Exploring Hydrogen-Enriched Fuels and the Promise of HCNG in Industrial Dual-Fuel Engines
Abstract
1. Introduction
Explanation of the Introduction
2. Materials and Methods
2.1. Methodology and Materials Selection
2.1.1. Explanation of the Selection of a Specific Fuel for Further Consideration
- hydrogen in pure form—compressed or liquefied;
- methanol, HVO, and e-fuels;
- hydrogen bound to nitrogen in the form of ammonia;
- hydrogen is mixed with NG/methane to a compressed form of fuel named HCNG, H2CNG, or “hythane”.
2.1.2. Hydrogen in Its Pure Form
- The low energy density of hydrogen—hydrogen has a very low energy density, which means that much larger quantities of hydrogen are required to obtain the same amount of energy as from other fuels. In practice, required hydrogen storage tanks take up more space than tanks storing other hydrocarbon fuels, complicating the practical use of hydrogen for fueling ICEs [3].
- Safety—hydrogen, in its pure form, is highly flammable and explosive. For this reason, it requires special safety measures during storage, transport, and use. This requires high costs and additional procedures, which increases costs and hinders its widespread use, but keep in mind that safety procedures apply to all hydrocarbon fuels [61].
- Reactivity—hydrogen is a highly reactive fuel. It readily reacts chemically with other elements, including oxygen, and causes the so-called hydrogen sickness in steel, i.e., the chemical corrosion related to the high permeability of this element, which penetrates steel structures and causes internal damage. This involves the need to use unique materials resistant to this phenomenon and the fact that hydrogen, as the lightest and smallest of all the elements, freely penetrates the structures of other substances. Hence, there is a permanent loss of hydrogen in the tank in which it is stored. This phenomenon cannot be avoided in the case of hydrogen stored in pure form [62]. Hydrogen reactivity is visualized in cartoon form in Figure 5.
- Difficulty in distributing hydrogen—due to the lack of suitable infrastructure and the high reactivity of hydrogen, which causes hydrogen disease in steel in contact with it, as well as its permeability, as it can pass through structures that are not permeable to other fuels, such as steel pressure vessels, among others. For its mass distribution, tankers and pipelines will also be needed, and transport in its pure form cannot be based on the use of materials that are used to transport other liquefied or compressed hydrocarbon fuels [63].
- Storage difficulties—hydrogen gas requires special storage conditions. Tanks should withstand high pressure, and their material should resist hydrogen permeation and the hydrogen sickness it causes. Appropriate materials and technology must be used to ensure safety and optimum storage conditions. In its liquefied form, hydrogen requires very low storage temperatures and constant cooling due to the orthopnea transformations that occur in hydrogen, which gives off heat in this process. An additional factor in favor of the liquefaction method of stored hydrogen is the possibility of improving its purity with this method and the significantly higher potential for deifying the energy it contains. This is because the process of compressing hydrogen does not proceed as in the case of the compression of an ideal gas—as the pressure under which the compressed hydrogen is stored increases, the increase in its density decreases—thus increasing the amount of energy that must be put into further increasing the density of the compressed hydrogen. It is crucial to know that CNG is compressed under 200 atmospheres—compressed hydrogen is used under a pressure of about 300–700 atmospheres [64,65,66]. Compressing or liquefying hydrogen under such considerable pressures or low temperatures is expensive. The phenomena of the differences between hydrogen and ideal gas compression process can be seen in the graph in Figure 6 below, where hydrogen is compared with ideal gas [58].
- Lack of infrastructure—the distribution problem, as mentioned earlier, also translates into the lack of a hydrogen distribution network, both on a local and global scale. All investments aimed at the mass transmission of hydrogen over longer distances are still only in the planning stage [67]. The local distribution network is deficient, and hydrogen can currently only be delivered to selected locations [68], often creating only temporary, local refueling stations [69].
- Difficulty of production—hydrogen in its pure form is not found in natural sources, so it must be produced. Currently, the most common processes for producing hydrogen from hydrocarbon feedstocks involve greenhouse gas emissions and negative environmental impacts. All standard hydrogen production methods also involve the production of other substances (Figure 3). Pure hydrogen must, therefore, be separated from other products. Producing pure hydrogen is expensive but represents a milestone in organizing a zero-carbon economy. Product liquefaction is the most effective method of purifying the produced hydrogen from impurities, which involves cooling the produced gas below 20.4 K [49].
2.1.3. Methanol, HVO and E-Fuels
2.1.4. Ammonia Used as a Fuel for ICEs
- High chemical reactivity, causing corrosion of steel;
- Harmful in contact with living organisms;
- High volatility under normal conditions, posing a danger to humans who inhale ammonia vapor;
- High freezing point, causing ammonia to solidify when it is used in winter conditions;
- Higher N2O emission from ICE using ammonia as a fuel [117];
- Low energy density relative to other liquid motor fuels;
2.1.5. HCNG, H2CNG, Hythane
3. Results
3.1. Basic Properties of HCNG
3.1.1. Quantum Hydrogen Fuel Properties, and Why Is There no “HLNG” Fuel?
3.1.2. Characteristics of HCNG Properties Relevant to Its Use as Engine Fuel
3.2. Discrepancies in HCNG Data
4. Discussion
- Density of NG used in the European Union (EU);
- Air hydrogen fuel ratio;
- Wobbe index;
- Combustion temperature;
- Parameters determining the safety of using HCNG;
- Emission of harmful components in the exhaust gas.
- Firstly, it was impossible to review all the available literature, so the authors may have omitted some essential sources;
- Secondly, it was not possible to determine in a simple, computational way many parameters that would be important from the point of view of using HCNG in DF ICEs;
- Some of the parameters should be examined experimentally or numerically in a more advanced way and with greater accuracy than in the existing, widely available literature;
- Another limitation is the knowledge of the exact chemical composition of natural gas, which is highly variable, and adding its variability to the calculations would lead to a solid dispersion in the results [14].
5. Conclusions
- The NG distribution infrastructure allows the distribution of HCNG without incurring additional significant expansion costs—the costs of modifying it are a fraction of the costs associated with creating a supply infrastructure for other hydrogen fuels;
- Using HCNG with a hydrogen concentration of no more than 50% of the fuel content in industrial DF engines allows lower CO2 emissions than in SI engines. This will also be the case for smaller CI engines if the degree of substitution achieved in this engine is sufficiently high. Therefore, this fuel fits in very well with current trends in the use of DF engines in industry and transport;
- Carbon dioxide emissions can be reduced to zero if HCNG contains nearly 100% hydrogen;
- The decrease in energy density of HCNG with increasing hydrogen content in the fuel can be compensated for by increasing the storage pressure of the fuel.
- The non-linear change in the density of HCNG depending on the hydrogen content in the mixture is consistent with the available data on this subject because hydrogen has properties different from those of an ideal gas;
- The decrease in the Wobbe index is only initially above a particular value; this coefficient starts to increase, which is a good indication of the stability of this fuel. The Wobbe index reaches its minimum value at a certain hydrogen content in the HCNG mixture;
- The increase in combustion temperature with increasing hydrogen content will not pose a problem for using this fuel in the engine, as these values are not significantly higher than the base values;
- Adding hydrogen to CNG improves the value of the adiabatic exponent, which affects the efficiency of the ICE. However, its effect cannot be precisely determined using the divergent data from scientific publications. This property has excellent potential for further research on this topic;
- An analysis of the literature revealed a lack of specific standards for HCNG fuel. In the available literature, a vast discrepancy was found in the data on hydrogen’s physical and chemical properties. Information in the popular science literature also contains discrepancies to mislead the reader into a particular reaction to the information read. These cases, in the authors’ opinion, unambiguously indicate the need for verification and further analysis of the properties of HCNG fuel, defining in which range of values of specified parameters the properties of this fuel should be contained.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BLEVE | boiling liquid expanding vapor explosion |
| CI | compression ignition |
| CH4 | methane |
| CNG | compressed natural gas |
| CO | carbon monoxide |
| CO2 | carbon dioxide |
| DF | dual-fuel |
| EU | European Union |
| GHG | greenhouse gas(es) |
| GWP | global warming potential |
| H2 | hydrogen |
| H2CNG | hydrogen to compressed natural gas |
| HCCI | homogeneous charge compression ignition |
| HCNG | hydrogen enriched compressed natural gas |
| HDO | hydrodeoxy-generated fuel |
| HLNG | hydrogen-enriched liquefied natural gas |
| HVO | hydrotreated vegetable oil |
| IC | internal combustion |
| ICE | internal combustion engine(s) |
| LNG | liquefied natural gas |
| LPG | liquefied petroleum gas |
| LTC | low-temperature combustion |
| NOX | nitrogen oxide |
| RCCI | reactivity-controlled compression ignition |
| RES | renewable energy sources |
| SI | spark ignition |
| TSO | transmission system operators |
| UOP | fuel name adopted from the name of the enterprise |
| US | United States |
| WTW | well-to-wheel |
References
- Collis, J.; Schomäcker, R. Determining the Production and Transport Cost for H2 on a Global Scale. Front. Energy Res. 2022, 10, 909298. [Google Scholar] [CrossRef]
- Breeze, P. Chapter 8—Hydrogen Energy Storage. In Power System Energy Storage Technologies; Breeze, P., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 69–77. ISBN 978-0-12-812902-9. [Google Scholar]
- Surygała, J. Wodór Jako Paliwo; Wydawnictwo WNT: Warsaw, Poland, 2007; ISBN 978-83-204-3457-6. [Google Scholar]
- Climstra, J.; Hotakainen, M. Smart Power Generation; Avain: Finland, Helsinki, 2011; ISBN 978-951-692-846-6. [Google Scholar]
- Sanchez, A.G.; Rizzato Lede, A.; Bärwaldt, G.; Molina, M. Análisis Regional de La Red Eléctrica de Alemania En Relación Con La Creciente Penetración de Generación Solar Fotovoltaica Distribuida. Rev. Cienc. Tecnol. 2012, 18, 21–27. [Google Scholar]
- Kath, C.; Nitka, W.; Serafin, T.; Weron, T.; Zaleski, P.; Weron, R. Balancing Generation from Renewable Energy Sources: Profitability of an Energy Trader. Energies 2020, 13, 205. [Google Scholar] [CrossRef]
- Hydrogen Energy Storage. Energy Storage Association. Available online: https://www.fchea.org/hydrogen-as-storage (accessed on 25 March 2023).
- Is Hydrogen Internal Combustion a Better Idea than Fuel Cells? Available online: https://www.greencarreports.com/news/1122364_is-hydrogen-internal-combustion-a-better-idea-than-fuel-cells-engineering-explained (accessed on 10 April 2023).
- Hydrogen Internal Combustion Engines and Hydrogen Fuel Cells. Available online: https://www.cummins.com/news/2022/01/27/hydrogen-internal-combustion-engines-and-hydrogen-fuel-cells (accessed on 10 April 2023).
- Lambert, F. Study Confirms What Common Sense Has Made Clear for Years: Hydrogen Fuel Cells Cannot Catch up to Battery-Electric Vehicles. Available online: https://electrek.co/2022/02/15/study-hydrogen-fuel-cells-cannot-catch-up-battery-electric-vehicles/ (accessed on 10 April 2023).
- Lalsangi, S.; Yaliwal, V.; Banapurmath, N.; Soudagar, M.E.M.; Balasubramanian, D.; Sonthalia, A.; Varuvel, E.G.; Wae-Hayee, M. Influence of Hydrogen Injection Timing and Duration on the Combustion and Emission Characteristics of a Diesel Engine Operating on Dual Fuel Mode Using Biodiesel of Dairy Scum Oil and Producer Gas. Int. J. Hydrogen Energy 2023, 48, 21313–21330. [Google Scholar] [CrossRef]
- Szamrej, G.; Karczewski, M.; Chojnowski, J. A Review of Technical Solutions for RCCI Engines. Combust. Engines 2022, 189, 36–46. [Google Scholar] [CrossRef]
- Karczewski, M.; Szamrej, G.; Chojnowski, J. Experimental Assessment of the Impact of Replacing Diesel Fuel with CNG on the Concentration of Harmful Substances in Exhaust Gases in a Dual Fuel Diesel Engine. Energies 2022, 15, 4563. [Google Scholar] [CrossRef]
- Karczewski, M.; Chojnowski, J.; Szamrej, G. A Review of Low-CO2 Emission Fuels for a Dual-Fuel RCCI Engine. Energies 2021, 14, 5067. [Google Scholar] [CrossRef]
- Karczewski, M.; Szamrej, G. Experimental Evaluation of the Effect of Replacing Diesel Fuel by CNG on the Emission of Harmful Exhaust Gas Components and Emission Changes in a Dual-Fuel Engine. Energies 2023, 16, 475. [Google Scholar] [CrossRef]
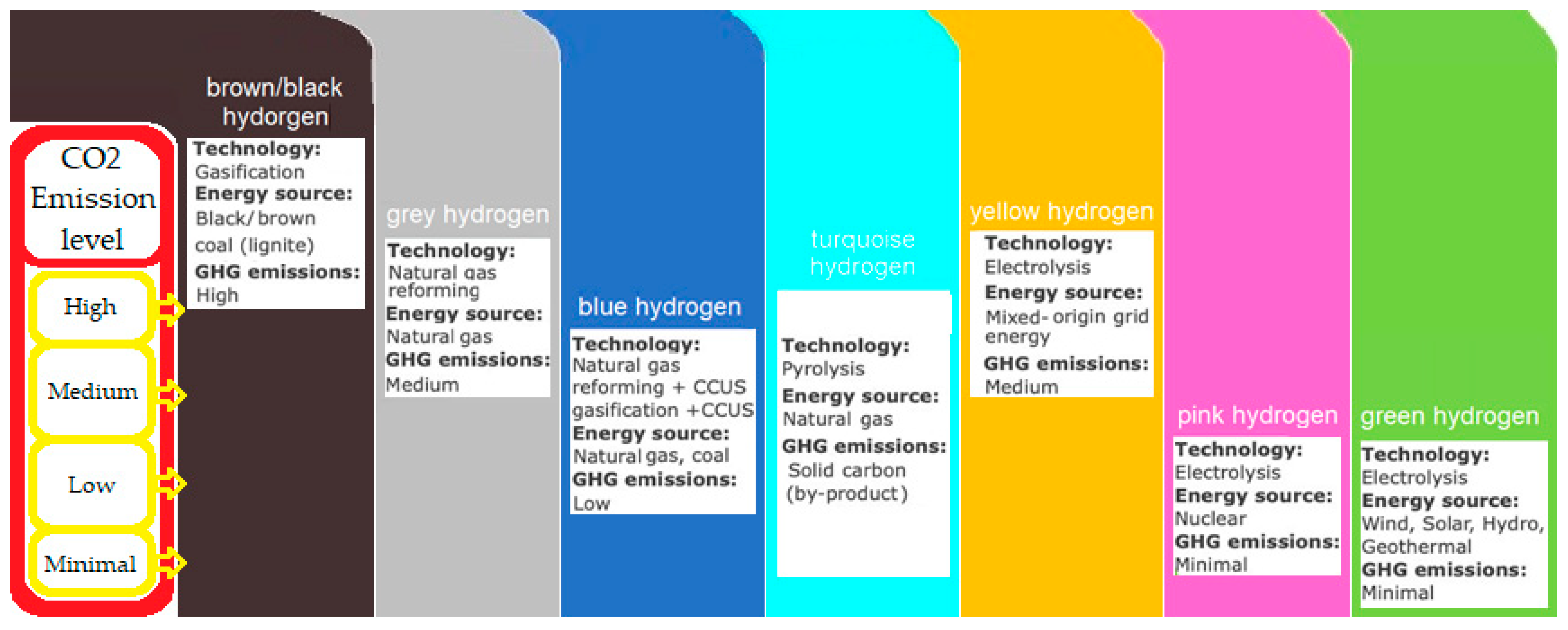
- Arcos, J.M.M.; Santos, D.M.F. The Hydrogen Color Spectrum: Techno-Economic Analysis of the Available Technologies for Hydrogen Production. Gases 2023, 3, 25–46. [Google Scholar] [CrossRef]
- What Is ‘White Hydrogen’ and Could It Be a Fuel of the Future? Available online: https://www.euronews.com/green/2023/11/05/what-is-white-hydrogen-the-pros-and-cons-of-europes-latest-clean-energy-source (accessed on 1 February 2024).
- France’s Bold Quest for “White Hydrogen” Reservoirs—Green Hydrogen News. Available online: https://energynews.biz/frances-bold-quest-for-white-hydrogen-reservoirs/ (accessed on 1 February 2024).
- France Uncovers Massive White Hydrogen Deposit|OilPrice.Com. Available online: https://oilprice.com/Energy/Energy-General/France-Uncovers-Massive-White-Hydrogen-Deposit.html (accessed on 1 February 2024).
- Pokatina, A.; Hydrogen Europe. Hydrogen Europe. 2023. Available online: https://hydrogeneurope.eu/excitement-grows-about-natural-h2-as-huge-reserves-found-in-france/ (accessed on 12 March 2023).
- Cooper, J.; Dubey, L.; Bakkaloglu, S.; Hawkes, A. Hydrogen Emissions from the Hydrogen Value Chain-Emissions Profile and Impact to Global Warming. Sci. Total Environ. 2022, 830, 154624. [Google Scholar] [CrossRef]
- Zasady Monitorowania Emisji Gazów Cieplarnianych z Dodatkowych Działalności Włączonych do EU ETS. Available online: https://www.kobize.pl/uploads/materialy/materialy_do_pobrania/opracowania/Zasady_monitorowania_emisji_gazow_cieplarnianych_z_dodatkowych_dzialalnosci_wlaczonych_do_EU_ETS.pdf (accessed on 12 March 2023).
- Smol, E.; Jeszke, R.; Sikora, P.; Błachowicz, A. Metodyka Wraz z Przykładowym Obliczeniem “Limitu” Krajowej Emisji Gazów Cieplarnianych dla Polski na Lata 2013–2020; KASHUE-KOBiZE: Warsaw, Poland, 2010. [Google Scholar]
- Sand, M.; Skeie, R.B.; Sandstad, M.; Krishnan, S.; Myhre, G.; Bryant, H.; Derwent, R.; Hauglustaine, D.; Paulot, F.; Prather, M.; et al. A Multi-Model Assessment of the Global Warming Potential of Hydrogen. Commun. Earth Environ. 2023, 4, 203. [Google Scholar] [CrossRef]
- Parkes, R. Hydrogen Is a More Potent Greenhouse Gas than Previously Reported, New Study Reveals. Available online: https://www.hydrogeninsight.com/analysis/hydrogen-is-a-more-potent-greenhouse-gas-than-previously-reported-new-study-reveals/2-1-1463495 (accessed on 4 February 2024).
- Warwick, N.; Griffiths, P.; Keeble, J.; Archibald, A.; Pyle, J. Atmospheric Implications of Increased Hydrogen Use; University of Cambridge and NCAS Keith Shine, University of Reading. April 2022. Available online: https://www.gov.uk/government/publications/atmospheric-implications-of-increased-hydrogen-use (accessed on 21 May 2023).
- Erbach, G.; Liselotte, J. BRIEFING Towards climate neutrality; European Parliament; EU Hydrogen Policy Hydrogen as an Energy Carrier for a Climate-Neutral Economy; Climate Action Research and Tracking Service, Members’ Research Service; PE 689.332. April 2021. Available online: https://www.europarl.europa.eu/RegData/etudes/BRIE/2021/689332/EPRS_BRI(2021)689332_EN.pdf (accessed on 21 May 2023).
- Mehrzad, K.; Saint-Just, J.; Hulteberg, C.; Corfitz, N. HCNG—A Dead End or a Bridge to the Future? In Proceedings of the 18th World Hydrogen Energy Conference, Germany, Essen, 16–21 May 2010. [Google Scholar]
- Green & Blue Hydrogen: Current Levelized Cost of Production & Outlook|GEP Blog. Available online: https://www.gep.com/blog/strategy/Green-and-blue-hydrogen-current-levelized-cost-of-production-and-outlook (accessed on 3 February 2024).
- Martínez de León, C.; Ríos, C.; Brey, J.J. Cost of Green Hydrogen: Limitations of Production from a Stand-Alone Photovoltaic System. Int. J. Hydrogen Energy 2023, 48, 11885–11898. [Google Scholar] [CrossRef]
- Estimated Investment & Cost|EHB European Hydrogen Backbone. Available online: https://ehb.eu/page/estimated-investment-cost (accessed on 10 April 2023).
- Ordóñez, D.F.; Ganzer, C.; Halfdanarson, T.; Garay, A.G.; Patrizio, P.; Bardow, A.; Guillén-Gosálbez, G.; Shah, N.; Dowell, N.M. Quantifying Global Costs of Reliable Green Hydrogen. Energy Adv. 2023, 2, 2042–2054. [Google Scholar] [CrossRef]
- Europe to Find out True Price of Renewable Hydrogen—Cipher News. Available online: https://ciphernews.com/articles/europe-to-find-out-true-price-of-renewable-hydrogen/ (accessed on 3 February 2024).
- Executive Summary—Global Hydrogen Review 2021—Analysis. Available online: https://www.iea.org/reports/global-hydrogen-review-2021/executive-summary (accessed on 3 February 2024).
- Katebah, M.; Al-Rawashdeh, M.; Linke, P. Analysis of Hydrogen Production Costs in Steam-Methane Reforming Considering Integration with Electrolysis and CO2 Capture. Clean. Eng. Technol. 2022, 10, 100552. [Google Scholar] [CrossRef]
- Zang, G.; Graham, E.J.; Mallapragada, D. H2 Production through Natural Gas Reforming and Carbon Capture: A Techno-Economic and Life Cycle Analysis Comparison. Int. J. Hydrogen Energy 2024, 49, 1288–1303. [Google Scholar] [CrossRef]
- lhancox How Biomass Gasification Can Boost Hydrogen Production. Available online: https://eqtec.com/how-biomass-gasification-can-help-boost-global-green-hydrogen-production/ (accessed on 4 February 2024).
- Demusiak, G. Otrzymywanie Paliwa Wodorowego Metodą Reformowania Gazu Ziemnego Dla Ogniw Paliwowych Małej Mocy. Nafta-Gaz 2012, 10, 661–673. [Google Scholar]
- Kumar, S.S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Reports 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Zgazowanie Węgla Szansą Dla Górnictwa. Available online: http://www.giph.com.pl/biuletyn-gorniczy/biuletyn-gorniczy-nr-4-6-272-274-kwiecien-maj-czerwiec-2018-r/zgazowanie-wegla-szansa-dla-gornictwa-246/ (accessed on 10 April 2023).
- Valverde, M. Hydrogen from Coal. Available online: https://www.coalage.com/features/hydrogen-from-coal/ (accessed on 10 April 2023).
- Allen, J. Explainer: How Do We Make Hydrogen from Coal, and Is It Really a Clean Fuel? Available online: http://theconversation.com/explainer-how-do-we-make-hydrogen-from-coal-and-is-it-really-a-clean-fuel-94911 (accessed on 10 April 2023).
- What Are the Colours of Hydrogen and What Do They Mean? Available online: https://www.acciona.com.au/updates/stories/what-are-the-colours-of-hydrogen-and-what-do-they-mean/ (accessed on 17 April 2023).
- Hydrogen Cost and Sales Prices|H2Valleys. Available online: https://h2v.eu/analysis/statistics/financing/hydrogen-cost-and-sales-prices (accessed on 3 February 2024).
- Blue or Green Hydrogen? What Colour Will the Fuel of the Future Be?|Costain. Available online: https://www.costain.com/news/insights/blue-or-green-hydrogen-what-colour-will-the-fuel-of-the-future-be/ (accessed on 17 April 2023).
- Upadhyay, G. ⚡ The Reality Behind Green Hydrogen. ReadOn. 2022. Available online: https://readon.substack.com/p/-the-reality-behind-green-energy (accessed on 20 April 2023).
- Hydrogen Present and Future. Part 2—Técnicas Reunidas. Available online: https://www.tecnicasreunidas.es/articulo/hydrogen-present-and-future-part-2/ (accessed on 12 April 2023).
- AG, E. The Colors of Hydrogen: An Overview. Available online: https://www.ewe.com/en/shaping-the-future/hydrogen/the-colours-of-hydrogen (accessed on 17 April 2023).
- The “Colors” of Hydrogen. Available online: https://aeclinic.org/aec-blog/2021/6/24/the-colors-of-hydrogen (accessed on 10 April 2023).
- Cheng, W.; Lee, S. How Green Are the National Hydrogen Strategies? Sustainability 2022, 14, 1930. [Google Scholar] [CrossRef]
- The Hydrogen Rainbow: More than Blue and Green|WSP. Available online: https://www.wsp.com/en-gb/insights/the-hydrogen-rainbow-more-than-blue-and-green (accessed on 17 April 2023).
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Multi-stage Flexible Planning of Regional electricity-HCNG-integrated Energy System Considering Gas Pipeline Retrofit and Expansion—Qiu—2022—IET Renewable Power Generation—Wiley Online Library. Available online: https://ietresearch.onlinelibrary.wiley.com/doi/full/10.1049/rpg2.12586 (accessed on 11 April 2023).
- Evanko, J. Chart Industries. 2020. Available online: https://www.chartindustries.com/Products/Hydrogen-Energy (accessed on 20 May 2023).
- Hydrogen Production Technologies: From Fossil Fuels toward Renewable Sources. A Mini Review|Energy & Fuels. Available online: https://pubs.acs.org/doi/10.1021/acs.energyfuels.1c02501 (accessed on 11 April 2023).
- Hydrogen in Industrial Applications. Available online: https://www.fchea.org/hydrogen-in-industrial-applications (accessed on 11 April 2023).
- Cox, R. Waste/By-Product Hydrogen. Available online: https://www.energy.gov/eere/fuelcells/articles/wasteby-product-hydrogen (accessed on 10 April 2023).
- Paweł, D.; Marek, I.; Maciej, B.; Wojciech, K. Perspektywa Progresu Wskaźników Ekologicznych Silnika Badawczego Zasilanego Olejem Napędowym Z Domieszką Wodoru. Available online: https://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-d2be508c-5a90-486b-bedb-b938a8d753ce (accessed on 12 April 2023).
- Czepirski, L. Magazynowanie wodoru w porowatych materiałach węglowych. Biul. Polskiego Stowarzyszenia Wodoru Ogniw Paliw. 2007, 2, 36–40. [Google Scholar]
- Wodorki Metalu Do Magazynowania i Sprężania Wodoru|ATLAS-H2 Project|Results in Brief|FP7|CORDIS|European Commission. Available online: https://cordis.europa.eu/article/id/188584-metal-hydrides-for-hydrogen-storage-and-hydrogen-compression/pl (accessed on 13 April 2023).
- Leachman, J. So Just How Dangerous Is Hydrogen Fuel? In HYdrogen Properties for Energy Research (HYPER) Laboratory; Washington State University: Pullman, WA, USA, 2017. [Google Scholar]
- Hydrogen—Production and Applications of Hydrogen|Britannica. Available online: https://www.britannica.com/science/hydrogen/Reactivity-of-hydrogen (accessed on 11 April 2023).
- Steen, M. 12—Building a Hydrogen Infrastructure in the EU. In Compendium of Hydrogen Energy; Ball, M., Basile, A., Veziroğlu, T.N., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing: Oxford, UK, 2016; pp. 267–292. ISBN 978-1-78242-364-5. [Google Scholar]
- Barthélémy, H. Hydrogen Storage—Industrial Prospectives. Int. J. Hydrogen Energy 2012, 37, 17364–17372. [Google Scholar] [CrossRef]
- What Is Hydrogen Storage and How Does It Work? Available online: https://www.twi-global.com/technical-knowledge/faqs/what-is-hydrogen-storage.aspx (accessed on 13 April 2023).
- HOW TO STORE HYDROGEN? CIRO Project. Available online: http://ciroproject.com/how-to-store-hydrogen#:~:text=Storage%20of%20hydrogen%20as%20a,to%20bring%20down%20the%20temperature (accessed on 11 April 2023).
- Alternative Fuels Data Center: Hydrogen Fueling Infrastructure Development. Available online: https://afdc.energy.gov/fuels/hydrogen_infrastructure.html (accessed on 11 April 2023).
- MOBILE HYDROGEN TRANSPORT UNITS—Powertech Labs. Available online: https://powertechlabs.com/mobile-hydrogen-transport-units/ (accessed on 11 April 2023).
- ORLEN Will Build the First Hydrogen Refueling Stations in Poland. Hydrogen Central. 2021. Available online: https://www.orlen.pl/en/sustainability/transition-projects/ORLEN-Group-Hydrogen-Strategy-2030/News-about-hydrogen/ORLEN-will-build-the-first-hydrogen-refueling-stations (accessed on 21 April 2023).
- Kurmayer, N.J. EU Eyes CO2-Hydrogen Combination to Make Synthetic Fuels. Available online: https://www.euractiv.com/section/energy-environment/news/eu-eyes-co2-hydrogen-combination-to-make-synthetic-fuels/ (accessed on 11 April 2023).
- Energies|Free Full-Text|An Overview of Major Synthetic Fuels. Available online: https://www.mdpi.com/1996-1073/16/6/2834 (accessed on 11 April 2023).
- Hydrogen Storage. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-storage (accessed on 11 April 2023).
- World First for Liquid Hydrogen Transportation. Available online: https://www.lr.org/en/insights/articles/world-first-for-liquid-hydrogen-transportation/ (accessed on 14 April 2023).
- SAG Develops First LH2 Cryogenic Tank System for Trucks—Weltmarktführer Bei Aluminium-Tanks|SAG. Available online: https://www.sag.at/en/sag-develops-first-lh2-cryogenic-tank-system-for-trucks/ (accessed on 14 April 2023).
- Boehm, M. Hydrogen On-Board Storage Options for Rail Vehicles. In Proceedings of the European Hydrogen Energy Conference 2022, Spain, Madrid, 18–20 May 2022. [Google Scholar]
- Ahluwalia, R.K.; Roh, H.-S.; Peng, J.-K.; Papadias, D.; Baird, A.R.; Hecht, E.S.; Ehrhart, B.D.; Muna, A.; Ronevich, J.A.; Houchins, C.; et al. Liquid Hydrogen Storage System for Heavy Duty Trucks: Configuration, Performance, Cost, and Safety. Int. J. Hydrogen Energy 2023, 48, 13308–13323. [Google Scholar] [CrossRef]
- Mobilizing Buses and Trains with H2. Available online: https://www.linde-engineering.com/en/about-linde-engineering/success-stories/mobilizing-public-buses-and-trains-with-h2.html (accessed on 14 April 2023).
- Ohira, K. 3—Slush Hydrogen Production, Storage, and Transportation. In Compendium of Hydrogen Energy; Gupta, R.B., Basile, A., Veziroğlu, T.N., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing: Oxford, UK, 2016; pp. 53–90. ISBN 978-1-78242-362-1. [Google Scholar]
- Suda, S. Hydrogen–Metal Systems: Applications of Gas–Solid Reactions. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2001; pp. 3895–3905. ISBN 978-0-08-043152-9. [Google Scholar]
- Kohlmann, H. Metal Hydrides. In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: New York, NY, USA, 2003; pp. 441–458. ISBN 978-0-12-227410-7. [Google Scholar]
- Metal Hydrides—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/B9780124095472058947 (accessed on 14 April 2023).
- Allevi, C.; Collodi, G. 12—Hydrogen Production in IGCC Systems. In Integrated Gasification Combined Cycle (IGCC) Technologies; Wang, T., Stiegel, G., Eds.; Woodhead Publishing: Oxford, UK, 2017; pp. 419–443. ISBN 978-0-08-100167-7. [Google Scholar]
- Liquefied Natural Gas (LNG) Energy KnowledgeBase. Available online: https://energyknowledgebase.com/topics/liquefied-natural-gas.asp (accessed on 4 February 2024).
- Blanke, W.; Thomas, W. The Temperatures of the Triple Points of Methane and Argon on IPTS-68. Measurement 1983, 1, 105–108. [Google Scholar] [CrossRef]
- Monterey, G. Energy Requirements for Hydrogen Gas Compression and Liquefaction as Related to Vehicle Storage Needs; US Department of Energy (DOE): Washington, DC, USA, 2009.
- Waqar, M.; Ishaq, H.; Jamil, S.R. Energetically Enhanced Natural Gas Liquefaction Process with CO2 Precooling. Energy Convers. Manag. X 2022, 14, 100200. [Google Scholar] [CrossRef]
- Franco, A.; Casarosa, C. Thermodynamic Analysis of Direct Expansion Configurations for Electricity Production by LNG Cold Energy Recovery. Appl. Therm. Eng. 2015, 78, 649–657. [Google Scholar] [CrossRef]
- What Are eFuels?—eFuel Alliance. Available online: https://www.efuel-alliance.eu/efuels/what-are-efuels (accessed on 11 April 2023).
- Low Carbon Pulse—Edition 34. Available online: https://www.ashurst.com/en/news-and-insights/insights/low-carbon-pulse-edition-34/ (accessed on 17 April 2023).
- Direct Air Capture CO2 for Aviation E-Fuels Production Faces Many Obstacles, Finds E4Tech Study—GreenAir News. Available online: https://www.greenairnews.com/?p=1334 (accessed on 11 May 2023).
- E-Methanol—The Future Fuel?|Wallenius Sol. Available online: https://wallenius-sol.com/en/enabler-magazine/e-methanol-future-fuel (accessed on 11 April 2023).
- Wijayapala, R.; Karunanayake, A.G.; Proctor, D.; Yu, F.; Pittman, C.U.; Mlsna, T.E. Hydrodeoxygenation (HDO) of Bio-Oil Model Compounds with Synthesis Gas Using a Water Gas Shift Catalyst with a Mo/Co/K Catalyst. In Handbook of Climate Change Mitigation and Adaptation; Chen, W.-Y., Suzuki, T., Lackner, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1903–1935. ISBN 978-3-319-14409-2. [Google Scholar]
- Kumar, P.; Verma, D.; Sibi, M.G.; Butolia, P.; Maity, S.K. Chapter 4—Hydrodeoxygenation of Triglycerides for the Production of Green Diesel: Role of Heterogeneous Catalysis. In Hydrocarbon Biorefinery; Maity, S.K., Gayen, K., Bhowmick, T.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 97–126. ISBN 978-0-12-823306-1. [Google Scholar]
- Hydrodeoxygenation—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/engineering/hydrodeoxygenation (accessed on 11 April 2023).
- Meeting the Future’s Energy Challenges with Green Fuels. Available online: https://uop.honeywell.com/content/uop/en/us/home/industry-solutions/renewable-fuels.html (accessed on 11 April 2023).
- What Is HVO100? Available online: https://www.neste.be/en/neste-my-renewable-diesel-be/product-information/what-is-hvo (accessed on 11 April 2023).
- HVO—Fossil Free Diesel—90% CO2 Reduction. Biofuel Express. Available online: https://biofuel-express.com/products/hvo100-renewable-diesel/ (accessed on 11 April 2023).
- Howey, W. EU Decision on E-Fuels Unblocks Electric-Vehicle Targets. Available online: https://www.eiu.com/n/eu-decision-on-e-fuels-unblocks-electric-vehicle-targets/ (accessed on 13 April 2023).
- E-Fuels Could Keep Combustion Engine Cars on the Road in the EU Past Its 2035 Climate Deadline—The Verge. Available online: https://www.theverge.com/2023/3/28/23660005/e-fuels-internal-combustion-engine-car-european-union-2035-climate-deadline (accessed on 13 April 2023).
- In Win for Germany, EU Agrees to Exempt e-Fuels from 2035 Ban on New Sales of Combustion-Engine Cars|Euronews. Available online: https://www.euronews.com/my-europe/2023/03/28/in-win-for-germany-eu-agrees-to-exempt-e-fuels-from-2035-ban-on-new-sales-of-combustion-en (accessed on 13 April 2023).
- Fit for 55. Available online: https://www.consilium.europa.eu/en/policies/green-deal/fit-for-55-the-eu-plan-for-a-green-transition/ (accessed on 17 April 2023).
- SAE MOBILUS. Available online: https://saemobilus.sae.org/content/2008-01-2500/ (accessed on 11 April 2023).
- Kuronen, M.; Mikkonen, S.; Aakko, P.; Murtonen, T. Hydrotreated Vegetable Oil as Fuel for Heavy Duty Diesel Engines; SAE International: USA, Warrendale, PA, 2007. [Google Scholar]
- Dimitriadis, A.; Natsios, I.; Dimaratos, A.; Katsaounis, D.; Samaras, Z.; Bezergianni, S.; Lehto, K. Evaluation of a Hydrotreated Vegetable Oil (HVO) and Effects on Emissions of a Passenger Car Diesel Engine. Front. Mech. Eng. 2018, 4, 7. [Google Scholar] [CrossRef]
- Sugiyama, K.; Goto, I.; Kitano, K.; Mogi, K.; Honkanen, M. Effects of Hydrotreated Vegetable Oil (HVO) as Renewable Diesel Fuel on Combustion and Exhaust Emissions in Diesel Engine. SAE Int. J. Fuels Lubr. 2011, 5, 205–217. [Google Scholar] [CrossRef]
- Aatola, H.; Larmi, M.; Sarjovaara, T.; Mikkonen, S. Hydrotreated Vegetable Oil (HVO) as a Renewable Diesel Fuel: Trade-off between NOx, Particulate Emission, and Fuel Consumption of a Heavy Duty Engine. SAE Int. J. Engines 2008, 1, 1251–1262. [Google Scholar] [CrossRef]
- No, S.-Y. Application of Hydrotreated Vegetable Oil from Triglyceride Based Biomass to CI Engines—A Review. Fuel 2014, 115, 88–96. [Google Scholar] [CrossRef]
- Bioenergy Association. Available online: https://www.liquidbiofuels.org.nz/resource/neste-renewable-diesel-handbook (accessed on 11 April 2023).
- Chojnowski, J.; Nogas, P. The Potential of HVO as a Highly Reactive Biofuel in Dual Fuel Systems. Biul. Wojsk. Akad. Tech. 2021, 70, 73–87. [Google Scholar] [CrossRef]
- Roque, L.F.A.; da Costa, R.B.R.; de Souza, T.A.Z.; Coronado, C.J.R.; Pinto, G.M.; Cintra, A.J.A.; Raats, O.O.; Oliveira, B.M.; Frez, G.V.; Alves, L.F.R. Experimental Analysis and Life Cycle Assessment of Green Diesel (HVO) in Dual-Fuel Operation with Bioethanol. J. Clean. Prod. 2023, 389, 135989. [Google Scholar] [CrossRef]
- Breaking Ammonia: A New Catalyst to Generate Hydrogen from Ammonia at Low Temperatures. Available online: https://www.sciencedaily.com/releases/2021/08/210830140251.htm (accessed on 11 April 2023).
- Herbinet, O.; Bartocci, P.; Grinberg Dana, A. On the Use of Ammonia as a Fuel—A Perspective. Fuel Commun. 2022, 11, 100064. [Google Scholar] [CrossRef]
- Green Ammonia as Fuel of the Future—Production, Supply Chain, and Usage|Frontiers Research Topic. Available online: https://www.frontiersin.org/research-topics/44458/green-ammonia-as-fuel-of-the-future--production-supply-chain-and-usage (accessed on 11 April 2023).
- Butterworth, P. Green Ammonia Fuel Faces Three Big Challenges. Available online: https://sustainability.crugroup.com/article/green-ammonia-fuel-faces-three-big-challenges (accessed on 11 April 2023).
- Erdemir, D.; Dincer, I. A Perspective on the Use of Ammonia as a Clean Fuel: Challenges and Solutions. Int. J. Energy Res. 2021, 45, 4827–4834. [Google Scholar] [CrossRef]
- Tornatore, C.; Marchitto, L.; Sabia, P.; De Joannon, M. Ammonia as Green Fuel in Internal Combustion Engines: State-of-the-Art and Future Perspectives. Front. Mech. Eng. 2022, 8, 944201. [Google Scholar] [CrossRef]
- Yousefi, A.; Guo, H.; Dev, S.; Lafrance, S.; Liko, B. A Study on Split Diesel Injection on Thermal Efficiency and Emissions of an Ammonia/Diesel Dual-Fuel Engine. Fuel 2022, 316, 123412. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Z.; Liu, J. The Methodology of Decoupling Fuel and Thermal Nitrogen Oxides in Multi-Dimensional Computational Fluid Dynamics Combustion Simulation of Ammonia-Hydrogen Spark Ignition Engines. Int. J. Hydrogen Energy 2024, 55, 300–318. [Google Scholar] [CrossRef]
- Hunicz, J. System Spalania Niskotemperaturowego w Silniku Tłokowym; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2023; ISBN 978-83-01-22962-7. [Google Scholar]
- Crolet, J.-L. Detailed Mechanisms of Hydrogen Charging and Hydrogen Stress Cracking of Steel in Liquid Ammonia Storage—I. Reminder of the Past and Specificity of the Corrosive Medium. Matér. Tech. 2019, 107, 401. [Google Scholar] [CrossRef]
- Mallouppas, G.; Ioannou, C.; Yfantis, E.A. A Review of the Latest Trends in the Use of Green Ammonia as an Energy Carrier in Maritime Industry. Energies 2022, 15, 1453. [Google Scholar] [CrossRef]
- Technology—Amogy. Available online: https://amogy.co/technology/ (accessed on 11 April 2023).
- Yara Clean Ammonia|Enabling the Hydrogen Economy|Yara International. Available online: https://www.yara.com/yara-clean-ammonia/ (accessed on 11 April 2023).
- Amogy Moving the Maritime Industry Closer to Clean Energy, Amogy Is Building the World’s First Ammonia-Powered, Zero-Emission Ship. Available online: https://www.prnewswire.com/news-releases/moving-the-maritime-industry-closer-to-clean-energy-amogy-is-building-the-worlds-first-ammonia-powered-zero-emission-ship-301762430.html (accessed on 11 April 2023).
- How Ammonia Could Help Clean up Global Shipping|MIT Technology Review. Available online: https://www.technologyreview.com/2022/08/31/1058791/ammonia-fuel-clean-up-global-shipping/ (accessed on 11 April 2023).
- Speeding Up Clean Ammonia in Shipping. Hydrogen x REVOLVE. Available online: https://hydrogen.revolve.media/2022/case-studies/speeding-up-clean-ammonia-in-shipping/ (accessed on 14 April 2023).
- Ammonia Takes Centre Stage as a Clean Marine Fuel. Available online: https://www.rivieramm.com/news-content-hub/news-content-hub/ammonia-takes-centre-stage-as-a-clean-marine-fuel-74978 (accessed on 11 April 2023).
- Is Ammonia the Future for Clean Shipping? Available online: https://www.euronews.com/2022/11/07/how-the-shipping-industry-is-betting-on-ammonia-as-part-of-a-cleaner-energy-mix (accessed on 11 April 2023).
- Exploration of Environmentally Friendly Marine Power Technology-Ammonia/Diesel Stratified Injection. J. Clean. Prod. 2022, 380, 135014. [CrossRef]
- Industry Leaders Join Forces on Ammonia-Fuelled Tanker Project. Available online: https://www.lr.org/en/marine-shipping/maritime-decarbonisation-hub/decarbonising-shipping-the-global-challenge/industry-leaders-join-forces-ammonia-fuelled-tanker-project-misc-berhad-samsung-heavy-industries/ (accessed on 14 April 2023).
- IRENA. A Pathway to Decarbonise the Shipping Sector by 2050; International Renewable Energy Agency: Masdar City, United Arab Emirates, 2021. [Google Scholar]
- Cars, Planes, Trains: Where Do CO2 Emissions from Transport Come From? Available online: https://ourworldindata.org/co2-emissions-from-transport (accessed on 17 April 2023).
- Visualization for World Merchant Fleet Data (2016–2021). infomaritime.eu 2021. Available online: https://www.pinterest.com.au/pin/637400153487754854/ (accessed on 23 April 2023).
- Frost, J.; Tall, A.; Sheriff, A.M.; Schönborn, A.; Hellier, P. An Experimental and Modelling Study of Dual Fuel Aqueous Ammonia and Diesel Combustion in a Single Cylinder Compression Ignition Engine. Int. J. Hydrogen Energy 2021, 46, 35495–35510. [Google Scholar] [CrossRef]
- The Facts About Ammonia. Available online: https://www.health.ny.gov/environmental/emergency/chemical_terrorism/ammonia_general.htm (accessed on 11 April 2023).
- Ammonia Production. Available online: https://encyclopedia.pub/entry/1129 (accessed on 11 April 2023).
- Liu, J.; Liu, Z. In-Cylinder Thermochemical Fuel Reforming for High Efficiency in Ammonia Spark-Ignited Engines through Hydrogen Generation from Fuel-Rich Operations. Int. J. Hydrogen Energy 2024, 54, 837–848. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Amer-Hatem, F.; Azad, A.K.; Dedoussi, I.C.; de Joannon, M.; Fernandes, R.X.; Glarborg, P.; Hashemi, H.; He, X.; Mashruk, S.; et al. Review on Ammonia as a Potential Fuel: From Synthesis to Economics. Energy Fuels 2021, 35, 6964–7029. [Google Scholar] [CrossRef]
- Full-Scale Ammonia Engine Test Announced. Available online: https://www.dieselgasturbine.com/news/full-scale-ammonia-engine-test-announced/7011497.article (accessed on 11 April 2023).
- The Case for Two-Stroke Ammonia Engines. Available online: https://www.man-es.com/discover/two-stroke-ammonia-engine (accessed on 11 April 2023).
- redactoramexico. Hydrogen Engine—Cummins Unveils Industry-First Fuel-Agnostic Internal Combustion Powertrain Solutions, Helping Fleets Decarbonize with Low-Carbon Fuels; Hydrogen Central: Hydrogen Industry News & Market Intelligence. February 2022. Available online: https://hydrogen-central.com/hydrogen-engine-cummins-industry-first-fuel-agnostic-internal-combustion-powertrain/ (accessed on 1 February 2024).
- Ammonia Energy Association. Ammonia-Diesel Dual Fuel. Available online: https://www.ammoniaenergy.org/wp-content/uploads/2019/12/nh3fa-2014-youngmin-woo.pdf (accessed on 22 May 2023).
- Nanthagopal, K.; Subbarao, R.; Elango, T.; Baskar, P.; Annamalai, K. Hydrogen enriched compressed natural gas—A futuristic fuel for internal combustion engines. Therm. Sci. 2011, 15, 1145–1154. [Google Scholar] [CrossRef]
- Saaidia, R.; Almusallm, H. CFD Analysis of the Effect of H2CNG Blend Nature on In-Cylinder Flow. 2020. Available online: https://www.researchgate.net/publication/344377317_CFD_analysis_of_the_effect_of_H2CNG_blend_nature_on_in-cylinder_flow (accessed on 23 April 2023).
- gazeo.pl Silnik Euro 6 zasilany hytanem (HCNG)|cng-lng.pl. Available online: http://cng-lng.pl/motoryzacja/technika/Silnik-Euro-6-zasilany-hytanem-HCNG,artykul,8837.html (accessed on 11 April 2023).
- Hora, T.; Agarwal, A. Compressed Natural Gas and Hythane for On-Road Passenger and Commercial Vehicles. In Prospects of Alternative Transportation Fuels; Springer: Singapore, 2018; pp. 79–106. ISBN 978-981-10-7517-9. [Google Scholar]
- Melaina, M.W.; Antonia, O.; Penev, M. Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues; National Renewable Energy Laboratory: USA, Golden, CO, 2013. [Google Scholar]
- Foute, S.J.; Kielian, D. Technical Comparison between Hythane, CNG and Gasoline Fueled Vehicles; Office of Scientific & Technical Information Technical Reports; A Project of the Urban Consortium Energy Task Force of Public Technology, Inc.; City and County of Denver; Urban Consortium Energy Task Force. 1996. Available online: https://www.osti.gov/servlets/purl/258149 (accessed on 2 February 2024).
- Topolski, K.; Reznicek, E.P.; Erdener, B.C.; San Marchi, C.W.; Ronevich, J.A.; Fring, L.; Simmons, K.; Fernandez, O.J.G.; Hodge, B.-M.; Chung, M. Hydrogen Blending into Natural Gas Pipeline Infrastructure: Review of the State of Technology; National Renewable Energy Laboratory: Golden, CO, USA, 2022. [Google Scholar]
- gazeo.pl. GasShow 2022—Konferencja LPG, CNG, LNG|gazeo.pl. Available online: https://gazeo.pl/informacje/wiadomosci/GasShow-2022-konferencja-LPG-CNG-LNG,wiadomosc,10955.html (accessed on 11 April 2023).
- PRF, Gás, Tecnologia e Construção. Available online: https://www.prf.pt/ (accessed on 12 April 2023).
- Jones, A.D.S.; Yen, K.-H. Hydrogen Use in Natural Gas Pipeline. 2023. Available online: https://www.ul.com/sites/g/files/qbfpbp251/files/2023-02/Hydrogen%20Gas_White%20Paper%202023.pdf (accessed on 23 April 2023).
- Mahajan, D.; Tan, K.; Venkatesh, T.; Kileti, P.; Clayton, C.R. Hydrogen Blending in Gas Pipeline Networks—A Review. Energies 2022, 15, 3582. [Google Scholar] [CrossRef]
- Welcome to Sewerin. Available online: https://www.sewerin.com/pl/ (accessed on 12 April 2023).
- Makaryan, I.A.; Sedov, I.V.; Salgansky, E.A.; Arutyunov, A.V.; Arutyunov, V.S. A Comprehensive Review on the Prospects of Using Hydrogen–Methane Blends: Challenges and Opportunities. Energies 2022, 15, 2265. [Google Scholar] [CrossRef]
- Wayback Machine. Available online: https://web.archive.org/web/20140222021809/http://www.statoileconordic.dk/mar/svg01185.nsf/f53d31ce5fbe93fe4125667c0051b703/68398d9262846974c1256ebe004b0a39/$FILE/hydrogen_folder.pdf (accessed on 12 April 2023).
- Experimental and Artificial Neural Network (ANN) Study of Hydrogen Enriched Compressed Natural Gas (HCNG) Engine under Various Ignition Timings and Excess Air Ratios|Request PDF. Available online: https://www.researchgate.net/publication/326246154_Experimental_and_artificial_neural_network_ANN_study_of_hydrogen_enriched_compressed_natural_gas_HCNG_engine_under_various_ignition_timings_and_excess_air_ratios (accessed on 12 April 2023).
- Jamrozik, A.; Tutak, W.; Grab-Rogaliński, K. An Experimental Study on the Performance and Emission of the Diesel/CNG Dual-Fuel Combustion Mode in a Stationary CI Engine. Energies 2019, 12, 3857. [Google Scholar] [CrossRef]
- Hydrocarbon|Definition, Types, & Facts|Britannica. Available online: https://www.britannica.com/science/hydrocarbon (accessed on 11 April 2023).
- CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data|WorldCat.Org. Available online: https://www.worldcat.org/title/crc-handbook-of-chemistry-and-physics-a-ready-reference-book-of-chemical-and-physical-data/oclc/930681942 (accessed on 17 April 2023).
- Babalola, O.; Sanni, S.; Ibegbu, A.; Aduojo, A.A. Experimental Optimization of Engine Performance of a Dual-Fuel Compression-Ignition Engine Operating on Hydrogen-Compressed Natural Gas and Moringa Biodiesel. Energy Rep. 2021, 7, 607–619. [Google Scholar] [CrossRef]
- Lee, S.-W.; Lee, H.-S.; Park, Y.-J.; Cho, Y. Combustion and Emission Characteristics of HCNG in a Constant Volume Chamber. J. Mech. Sci. Technol. 2011, 25, 489–494. [Google Scholar] [CrossRef]
- Ewan, B.C.R.; Moodie, K.; Hawksworth, S. Chapter 4—Accident Consequences. In Hydrogen Safety for Energy Applications; Kotchourko, A., Jordan, T., Eds.; Butterworth-Heinemann: Oxford, UK, 2022; pp. 195–261. ISBN 978-0-12-820492-4. [Google Scholar]
- Publikacja Informacyjna 11/I Bezpieczne Wykorzystywanie Wodoru Jako Paliwa w Komercyjnych Zastosowaniach Przemysłowych. 2021. Available online: https://www.prs.pl/uploads/p11i_pl.pdf (accessed on 23 April 2023).
- Grabner, P.; Wimmer, A.; Gerbig, F.; Krohmer, A. Hydrogen as a Fuel for Internal Combustion Engines—Properties, Problems and Chances: 5th International Colloquium FUELS. In Proceedings of the 5th International Colloquium FUELS, Esslingen, Germany, 12–13 January 2005; pp. 3–13. [Google Scholar]
- Methane—Thermophysical Properties. Available online: https://www.engineeringtoolbox.com/methane-d_1420.html (accessed on 12 April 2023).
- Basic Hydrogen Properties|Hydrogen Tools. Available online: https://h2tools.org/hyarc/hydrogen-data/basic-hydrogen-properties (accessed on 12 April 2023).
- Fossil and Alternative Fuels—Energy Content. Available online: https://www.engineeringtoolbox.com/fossil-fuels-energy-content-d_1298.html (accessed on 12 April 2023).
- Leachman, J. Is It Parahydrogen or Para-Hydrogen? In HYdrogen Properties for Energy Research (HYPER) Laboratory; Washington State University: Pullman, WA, USA, 2020. [Google Scholar]
- ICSC 0291—METAN. Available online: https://www.ilo.org/dyn/icsc/showcard.display?p_lang=pl&p_card_id=0291&p_version=2 (accessed on 14 April 2023).
- LNG w Pigułce. Available online: http://www.gaz-system.pl:8080/pl/terminal-lng/lng-w-pigulce.html (accessed on 14 April 2023).
- Gnutek, Z.; Pomorski, M. Korzyści i zagrożenia Wynikające z Dostarczania Gazu Ziemnego w Postaci Skroplonej. Oficyna Wydawnicza Energia [Stowarzyszenie Elektryków Polskich—COSiW]. Energetyka 2008, 10, 664–667. Available online: https://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-article-BPS1-0032-0023 (accessed on 9 January 2024).
- Hydrogen—Density and Specific Weight vs. Temperature and Pressure. Available online: https://www.engineeringtoolbox.com/hydrogen-H2-density-specific-weight-temperature-pressure-d_2044.html (accessed on 14 April 2023).
- Technologie Kriogeniczne Konspekt Do Wykładu Maciej Chorowski. Produkcja i Zastosowania Helu—PDF Free Download. Available online: https://docplayer.pl/12247762-Technologie-kriogeniczne-konspekt-do-wykladu-maciej-chorowski-produkcja-i-zastosowania-helu.html (accessed on 12 April 2023).
- Matveev, K.I.; Leachman, J.W. Modeling of Liquid Hydrogen Tank Cooled with Para-Orthohydrogen Conversion. Hydrogen 2023, 4, 146–153. [Google Scholar] [CrossRef]
- Nejat Veziroglu, T.; Sherif, S.A.; Barbir, F. Chapter 7—Hydrogen Energy Solutions. In Environmental Solutions; Agardy, F.J., Nemerow, N.L., Eds.; Academic Press: Burlington, NJ, USA, 2005; pp. 143–180. ISBN 978-0-12-088441-4. [Google Scholar]
- Properties of Hydrogen Relevant to Safety, Level IV. Specialist Officer. 2023. Available online: https://hyresponder.eu/wp-content/uploads/2023/05/L2_HyResponder_Level4_2302201.pdf (accessed on 17 April 2023).
- Ortho and Para Hydrogen. Available online: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Quantum_Mechanics/11%3A_Molecules/Ortho_and_Para_hydrogen (accessed on 12 April 2023).
- Zhang, X.; Karman, T.; Groenenboom, G.C.; van der Avoird, A. Para-Ortho Hydrogen Conversion: Solving a 90-Year Old Mystery. Nat. Sci. 2021, 1, e10002. [Google Scholar] [CrossRef]
- ArenaRed. Available online: https://www.arenared.com (accessed on 17 April 2023).
- Xia, L.; Willems, R.; de Jager, B.; Willems, F. Constrained Optimization of Fuel Efficiency for RCCI Engines ⁎⁎This Work Is Supported by the Research Programme “Towards a HiEff Engine” through the Netherlands Organisation for Scientific Research under STW Project 14927. IFAC Pap. 2019, 52, 648–653. [Google Scholar] [CrossRef]
- Splitter, D.; Wissink, M.; DelVescovo, D.; Reitz, R.D. RCCI Engine Operation Towards 60% Thermal Efficiency; SAE International: Warrendale, PA, USA, 2013. [Google Scholar]
- Reitz, R.D.; Duraisamy, G. Review of High Efficiency and Clean Reactivity Controlled Compression Ignition (RCCI) Combustion in Internal Combustion Engines. Prog. Energy Combust. Sci. 2015, 46, 12–71. [Google Scholar] [CrossRef]
- Ahlgren, W.L. The Dual-Fuel Strategy: An Energy Transition Plan. Proc. IEEE 2012, 100, 3001–3052. [Google Scholar] [CrossRef]
- Performance and Emissions of a Diesel Engine Converted to Dual Diesel-CNG Fuelling|Request PDF. Available online: https://www.researchgate.net/publication/279545131_Performance_and_emissions_of_a_Diesel_engine_converted_to_dual_Diesel-CNG_fuelling (accessed on 17 April 2023).
- Vavra, J.; Bortel, I.; Takats, M. A Dual Fuel Hydrogen—Diesel Compression Ignition Engine and Its Potential Application in Road Transport; SAE International: Warrendale, PA, USA, 2019. [Google Scholar]
- Falfari, S.; Cazzoli, G.; Mariani, V.; Bianchi, G.M. Hydrogen Application as a Fuel in Internal Combustion Engines. Energies 2023, 16, 2545. [Google Scholar] [CrossRef]
- Mariani, A. Hydrogen—Natural Gas (HCNG) Mixtures as Fuels in Internal Combustion Engines; International Workshop of Hydrogen and Fuel Cells: Orlean, France, 2012. [Google Scholar]
- Wobbe_index. Available online: https://www.chemeurope.com/en/encyclopedia/Wobbe_index.html (accessed on 12 April 2023).
- Significance of Wobbe Index in Gas Turbine Operation and Control. Available online: https://www.linkedin.com/pulse/significance-wobbe-index-gas-turbine-operation-control-magesh-john (accessed on 13 April 2023).
- Fuel Gases—Wobbe Index. Available online: https://www.engineeringtoolbox.com/wobbe-index-d_421.html (accessed on 12 April 2023).
- Šola, H. Mogući Sadržaj Vodika u Smjesi Prirodnog Plina u Transportnom Sustavu i Izmjene Regulative Vezane uz Vodik. Doctoral Dissertation, University of Zagreb, Zagreb, Croatia, 2022. [Google Scholar]
- Wobbe Index—Wikipedia. Available online: https://cs2cs.wiki/wiki/Wobbe_index (accessed on 12 April 2023).
- Wiki Wobbe Index|PDF. Available online: https://www.scribd.com/doc/296076790/En-wikipedia-org-Wiki-Wobbe-Index (accessed on 12 April 2023).
- Wobbe Index Calorific Value Handout Natural Gas|PDF|Natural Gas|Combustion. Available online: https://www.scribd.com/document/343184100/Wobbe-Index-Calorific-Value-Handout-Natural-Gas (accessed on 13 April 2023).
- Zachariah-Wolff, J.; Egyedi, T.; Hemmes, K. From Natural Gas to Hydrogen via the Wobbe Index: The Role of Standardized Gateways in Sustainable Infrastructure Transitions. Int. J. Hydrogen Energy 2007, 32, 1235–1245. [Google Scholar] [CrossRef]
- Calorific Value of Natural Gas (MJ/M3 and BTU/SCF). Available online: https://group.met.com/en/media/energy-insight/calorific-value-of-natural-gas (accessed on 13 April 2023).
- Gaz Ziemny i Skroplony Gaz Ziemny LNG—Portal Korporacyjny. Available online: https://pgnig.pl/odolanow/produkty/gaz-ziemny-i-skroplony-gaz-ziemny-lng (accessed on 13 April 2023).
- Czyżewski, P.; Goraj, R.; Ślefarski, R. The Impact of Hydrogen Addition into Natural Gas on Combustion Characteristics and Operational Parameters of Public Natural Gas Distribution Network. J. Mech. Transp. Eng. 2019, 71, 17–29. [Google Scholar]
- Ingo, C.; Tuuf, J.; Björklund-Sänkiaho, M. Impact of Hydrogen on Natural Gas Compositions to Meet Engine Gas Quality Requirements. Energies 2022, 15, 7990. [Google Scholar] [CrossRef]
- Wojtowicz, R.; Strugała, A. Possibilities of diversification of natural gas supply to Poland in view of domestic gasquality requirements. Polityka Energ. Energy Policy J. 2011, 14, 297–313. [Google Scholar]
- Serrato, D.; Zapata-Mina, J.; Restrepo, Á.; Torres, J. Assessment of Liquefied Natural Gas (LNG) Regasified through Gas Interchangeability in Energy Consumption Sectors. Energy Rep. 2021, 7, 2526–2533. [Google Scholar] [CrossRef]
- Levinsky, H.; Gersen, S.; Kiewiet, B. Requirements for Gas Quality and Gas Appliances; DNV-GL; University of Groningen: The Netherlands, Groningen, 2015. [Google Scholar]
- Types of Gas and the Corresponding Supply Pressures According to Article 4(1) of Regulation (EU) 2016/426 of the European Parliament and of the Council on Appliances Burning Gaseous Fuels and Repealing Directive 2009/142/EC (This Publication Is Based on Information Received by the Commission from the Member States). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52019XC0121(02)&rid=1 (accessed on 17 April 2023).
- Diderich, G.; Scherm, P. Requirements on the Quality of Natural Gas. 2017. Available online: https://www.euromot.eu/wp-content/uploads/2018/02/EUROMOT-Position-Gas-Quality-2017-11-09-.pdf (accessed on 17 April 2023).
- European Network of Transmission System Operators for Gas; Ten-Year Network Development Plan; Annex F—Gas Quality Outlook. 2020. Available online: https://tyndp2020.entsog.eu/downloads/#introduction (accessed on 17 April 2023).
- Leicher, J.; Carpentier, S. Testing Hydrogen Admixture for Gas Applications. In Proceedings of the THyGA Workshop, No. 1, Online, 6 May 2020; GERG Brussels Office: Brussels, Belgium, 2020. [Google Scholar]
- Mitianiec, W. Factors Determing Ignition and Efficient Combustion in Modern Engines Operating on Gaseous Fuels; InTech: Rzeszów, Poland, 2012; ISBN 978-953-51-0856-6. [Google Scholar]
- Wiadomości Wstępne. Available online: https://instsani.pl/technik-inzynierii-sanitarnej/vademecum-instalacji-sanitarnych/instalacje-gazowe/wiadomosci-wstepne-2-3-4-5-6-7-8-9-10/ (accessed on 14 April 2023).
- Sandfort, V.; Trabold, B.M.; Abdolvand, A.; Bolwien, C.; Russell, P.S.J.; Wöllenstein, J.; Palzer, S. Monitoring the Wobbe Index of Natural Gas Using Fiber-Enhanced Raman Spectroscopy. Sensors 2017, 17, 2714. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Xi, H.; Zhou, S.; Shreka, M.; Zhang, Z. Effects of Wobbe Index on the Combustion and Emission Characteristics of a Natural Gas/Diesel RCCI Engine. J. Eng. Gas Turbines Power 2023, 145, 061001. [Google Scholar] [CrossRef]
- (PDF) Experimental Assessment of the Combustion Performance of an Oven Burner Operated on Pipeline Natural Gas Mixed with Hydrogen. Available online: https://www.researchgate.net/publication/335642851_Experimental_assessment_of_the_combustion_performance_of_an_oven_burner_operated_on_pipeline_natural_gas_mixed_with_hydrogen/figures?lo=1 (accessed on 12 April 2023).
- Middha, P.; Engel, D.; Hansen, O.R. Can the Addition of Hydrogen to Natural Gas Reduce the Explosion Risk? Int. J. Hydrogen Energy 2011, 36, 2628–2636. [Google Scholar] [CrossRef]
- Witkowski, A.; Rusin, A.; Majkut, M.; Stolecka, K. Comprehensive Analysis of Hydrogen Compression and Pipeline Transportation from Thermodynamics and Safety Aspects. Energy 2017, 141, 2508–2518. [Google Scholar] [CrossRef]
- Hydrogen Explosion—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/engineering/hydrogen-explosion (accessed on 14 April 2023).
- Dagdougui, H.; Sacile, R.; Bersani, C.; Ouammi, A. Chapter 7—Hydrogen Logistics: Safety and Risks Issues. In Hydrogen Infrastructure for Energy Applications; Dagdougui, H., Sacile, R., Bersani, C., Ouammi, A., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 127–148. ISBN 978-0-12-812036-1. [Google Scholar]
- ICSC 0001—WODÓR. Available online: https://www.ilo.org/dyn/icsc/showcard.display?p_card_id=1&p_version=1&p_lang=pl (accessed on 14 April 2023).
- Lanz, W. Hydrogen Properties. Hydrog. Fuel 2001, 47. [Google Scholar]
- Sir/Madam, D.; Davazdah Emami, S.; Sulaiman, S.; Kasmani, R.; Hamid, M.; Hassan, C. Effect of Pipe Configurations on Flame Propagation of Hydrocarbons-Air and Hydrogen-Air Mixtures in a Constant Volume. J. Loss Prev. Process Ind. 2015, 39, 141–151. [Google Scholar] [CrossRef]
- Guarco, J.; Langstine, B.; Turner, M. Practical Considerations for Firing Hydrogen Versus Natural Gas; Combustion Engineering Association: Sedgefield, UK, 2022. [Google Scholar]
- Haghighi, K.; McTaggart-Cowan, G.P. Modelling the Impacts of Hydrogen–Methane Blend Fuels on a Stationary Power Generation Engine. Energies 2023, 16, 2420. [Google Scholar] [CrossRef]
- Dong, C.; Zhou, Q.; Zhang, X.; Zhao, Q.; Xu, T.; Hui, S. Experimental Study on the Laminar Flame Speed of Hydrogen/Natural Gas/Air Mixtures. Front. Chem. Eng. China 2010, 4, 417–422. [Google Scholar] [CrossRef]
- Zareei, J.; Alvarez, J.R.N.; Albuerne, Y.L.; Gámez, M.R.; Linzan, Á.R.A. A Simulation Study of the Effect of HCNG Fuel and Injector Hole Number along with a Variation of Fuel Injection Pressure in a Gasoline Engine Converted from Port Injection to Direct Injection. Processes 2022, 10, 2389. [Google Scholar] [CrossRef]
- Paykani, A.; Kakaee, A.; Rahnama, P.; Reitz, R. Progress and Recent Trends in Reactivity-Controlled Compression Ignition Engines. Int. J. Engine Res. 2015, 17, 481–524. [Google Scholar] [CrossRef]
- Motyl, K.; Rychter, T.J. HCCI Engine—A Preliminary Analysis. J. KONES 2003, 10, 217–225. [Google Scholar]
- Amirante, R.; Distaso, E.; Tamburrano, P.; Reitz, R.D. Laminar Flame Speed Correlations for Methane, Ethane, Propane and Their Mixtures, and Natural Gas and Gasoline for Spark-Ignition Engine Simulations. Int. J. Engine Res. 2017, 18, 951–970. [Google Scholar] [CrossRef]
- Winkier, R. Karta Charakterystyki dla CNG Zgodna z Wzorem Określonym w Rozporządzeniu REACH ze zm. Rozporządzeniem 453/2010. 2014. Available online: https://pgnig.pl/documents/10184/2091995/Gaz_Ziemny_niskie_cisnienie_ver_1_5.pdf/3c951b0d-baf3-4cec-b7b6-91f0fb9c0a44 (accessed on 23 May 2023).
- Robinson, C.; Smith, D.B. The Auto-Ignition Temperature of Methane. J. Hazard. Mater. 1984, 8, 199–203. [Google Scholar] [CrossRef]
- Alke, A. Minimum Ignition Energies of Natural Gases and Natural Gas/LPG/Air Mixtures Distributed by Public Gas Supply Networks. GWF 2001, 142, 336, 368–373. [Google Scholar]
- ISO 10156:2010; Gases and Gas Mixtures: Determination of Fire Potential and Oxidizing Ability for the Selection of Cylinder Valve Outlets. ISO: Geneva, Switzerland. Available online: https://www.iso.org/standard/44817.html (accessed on 3 February 2024).
- Wilkes, J.; McBain, M.; Kurz, R.; Goldmeer, J.; Callahan, T.; Wygant, K.; Singh, J.; Geswein, B.; Freund, S. Chapter 10—Power Generation and Mechanical Drivers. In Machinery and Energy Systems for the Hydrogen Economy; Brun, K., Allison, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 425–473. ISBN 978-0-323-90394-3. [Google Scholar]
- Arnold, K.; Stewart, M. Chapter 17—Electrical Systems**Reviewed for the 1999 Edition by Dinesh P. Patel of Paragon Engineering Services, Inc. In Surface Production Operations: Design of Gas-Handling Systems and Facilities, 2nd ed.; Arnold, K., Stewart, M., Eds.; Gulf Professional Publishing: Woburn, UK, 1999; pp. 493–552. ISBN 978-0-88415-822-6. [Google Scholar]
- National Research Council (US) Committee on Toxicology. METHANE. In Emergency and Continuous Exposure Limits for Selected Airborne Contaminants: Volume 1; National Academies Press: Washington, DC, USA, 1984. [Google Scholar]
- PN-EN 60079-20-1:2010; Atmosfery Wybuchowe—Część 20-1: Właściwości Materiałowe Dotyczące Klasyfikacji Gazów i Par—Metody Badań i Dane Tabelaryczne. Wersja Angielska; Polski Komitet Normalizacyjny: Warsaw, Poland. Available online: https://sklep.pkn.pl/pn-en-60079-20-1-2010e.html (accessed on 3 February 2024).
- Miao, H.; Lu, L. Flammability Limits of Hydrogen-Enriched Natural Gas. Fuel Energy Abstr. 2011, 36, 6937–6947. [Google Scholar] [CrossRef]
- Ma, F.; Mehra, R.K.; Ma, F.; Mehra, R.K. Study of Quasi-Dimensional Combustion Model of Hydrogen-Enriched Compressed Natural Gas (HCNG) Engines; IntechOpen: London, UK, 2016; ISBN 978-953-51-2840-3. [Google Scholar]
- ISO/TR 15916:2004; Basic Considerations for the Safety of Hydrogen Systems. ISO: Geneva, Switzerland, 2004. Available online: https://www.iso.org/standard/29145.html (accessed on 14 April 2023).
- Hydrogen Use In Internal Combustion Engine: A Review. Available online: https://www.researchgate.net/publication/267711346_HYDROGEN_USE_IN_INTERNAL_COMBUSTION_ENGINE_A_REVIEW/figures?lo=1&utm_source=google&utm_medium=organic (accessed on 14 April 2023).
- Mahuthannan, A.M.; Damazo, J.S.; Kwon, E.; Roberts, W.L.; Lacoste, D.A. Effect of Propagation Speed on the Quenching of Methane, Propane and Ethylene Premixed Flames between Parallel Flat Plates. Fuel 2019, 256, 115870. [Google Scholar] [CrossRef]
- Guiberti, T.F.; Belhi, M.; Damazo, J.S.; Kwon, E.; Roberts, W.L.; Lacoste, D.A. Quenching Distance of Laminar Methane-Air Flames at Cryogenic Temperatures and Implications for Flame Arrester Design. Appl. Energy Combust. Sci. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Palecka, J.; Julien, P.; Goroshin, S.; Bergthorson, J.M.; Frost, D.L.; Higgins, A.J. Quenching Distance of Flames in Hybrid Methane–Aluminum Mixtures. Proc. Combust. Inst. 2015, 35, 2463–2470. [Google Scholar] [CrossRef]
- Mahuthannan, A.M.; Lacoste, D.A.; Damazo, J.; Kwon, E.; Roberts, W.L. Flame Quenching Dynamics of High Velocity Flames in Rectangular Cross-Section Channels. In Proceedings of the 55th AIAA Aerospace Sciences Meeting, Grapevine, TX, USA, 9–13 January 2017; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2017. [Google Scholar]
- Liu, D.; Liu, Z.; Xiao, H. Flame Acceleration and Deflagration-to-Detonation Transition in Narrow Channels Filled with Stoichiometric Hydrogen-Air Mixture. Int. J. Hydrogen Energy 2022, 47, 11052–11067. [Google Scholar] [CrossRef]
- Shamshin, I.O.; Kazachenko, M.V.; Frolov, S.M.; Basevich, V.Y. Deflagration-to-Detonation Transition in Stochiometric Mixtures of the Binary Methane–Hydrogen Fuel with Air. Int. J. Hydrogen Energy 2021, 46, 34046–34058. [Google Scholar] [CrossRef]
- Wójcicki, S. Spalanie; Wydawnictwo Naukowo-Techniczne: Warsaw, Poland, 1969. [Google Scholar]
- Kyle, B.G. Chemicaf and Process Thertmodynamics, 3rd ed.; Adaptedby permission of PearsonEducation, Inc.: Upper Saddle River, NJ, USA, 2000. [Google Scholar]
- Properties of Various Ideal Gases (at 300 K). Available online: https://www.ohio.edu/mechanical/thermo/property_tables/gas/idealGas.html (accessed on 14 April 2023).
- Laibuta, R. Maximizing the Adiabatic Flame Temperature of Biogas Using Hydrogen. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2014. [Google Scholar]
- Skarvelis, G.V. Containerized Compressed Natural Gas Shipping. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2013. [Google Scholar]
- Podstawowe Właściwości Substancji Czystych i Powietrza. Właściwości Osobliwe oraz Ciepło Spalania i Wartość Opałowa. Available online: https://Home.Agh.Edu.Pl/~kepw/Student/Plik/Parametry.Pdf (accessed on 2 February 2024).
- Walentynowicz, J. Termodynamika Techniczna i Jej Zastosowania; WAT: Warsaw, Poland, 2009. [Google Scholar]
- Abdulagatov, I.M.; Levina, L.N.; Zakaryaev, Z.R.; Mamchenkova, O.N. The Two-Phase Specific Heat at Constant Volume of Propane in the Critical Region. Fluid Ph. Equilibria 1997, 127, 205. [Google Scholar] [CrossRef]
- U.S. Department of Commerce National Bureau of Standards Report. Preliminary Report on the Thermodynamic Properties of Selected Light-Element and Some Related Compounds; U.S. Department of Commerce National Bureau of Standards Report: Washington, DC, USA, 1964.
- Gases—Ratios of Specific Heat. Available online: https://www.engineeringtoolbox.com/specific-heat-ratio-d_608.html (accessed on 15 April 2023).
- Wydział Energetyki i Paliw. Katedra Technologii Paliw Paliwa Stałe, Ciekłe i Gazowe Podstawowe Właściwości Paliw Gazowych. Available online: https://home.agh.edu.pl/~kepw/student/plik/sg_w1.pdf (accessed on 22 May 2023).
- Wiśniewski, S. Termodynamika Techniczna; Wydawnictwo WNT: Warsaw, Poland, 2012. [Google Scholar]
- Szargut, J. Termodynamika Techniczna. Cz. 1; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 1959. [Google Scholar]
- Methane Gas—Specific Heat, vs. Temperature. Available online: https://www.engineeringtoolbox.com/methane-d_980.html (accessed on 15 April 2023).
- Hydrogen—Specific Heat. Available online: https://www.engineeringtoolbox.com/hydrogen-d_976.html (accessed on 15 April 2023).
- Air—Specific Heat, vs. Temperature at Constant Pressure. Available online: https://www.engineeringtoolbox.com/air-specific-heat-capacity-d_705.html (accessed on 16 April 2023).
- Sikora, M.; Orliński, P.; Bednarski, M.; Wojs, M.K. Theoretical Analysis of Determining the Thermal Efficiency in Engine with Atkinson Cycle. Zesz. Nauk. Inst. Pojazdów 2019, 1, 43–53. [Google Scholar]
- Szybist, J.P.; Busch, S.; McCormick, R.L.; Pihl, J.A.; Splitter, D.A.; Ratcliff, M.A.; Kolodziej, C.P.; Storey, J.M.E.; Moses-DeBusk, M.; Vuilleumier, D.; et al. What Fuel Properties Enable Higher Thermal Efficiency in Spark-Ignited Engines? Prog. Energy Combust. Sci. 2021, 82, 100876. [Google Scholar] [CrossRef]
- Paluch, M.; Noga, M.; Lisowska, A. Increasing the Theoretical Efficiency of The Spark-Ignition Engine Cycle by Adding Inert Gas at Part-Load. J. Phys. Conf. Ser. 2021, 1888, 012009. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals; McGraw-Hill, Inc.: New York, NY, USA, 1988. [Google Scholar]
- Silniki Tłokowe o Spalaniu Wewnętrznym/Obiegi Teoretyczne/Obieg Diesla—Wikibooks, Biblioteka Wolnych Podręczników. Available online: https://pl.wikibooks.org/wiki/Silniki_t%C5%82okowe_o_spalaniu_wewn%C4%99trznym/Obiegi_teoretyczne/Obieg_Diesla (accessed on 16 April 2023).
- Foody, G. Explaining the Unsuitability of the Kappa Coefficient in the Assessment and Comparison of the Accuracy of Thematic Maps Obtained by Image Classification. Remote Sens. Environ. 2020, 239, 111630. [Google Scholar] [CrossRef]
- Kuiken, K. Gas- and Dual-Fuel Engines for Ship Propulsion, Power Plants and Cogeneration. Book III: Operation and Maintenance; Target Global Energy Training: Onnen, The Netherlands, 2016. [Google Scholar]
- Dziubak, T. Experimental Investigation of Possibilities to Improve Filtration Efficiency of Tangential Inlet Return Cyclones by Modification of Their Design. Energies 2022, 15, 3871. [Google Scholar] [CrossRef]
- Saravanan, N.; Nagarajan, G. An Experimental Investigation on Hydrogen Fuel Injection in Intake Port and Manifold with Different EGR Rates. Int. J. Energy Environ. 2010, 1, 221–248. [Google Scholar]
- Marikatti, M.; Banapurmath, N.R.; Yaliwal, V.S.; Basavarajappa, Y.H.; Soudagar, M.E.M.; Márquez, F.P.G.; Mujtaba, M.; Fayaz, H.; Naik, B.; Khan, T.M.Y.; et al. Hydrogen Injection in a Dual Fuel Engine Fueled with Low-Pressure Injection of Methyl Ester of Thevetia Peruviana (METP) for Diesel Engine Maintenance Application. Energies 2020, 13, 5663. [Google Scholar] [CrossRef]
- Chapter 11: Combustion (Updated 5/31/10). Available online: https://www.ohio.edu/mechanical/thermo/Applied/Chapt.7_11/Chapter11.html (accessed on 16 April 2023).
- x-engineer.org Air Fuel Ratio. Available online: https://x-engineer.org/air-fuel-ratio/ (accessed on 28 May 2023).
- Mohammed, M.K.; Rahman, M.; Abu Bakar, R. Effects of Air Fuel Ratio on the Performance of Hydrogen Fuel Port Injection Engine. In Proceedings of the EnCon2008 2nd Engineering Conference on Sustainable Engineering Infrastructures Development & Management, Kuching, Malaysia, 18–19 December 2008. [Google Scholar]
- AFR Calculator|Air-Fuel Ratio Calculator. Available online: https://www.omnicalculator.com/chemistry/air-fuel-ratio-afr (accessed on 16 April 2023).
- Stoichiometric Air Fuel Ratio: Definition, Formula, Calculation, Examples [with Pdf]—Mech Content. 2022. Available online: http://fluid.wme.pwr.wroc.pl/~spalanie/dydaktyka/combustion_MiBM/fund/Stoichiometry.pdf (accessed on 12 April 2023).
- Lynch, D.; Smith, W.J. Comparison of AFR Calculation Methods Using Gas Analysis and Mass Flow Measurement. SAE Trans. 1997, 106, 311–321. [Google Scholar]
- Introduction to Fuel and Energy. Available online: http://eyrie.shef.ac.uk/eee/cpe630/comfun1.html (accessed on 16 April 2023).
- Lhuillier, C.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C. Experimental Study on Ammonia/Hydrogen/Air Combustion in Spark Ignition Engine Conditions. Fuel 2020, 269, 117448. [Google Scholar] [CrossRef]
- Air Fuel Ratio Study for Mixture of Biogas and Hydrogen on Mild Combustion—ProQuest. Available online: https://www.proquest.com/openview/25f5ad134d8c527ab0bdb4fcda2f0230/1?pq-origsite=gscholar&cbl=2032193 (accessed on 16 April 2023).
- Stoichiometric Combustion. Available online: https://www.engineeringtoolbox.com/stoichiometric-combustion-d_399.html (accessed on 16 April 2023).
- Air-Fuel_ratio. Available online: https://www.chemeurope.com/en/encyclopedia/Air-fuel_ratio.html (accessed on 16 April 2023).
- GEK Wiki/Stoichiometric Combustion Ratios. Available online: http://wiki.gekgasifier.com/w/page/6123822/Stoichiometric%20Combustion%20Ratios (accessed on 16 April 2023).
- Air-Fuel Ratio Calculation for a Natural Gas Fuelled Spark Ignition Engine|Request PDF. Available online: https://www.researchgate.net/publication/288424817_Air_-Fuel_Ratio_Calculation_for_a_Natural_Gas_Fuelled_Spark_Ignition_Engine (accessed on 16 April 2023).
- Ochwat, S.; Pawlak, K. Konstrukcja Silników Spalinowych, Materiały Do Ćwiczeń Obliczeniowych I Projektowania; WAT wewn. 1678/87; Wydział Wydawniczy WAT: Warsaw, Poland, 1987; Volume I. [Google Scholar]
- Falkowski, H.; Hausner, G.; Janiszewski, T.; Jaskuła, A. Układy Wtryskowe Silników Wysokoprężnych. Konstrukcja, Domelowanie, Sterowanie. Teoria Samochodu; Wydawnictwo Komunikacji i Łączności: Warsaw, Poland, 1989. [Google Scholar]
- Bernhardt, M.; Dobrzyński, S.; Loth, E. Silniki Samochodowe; Wydawnictwo Komunikacji i Łączności: Warsaw, Poland, 1978. [Google Scholar]
- Karim, G.A. Dual-Fuel Diesel Engines; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-1-4987-0309-3. [Google Scholar]
- Wajand, J.A. Silniki o Zapłonie Samoczynnym (Silniki Diesla); czwarte; Wydawnictwo Komunikacji i Łączności: Polska, Warszawa, 1988. [Google Scholar]
- Stone, R. Introduction to Internal Combustion Engines, 3rd ed.; Sae Intl, Macmillan Education: London, UK, 1999. [Google Scholar]
- Szawłowski, K. Silniki Wysokoprężne Dużej Mocy Okrętowe i Przemysłowe; Drugie Poprawione i Uzupełnione; Wydawnictwo Komunikacji i Łączności: Warsaw, Poland, 1969. [Google Scholar]
- Winterbone, D.E.; Turan, A. Chapter 3—Engine Cycles and Their Efficiencies. In Advanced Thermodynamics for Engineers, 2nd ed.; Winterbone, D.E., Turan, A., Eds.; Butterworth-Heinemann: Boston, MA, USA, 2015; pp. 35–59. ISBN 978-0-444-63373-6. [Google Scholar]
- Hänggi, S.; Moretto, G.; Albin, T.; Onder, C. The Potential of Heat Release Rate and Cylinder Pressure Feedback Control for Conventional and Premixed Charge Compression Ignition Combustion. Int. J. Engine Res. 2021, 22, 3080–3100. [Google Scholar] [CrossRef]
- Calculation of Heat Release in Direct Injection Diesel Engines on JSTOR. Available online: https://www.jstor.org/stable/44743361 (accessed on 16 April 2023).
- Brunt, M.F.J.; Rai, H.; Emtage, A.L. The Calculation of Heat Release Energy from Engine Cylinder Pressure Data. SAE Trans. 1998, 107, 1596–1609. [Google Scholar]
- Alrazen, H.A.; Ahmad, K.A. HCNG Fueled Spark-Ignition (SI) Engine with Its Effects on Performance and Emissions. Renew. Sustain. Energy Rev. 2018, 82, 324–342. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Q.; Liang, J.; Yang, C. The Performance and Emissions Characteristics of Diesel/Biodiesel/Alcohol Blends in a Diesel Engine. Energy Rep. 2021, 7, 1016–1024. [Google Scholar] [CrossRef]
- Karrthik, R.S.; Venkatachalam, S.; Dinesh, C.; Baskaran, S.; Shanmugaraj; Karthick, P. Cylinder Pressure and Heat Release Rate Analysis for Hydrogen Induction with Injected Biodiesel (Pongamia Pinnata) Operated CI Engine. AIP Conf. Proc. 2019, 2128, 050009. [Google Scholar] [CrossRef]
- Lee, B.T. Heat Release Rate Characteristics of Some Combustible Fuel Sources in Nuclear Power Plants; National Bureau of Standards: Gaithersburg, MD, USA, 1985; p. NBSIR 85-3195.
- Ranga Dinesh, K.K.J.; Shalaby, H.; Luo, K.H.; van Oijen, J.A.; Thévenin, D. Heat Release Rate Variations in High Hydrogen Content Premixed Syngas Flames at Elevated Pressures: Effect of Equivalence Ratio. Int. J. Hydrogen Energy 2017, 42, 7029–7044. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Bai, Y.; Li, S.; Kobayashi, N. Combustion and Heat Release Characteristics of Hydrogen/Air Diffusion Flame on a Micro-Jet Array Burner. Int. J. Hydrogen Energy 2018, 43. [Google Scholar] [CrossRef]
- Park, C.; Lee, S.; Lim, G.; Choi, Y.; Kim, C. Full Load Performance and Emission Characteristics of Hydrogen-Compressed Natural Gas Engines with Valve Overlap Changes. Fuel 2014, 123, 101–106. [Google Scholar] [CrossRef][Green Version]
- Tang, C.; Zhang, Y.; Huang, Z. Progress in Combustion Investigations of Hydrogen Enriched Hydrocarbons. Renew. Sustain. Energy Rev. 2014, 30, 195–216. [Google Scholar] [CrossRef]
- Lyon, R.E. Heat Release Rate Estimation Device for Internal Combustion Engine. Patent WO2012035647A1, 22 March 2012. [Google Scholar]
- Shiao, Y.; Moskwa, J.J. Cylinder Pressure and Combustion Heat Release Estimation for SI Engine Diagnostics Using Nonlinear Sliding Observers. IEEE Trans. Contr. Syst. Technol. 1995, 3, 70–78. [Google Scholar] [CrossRef]
- Rajkumar, M. Heat Release Analysis and Modeling for a Common-Rail Diesel Engine. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 2002. [Google Scholar]
- Modelling the Rate of Heat Release in Common Rail Diesel Engines: A Soft Computing Approach. Available online: https://www.researchgate.net/publication/290615657_Modelling_the_Rate_of_Heat_Release_in_Common_Rail_Diesel_Engines_a_Soft_Computing_Approach (accessed on 16 April 2023).
- Olsen, R.A. Analysis and Simulation of the Rate of Heat Release (ROHR) in Diesel Engines. Master’s Thesis, Institutt for Marin Teknikk, Trondheim, Norway, 2013. [Google Scholar]
- Zhang, Y.; Yan, Y.; Yang, R.; Wang, Q.; Zhang, B.; Gan, Q.; Liu, Z.; Fu, J. Study of In-Cylinder Heat Transfer Boundary Conditions for Diesel Engines Under Variable Altitudes Based on the CHT Model. Front. Energy Res. 2022, 10, 828215. [Google Scholar] [CrossRef]
- Mauro, S.; Şener, R.; Gül, M.Z.; Lanzafame, R.; Messina, M.; Brusca, S. Internal Combustion Engine Heat Release Calculation Using Single-Zone and CFD 3D Numerical Models. Int. J. Energy Environ. Eng. 2018, 9, 215–226. [Google Scholar] [CrossRef]
- Ding, Y.; Stapersma, D.; Grimmelius, H.T. Simulation Techniques for Heat Release Calculation of Diesel Engines. In Proceedings of the 2011 Asia-Pacific Power and Energy Engineering Conference, China, Wuhan, 25–28 March 2011; pp. 1–4. [Google Scholar]
- Liu, X.; Yao, C.; Chen, J.; Song, E. Modeling of 0-D Heat Release Rate of Heavy-Duty Diesel Engine Based on Classification Algorithm. Int. J. Engine Res. 2023, 24, 2437–2450. [Google Scholar] [CrossRef]
- Anderton, D. Rate of Heat Release in Diesel Engines; Raport NO. DOT-TSC-OST-77-76; Bureau of Transportation Statistics: Washington DC, USA, 1977.
- Oishi, Y.; Situmorang, R.S.; Sembiring, R.A.; Kawai, H.; Ambarita, H. Performance, Rate of Heat Release, and Combustion Stability of Dual-Fuel Mode in a Small Diesel Engine. Energy Sci. Eng. 2019, 7, 1333–1351. [Google Scholar] [CrossRef]
- Vipavanich, C.; Chuepeng, S.; Skullong, S. Heat Release Analysis and Thermal Efficiency of a Single Cylinder Diesel Dual Fuel Engine with Gasoline Port Injection. Case Stud. Therm. Eng. 2018, 12, 143–148. [Google Scholar] [CrossRef]
- Pandey, V.; Badruddin, I.A.; Khan, T.M.Y. Effect of H2 Blends with Compressed Natural Gas on Emissions of SI Engine Having Modified Ignition Timings. Fuel 2022, 321, 123930. [Google Scholar] [CrossRef]
- Karagöz, Y.; Sandalcı, T.; Yüksek, L.; Dalkilic, A.; Wongwises, S. Effect of Hydrogen–Diesel Dual-Fuel Usage on Performance, Emissions and Diesel Combustion in Diesel Engines. Adv. Mech. Eng. 2016, 8, 1687814016664458. [Google Scholar] [CrossRef]
- ARTFuels. Available online: https://artfuelsforum.eu/ (accessed on 17 April 2023).
- Sikora, M.; Orliński, P.; Matej, J. Hydro-Treated Vegetable Oil as a Potential Biofuel for Self-Ignition Engines. Transp. Samoch. Pol. Warszawa 2022, 65, 14–19. [Google Scholar] [CrossRef]
- Brake-Specific Fuel Consumption. Wikipedia. 2022. Available online: https://en.wikipedia.org/wiki/Brake-specific_fuel_consumption#:~:text=Brake%2Dspecific%20fuel%20consumption%20(BSFC,divided%20by%20the%20power%20produced (accessed on 22 May 2023).
- Natural Gas Engines. Available online: https://dieselnet.com/tech/engine_natural-gas.php (accessed on 17 April 2023).
- Solouk, A.; Shahbakhti, M. Energy Optimization and Fuel Economy Investigation of a Series Hybrid Electric Vehicle Integrated with Diesel/RCCI Engines. Energies 2016, 9, 1020. [Google Scholar] [CrossRef]
- MAN Energy Solutions SE. MAN B&W Two-Stroke Engine Operating on Ammonia, 1st ed.; MAN Energy Solutions Future in the Making: Munich, Denmark, 2020. [Google Scholar]
- LNG Binnenvaart—Breakthrough LNG Deployment in Inland Waterway Transport. Available online: https://lngbinnenvaart.eu/ (accessed on 17 April 2023).
- Scania Technical Engine Data. Available online: https://www.scania.com/content/dam/scanianoe/market/master/products-and-services/engines/pdf/specs/power-gen/DC1678A_620-680kW.pdf (accessed on 2 May 2023).
- Wartości Opałowe (WO) i Wskaźniki Emisji CO2 (WE) w Roku 2018 Do Raportowania w Ramach Systemu Handlu Uprawnieniami do Emisji za Rok 2021—KOBIZE. Available online: https://www.kobize.pl/pl/article/aktualnosci-2020/id/1783/wartosci-opalowe-wo-i-wskazniki-emisji-co2-we-w-roku-2018-do-raportowania-w-ramach-systemu-handlu-uprawnieniami-do-emisji-za-rok-2021 (accessed on 17 April 2023).
- Luo, S.; Ma, F.; Mehra, R.; Huang, Z. Deep Insights of HCNG Engine Research in China. Fuel 2019, 263, 116612. [Google Scholar] [CrossRef]
- Pathak, A.; News, I.T. Delhi Transport Minister Inaugurates HCNG Plant and Dispensing Station, to Reduce Pollution by 70%. Available online: https://www.indiatvnews.com/news/india/ashok-gahlot-inaugurates-hcng-plant-and-dispensing-station-hcng-to-reduce-pollution-by-70-per-cent-658548 (accessed on 17 April 2023).
- Hydrogen Enriched CNG India’s First HCNG Buses in Delhi, Current Affairs; India, Delhi. Available online: https://www.youtube.com/watch?v=tYDqz599-cM (accessed on 17 April 2023).
- RSTV Vishesh—HCNG: Fuel of The Future I.; India, Delhi. Available online: https://www.youtube.com/watch?v=WIA8p056bzE (accessed on 17 April 2023).
- HCNG. Wikipedia. 2023. Available online: https://en.wikipedia.org/wiki/HCNG (accessed on 16 April 2023).
- In Depth—HCNG: Fuel of the Future. 2018. India, Delhi. Available online: https://www.insightsonindia.com/2018/09/11/rajya-sabha-tv-in-depth-hcng-fuel-of-the-future (accessed on 16 April 2023).
- Kokjohn, S.L.; Hanson, R.M.; Splitter, D.A.; Reitz, R.D. Fuel Reactivity Controlled Compression Ignition (RCCI): A Pathway to Controlled High-Efficiency Clean Combustion. Int. J. Engine Res. 2011, 12, 209–226. [Google Scholar] [CrossRef]
- DelVescovo, D.A. The Effects of Fuel Stratification and Heat Release Rate Shaping in Reactivity Controlled Compression Ignition (RCCI) Combustion. Ph.D. Thesis, The University of Wisconsin-Madison, Madison, WI, USA, 2016. [Google Scholar]
- Dziubak, T.; Karczewski, M. Experimental Studies of the Effect of Air Filter Pressure Drop on the Composition and Emission Changes of a Compression Ignition Internal Combustion Engine. Energies 2022, 15, 4815. [Google Scholar] [CrossRef]
- Kuiken, K. Gas- and Dual-Fuel Engines for Ship Propulsion, Power Plants and Cogeneration. Book I: Engine Systems and Environment; Target Global Energy Training: Onnen, The Netherlands, 2016. [Google Scholar]
- Searle, S.; Bieker, G.; Baldino, C. Decarbonizing Road Transport by 2050: Zero-Emission Pathways for Passenger Vehicles; International Council on Clean Transportation: Washington, DC, USA, 2021. [Google Scholar]
- Fuel Cell Basics. Available online: https://www.fchea.org/fuelcells (accessed on 17 April 2023).
- Fuel Cell Benefits—Plug Power. Available online: https://www.plugpower.com/fuel-cell-power/fuel-cell-benefits/ (accessed on 17 April 2023).
- Patel, S. GE Unveils New H-Class Gas Turbine—And Already Has a First Order. POWER Magazine, 2 October 2019. [Google Scholar]
- What Are the Pros and Cons of Hydrogen Fuel Cells? Available online: https://www.twi-global.com/technical-knowledge/faqs/what-are-the-pros-and-cons-of-hydrogen-fuel-cells.aspx (accessed on 10 April 2023).
- Sonal, P. Hydrogen Fuel Cell Technology—Advantages and Disadvantages|Knauf; Knauf Industries Automotive; POWER; News & Technology for the Global Energy Industry. Available online: https://knaufautomotive.com/hydrogen-fuel-cells-technology-advantages-disadvantages-and-applications (accessed on 10 April 2023).
- Hydrogen Fuel Cell Advantages and Disadvantages in Material Handling. Available online: https://www.fluxpower.com/blog/hydrogen-fuel-cell-advantages-and-disadvantages-in-material-handling (accessed on 10 April 2023).
- Collins, L. Hydrogen v Battery Trucks|UK Launches $240m Competition to Find out Which Is Best for Zero-Emissions Haulage. Available online: https://www.hydrogeninsight.com/transport/hydrogen-v-battery-trucks-uk-launches-240m-competition-to-find-out-which-is-best-for-zero-emissions-haulage/2-1-1335064 (accessed on 10 April 2023).
- Hydrogen Pipelines’ CAPEX by Type. 2021. Available online: https://www.statista.com/statistics/1220856/capex-new-retrofitted-h2-pipelines-by-type/ (accessed on 10 April 2023).
- DeSantis, D.; James, B.D.; Houchins, C.; Saur, G.; Lyubovsky, M. Cost of Long-Distance Energy Transmission by Different Carriers. iScience 2021, 24, 103495. [Google Scholar] [CrossRef]
- Brown, D.; Reddi, K.; Elgowainy, A. The Development of Natural Gas and Hydrogen Pipeline Capital Cost Estimating Equations. Int. J. Hydrogen Energy 2022, 47, 33813–33826. [Google Scholar] [CrossRef]
- INGAA Foundation, Inc. North America Midstream Infrastructure through 2035: Capitalizing on Our Energy Abundance; INGAA Foundation, Inc.: Washington, DC, USA, 2014. [Google Scholar]
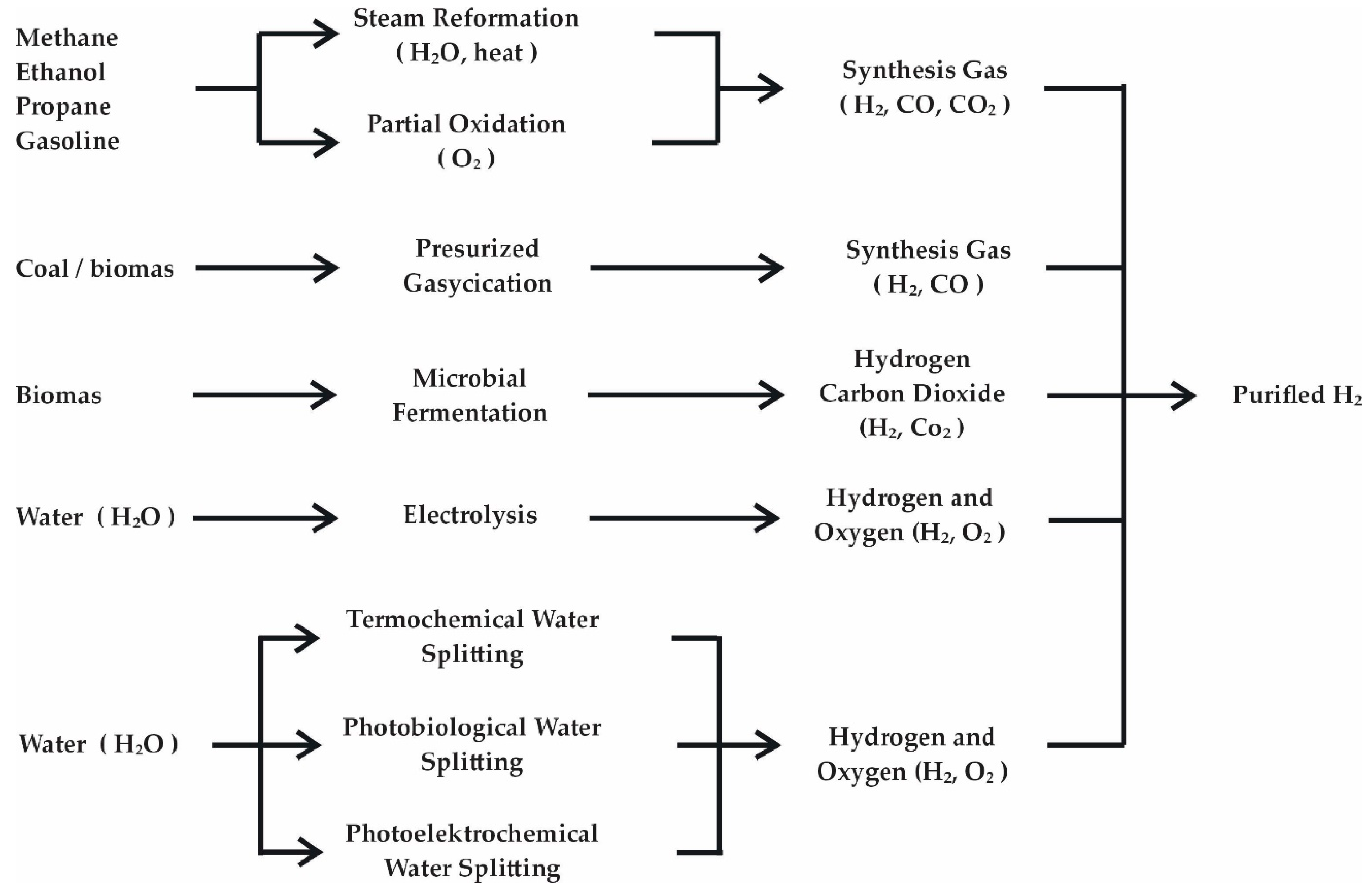

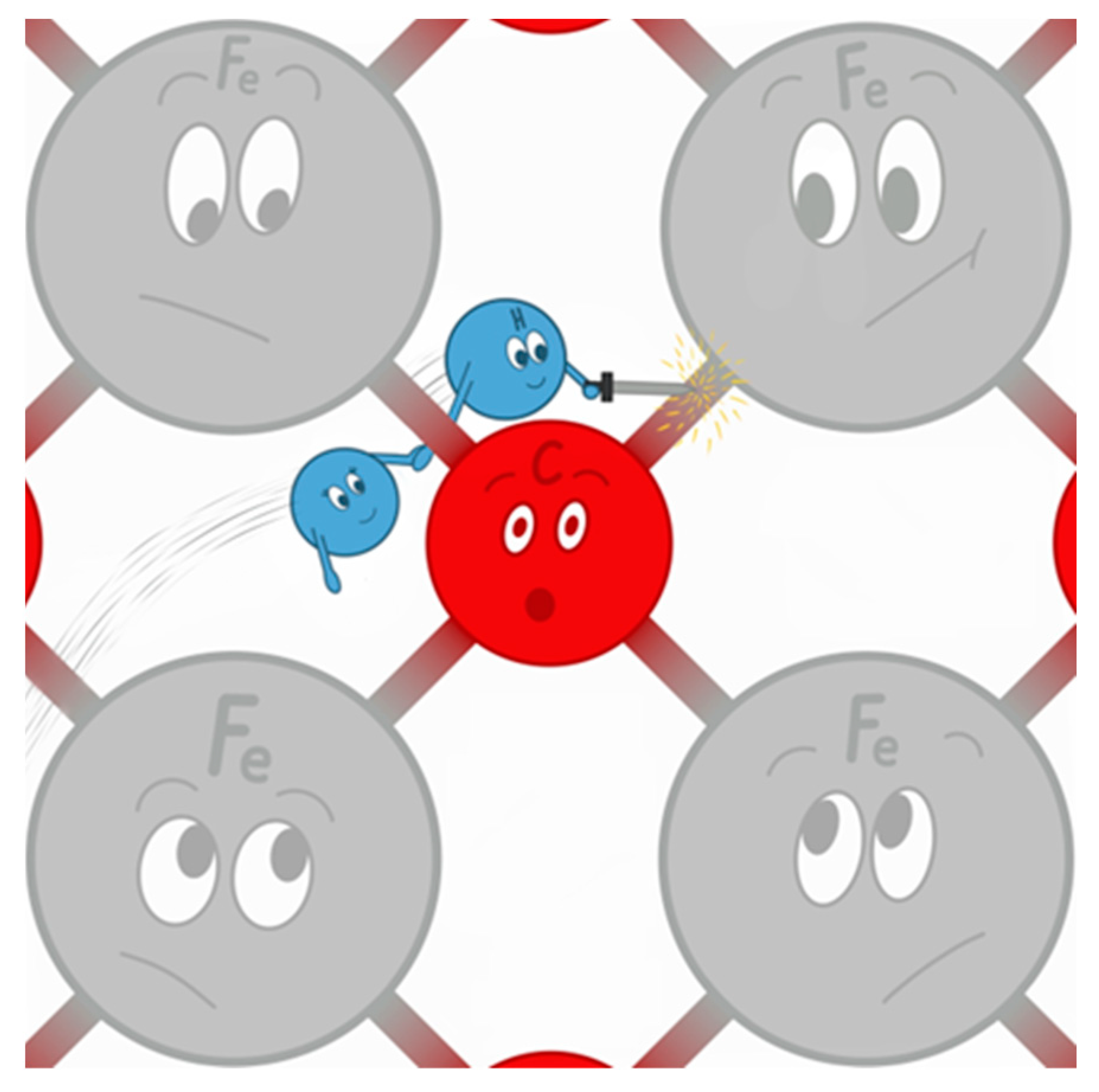
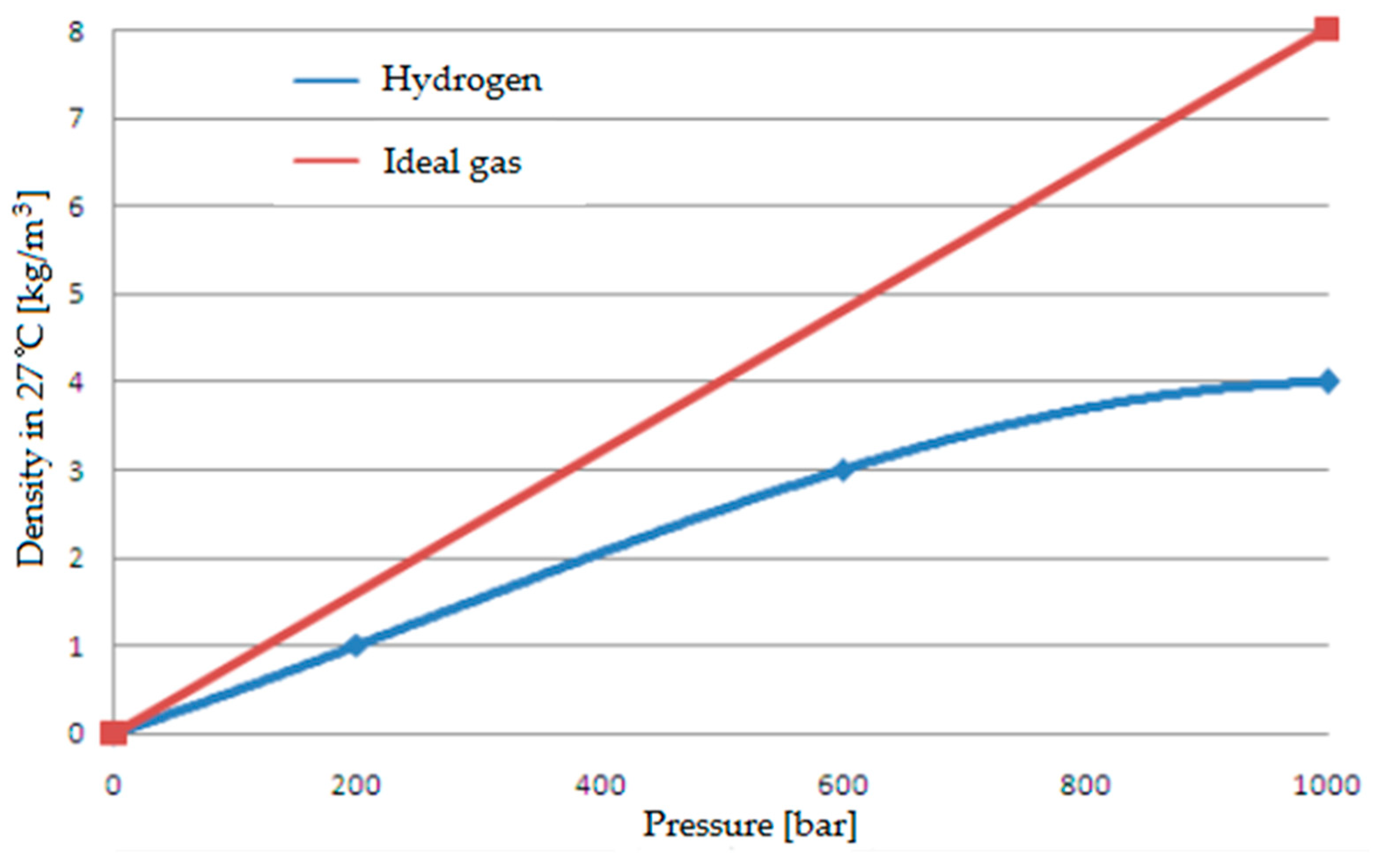
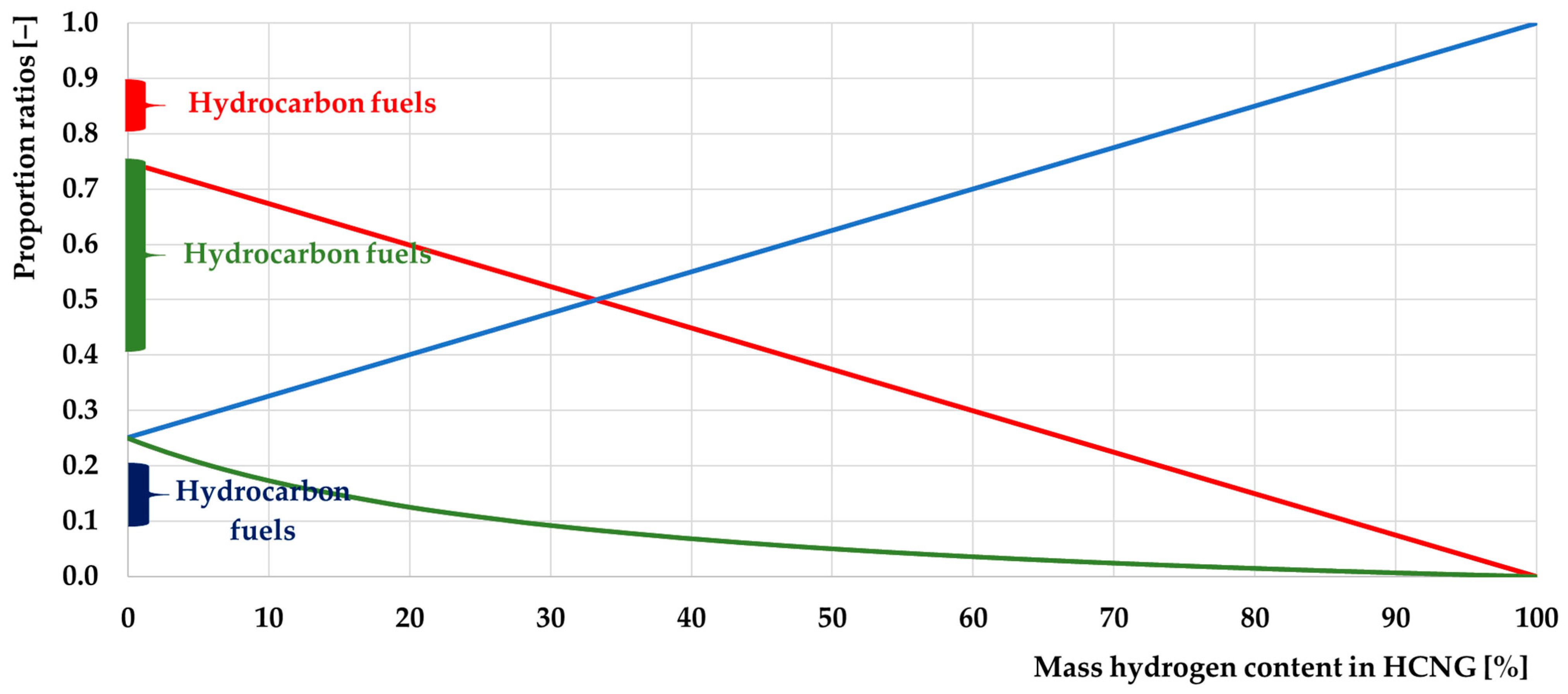
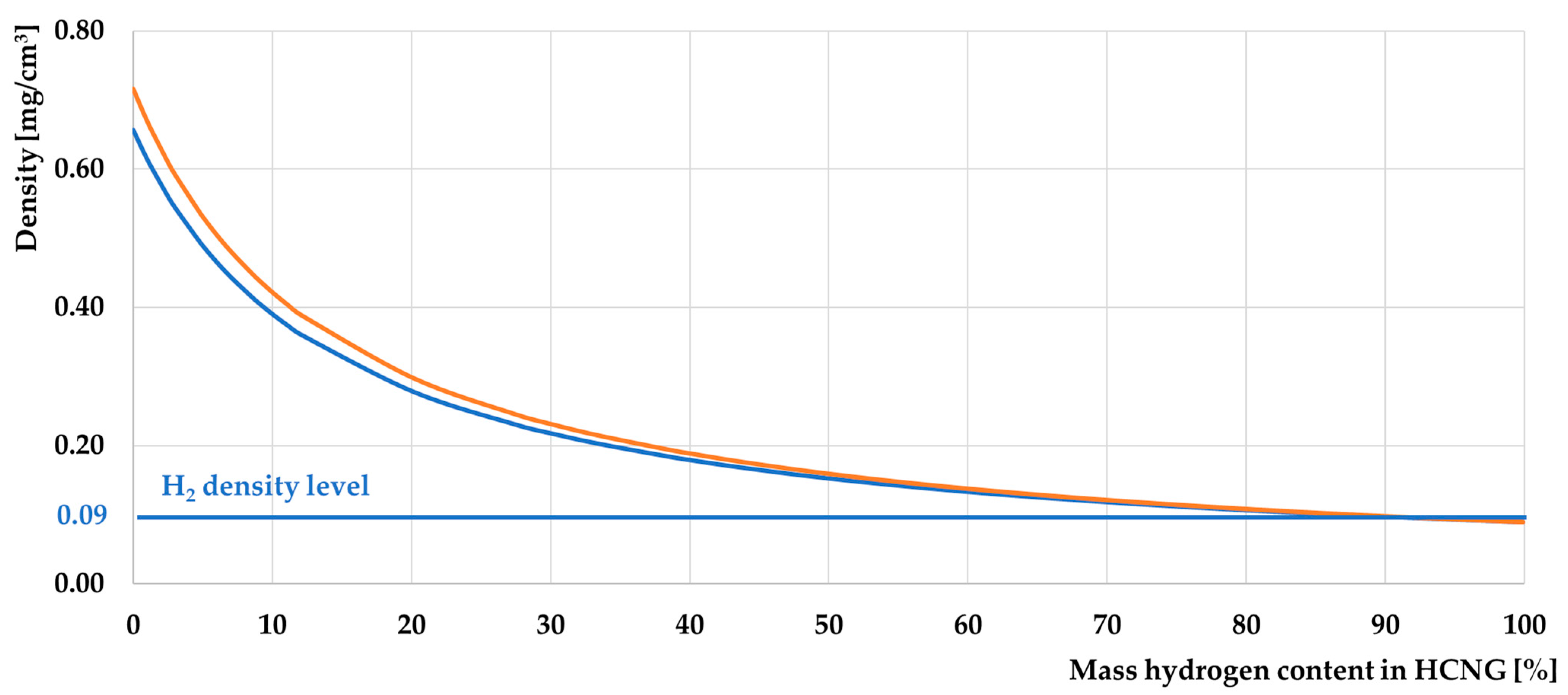
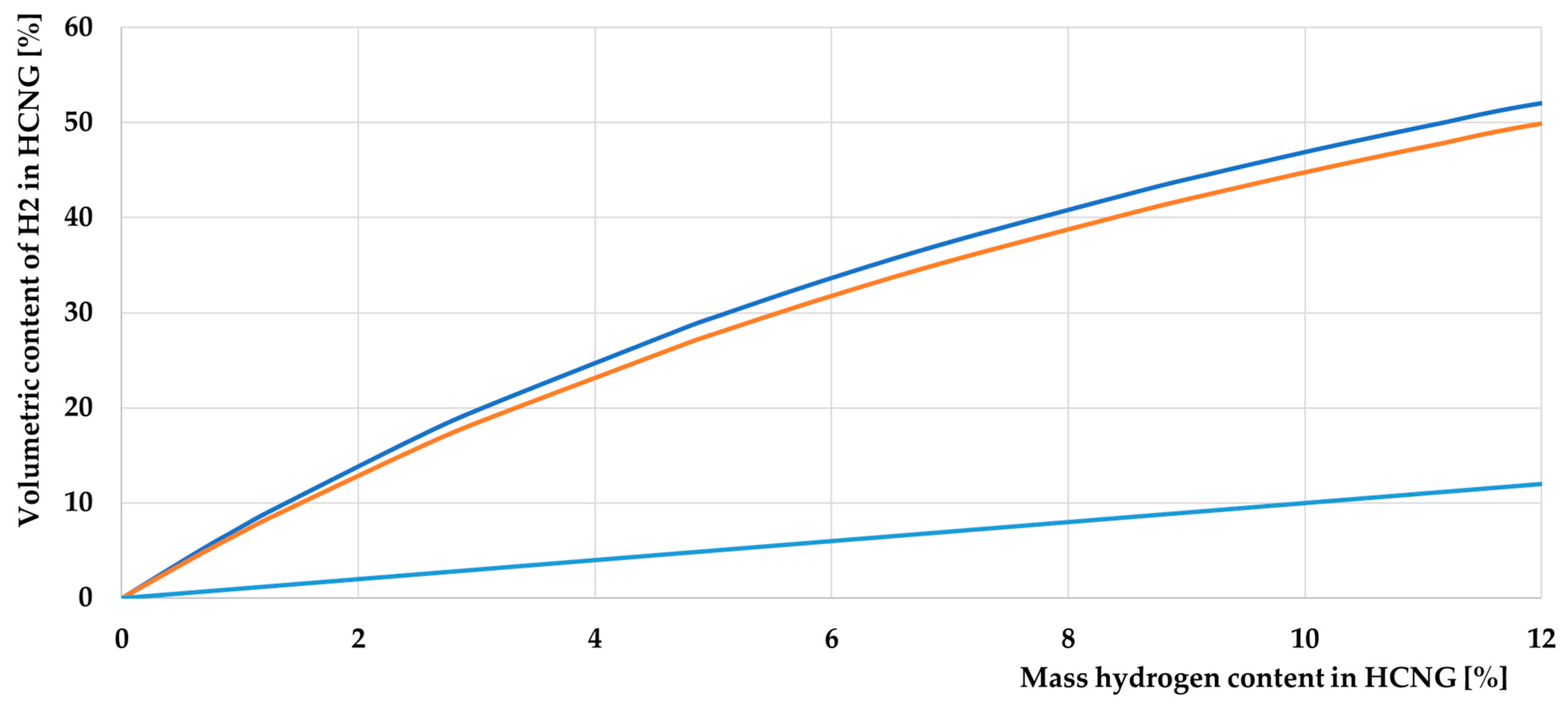
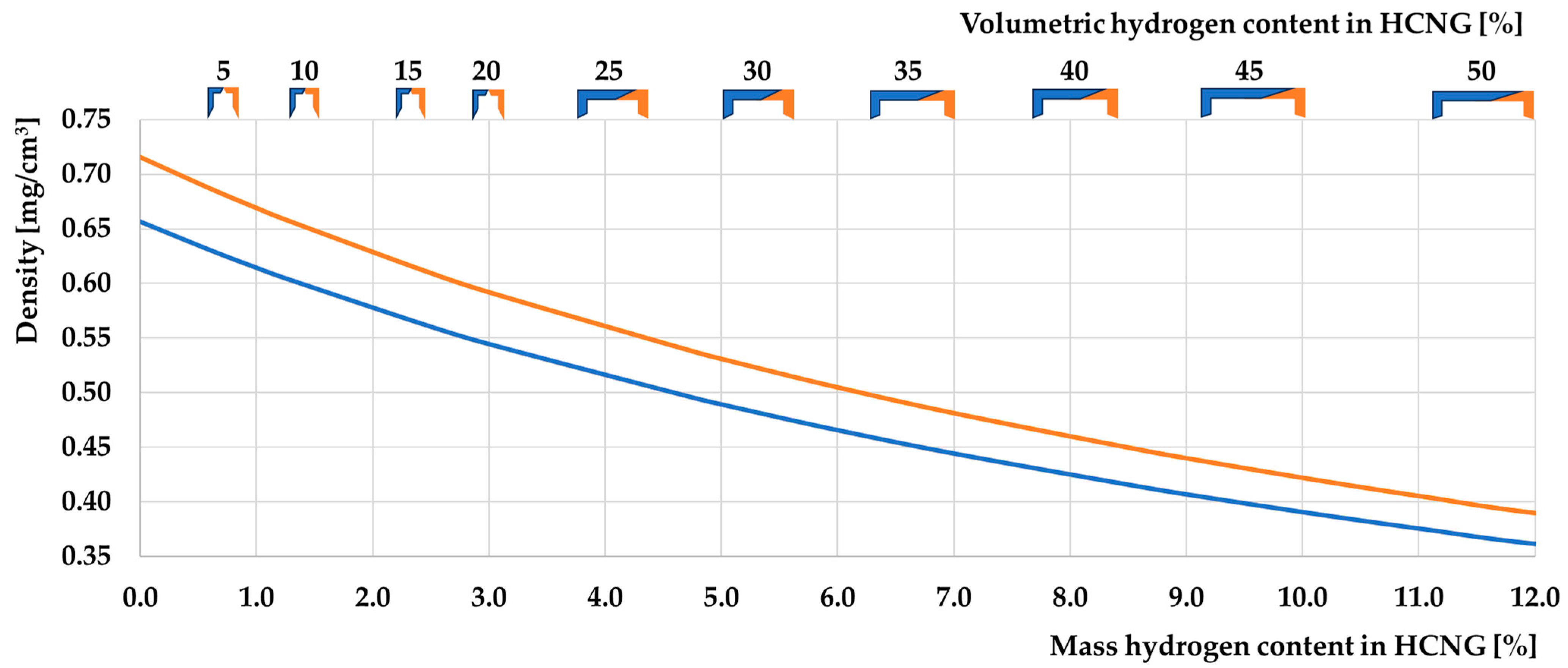
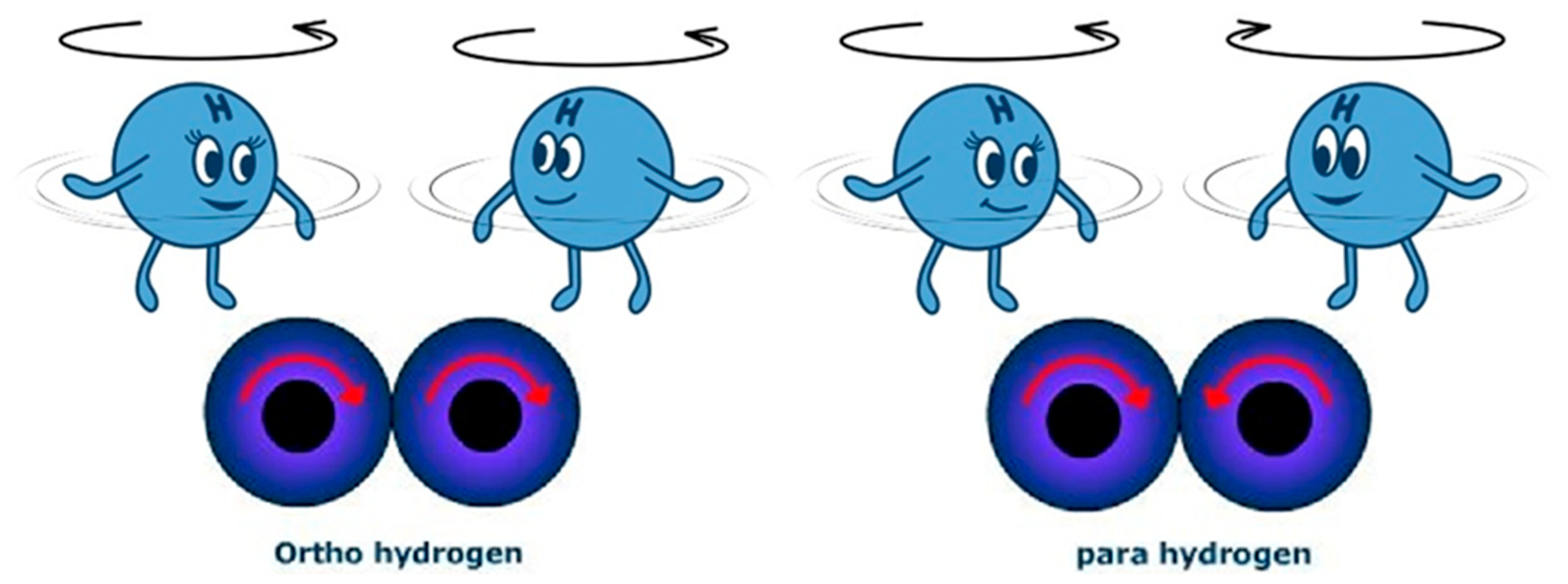


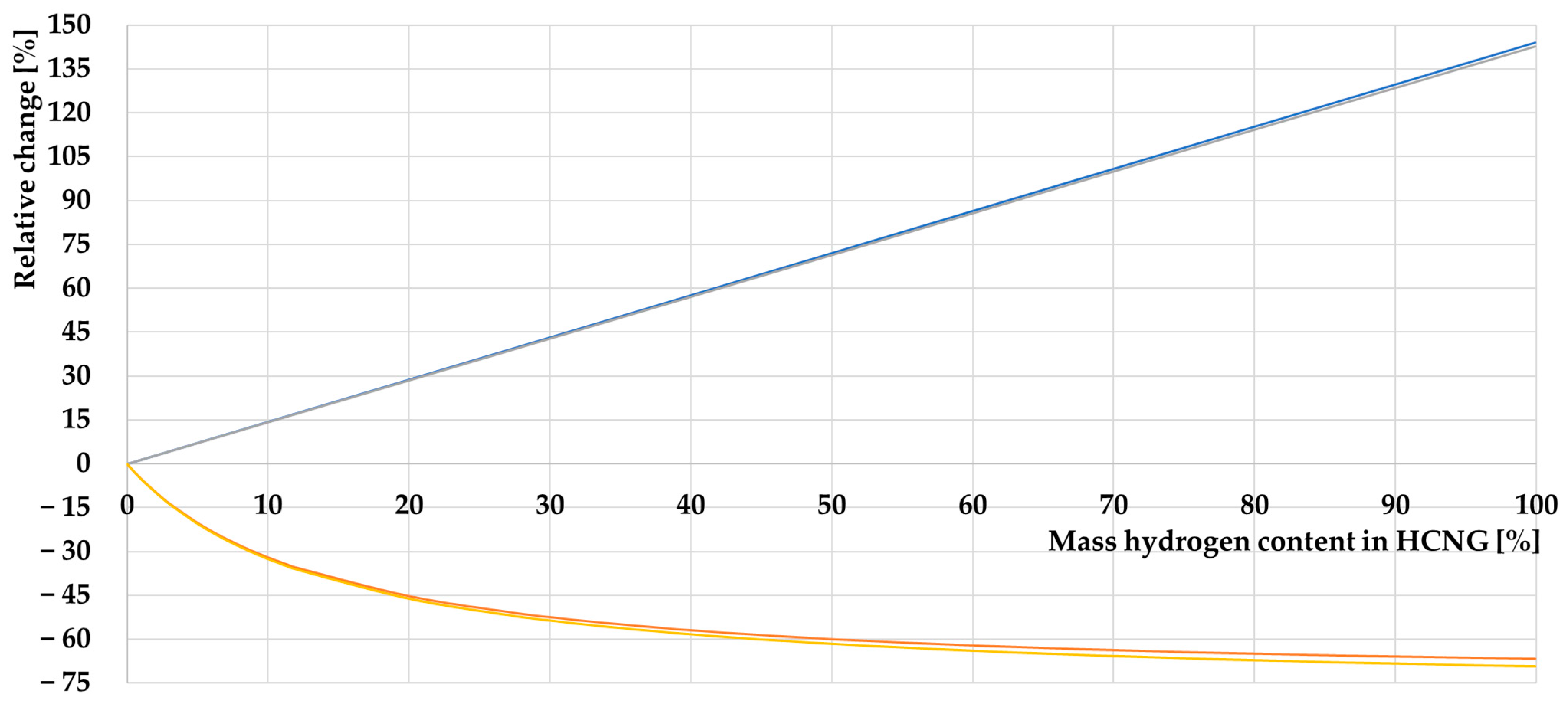
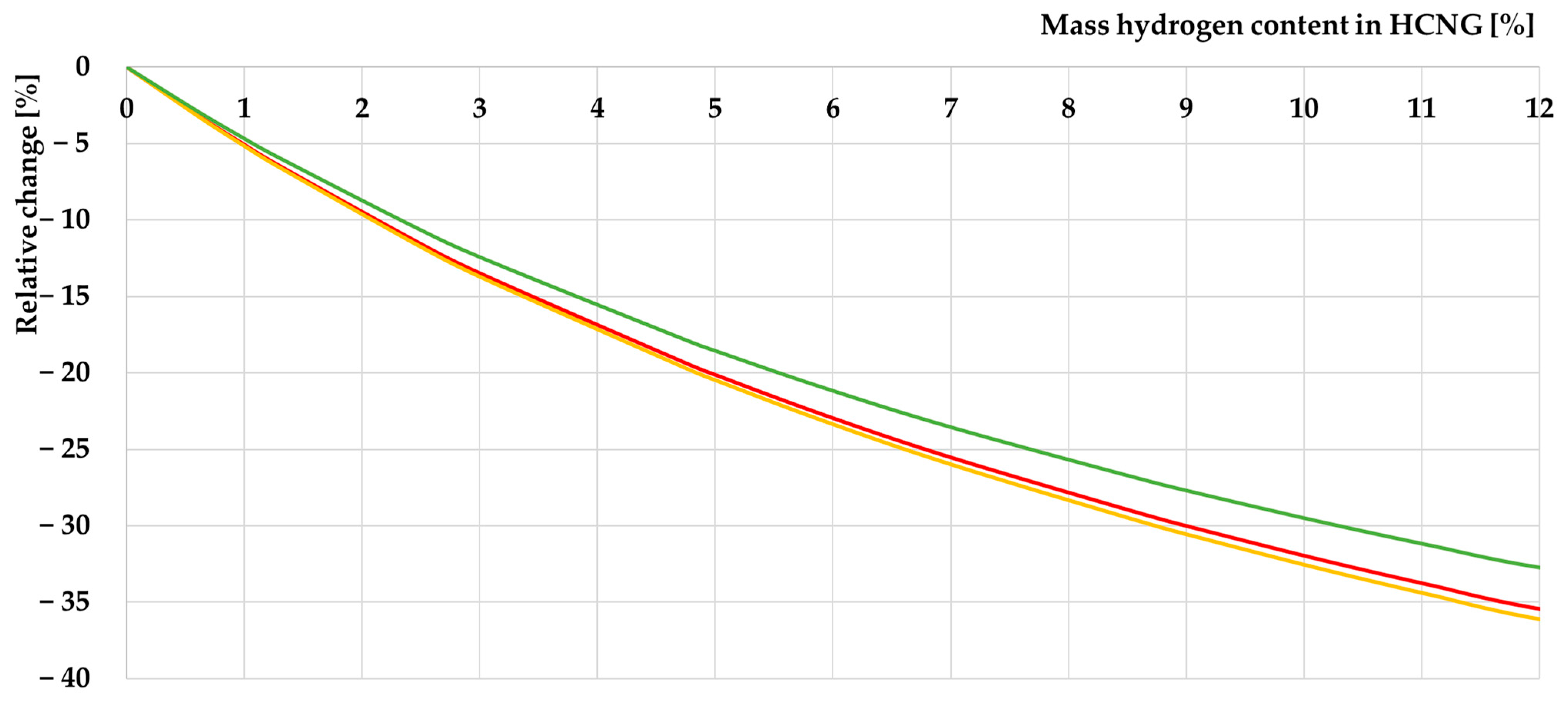
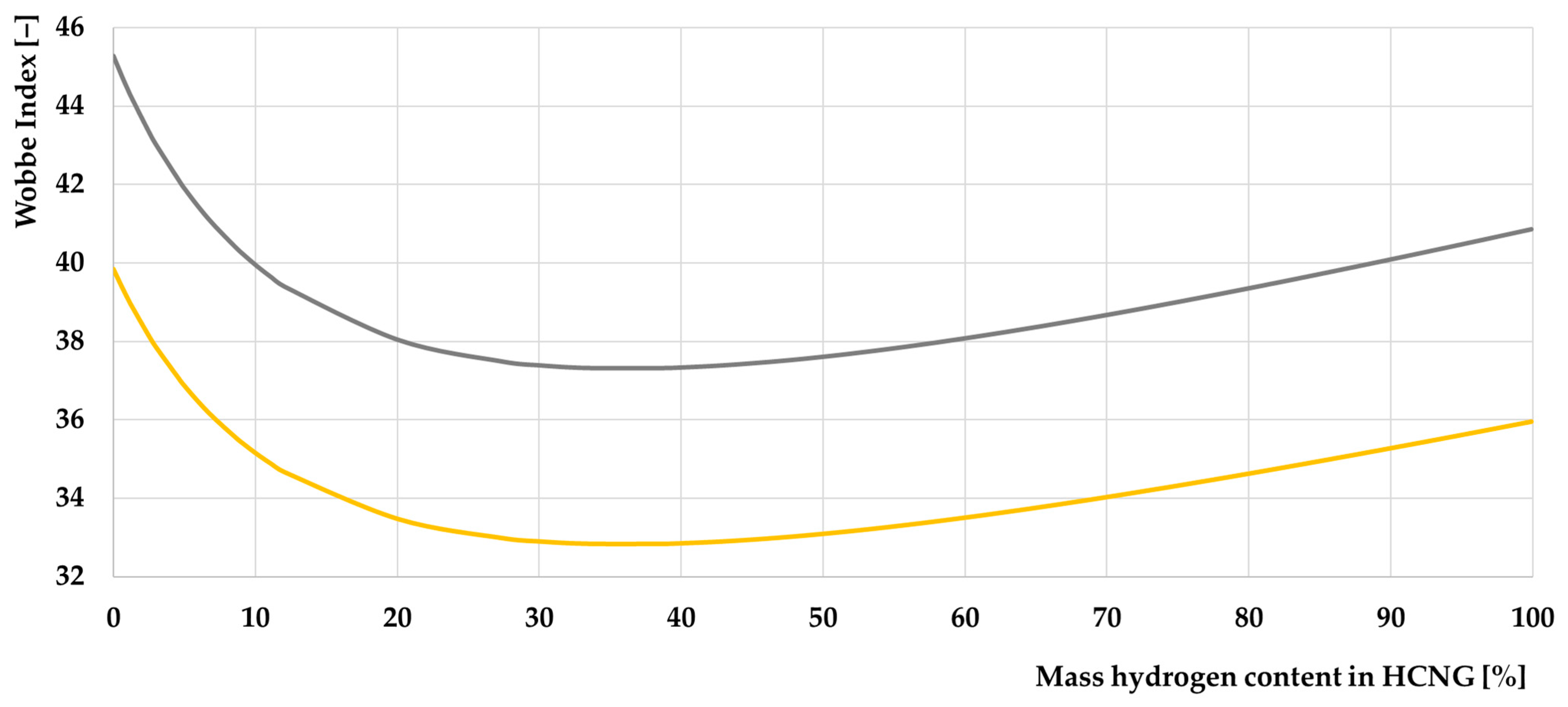

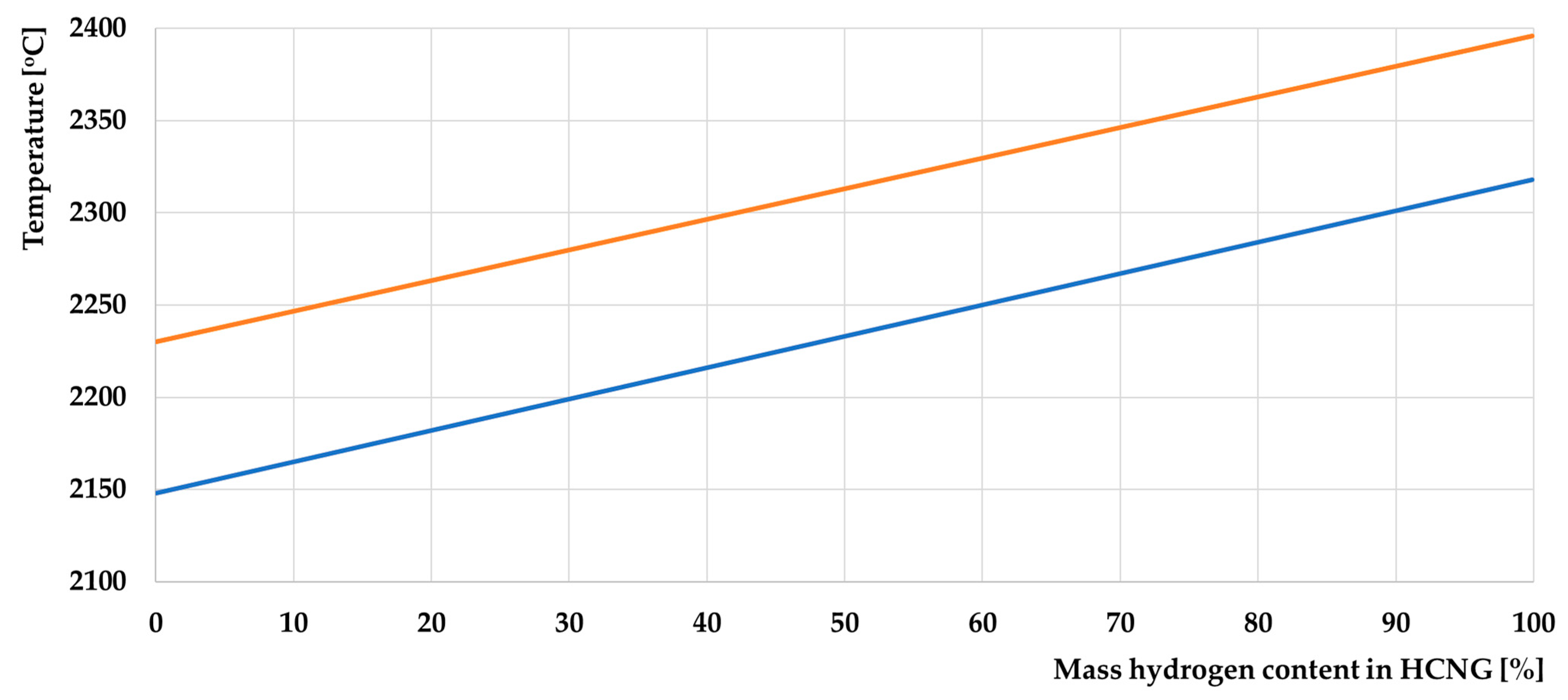
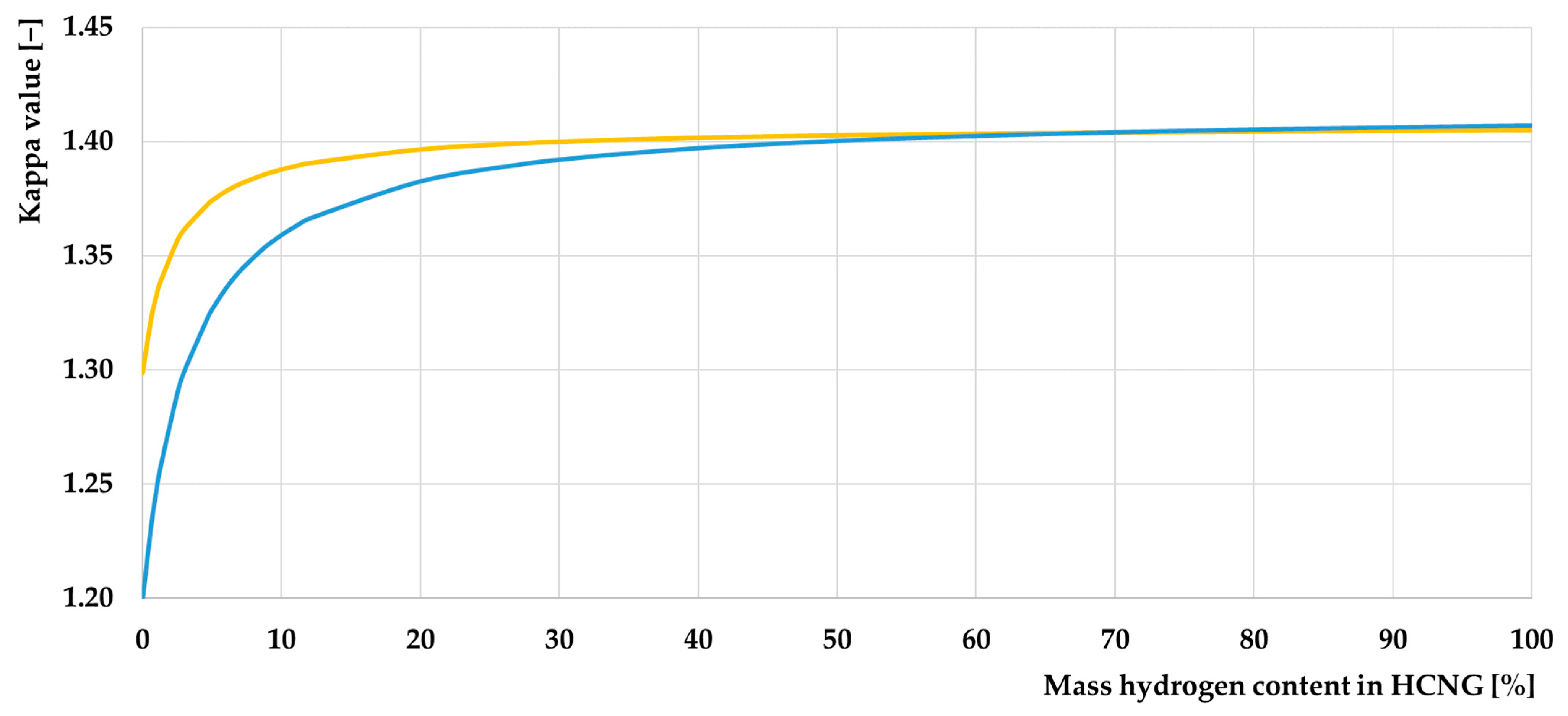

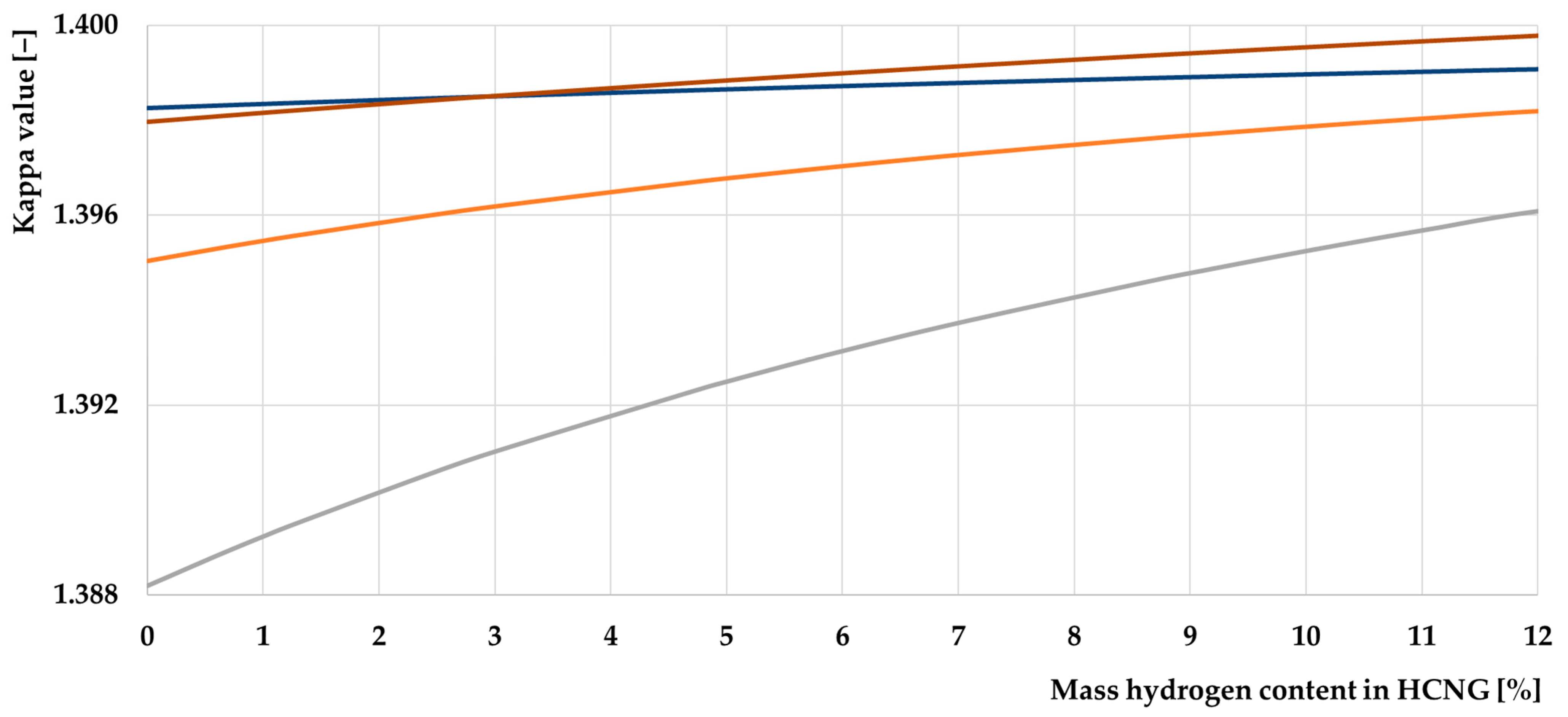


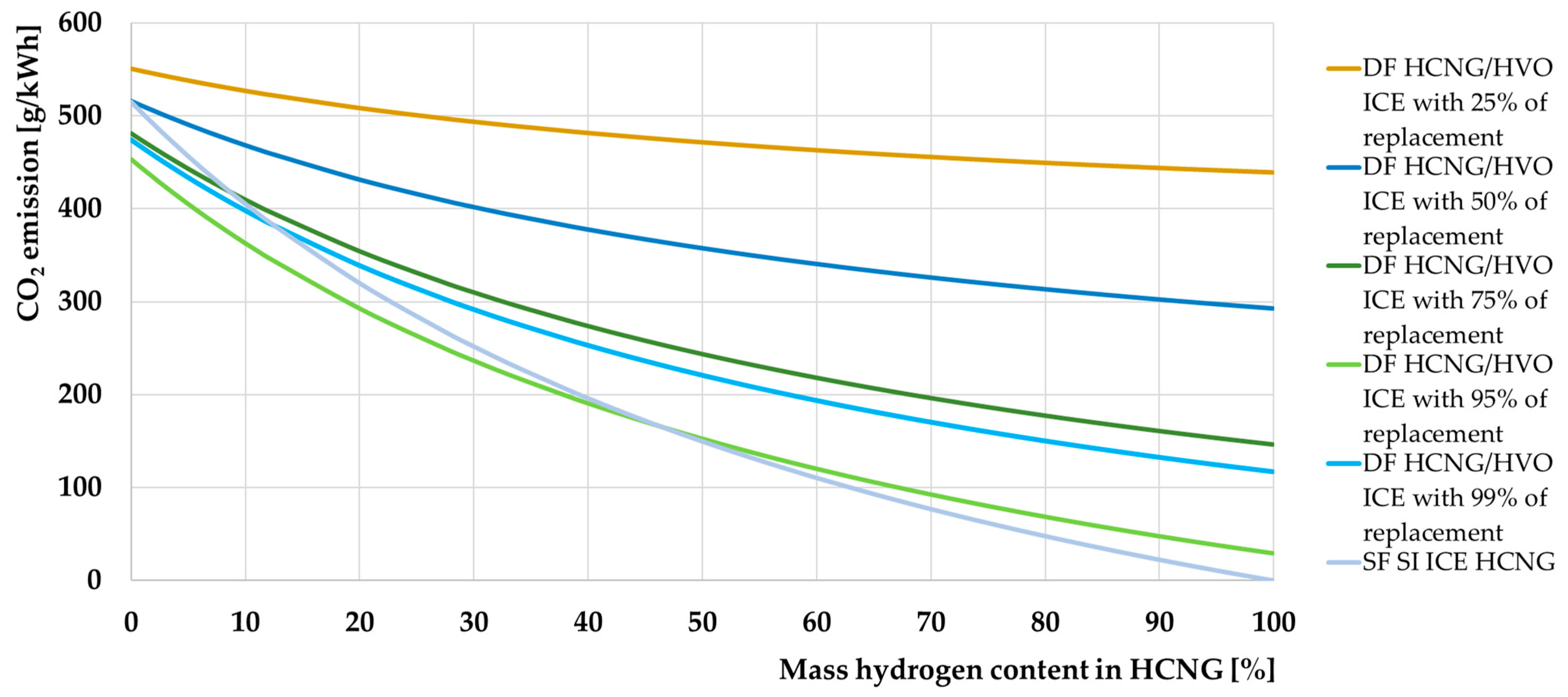
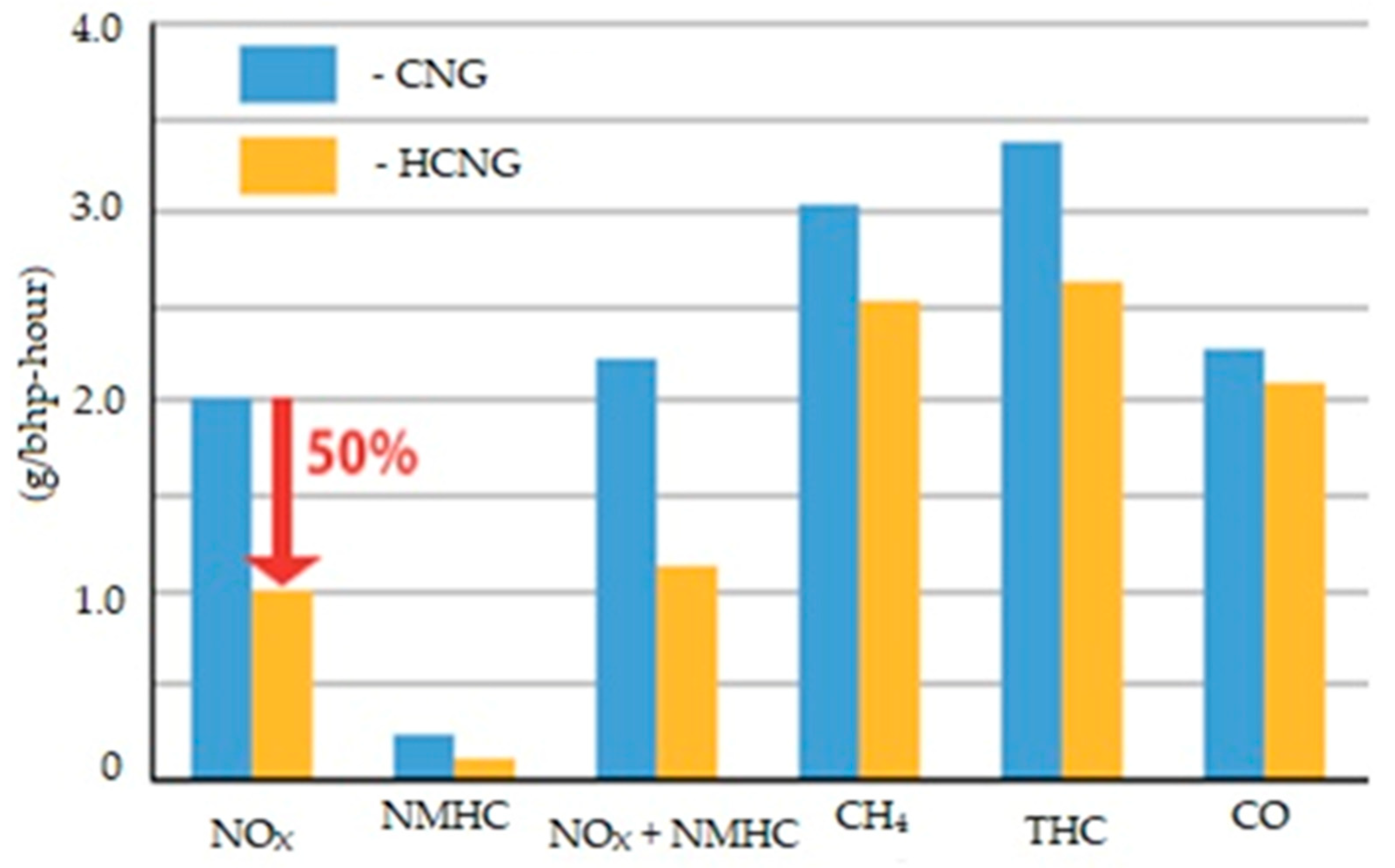
| H2 content | [%] | 100% | 8% | 20% | 25% | 30% |
| Electrolysis green electricity | 66 | USD 3–6/kg [29,30,32] | 64 | 62 | 60 | 59 |
| CO2 change | [%] | - | −2.4% | −6.4% | −8.3% | −10% |
| Electrolysis EU-mix electricity | 66 | USD 4–6/kg or EUR 12/kg [33] | 70 | 77 | 80 | 84 |
| CO2 change | [%] | - | +6.1% | +17% | +22% | +27% |
| Reforming natural gas | 66 | USD 0.5–2/kg [34,35,36] | 68 | 72 | 74 | 76 |
| CO2 change | [%] | - | +3.5% | +9.7% | +13% | +16% |
| Natural Gas | Hydrogen | Source | |
|---|---|---|---|
| Hydrogen to carbon ratio | 4:1 | - | [161] |
| Energy density [MJ/kg] | 48–50 | 120 | [161] |
| 49.15 | 119.9 | [158] | |
| 50.02 | 119.93 | [58] | |
| Energy density [MJ/dm3] | 12.6 | 3.0 | [162] |
| ] | 903 | 793 | [163] |
| 813 | 858 | [161] | |
| 810 | 858 | [157,164,165] | |
| ] | 0.29 | 0.02 | [161] |
| 0.274 | 0.017 | [163,164] | |
| ] | 0.29 | 0.02 | [162] |
| Octane rating | 120+ | 130+ | [161] |
| Octane number | RON 120 | - | [162] |
| Flammability limits [%] | 5.3–15% | 4–75% | [163] |
| 5.3–14% | 4–70% | [161] | |
| 5.3–17% | 4–70% | [164] | |
| Stoichiometric Air/fuel ratio | 9.48 | 29.53 | [162] |
| /] | 0.058 | 0.029 | [161] |
| 47.91–53.28 | 40.65–48.23 | [161] | |
| Flame velocity in the air at NTP [cm/s] | 37–45 | 265–325 | [161] |
| 38.5 | 315 | [164] | |
| Flame temperature [K] | 2148 | 2318 | [161,163] |
| 2230 | 2396 | [164] | |
| /] | 0.7 | 0.07 | [161] |
| 0.657 | 0.8988 | [166] | |
| - | 0.08375 | [167] | |
| 0.668 | 0.084 | [164] | |
| /] | 0.716 | 0.09 | [162] |
| Mainly emissions | , , , , | , | [161] |
| Energy Density [MJ/Lts] | 25.3 | 2.9 at 350 bars | [161] |
| /] | 16.043 | 2.016 | [162] |
| /] | 50 | 120 | [162] |
| Higher heating value | 50.02 | 141.86 | |
| 55.9 | 142.2 | [168] | |
| /] | ≈42 | ≈230 | [162] |
| Lower explosive limit [%] | 6.3 | 18.3 | [164] |
| Higher explosive limit [%] | 13.5 | 59 | [164] |
| Properties | Hydrogen | |
|---|---|---|
| Para-Hydrogen | 75% of Ortho- + 25% of Para-H2 | |
| Density in 0 °C, 103 mol/cm3 | 0.0546 | 0.0446 |
| Cp in 0 °C, J/(molxK) | 30.35 | 28.59 |
| Cv in 0 °C, J/(molxK) | 21.87 | 20.3 |
| Enthalpy in 0 °C, J/mol | 7656.6 | 7749.2 |
| Internal energy in 0 °C, J/mol | 5384.5 | 5477.1 |
| Entropy in 0 °C, J/(molxK) | 127.77 | 139.59 |
| Thermal conductivity in 0 °C, mW/(cmxK) | 1.841 | 1.74 |
| Temperature [K] | 20.39 | 30 | 40 | 70 | 120 | 200 | 250 | 300 |
| Para-hydrogen in hydrogen in % | 99.80 | 97.02 | 88.73 | 55.88 | 32.96 | 25.97 | 25.26 | 25.07 |
| Natural Gas | Methane | Hydrogen | HCNG50 | |
|---|---|---|---|---|
| Lower flammability volumetric limit [%] | 5.3/4.4 [234] | 5 [230] | 4 | ~5 |
| Higher flammability volumetric limit [%] | 17 | 14.3 [230] | 75 | ~24 |
| Lower explosive volumetric limit [%] | 6.3 | 5 [233] | 18.3 | ~7 |
| Higher explosive volumetric limit [%] | 13.5 | 15 [233] | 59 | ~20 |
| Maximal flame speed [m/s] | 0.374 [231] | 0.385 | 3.15/2.933 [231] | ~0.68 |
| Minimal ignition energy [mJ] | 0.31 [229] | 0.274 | 0.017 | ~0.27 |
| Self-ignition temperature [°C] | 582 [232] | 537/540 [228] | 585 | ~583 |
| CR | AIR | CNG | HCNG | Diff.: | CR | AIR | CNG | HCNG | Diff.: |
|---|---|---|---|---|---|---|---|---|---|
| 6 | 51.1% | 51.0% | 51.1% | −0.31% | 16 | 72.2% | 72.0% | 72.2% | −0.25% |
| 7 | 54.1% | 53.9% | 54.1% | −0.30% | 17 | 73.0% | 72.8% | 73.0% | −0.25% |
| 8 | 56.4% | 56.3% | 56.5% | −0.29% | 18 | 73.8% | 73.6% | 73.8% | −0.24% |
| 9 | 58.5% | 58.3% | 58.5% | −0.28% | 19 | 74.5% | 74.3% | 74.5% | −0.24% |
| 10 | 60.2% | 60.0% | 60.2% | −0.28% | 20 | 75.2% | 75.0% | 75.2% | −0.24% |
| 11 | 61.7% | 61.5% | 61.7% | −0.27% | 21 | 75.8% | 75.6% | 75.8% | −0.23% |
| 12 | 63.0% | 62.8% | 63.0% | −0.27% | 22 | 76.4% | 76.2% | 76.4% | −0.23% |
| 13 | 64.1% | 64.0% | 64.1% | −0.26% | 23 | 77.0% | 76.8% | 77.0% | −0.23% |
| 14 | 65.2% | 65.0% | 65.2% | −0.26% | 24 | 77.5% | 77.3% | 77.5% | −0.23% |
| Average difference: | −0.28% | Average difference: | −0.24% | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szamrej, G.; Karczewski, M. Exploring Hydrogen-Enriched Fuels and the Promise of HCNG in Industrial Dual-Fuel Engines. Energies 2024, 17, 1525. https://doi.org/10.3390/en17071525
Szamrej G, Karczewski M. Exploring Hydrogen-Enriched Fuels and the Promise of HCNG in Industrial Dual-Fuel Engines. Energies. 2024; 17(7):1525. https://doi.org/10.3390/en17071525
Chicago/Turabian StyleSzamrej, Grzegorz, and Mirosław Karczewski. 2024. "Exploring Hydrogen-Enriched Fuels and the Promise of HCNG in Industrial Dual-Fuel Engines" Energies 17, no. 7: 1525. https://doi.org/10.3390/en17071525
APA StyleSzamrej, G., & Karczewski, M. (2024). Exploring Hydrogen-Enriched Fuels and the Promise of HCNG in Industrial Dual-Fuel Engines. Energies, 17(7), 1525. https://doi.org/10.3390/en17071525







