Eng. Sci. Technol. Int. J. 2017, 20, 511–517. [Google Scholar] [CrossRef]
- Alptekin, E.; Canakci, M. Determination of the density and the viscosities of biodiesel-diesel fuel blends. Renew. Energy 2008, 33, 2623–2630. [Google Scholar] [CrossRef]
- Setiawan, A.; Setiani, V.; Mazdhatina, O.S. Characterization of fuel oil from pyrolysis waste light density polyethylene (LDPE) and polypropylene (PP). IOP Conf. Ser. Mater. Sci. Eng. 2021, 1034, 012069. [Google Scholar] [CrossRef]
- Kartik, S.; Balsora, H.K.; Sharma, M.; Saptoro, A.; Jain, R.K.; Joshi, J.B.; Sharma, A. Valorization of plastic wastes for production of fuels and value-added chemicals through pyrolysis—A review. Therm. Sci. Eng. Prog. 2022, 32, 101316. [Google Scholar] [CrossRef]
- Oni, B.A.; Sanni, S.E.; Olabode, O.S. Production of fuel-blends from waste tyre and plastic by catalytic and integrated pyrolysis for use in compression ignition (CI) engines. Fuel 2021, 297, 120801. [Google Scholar] [CrossRef]
- Shehata, M.; Okeily, M.A.; Hammad, A.S. Valorisation of shredded waste tyres through sequential thermal and catalytic pyrolysis for the production of diesel-like fuel. Results Eng. 2024, 21, 101718. [Google Scholar] [CrossRef]
- Ghurri, A.; Kim, J.D.; Kim, H.G.; Jung, J.Y.; Song, K.K. The effect of injection pressure and fuel viscosity on the spray characteristics of biodiesel blends injected into an atmospheric chamber. J. Mech. Sci. Technol. 2012, 26, 2941–2947. [Google Scholar] [CrossRef]
- Knothe, G.; Steidley, K.R. Kinematic viscosity of biodiesel fuel components and related compounds. Influence of compound structure and comparison to petrodiesel fuel components. Fuel 2005, 84, 1059–1065. [Google Scholar] [CrossRef]
- Tate, R.E.; Watts, K.C.; Allen, C.A.W.; Wilkie, K.I. The viscosities of three biodiesel fuels at temperatures up to 300 °C. Fuel 2006, 85, 1010–1015. [Google Scholar] [CrossRef]
- Tesfa, B.; Mishra, R.; Gu, F.; Powles, N. Prediction models for density and viscosity of biodiesel and their effects on fuel supply system in CI engines. Renew. Energy 2010, 35, 2752–2760. [Google Scholar] [CrossRef]
- Cristofaro, M.; Edelbauer, W.; Koukouvinis, P.; Gavaises, M. Influence of Diesel Fuel Viscosity on Cavitating Throttle Flow Simulations under Erosive Operation Conditions. ACS Omega 2020, 5, 7182–7192. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Nik-Ghazali, N.N.; Abul Kalam, M.; Badruddin, I.A.; Banapurmath, N.R.; Akram, N. The effect of nano-additives in diesel-biodiesel fuel blends: A comprehensive review on stability, engine performance and emission characteristics. Energy Convers. Manag. 2018, 178, 146–177. [Google Scholar] [CrossRef]
- Schaschke, C.; Fletcher, I.; Glen, N. Density and viscosity measurement of diesel fuels at combined high pressure and elevated temperature. Processes 2013, 1, 30–48. [Google Scholar] [CrossRef]
- Rowane, A.J.; Mahesh Babu, V.; Rokni, H.B.; Moore, J.D.; Gavaises, M.; Wensing, M.; Gupta, A.; McHugh, M.A. Effect of Composition, Temperature, and Pressure on the Viscosities and Densities of Three Diesel Fuels. J. Chem. Eng. Data 2019, 64, 5529–5547. [Google Scholar] [CrossRef]
- Bietresato, M.; Bolla, A.; Caligiuri, C.; Renzi, M.; Mazzetto, F. The kinematic viscosity of conventional and bio-based fuel blends as a key parameter to indirectly estimate the performance of compression-ignition engines for agricultural purposes. Fuel 2021, 298, 120817. [Google Scholar] [CrossRef]
- Robertson, L.X.; Schaschke, C.J. Combined high pressure and low temperature viscosity measurement of biodiesel. Energy Fuels 2010, 24, 1293–1297. [Google Scholar] [CrossRef]
- Joshi, R.M.; Pegg, M.J. Flow properties of biodiesel fuel blends at low temperatures. Fuel 2007, 86, 143–151. [Google Scholar] [CrossRef]
- Ding, H.; Wang, C.; Zhu, X. Estimation of the kinematic viscosities of bio-oil/alcohol blends: Kinematic viscosity-temperature formula and mixing rules. Fuel 2019, 254, 115687. [Google Scholar] [CrossRef]
- Sudan Reddy Dandu, M.; Nanthagopal, K. Tribological aspects of biofuels—A review. Fuel 2019, 258, 116066. [Google Scholar] [CrossRef]
- Shatrov, M.G.; Golubkov, L.N.; Dunin, A.U.; Yakovenko, A.L.; Dushkin, P.V. Influence of high injection pressure on fuel injection perfomances and diesel engine worcking process. Therm. Sci. 2015, 19, 2245–2253. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, J.; Markov, V.; Wu, T. Study on precise fuel injection under multiple injections of high pressure common rail system based on deep learning. Energy 2024, 307, 132784. [Google Scholar] [CrossRef]
- Petrea, N.D.; Bujoreanu, C. Importance of Fuel Injection System for Low Emissions, Combustion Noise and Low Fuel Consumption. IOP Conf. Ser. Mater. Sci. Eng. 2018, 444, 042020. [Google Scholar]
- Hazrat, M.A.; Rasul, M.G.; Khan, M.M.K. Lubricity Improvement of the Ultra-low Sulfur Diesel Fuel with the Biodiesel. Energy Procedia 2015, 75, 111–117. [Google Scholar] [CrossRef]
- Barbour, R.H.; Rickeard, D.J.; Elliott, N.G. Understanding Diesel Lubricity; SAE Technical Papers; SAE International: Warrendale, PA, USA, 2000. [Google Scholar]
- Zulkifli, N.W.M.; Kalam, M.A.; Masjuki, H.H.; Shahabuddin, M.; Yunus, R. Wear prevention characteristics of a palm oil-based TMP (trimethylolpropane) ester as an engine lubricant. Energy 2013, 54, 167–173. [Google Scholar] [CrossRef]
- Kaminski, P. Investigation Lubricity Performance of Lubricating Oil Used in Marine Diesel Engine—Fuel Injection Pump. Appl. Sci. 2024, 14, 6148. [Google Scholar] [CrossRef]
- Chodkiewicz, A.; Kałdoński, T. Analysis of standards for testing lubricity properties on a four-ball apparatus used in Poland. Bull. Mil. Univ. Technol. 2020, 69, 129–152. [Google Scholar] [CrossRef]
- Szpica, D.; Czaban, J.; Banaszuk, P.; Weresa, E. The diesel and the vegetable oil properties assessment in terms of pumping capability and cooperation with internal combustion engine fuelling system. Acta Mech. Autom. 2015, 9, 14–18. [Google Scholar] [CrossRef]
- Kuszewski, H.; Jakubowski, M.; Jaworski, A.; Lubas, J.; Balawender, K. Effect of temperature on tribological properties of 1-butanol–diesel fuel blends—Preliminary experimental study using the HFRR method. Fuel 2021, 296, 120700. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Moore, C.M.; Semelsberger, T.A.; Chuck, C.J.; Gordon, J.C.; Sutton, A.D. The Effect of Functional Groups in Bio-Derived Fuel Candidates. ChemSusChem 2016, 9, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Priyan, M.S.; Hariharan, P. Abrasive wear modes in ball-cratering test conducted on Fe73Si15 Ni10Cr2 alloy deposited specimen. Tribol. Ind. 2014, 36, 97–106. [Google Scholar]
- Gee, M.G.; Gant, A.; Hutchings, I.M.; Bethke, R.; Schiffman, K.; Van Acker, K.; Poulat, S.; Gachon, Y.; von Stebut, J. Measurement Good Practice Guide No 57—Ball Cratering or Micro-Abrasion Wear Testing of Coatings; Measurement Good Practice Guide; NPL Publications: Teddington, UK, 2002. [Google Scholar]
- Borawski, A. Impact of operating time on selected tribological properties of the friction material in the brake pads of passenger cars. Materials 2021, 14, 884. [Google Scholar] [CrossRef] [PubMed]
- Borawski, A.; Szpica, D.; Mieczkowski, G. Research on Tribological Features of Brake Friction Materials-Comparison of the Results Obtained with the Pin-On-Disc and Ball-Cratering Methods. Mechanika 2022, 28, 317–322. [Google Scholar] [CrossRef]
- Mikulski, M.; Hunicz, J.; Duda, K.; Kazimierski, P.; Suchocki, T.; Rybak, A. Tyre pyrolytic oil fuel blends in a modern compression ignition engine: A comprehensive combustion and emissions analysis. Fuel 2022, 320, 123869. [Google Scholar] [CrossRef]
- EN 116; Diesel and Domestic Heating Fuels-Determination of Cold Filter Plugging Point-Stepwise Cooling Bath Method. European Committee for Standardization (CEN): Brussels, Belgium, 2015.
- ASTM D4052; Standard Test Method for Density, Relative Density, and API Gravity of Liquids by Digital Density Meter. American Society for Testing and Materials: West Conshohocken, PA, USA, 2022.
- ISO 2555; Plastics—Resins in the Liquid State or as Emulsions or Dispersions-Determination of Apparent Viscosity Using a Single Cylinder Type Rotational Viscometer Method. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 20623; Petroleum and Related Products—Determination of the Extreme-Pressure and Anti-Wear Properties of Lubricants—Four-Ball Method (European Conditions). International Organization for Standardization: Geneva, Switzerland, 2017.
- ASTM D 2783; Standard Test Method for Measurement of Extreme-Pressure Properties of Lubricating Fluids (Four-Ball Method). American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- ISO 12156-1; Diesel Fuel—Assessment of Lubricity Using the High-Frequency Reciprocating Rig (HFRR)—Part 1: Test Method. ISO: Geneva, Switzerland, 2023.
- ASTM D975; Standard Specification for Diesel Fuel. American Society for Testing and Materials: West Conshohocken, PA, USA, 2023.
- Kuszewski, H.; Jaworski, A.; Ustrzycki, A. Lubricity of ethanol–diesel blends—Study with the HFRR method. Fuel 2017, 208, 491–498. [Google Scholar] [CrossRef]
- EN ISO 3104; Petroleum Products—Transparent and Opaque Liquids—Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity. International Organization for Standardization: Geneva, Switzerland, 2023.
- Hartikka, T.; Kuronen, M.; Kiiski, U. Technical Performance of HVO (Hydrotreated Vegetable Oil) in Diesel Engines; SAE Technical Papers; SAE International: Warrendale, PA, USA, 2012; Volume 9. [Google Scholar]
- Malinowska, M.; Zera, D. Analiza zmian smarności oleju silnikowego stosowanego w silniku Cegielski-Sulzer 3AL25/30. Zesz. Nauk. Akad. Morskiej W Gdyni 2016, 96, 93–104. [Google Scholar]
- Lewalski, M.; Muślewski, Ł.; Landowski, B.; Gniot, M. Optymalizacja doboru _rodka smarnego na podstawie diagnozy stanu wybranego układu mechanicznego. Stud. Proc. Pol. Assoc. Knowl. Manag. 2016, 79, 132–144. [Google Scholar]
- Szczypiński-Szala, W. Ocena własności smarnych paliw rzepakowych. Czas. Techniczne. Mech. 2012, 109, 279–286. [Google Scholar]
- Dodos, G.S.; Vassileiou, F.; Karonis, D. Lubricity of Diesel Fuel Hydrocarbons and Surrogate Fuels; SAE Technical Papers; SAE International: Warrendale, PA, USA, 2017. [Google Scholar]
- Worldwide Fuel Charter 2019. In Gasoline and Diesel Fuel, 6th ed.; ACEA: Brussels, Belgium, 2019.
- Song, R.; Yu, H.; Song, H. Tribological Behavior of Biomass Fast Pyrolysis Fuel and Diesel Blends. Appl. Sci. 2022, 12, 2540. [Google Scholar] [CrossRef]
- Gil, L.; Pieniak, D.; Kozłowski, E.; Selech, J. Impact of selected biofuels and diesel as lubricants on the statistical distribution and course of sliding friction coefficients for the kinematic pair 100Cr6-100Cr6. Tribologia 2019, 288, 17–23. [Google Scholar] [CrossRef]
- Huynh, K.K.; Pham, S.T.; Tieu, K.A.; Wan, S. Tribocatalysis Induced Carbon-Based Tribofilms—An Emerging Tribological Approach for Sustainable Lubrications. Lubricants 2023, 11, 327. [Google Scholar] [CrossRef]
- EN 14078; Liquid Petroleum Products-Determination of Fatty Acid Methyl Ester (FAME) Content in Middle Distillates-Infrared Spectrometry Method. European Committee for Standardization: Brussels, Belgium, 2014.
- EN 61326-1; Electrical Equipment for Measurement, Control and Laboratory Use-EMC Requirements-Part 1: General Requirements. European Committee for Standardization: Brussels, Belgium, 2006.
- ASTM D7668; Standard Test Method for Determination of Derived Cetane Number (DCN) of Diesel Fuel Oils—Ignition Delay and Combustion Delay Using a Constant Volume Combustion Chamber Method. American Society for Testing and Materials: West Conshohocken, PA, USA, 2010.
- ASTM D240; Standard Test Method for Heat of Combustion of Liquid Hydrocarbon Fuels by Bomb Calorimeter. American Society for Testing and Materials: West Conshohocken, PA, USA, 2019.
- ASTM D4809; Standard Test Method for Heat of Combustion of Liquid Hydrocarbon Fuels by Bomb Calorimeter (Precision Method). American Society for Testing and Materials: West Conshohocken, PA, USA, 2013.
- ASTM D 93; Standard Test Methods for Flash Point by Pensky-Martens Closed Cup Tester. American Society for Testing and Materials: West Conshohocken, PA, USA, 2020.
- ASTM D6079; Standard Test Method for Evaluating Lubricity of Diesel Fuels by the High-Frequency Reciprocating Rig (HFRR). ASTM International: West Conshohocken, PA, USA, 2022.


| Parameter | Unit | DF | HVO | PPO | PSO | TPO |
|---|---|---|---|---|---|---|
| Nitrogen content | mg/kg | 57.00 | 24.00 | 600.00 | 932.00 | 7763.00 |
| Sulphur content | mg/kg | 7.51 | <1.00 | 16.79 | 13.04 | 5000.00 |
| Carbon content | %wt. | 86.70 | 83.99 | 84.60 | 91.10 | 95.76 |
| Hydrogen content | %wt. | 13.10 | 14.21 | 15.20 | 8.20 | 5.40 |
| Total aromatics content | %wt. | 26.70 | <1.00 | 1.50 | 98.00 | 54.80 |
| FAME content | %v | 6.35 | 0.38 | 0.00 | 0.00 | 0.00 |
| Water content | ppm | 38.00 | 17.00 | 123.00 | 576.00 | 827.00 |
| Derived cetane number | - | 55.29 | 74.70 | 27.54 | <15.00 | 32.33 |
| Lower heating value | MJ/kg | 42.55 | 43.90 | 44.86 | 40.55 | 42.58 |
| Flash point | °C | 66.80 | 78.20 | <24.00 | 32.00 | 57.50 |
| Fuel Sample | Coefficients of the Trend Function | ||
|---|---|---|---|
| a | b | R2 | |
| DF | −0.0007 | 0.8468 | 0.9993 |
| HVO | −0.0007 | 0.7942 | 0.9998 |
| PPO | −0.0008 | 0.7948 | 0.9965 |
| PSO | −0.0008 | 0.9706 | 0.9992 |
| TPO | −0.0007 | 0.9206 | 0.9998 |
| Fuel Sample | Coefficients of the Trend Function | |||
|---|---|---|---|---|
| a | b | c | R2 | |
| DF | 0.0009 | −0.1229 | 6.4341 | 0.9894 |
| HVO | 0.0005 | −0.0816 | 5.6386 | 0.9757 |
| PPO | 0.0005 | −0.0680 | 2.8505 | 0.9536 |
| PSO | 0.0008 | −0.1427 | 7.9513 | 0.9888 |
| TPO | 0.0003 | −0.0486 | 3.4874 | 0.9906 |
| Fuel Sample | Coefficients of the Trend Function | |||
|---|---|---|---|---|
| a | b | c | R2 | |
| DF | 0.0010 | −0.1408 | 7.6007 | 0.9886 |
| HVO | 0.0006 | −0.0973 | 7.0969 | 0.9742 |
| PPO | 0.0007 | −0.0836 | 3.5845 | 0.9519 |
| PSO | 0.0002 | −0.0474 | 3.5924 | 0.9894 |
| TPO | 0.0008 | −0.1502 | 8.6423 | 0.9875 |
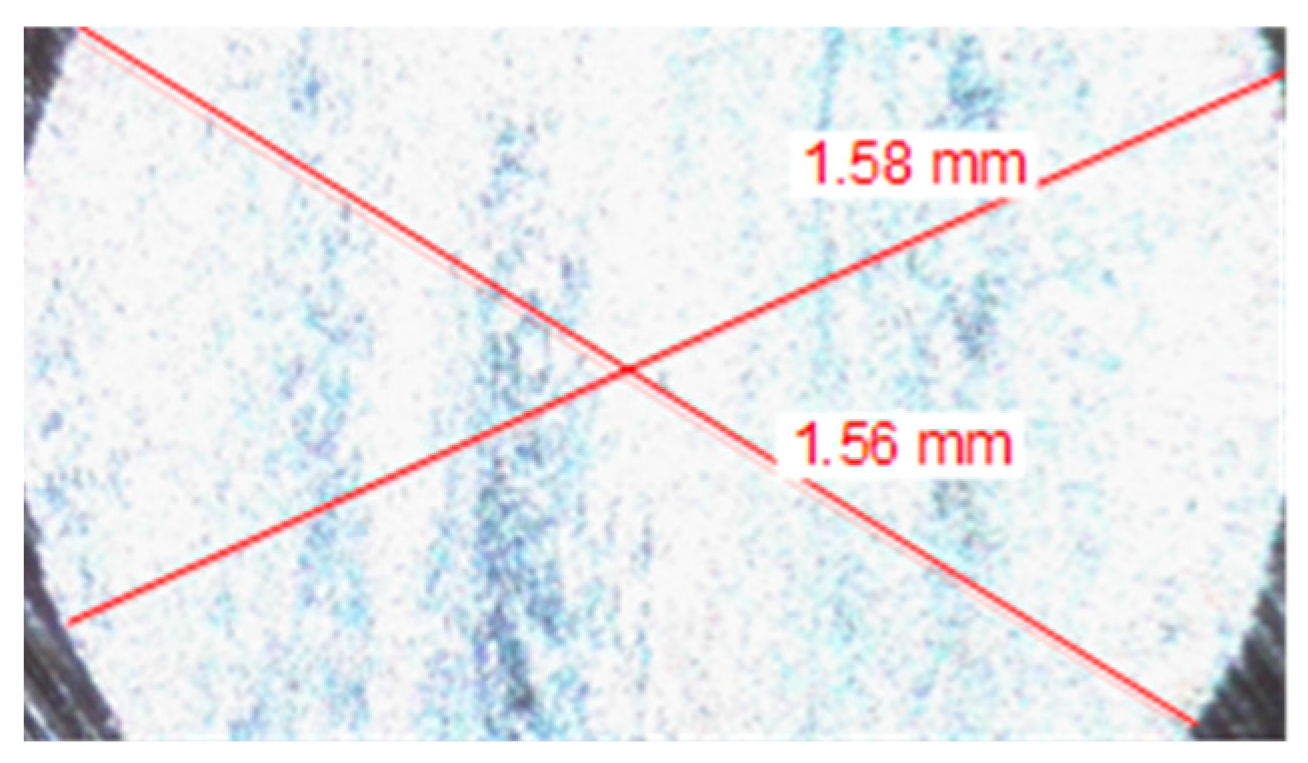
| Fuel Sample | b1, mm | b2, mm | ba, m | Kc, m3/(N × m) |
|---|---|---|---|---|
| DF | 0.25 | 0.22 | 0.000235 | 4.17 × 10−18 |
| HVO | 0.28 | 0.24 | 0.000260 | 6.25 × 10−18 |
| PPO | 0.42 | 0.63 | 0.000525 | 1.04 × 10−16 |
| PSO | 0.22 | 0.26 | 0.000240 | 4.54 × 10−18 |
| TPO | 0.23 | 0.33 | 0.000280 | 8.40 × 10−18 |
| Dry | 1.56 | 1.58 | 0.001570 | 8.31 × 10−15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szpica, D.; Borawski, A.; Mieczkowski, G.; Kuszewski, H.; Jaworski, A.; Hunicz, J. Comparative Evaluation of Performance Parameters of Conventional and Waste Fuels for Diesel Engines Towards Sustainable Transport. Energies 2025, 18, 5081. https://doi.org/10.3390/en18195081
Szpica D, Borawski A, Mieczkowski G, Kuszewski H, Jaworski A, Hunicz J. Comparative Evaluation of Performance Parameters of Conventional and Waste Fuels for Diesel Engines Towards Sustainable Transport. Energies. 2025; 18(19):5081. https://doi.org/10.3390/en18195081
Chicago/Turabian StyleSzpica, Dariusz, Andrzej Borawski, Grzegorz Mieczkowski, Hubert Kuszewski, Artur Jaworski, and Jacek Hunicz. 2025. "Comparative Evaluation of Performance Parameters of Conventional and Waste Fuels for Diesel Engines Towards Sustainable Transport" Energies 18, no. 19: 5081. https://doi.org/10.3390/en18195081
APA StyleSzpica, D., Borawski, A., Mieczkowski, G., Kuszewski, H., Jaworski, A., & Hunicz, J. (2025). Comparative Evaluation of Performance Parameters of Conventional and Waste Fuels for Diesel Engines Towards Sustainable Transport. Energies, 18(19), 5081. https://doi.org/10.3390/en18195081










