Carbon Mineralization in Basaltic Rocks: Mechanisms, Applications, and Prospects for Permanent CO2 Sequestration
Abstract
1. Introduction
1.1. Geological Background of Basalt
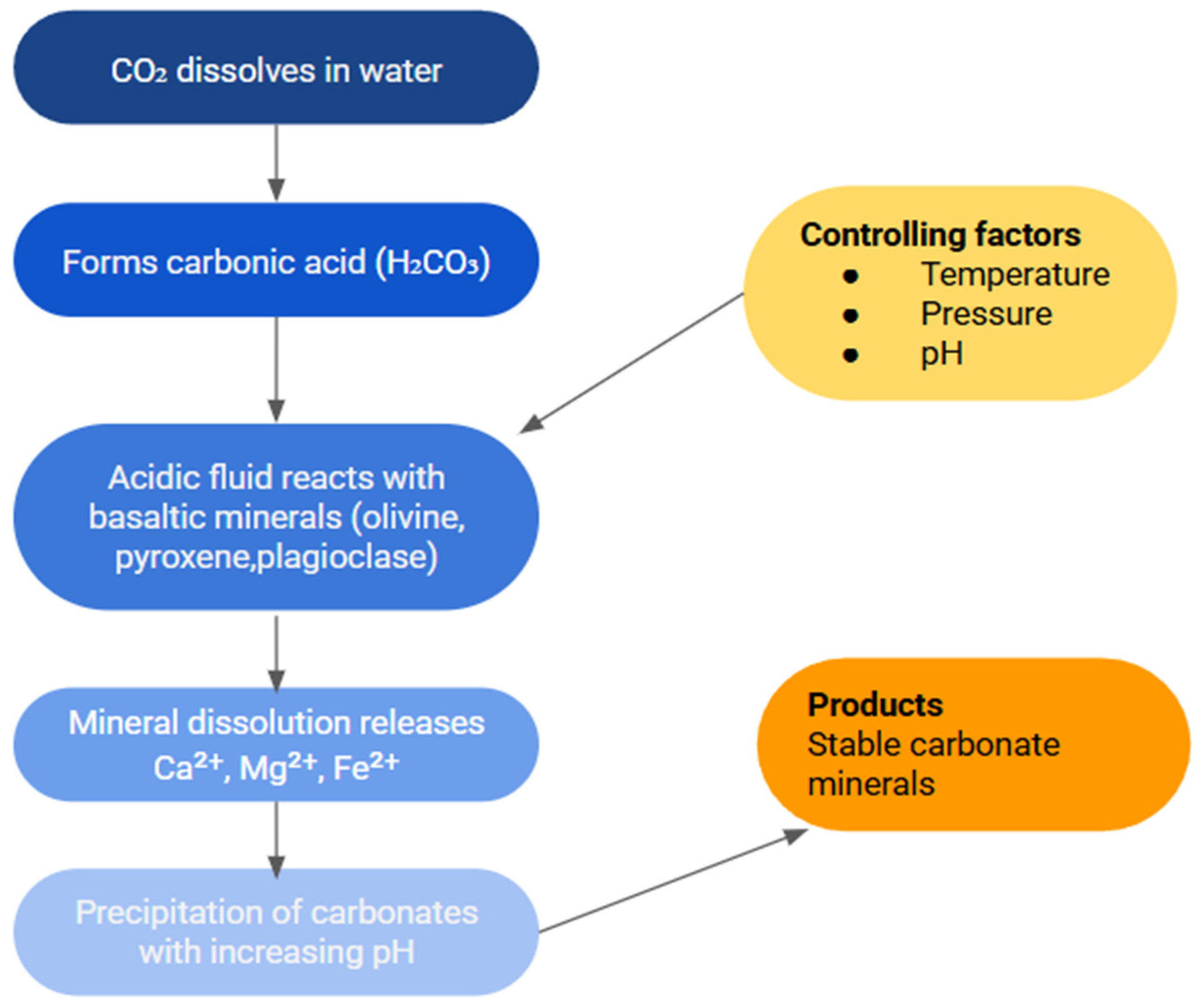
1.2. Mechanism of CO2 Mineralization in Basalt
1.2.1. Role of Water–Rock Interactions
1.2.2. Geomechanical Impacts of Basalt Carbon Mineralization
1.3. Overview and Future Prospects of Basaltic Rock Utilization
2. Current Utilization of Basalt in Carbon Mineralization
2.1. In Situ Carbon Dioxide Mineralization
2.2. Ex Situ Carbon Dioxide Mineralization
2.3. Case Studies and Real-World Applications
2.3.1. CarbFix Project
2.3.2. Wallula Basalt Pilot Project
2.3.3. Global Overview of Basalt CO2 Mineralization Efforts
2.4. Fundamental Studies
3. Kinetics and Thermodynamics of Mineralization
3.1. Effect of Basalt Mineral Composition on Carbon Mineralization
3.2. Effect of pH on Kinetics of Dissolution and Precipitation
3.3. Effect of Pressure on the Kinetics of Carbon Mineralization
3.4. Effect of Temperature on the Kinetics of Carbon Mineralization
4. Challenges and Limitations
5. Conclusions
Funding
Conflicts of Interest
List of Acronyms
| Acronyms | Meaning |
| CCS | Carbon Capture and Storage |
| CNRS | National Centre for Scientific Research |
| DAS | Distributed Acoustic Sensing |
| DAS-VSP | Distributed Acoustic Sensing Vertical Seismic Profiling |
| DSS | Distributed Strain Sensing |
| DTS | Distributed Temperature Sensing |
| EDS-WDS | Energy Dispersive Spectroscopy—Wavelength Dispersive X-ray Spectroscopy |
| EOR | Enhance Oil Recovery |
| InSAR | Interferometric Synthetic Aperture Radar |
| PNNL | Pacific Northwest National Laboratory |
| RITE | Research Institute of Innovative Technology for the Earth |
| RTE | Red Trail Energy |
| SASSA | Scalable Automated Semi-Permanent Seismic Array |
| SEM | Scanning Electron Microscope |
| SOV | Surface Orbital Vibrators |
References
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change. In Climate Change 2021: The Physical Science Basis; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; Volume 2, p. 2391. [Google Scholar]
- Wu, J.; Hu, S.; Jessen, K.; Tsotsis, T. A Study of Capillary Condensation Phenomena in Mesoporous Materials. In Proceedings of the 2022 AIChE Annual Meeting, Phoenix, AZ, USA, 13–18 November 2022. [Google Scholar]
- Simeski, F. Molecular Simulations of Adsorption, Phase Behavior, and Reactivity in Subsurface Fluid-Solid Systems. Ph.D. Thesis, Stanford University, Stanford, CA, USA, 2023. [Google Scholar]
- Lee, J.J. NASA Analysis Shows Unexpected Amount of Sea Level Rise in 2024. 2025. Available online: https://www.nasa.gov/missions/jason-cs-sentinel-6/sentinel-6-michael-freilich/nasa-analysis-shows-unexpected-amount-of-sea-level-rise-in-2024/ (accessed on 30 April 2025).
- Friedlingstein, P.; O’sullivan, M.; Jones, M.W.; Andrew, R.M.; Gregor, L.; Hauck, J.; Le Quéré, C.; Luijkx, I.T.; Olsen, A.; Peters, G.P. Global carbon budget 2022. Earth Syst. Sci. Data 2022, 14, 4811–4900. [Google Scholar] [CrossRef]
- Smil, V. Energy: A Beginner’s Guide; Simon and Schuster: New York, NY, USA, 2017. [Google Scholar]
- Yergin, D. The Prize: The Epic Quest for Oil, Money, and Power; Simon & Schuster: New York, NY, USA, 1991; pp. 570–594. [Google Scholar]
- Arrhenius, S. XXXI. On the influence of carbonic acid in the air upon the temperature of the ground. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1896, 41, 237–276. [Google Scholar] [CrossRef]
- Metz, B.; Davidson, O.; De Coninck, H.; Loos, M.; Meyer, L. IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Matter, J.M.; Stute, M.; Snæbjörnsdottir, S.Ó.; Oelkers, E.H.; Gislason, S.R.; Aradottir, E.S.; Sigfusson, B.; Gunnarsson, I.; Sigurdardottir, H.; Gunnlaugsson, E.; et al. Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions. Science 2016, 352, 1312–1314. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D.; Jarvis, M.; Hitchins, T. Kinetics and mechanism of mineral carbonation of olivine for CO2 sequestration. Miner. Eng. 2019, 131, 185–197. [Google Scholar] [CrossRef]
- Kim, K.; Kim, D.; Na, Y.; Song, Y.; Wang, J. A review of carbon mineralization mechanism during geological CO2 storage. Heliyon 2023, 9, e23135. [Google Scholar] [CrossRef]
- Gislason, S.R.; Oelkers, E.H. Mechanism, rates, and consequences of basaltic glass dissolution: II. An experimental study of the dissolution rates of basaltic glass as a function of pH and temperature. Geochim. Cosmochim. Acta 2003, 67, 3817–3832. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Gislason, S.R. The mechanism, rates and consequences of basaltic glass dissolution: I. An experimental study of the dissolution rates of basaltic glass as a function of aqueous Al, Si and oxalic acid concentration at 25 °C and pH = 3 and 11. Geochim. Cosmochim. Acta 2001, 65, 3671–3681. [Google Scholar] [CrossRef]
- Klein, E.M. Geochemistry of the igneous oceanic crust. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Rudnick, R.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 3, Chapter 3.13. [Google Scholar]
- Fitton, J.; Saunders, A.; Norry, M.; Hardarson, B.; Taylor, R. Thermal and chemical structure of the Iceland plume. Earth Planet. Sci. Lett. 1997, 153, 197–208. [Google Scholar] [CrossRef]
- Arndt, N.; Lesher, M.C.; Barnes, S.J. Komatiite; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Oelkers, E.H.; Gislason, S.R.; Eiriksdottir, E.S.; Jones, M.; Pearce, C.R.; Jeandel, C. The role of riverine particulate material on the global cycles of the elements. Appl. Geochem. 2011, 26, S365–S369. [Google Scholar] [CrossRef]
- Beerling, D.J.; Leake, J.R.; Long, S.P.; Scholes, J.D.; Ton, J.; Nelson, P.N.; Bird, M.; Kantzas, E.; Taylor, L.L.; Sarkar, B.; et al. Farming with crops and rocks to address global climate, food and soil security. Nat. Plants 2018, 4, 138–147. [Google Scholar] [CrossRef]
- Kelemen, P.; Benson, S.M.; Pilorgé, H.; Psarras, P.; Wilcox, J. An Overview of the Status and Challenges of CO2 Storage in Minerals and Geological Formations. Front. Clim. 2019, 1, 9. [Google Scholar] [CrossRef]
- Snæbjörnsdóttir, S.Ó.; Sigfússon, B.; Marieni, C.; Goldberg, D.; Gislason, S.R.; Oelkers, E.H. Carbon dioxide storage through mineral carbonation. Nat. Rev. Earth Environ. 2020, 1, 90–102. [Google Scholar] [CrossRef]
- Luhmann, A.J.; Tutolo, B.M.; Bagley, B.C.; Mildner, D.F.R.; Seyfried Jr, W.E.; Saar, M.O. Permeability, porosity, and mineral surface area changes in basalt cores induced by reactive transport of CO2-rich brine. Water Resour. Res. 2017, 53, 1908–1927. [Google Scholar] [CrossRef]
- Rasool, M.H.; Ahmad, M. Reactivity of basaltic minerals for CO2 sequestration via in situ mineralization: A review. Minerals 2023, 13, 1154. [Google Scholar] [CrossRef]
- Snæbjörnsdóttir, S.Ó.; Tómasdóttir, S.; Sigfússon, B.; Aradóttir, E.S.; Gunnarsson, G.; Niemi, A.; Basirat, F.; Dessirier, B.; Gislason, S.R.; Oelkers, E.H. The geology and hydrology of the CarbFix2 site, SW-Iceland. Energy Procedia 2018, 146, 146–157. [Google Scholar] [CrossRef]
- Alfredsson, H.A.; Oelkers, E.H.; Hardarsson, B.S.; Franzson, H.; Gunnlaugsson, E.; Gislason, S.R. The geology and water chemistry of the Hellisheidi, SW-Iceland carbon storage site. Int. J. Greenh. Gas Control 2013, 12, 399–418. [Google Scholar] [CrossRef]
- Sandalow, D.; Aines, R.; Friedmann, J.; Kelemen, P.; McCormick, C.; Power, I.; Schmidt, B.; Wilson, S.; (Lawrence Livermore National Lab. (LLNL), Livermore, CA (United States)). Carbon Mineralization Roadmap Draft October 2021; Lawrence Livermore National Lab. (LLNL): Livermore, CA, USA, 2021. [Google Scholar]
- Menefee, A.H.; Giammar, D.E.; Ellis, B.R. Permanent CO2 trapping through localized and chemical gradient-driven basalt carbonation. Environ. Sci. Technol. 2018, 52, 8954–8964. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Jenkins, J.D. US EPA’s power plant rules reduce CO2 emissions but can achieve more cost-efficient and deeper reduction by regulating existing gas-fired plants. One Earth 2025, 8, 101230. [Google Scholar] [CrossRef]
- Sigfusson, B.; Gislason, S.R.; Matter, J.M.; Stute, M.; Gunnlaugsson, E.; Gunnarsson, I.; Aradottir, E.S.; Sigurdardottir, H.; Mesfin, K.; Alfredsson, H.A.; et al. Solving the carbon-dioxide buoyancy challenge: The design and field testing of a dissolved CO2 injection system. Int. J. Greenh. Gas Control 2015, 37, 213–219. [Google Scholar] [CrossRef]
- Gao, F.; Cheng, Y.; Guo, R.; Liu, X.; Liu, Z. The Late Carboniferous Mafic–Ultramafic Complex Induced by Slab Breakoff in Eastern North Tianshan, Central Asian Orogenic Belt. Minerals 2023, 13, 1293. [Google Scholar] [CrossRef]
- Hellevang, H.; Aagaard, P.; Oelkers, E.H.; Kvamme, B. Can Dawsonite Permanently Trap CO2? Environ. Sci. Technol. 2005, 39, 8281–8287. [Google Scholar] [CrossRef]
- Giacomel, P.; Spagnuolo, E.; Nazzari, M.; Marzoli, A.; Passelegue, F.; Youbi, N.; Di Toro, G. Frictional Instabilities and Carbonation of Basalts Triggered by Injection of Pressurized H2O- and CO2-Rich Fluids. Geophys. Res. Lett. 2018, 45, 6032–6041. [Google Scholar] [CrossRef]
- Elshall, A. Experimental and Geochemical Modeling Evidences of Mineral Sequestration of CO2 in Saline Siliciclastic aquifers. Earth ArXiv 2018. [Google Scholar] [CrossRef]
- McGrail, B.P.; Schaef, H.T.; Spane, F.A.; Cliff, J.B.; Qafoku, O.; Horner, J.A.; Thompson, C.J.; Owen, A.T.; Sullivan, C.E. Field Validation of Supercritical CO2 Reactivity with Basalts. Environ. Sci. Technol. Lett. 2017, 4, 6–10. [Google Scholar] [CrossRef]
- Latifah, N.; Febrianto, S.; Wirasatriya, A.; Endrawati, H.; Zainuri, M.; Suryanti, S.; Hidayat, A.N. Air-Sea Flux of CO2 in the Waters of Karimunjawa Island, Indonesia. Saintek Perikan. Indones. J. Fish. Sci. Technol. 2020, 16, 171–178. [Google Scholar] [CrossRef]
- Xu, G.X.; Wang, S.Z. Water-Rock-CO2 Interaction in Hot Dry Rock—A Review. Key Eng. Mater. 2017, 748, 446–450. [Google Scholar] [CrossRef]
- Ajayi, T. Investigation of PH effect in a mixture of basalt and iron on CO2 sequestration in synthetic brines. Int. J. Adv. Geosci. 2019, 7, 112. [Google Scholar] [CrossRef]
- Rohmer, J.; Pluymakers, A.; Renard, F. Mechano-chemical interactions in sedimentary rocks in the context of CO2 storage: Weak acid, weak effects? Earth-Sci. Rev. 2016, 157, 86–110. [Google Scholar] [CrossRef]
- Rutqvist, J.; Birkholzer, J.; Cappa, F.; Tsang, C.-F. Estimating maximum sustainable injection pressure during geological sequestration of CO2 using coupled fluid flow and geomechanical fault-slip analysis. Energy Convers. Manag. 2007, 48, 1798–1807. [Google Scholar] [CrossRef]
- Benson, S.M.; Cole, D.R. CO2 sequestration in deep sedimentary formations. Elements 2008, 4, 325–331. [Google Scholar] [CrossRef]
- Rutqvist, J.; Zheng, L.; Chen, F.; Liu, H.-H.; Birkholzer, J. Modeling of coupled thermo-hydro-mechanical processes with links to geochemistry associated with bentonite-backfilled repository tunnels in clay formations. Rock Mech. Rock Eng. 2014, 47, 167–186. [Google Scholar] [CrossRef]
- Aradóttir, E.S.; Sigurdardóttir, H.; Sigfússon, B.; Gunnlaugsson, E. CarbFix: A CCS pilot project imitating and accelerating natural CO2 sequestration. Greenh. Gases Sci. Technol. 2011, 1, 105–118. [Google Scholar] [CrossRef]
- Gislason, S.R.; Wolff-Boenisch, D.; Stefansson, A.; Oelkers, E.H.; Gunnlaugsson, E.; Sigurdardottir, H.; Sigfusson, B.; Broecker, W.S.; Matter, J.M.; Stute, M.; et al. Mineral sequestration of carbon dioxide in basalt: A pre-injection overview of the CarbFix project. Int. J. Greenh. Gas Control 2010, 4, 537–545. [Google Scholar] [CrossRef]
- Winter, J.D. Principles of Igneous and Metamorphic Petrology; Pearson education: Harlow, UK, 2014; Volume 2. [Google Scholar]
- Mielenz, R.C. Petrographic Examination; ASTM International: West Conshohocken, PA, USA, 1956. [Google Scholar]
- Mindess, S.; Young, F.; Darwin, D. Technical Documents. Concrete, 2nd ed.; Prentice Hall: Hoboken, NJ, USA, 2003; Volume 585. [Google Scholar]
- Fiore, V.; Scalici, T.; Di Bella, G.; Valenza, A. A review on basalt fibre and its composites. Compos. Part B Eng. 2015, 74, 74–94. [Google Scholar] [CrossRef]
- Brandt, A.M. Fibre reinforced cement-based (FRC) composites after over 40 years of development in building and civil engineering. Compos. Struct. 2008, 86, 3–9. [Google Scholar] [CrossRef]
- Sim, J.; Park, C. Characteristics of basalt fiber as a strengthening material for concrete structures. Compos. Part B Eng. 2005, 36, 504–512. [Google Scholar] [CrossRef]
- Wu, C.; Yin, A.; Zuza, A.V.; Zhang, J.; Liu, W.; Ding, L. Pre-Cenozoic geologic history of the central and northern Tibetan Plateau and the role of Wilson cycles in constructing the Tethyan orogenic system. Lithosphere 2016, 8, 254–292. [Google Scholar] [CrossRef]
- Self, S.; Widdowson, M.; Thordarson, T.; Jay, A.E. Volatile fluxes during flood basalt eruptions and potential effects on the global environment: A Deccan perspective. Earth Planet. Sci. Lett. 2006, 248, 518–532. [Google Scholar] [CrossRef]
- Spear, F.S. Metamorphic Phase Equilibria and Pressure–Temperature–Time Paths; Mineralogical Society of America: Chantilly, VA, USA, 1993; Volume 352. [Google Scholar]
- van Straaten, O.; Veldkamp, E.; Corre, M.D. Simulated drought reduces soil CO2 efflux and production in a tropical forest in Sulawesi, Indonesia. Ecosphere 2011, 2, 1–22. [Google Scholar] [CrossRef]
- Swoboda, P.; Döring, T.F.; Hamer, M. Remineralizing soils? The agricultural usage of silicate rock powders: A review. Sci. Total Environ. 2022, 807, 150976. [Google Scholar] [CrossRef]
- Park, K.; Kim, C.Y.; Kirk, M.F.; Chae, G.; Kwon, M.J. Effects of natural non-volcanic CO2 leakage on soil microbial community composition and diversity. Sci. Total Environ. 2023, 862, 160754. [Google Scholar] [CrossRef] [PubMed]
- Borhan, T.M. Properties of glass concrete reinforced with short basalt fibre. Mater. Des. 2012, 42, 265–271. [Google Scholar] [CrossRef]
- Snæbjörnsdóttir, S.Ó.; Wiese, F.; Fridriksson, T.; Ármansson, H.; Einarsson, G.M.; Gislason, S.R. CO2 storage potential of basaltic rocks in Iceland and the oceanic ridges. Energy Procedia 2014, 63, 4585–4600. [Google Scholar] [CrossRef]
- Xiong, W.; Wells, R.K.; Horner, J.A.; Schaef, H.T.; Skemer, P.A.; Giammar, D.E. CO2 Mineral Sequestration in Naturally Porous Basalt. Environ. Sci. Technol. Lett. 2018, 5, 142–147. [Google Scholar] [CrossRef]
- Earle, S. Physical Geology; BCcampus: Victoria, BC, Canada, 2015. [Google Scholar]
- Reidel, S.P.; Spane, F.A.; Johnson, V.G.; (Pacific Northwest National Lab. (PNNL), Richland, WA (United States)). Potential for Natural Gas Storage in Deep Basalt Formations at Canoe Ridge, Washington State: A Hydrogeologic Assessment; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2005. [Google Scholar]
- Snæbjörnsdóttir, S.Ó.; Gislason, S.R. CO2 Storage Potential of Basaltic Rocks Offshore Iceland. Energy Procedia 2016, 86, 371–380. [Google Scholar] [CrossRef]
- Takaya, Y.; Nakamura, K.; Kato, Y. Geological, geochemical and social-scientific assessment of basaltic aquifers as potential storage sites for CO2. Geochem. J. 2013, 47, 385–396. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, R.; Li, F.; Li, C.; Sun, Y.; Yang, H. Assessment of CO2 mineral storage potential in the terrestrial basalts of China. Fuel 2023, 348, 128602. [Google Scholar] [CrossRef]
- Gislason, S.R.; Oelkers, E.H. Carbon storage in basalt. Science 2014, 344, 373–374. [Google Scholar] [CrossRef]
- Matter, J.M.; Broecker, W.; Gislason, S.; Gunnlaugsson, E.; Oelkers, E.; Stute, M.; Sigurdardóttir, H.; Stefansson, A.; Alfreðsson, H.; Aradóttir, E. The CarbFix Pilot Project–storing carbon dioxide in basalt. Energy Procedia 2011, 4, 5579–5585. [Google Scholar] [CrossRef]
- McGrail, B.P.; Schaef, H.T.; Ho, A.M.; Chien, Y.; Dooley, J.J.; Davidson, C.L. Potential for carbon dioxide sequestration in flood basalts. J. Geophys. Res. 2006, 111, B12201. [Google Scholar] [CrossRef]
- Matter, J.M.; Kelemen, P.B. Permanent storage of carbon dioxide in geological reservoirs by mineral carbonation. Nat. Geosci. 2009, 2, 837–841. [Google Scholar] [CrossRef]
- McGrail, B.; Spane, F.; Sullivan, E.; Bacon, D.; Hund, G. The Wallula basalt sequestration pilot project. Energy Procedia 2011, 4, 5653–5660. [Google Scholar] [CrossRef]
- McGrail, B.P.; Spane, F.A.; Amonette, J.E.; Thompson, C.; Brown, C.F. Injection and monitoring at the Wallula basalt pilot project. Energy Procedia 2014, 63, 2939–2948. [Google Scholar] [CrossRef]
- Schaef, H.T.; McGrail, B.P.; Owen, A.T. Carbonate mineralization of volcanic province basalts. Int. J. Greenh. Gas Control 2010, 4, 249–261. [Google Scholar] [CrossRef]
- McGrail, B.P.; Schaef, H.T.; Spane, F.A.; Horner, J.A.; Owen, A.T.; Cliff, J.B.; Qafoku, O.; Thompson, C.J.; Sullivan, E.C. Wallula basalt pilot demonstration project: Post-injection results and conclusions. Energy Procedia 2017, 114, 5783–5790. [Google Scholar] [CrossRef]
- Matter, J.M.; Takahashi, T.; Goldberg, D. Experimental evaluation of in situ CO2-water-rock reactions during CO2 injection in basaltic rocks: Implications for geological CO2 sequestration. Geochem. Geophys. Geosyst. 2007, 8, Q02001. [Google Scholar] [CrossRef]
- Burton, M.; Bryant, S.L. Eliminating buoyant migration of sequestered CO2 through surface dissolution: Implementation costs and technical challenges. SPE Reserv. Eval. Eng. 2009, 12, 399–407. [Google Scholar] [CrossRef]
- Steefel, C.I.; Lasaga, A.C. A coupled model for transport of multiple chemical species and kinetic precipitation/dissolution reactions with application to reactive flow in single phase hydrothermal systems. Am. J. Sci. 1994, 294, 529–592. [Google Scholar] [CrossRef]
- Saif, M.; Kiran, R.; Rajak, V.K.; Verma, R.K. Investigation of an Indian Site with Mafic Rock for Carbon Sequestration. ACS Omega 2024, 9, 30270–30280. [Google Scholar] [CrossRef]
- Milani, D.; McDonald, R.; Fawell, P.; Weldekidan, H.; Puxty, G.; Feron, P. Ex-situ mineral carbonation process challenges and technology enablers: A review from Australia’s perspective. Miner. Eng. 2025, 222, 109124. [Google Scholar] [CrossRef]
- Gysi, A.P.; Stefánsson, A. CO2-water–basalt interaction. Low temperature experiments and implications for CO2 sequestration into basalts. Geochim. Cosmochim. Acta 2012, 81, 129–152. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Gislason, S.R. 4. Carbon capture and storage (CCS). Geochem. Perspect. 2023, 12, 240–310. [Google Scholar]
- Luhmann, A.J.; Tutolo, B.M.; Tan, C.; Moskowitz, B.M.; Saar, M.O.; Seyfried, W.E. Whole rock basalt alteration from CO2-rich brine during flow-through experiments at 150 °C and 150 bar. Chem. Geol. 2017, 453, 92–110. [Google Scholar] [CrossRef]
- Voigt, M.; Marieni, C.; Baldermann, A.; Galeczka, I.M.; Wolff-Boenisch, D.; Oelkers, E.H.; Gislason, S.R. An experimental study of basalt–seawater–CO2 interaction at 130 °C. Geochim. Cosmochim. Acta 2021, 308, 21–41. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Declercq, J.; Saldi, G.D.; Gislason, S.R.; Schott, J. Olivine dissolution rates: A critical review. Chem. Geol. 2018, 500, 1–19. [Google Scholar] [CrossRef]
- Stockmann, G.J.; Wolff-Boenisch, D.; Gislason, S.R.; Oelkers, E.H. Do carbonate precipitates affect dissolution kinetics? 1: Basaltic glass. Chem. Geol. 2011, 284, 306–316. [Google Scholar] [CrossRef]
- Kelemen, P.B.; Matter, J.; Streit, E.E.; Rudge, J.F.; Curry, W.B.; Blusztajn, J. Rates and mechanisms of mineral carbonation in peridotite: Natural processes and recipes for enhanced, in situ CO2 capture and storage. Annu. Rev. Earth Planet. Sci. 2011, 39, 545–576. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y. Olivine dissolution in basaltic melt. Geochim. Cosmochim. Acta 2008, 72, 4756–4777. [Google Scholar] [CrossRef]
- Cao, X.; Li, Q.; Xu, L.; Tan, Y. A review of in situ carbon mineralization in basalt. J. Rock Mech. Geotech. Eng. 2024, 16, 1467–1485. [Google Scholar] [CrossRef]
- Clark, D.E.; Oelkers, E.H.; Gunnarsson, I.; Sigfússon, B.; Snæbjörnsdóttir, S.Ó.; Aradottir, E.S.; Gíslason, S.R. CarbFix2: CO2 and H2S mineralization during 3.5 years of continuous injection into basaltic rocks at more than 250 C. Geochim. Cosmochim. Acta 2020, 279, 45–66. [Google Scholar] [CrossRef]
- Hellevang, H.; Haile, B.G.; Tetteh, A. Experimental study to better understand factors affecting the CO2 mineral trapping potential of basalt. Greenh. Gases Sci. Technol. 2017, 7, 143–157. [Google Scholar] [CrossRef]
- Chen, L.; Liu, K.; Jiang, S.; Huang, H.; Tan, J.; Zuo, L. Effect of adsorbed phase density on the correction of methane excess adsorption to absolute adsorption in shale. Chem. Eng. J. 2021, 420, 127678. [Google Scholar] [CrossRef]
- Duan, Z.; Sun, R. An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. Chem. Geol. 2003, 193, 257–271. [Google Scholar] [CrossRef]
- Halldorsson, S.A.; Oskarsson, N.; Gronvold, K.; Sigurdsson, G.; Sverrisdottir, G.; Steinthorsson, S. Isotopic-heterogeneity of the Thjorsa lava—Implications for mantle sources and crustal processes within the Eastern Rift Zone, Iceland. Chem. Geol. 2008, 255, 305–316. [Google Scholar] [CrossRef]
- Guy, C. Mechanisms of Dissolution of Solids in Hydrothermal Solutions Inferred from the Behavior of Basaltic Glasses and Deformed Calcites. Ph.D. Thesis, Paul Sabatier University, Toulouse, France, 1989. [Google Scholar]
- Berger, G.; Clarapols, C.; Guy, C.; Daux, V. Dissolution rate of basaltic glass in silica-rich solutions: Implications for long term alteration. Geochim. Cosmochim. Acta 1994, 58, 4875–4886. [Google Scholar] [CrossRef]
- Daux, V.; Guy, C.; Advocat, T.; Crovisier, J.; Stille, M. Kinetic Aspects of basaltic glass dissolution at 90 °C: Role of silicon and aluminum. Chem. Geol. 1997, 142, 109–128. [Google Scholar] [CrossRef]
- Carroll, K.C.; Tsai, C.-H.; Rucker, D.F.; Brooks, S.C.; Ginn, T.R. Transient Storage Model Parameter Optimization Using the Simulated Annealing Method. In Proceedings of the AGU Fall Meeting Abstracts, Chicago, IL, USA and Online, 12–16 December 2022; p. H42D–1267. [Google Scholar]
- Morse, J.W.; Arvidson, R.S. The dissolution kinetics of major sedimentary carbonate minerals. Earth-Sci. Rev. 2002, 58, 51–84. [Google Scholar] [CrossRef]
- Gadikota, G.; Matter, J.; Kelemen, P.; Brady, P.V.; Park, A.-H.A. Elucidating the differences in the carbon mineralization behaviors of calcium and magnesium bearing alumino-silicates and magnesium silicates for CO2 storage. Fuel 2020, 277, 117900. [Google Scholar] [CrossRef]
- Otabir, P.N.; Khanal, A.; Nath, F. Geochemical Impacts of CO2 Mineralization in Carbonate and Basalt Formations: A Critical Review on Challenges and Future Outlook. Energy Fuels 2025, 39, 1226–1251. [Google Scholar] [CrossRef]
- Nooraiepour, M.; Polański, K.; Masoudi, M.; Kuczyński, S.; Derluyn, H.; Nogueira, L.P.; Bohloli, B.; Nagy, S.; Hellevang, H. Potential for 50% Mechanical Strength Decline in Sandstone Reservoirs Due to Salt Precipitation and CO2–Brine Interactions During Carbon Sequestration. Rock Mech. Rock Eng. 2025, 58, 1239–1269. [Google Scholar] [CrossRef]
- Lu, P.; Apps, J.; Zhang, G.; Gysi, A.; Zhu, C. Knowledge gaps and research needs for modeling CO2 mineralization in the basalt-CO2-water system: A review of laboratory experiments. Earth-Sci. Rev. 2024, 254, 104813. [Google Scholar] [CrossRef]
- Holdsworth, C.; John, C.M.; Snæbjörnsdóttir, S.Ó.; Johnson, G.; Sigfússon, B.; Leslie, R.; Haszeldine, R.S.; Gilfillan, S. Reconstructing the temperature and origin of CO2 mineralisation in CarbFix calcite using clumped, carbon and oxygen isotopes. Appl. Geochem. 2024, 162, 105925. [Google Scholar] [CrossRef]
- Balch, R.; McPherson, B.; Cather, M.; Esser, R. The Carbon Utilization and Storage Partnership of the Western United States. In Proceedings of the 16th Greenhouse Gas Control Technologies Conference (GHGT-16), Lyon, France, 23–27 October 2022; pp. 23–24. [Google Scholar]
- Krishna, A.; Shenoy, R.; Jha, B.; Liu, Z.; Paul, D.L.; Ershaghi, I. Repurposing idle oil and gas wells for large-scale subsurface energy storage in saline aquifers. In Proceedings of the SPE Western Regional Meeting, Bakersfield, CA, USA, 26–28 April 2022; p. D011S005R005. [Google Scholar]
- Manjunath, G.; Liu, Z.; Jha, B. Multi-stage hydraulic fracture monitoring at the lab scale. Eng. Fract. Mech. 2023, 289, 109448. [Google Scholar] [CrossRef]



| Project | Location | CO2 Source | Time Period | CO2 Injection Rate (t/yr) | Summary |
|---|---|---|---|---|---|
| CarbFix Phase I | Iceland | Geothermal power plant | 2012–2016 | ~200 t/yr | Pilot-scale injection of CO2 dissolved in water into basalt; ~95% mineralized into calcite within 2 years at 400–800 m depth. |
| CarbFix Phase II | Iceland | CO2 and H2S from Hellisheiði plant | 2014–present | ~12,000 t/yr CO2 + 5000 t/yr H2S | Full-scale operation with co-injection of gases dissolved in water; rapid in situ mineralization; supports the Coda Terminal export project. |
| Wallula Basalt Pilot | Washington State, United States | supercritical CO2 | 2009–2013 | ~977 t total (≈326 t/yr equivalent) | CO2 injected into Columbia River Basalt Group at 828–875 m; ankerite formation confirmed; ~60–65% mineralized within 2 years. |
| Basalt Screening—China | China | Hypothetical; modeled scenarios | Not yet implemented | Simulated: 607.8–1121.4 Gt total capacity | Nationwide study estimated 46,948 Gt theoretical capacity; recommended carbonated water injection due to improved mineralization efficiency. |
| CSIRO Basalt Mineralization Toolkit | Australia | Industrial CO2 (planned) | Ongoing R&D | Not yet injected | Focused on in situ mineralization site selection and ex situ carbonation of ultramafic waste; explored integration with DAC. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owusu, E.A.; Wu, J.; Appiah, E.A.; Marfo, W.A.; Yuan, N.; Ge, X.; Ling, K.; Wang, S. Carbon Mineralization in Basaltic Rocks: Mechanisms, Applications, and Prospects for Permanent CO2 Sequestration. Energies 2025, 18, 3489. https://doi.org/10.3390/en18133489
Owusu EA, Wu J, Appiah EA, Marfo WA, Yuan N, Ge X, Ling K, Wang S. Carbon Mineralization in Basaltic Rocks: Mechanisms, Applications, and Prospects for Permanent CO2 Sequestration. Energies. 2025; 18(13):3489. https://doi.org/10.3390/en18133489
Chicago/Turabian StyleOwusu, Ernest Ansah, Jiyue Wu, Elizabeth Akonobea Appiah, William Apau Marfo, Na Yuan, Xiaojing Ge, Kegang Ling, and Sai Wang. 2025. "Carbon Mineralization in Basaltic Rocks: Mechanisms, Applications, and Prospects for Permanent CO2 Sequestration" Energies 18, no. 13: 3489. https://doi.org/10.3390/en18133489
APA StyleOwusu, E. A., Wu, J., Appiah, E. A., Marfo, W. A., Yuan, N., Ge, X., Ling, K., & Wang, S. (2025). Carbon Mineralization in Basaltic Rocks: Mechanisms, Applications, and Prospects for Permanent CO2 Sequestration. Energies, 18(13), 3489. https://doi.org/10.3390/en18133489







