A Review of the State of the Art on Ionic Liquids and Their Physical Properties During Heat Transfer
Abstract
1. Introduction
2. Ionic Liquids
2.1. General Characteristics of Ionic Liquids
2.2. Classification of Ionic Liquids
- cationic: imidazolium (i.e., [BmIm][BF4], [BmIm][PF6]), pyridinium, ammonium, phosphonium, sulfonium;
- anionic: fluorinated (np.: [BF4]−, [PF6]−); organic (i.e., [Tf2N]−, [OTf]−); inorganic (i.e., Cl−, NO3−, SCN−).
3. Selected Physical, Thermal and Hydrodynamic Properties of Ionic Liquids
3.1. Melting Point (Tm) of Ionic Liquids
3.2. Density
3.3. The Thermal Conductivity Coefficient
3.4. Viscosity
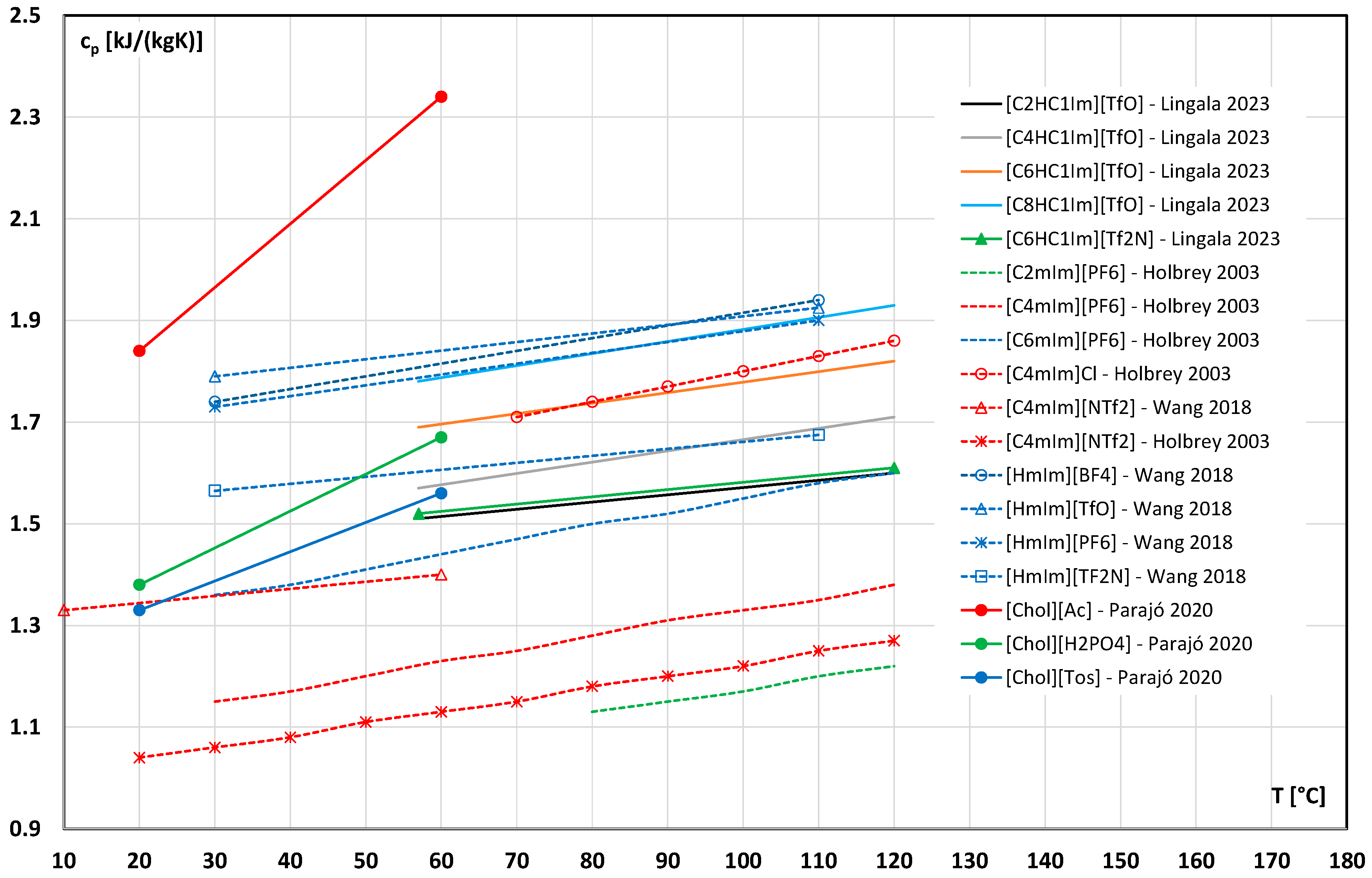
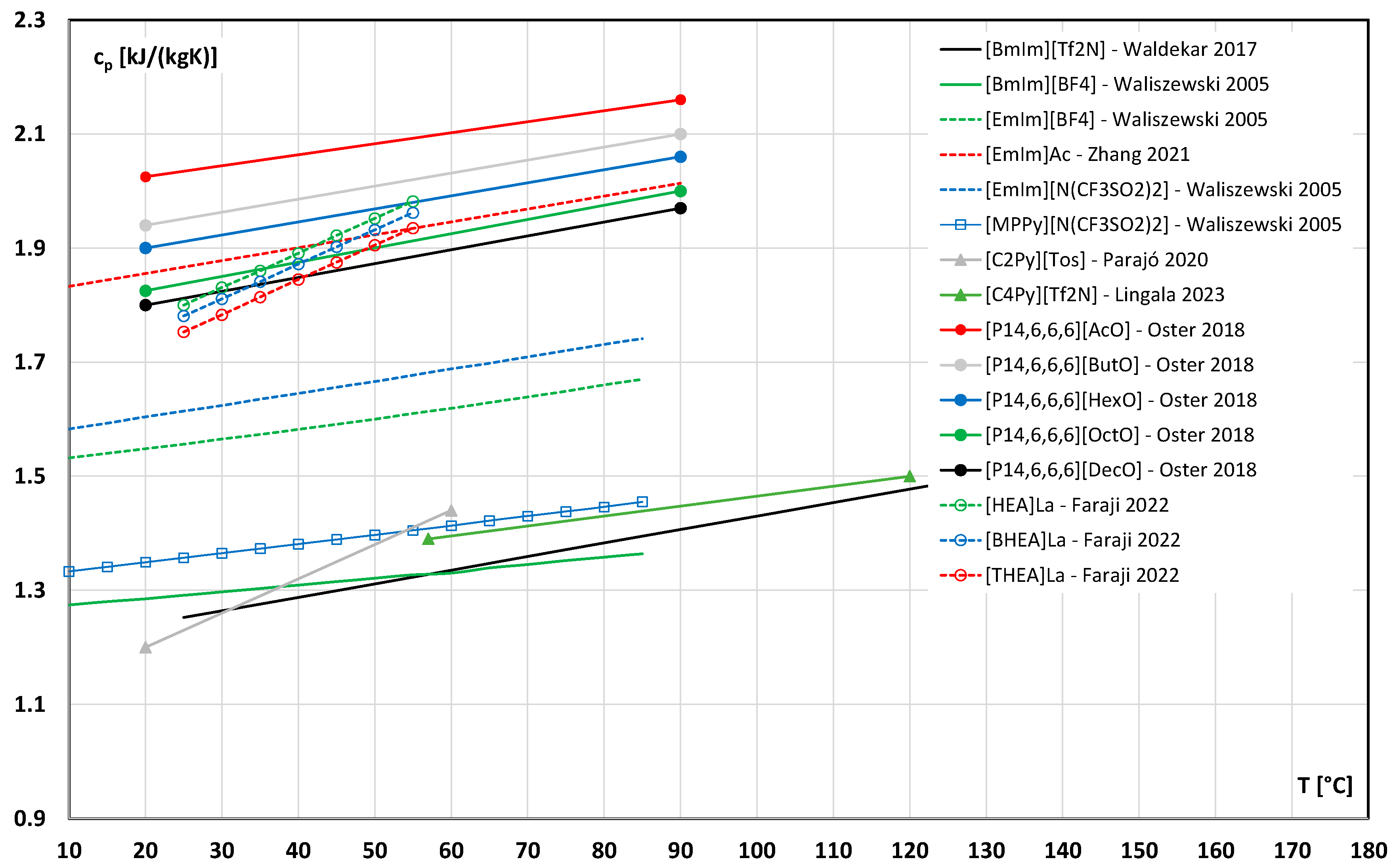
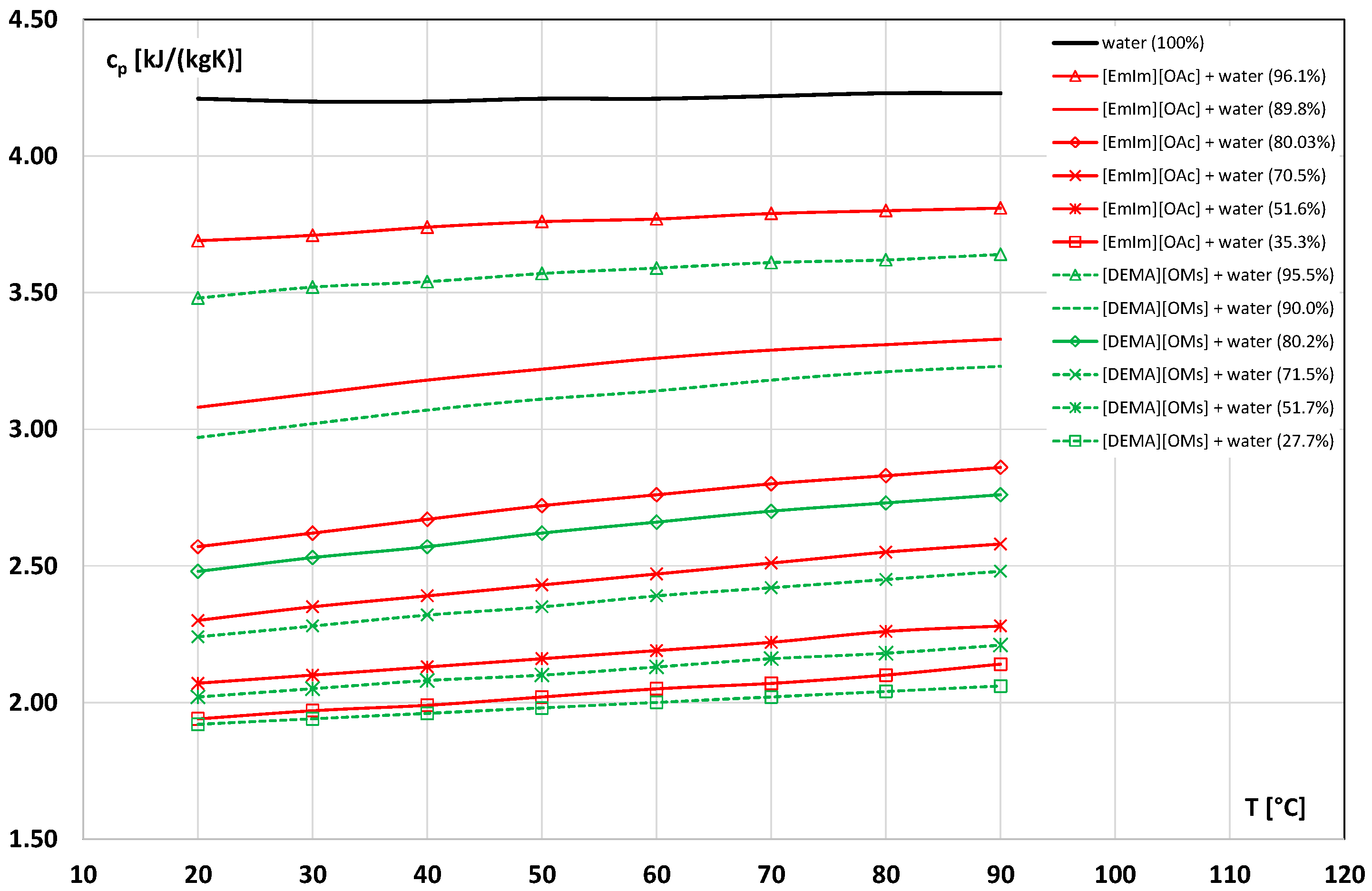
3.5. Specific Heat
4. Application of Ionic Liquids in Thermal Technology
- 1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([BmIm][NTf2]) and N-Butyl-N-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide ([BMPyrr][NTf2]). These substances are considered to be among the most suitable for use as heat transfer fluids. Their thermophysical properties are similar to those of commonly used organic and organosilicon fluids;
- ionic liquids with anions such as [NTf2]−, [OTf]− or [PF6]−. These liquids are characterized by high thermal stability and low viscosity;
- Ionic liquids have low vapour pressure and high thermal stability. These liquids can be used at higher temperatures (>200 °C) without the risk of explosion or high volatility, which is particularly important in industrial applications.
5. Summary and Conclusions
- it is necessary to systematize the current state of knowledge in the field of thermodynamic properties of ionic liquids, taking into account the largest possible number of substances produced so far;
- due to the scarcity of knowledge and in the context of the synthesis of new, previously unknown ionic liquids, there is a need to carry out extensive research on the fundamental physical properties of ILs in the field of thermal engineering;
- it is necessary to conduct applied research using ionic liquids, including the determination of heat transfer coefficients and flow resistances;
- numerical study is required to predict the behavior of ionic liquids in heat exchange systems, validated by reliable results of experimental studies.
Funding
Conflicts of Interest
References
- Shahin, M.B.; Liaqat, S.; Nancarrow, P.; McCormack, S.J. Crystal Phase Ionic Liquids for Energy Applications: Heat Capacity Prediction via a Hybrid Group Contribution Approach. Molecules 2024, 29, 2130. [Google Scholar] [CrossRef]
- Paredes, X.; Lourenço, M.J.; de Castro, C.N.; Wakeham, W. Thermal conductivity of ionic liquids and ionanofluids. Can molecular theory help? Fluids 2021, 6, 116. [Google Scholar] [CrossRef]
- Xue, Z.; Qin, L.; Jiang, J.; Mu, T.; Gao, G. Thermal, electrochemical and radiolytic stabilities of ionic liquids. Phys. Chem. Chem. Phys. 2018, 20, 8382–8402. [Google Scholar] [CrossRef] [PubMed]
- Kar, M.; Plechkova, N.V.; Seddon, K.R.; Pringle, J.M.; MacFarlane, D.R. Ionic Liquids-Further Progress on the Fundamental Issues. Aust. J. Chem. 2019, 72, 3–10. [Google Scholar] [CrossRef]
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhuang, Q.; Zhang, M.; Wang, H.; Gao, Z.; Sun, J.K.; Yuan, J. Poly(ionic liquid) composites. Chem. Soc. Rev. 2020, 49, 1726–1755. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Zhai, C.; Lin, H.; Wu, W. How to rationally screen refrigerant/ionic liquids for thermal cooling: A multi-criteria approach based on machine learning. Energy Convers. Manag. 2023, 282, 116853. [Google Scholar] [CrossRef]
- Matsumoto, Y. Recent Progress in Vacuum Engineering of Ionic Liquids. Molecules 2023, 28, 1991. [Google Scholar] [CrossRef]
- Cao, B.; Yin, Y.; Xu, G.; Cheng, X.; Li, W.; Ji, Q.; Chen, W. A proposed method of bubble absorption-based deep dehumidification using the ionic liquid for low-humidity industrial environments with experimental performance. Appl. Energy 2023, 348, 121534. [Google Scholar] [CrossRef]
- Asensio-Delgado, S.; Jovell, D.; Zarca, G.; Urtiaga, A.; Llovell, F. Thermodynamic and process modeling of the recovery of R410A compounds with ionic liquids. Int. J. Refrig. 2020, 118, 365–375. [Google Scholar] [CrossRef]
- Zhai, C.; Sui, Y.; Sui, Z.; Wu, W. Ionic liquids for microchannel membrane-based absorption heat pumps: Performance comparison and geometry optimization. Energy Convers. Manag. 2021, 239, 114213. [Google Scholar] [CrossRef]
- Asensio-Delgado, J.M.; Asensio-Delgado, S.; Zarca, G.; Urtiaga, A. Analysis of hybrid compression absorption refrigeration using low-GWP HFC or HFO/ionic liquid working pairs. Int. J. Refrig. 2022, 134, 232–241. [Google Scholar] [CrossRef]
- Kallitsis, K.; Koulocheris, V.; Pappa, G.; Voutsas, E. Evaluation of water + imidazolium ionic liquids as working pairs in absorption refrigeration cycles. Appl. Therm. Eng. 2023, 233, 121201. [Google Scholar] [CrossRef]
- Bender, C.R.; Kuhn, B.L.; Farias, C.A.A.; Ziembowicz, F.I.; Beck, T.S.; Frizzo, C.P. Thermal stability and kinetic of decomposition of mono- and dicationic imidazolium-based ionic liquids. J. Braz. Chem. Soc. 2019, 30, 2199–2209. [Google Scholar] [CrossRef]
- Lingala, S.S. Ionic-Liquid-Based Nanofluids and Their Heat-Transfer Applications: A Comprehensive Review. ChemPhysChem 2023, 24, e202300191. [Google Scholar] [CrossRef]
- Chen, Y.; Kontogeorgis, G.M.; Woodley, J.M. Group Contribution Based Estimation Method for Properties of Ionic Liquids. Ind. Eng. Chem. Res. 2019, 58, 4277–4292. [Google Scholar] [CrossRef]
- Nardelli, F.; Bramanti, E.; Lavacchi, A.; Pizzanelli, S.; Campanella, B.; Forte, C.; Berretti, E.; Freni, A. Thermal Stability of Ionic Liquids: Effect of Metals. Appl. Sci. 2022, 12, 1652. [Google Scholar] [CrossRef]
- Kumar, N.; Raza, M.Q.; Seth, D.; Raj, R. Aqueous ionic liquid solutions for boiling heat transfer enhancement in the absence of buoyancy induced bubble departure. Int. J. Heat. Mass. Transf. 2018, 122, 354–363. [Google Scholar] [CrossRef]
- Bendová, M.; Čanji, M.; Wagner, Z.; Bogdanov, M.G. Ionic Liquids as Thermal Energy Storage Materials: On the Importance of Reliable Data Analysis in Assessing Thermodynamic Data. J. Solut. Chem. 2019, 48, 949–961. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Li, B.; Sundén, B. A review on molten-salt-based and ionic-liquid-based nanofluids for medium-to-high temperature heat transfer. J. Therm. Anal. Calorim. 2019, 136, 1037–1051. [Google Scholar] [CrossRef]
- Li, Q.; Yang, C.; Wang, S.; Zhou, M.; Xie, H.; Qiao, G.; Du, Y.; Li, C.; Wu, Y. Challenges and strategies for imidazolium ionic liquids as novel phase change materials for low and medium temperature thermal energy storage: A critical review. J. Mol. Liq. 2024, 395, 123812. [Google Scholar] [CrossRef]
- Minea, A.A. Overview of Ionic Liquids as Candidates for New Heat Transfer Fluids. Int. J. Thermophys. 2020, 41, 151. [Google Scholar] [CrossRef]
- Piper, S.L.; Kar, M.; MacFarlane, D.R.; Matuszek, K.; Pringle, J.M. Ionic liquids for renewable thermal energy storage—A perspective. Green Chem. 2022, 24, 102–117. [Google Scholar] [CrossRef]
- Xu, C.; Cheng, Z. Thermal stability of ionic liquids: Current status and prospects for future development. Processes 2021, 9, 337. [Google Scholar] [CrossRef]
- Nieto de Castro, C.A.; Lourenço, M.J.V. Towards the correct measurement of thermal conductivity of ionic melts and nanofluids. Energies 2019, 13, 99. [Google Scholar] [CrossRef]
- Najafabadi, M.S.; Lay, E.N. An empirical correlation for predicting vapor pressure of ionic liquids. J. Ion. Liq. 2022, 2, 100035. [Google Scholar] [CrossRef]
- Cao, B.; Yin, Y.; Xu, G.; Li, W.; Dai, S.; Chen, W.; Ji, Q.; Zhang, F. Experimental and modeling study of bubble absorption-based deep dehumidification using the ionic liquid: Parametric analysis on heat and mass transfer. Energy Convers. Manag. 2023, 290, 117169. [Google Scholar] [CrossRef]
- Tariq, H.A.; Bourouis, M.; Coronas, A. Heat and mass transfer characteristics of a horizontal tube falling film absorber working with water/lithium bromide and ionic liquid as an anti-crystallization additive. Int. J. Heat Mass Transf. 2025, 242, 126859. [Google Scholar] [CrossRef]
- Das, L.; Habib, K.; Saidur, R.; Aslfattahi, N.; Yahya, S.M.; Rubbi, F. Improved thermophysical properties and energy efficiency of aqueous ionic liquid/mxene nanofluid in a hybrid pv/t solar system. Nanomaterials 2020, 10, 1372. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, N.; He, X.; Lu, X.; Zhang, X. Physical properties of ionic liquids: Database and evaluation. J. Phys. Chem. Ref. Data 2006, 35, 1475–1517. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, Y.; Wang, Y.; Wang, X. Viscosity of Typical Room-Temperature Ionic Liquids: A Critical Review. J. Phys. Chem. Ref. Data 2019, 48, 033101. [Google Scholar] [CrossRef]
- Gonçalves, A.R.P.; Paredes, X.; Cristino, A.F.; Santos, F.J.V.; Queirós, C.S.G.P. Ionic liquids—A review of their toxicity to living organisms. Int. J. Mol. Sci. 2021, 22, 5612. [Google Scholar] [CrossRef]
- Said, Z.; Sharma, P.; Aslfattahi, N.; Ghodbane, M. Experimental analysis of novel ionic liquid-MXene hybrid nanofluid’s energy storage properties: Model-prediction using modern ensemble machine learning methods. J. Energy Storage 2022, 52, 104858. [Google Scholar] [CrossRef]
- Richter, J.; Ruck, M. Synthesis and dissolution of metal oxides in ionic liquids and deep eutectic solvents. Molecules 2020, 25, 78. [Google Scholar] [CrossRef]
- De Castro, C.A.N.; Lourenço, M.J.V.; Ribeiro, A.P.C.; Langa, E.; Vieira, S.I.C.; Goodrich, P.; Hardacre, C. Thermal properties of ionic liquids and IoNanoFluids of imidazolium and pyrrolidinium liquids. J. Chem. Eng. Data 2010, 55, 653–661. [Google Scholar] [CrossRef]
- Römich, C.; Merkel, N.C.; Valbonesi, A.; Schaber, K.; Sauer, S.; Schubert, T.J.S. Thermodynamic properties of binary mixtures of water and room-temperature ionic liquids: Vapor pressures, heat capacities, densities, and viscosities of water + 1-ethyl-3-methylimidazolium acetate and water + diethylmethylammonium methane sulfonate. J. Chem. Eng. Data 2012, 57, 2258–2264. [Google Scholar] [CrossRef]
- Zhang, F.F.; Li, X.Y.; Chen, G.; Wang, T.; Jin, T.X.; Cheng, C.X.; Li, G.; Zhang, L.; Zhang, B.; Zheng, F. Thermophysical properties and water sorption characteristics of 1-ethyl-3-methylimidazolium acetate ionic liquid and water binary systems. Int. Commun. Heat Mass Transf. 2021, 127, 105558. [Google Scholar] [CrossRef]
- Oster, K.; Goodrich, P.; Jacquemin, J.; Hardacre, C.; Ribeiro, A.P.C.; Elsinawi, A. A new insight into pure and water-saturated quaternary phosphonium-based carboxylate ionic liquids: Density, heat capacity, ionic conductivity, thermogravimetric analysis, thermal conductivity and viscosity. J. Chem. Thermodyn. 2018, 121, 97–111. [Google Scholar] [CrossRef]
- Greer, A.J.; Jacquemin, J.; Hardacre, C. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Feder-Kubis, J.; Tatarczak-Michalewska, M. Chiral ionic liquids: Structural diversity, properties and applications in selected separation techniques. Int. J. Mol. Sci. 2020, 21, 4253. [Google Scholar] [CrossRef]
- Macfarlane, D.R.; Tachikawa, N.; Forsyth, M.; Pringle, J.M.; Howlett, P.C.; Elliott, G.D.; Davis, J.H.; Watanabe, M.; Simon, P.; Angell, C.A. Energy applications of ionic liquids. Energy Environ. Sci. 2014, 7, 232–250. [Google Scholar] [CrossRef]
- Liu, F.; Zhong, X.; Xu, J.; Kamali, A.; Shi, Z. Temperature dependence on density, viscosity, and electrical conductivity of ionic liquid 1-ethyl-3-methylimidazolium fluoride. Appl. Sci. 2018, 8, 356. [Google Scholar] [CrossRef]
- Parajó, J.J.; Villanueva, M.; Troncoso, J.; Salgado, J. Thermophysical properties of choline and pyridinium based ionic liquids as advanced materials for energy applications. J. Chem. Thermodyn. 2020, 141, 105947. [Google Scholar] [CrossRef]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef]
- Khan, R.A.; Mohammed, H.A.; Sulaiman, G.M.; Al Subaiyel, A.; Karuppaiah, A.; Rahman, H.; Makhathini, S.; Ramburrun, P.; Choonara, Y.E. Molecule(s) of Interest: I. Ionic Liquids–Gateway to Newer Nanotechnology Applications: Advanced Nanobiotechnical Uses’, Current Status, Emerging Trends, Challenges, and Prospects. Int. J. Mol. Sci. 2022, 23, 14346. [Google Scholar] [CrossRef]
- Fumino, K.; Reimann, S.; Ludwig, R. Probing molecular interaction in ionic liquids by low frequency spectroscopy: Coulomb energy, hydrogen bonding and dispersion forces. Phys. Chem. Chem. Phys. 2014, 16, 21903–21929. [Google Scholar] [CrossRef]
- Hunt, P.A.; Ashworth, C.R.; Matthews, R.P. Hydrogen bonding in ionic liquids, Chem. Soc. Rev. 2015, 44, 1257–1288. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; You, T.; Leung, M. Screening of novel water/ionic liquid working fluids for absorption thermal energy storage in cooling systems. Int. J. Energy Res. 2020, 44, 9367–9381. [Google Scholar] [CrossRef]
- Soares, L.H.; Guirardello, R.; Rolemberg, M.P. A simple group contribution model to predict thermal conductivity of pure ionic liquids. Chem. Eng. Trans. 2019, 74, 1195–1200. [Google Scholar] [CrossRef]
- Fabre, E.; Murshed, S.M.S. A comprehensive review of thermophysical properties and prospects of ionanocolloids in thermal energy applications. Renew. Sustain. Energy Rev. 2021, 151, 111593. [Google Scholar] [CrossRef]
- Thasneema, K.K.; Thayyil, M.S.; Rosalin, T.; Elyas, K.K.; Dipin, T.; Sahu, P.K.; Kumar, N.K.; Saheer, V.; Messali, M.; Ben Hadda, T. Thermal and spectroscopic investigations on three phosphonium based ionic liquids for industrial and biological applications. J. Mol. Liq. 2020, 307, 112960. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, H.; Singla, M. Diverse applications of ionic liquids: A comprehensive review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Lopez-Morales, J.L.; Perez-Arce, J.; Serrano, A.; Dauvergne, J.L.; Casado, N.; Kottarathil, A.; Del Barrio, E.P.; Garcia-Suarez, E.J. Protic dialkylammonium-based ionic liquids as promising solid-solid phase change materials for thermal energy storage: Synthesis and thermo-physical characterization. J. Energy Storage 2023, 72, 108379. [Google Scholar] [CrossRef]
- Faraji, S.; Shekaari, H.; Zafarani-Moattar, M.T.; Mokhtarpour, M. Experimental studies on thermophysical properties of protic ionic liquids for thermal energy storage systems. J. Energy Storage 2022, 54, 105251. [Google Scholar] [CrossRef]
- Deferm, C.; Van Den Bossche, A.; Luyten, J.; Oosterhof, H.; Fransaer, J.; Binnemans, K. Thermal stability of trihexyl(tetradecyl)phosphonium chloride. Phys. Chem. Chem. Phys. 2018, 20, 2444–2456. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.K.; Moshikur, R.M.; Wakabayashi, R.; Tahara, Y.; Moniruzzaman, M.; Kamiya, N.; Goto, M. Synthesis and characterization of choline–fatty-acid-based ionic liquids: A new biocompatible surfactant. J. Colloid. Interface Sci. 2019, 551, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Jacquemin, J.; Oozeerally, R.; Degirmenci, V. New method for the estimation of viscosity of pure and mixtures of ionic liquids based on the UNIFAC-VISCO Model. J. Chem. Eng. Data 2016, 61, 2160–2169. [Google Scholar] [CrossRef]
- Clarke, C.J.; Clarke, C.J.; Bui-Le, L.; Hallett, J.P.; Licence, P. Thermally-stable imidazolium dicationic ionic liquids with pyridine functional groups. ACS Sustain. Chem. Eng. 2020, 8, 8762–8772. [Google Scholar] [CrossRef]
- Alammar, T.; Slowing, I.I.; Anderegg, J.; Mudring, A.V. Ionic-Liquid-Assisted Microwave Synthesis of Solid Solutions of Sr1−xBaxSnO3 Perovskite for Photocatalytic Applications. ChemSusChem 2017, 10, 3387–3401. [Google Scholar] [CrossRef]
- Ansarpour, M.; Danesh, E.; Mofarahi, M. Investigation the effect of various factors in a convective heat transfer performance by ionic liquid, ethylene glycol, and water as the base fluids for Al2O3 nanofluid in a horizontal tube: A numerical study. Int. Commun. Heat Mass Transf. 2020, 113, 104556. [Google Scholar] [CrossRef]
- Wei, J.; Ren, C.; Zhang, Y.; Liang, K.; Fang, D.; Gao, P. A strategy of imidazole ionic liquids containing metallic element [Cneim][SbF6] (n = 4,5) as innovative media in sustainable heat transfer processes. Int. Commun. Heat Mass Transf. 2023, 140, 106541. [Google Scholar] [CrossRef]
- Liang, K.; Yao, H.; Qiao, J.; Gao, S.; Zong, M.; Liu, F.; Yang, Q.; Liang, L.; Fang, D. Thermodynamic Evaluation of Novel 1,2,4-Triazolium Alanine Ionic Liquids as Sustainable Heat-Transfer Media. Molecules 2024, 29, 5227. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Kumar, B.; Kumar, N.; Raj, R. Simultaneous enhancement of critical heat flux and heat transfer coefficient via in-situ deposition of ionic liquids during pool boiling. Int. J. Heat Mass Transf. 2023, 208, 124066. [Google Scholar] [CrossRef]
- Lexow, M.; Maier, F.; Steinrück, H.P. Ultrathin ionic liquid films on metal surfaces: Adsorption, growth, stability and exchange phenomena. Adv. Phys. 2020, 5, 1761266. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Reichert, W.M.; Reddy, R.G.; Rogers, R.D. Heat Capacities of Ionic Liquids and Their Applications as Thermal Fluids; ACS Symposium Series; American Chemical Society: New York, NY, USA, 2003; Volume 856, pp. 121–133. [Google Scholar] [CrossRef]
- Valkenburg, M.E.V.; Vaughn, R.L.; Williams, M.; Wilkes, J.S. Thermochemistry of ionic liquid heat-transfer fluids. Thermochim. Acta 2005, 425, 181–188. [Google Scholar] [CrossRef]
- Chaudoy, V.; Jacquemin, J.; Tran-Van, F.; Deschamps, M.; Ghamouss, F. Effect of mixed anions on the transport properties and performance of an ionic liquid-based electrolyte for lithium-ion batteries. Pure Appl. Chem. 2019, 91, 1361–1381. [Google Scholar] [CrossRef]
- Koller, T.M.; Lenahan, F.D.; Schmidt, P.S.; Klein, T.; Mehler, J.; Maier, F.; Rausch, M.H.; Wasserscheid, P.; Steinrück, H.-P.; Fröba, A.P. Surface Tension and Viscosity of Binary Mixtures of the Fluorinated and Non-fluorinated Ionic Liquids [PFBMIm][PF6] and [C4C1Im][PF6] by the Pendant Drop Method and Surface Light Scattering. Int. J. Thermophys. 2020, 41, 143–167. [Google Scholar] [CrossRef]
- Bablee, A.; Amarasekara, A.; Gabitto, J.; Bhuiyan, A.; Shamim, N. A Comprehensive Review of the Thermophysical Properties of Energetic Ionic Liquids. Energies 2025, 18, 267. [Google Scholar] [CrossRef]
- Pereira, J.; Souza, R.; Moita, A. A Review of Ionic Liquids and Their Composites with Nanoparticles for Electrochemical Applications. Inorganics 2024, 12, 186. [Google Scholar] [CrossRef]
- Krasovskiy, V.G.; Kapustin, G.I.; Gorbatsevich, O.B.; Glukhov, L.M.; Chernikova, E.A.; Koroteev, A.A.; Kustov, L.M. Properties of dicationic disiloxane ionic liquids. Molecules 2020, 25, 2949. [Google Scholar] [CrossRef]
- Paul, T.C.; Morshed, A.K.M.M.; Fox, E.B.; Visser, A.E.; Bridges, N.J.; Khan, J.A. Thermal performance of ionic liquids for solar thermal applications. Exp. Therm. Fluid. Sci. 2014, 59, 88–95. [Google Scholar] [CrossRef]
- Varela, R.J.; Giannetti, N.; Saito, K.; Wang, X.; Nakayama, H. Experimental performance of a three-fluid desiccant contactor using a novel ionic liquid. Appl. Therm. Eng. 2022, 210, 118343. [Google Scholar] [CrossRef]
- Boldoo, T.; Lee, M.; Cho, H. Numerical investigation on thermal performance of absorption refrigeration system using MWCNT nanoparticle-enhanced 1-hexyl-3-methylimidazolium cation-based ionic liquids. Appl. Therm. Eng. 2022, 206, 118093. [Google Scholar] [CrossRef]
- Heidarshenas, A.; Azizi, Z.; Peyghambarzadeh, S.M.; Sayyahi, S. Experimental investigation of heat transfer enhancement using ionic liquid-Al2O3 hybrid nanofluid in a cylindrical microchannel heat sink. Appl. Therm. Eng. 2021, 191, 116879. [Google Scholar] [CrossRef]
- Cherecheş, E.I.; Minea, A.A.; Sharma, K.V. A complex evaluation of [C2mim][CH3SO3]–alumina nanoparticle enhanced ionic liquids internal laminar flow. Int. J. Heat Mass Transf. 2020, 154, 119674. [Google Scholar] [CrossRef]
- Meikandan, M.; Ganesh Kumar, P.; Sundarraj, M.; Yogaraj, D. Numerical analysis on heat transfer characteristics of ionic liquids in a tubular heat exchanger. Int. J. Ambient. Energy 2020, 41, 911–917. [Google Scholar] [CrossRef]
- Abumandour, E.S.; Mutelet, F.; Alonso, D. Thermodynamic properties assessment of working mixtures {water + alkylphosphonate based ionic liquids} as innovative alternatives working pairs for absorption heat transformers. Appl. Therm. Eng. 2020, 181, 115943. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Zhang, Y.; Li, B.; Sundén, B. Thermophysical properties and convection heat transfer behavior of ionic liquid [C4mim][NTf2] at medium temperature in helically corrugated tubes. Appl. Therm. Eng. 2018, 142, 457–465. [Google Scholar] [CrossRef]
- Rodríguez, H.; Brennecke, J.F. Temperature and composition dependence of the density and viscosity of binary mixtures of water + ionic liquid. J. Chem. Eng. Data 2006, 51, 2145–2155. [Google Scholar] [CrossRef]
- Paul, T.C.; Morshed, A.K.M.M.; Fox, E.B.; Khan, J.A. Thermal performance of Al2O3 Nanoparticle Enhanced Ionic Liquids (NEILs) for Concentrated Solar Power (CSP) applications. Int. J. Heat. Mass. Transf. 2015, 85, 585–594. [Google Scholar] [CrossRef]
- Paul, T.C.; Morshed, A.K.M.M.; Fox, E.B.; Khan, J.A. Experimental investigation of natural convection heat transfer of Al2O3 Nanoparticle Enhanced Ionic Liquids (NEILs). Int. J. Heat Mass Transf. 2015, 83, 753–761. [Google Scholar] [CrossRef]
- Xie, Y.; Ma, C.; Lu, X.; Ji, X. Evaluation of imidazolium-based ionic liquids for biogas upgrading. Appl. Energy 2016, 175, 69–81. [Google Scholar] [CrossRef]
- Wadekar, V.V. Ionic liquids as heat transfer fluids—An assessment using industrial exchanger geometries. Appl. Therm. Eng. 2017, 111, 1581–1587. [Google Scholar] [CrossRef]
- The National Institute of Standards and Technology. NIST Chemistry WebBook. Available online: https://webbook.nist.gov/chemistry/fluid (accessed on 17 May 2025).
- Jóźwiak, B.; Dzido, G.; Kolanowska, A.; Jędrysiak, R.G.; Zorębski, E.; Greer, H.F.; Dzida, M.; Boncel, S. From lab and up: Superior and economic heat transfer performance of ionanofluids containing long carbon nanotubes and 1-ethyl-3-methylimidazolium thiocyanate. Int. J. Heat Mass Transf. 2021, 172, 121161. [Google Scholar] [CrossRef]
- Jozwiak, B.; Dzido, G.; Zorebski, E.; Kolanowska, A.; Jedrysiak, R.; Dziadosz, J.; Libera, M.; Boncel, S.; Dzida, M.; Jedrysiak, R.G. Remarkable Thermal Conductivity Enhancement in Carbon-Based Ionanofluids: Effect of Nanoparticle Morphology. ACS Appl. Mater. Interfaces 2020, 12, 38113–38123. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zou, C.; Li, X. An investigation into the thermophysical and optical properties of SiC/ionic liquid nanofluid for direct absorption solar collector. Sol. Energy Mater. Sol. Cells 2017, 163, 157–163. [Google Scholar] [CrossRef]
- Zaripov, Z.I.; Nakipov, R.R.; Gumerov, F.M.; Boncel, S.; Dzida, M.; Abdulagatov, I.M. Measurements of the thermal conductivity of 1-ethyl-3-methylimidazolium thiocyanate at temperatures from (296 to 365) K and at pressures up to 30 MPa. J. Mol. Liq. 2022, 357, 119091. [Google Scholar] [CrossRef]
- Minea, A.A.; Cherecheş, E.I. Experimental studies on thermal conductivity and heat transfer of 1-Butyl-3-methylimidazolium tetrafluoroborate ionic liquid and its nanocolloids. Int. Commun. Heat Mass Transf. 2024, 154, 107406. [Google Scholar] [CrossRef]
- França, J.M.P.M.; Lourenço, M.J.V.; Murshed, S.M.S.; Pádua, A.A.H.; Nieto Nieto de Castro, C.A. Thermal Conductivity of Ionic Liquids and IoNanofluids and their Feasibility as Heat Transfer Fluids. Ind. Eng. Chem. Res. 2018, 57, 6516–6529. [Google Scholar] [CrossRef]
- Kanti, P.K.; Sharma, K.V.; H N, A.R.; Karbasi, M.; Said, Z. Experimental investigation of synthesized Al2O3 Ionanofluid’s energy storage properties: Model-prediction using gene expression programming. J. Energy Storage 2022, 55, 105718. [Google Scholar] [CrossRef]
- Li, K.; Wu, W.; Wu, J.; Liang, H.; Zhang, H. Experiments on vapour-liquid equilibrium of CO2-ionic liquid under flow conditions and influence on its refrigeration cycle. Appl. Therm. Eng. 2020, 180, 115865. [Google Scholar] [CrossRef]
- He, G.D.; Fang, X.M.; Xu, T.; Zhang, Z.G.; Gao, X.N. Forced convective heat transfer and flow characteristics of ionic liquid as a new heat transfer fluid inside smooth and microfin tubes. Int. J. Heat Mass Transf. 2015, 91, 170–177. [Google Scholar] [CrossRef]
- Chernikova, E.A.; Glukhov, L.M.; Krasovskiy, V.G.; Kustov, L.M.; Vorobyeva, M.G.; Koroteev, A.A. Ionic liquids as heat transfer fluids: Comparison with known systems, possible applications, advantages and disadvantages. Russ. Chem. Rev. 2015, 84, 875–890. [Google Scholar] [CrossRef]
- Waliszewski, D.; Stȩpniak, I.; Piekarski, H.; Lewandowski, A. Heat capacities of ionic liquids and their heats of solution in molecular liquids. Thermochim. Acta 2005, 433, 149–152. [Google Scholar] [CrossRef]
- Lopez-Morales, J.L.; Centeno-Pedrazo, A.; Serrano, A.; Perez-Arce, J.; Dauvergne, J.L.; Casado, N.; Del Barrio, E.P.; Garcia-Suarez, E.J. Alkali alkanoate ionic liquids for thermal energy storage at mid-to-high temperature: Synthesis and thermal–physical characterization. J. Mol. Liq. 2024, 413, 125912. [Google Scholar] [CrossRef]
- Li, Q.; Wang, S.; Zhou, M.; Lu, X.; Qiao, G.; Li, C.; Wu, Y. A review of imidazolium ionic liquid-based phase change materials for low and medium temperatures thermal energy storage and their applications. Green Energy Resour. 2023, 1, 100010. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, M. Ionic liquids toxicity—Benefits and threats. Int. J. Mol. Sci. 2020, 21, 6267. [Google Scholar] [CrossRef]
- Mika, Ł.; Radomska, E.; Sztekler, K.; Gołdasz, A.; Zima, W. Review of Selected PCMs and Their Applications in the Industry and Energy Sector. Energies 2025, 18, 1233. [Google Scholar] [CrossRef]
- Urikhinbam, S.S.; Shagolsem, L.S. Effect of ion size disparity on the thermal hysteresis of ionic liquids. Mater. Today Proc. 2022, 50, A34–A38. [Google Scholar] [CrossRef]





| Full Name | Abbreviation | Scheme |
|---|---|---|
| ammonium | ||
| trimethylpropylammonium [30] | [N1,1,1,3]+ |  |
| triethylammonium [31] | [N2,2,2,H]+ |  |
| tributylammonium [31] | [N4,4,4,H]+ | 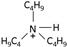 |
| tetrabutylammonium [31] | [N4,4,4,4]+ | 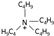 |
| guanidinium | ||
| 1,1,3,3-tetramethylguanidinium [32] | HTBDH+ |  |
| imidazolium | ||
| 1-methylimidazolium [3] | [mIm]+ | 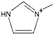 |
| 1-ethylimidazolium [3] | [C2Im]+ |  |
| 1-Alkyl-3-methylimidazolium | ||
| 1-allyl-3-methylimidazolium [3] | [AmIm]+ | 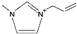 |
| 1-butyl-3-methylimidazolium [3] | [BmIm]+ | 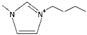 |
| 1-methyl-3-methylimidazolium [3] | [DmIm]+ |  |
| 1-ethyl-3-methylimidazolium [3] | [EmIm]+ |  |
| 1-hexyl-3-methylimidazolium [3] | [HmIm]+ |  |
| 1-octyl-3-methylimidazolium [3] | [OmIm]+ | 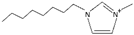 |
| 1-propyl-3-methylimidazolium [3] | [PmIm]+ | 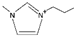 |
| 1-hexadecyl-3-methylimidazolium [3] | [C16mIm]+ |  |
| 1-Alkyl-2,3-dimethylimidazolium | ||
| 1-butyl-2,3-dimethylimidazolium [33] | [BdmIm]+ |  |
| 1-ethyl-2,3-dimethylimidazolium [34] | [EdmIm]+ | 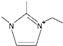 |
| 1-propyl-2,3-dimethylimidazolium [34] | [PdmIm]+ |  |
| 1,1′-(butane-1,4-diyl)-bis(3-methylimidazolium) [34] | C4(mim)2+ | 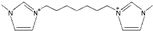 |
| 1,1′-(pentane-1,5-diyl)-bis(3-methylimidazolium) [34] | C5(mim)2+ | 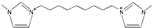 |
| 1,1′-(hexane-1,6-diyl)-bis(3-methylimidazolium) [34] | C6(mim)2+ | 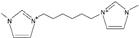 |
| morpholinium | ||
| 4-methylmorpholinium [31] | MmMor+ | 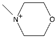 |
| 4-ethyl-4-methylmorpholinium [31] | EmMor+ |  |
| 4-(2-methoxyethyl)-4-methylmorpholinium [31] | MoemMor+ |  |
| phosphonium | ||
| trihexyltetradecylphosphonium [15] | [P6,6,6,14]+ |  |
| Triphenylphosphonium [15] | Ph3PH+ |  |
| pyridinium | ||
| 1-ethylpyridinium [31] | [Epy]+ | 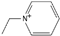 |
| 1-butylpyridinium [31] | [Bpy]+ | 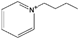 |
| piperidinium | ||
| 1-methylpiperidinium [31] | [MPip]+ | 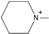 |
| 1-ethylpiperidinium [31] | [EPip]+ | 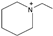 |
| 1-methyl-1-propylpiperidinium [31] | [MPPip]+ |  |
| 1-methyl-1-butylpiperidinium [31] | [MBPip]+ |  |
| pyrrolidinium | ||
| 1-butyl-1-methylpyrrolidinium [35] | [BMPyrr]+ |  |
| 1-n-nonyl-1-butylpyrrolidinium [35] | [C9(Bpyrr)2]+ |  |
| 1-n-nonyl-1-methylpyrrolidinium [35] | [C9(Mpyrr)2]+ |  |
| trialkylsulfonium | ||
| Trimetylsulfonium [31] | [S111]+ | 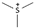 |
| Triethylsulfonium [31] | [S222]+ |  |
| Full Name | Abbreviation | Scheme |
|---|---|---|
| acetate [36,37,38] | [OAc]−, [Ac]−, [AcO]− | 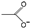 |
| bis(fluorosulfonyl)imide [39] | [fsi]− or [N(SO2F)2]− |  |
| bis(trifluromethylsulfonyl)imide [40] | [NTf2]− or [N(SO2CF3)2]− | 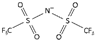 |
| bromide [39] | [Br]− | |
| chloride [39] | [Cl]− | |
| dicyanamide [41] | [DCA]− | 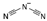 |
| dihydrogenphosphate [4] | [DHP]− or [H2PO4]− |  |
| dimethylphosphate [31] | [DMP]− | 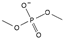 |
| ethyl sulfate [31] | [EtSO4]− |  |
| fluoride [42] | [F]− | |
| fluoroalkylphosphates [4] | [fap]−, [efap]−, etc. | 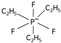 |
| fluorohydrogenate [34] | [F(HF)2] − | 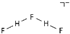 |
| glutamate [3] | [Glu]− | 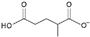 |
| glycinate [3] | [Gly]− | 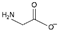 |
| hexafluorophosphate [39] | [PF6]− |  |
| hydrogen carbonate [3] | [HCO3]− | 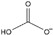 |
| hydrogensulfate [4] | [HSO4]− |  |
| hydroxide [4] | [OH]− |  |
| iodide [40] | [I]− | |
| nitrate [40] | [NO3]− | 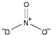 |
| octenylsuccinate/organosulfonate [3] | [OSA]− |  |
| p-toluenesulfonate or tosylate [43] | [Tos]− | 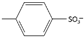 |
| perchlorate [31] | [ClO4]− | 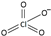 |
| phenylalaninate [3] | [Phe]− | 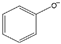 |
| prolinate [3] | [Pro]− |  |
| serinate [3] | [Ser]− |  |
| tetrachloroaluminate [39] | [AlCl4]− |  |
| tetracyanoborate [4] | [B(CN)4]− |  |
| tetrafluoroborate [39] | [BF4]− |  |
| tetrahydroborate (borohydride) [4] | [BH4]− |  |
| trifluoroacetate [39] | CF3COO−, [tfa]− | 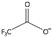 |
| trifluoromethylsulfate [40] | [CF3SO3]− |  |
| trifluoromethanesulfonate [39] | [OTf]− or [CF3SO3]− | 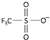 |
| trifluoromethylsulfate [4] | [TfO]− | 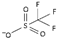 |
| triocyanate [31] | [SCN]− |  |
| trifluoroethanoate (acetate) [39] | [tfa]− |  |
| Ionic Liquid | Full Name |
|---|---|
| [BmIm] [BF4] (or [C4mIm] [BF4]) | 1-butyl-3-methylimidazolium tetrafluoroborate [30,48] |
| [BmIm] [Br] | 1-butyl-3-methylimidazolium bromide [48] |
| [BmIm] [DBP] | 1-butyl-3-methylimidazolium dibutylphosphate [48] |
| [C10mIm] [B(CN)4] | 1-decyl-3-methylimidazolium tetracyanoborate [49] |
| [C10mIm] [C(CN)3] | 1-decyl-3-methylimidazolium tricyanomethanide [49] |
| [C10mIm] [TF2N] | 1-decyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide [49] |
| [C2mIm] [Ac] (or: [C2mIm] [CH3COO], [EmIm] [Ac]) | 1-ethyl-3-methylimidazolium acetate [48,49,50] |
| [C2mIm] [BF4] | 1-ethyl-3-methylimidazolium tetrafluoroborate [49] |
| [C2mIm] [C(CN)3] | 1-ethyl-3-methylimidazolium tricyanomethanide [50] |
| [C2mIm] [C2SO4] | 1-ethyl-3-methylimidazolium ethylsulfate [50] |
| [C2mIm] [CF3SO3] (or [EmIm] [TfO]) | 1-ethyl-3-methylimidazolium trifluoromethanesulfonate [48,49] |
| [C2mIm] [CH3SO3] | 1-ethyl-3-methylimidazolium methanesulfonate [50] |
| [C2mIm] [DCA] | 1-ethyl-3-methylimidazolium dicyanamide [50] |
| [C2mIm] [DEP] (or [EmIm] [DEP]) | 1-ethyl-3-methylimidazolium diethylphosphate [48,50] |
| [C2mIm] [EtSO4] (or [EmIm] [EtSO4]) | 1-ethyl-3-methylimidazolium ethyl sulfate [48,49] |
| [C2mIm] [MeOHPO2] | 1-ethyl-3-methylimidazolium methyl phosphonate [49] |
| [C2mIm] [SCN] | 1-ethyl-3-methylimidazolium thiocyanate [50] |
| [C2mIm] [TF2N] | 1-ethyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide [49] |
| [C4mIm] [C(CN)3] | 1-butyl-3-methylimidazolium tricyanomethane [36] |
| [C4mIm] [CF3SO3] | 1-butyl-3-methylimidazolium trifluoromethanesulfonate [36] |
| [C4mIm] [DCA] | 1-butyl-3-methylimidazolium dicyanamide [49] |
| [C4mIm] [PF6] | 1-butyl-3-methylimidazolium hexafluorophosphate [49] |
| [C4mIm] [SCN] | 1-butyl-3-methylimidazolium thiocyanate [49] |
| [C4mIm] [TF2N] (or [C4mIm] [NTf2]) | 1-butyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide [49,50] |
| [C4mPyr] [DCA] | 1-butyl-1-methylpyrrolidinium dicyanamide [49] |
| [C4mPyr] [FAP] | 1-butyl-1-methylpyrrolidinium tris(pentafluoroethyl)trifluorophosphate [49] |
| [C4mPyr] [TF2N] | 1-butyl-1-methylpyrrolidinium bis[(trifluoromethyl)sulfonyl]imide [49] |
| [C6mIm] [B(CN)4] | 1-hexyl-3-methylimidazolium tetracyanoborate [49] |
| [C6mIm] [BF4] | 1-hexyl-3-methyl imidazolium tetrafluoroborate [50] |
| [C6mIm] [PF6] | 1-hexyl-3-methylimidazolium hexafluorophosphate [30] |
| [C6mIm] [TF2N] | 1-hexyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide [49] |
| [C8mIm] [C(CN)3] | 1-octyl-3-methylimidazolium tricyanomethanide [49] |
| [C8mIm] [PF6] | 1-octyl-3-methylimidazolium hexafluorophosphate [49] |
| [C8mIm] [TF2N] | 1-octyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide [49] |
| [DEMA] [OMs] | diethylmethylammonium methane sulfonate [48] |
| [DmIm] [BF4] | 1,3-dimethylimidazolium tetrafluoroborate [48] |
| [DmIm] [Cl] | 1,3-dimethylimidazolium chloride [48] |
| [DmIm] [DMP] | 1,3-dimethylimidazolium dimethylphosphate [48] |
| [EEIM] [DEP] | 1-ethyl-3-ethylimidazolium diethylphosphate [48] |
| [EmIm] [DMP] | 1-ethyl-3-methylimidazolium dimethylphosphate [48] |
| [EmIm] [TFA] | 1-ethyl-3-methylimidazolium trifluoroacetate [48] |
| [N4,1,1,1] [NTf2] | butyltrimethylammonium bis(trifluoromethylsulfonyl)imide [50] |
| [P14,6,6,6] [AcO] | trihexyl(tetradecyphosphonium) acetate [50] |
| [P14,6,6,6] [ButO] | trihexyl(tetradecyphosphonium) butanoate [50] |
| [P14,6,6,6] [DecO] | trihexyl(tetradecyphosphonium) decanoate [50] |
| [P14,6,6,6] [HexO] | trihexyl(tetradecyphosphonium) hexanoate [50] |
| [P14,6,6,6] [OctO] | trihexyl(tetradecyphosphonium) octanoate [50] |
| [P6,6,6,14] [Cl] | trihexyltetradecylphosphonium chloride [50] |
| [P6,6,6,14] [NTf2] | trihexyl(tetradecylphosphonium) bis((trifluoromethyl)sulfonyl)imide [50] |
| [P6,6,6,14] [Phosph] | trihexyl(tetradecylphosphonium) phosphinate [50] |
| [TBPh] [CYS] | tetrabutylphosphonium L-cysteinate [49] |
| [TBPh] [LYS] | tetrabutylphosphonium L-lysinate [49] |
| [TBPh] [PRO] | tetrabutylphosphonium L-prolinate [49] |
| [TBPh] [SER] | etrabutylphosphonium L-serinate [49] |
| [TBPh] [TAU] | tetrabutylphosphonium 2-aminoethanesulfonate [49] |
| [TBPh] [THR] | tetrabutylphosphonium L-threoninate [49] |
| [TBPh] [VAL] | tetrabutylphosphonium L-valinate [49] |
| [THTDPh] [Cl] | trihexyl(tetradecyl)phosphonium chloride [49] |
| [THTDPh] [TF2N] | trihexyl(tetradecyl)phosphonium bis[(trifluoromethyl)sulfonyl]imide [49] |
| Ionic Liquid | Density [kg/m3] at 20 °C | Ionic Liquid | Density [kg/m3] at 25 °C | Ionic Liquid | Density [kg/m3] at 30 °C |
|---|---|---|---|---|---|
| [C2mIm][CH3SO3]—[76] | 1243 | [AmIm][BF4]—[45] | 1231 | [bdmIm][BF4]—[73] | 1094 |
| [DmIm][MPh]—[78] | 1180 | [BmIm][(CF3SO2)2N]—[45] | 1420 | [C2mIm][OTf]—[73] | 1370 |
| [EmIm][EPh]—[78] | 1130 | [BmIm][BF4]—[73] | 1201 | [C2mIm][SCN]—[73] | 1114 |
| [HmIm][BF4]—[74] | 1200 | [BmIm][BF4]—[45] | 1208 | [C2mIm][Tf2N]—[73] | 1514 |
| [HmIm][PF6]—[74] | 1304 | [BmIm][BF4]—[77] | 1120 | ||
| [HmIm][Tf2N]—[74] | 1370 | [BmIm][CF3SO3]—[45] | 1290 | ||
| [HmIm][TfO]—[74] | 1240 | [BmIm][dca]—[73] | 1058 | ||
| [BmIm][NTfO2]—[45] | 1404 | ||||
| [BmIm][PF6]—[82] | 1373 | ||||
| [BmIm][Tf2N]—[73] | 1439 | ||||
| [BmIm]Br—[45] | 1134 | ||||
| [BmIm]Cl—[83] | 1120 | ||||
| [BMmIm][PF6]—[73] | 1242 | ||||
| [BMmIm][PF6]—[77] | 1360 | ||||
| [BMPyrrol][NTfO2]—[45] | 1400 | ||||
| [bpy][BF4]—[73] | 1214 | ||||
| [C2mIm][EtSO4]—[72] | 1241 | ||||
| [C4mIm][BF4]—[75] | 1210 | ||||
| [C4mIm]Br—[22] | 1293 | ||||
| [C4mIm]Cl—[79] | 1086 | ||||
| [C4mIm]I—[22] | 1489 | ||||
| [EmIm][BF4]—[45] | 1248 | ||||
| [EmIm][ETSO4]—[77] | 1242 | ||||
| [EmIm][MeSO4]—[73] | 1280 | ||||
| [EmIm][PF6]—[45] | 1373 | ||||
| [EmIm][Tf2N]—[73] | 1521 | ||||
| [EmmIm][Tf2N]—[73] | 1491 | ||||
| [HmIm][BF4]—[80] | 1075 | ||||
| [HmIm][PF6]—[45] | 1304 | ||||
| [MPPyr][NTfO2]—[45] | 1440 | ||||
| [OmIm][BF4]—[81] | 1110 | ||||
| [OmIm]Cl—[84] | 1000 | ||||
| BAF—[45] | 990 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutkowski, K.; Kruzel, M.; Smuga-Kogut, M.; Walczak, M. A Review of the State of the Art on Ionic Liquids and Their Physical Properties During Heat Transfer. Energies 2025, 18, 4053. https://doi.org/10.3390/en18154053
Dutkowski K, Kruzel M, Smuga-Kogut M, Walczak M. A Review of the State of the Art on Ionic Liquids and Their Physical Properties During Heat Transfer. Energies. 2025; 18(15):4053. https://doi.org/10.3390/en18154053
Chicago/Turabian StyleDutkowski, Krzysztof, Marcin Kruzel, Małgorzata Smuga-Kogut, and Marcin Walczak. 2025. "A Review of the State of the Art on Ionic Liquids and Their Physical Properties During Heat Transfer" Energies 18, no. 15: 4053. https://doi.org/10.3390/en18154053
APA StyleDutkowski, K., Kruzel, M., Smuga-Kogut, M., & Walczak, M. (2025). A Review of the State of the Art on Ionic Liquids and Their Physical Properties During Heat Transfer. Energies, 18(15), 4053. https://doi.org/10.3390/en18154053











