Study of Three-Component Fe2O3/TiO2/rGO Nanocomposite Thin Films Anode for Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Suspensions and Electrophoretic Deposition
2.2. Material Characterization
2.3. Electrochemical Characterization
3. Results and Discussion
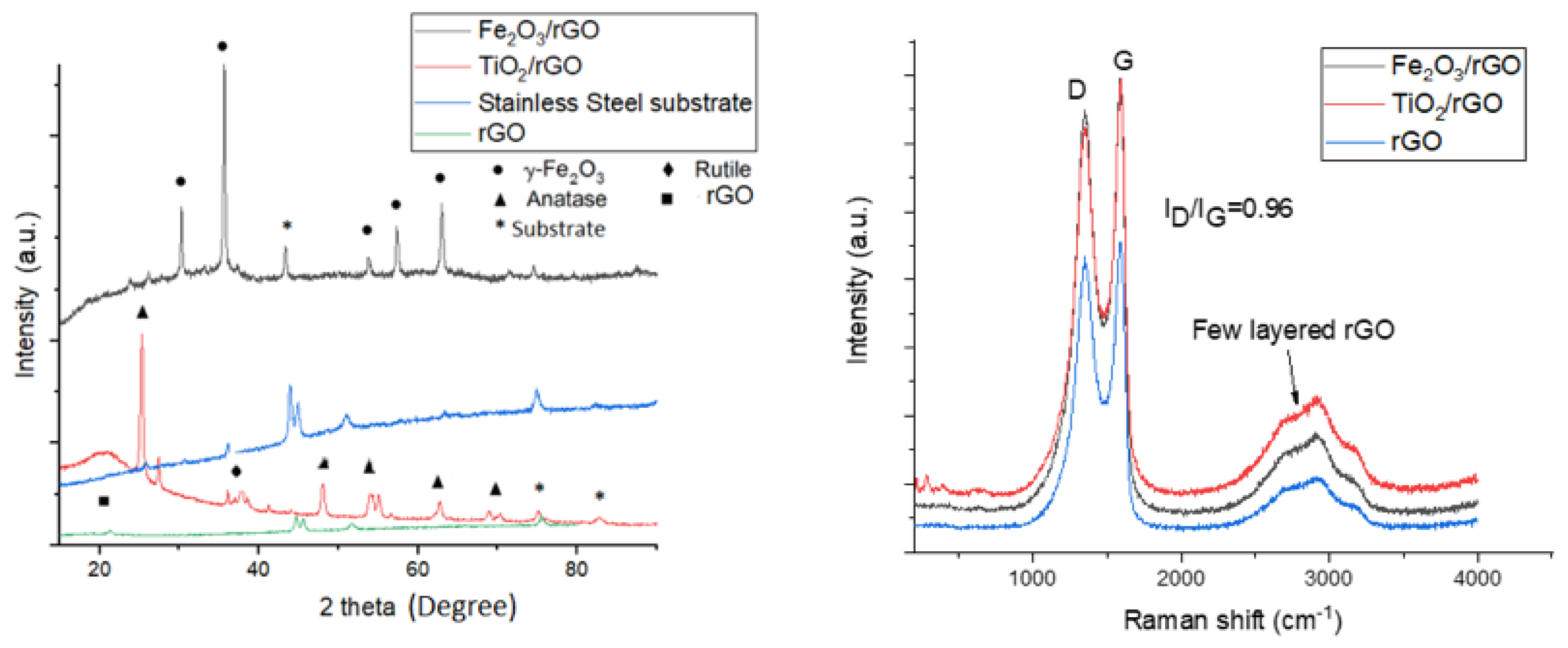
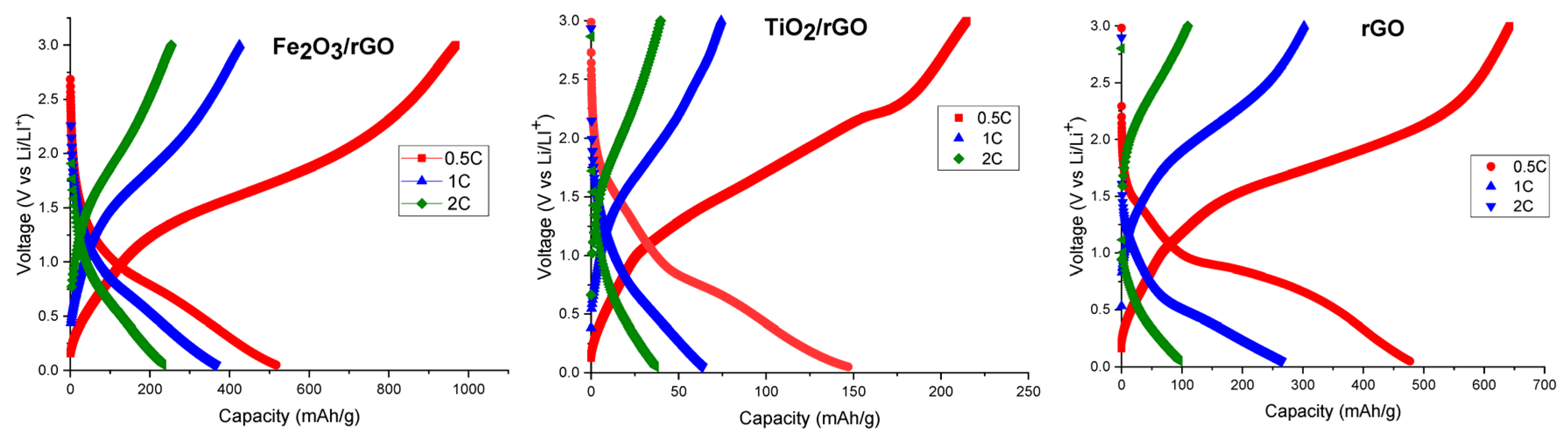
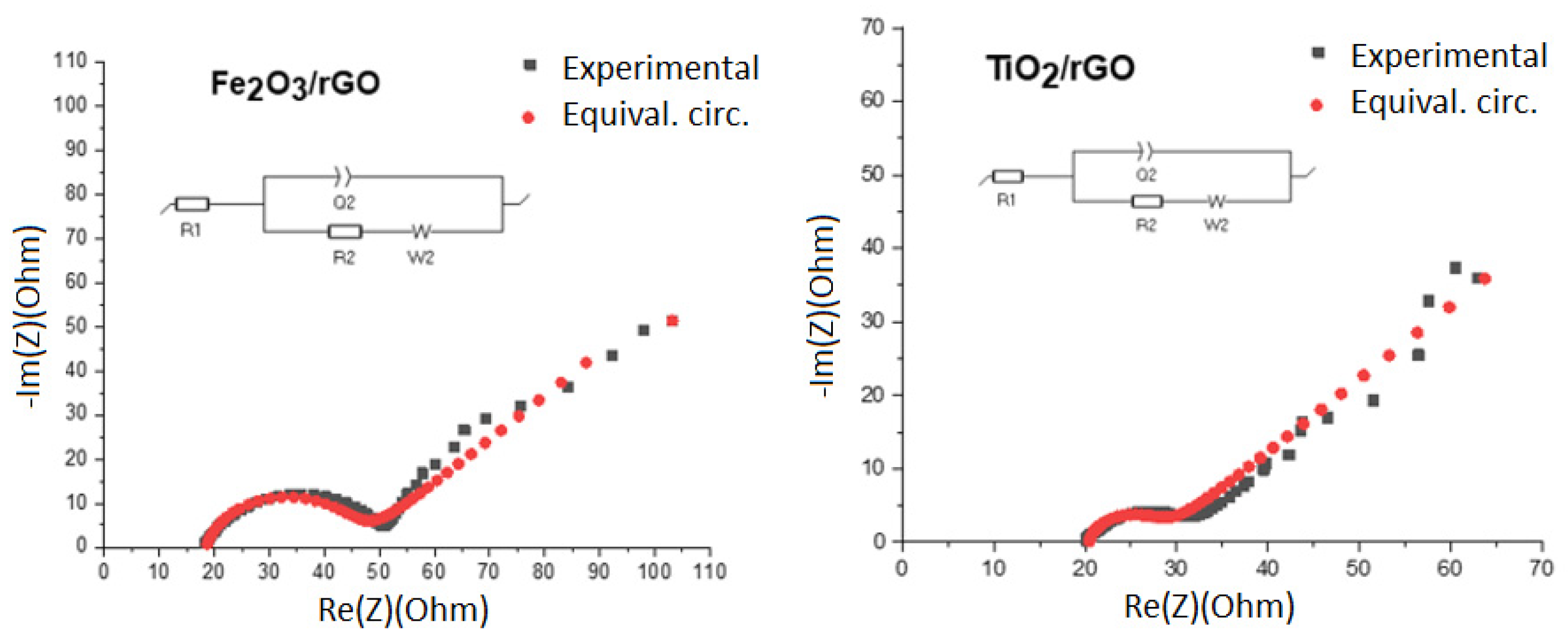
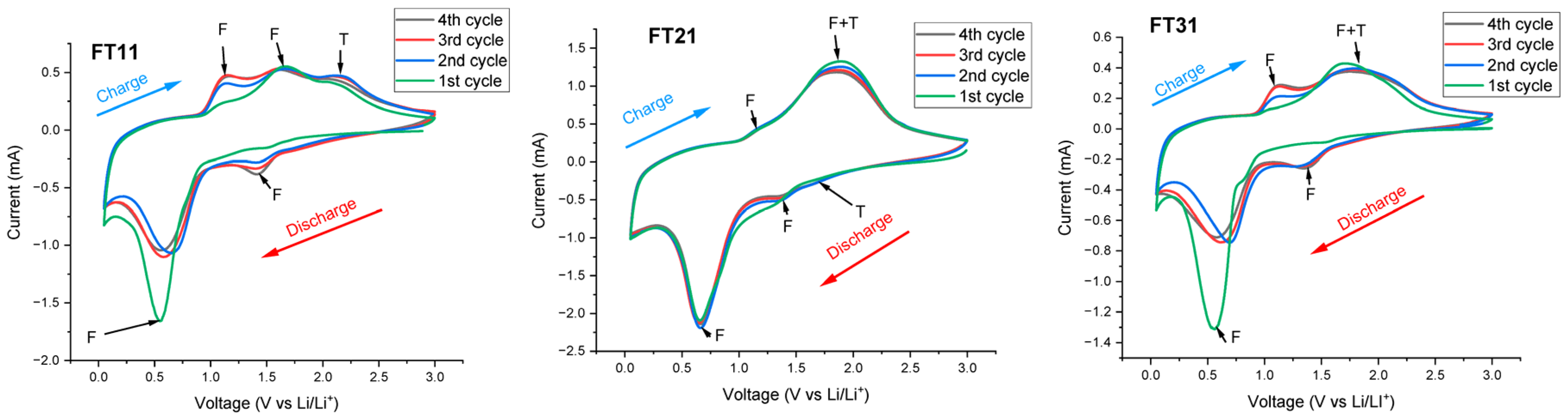
3.1. Study of Electrophoretically Deposited Iron Oxide, Titanium Dioxide, and Reduced Graphene Oxide
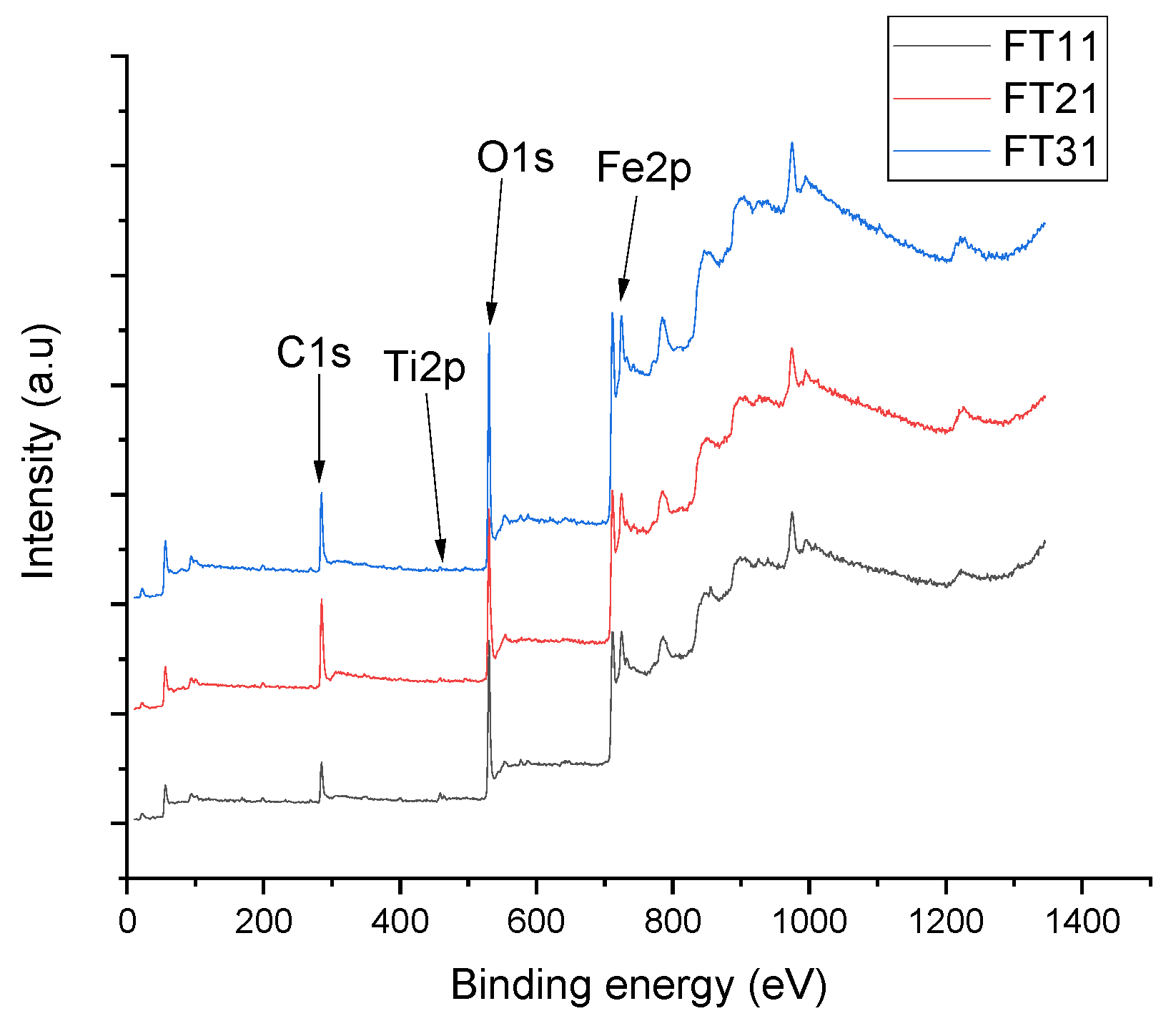
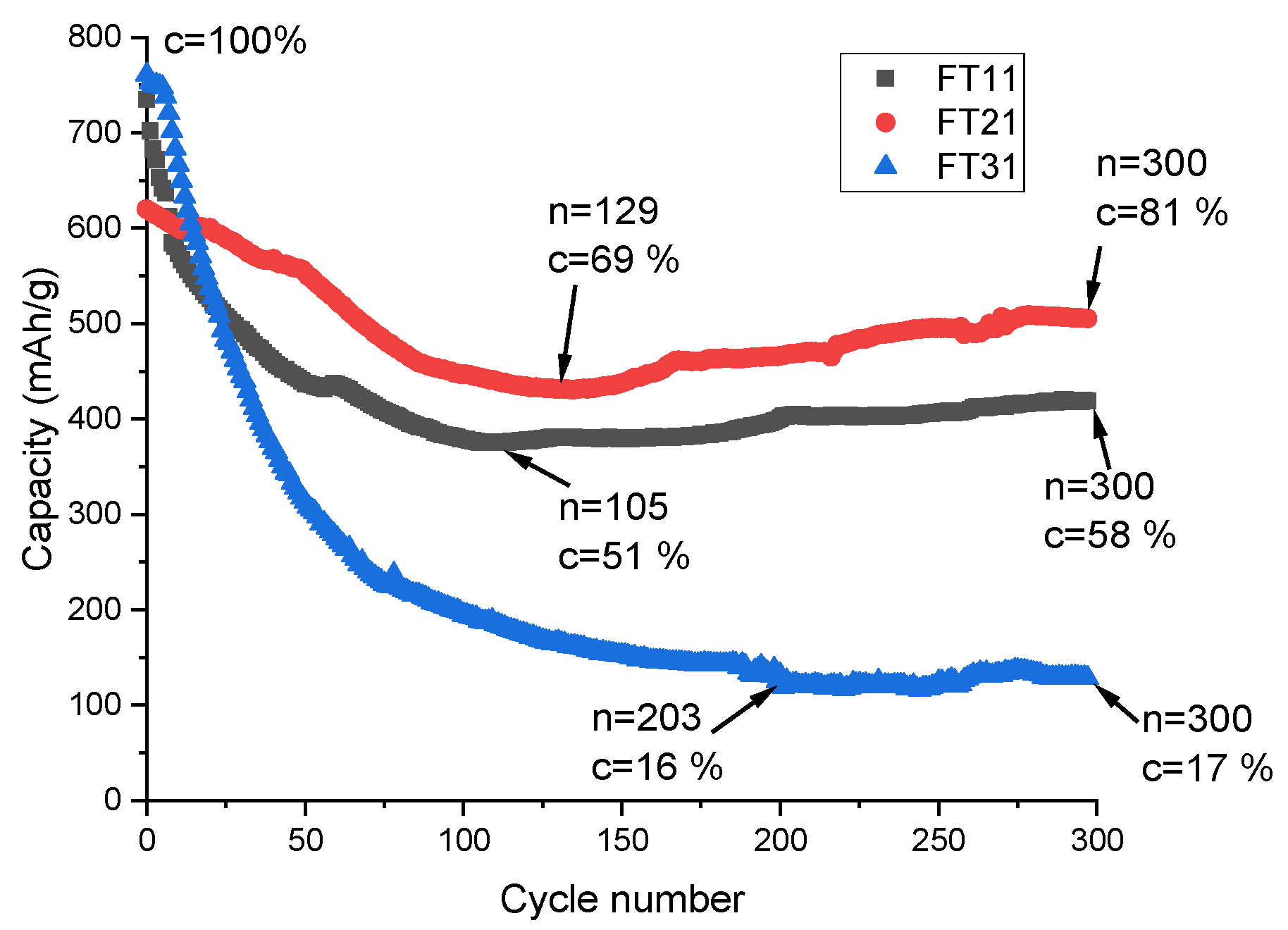
3.2. Study of Different Quantitative Compositions of Iron Oxide and Titanium Dioxide Electrode Materials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qi, W.; Shapter, J.G.; Wu, Q.; Yin, T.; Gao, G.; Cui, D. Nanostructured Anode Materials for Lithium-Ion Batteries: Principle, Recent Progress and Future Perspectives. J. Mater. Chem. A 2017, 5, 19521–19540. [Google Scholar] [CrossRef]
- Smith, J.; Zhao, L. Iron Oxide-Based Composites for Energy Storage. Electrochim. Acta 2017, 231, 247–254. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Baker-Fales, M.; Amama, P.B. 3D Graphene-Based Anode Materials for Li-Ion Batteries. Curr. Opin. Chem. Eng. 2016, 13, 124–132. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, A. Novel Fe2O3 Anodes for Lithium Batteries. J. Power Sources 2011, 196, 6425. [Google Scholar] [CrossRef]
- Mishra, A.K.; Monika; Patial, B.S. A Review on Recent Advances in Anode Materials in Lithium Ion Batteries. Mater. Today Proc. 2024, 7, 100089. [Google Scholar] [CrossRef]
- Khan, B.M.; Oh, W.C.; Ullah, K. Development of Nanocomposites in Lithium-Ion Batteries. In Nanocomposites—Advanced Materials for Energy and Environmental Aspects; Khan, M.E., Aslam, J., Verma, C., Eds.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Cambridge, UK, 2023; pp. 189–208. [Google Scholar] [CrossRef]
- Kurc, B.; Siwińska-Stefańska, K.; Jakóbczyk, P.; Jesionowski, T. Titanium Dioxide/Graphene Oxide Composite and Its Application as an Anode Material in Non-Flammable Electrolyte Based on Ionic Liquid and Sulfolane. J. Solid State Electrochem. 2016, 20, 1971–1981. [Google Scholar] [CrossRef]
- Shchegolkov, A.V.; Lipkin, M.S.; Shchegolkov, A.V. Preparation of WO3 Films on Titanium and Graphite Foil for Fuel Cell and Supercapacitor Applications by Electrochemical (Cathodic) Deposition Method. Russ. J. Gen. Chem. 2022, 92, 1161–1167. [Google Scholar] [CrossRef]
- Madian, M.; Eychmüller, A.; Giebeler, L. Current Advances in TiO2-Based Nanostructure Electrodes for High Performance Lithium Ion Batteries. Batteries 2018, 4, 7. [Google Scholar] [CrossRef]
- Perreault, L.L.; Colo, F.; Meligrana, G.; Kim, K.; Fiorilli, S.; Bella, F.; Nair, J.R.; Vitale-Brovarone, C.; Florek, J.; Kleitz, F.; et al. Spray-Dried Mesoporous Mixed Cu-Ni Oxide@Graphene Nanocomposite Microspheres for High Power and Durable Li-Ion Battery Anodes. Adv. Energy Mater. 2018, 8, 1802438. [Google Scholar] [CrossRef]
- Ulusoy Ghobadi, T.G.; Kunduraci, M.; Yilmaz, E. Improved Lithium-Ion Battery Anode Performance via Multiple Element Approach. J. Alloys Compd. 2018, 730, 96–102. [Google Scholar] [CrossRef]
- Kucinskis, G.; Bajars, G.; Kleperis, J. Graphene in Lithium Ion Battery Cathode Materials: A Review. J. Power Sources 2013, 240, 66–79. [Google Scholar] [CrossRef]
- Yu, S.; Guo, B.; Zeng, T.; Qu, H.; Yang, J.; Bai, J. Graphene-Based Lithium-Ion Battery Anode Materials Manufactured by Mechanochemical Ball Milling Process: A Review and Perspective. Compos. Part B Eng. 2022, 246, 110232. [Google Scholar] [CrossRef]
- Wang, D.; Choi, D.; Li, J.; Yang, Z.; Nie, Z.; Kou, R.; Hu, D.; Wang, C.; Saraf, L.V.; Zhang, J.; et al. Self-assembled TiO2–graphene hybrid nanostructures for enhanced Li-ion insertion. ACS Nano 2009, 3, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.B.; Chen, J.S.; Hng, H.H.; Lou, X.W. Nanostructured metal oxide-based materials as advanced anodes for lithium-ion batteries. Nanoscale 2012, 4, 2526–2542. [Google Scholar] [CrossRef]
- Kaprans, K.; Mateuss, J.; Dorondo, A.; Bajars, G.; Kucinskis, G.; Lesnicenoks, P.; Kleperis, J. Electrophoretically Deposited α-Fe2O3 and TiO2 Composite Anchored on rGO with Excellent Cycle Performance as Anode for Lithium Ion Batteries. Solid State Ionics 2018, 319, 1–6. [Google Scholar] [CrossRef]
- Wu, H.; Cui, Y. Designing Nanostructured Si Anodes for High Energy Lithium Ion Batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, B.; Pan, A.; Liang, S.; Tang, Y.; Wu, X. Facile Synthesis of Fe2O3/TiO2/rGO Composite with Enhanced Lithium Storage Performance. J. Alloys Compd. 2017, 723, 1006–1013. [Google Scholar] [CrossRef]
- Guo, S.; Wang, Y.; Chen, L.; Pan, D.; Guo, Z.; Xia, S. Porous TiO2–FeTiO3@Carbon Nanocomposites as Anode for High-Performance Lithium-Ion Batteries. J. Alloys Compd. 2021, 858, 157635. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium Batteries: Status, Prospects and Future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the Development of Advanced Li-Ion Batteries: A Review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Qi, C.; Liu, C.; Meng, K.; Yu, X.; Zhang, Y.; Liu, J.; Jin, X.; Jin, L. Enhanced Performance of TiO2/α-Fe2O3 Nanostructure as Anode Material for Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2018, 13, 265–274. [Google Scholar] [CrossRef]
- Li, H.; Richter, G.; Maier, J. Reversible Formation and Decomposition of LiF Clusters Using Transition Metal Fluorides as Precursors and Their Application in Rechargeable Li Batteries. Adv. Mater. 2003, 15, 736–739. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, F.; Zhao, Y.; Ding, M.; Wang, J.; Zheng, X.; Wang, H.; Record, M.-C.; Boulet, P. Lychee-like TiO2@Fe2O3 Core-Shell Nanostructures with Improved Lithium Storage Properties as Anode Materials for Lithium-Ion Batteries. Materials 2023, 16, 1945. [Google Scholar] [CrossRef]
- Li, W.; Yang, Z.; Wang, J.; Wang, Y.; Wang, J.; Li, X.; Wang, Y. A Review on Recent Progress of Nanostructured Anode Materials for Lithium-Ion Batteries. J. Mater. Sci. Technol. 2022, 99, 9–17. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.B.; Lou, X.W. Iron-Oxide-Based Advanced Anode Materials for Lithium-Ion Batteries. Adv. Energy Mater. 2014, 4, 1300958. [Google Scholar] [CrossRef]
- Xia, H.; Xiong, W.; Lim, C.K.; Yao, Q.; Wang, Y.; Xie, J. Hierarchical TiO2-B Nanowire@α-Fe2O3 Nanothorn Core-Branch Arrays as Superior Electrodes for Lithium-Ion Microbatteries. Nano Res. 2014, 7, 1797–1808. [Google Scholar] [CrossRef]
- Zuniga, L.; Gonzalez, G.; Orrostieta Chavez, R.; Myers, J.C.; Lodge, T.P.; Alcoutlabi, M. Centrifugally Spun α-Fe2O3/TiO2/Carbon Composite Fibers as Anode Materials for Lithium-Ion Batteries. Appl. Sci. 2019, 9, 4032. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Laruelle, S.; Grugeon, S.; Poizot, P.; Dolle, M.; Dupont, L.; Tarascon, J.M. On the Origin of the Extra Electrochemical Capacity Displayed by MO/Li Cells at Low Potential. J. Electrochem. Soc. 2002, 149, A627–A634. [Google Scholar] [CrossRef]
- Nguyen, T.-A.; Halim, M.; Lee, J.K.; Lee, S.-W. Facile Synthesis of Nanoporous Fe2O3 with Internal Nanocavities for Highly Reversible Lithium Storage. Acta Mater. 2017, 140, 290–299. [Google Scholar] [CrossRef]
- Beaulieu, L.Y.; Larcher, D.; Dunlap, R.A.; Dahn, J.R. Reaction of Li with Grain-Boundary Atoms in Nanostructured Compounds. J. Electrochem. Soc. 2000, 147, 3206–3212. [Google Scholar] [CrossRef]
- Balaya, P.; Li, H.; Kienle, L.; Maier, J. Fully Reversible Homogeneous and Heterogeneous Li Storage in RuO2 with High Capacity. Adv. Funct. Mater. 2003, 13, 621–625. [Google Scholar] [CrossRef]
- Li, H.; Balaya, P.; Maier, J. Li-Storage via Heterogeneous Reaction in Selected Binary Metal Fluorides and Oxides. J. Electrochem. Soc. 2004, 151, A1878–A1885. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B. Hierarchical Nanostructures for High-Performance Lithium-Ion Batteries. Adv. Energy Mater. 2013, 3, 737–743. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, Q.; Tian, J.; Jiang, F. TiO2 Nanobelt@Co9S8 Composites as Promising Anode Materials for Lithium and Sodium Ion Batteries. Nanomaterials 2017, 7, 252. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Huang, J. Three-Dimensional TiO2 Nanotubes Immobilized with Fe2O3 Nanoparticles as an Anode Material for Lithium-Ion Batteries. J. Alloys Compd. 2019, 783, 793–800. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, W.; Zhang, X.; Wang, C.; Yuan, Y.; Huang, Y.; Ye, Y.; Qiu, Z.; Tang, Y. A Review on FexOy-Based Materials for Advanced Lithium-Ion Batteries. Renew. Sustain. Energy Rev. 2020, 127, 109884. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The Chemistry of Graphene Oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Li, W.; Gou, X. α-Fe2O3 Nanotubes as a Chemically Stable and Comb-Like Host for Lithium Storage with Superior Rate Capability and Long Cycling Life. Electrochim. Acta 2016, 211, 902–910. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Wang, T.; Chen, Y.; Zhao, X. Improving Effect of Graphene on Electrochemical Properties of Fe2O3 Anode Materials. Metals 2022, 12, 593. [Google Scholar] [CrossRef]
- Zhang, S. Chemomechanical modeling of lithiation-induced failure in high-volume-change electrode materials for lithium ion batteries. npj Comput. Mater. 2017, 3, 7. [Google Scholar] [CrossRef]
- Liang, M.; Zou, J.; Zeng, X.; Ding, W. Nanostructured Fe2O3-Based Composites Prepared through Arc Plasma Method as Anode Materials in the Lithium-Ion Battery. J. Nanomater. 2016, 2016, 1207907. [Google Scholar] [CrossRef]
- Powell, C.J.; Jablonski, A. Surface Sensitivity of X-ray Photoelectron Spectroscopy. Nucl. Instrum. Methods Phys. Res. Sect. A 2009, 601, 54–65. [Google Scholar] [CrossRef]
- Guo, S.; Wang, S.; Wu, N.; Liu, J.; Nic, Y.; Liu, W. Facile Synthesis of Porous Fe2TiO5 Microparticulates Serving as Anode Material with Enhanced Electrochemical Performances. RSC Adv. 2015, 5, 103767–103775. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.; Cao, M.; Ju, Q.; Wang, H.; Fu, R.; Ji, H.; Yang, G. Enhanced Electrochemical Performance of α-Fe2O3 Grains Grafted onto TiO2-Carbon Nanofibers via a Vapor-Solid Reaction as Anode Materials for Li-Ion Batteries. Appl. Surf. Sci. 2019, 463, 322–330. [Google Scholar] [CrossRef]
- Zhao, W.; Fei, P.; Zhang, X.; Zhang, Y.; Qin, C.; Wang, Z. Porous TiO2/Fe2O3 Nanoplate Composites Prepared by De-Alloying Method for Li-Ion Batteries. Mater. Lett. 2018, 211, 254–257. [Google Scholar] [CrossRef]
- Zhong, Y.; Ma, Y.; Guo, Q.; Liu, J.; Wang, Y.; Yang, M.; Xia, H. Controllable Synthesis of TiO2@Fe2O3 Core-Shell Nanotube Arrays with Double-Wall Coating as Superb Lithium-Ion Battery Anodes. Sci. Rep. 2017, 7, 40927. [Google Scholar] [CrossRef]
- Kaprans, K.; Bajars, G.; Kleperis, J. Electrophoretic Nanocrystalline Graphene Film Electrode for Lithium Ion Battery. IOP Conf. Ser. Mater. Sci. Eng. 2015, 77, 012042. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J. Definitions of Pseudocapacitive Materials: A Brief Review. Energy Environ. Mater. 2019, 2, 30–37. [Google Scholar] [CrossRef]
- Redel, K.; Kulka, A.; Walczak, K.; Plewa, A.; Hanc, E.; Marzec, M.; Lu, L.; Molenda, J. Origin of Extra Capacity in Advanced Li–Rich Cathode Materials for Rechargeable Li–Ion Batteries. Chem. Eng. J. 2021, 424, 130293. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Zhitomirsky, I. Application of electrophoretic and electrolytic deposition techniques in ceramics processing. Curr. Opin. Solid State Mater. Sci. 2010, 14, 205–212. [Google Scholar] [CrossRef]
- Wang, X.; Wang, F.; Chen, Y.; Liu, J. A review on electrophoretic deposition for organic/inorganic nanocomposite coatings: Fundamentals, applications, and perspectives. Adv. Colloid Interface Sci. 2015, 221, 25–43. [Google Scholar] [CrossRef]
- Yin, Y.-J.; Hu, F.; Cheng, L.-H.; Wang, X.-D. Electrophoretic Deposition of Hybrid Organic-Inorganic PTFE/Al/CuO Energetic Film. Defence Technol. 2023, 22, 112–118. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Guo, Y.; Zhang, H.; Wang, Y.; Wang, Z.; Liu, H.; Wang, Y. Reduced Graphene Oxide as a Conductive Additive and Structural Buffer for High-Performance Lithium-Ion Battery Electrodes. Materials 2020, 13, 218. [Google Scholar] [CrossRef]







| Anode Material | Theoretical Capacity (mAh/g) | Advantages | Disadvantages |
|---|---|---|---|
| Graphite [20] | 372 | Commercial standard; high cycle life; stable SEI formation | Low capacity; limited rate performance |
| Silicon (Si) [21] | 4200 | Extremely high capacity; abundant | Severe volume expansion (~300%); poor cycle stability |
| Fe2O3 [18] | 1007 | High capacity; environmentally friendly; low cost | Large volume change; poor electronic conductivity |
| TiO2 (anatase) [22] | 335 | Safe; stable structure; fast Li+ kinetics | Low capacity; needs nanostructuring |
| SnO2 [23] | 790 | High capacity; good conductivity | Volume change; aggregation; unstable SEI |
| rGO [22] | ~500 | High surface area; good electrical conductivity | Needs combination with active material; restacking tendency |
| Fe2O3/TiO2/rGO [24] | Estimated ~600–800 | Synergistic effects; improved conductivity and stability | Requires careful optimization; complex interface |
| Sample | Rs, (Ω) | Rct, (Ω) | CPE-T (F) | CPE-P | W (Ω·s−1/2) | χ2 |
|---|---|---|---|---|---|---|
| Fe2O3/rGO | 12 | 38 | 3.0 × 10−5 | 0.85 | 20 | 1.2 × 10−3 |
| TiO2/rGO | 18 | 15 | 2.0 × 10−5 | 0.85 | 15 | 1.0 × 10−3 |
| Nanocomposite Material | Average Crystallite Size, nm | Chemical Element Content Determined by EDX, wt% | Chemical Element Content Determined by XPS, wt% | Quantities of Material Phases Calculated from XRD Data by the Rietveld Method, wt% | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Ti | O | Fe | Ti | O | C | Fe2O3 (maghemite) | TiO2 (anatase) | TiO2 (rutile) | ||
| FT11 | 37 ± 2 | 67 | 4 | 29 | 62 | 30 | 6 | 2 | 55 ± 0.01 | 35 ± 0.01 | 10 ± 0.01 |
| FT21 | 24 ± 2 | 72 | 5 | 23 | 71 | 23 | 3 | 3 | 79 ± 0.02 | 14 ± 0.01 | 7 ± 0.02 |
| FT31 | 32 ± 2 | 76 | 7 | 17 | 80 | 13 | 2 | 5 | 88 ± 0.02 | 9 ± 0.01 | 3 ± 0.01 |
| Synthesis | Rate Capability | Cycle Performance | |
|---|---|---|---|
| TiO2/Fe2O3/C [47] | Thermal alloying | Charge capacity of 558 mAhg−1 at a discharge current of 0.5C (500 mAg−1) | Charge capacity of 438 mAhg−1 after 400 charge/discharge cycles |
| TiO2/Fe2O3/CNT [46] | Hydrothermal synthesis | Charge capacity of 487 mAhg−1 at a discharge current of 0.5C (500 mAg−1) | Charge capacity of 436 mAhg−1 after 600 charge/discharge cycles |
| TiO2/Fe2O3/CNF [46] | Chemical synthesis | Charge capacity of 592 mAhg−1 at a discharge current of 500 mAg−1 | Charge capacity of 576 mAhg−1 after 200 charge/discharge cycles |
| TiO2/Fe2O3/C [19] | Atomic layer deposition | Charge capacity of 525 mAhg−1 at a discharge current of 400 mAg−1 | Charge capacity of 591 mAhg−1 after 200 charge/discharge cycles |
| TiO2/Fe2O3/rGO [this work] | Electrophoretic deposition | Charge capacity of 683 mAhg−1 at a discharge current of 0.5C (500 mAg−1) | Charge capacity of 508 mAhg−1 after 300 charge/discharge cycles |
| Cyclic Voltammetry, D, cm/s2 | Electrochemical Impedance Spectroscopy, D, cm2/s × 10−16 | |
|---|---|---|
| Fe2O3/rGO | 1.6 × 10−13 | 1 |
| TiO2/rGO | 1 × 10−14 | 5 |
| FT11 | 6 × 10−13 | 7 |
| FT21 | 1.2 × 10−12 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaprans, K.; Bajars, G.; Kucinskis, G. Study of Three-Component Fe2O3/TiO2/rGO Nanocomposite Thin Films Anode for Lithium-Ion Batteries. Energies 2025, 18, 3490. https://doi.org/10.3390/en18133490
Kaprans K, Bajars G, Kucinskis G. Study of Three-Component Fe2O3/TiO2/rGO Nanocomposite Thin Films Anode for Lithium-Ion Batteries. Energies. 2025; 18(13):3490. https://doi.org/10.3390/en18133490
Chicago/Turabian StyleKaprans, Kaspars, Gunars Bajars, and Gints Kucinskis. 2025. "Study of Three-Component Fe2O3/TiO2/rGO Nanocomposite Thin Films Anode for Lithium-Ion Batteries" Energies 18, no. 13: 3490. https://doi.org/10.3390/en18133490
APA StyleKaprans, K., Bajars, G., & Kucinskis, G. (2025). Study of Three-Component Fe2O3/TiO2/rGO Nanocomposite Thin Films Anode for Lithium-Ion Batteries. Energies, 18(13), 3490. https://doi.org/10.3390/en18133490











