Evaluation of Tire Pyrolysis Oil–HVO Blends as Alternative Diesel Fuels: Lubricity, Engine Performance, and Emission Impacts
Abstract
1. Introduction
2. Materials and Methods
- A total of 100% HVO—diesel fuel obtained by hydrogenation from vegetable oils and animal fats, wood, and industrial waste (HVO);
- A total of 100% tire pyrolysis oil (TPO);
- A total of 15% HVO and 85% tire pyrolysis oil (TPO15);
- A total of 30% HVO and 70% tire pyrolysis oil (TPO30);
- A total of 60% HVO and 40% tire pyrolysis oil (TPO60);
- DD—mineral diesel.
3. Results
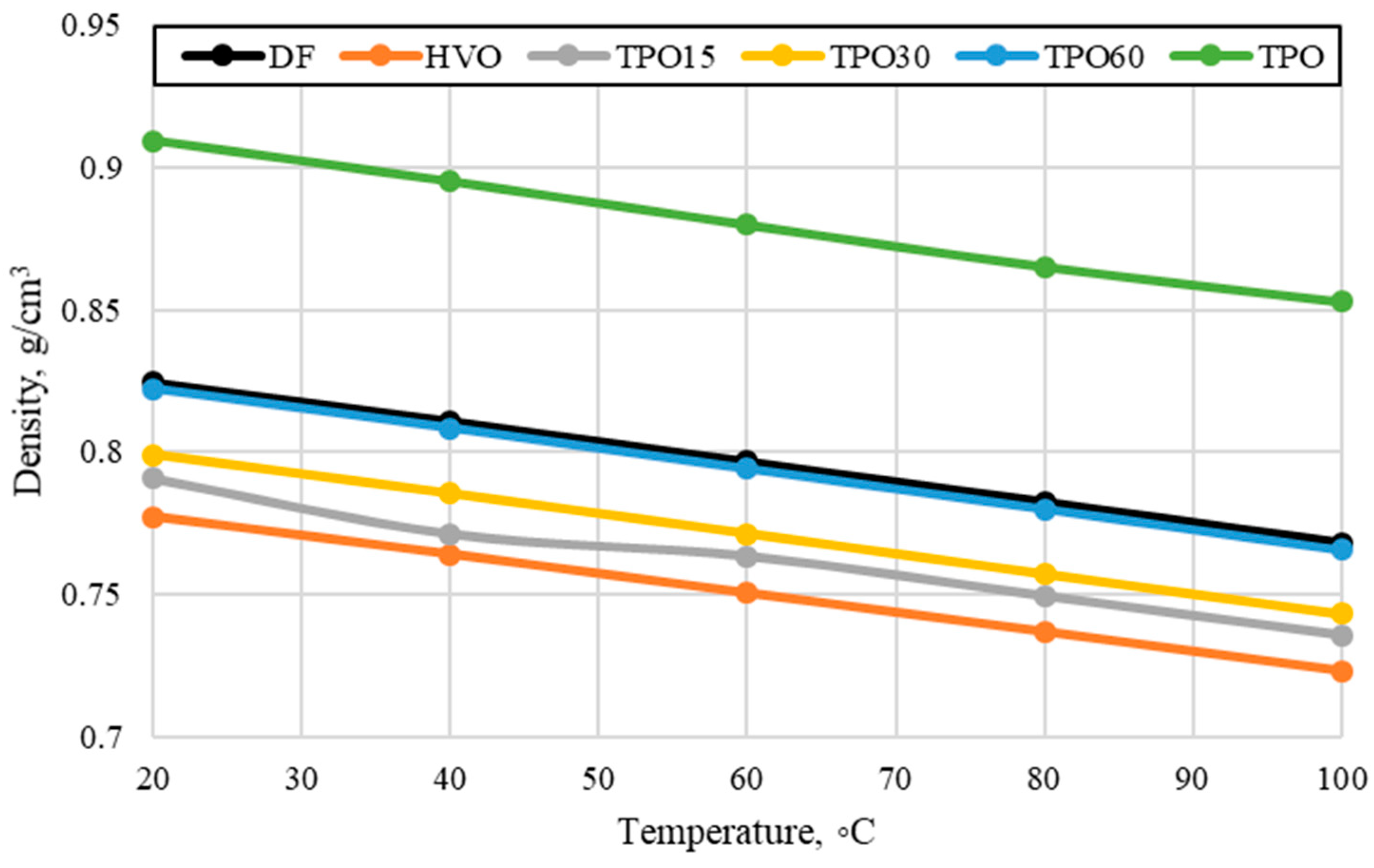
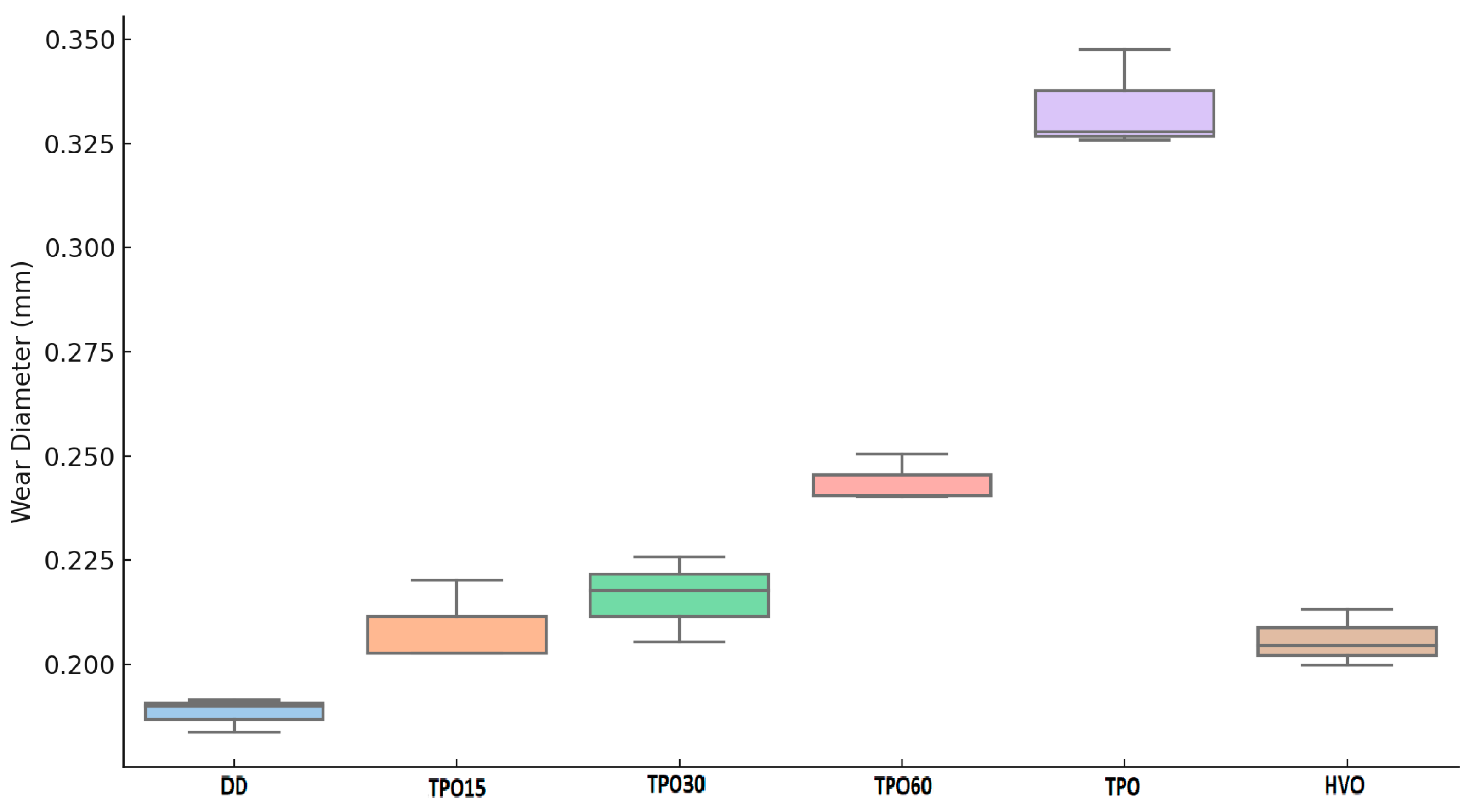
3.1. Influence of TPO–HVO Mixtures on Density, Viscosity, and Lubricity
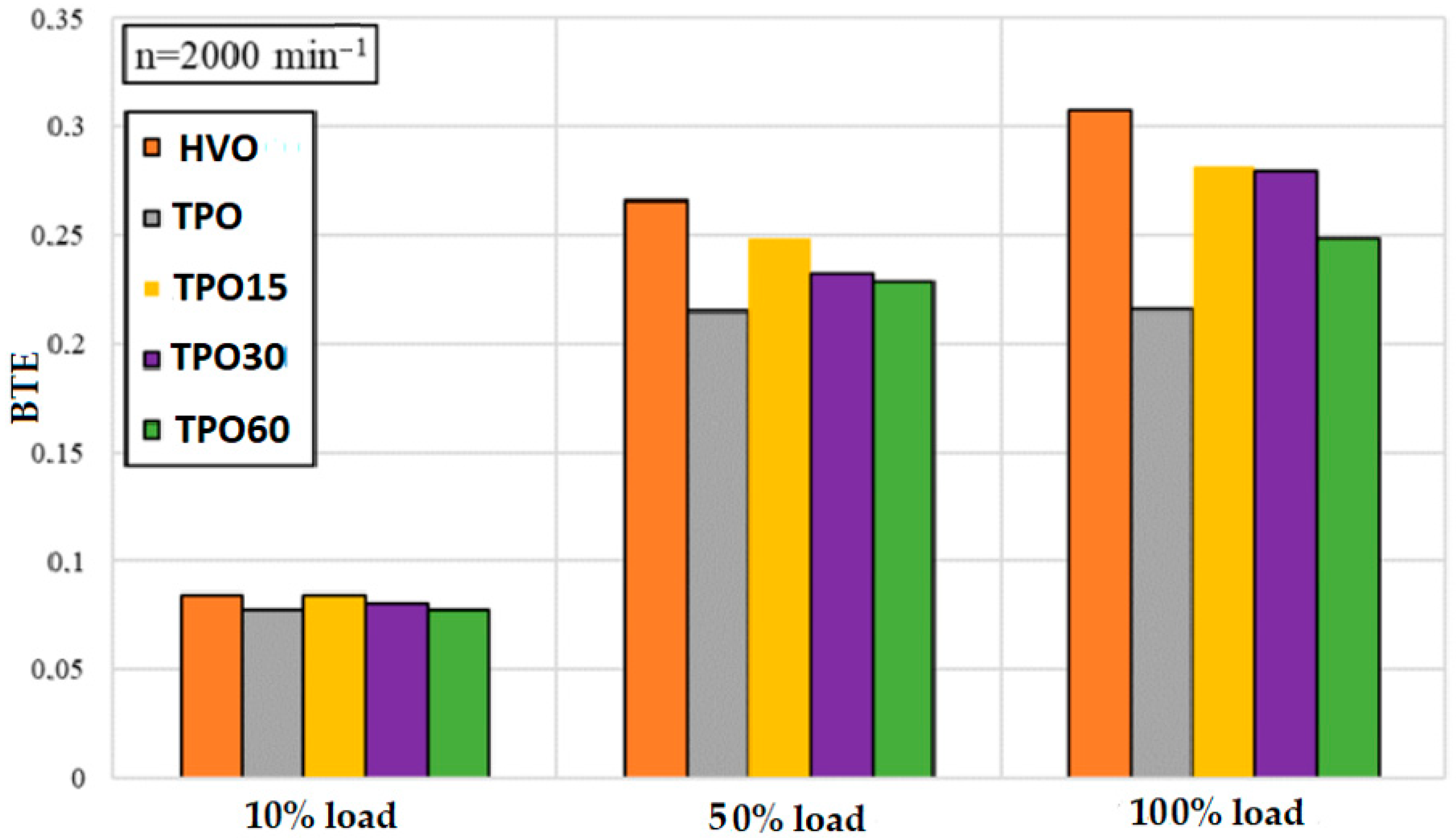
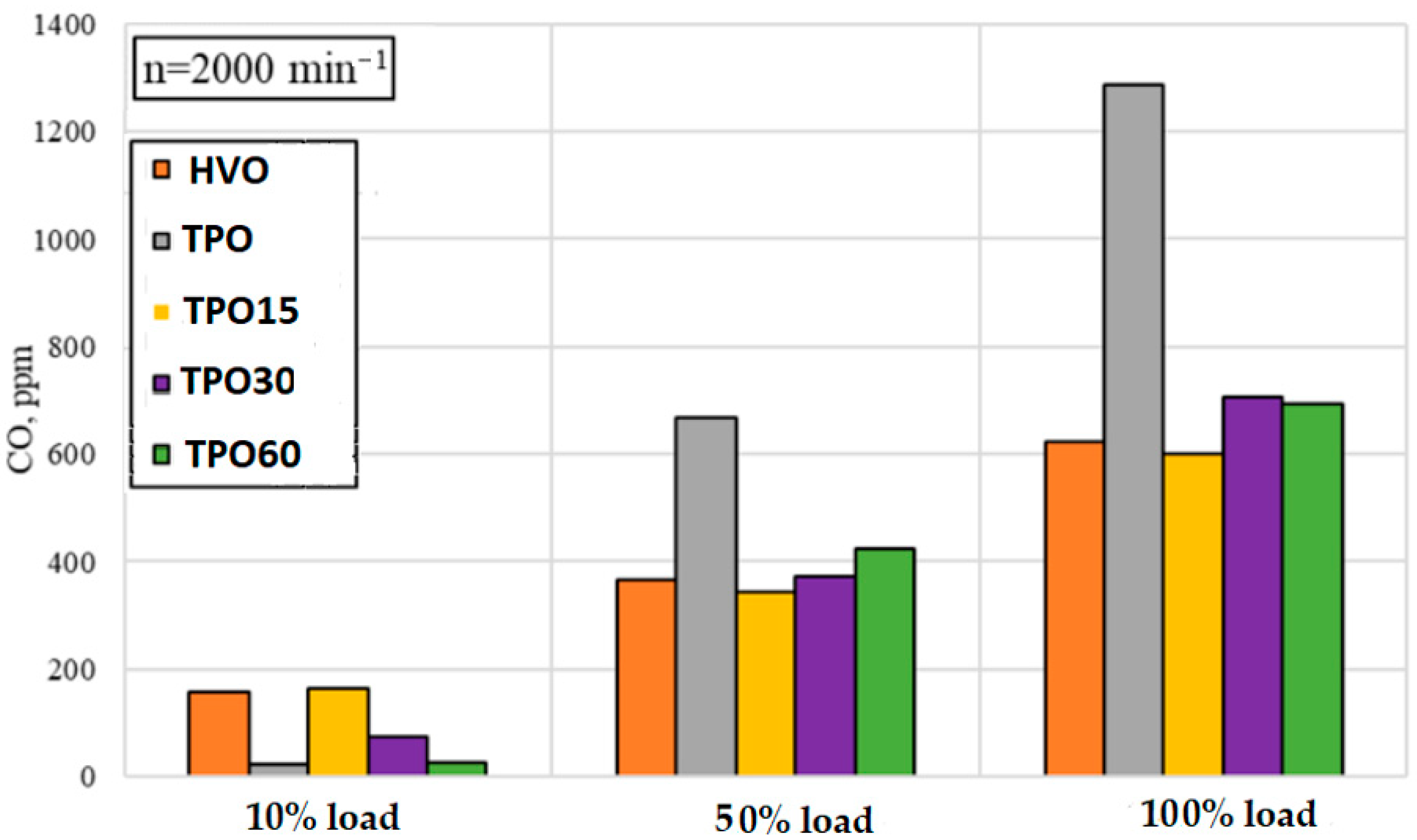
3.2. Engine Performance and Emission Analysis with TPO–HVO Blends
4. Discussion
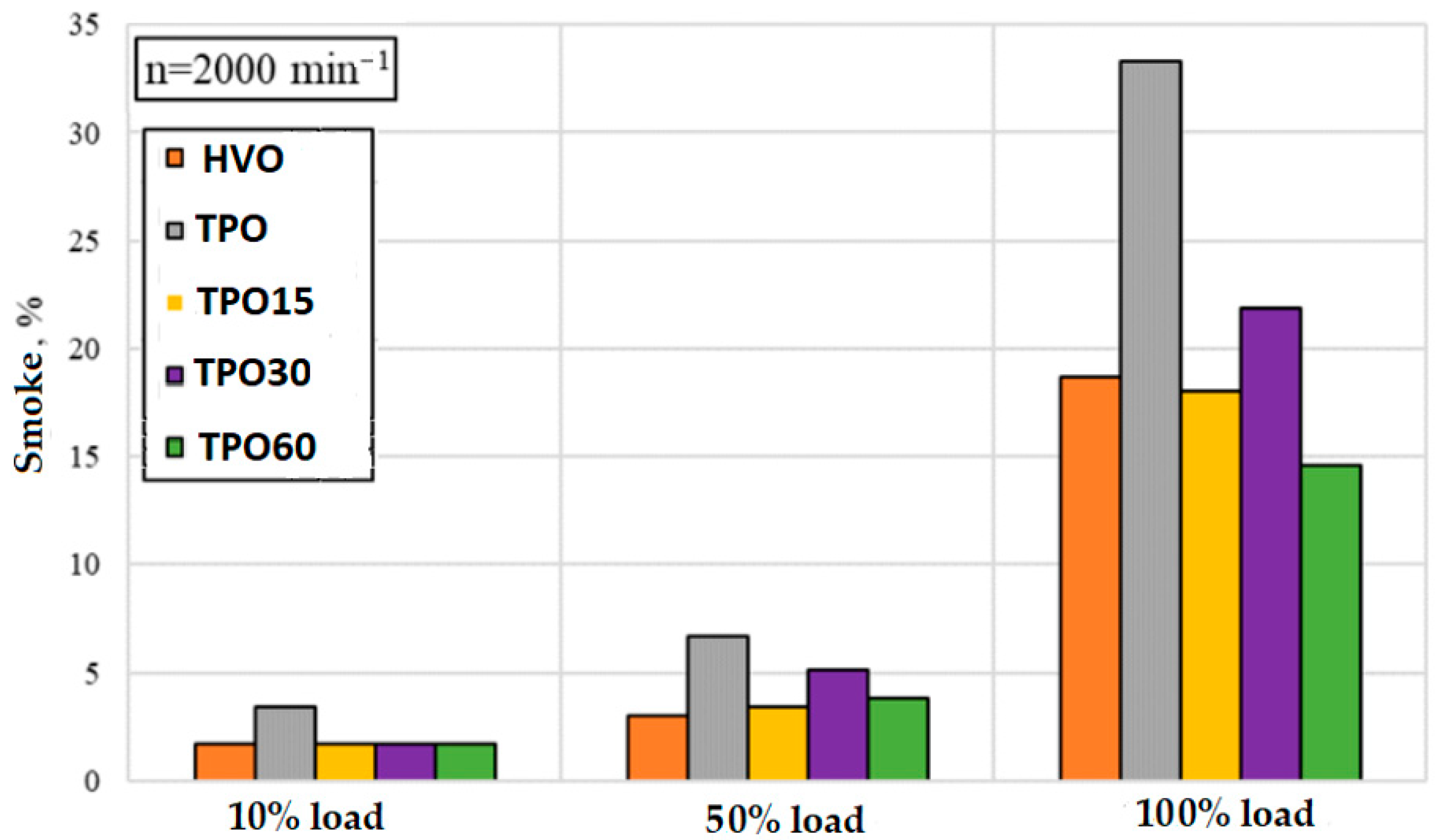
- CO and soot (smoke) emissions often decrease with increasing TPO content (up to a certain limit). This may be because TPO contains oxygen-rich compounds and fewer saturated (paraffinic) components, which tend to form more soot during combustion. A similar effect was reported by [27], who observed that oxygen-rich compounds may improve combustion and help reduce the formation of incomplete combustion products. In addition, if TPO contains more aromatic compounds, more intense pyrolytic combustion is possible, but with good oxidation reactions, soot formation may be somewhat lower—it is important that combustion does not continue at low temperatures.
- The changes in NOx emissions are rather ambiguous—in some regimes they increase, in others they decrease. Theoretically, aromatic compounds and a low cetane number usually delay ignition; therefore, if the delay time is increased, the peak temperature could sometimes decrease, thus reducing NOx emissions. However, if a certain part of the combustion takes place quickly and at high-temperature conditions (when a larger amount of fuel ignites immediately after the delay), NOx may increase. In addition, TPO contains sulfur and nitrogen impurities, which can promote the formation of not only NOx but also SOx. Our study showed that at high load (100% load mode), NOx increased significantly when the TPO content reached 60% and above. This observation is consistent with the findings of other publications that increased aromatic content of fuels and low cetane number can lead to more intense combustion at higher temperatures, especially at maximum load [18,28].
- SOx emissions were not directly measured in the results section of this work, but considering the sulfur content in TPO (~0.84% according to the table), it can be assumed that under real conditions without additional TPO desulfurization, such emissions would exceed the ultra-low sulfur limits required for road transport fuels today. This indicates that in order to practically apply a large proportion of TPO, it is necessary to perform additional refining or blend with ultra-low sulfur components (for example, HVO) so that the final sulfur content in the blend meets the standards (<10 ppm in the EU EN 590 standard [29]).
- A regular supply of waste tires is ensured;
- The resulting TPO is sold at an adequate price corresponding to its energy value;
- The planned additional processing steps (e.g., desulfurization) do not make the fuel too expensive;
- There is political/legal support (e.g., tax incentives for the development of waste recycling technologies).
- Blending with ultra-high cetane fuel components, e.g., HVO. HVO itself has a cetane number of ~78–80, so it could compensate for the low TPO indicator, as long as the total cetane number of the mixture does not fall below ~50.
- Biological desulfurization is still mainly a research topic [43], but in the future it could allow for sulfur reduction with lower energy costs.
5. Conclusions
- A small concentration of TPO in mixtures with HVO (up to ~20%) does not significantly affect the effective fuel consumption or power, but exceeding this limit significantly increases the ignition delay and increases the overall fuel consumption.
- Studies have shown that at higher temperatures (40–60 °C), the viscosity decreases but remains higher than that of diesel or HVO. This makes fuel spraying more difficult and may contribute to higher fuel consumption.
- In some load modes, NOx decreased due to the longer ignition delay, but at a high TPO fraction and maximum load, NOx increased significantly. This effect is determined not only by the decrease in cetane number but also by the sulfur and nitrogen impurities in the TPO composition.
- Due to the oxygen-enriched compounds and different balance of aromatic compounds in TPO, combustion becomes more intense, and some soot is oxidized more efficiently. This allowed the reduction of smoke, but the dependence on engine load remains high.
- Lubricity (HFRR method) is best when using conventional diesel or HVO, but wear diameter indicators obtained in blends with a limited amount of TPO (up to ~30%) still meet the requirements of EN 590. Employing a higher proportion of TPO may necessitate the incorporation of additional additives to improve lubricity indicators.
- The TPO exhibited a sulfur content of approximately 0.84% during the trial, significantly surpassing the ultra-low sulfur threshold of less than 10 ppm. Desulfurization techniques hold significant importance in industrial applications, as elevated sulfur concentrations lead to the production of acid gas emissions and can promote corrosion within fuel systems.
- The obtained short-term results confirm the potential for using TPO blends with HVO, but longer-term tests are necessary to assess the potential for deposit accumulation, changes in oil properties, and loads on gaskets or injectors.
- In order for TPO to become a sustainable, industrially acceptable alternative, efficient tire pyrolysis, refining (e.g., desulfurization, cetane number enhancement), and properly balanced blends with second-generation biofuels are required to ensure sufficient engine power, compliance with emission standards, and reliable fuel system operation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mikulski, M.; Ambrosewicz-Walacik, M.; Hunicz, J.; Nitkiewicz, S. Combustion Engine Applications of Waste Tyre Pyrolytic Oil. Prog. Energy Combust. Sci. 2021, 85, 100915. [Google Scholar] [CrossRef]
- Sarkan, B.; Stopka, O.; Gnap, J.; Caban, J. Investigation of Exhaust Emissions of Vehicles with the Spark Ignition Engine within Emission Control. Procedia Eng. 2017, 187, 775–782. [Google Scholar] [CrossRef]
- Fuc, P.; Lijewski, P.; Kurczewski, P.; Ziolkowski, A.; Dobrzynski, M. The Analysis of Fuel Consumption and Exhaust Emissions from Forklifts Fueled by Diesel Fuel and Liquefied Petroleum Gas (LPG) Obtained Under Real Driving Conditions. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition (ASME 2018), Tampa, FL, USA, 3–9 November 2017; Volume 6. [Google Scholar]
- Siwale, L.; Kristóf, L.; Adam, T.; Bereczky, A.; Mbarawa, M.; Penninger, A.; Kolesnikov, A. Combustion and Emission Characteristics of N-Butanol/Diesel Fuel Blend in a Turbo-Charged Compression Ignition Engine. Fuel 2013, 107, 409–418. [Google Scholar] [CrossRef]
- Singh, S.; Kulshrestha, M.J.; Rani, N.; Kumar, K.; Sharma, C.; Aswal, D.K. An Overview of Vehicular Emission Standards. MAPAN 2023, 38, 241–263. [Google Scholar] [CrossRef]
- Wierzbicki, S.; Śmieja, M. Use of Biogas to Power Diesel Engines with Common Rail Fuel Systems. MATEC Web Conf. 2018, 182, 01018. [Google Scholar] [CrossRef]
- Duda, K.; Wierzbicki, S.; Mikulski, M.; Konieczny, Ł.; Łazarz, B.; Letuń-Łątka, M. Emissions from a Medium-Duty CRDI Engine Fuelled with Diesel-Biodiesel Blends. Transp. Probl. 2021, 16, 39–49. [Google Scholar] [CrossRef]
- Borucka, A.; Kozłowski, E. Modeling the Dynamics of Changes in CO2 Emissions from Polish Road Transport in the Context of COVID-19 and Decarbonization Requirements. Combust. Engines 2023, 195, 63–70. [Google Scholar] [CrossRef]
- Maghrour Zefreh, M.; Torok, A. Theoretical Comparison of the Effects of Different Traffic Conditions on Urban Road Traffic Noise. J. Adv. Transp. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Surblys, V.; Žuraulis, V.; Sokolovskij, E. Estimation of Road Roughness from Data of On-Vehicle Mounted Sensors. EIN 2017, 19, 369–374. [Google Scholar] [CrossRef]
- Yaqoob, H.; Teoh, Y.H.; Jamil, M.A.; Gulzar, M. Potential of Tire Pyrolysis Oil as an Alternate Fuel for Diesel Engines: A Review. J. Energy Inst. 2021, 96, 205–221. [Google Scholar] [CrossRef]
- Kozłowski, E.; Zimakowska-Laskowska, M.; Dudziak, A.; Wiśniowski, P.; Laskowski, P.; Stankiewicz, M.; Šnauko, B.; Lech, N.; Gis, M.; Matijošius, J. Analysis of Instantaneous Energy Consumption and Recuperation Based on Measurements from SORT Runs. Appl. Sci. 2025, 15, 1681. [Google Scholar] [CrossRef]
- Graba, M.; Bieniek, A.; Prażnowski, K.; Hennek, K.; Mamala, J.; Burdzik, R.; Śmieja, M. Analysis of Energy Efficiency and Dynamics during Car Acceleration. Eksploat. I Niezawodn. 2023, 25, 17. [Google Scholar] [CrossRef]
- Yu, J.; Wang, D.; Sun, L. The Pyrolysis of Lignin: Pathway and Interaction Studies. Fuel 2021, 290, 120078. [Google Scholar] [CrossRef]
- Zerin, N.H.; Rasul, M.G.; Jahirul, M.I.; Sayem, A.S.M.; Haque, R. Physicochemical Properties of Pyrolytic Char and Oil from Mixed Tyre Using Batch Pyrolysis Process. Energy Convers. Manag. X 2025, 26, 100941. [Google Scholar] [CrossRef]
- Dewi, W.N.; Zhou, Q.; Mollah, M.; Yang, S.; Ilankoon, I.M.S.K.; Chaffee, A.; Zhang, L. Synergistic Interaction between Scrap Tyre and Plastics for the Production of Sulphur-Free, Light Oil from Fast Co-Pyrolysis. Waste Manag. 2024, 179, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Zhang, Y.; Jiang, X.; Yang, H.; Yan, K.; Han, M.; Li, W.; Zhong, H.; Tan, X.; Xia, L.; et al. Simulation and Techno-Economical Analysis on the Pyrolysis Process of Waste Tire. Energy 2022, 260, 125039. [Google Scholar] [CrossRef]
- Kumaravel, S.T.; Murugesan, A.; Kumaravel, A. Tyre Pyrolysis Oil as an Alternative Fuel for Diesel Engines—A Review. Renew. Sustain. Energy Rev. 2016, 60, 1678–1685. [Google Scholar] [CrossRef]
- Lévai, E.; Bereczky, Á. Analysis of Operational Parameters and Emissions in a Domestic Natural Gas Heating Appliance with Hydrogen Blending. Int. J. Hydrogen Energy 2025, 97, 950–958. [Google Scholar] [CrossRef]
- Hossain, F.M.; Nabi, M.N.; Rainey, T.J.; Bodisco, T.; Bayley, T.; Randall, D.; Ristovski, Z.; Brown, R.J. Novel Biofuels Derived from Waste Tyres and Their Effects on Reducing Oxides of Nitrogen and Particulate Matter Emissions. J. Clean. Prod. 2020, 242, 118463. [Google Scholar] [CrossRef]
- Heydari, A.; Fazeli, A.; Hallajisani, A. Enhanced Tire Oil Production through Hydrothermal Liquefaction Using Devulcanization Agents. Process Saf. Environ. Prot. 2025, 193, 957–975. [Google Scholar] [CrossRef]
- Pyshyev, S.; Lypko, Y.; Chervinskyy, T.; Fedevych, O.; Kułażyński, M.; Pstrowska, K. Application of Tyre Derived Pyrolysis Oil as a Fuel Component. S. Afr. J. Chem. Eng. 2023, 43, 342–347. [Google Scholar] [CrossRef]
- Hariharan, S.; Murugan, S.; Nagarajan, G. Effect of Diethyl Ether on Tyre Pyrolysis Oil Fueled Diesel Engine. Fuel 2013, 104, 109–115. [Google Scholar] [CrossRef]
- Karthickeyan, V.; Balamurugan, P.; Senthil, R. Experimental Investigation of Tyre Pyrolysis Oil (TPO) in Diesel Engine without Any Engine Modification. Jour. Biof. Bioen. 2015, 1, 170. [Google Scholar] [CrossRef]
- Kumar, K.S.; Razak, A.; Yadav, A.; Raghavendra Rao, P.S.; Majdi, H.S.; Khan, T.M.Y.; Almakayeel, N.; Singh, K. Experimental Analysis of Cycle Tire Pyrolysis Oil Doped with 1-Decanol + TiO2 Additives in Compression Ignition Engine Using RSM Optimization and Machine Learning Approach. Case Stud. Therm. Eng. 2024, 61, 104863. [Google Scholar] [CrossRef]
- Rajkumar, P.; Murugavelh, S. Co-Pyrolysis of Wheat Husk and Residual Tyre: Techno-Economic Analysis, Performance and Emission Characteristics of Pyro Oil in a Diesel Engine. Bioresour. Technol. Rep. 2022, 19, 101164. [Google Scholar] [CrossRef]
- Hunicz, J.; Mikulski, M.; Rybak, A. Tyre Pyrolytic Oil Blends in a State-of-the-Art Compression Ignition Engine: Towards Fuel-Optimised Combustion. Fuel 2023, 353, 129281. [Google Scholar] [CrossRef]
- Yaqoob, H.; Ali, H.M. Sustainability Analysis of Neat Waste Tire Oil Powered Diesel Engine: A Thermodynamics Approach. Process Saf. Environ. Prot. 2024, 182, 1121–1129. [Google Scholar] [CrossRef]
- Mikulski, M.; Hunicz, J.; Duda, K.; Kazimierski, P.; Suchocki, T.; Rybak, A. Tyre Pyrolytic Oil Fuel Blends in a Modern Compression Ignition Engine: A Comprehensive Combustion and Emissions Analysis. Fuel 2022, 320, 123869. [Google Scholar] [CrossRef]
- Öner, İ.V.; Atabani, A.E.; Durnagöl, T. Recycling of Waste Tires to Crude Pyrolytic Oil: Engine Performance, Combustion Characteristics and Emissions Analysis of Diesel-Butanol-Crude Pyrolytic Oil Blends in CI Diesel Engines. Sustain. Energy Technol. Assess. 2023, 56, 103023. [Google Scholar] [CrossRef]
- Uyumaz, A.; Aydoğan, B.; Solmaz, H.; Yılmaz, E.; Yeşim Hopa, D.; Aksoy Bahtli, T.; Solmaz, Ö.; Aksoy, F. Production of Waste Tyre Oil and Experimental Investigation on Combustion, Engine Performance and Exhaust Emissions. J. Energy Inst. 2019, 92, 1406–1418. [Google Scholar] [CrossRef]
- Karagöz, M.; Polat, F.; Sarıdemir, S.; Yeşilyurt, M.K.; Ağbulut, Ü. An Experimental Assessment on Dual Fuel Engine Behavior Powered by Waste Tire-Derived Pyrolysis Oil—Biogas Blends. Fuel Process. Technol. 2022, 229, 107177. [Google Scholar] [CrossRef]
- Nabi, N.; Hussam, W.K.; Afroz, H.M.M.; Rashid, A.B.; Islam, J.; Mukut, A.N.M.M.I. Investigation of Engine Performance, Combustion, and Emissions Using Waste Tire Oil-Diesel-Glycine Max Biodiesel Blends in a Diesel Engine. Case Stud. Therm. Eng. 2022, 39, 102435. [Google Scholar] [CrossRef]
- Polat, F. Experimental Evaluation of the Impacts of Diesel-Nanoparticles-Waste Tire Pyrolysis Oil Ternary Blends on the Combustion, Performance, and Emission Characteristics of a Diesel Engine. Process Saf. Environ. Prot. 2022, 160, 847–858. [Google Scholar] [CrossRef]
- Teoh, Y.H.; Yaqoob, H.; How, H.G.; Le, T.D.; Nguyen, H.T. Comparative Assessment of Performance, Emissions and Combustion Characteristics of Tire Pyrolysis Oil-Diesel and Biodiesel-Diesel Blends in a Common-Rail Direct Injection Engine. Fuel 2022, 313, 123058. [Google Scholar] [CrossRef]
- Abdullah, B.; Syed Muhammad, S.A.F.; Shokravi, Z.; Ismail, S.; Kassim, K.A.; Mahmood, A.N.; Aziz, M.M.A. Fourth Generation Biofuel: A Review on Risks and Mitigation Strategies. Renew. Sustain. Energy Rev. 2019, 107, 37–50. [Google Scholar] [CrossRef]
- Auti, S.M.; Rathod, W.S. Effect of Hybrid Blends of Raw Tyre Pyrolysis Oil, Karanja Biodiesel and Diesel Fuel on Single Cylinder Four Stokes Diesel Engine. Energy Rep. 2021, 7, 2214–2220. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Tabatabaei, M.; Mohammadi, P.; Pourvosoughi, N.; Nikbakht, A.M.; Goli, S.A.H. Improving Exergetic and Sustainability Parameters of a DI Diesel Engine Using Polymer Waste Dissolved in Biodiesel as a Novel Diesel Additive. Energy Convers. Manag. 2015, 105, 328–337. [Google Scholar] [CrossRef]
- Abdrassilova, A.; Vassilina, G.; Abdildina, K.; Briones, L.; Peral, A.; Escola, J.M. The Notable Features of Mesoporous Aluminosilicates as Catalytic Supports for Hydrodearomatization and Hydrodesulfurization of Fuels. Microporous Mesoporous Mater. 2025, 384, 113457. [Google Scholar] [CrossRef]
- Gholinejad, M.; Mohammadi, S.; Nayeri, S.; Sansano, J.M. Enhanced Catalytic Activity of Nickel-Gold Nanoprisms in Hydrodesulfurization and Reduction of 4-Nitrophenol and Organic Dyes. Inorg. Chem. Commun. 2025, 177, 114376. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, D.; Deng, C.; Niu, H.; Yang, H.; Yang, L.; Bai, L.; Yin, K.; Wei, D.; Chen, H. Dual Noble-Metal-Free Cocatalysts on Cobalt Nickel Oxide Nanocages for Boosting Photocatalytic Oxidation Desulfurization. J. Catal. 2025, 447, 116120. [Google Scholar] [CrossRef]
- Ahmed, H.R.; Ealias, A.M.; George, G. Advanced Oxidation Processes for Desulfurization: A Review of Heterogeneous Catalytic Systems. J. Ind. Eng. Chem. 2025, 150, 231–246. [Google Scholar] [CrossRef]
- Andler, R.; Cancino, C.; González-Arancibia, F.; Rojas, J.; Castro, R.; Kasai, D.; Sanhueza, V. Bio-Desulfurization of Different Vulcanized Rubber Blends: An Ecofriendly Approach for End-of-Life Tires Waste Pollution. J. Environ. Chem. Eng. 2025, 13, 115598. [Google Scholar] [CrossRef]



| Properties | Evaluation Method | DD | HVO | TPO | EN590 |
|---|---|---|---|---|---|
| Density at 15 °C, kg/m3 | EN ISO 12185:1999 | 832.7 | 779.8 | 910 | 800–845 |
| Kinematic viscosity at 40 °C, mm2/s | EN ISO 3104+AC:2000 | 2.13 | 2.92 | 3.77 | 1.5–4 |
| Lubricity properties adjusted for diameter wear (HFRR), (wsd 1.4) at 60 °C, µm | EN ISO 12156-1 | - | - | - | Max: 460 |
| Cetane number | EN ISO 5165:1999 | 51.4 | 78.9 | 39 | Min: 51 |
| Oxygen content, max wt% | - | 0 | 0 | 1.76 | |
| Carbon-to-hydrogen mass ratio (C/H) | - | 6.62 | 5.5 | 8.26 |
| Testing Fuels | HVO Amount Vol % | TPO Amount Vol % |
|---|---|---|
| HVO | 100 | 0 |
| TPO15 | 85 | 15 |
| TPO30 | 70 | 30 |
| TPO60 | 40 | 60 |
| TPO | 0 | 100 |
| Properties | Measurement Method | TPO | HVO |
|---|---|---|---|
| Flash point in open cup (FP), °C | EN ISO 2719:2000 | 43 | 79.5 |
| Stoichiometric air–fuel ratio, kg/kg | - | 13.46 | 15.1 |
| Normal calorific value, MJ/kg | EN ISO 8217:2012 | 40.49 | 43 |
| Cetane number | EN ISO 5165:1999 | 39.94 | 78.9 |
| Carbon (%) | 86.68 | 84.6 | |
| Hydrogen (%) | 10.49 | 15.39 | |
| Oxygen (%) | 1.29 | − | |
| Nitrogen (%) | 0.48 | − | |
| Sulfur (%) | 0.84 | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mickevičius, T.; Dudziak, A.; Matijošius, J.; Rimkus, A. Evaluation of Tire Pyrolysis Oil–HVO Blends as Alternative Diesel Fuels: Lubricity, Engine Performance, and Emission Impacts. Energies 2025, 18, 4389. https://doi.org/10.3390/en18164389
Mickevičius T, Dudziak A, Matijošius J, Rimkus A. Evaluation of Tire Pyrolysis Oil–HVO Blends as Alternative Diesel Fuels: Lubricity, Engine Performance, and Emission Impacts. Energies. 2025; 18(16):4389. https://doi.org/10.3390/en18164389
Chicago/Turabian StyleMickevičius, Tomas, Agnieszka Dudziak, Jonas Matijošius, and Alfredas Rimkus. 2025. "Evaluation of Tire Pyrolysis Oil–HVO Blends as Alternative Diesel Fuels: Lubricity, Engine Performance, and Emission Impacts" Energies 18, no. 16: 4389. https://doi.org/10.3390/en18164389
APA StyleMickevičius, T., Dudziak, A., Matijošius, J., & Rimkus, A. (2025). Evaluation of Tire Pyrolysis Oil–HVO Blends as Alternative Diesel Fuels: Lubricity, Engine Performance, and Emission Impacts. Energies, 18(16), 4389. https://doi.org/10.3390/en18164389






