1. Introduction
Biomass is renewable and sustainable in nature; therefore, research on biomass conversion has been gaining a lot of interest recently. Most review papers on biomass conversion are devoted to thermochemical processes, like torrefaction, pyrolysis, liquefaction, gasification, and combustion [
1,
2,
3,
4], or the combined thermo- and biochemical treatment of biomass [
5,
6,
7,
8]. These articles cite original research on the subject. However, I believe that it is impossible to gain the necessary knowledge in this area by reading even these review articles, especially since they mostly concern thermochemical processes of biomass conversion and omit or only touch on the newest achievements in the field of biotechnology. Therefore, I have decided to write an article providing a review of the latest developments in biomass conversion, excluding thermochemical processes. It is well known that, in addition to thermochemical processes, which require higher energy input, biochemical methods of LCB processing play an important role in the production of renewable fuels and value-added products. Biochemical conversion involves the use of enzymes and microorganisms as catalysts in the breakdown of biomass into biofuels. The main biochemical conversion routes are anaerobic digestion (AD), fermentation, photobiological hydrogen production, and bioelectrochemical processes that take place in microbial electrolysis cells (MEC). The biochemical processes are less efficient at breaking down recalcitrant biomass materials and take a long time to complete. For this reason, a combination of thermochemical and biochemical routes may be promising, taking advantage of both methods for the processing of biofuels. However, due to limited space in this review, we will be limited to a description of the biochemical processes involved in biorefinery production.
The four main routes for biochemical conversion of LCB are considered in this review:
Digestion (anaerobic);
Fermentation (syngas, ethanol, butanol, and dark fermentation);
Photobiological hydrogen production;
Bioelectrochemical processes in MEC.
This review aims to provide a basic understanding of the potential routes to complete biomass conversion in the most important bioprocesses. It highlights recent developments in well-known bioprocesses, such as anaerobic digestion and alcoholic fermentation, as well as in newly explored processes, such as bioelectrochemical fermentation, and considers their future prospects. The review focuses mainly on the last 5 years of literature on the subject, in order to encourage collaboration between researchers and industry stakeholders for the development and successful implementation of biomass resources, and to encourage research efforts to focus on the biochemical engineering and the modern biotechnology of biomass conversion processes, as thermochemical processes are much better understood and developed.
2. Biochemical Characterisation of Biomass
Biomass is considered a renewable energy source due to the manner in which it is extracted. It consists of various chemical compounds, including cellulose, hemicellulose, lignin, fats, starches, water-soluble sugars, and other substances. Higher-molecular-weight polysaccharides account for around 70% of all biomass components and, together with lignin, form its lignocellulosic structure.
Cellulose, an unbranched polysaccharide built linearly from 3000–14,000 D-glucose molecules linked by β-1,4-glycosidic bonds, is the world’s most widespread biopolymer. β-bonding contributes to the formation of stiff, long strands that stack in parallel. These strands are further stabilised by hydrogen bonds. Cellulose is the major crystalline compound in biomass, accounting for about 50% of its content. The crystalline structure of cellulose fibres makes lignocellulose highly resistant to attack by hydrolytic enzymes, whereas amorphous cellulose microfibres are more susceptible to enzymatic degradation.
Hemicelluloses are a heterogeneous group of polysaccharides that are linear or branched heteropolymers. They are linked by β-glycosidic bonds to form branched chains. They are one of the main components of plant cell walls and can account for around 20% of their dry weight. Unlike cellulose, hemicellulose macromolecules are composed not only of glucose residues but also of other C6 monosaccharides such as mannose and galactose; C5 sugars such as xylose and arabinose; and uronic acid molecules.
Hemicelluloses are plant-derived branched heteropolymeric amorphous structures, mostly bound to cellulose with pectin and linked to lignin. Hemicelluloses have a lower degree of polymerisation and structure order than cellulose, making them less resistant to degradation. All hemicelluloses are characterised by their good solubility in dilute alkalis.
Lignin is a complex, amorphous, heterogeneous organic polymer found in all green plant cell walls, bark and wood. It contains approximately 21–30% non-fossil organic carbon. It is one of the most important components of wood and other plant fibres. It plays a vital role in maintaining the structure of plant cells and provides a barrier to hydrolysis and delignification [
9]. Lignin is a polymer formed from coniferyl alcohol and other phenylpropanoid compounds derived from the aromatic amino acids phenylalanine and tyrosine. The lignin surface adsorbs cellulolytic enzymes, resulting in their inactivation. Each type of LCB has a different structure and a differentiated composition of individual components. Srivastava et al. [
10] reviewed the classification, components, and structure of biomass, discussing its key role as a substrate in bioenergy production.
Less important components of biomass include volatile and extractive substances, such as water-soluble sugars and proteins, as well as ethanol-soluble substances, such as chlorophyll and waxes. The final conversion process can also be affected by inorganic matter, such as ash, which contains material such as calcium and potassium ions. In addition, sand is often present, especially in agricultural waste, which is accumulated in biomass during harvesting [
11]. These authors tabulated the average composition of specific woody and non-wood feedstocks and also included selected wastes such as MSW and woody residues. The composition of woody biomass can vary significantly. For instance, hardwood contains a high proportion of cellulose, whereas bark contains a large amount of lignin. Deciduous trees mainly contain xylan (35%) in their hemicellulose, whereas coniferous biomass mainly contains glucomannan (28%). All reported proximate and ultimate values have been compiled by the Idaho National Laboratory and are available in the Bioenergy Feedstock Library [
12].
3. Pretreatment Techniques for LCB
The complex structure of lignocellulosic biomass (LCB) hinders the release of fermentable sugars that are necessary for further biotechnological processes. The purpose of pretreatment is to overcome biomass recalcitrance by disrupting the lignin structure and decrystallising and depolymerising the cellulose, thereby increasing its reactivity and the yield of fermentable sugars.
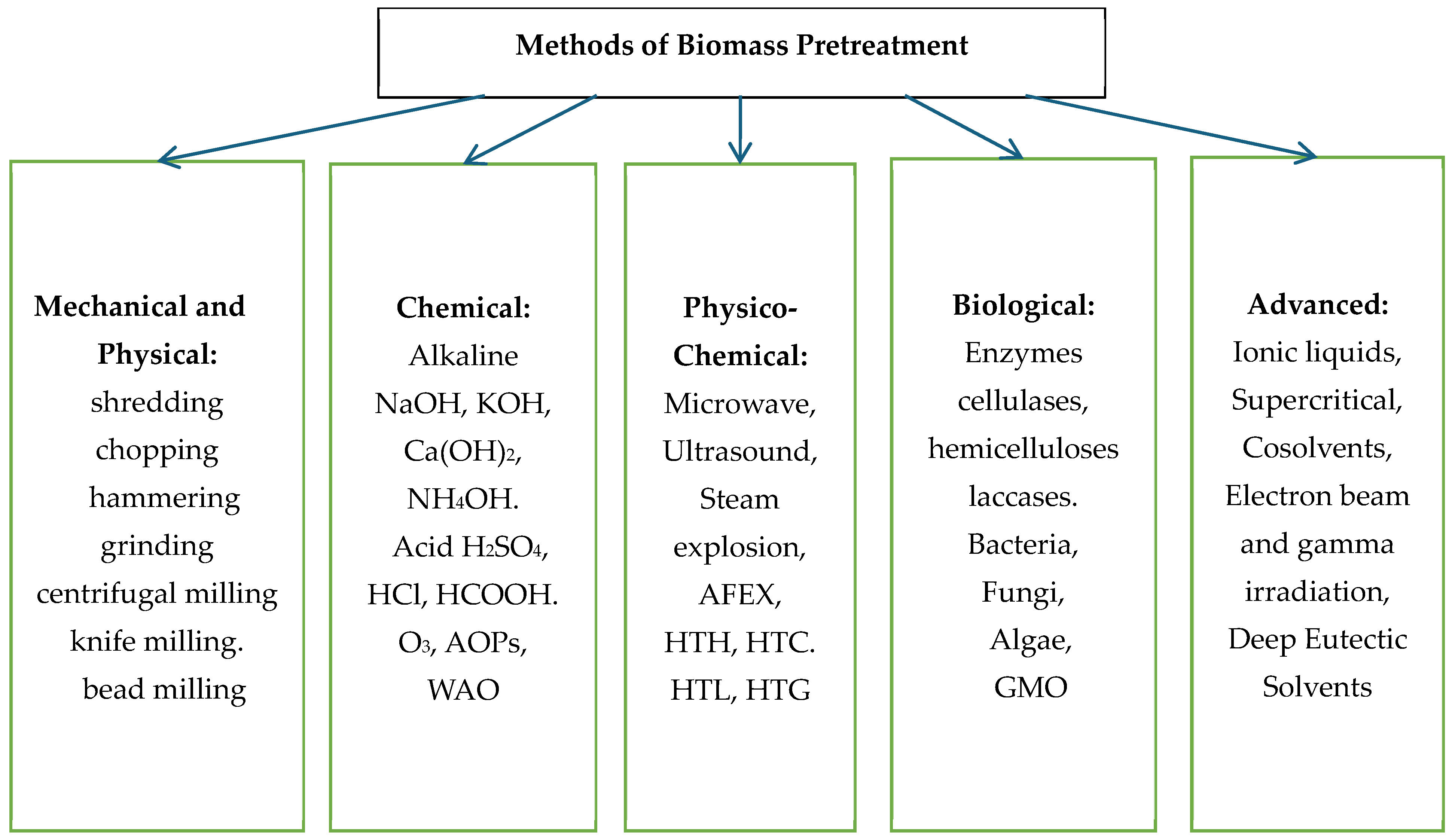
Conventional pretreatment methods commonly used include mechanical methods (e.g., milling and grinding), physical (microwave, ultrasound, pyrolysis), chemical (dilute acid, base, ozone, organic solutions), and biological (biodelignification by laccase, enzymatic saccharification by cellulase and hemicellulose).
Figure 1 illustrates different pretreatment techniques for LCB.
Pretreatment is considered one of the most costly unit operations in biomass conversion, accounting for over 20% of the total cost at USD 70–150/tonne of biomass [
13].
The mechanical method aims to reduce the particle size of LCB and break down its main components using machines such as bead and knife mills, centrifuges, hammers, and vibrators. High pressure is used for LCB, but, unfortunately, delignification is expressly limited. None of these techniques use chemicals, and they do not interfere with the hydrolysis process.
Chemical pretreatment methods use alkalis, acids and other chemical mixtures. Alkaline treatment methods using calcium hydroxide (Ca(OH)
2), sodium hydroxide (NaOH) and potassium hydroxide (KOH) are effective at degrading the lignin content. These methods swell the lignocellulosic biomass, reducing its crystallinity and degree of polymerisation. Acid pre-treatment (HCl, H
2SO
4) breaks van der Waals interactions and hydrogen bonds of hemicellulose, dilute acid effectively increases the reaction rate and cellulose hydrolysis; however, it produces inhibitors that can hinder downstream fermentation. The ozonolysis process uses the powerful oxidant O
3 for delignification (80%) but alters the nature of the biomass. Ozonolysis released 75% of the sugars in the hydrolysate, which were then used to produce second-generation (2G) fuels. Conversely, the short carboxylic acids that were also formed are the main inhibiting compounds, but these can be adequately removed by washing with water. Fortunately, the most common inhibiting compounds that occur as a result of the chemical pretreatment of LCB, such as furfural or 5-hydroxymethylfurfural, are not present in ozone-treated hydrolysates [
14].
Advanced Oxidation Processes (AOPs) are powerful processes that have traditionally been used to treat hazardous materials. M’Arimi et al. [
15] reviewed the current status and future prospects of AOPs application as the pretreatment of LCB, excess sludge, organic wastewater, solid waste and others. Wet air oxidation has high potential for LCB pretreatment. Sonolysis is most effective for the pretreatment of biosolids. Ozonolysis [
16] and photocatalysis are most commonly used for the selective removal of colourants in organic effluents [
17]; however, the main limitation to the application of AOPs in the biofuels sector is the high demand for electricity.
Among the physicochemical methods of LCB pretreatment are the action of microwaves, ultrasound, steam explosion, CO
2 explosion, ammonia fibre expansion and the hot water method. Microwaves interact with the dielectric properties of water, releasing heat in a one-step process involving hydrolysis. However, this method is only effective on a small scale. Ultrasound creates bubbles of steam through cavitation, which break up the cells, releasing the desired sugars and causing hydrolysis. Steam explosion is a thermal process in which the cells are exploded in a reactor that is heated to 260 °C at a pressure of 5 MPa. Ammonia fibre expansion (AFEX) is one of the most extensively tested methods for treating LCB to produce fermentable sugars and has been used quite frequently recently [
18]. This article provides a comprehensive review of studies focusing on AFEX pretreated biomass for the production of 2G biofuels and bioproducts such as enzymes, lipids and proteins. Anhydrous or highly concentrated ammonia is added to the wet substrate at a moderate temperature and high pressure. When the pressure is released, the ammonia evaporates and can be collected and recycled. AFEX pretreatment is best suited to prevent cellulase adsorption on lignin. The hot water process is a hydrothermal process, such as hydrothermal carbonisation, where high temperatures of from 150 to 250 °C are used and the pressure is equal to the vapour pressure at these temperatures.
Biological methods use fewer chemicals or more environmentally friendly pretreatment and use microbes or enzymes such as laccases to break down the lignin. Biological hydrolysis of lignocellulose (LCB) is considered more effective than chemical methods because enzymes exhibit better catalytic activity, selectivity and specificity than chemical catalysts. They are, therefore, used to degrade LCB into simple sugars. As bacteria cannot degrade lignin, white- and brown-rot fungi, such as
Phanerochaete chrysosporium and
Trametes versicolor, are mainly used for LCB hydrolysis. These fungi can degrade lignin because they secrete lignin-degrading enzymes, including laccase, lignin peroxidase and manganese peroxidase. The use of white-rot fungi is a very selective method of degrading lignin. However, it is sensitive to environmental conditions and has a long processing time [
19]. Cellulases used commercially are produced by various bacterial species, including
Bacillus,
Cellulomonas,
Thermomonospora and
Clostridium, as well as fungal strains such as
Trichoderma,
Penicillium and
Aspergillus [
20]. Enzymatic hydrolysis is clearly preferable to acid hydrolysis because it takes place under ambient conditions, has a higher degradation rate, and is more environmentally friendly. However, enzyme production costs increase the overall cost of the process. To make LCB hydrolysis more economical, a new approach to producing biocatalysts on nanomaterials (NPs) has been introduced, involving the reuse of enzymes in LCB hydrolysis. This is made possible by immobilising enzymes on NPs/nanomaterials [
21]. Due to the increased surface area of nanomaterials, larger amounts of enzymes can be loaded, and the mass transfer resistance of the substrate is reduced. This significantly increases the lifetime of the biocatalyst. The integration of enzyme immobilisation and hybrid approaches combining acid and enzymatic hydrolysis is a promising area.
One of the main problems and challenges of biomass pretreatment in commercial biorefineries is the resistance of lignocellulose (LCB) to delignification. Therefore, it is necessary to break down the rigid carbohydrate–lignin structure effectively. Various methods of pretreating LCB have been employed for this purpose, including biological, chemical and physical methods, as well as combinations of these methods [
22,
23]. To pretreat sorghum straw under mild conditions, the authors [
23] used a combination of chemicals (formic acid, sodium chlorite and hydrogen peroxide in an alkaline environment) and enzymes. The lignin removal by alkaline pretreatment was the highest, 94.97%, as was the hydrolysis yield, 90.76%. The results of this work suggest that the NaOH pretreatment and its efficient enzymatic hydrolysis make the whole process more cost-effective.
The review paper [
24] discusses environmentally friendly techniques for the pretreatment of LCB. Furthermore, the paper provides insight into the reaction mechanisms involved in technologies, such as microwave, ultrasound, deep eutectic solvent, irradiation and high-force-assisted pretreatment methods, to enable the effective valorisation of LCB. Microwave pretreatment shows significant potential for the treatment of LCB due to the dielectric properties of biomass. The hydroxyl groups in LCB contribute to its polarity, and the presence of non-polar lignocellulosic fibres results in the formation of dipoles. LCB materials with stronger dipolar properties exhibit greater dielectric properties. When microwaves are applied, this generates significant heat in the biomass. This heat does not pass through the surface of the sample but increases the temperature throughout its volume. This makes it a more energy-efficient process than conventional heating methods. Unfortunately, this technique only works well at a laboratory scale.
The use of ultrasound technology in biomass pretreatment has increased in recent years. Ultrasound is a mechanical wave with various frequencies ranging from 20 kHz to almost 1 GHz that transforms energy in the liquid reaction medium through acoustic cavitation. This phenomenon involves the formation and collapse of microbubbles. The collapse of these microbubbles creates microjets that travel at high speed towards solid surfaces, resulting in extreme pressures of up to 50 MPa and temperatures of around 5000 °C [
24]. These high temperatures and pressures destroy the crystal structure of cellulose and cause the formation of *H and *OH radicals from the decomposition of water molecules. These highly reactive radicals then cause the deconstruction of the lignocellulose matrix by cleaving glycosidic bonds.
Since its inception in 2003, deep eutectic solvents (DESs) have been widely adopted in bioprocess engineering [
24]. These mixtures of two different constituents are observed to melt at a temperature lower than the boiling point of the components individually. One constituent of the DES acts as a hydrogen bond donor (HBD), while the other acts as a hydrogen bond acceptor (HBA). The charge delocalisation between DES species differentiates them from the more expensive ionic liquids. The performance of biomass deconstruction depends significantly on the HBD and HBA of the DES. Choline chloride (ChCl) is the most common HBA, while glycerol and ethylene glycol are the most widely studied HBDs for lignin removal during biomass pretreatment. However, acids (e.g., acetic acid or formic acid), bases (e.g., amines or imidazoles) and phenols are also potential HBDs [
25]. The properties of DES, combined with their delignification and hemicellulose removal capabilities, favour their use for biomass fractionation. The use of DES reduces the crystallinity of cellulose, generating microbubbles and cracks in pretreated solids, and improves further LCB conversion. Combining low-cost ChCl with organic acids yields higher degrees of delignification than can be achieved with other HBAs. Pretreatment of DES at temperatures up to 175 °C and durations ranging from a few minutes to a few hours while using microwaves or conventional heating has proven very effective. Unfortunately, DESs are relatively more expensive than the typical acids and bases used in pretreatment processes, but, if efficient recycling and reuse are employed, they will succeed in reducing process costs.
Similar to the action of ultrasound, irradiating biomass with electron beams or gamma rays generates short-lived ions and radicals that cause biomass fragmentation [
24], consequently facilitating enzyme action. Cellulose in its polymeric form is highly susceptible to radiation-induced degradation, which results in the cleavage of β-1,4-glycosidic bonds. Additionally, radiation generates a significant amount of heat and energy. As a result of thermal induction, the generated free electron interacts with the cation (neutralisation) formed in one of the monomeric units, thereby becoming excited and subsequently being removed from the C-H bonds at the C1 or C4 site of the cellulose structure. As the radiation dose increases, the content of carbonyl and carboxyl groups increases due to the breakdown of cellulose polymer chains. However, an increased dose will result in further oxidative decomposition of the furfural, thereby reducing its inhibitory effect [
26].
Many of the combined LCB pretreatment technologies are still in the pilot stage and require further scale-up. In addition, the environmental impact of these LCB pretreatment methods should be further investigated. The difficulties of implementing LCB pretreatment at pilot scale have been highlighted in another review [
27]. Unresolved problems such as the production of inhibitors, reduced hydrolysis capacity, high disposal costs, inferior fermentation performance, insufficient separation of lignin and cellulose, etc., also need to be addressed. Before concrete implementation takes place, an energy balance and environmental audit of the process should be carried out, as well as an analysis of the total costs.
Over the last five years, more than 2000 papers containing the keywords ‘biomass pretreatment’ have been published. A similar search for publications on newly developed ionic liquids or deep eutectic solvents reveals a steady increase from almost none in 2010 to around 500 papers a year today. Many papers concern the comparison of various pretreatment techniques to enhance the biodegradability of lignocellulosic biomass [
28,
29,
30,
31,
32,
33,
34]. However, the wide variety of lignocellulosic materials and the numerous pretreatment methods make it difficult to establish a standardised biorefinery process design. Therefore, determining the optimal pretreatment method is challenging, and any recommendation must be based on thorough techno-economic evaluations and life-cycle analyses.
4. Anaerobic Digestion
Anaerobic digestion (AD) is a sequence of biological processes in which microorganisms, including acetic acid-producing bacteria (acetogens) and methane-producing archaea (methanogens), break down biodegradable material in the absence of oxygen. The process consists of four stages: hydrolysis, acidogenesis, acetogenesis and methanogenesis. Hydrolysis—the first step, wherein insoluble organic polymers, such as carbohydrates, are broken down into soluble derivatives that are available to other bacteria. Acidogenesis is a biological reaction that converts simple monomers into volatile fatty acids (VFAs); in acetogenesis, bacteria convert the VFAs to acetic acid, along with additional ammonia, H
2 and CO
2. Finally, methanogens convert these products to CH
4 and CO
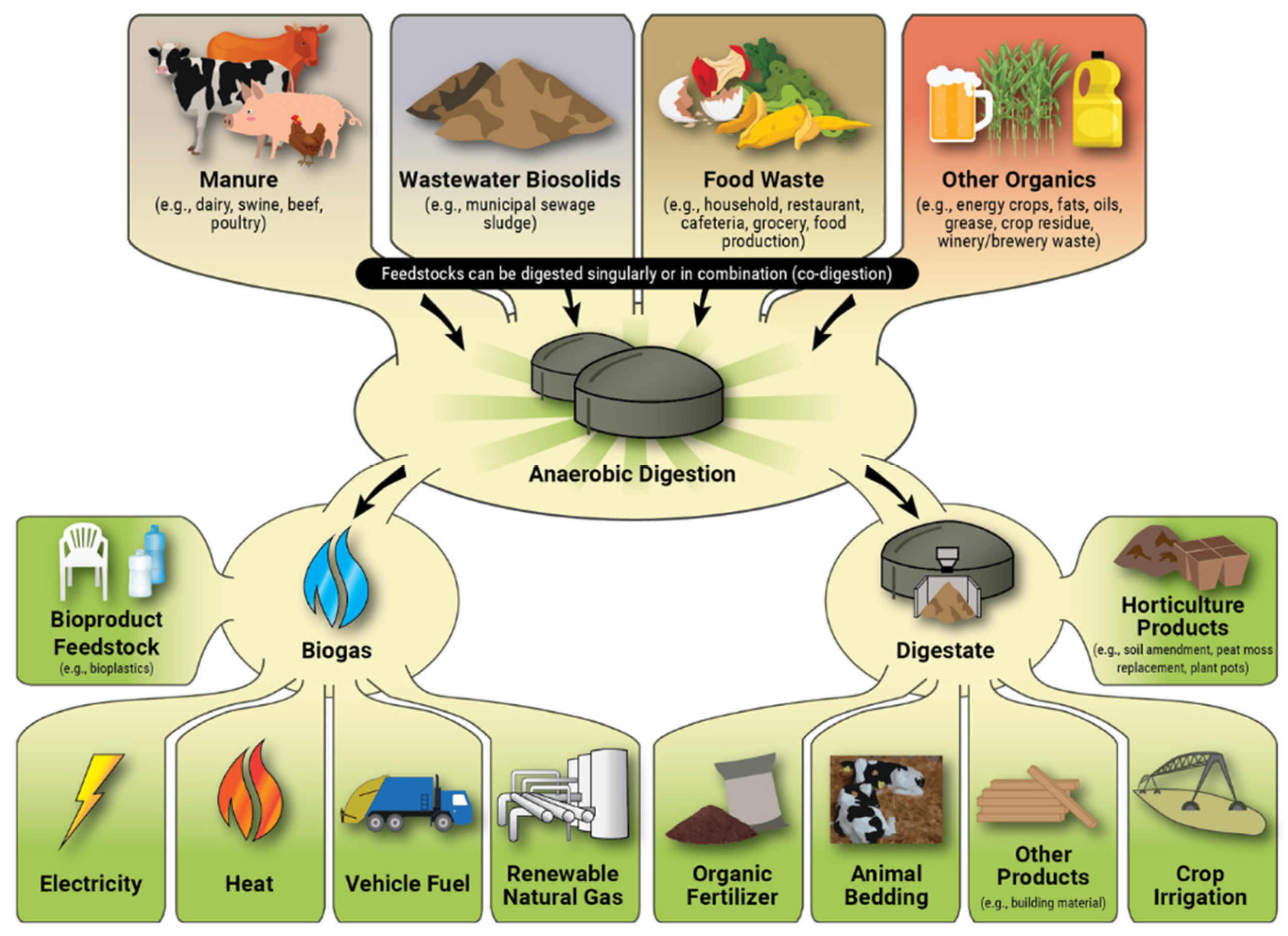
2, which are the main products, along with liquid and solid residues called digestate. AD technology is widely used as a renewable energy source, because biogas can be directly burned in cogeneration gas engines or upgraded to biomethane, and the digestate by-product, which is rich in nutrients, can also be used as a fertiliser and soil improver. Biogas upgrading is increasingly used because, after removing CO
2 from biogas to a minimum of 95% CH
4, it can be injected into natural gas distribution lines. The pathways for converting different feedstocks into bioproducts and their use are illustrated in
Figure 2.
Biogas can be produced from locally available biomass such as agricultural residues, municipal/industrial biowaste, etc.; however, in many cases, the methane yields obtained from the conventional AD process are considered to be of limited economic viability. In their review [
36], the authors summarised the current knowledge on the different strategies to increase the efficiency of AD and the existing methods to overcome the current barriers to biogas production. Special attention was given to co-digestion and pre-treatment of single/mixed substrates. The authors [
36] claim that it is necessary to implement appropriate monitoring and control systems to produce biogas more efficiently from available biomass. Therefore, they postulate the need for laboratory-scale research, and then pilot-scale research, to properly assess the type of substrate or co-substrate and select an effective method of raw material pretreatment.
AD research has recently been moving towards the co-fermentation of two or more biomass waste streams. For example, AD co-fermentation of sewage sludge, food waste and garden waste has resulted in high methane yields with full system stability [
37]. The results of the studies on anaerobic co-fermentation of municipal organic waste (food waste, sewage sludge and garden waste) have been more promising than AD mono-fermentation of each of the individual substrates. Karki et al. [
38] presented the results of modelling the kinetics of the AD process. Nine different kinetic models (five conventional mono-fermentation models and four co-fermentation models) were compared and evaluated. It was concluded that the type of feedstock has a great influence on the selection of the appropriately matched model. The results of research and development efforts have highlighted several inherent advantages of co-digestion, including improved digestibility due to synergistic effects of co-substrates, better process stability and higher nutritional value of the co-digestate produced [
39]. This review highlights the limitations of monodigestion and critically evaluates the benefits of co-digestion. The authors [
39] demonstrated the synergistic effect of co-substrates, described the characteristics of microbial communities and suggested future research directions. Dar et al. [
40] summarised the literature from 2010 to 2020 on anaerobic digestion of agricultural wastes. The results of this study allow for understanding the key aspects of AD technology and suggest further research towards biomethanation. Raw biogas typically contains 60% CH
4 and 40% CO
2, with some contaminants such as H
2O vapor, H
2S, O
2, N
2 and NH
3. The ultimate goal should be the production of biomethane (over 95% CH
4), with a much higher energy density, which allows for injection into gas pipelines and use as a vehicle fuel. Several methods are used for biogas upgrading, including physicochemical absorption, high-pressure water washing, amine absorption, pressure swing adsorption, membrane permeation/separation and other methods. Swinbourn et al. [
41] discussed general guidelines for the techno-economic assessment of bio-CH
4 production, presenting life-cycle assessment (LCA) and production costs. Despite these promising prospects, the cost of biomethane production through methanation remains a problem and is limited by high capital and operating costs, as well as raw material costs. At the moment, biomethane production from biogas is less profitable compared to current natural gas prices.
5. Syngas Fermentation
Syngas is a gaseous mixture containing H
2, CO and/or CO
2 produced by LCB thermochemical conversion, e.g., pyrolysis and gasification of biomass waste, coal, MSW and other materials. Considering that modern biorefineries integrate sugar and syngas platforms, it is reasonable to focus on syngas fermentation (SNF), which has recently been developed for the production of biofuels and chemicals. The syngas mixture is converted by acetogenic bacteria into ethanol, butanol, acetic acid, butyric acid and methane. Various mesophilic and thermophilic microorganisms are suitable for SNF processes. There are several microorganisms that can produce fuels and chemicals from syngas. These microorganisms are mostly known as acetogens and include bacteria of the genera
Clostridium,
Eubacterium, and
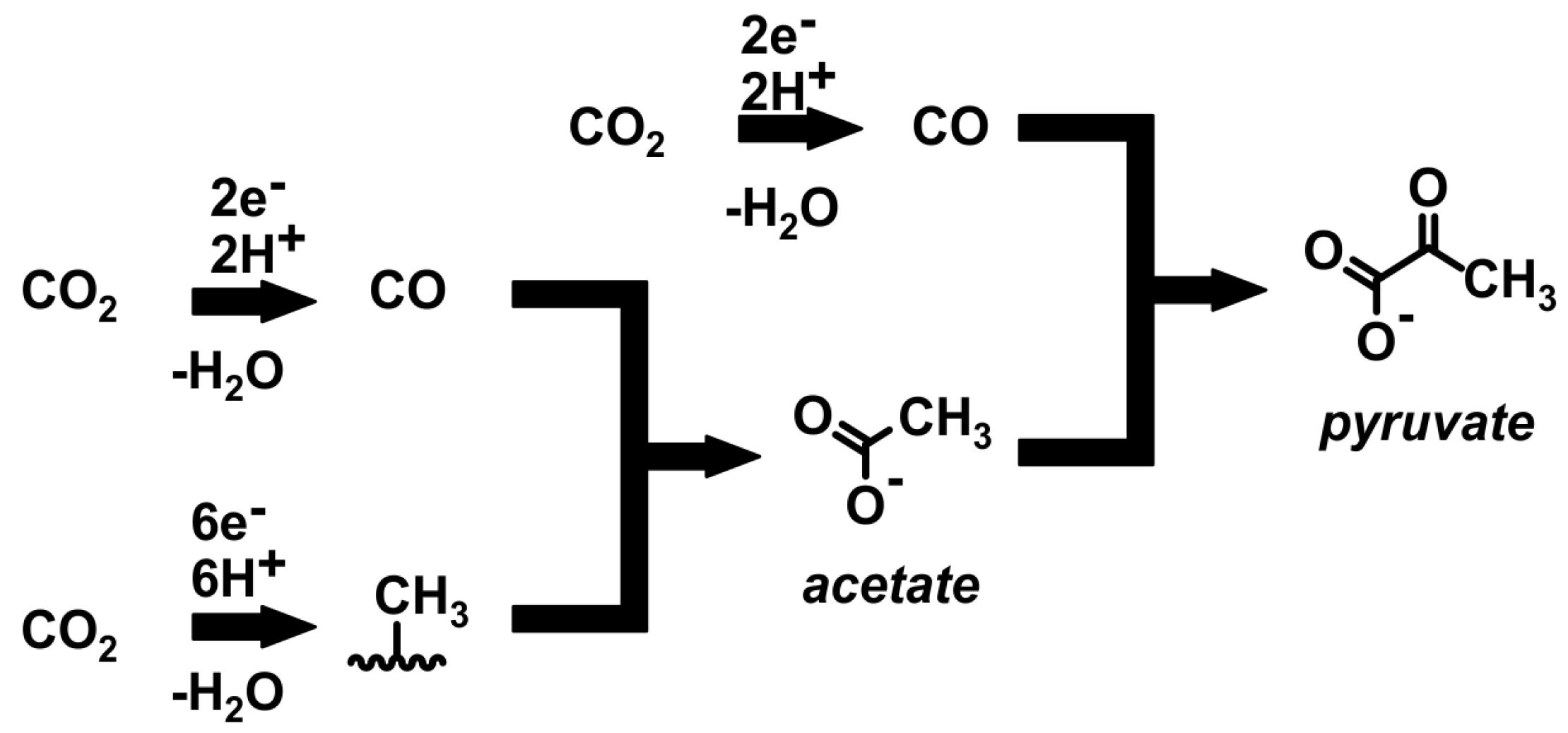
Streptococcus. Most microorganisms use the Wood–Ljungdahl pathway (WLP) under anaerobic conditions, involving two enzymes: CO dehydrogenase and acetyl-CoA synthase. In the WL pathway, CO
2 is reduced to CO and HCOOH or to a formyl group and the formyl group is reduced to a methyl group, then combined with CO and coenzyme A to form acetyl-CoA. The simplified Wood–Ljungdahl is shown in
Figure 3.
Two pathways can be noted in the Wood–Ljungdahl pathway: methyl and carbonyl. If CO is the only substrate, one CO molecule enters the direct carbonyl pathway, and the other is oxidised to CO2 by the enzyme carbon monoxide dehydrogenase (CODH). On the other hand, if CO2 is the only substrate, it is first reduced to CO by the CO dehydrogenase/acetyl-CoA synthase (CODH/ACS) complex, and the second molecule is reduced to methyl via the methyl pathway. The CODH/ACS complex connects these two pathways, allowing the formation of acetyl-CoA, which is the precursor of various end products.
Kim et al. [
42] explained the mechanism of the WLP and its use for the production of C2 chemicals in detail. Acetate is the main product of acetogenic bacteria, and some acetogens can also produce other C2 molecules, such as ethanol, but also organic compounds with longer carbon chains up to C4—butyrate, butanol and butanediol.
The SNF process does not require high temperatures or pressures, reducing operating costs. The microorganisms are not particularly sensitive to impurities in syngas, and the H
2:CO ratio is not important. A review article [
43] describes in detail syngas fermentation processes for the production of biofuels and chemicals with a detailed description of the microorganisms, the media used, gas-liquid mass transfer problems, reactor design and techno-economic analysis. This worthy review provides an indication of future research directions leading to the commercialisation of this technology.
The syngas components CO and H2 are poorly soluble in water and even worse in the medium, making gas transfer to microbial metabolic sites very difficult, but the main resistance to mass transfer lies on the liquid side at the gas-liquid interface. This limited gas–liquid mass transfer, as well as low fermentation efficiency, is one of the significant drawbacks of this technology and raises the cost of producing C2 and C4 biochemicals
A review of the current state of the art in SNF technology and a discussion of possible concepts for industrial-scale application have been given by Stoll et al. [
44]. Due to the high mass transport resistance in the gas–liquid system, which limits the speed of the overall process, research is being conducted to determine the volumetric mass transfer coefficient k
La in various bioreactor configurations. These types of mass exchangers are used for gases that are poorly soluble in liquids, that is, in bubble columns and air-lift bioreactors. It must be admitted that there are few publications on syngas fermentation using these types of reactors. It is possible to increase the driving force of mass transfer by increasing the pressure, because the solubility of gases increases at higher pressure, and the availability of the substrate for the enzymes will be improved.
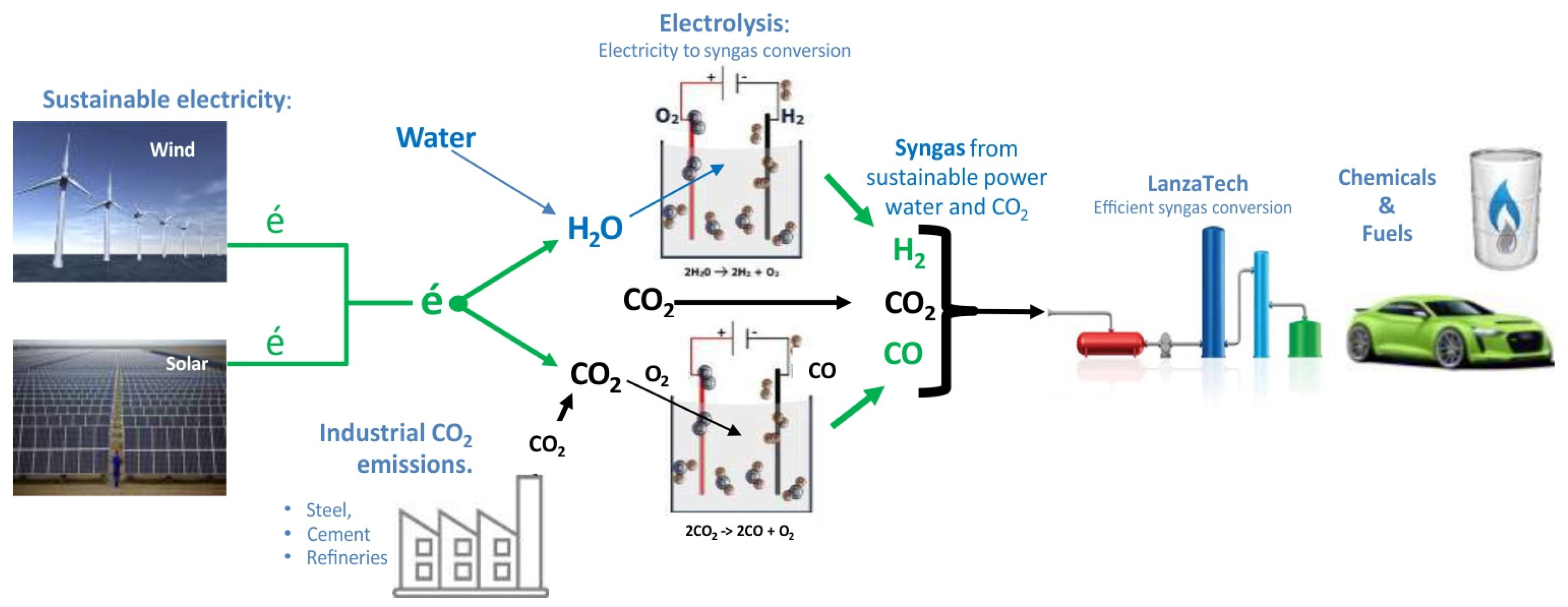
It is worth mentioning the power-to-x (P2X) technologies, in particular, one of the pathways combining electrolysis using green electricity and biotechnological CO
2 fixation via fermentation as one of the sustainable alternatives. A next step in this direction, as postulated by Stoll et al. [
44], is a satellite of Kopernikus P2X, Rheticus project. In 2018, Evonik and Siemens launched a EUR 2.8 million joint research project to produce valuable chemicals from CO
2 and using green electricity. In the Rheticus project, two steps—electrolysis and SNF fermentation—were combined in a laboratory-scale pilot plant. Several companies have been involved in scaling up the SNF process and commercialising it, with LanzaTech leading the way as a pioneer in SNF fermentation and continuing to be successful on a larger scale [
45].
Figure 4 shows LanzaTech’s idea of a future fuel from CO
2. Despite the progress made in commercialising SNF technology, further research is still needed to increase the efficiency of SNF. The use of genetically modified microorganisms, the selection of appropriate fermentation media, the choice of an optimal bioreactor and proper process control—all of these will increase product yields and significantly reduce production costs [
46].
6. Alcoholic Fermentation
As a valuable substitute for renewable energy sources, bioethanol is commonly used as a biofuel, blended with gasoline at a concentration of 5%. This ethanol fermentation, which has been known for centuries, is one of the most widely recommended technologies for producing renewable fuels by converting LCB into 2G-ethanol. However, the conversion of LCB into bioethanol requires various types of pretreatment technologies depending on the feedstock used. On the other hand, in the hydrolysis of pretreated biomass, enzymes are needed to destroy cellulose and hemicellulose into simple sugars, which further undergo fermentation. The main product is bioethanol, but biobutanol, methane and some other compounds are also produced.
Alcoholic fermentation follows the Embden–Meyerhof–Parnas (glycolytic) pathway. Glycolysis of hexose results in the formation of pyruvate. Initially, pyruvate undergoes decarboxylation, during which carbon dioxide is detached from its molecule. As a result, a two-carbon molecule of acetaldehyde is formed. The acetaldehyde is then reduced to ethanol by NADH, which thereby undergoes oxidation to NAD+ and allows ATP synthesis. Regeneration allows glycolysis to continue and the oxidation of the next glucose molecule. The overall reaction of bioethanol fermentation from glucose is as follows:
Alcoholic fermentation is carried out by a wide range of bacteria (
E. coli and
Zymomonas mobilis) and fungi (
Rhizopus and
Mucor). The yeast
Saccharomyces cerevisiae and the bacteria
Zymomonas mobilis are the most commonly used to produce bioethanol from LCB. The widespread use of
S. cerevisiae is due to its robustness and suitability for fermenting glucose from LCB, while
Z. mobilis is used less frequently because it has a lower yield and is more susceptible to contamination. In order to improve inhibitor resistance and increase bioethanol yields, microorganisms are being genetically modified. For example [
47], some genetic manipulation of
S. cerevisiae has been carried out to improve the utilisation of xylose, which is much more difficult to ferment than glucose. Improved utilisation of pentose through genetic manipulation is still needed.
Improving tolerance to toxicity, for example, carboxylic acids, which are enzyme inhibitors, and tolerance to high concentrations of ethanol in microorganisms is a major challenge for commercialisation. Reprogramming of gene transcription in
S. cerevisiae has been shown to increase ethanol tolerance and increase xylose utilisation efficiency [
48]. In recent years, review articles have been published outlining strategies for improving yeast surface display systems [
49,
50,
51,
52]. This cell surface display technology involves immobilising functional proteins/peptides on the surface of microorganisms and thereby conferring specific functions to microbial cells. Functional proteins/peptides are fused to anchor protein genes and are expressed on the cell surface under the influence of signal peptides. For example, as reported by [
50], due to surface expression of cellulase or other proteins, ethanol was formed in one step, eliminating the need for enzyme purification and saccharification. Tang et al. [
51,
52] developed a complex, multicomponent assembly system in which multiple enzymes were co-expressed on the cell surface. This eliminated the need for multiple steps in the reaction, resulting in greater efficiency.
The saccharification and fermentation stages, which occur in the production of bioethanol, can be carried out in different configurations [
53]. These include a two-step process and a one-step process, in which saccharification and fermentation occur simultaneously (SSF). The SSF process is more economical and has a higher ethanol yield, as enzymatic hydrolysis and fermentation occur simultaneously, thus avoiding cellulase feedback inhibition [
54].
An overview of current available bioethanol technologies and targets for future innovation has been provided in a review [
55]. This review compares 2G-ethanol production from a range of feedstocks, and ethanol yield is highly variable and feedstock-dependent. Third-generation bioethanol production uses microalgae and macroalgae as the primary feedstock. Algae absorb CO
2, accumulate high concentrations of lipids and carbohydrates more quickly, and are easier to grow than terrestrial plants, as they do not require cultivated land. At present, macroalgae (seaweeds) are also reported to be one of the best candidates for use as a 3G-bioethanol feedstock [
56].
There are 4G ethanol production processes using genetically modified organisms (e.g., yeast and algae) that are under development. The use of metabolic engineering of algae to produce bioethanol from photoautotrophs and technological advances in these production processes are outlined in a review [
57], together with a look at future prospects. The most important development in 4G bioethanol production is the creation of new bacteria that enable direct bioethanol production in natural micro-scale bioreactors, known as green cell factories. Genetically modified algae have a significantly higher energy content than their wild counterparts. For instance,
Chlorella vulgaris transformed with a codon-optimised
E. coli glgC gene produced ethanol at a yield of 82.82 mg/L, which is significantly higher than the yield of 54.41 mg/L of its wild counterpart [
58].
There are three main strategies for industrial-scale fermentation: submerged liquid fermentation, solid-state fermentation, and ultra-high gravity fermentation [
59]. While submerged/liquid fermentation takes place in the liquid phase and solid fermentation uses organisms growing on insoluble solid substrates, very high gravity fermentation uses a much higher concentration of sugar substrate to increase the final ethanol concentration. The production of 2G bioethanol often involves the solid-state fermentation of waste and other feedstocks, such as agricultural waste [
60]. However, this process requires large-volume bioreactors and produces significant amounts of waste, including distillers’ stock and wet distillers’ grain. Combining solid-state fermentation to produce the enzyme with hydrolysis on a second medium in submerged fermentation has resulted in better sequential performance, and combining these two technologies can provide additional benefits [
61]. In very high gravity fermentation, sugar concentrations for ethanol production are above 250 g/L total sugars, while, in normal gravity, they are below 180 g/L total sugars [
61].
There are three types of submerged fermentation: batch, fed-batch and continuous fermentation. The type of fermentation used depends on the kinetics of the process and the microorganisms and raw material used. In fed-batch fermentation, the microorganisms are inoculated into a fixed volume of nutrient solution in the fermenter. When the nutrients are exhausted, the fermentation process ends. In fed-batch fermentation, nutrients are added gradually to the fermenter during the process, whereas, in continuous fermentation, fresh nutrients are continuously added at the same rate at which ethanol and by-products are removed from the bioreactor. Traditionally, ethanol was distilled from fermented media by heating, which would have destroyed the microbial culture. To avoid this, methods such as filtering the yeast from the product stream before distillation or vacuum distillation at fermentation temperature have been proposed so that the yeast can be recycled [
62]. A comprehensive review [
63] by Robak and Balcerek highlighted recent progress in the production of 2G-bioethanol from LCB. Particular attention was paid to each of the production steps, namely, pre-treatment, enzymatic hydrolysis, fermentation and distillation. Additionally, the development of microbial engineering to make enzymatic hydrolysis more cost-effective and overcome the limitations of using natural fermentation bacteria and yeast is discussed.
Another alcohol produced from the fermentation of LCB is biobutanol, which is gaining increasing attention as an alternative renewable fuel due to high energy content, low volatility, low corrosivity and low hygroscopicity. Biobutanol is a potential biofuel that is far superior to bioethanol. Biobutanol is also a drop-in biofuel that can be blended up to 85% with petrol and used in vehicles without modification. In ABE fermentation, sugar-rich biomass is subjected to anaerobic fermentation by
Clostridia bacteria to produce acetone, butanol and ethanol in a 3:6:1 ratio. These bio-products are produced through a process called solventogenesis, preceded by acidogenesis in which
Clostridia species synthesise butyric acid and acetic acid. In their review [
64], Guo et al. reported on recent developments in butanol production from LCB, including the pretreatment and hydrolysis of hemicellulose and cellulose. The authors believe that an ideal strain for producing lignocellulosic butanol should exhibit high butanol activity and utilise fermentable mixed sugars, as well as demonstrating good substrate tolerance. This could help to improve fermentation yields and reduce process costs. Tsai et al. [
65] used renewable feedstocks such as rice straw, sugarcane bagasse, and microalgae hydrolysate for ABE fermentation. Using immobilised cells, the authors achieved high biobutanol titers, productivity and yields of 13.80 g/L, 0.90 g/L/h and 0.58 mol biobutanol/mol glucose, respectively. The butanol product is cytotoxic, so attempts are being made to reduce its inhibition by combining different methods of in situ butanol separation during ABE fermentation. These methods usually involve liquid–liquid extraction, gas desorption, adsorption and pervaporation [
66]. The review also covers cocultivation technology and other methods that allow for butanol fermentation under non-strict anaerobic conditions.
7. Dark Fermentation
Biohydrogen (bioH
2) is an environmentally friendly, sustainable and reliable source of energy with zero carbon emissions. Under natural conditions, the hydrogen produced during the acidogenesis stage of AD is immediately used by microorganisms to produce methane, with carbon dioxide as a by-product. For hydrogen recovery, the hydrolysis and acidogenesis steps can be separated in time and space from the acetogenesis and methanogenesis. The methanation process can be inhibited by introducing a suitable set of microorganisms or by eliminating methane-forming bacteria. This makes bioH
2 the end product of dark fermentation (DF). In DF, anaerobic bacteria and some green algae grown on carbohydrate-rich media can produce hydrogen in the dark at a temperature range of 30–70 °C. The most commonly used anaerobic bacteria for DF are
Clostridium,
Enterobacter and
Bacillus strains [
67].
A review of the literature on DF shows that a lot of research is being performed to obtain more efficient bacterial strains by genetic manipulation [
67] and to intensify the production of bioH
2. A comprehensive article by Łukajtis et al. [
68] summarises the most important information on bacterial strains used for H
2 production from LCB agricultural and food wastes. The influence of the type of raw material, the method of inoculum preparation, the type of reactor and process parameters such as temperature, pH, substrate concentration, partial hydrogen pressure and hydraulic retention time on DF efficiency was discussed. The metabolic pathways of the main hydrogen-producing fermenting microorganisms are now well understood, including methane fermentation and the action of the enzyme hydrogenase. In glycolysis, glucose is converted to pyruvate, and then pyruvate is oxidised to acetyl-coenzyme A, with concomitant reduction of ferredoxin. In the third step, ferredoxin is oxidised to H
2 by the enzyme hydrogenase. The stoichiometric, theoretical maximum amount of H
2 per mole of glucose can be up to 12 moles of H
2, according to the following equation:
However, the formation of various end products, such as acetic acid, propionic acid and butyric acid, as well as methanol or butanol, reduces the amount of H
2 produced, as can be seen from the following equations:
Exceeding the metabolic barrier of 4 moles H
2/mole glucose is a serious performance limitation, as the substrates are not fully converted to H
2, and the VFAs formed further reduce the efficiency of bioH2 production. The choice of feedstock for bioH
2 production depends on its availability and cost, carbohydrate content and fermentability. BioH
2 production from biomass, similar to biorefinery classification, can be divided into three generations based on raw materials used, such as 1G (agricultural/food waste), 2G (lignocellulosic waste) and 3G biomass (algae). Fermentative production of bioH
2, especially from LCB waste, requires some pretreatment. For example, bioH
2 production from water hyacinth was increased by about 350% by integrating ultrasound-assisted pretreatment into DF [
69].
Various bioreactors are used for bioH
2 production, including anaerobic fluidised bed, anaerobic upflow reactors, continuous stirred tank and anaerobic sequential batch reactors [
70]. Scaling up production may be facilitated by the use of CFD modelling and machine learning algorithms. Wodołażski and Smoliński [
71] modelled an anaerobic DF of biomass for bio-H
2 production in a continuous plug flow reactor. A CFD multiphase full transient model in long-term horizons was employed to simulate the DF process in continuous mode. The effects of HRT, pH and feed rate on bioH
2 yield and production rates were investigated. Frascari et al. [
72] used an Andrew kinetic model of substrate inhibition in bioH
2 production from glucose by
Thermotoga neapolitana cells. Numerical simulations were performed using ANSYS Fluent 2016 software. The bioH
2 production rate was 0.2–0.68 mg/L/h for a period of 4–6 days, after which it began to decline. The highest bioH
2 concentration occurred during this period. The reduced bioH
2 production may be due to VFA accumulation and a subsequently lower pH.
Although it is commonly believed that DF is the most efficient method of producing bioH
2, the significant amounts of fatty acids (VFAs), particularly acetic and butyric acids, that are produced reduce the yield of bioH
2. Other factors hindering the commercialisation of bioH
2 production include inorganic inhibitors (heavy metal ions, ammonia), organic inhibitors, such as VFAs, furan derivatives and phenolic compounds and bacteriocins used as food preservatives [
73]. Disadvantages of DF include low substrate conversion, thermodynamic limitations, low hydrogen yields and a mixture of H
2 and CO
2 products that require separation. These technical issues can be addressed by process modification, improved H
2-producing bioreactor design, feedstock selection, microbial strain selection and immobilisation, and the use of additives such as metal (Fe, Ni) oxides, or L-cysteine and others [
74].
There have been several reviews on DF; one of the most recent [
75] discusses the challenges of transferring bioH
2 technology from the laboratory to industry. Another review on the DF process [
76] postulated that although various types of DF research have been carried out, there are still many problems with microbial consortia, which, unfortunately, result in a shift towards CH
4 production. Currently, the DF process cannot be scaled up sufficiently as a standalone technology, so further research is needed to improve process efficiency and overall cost-effectiveness. [
77]. This paper also discusses bioH
2 purification methods and storage techniques.
Finally, the current gaps in the progress of the DF process development may be highlighted through a SWOT analysis of bioH
2 production (
Table 1):
8. Photobiological Hydrogen Production
Photofermentation (PF) is the fermentative decomposition of volatile fatty acids (VFAs) and sugars into hydrogen (H
2) and carbon dioxide (CO
2) in the presence of light by a group of photosynthetic bacteria. Phototrophic bacteria produce H
2 in a series of biochemical reactions involving steps similar to those in anaerobic conversion, except that the bioH
2 is derived from organic compounds rather than water. This can be seen in the following equations:
Unlike plants, the electron donor in photosynthesis is not water but organic compounds, and the end product is not oxygen—hence the name anoxygenic photosynthesis. The process is catalysed by two enzymes, hydrogenase and nitrogenase, but in the absence of nitrogen in the system. The release of bioH
2 occurs during the reduction of molecular nitrogen as a result of the activity of nitrogenase, which, as in cyanobacteria, also reduces protons to molecular hydrogen. In the presence of light, purple non-sulfur bacteria, including species of
Rhodobacter,
Rhodospirillum and
Rhodopseudomonas, produce bioH2 from organic substrates, such as acetic, butyric and malic acids, as well as sugars, such as glucose and fructose. These bacteria demonstrate high substrate conversion efficiency and are relatively insensitive to oxygen [
78]. Most of these bacteria produce hydrogen via PF using the enzyme nitrogenase and organic acids. The production of hydrogen in photoautotrophic bacteria depends on several factors, including the intensity and wavelength of light radiation and the light pattern. While hydrogen production initially increases with light intensity, too much light can cause photoinhibition, which manifests differently depending on the type of microorganism. Gupta et al. [
79] compared the hydrogen production rates of several bacterial strains in PF under optimised conditions, including the appropriate substrate concentration, bioreactor type and volume, and light source intensity. For instance,
R. palustris produced hydrogen at a maximum rate of 3.2 mM H
2/h and
R. sphaeroides at up to 8.7 mM H
2/h.
A review [
80] has discussed advanced biotechnological approaches to improving bioH2 production in PF. Optimising the media, considering abiotic factors, adjusting the lighting regime, using immobilisation techniques, employing photoluminescent nanomaterials and applying genetic engineering have all been shown to increase photohydrogen production by purple non-sulfur bacteria. pH control in PF is very important. The results of the work of Guo et al. [
81] show that hydrogen production was lower at pH 6.5 than at pH 6.0, which was provided by a phosphate buffer.
Unfortunately, PF has the disadvantages of a low H
2 synthesis rate and a low light conversion efficiency and nitrogenase sensitivity to O
2. Therefore, to enhance bioH
2 production, various LCB pretreatment procedures have been developed, including physical, chemical and biological methods. Several key factors, such as pH, mixing and lighting conditions, substrate concentration, and different fermentation modes, have also been analysed in [
82].
Typical bioH
2 production by PF requires a thermostatically controlled photoreactor of sufficient volume with appropriate light intensity and pH control. It is well-known that light is necessary to provide the energy required for electron transport and hydrogen production. It is important to realise that light intensity must be distributed evenly throughout the system. In the PF reaction zone, three sub-areas are distinguished: the light inhibition zone, the light growth zone, and the light limitation zone. Unfortunately, these three zones do not provide similar efficiency in terms of light utilisation and energy consumption. This results in insufficient light intensity for bioH
2 production. It should also be noted that residual substrate and wastewater can shade the area, further reducing light penetration. The PF process has a high energy demand due to the high activation energy of nitrogenase enzymes. Zhang et al. [
82] determined the optimal mixing speed and light intensity by investigating the impact of mixing on lighting under various conditions. It was found that, using dynamic mixing and dynamic light intensity, corncob PF showed maximum hydrogen yield (84.7 mL H
2/g TS).
There are three main types of photobioreactor (PBR): plate, ring and tubular. Palamae et al. [
83] compared PBRs of the same volume but with different surface areas and found that those constructed of long, transparent glass U-tubes had the best H
2 production. Tubular reactors with a large surface area can be horizontal or vertical, depending on the design. Horizontal reactors allow for better light incidence than vertical designs. Flat photobioreactors made of glass, plexiglass or polycarbonate consist of a transparent rectangular cuboid that allows complete light penetration. While the height and width can be varied to some extent, the depth is limited to 5 cm. Like vertical tubular reactors, flat panel reactors have a similarly high surface area to volume ratio. Unfortunately, flat panel reactors have high energy consumption for aeration and rapid heat accumulation, which are significant drawbacks.
In [
84], a 4 m
3 continuous-flow baffle photoreactor comprising four consecutive chambers was tested to investigate the production of hydrogen from wastewater containing 10 g/L of glucose. The experiment was conducted at 30 °C under an intensity of light of 3000 ± 200 lux, with a hydraulic retention time (HRT) of 24–72 h. Sunlight was collected by a concentrator and delivered to each chamber of the photoreactor via an optical fibre. The photoreactor temperature was maintained by a solar-powered thermostatic water system. The inoculum microflora consisted of
R. rubrum,
R. capsulate,
R. palustris,
R. sphaeroides and
R. capsulatus. To achieve an HRT of 24, 48 or 72 h, the appropriate feed rate was set for the reactor. The hydrogen production rate varied significantly with the flow direction. The total H
2 production rate at these HRTs (72, 48 and 24 h) was 234, 310 and 381 mol H
2/d, respectively; that is to say, it increased with decreasing HRT.
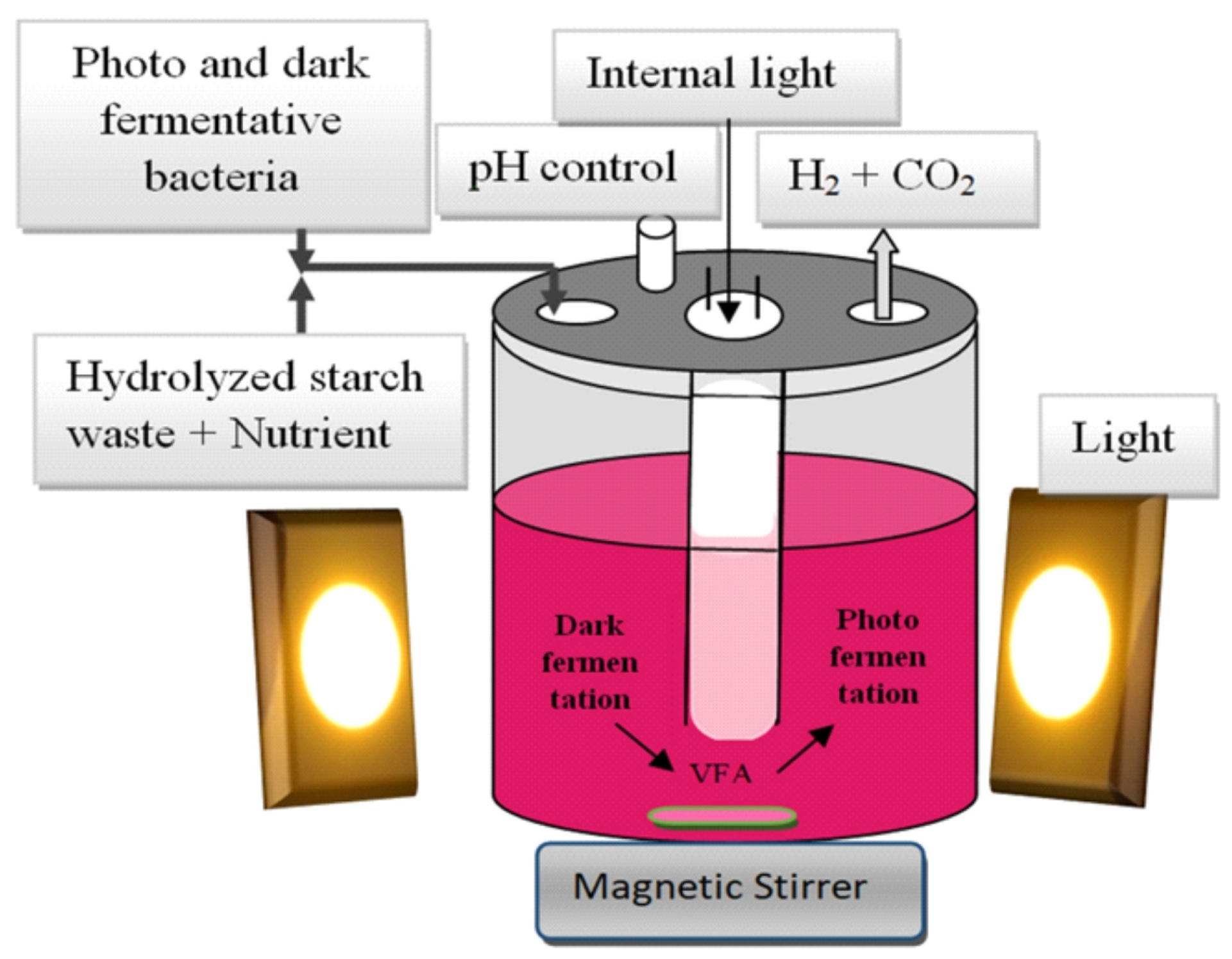
Single fermentation techniques have several disadvantages. In the case of PF, for example, complex carbohydrates cannot be used as a substrate, although VFA produced in the DF process can be used. However, this DF product decreased H2 yield. However, integrating these two fermentation techniques, e.g., sequential DF followed by PF or single-stage combined fermentation, can overcome these disadvantages.
9. Hybrid Biological Processes
The combination of DF and PF is a potential technique to improve the productivity of bioH2 production. The maximum theoretical yield of bioH2 production in DF is 4 mol H2/mol glucose. In contrast, PF using purple sulphur-free bacteria allows the by-products of DF (mainly butyric and acetic acids) to be used to produce additional amounts of hydrogen using solar energy. In hybrid fermentation, which combines DF and PF, the end products (organic acids and alcohols) produced by DF of carbohydrates can be further converted to bioH2 by photosynthetic bacteria.
As previously mentioned, there are two options for the integrated DF and PF process. The first is to use two separate reactors connected in series: one for the DF process and one for the PF process. The second option involves using a single reactor in which both processes take place, as shown in
Figure 5. This is known as a combined, hybrid, single-stage, mixed or co-culture system [
85]. The fatty acids produced during DF are utilised by PF and do not hinder hydrogen production. The total hydrogen yield is higher in the sequential two-stage system than in the mixed system. However, the single-stage hybrid DF and PF system is simpler to operate than the sequential two-stage fermentation system and eliminates the need for pumping the DF medium, making it more cost-effective for bioH
2 production. The total reactor volume can also be reduced due to the shorter fermentation time.
Nino-Navarro et al. [
86] mixed fruit and vegetable waste with cheese whey powder in order to vary the C/N ratio for two-step bioH
2 production. The DF process used a consortium consisting mainly of
Acetobacter lovaniensis,
Clostridium butyricum,
Enterobacter sp.,
Bifidobacterium and
Lactobacillus casei, while the PF process was dominated by
Rhodopseudomonas palustris. The lactate-rich effluent from the DF process was diluted and used for bioH
2 production via the PF process by
R. palustris bacteria. BioH
2 production was low during the DF step due to lactate production; however, when lactate was used as a substrate for PF, high H
2 production was enabled. Maximum total H
2 yields of 793.7 and 695.4 mL H
2/g COD were obtained at a dilution of 1:10 with C/N ratios of 60 and 70, respectively. These bioH
2 yields were higher than those achieved in single processes. A pilot-scale baffled bioreactor for sequential dark and photo-fermentation continuous hydrogen production was established [
87,
88], based on previous research results [
82,
84]. BioH
2 production in a pilot-scale fermenter depended on HTR and substrate concentration. The sustainability of pilot-scale hydrogen production systems was considered during the evaluation. It was estimated that the system would consume 171,530 MJ of energy and emit 9.37 tonnes of CO
2 equivalent for every tonne of hydrogen produced, with a payback period of 6.86 years. The authors’ analysis also suggests future avenues for the development of effective bioH
2 production technologies and their implementation.
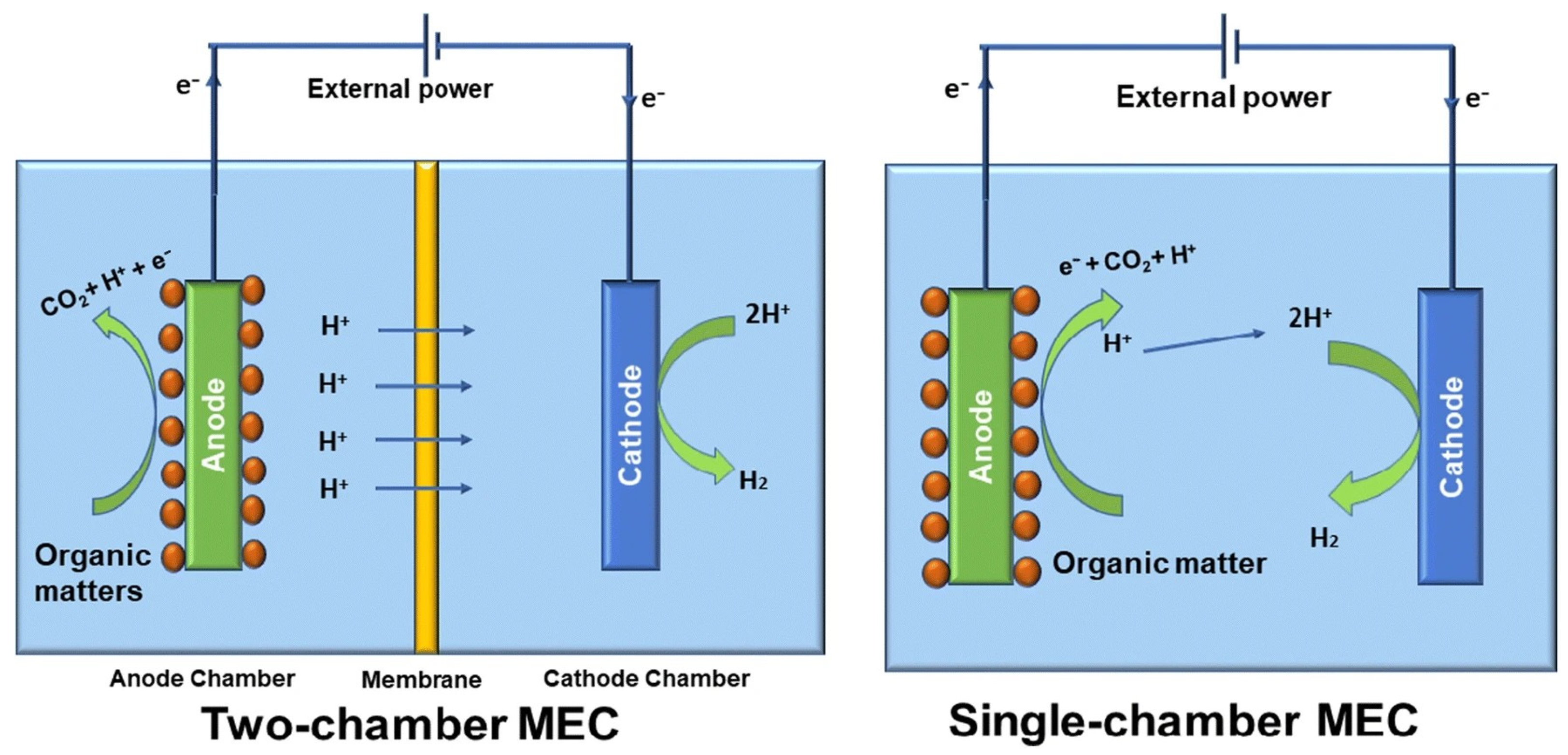
10. Bioelectrochemical Processes
Bioelectrochemical systems for H
2 production from LCB substrates using microbial electrolysis cells (MEC) are an emerging technology, also known as biocatalyzed electrolysis cells or electrofermentation, and could be a promising solution for future energy needs. Fuel cells operate based on redox reactions, whereby hydrogen (H
2) is oxidised at the anode and oxygen (O
2) is reduced at the cathode. In microbial fuel cells (MFCs), microorganisms convert organic matter into electricity through the microbial decomposition of organic compounds. In microbial electrolysis cells (MECs), however, the process is reversed: hydrogen (MEC-DF) or methane (MEC-AD) is produced from organic materials using an electric current. This process utilises the properties of electrogenic microorganisms, which catalyse the electrochemical oxidation of organic substrates, converting electrical energy into the chemical energy contained in H
2 produced by the electrochemical reduction of protons from an electrolyte medium—this medium may contain organic matter from wastewater. This is a valuable advantage of the bioH
2 production from wastewater using the bioelectrochemical method [
89].
The MEC has two electrodes, a cathode and an anode. These can be placed in the same chamber or in two separate chambers, which are separated by a proton exchange membrane (see
Figure 6). The anode chamber is filled with organic matter, while the cathode chamber is filled with a buffer solution. Electrons are generated by the oxidation of organic matter (acetic acid in this case) according to reaction 7 and are then transported to the anode. The electrons are then transferred to the cathode, where they reduce protons according to reaction (8), resulting in the formation of H
2 [
90]. Reaction (9) shows the general equation for the process occurring in MEC.
When we compare the processes that occur in an MEC with electrolysis for hydrogen production, the main advantage of the MEC is that water oxidation is replaced by the oxidation of organic compounds, which can occur at much lower redox potentials. The equilibrium voltage of an MEC cell is almost ten times lower (0.123 V) than the 1.23 V required for water electrolysis under standard conditions. This means that the energy consumption of the electrolytic cell, which is proportional to the cell voltage, is also lower.
Microorganisms that can transfer electrons from the cell to the anode are known as electrogens. Popular groups of electrogens include
Shewanella and
Geobacter species. Other exogenous species that have been described in recent studies include
Acetobacterium,
Ochrobactrum,
Sphingomonas,
Rhodopseudomonas palustris and
Rhodoferax ferrireducens. Rago et al. [
91] achieved an H
2 production efficiency of 2.6 l H
2/L/d in an alkaline environment (pH 9.3) using
Alkalibacter sp. in a microbial fuel cell (MFC).
The design and operation of an advanced electrochemical bioreactor (MEC) can be influenced by various factors, including reactor geometry and size, electrode material and configuration (e.g., the distance between the anode and cathode), the presence and type of membrane between the electrodes, the composition of the electrolyte medium, substrate concentration and the type of microorganisms in the anode space [
92]. Over the last five years, more than 20 review articles have been published on the study of bioelectrochemical processes and the development of MEC reactors. If the intrinsic conductivity of the electrodes is relatively low, such an MEC reactor must contain several electrical connections and large current collectors. Moreover, precise control procedures must be implemented.
An MEC process model was developed to estimate steady-state reactor performance and electrical efficiency. This model takes into account conservation laws, transport phenomena, microorganism kinetics, and a secondary current distribution model. Certain empirical correlations were also incorporated [
93]. A sensitivity analysis revealed that process efficiency depends strongly on the inlet fluid velocity. To maximise electrical efficiency, a gradient-free optimisation algorithm was employed. It was found that halving the hydraulic retention time (HRT), increasing the organic load by 35%, and slightly reducing the supply voltage increased electrical efficiency tenfold. This significant increase in productivity could be indicative of increased hydrogen production.
Some examples of the application of MEC for bioH
2 production from LCB are available in the literature. Bamboo biomass (
Bambusa bambos) was pre-treated with 10%
w/
w laccase, resulting in a 40% reduction in lignin content. This was followed by enzymatic hydrolysis using cellulase. After 96 h of incubation, the glucose solution (99.54 mg/dL) produced was subjected to further microbiological electrolysis in a single-chamber MEC, resulting in a maximum bioH
2 production of 224 mL H
2/gram of biomass [
94].
Wang et al. [
95] achieved another example of high hydrogen yield (91%) using lignocellulosic hydrolysate, with an H
2 production rate of 0.71 L H
2/L/d at an organic load of 0.4 g/d. They used a 10-litre single-chamber MEC with an electrode surface area-to-volume ratio of 66 m
2/m
3, consisting of four cathodes and seven anodes. The lignocellulosic hydrolysate was derived from raw Napier grass that had undergone preliminary enzymatic hydrolysis. The anode space was dominated by
Cloacibacillus spp.,
Tissierella spp.,
Enterococcus spp.,
Proteiniphilum spp. and
Desulfovibrio spp., while the cathode space was dominated by
Acetobacterium spp.,
Enterococcus spp.,
Methanobrevibacter spp. and
Anoxynatronum spp.
Another example of MEC application is provided by Gautman et al. [
96], who obtained 0.25 m
3 H
2/d using bagasse at an external supply voltage of 0.8 V, achieving an electrical energy efficiency of 97.47%. The bioanode was enriched with
Shewanella sp., which reduced the overpotential, resulting in a high current density of 62 A/m
2.
Traditional MEC designs incorporate membranes, but the membranes are costly and have limited membrane life due to biofilm fouling. Their poor stability and high cost have led researchers to develop various types of membrane-less electrolysis (MLEs) configurations with reduced cost. MEC structures without membranes (MLEs) can provide high hydrogen recovery efficiency and production rates [
97,
98]. Kumar et al. [
98] reviewed the latest MLEs research, comparing results obtained using different methods and presenting recommendations for future studies. MLEs can operate at low temperatures and high pressures, which facilitates the liquefaction of hydrogen for storage and transport. Various methods of operating without an electrolyte membrane have been described. For example, electrolyte flow can be used in gas diffusion electrodes via capillary action to directly extract gas as it is formed. Alternatively, O
2 evolution at the anode can be replaced with the use of more easily oxidisable compounds, such as methanol or ethanol. In this case, there is no need for a membrane as no oxygen is produced at the anode. This reduces energy requirements and allows for the production of carbon dioxide, protons and electrons, which are transferred to the electrode by exoelectrogenic bacteria.
Kilicaslan et al. [
99] proposed a new electrobioreactor for the production of bioH
2 using gas sparging with CO
2 and N
2. The study investigated the effect of various factors, including voltage changes, electrode materials, and gas sparging with CO
2 and N
2, on bioH
2 production. The results of the study showed that the type of electrode, the applied voltage, and the selection of the appropriate inoculum all significantly affect process efficiency when poplar leaves are used as a biomass source. Applying aluminium electrodes at a voltage of 2.0 V significantly increased bioH
2 production to 884 mL H
2/L within 11.25 min. Reduced partial pressure using CO
2 or N
2 gas sparging (at a volumetric flow rate of 400 mL/min) improved the bioH
2 production rate to 821 mL H
2/L within 4.25 min. The use of MEC has huge potential for the future, not only because it reduces pollutants in industrial wastewater, but also because it contributes to the recovery of energy resources from industrial wastewater in the form of bioH
2. Therefore, such a system can be described as both energy positive and carbon negative. The article [
100] proposes the integration of MEC with other processes, such as an integrated system with anaerobic digestion or an anaerobic membrane bioreactor with a thermoelectric microconverter.
11. Conclusions and Future Prospects
In the face of a changing energy economy based on fossil fuels, we need to move towards renewable energy sources, from a linear management of raw materials to a circular economy (CE). One such resource that meets these criteria is biomass. If we compare the reuse of materials in technical and biological cycles, we find that all materials used in production can be reused as nutrients in the biological cycle or as substrates in the technical production cycle. In a biological cycle, the nutrients are returned to the biosphere without harm, whereas in a technical cycle, the material is continuously reused and loses its value. For CE, not all products or waste can be converted into raw materials because transformations at each stage of the supply chain result in a decrease in energy quality due to the second law of thermodynamics, which gives a direction to the processes by generat-ing entropy that is irreversible. Lignocellulosic biomass is an inexpensive, renewable raw material that is readily available, making it the most attractive alternative to fossil fuels for energy production. As this review shows, LCB can be converted into various products, such as hydrogen, biogas, ethanol and butanol, through biological processes. In particular, the biological conversion of LCB to bioH2 has been reviewed, and improvement methods have been discussed, because bioH2 is a challenging candidate among green energy sources. If we want to achieve the Sustainable Development Goals by 2030, today, action to achieve the goals should be accelerated, and research should focus on improving LCB pretreatment methods and improving fermentation techniques. New research topics should include sustainability. The integration of thermochemical processes with biological conversion of LCBs, especially waste biomass, is an opportunity for further biorefinery development. Biomass obtained from cultivation or breeding is wet, and the use of thermochemical methods for its processing requires the dry form of biomass, and it is known that the drying process is energetically expensive. Therefore, the combination of biological methods that accept water content in biomass with hydrothermal methods seems to be the best solution proposed for biorefineries. Hydrothermal processes use water as a solvent and at the same time as a reactant at higher temperatures and under subcritical pressure. For example, the integration of traditional AD with thermochemical conversion processes, such as pyrolysis, or, better yet, with hydrothermal processes, such as hydrothermal carbonisation or hydrothermal liquefaction, allows the development of a closed-loop bioeconomy concept. Much hope is pinned on the use of advanced genetic modification techniques to ensure that microorganisms can adapt to different process conditions. However, 4G biorefineries are still in their infancy. Nevertheless, the technology readiness level (TRL) of the processes used in 2G and 3G biorefineries is close to 10, and there are many examples of their industrial application.
When we compare the profitability of producing biofuels with that of producing fuels derived from oil or natural gas, we still see an inequity that disadvantages biofuels. Although carbon neutrality is shifting in favour of biofuels, complete replacement of fossil fuels by biofuels is not yet possible. Additionally, there is a serious issue with waste biomass. Instead of being used, it is often disposed of in landfills, where it pollutes our atmosphere and increases the greenhouse effect. Therefore, it is necessary to process waste biomass—a free substrate—using biological and hydrothermal methods. It is therefore worth investing further in research into the biological processing of biomass into biofuels and other added-value products.