Electrochemical Synthesis of Ammonia via Nitrogen Reduction and Oxygen Evolution Reactions—A Comprehensive Review on Electrolyte-Supported Cells
Abstract
1. Introduction
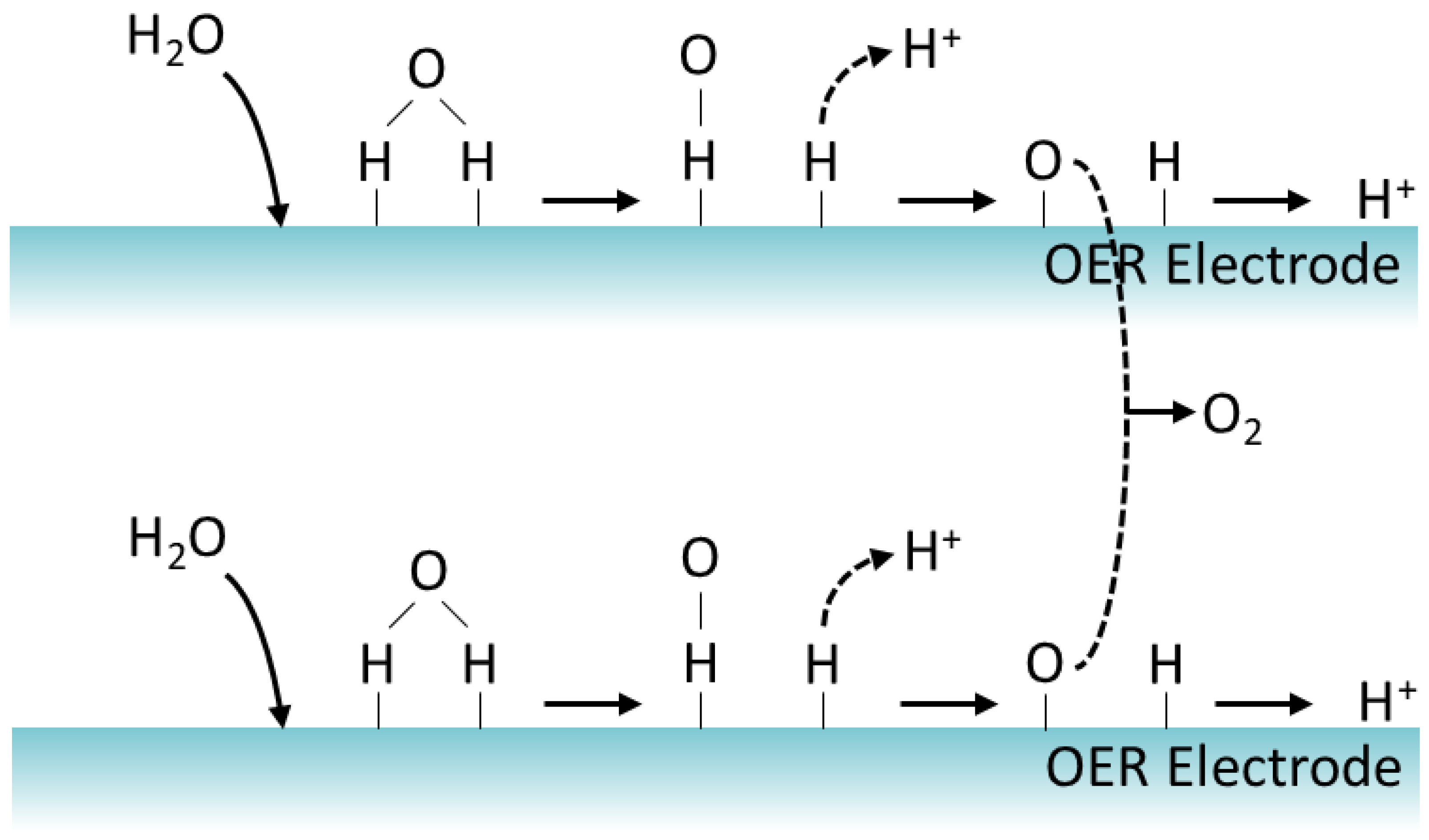
- Anode reaction
- ●
- At the anode, an electric current passes through water, thereby splitting water molecules into hydrogen (protons) and oxygen ions.
- ●
- Reaction: 2H2O → O2 + 4H+ + 4e−
- Electron and proton transport:
- ●
- Electrons generated at the anode during water splitting are transported through an external electric circuit.
- ●
- Protons generated at the anode during water splitting are transported through the electrolyte [18].
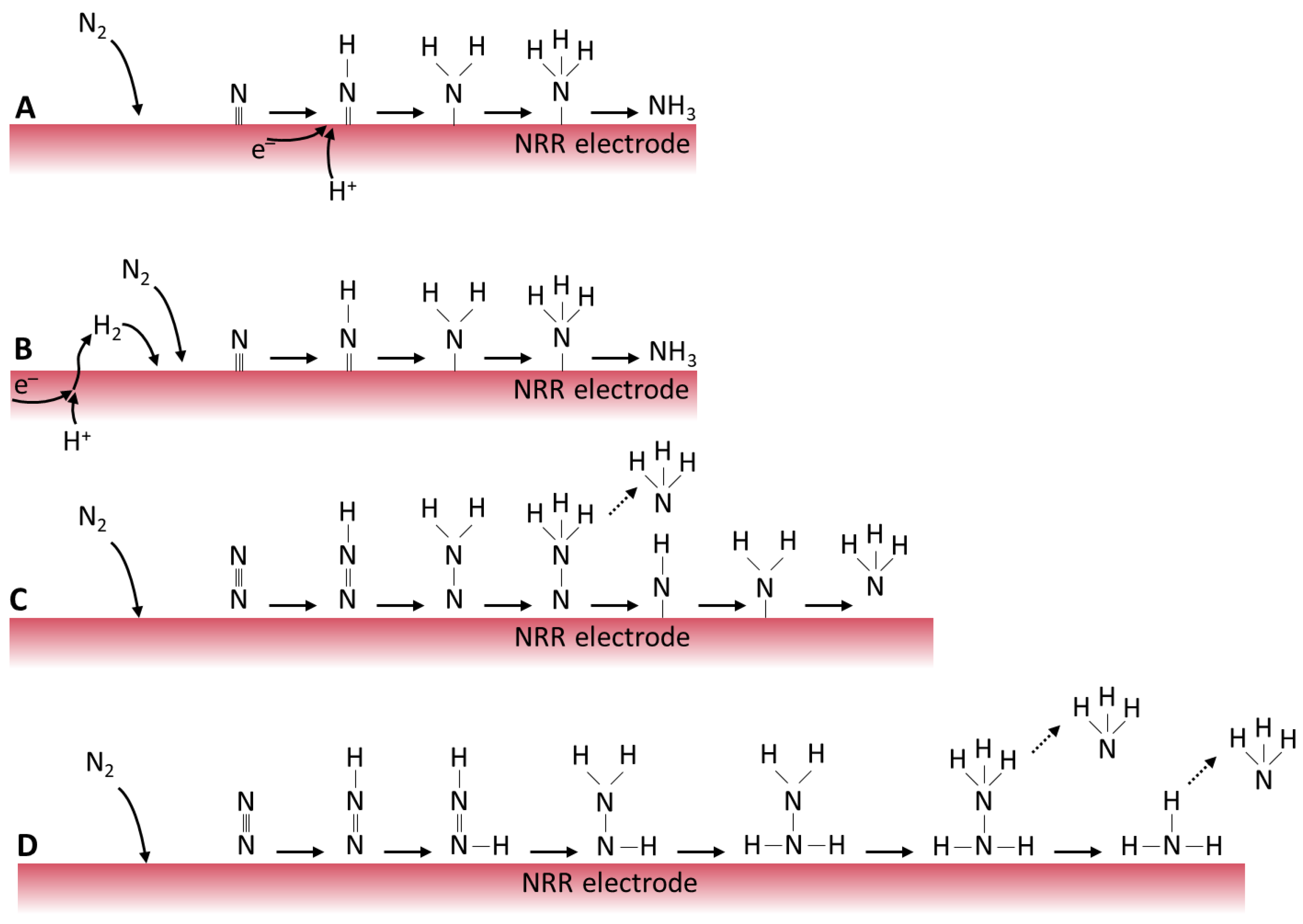
- Cathode reaction
- ●
- At the cathode, protons (H+) from the anode and nitrogen atoms react to produce NH3
- ●
- Reaction: N2 + 3H+ + 3e− → NH3
2. Reaction Mechanism
- (i)
- The reaction is initiated when a water molecule is adsorbed on the catalyst surface.
- (ii)
- Consequently, surface-bound hydroxyl species (HO*) are formed.
- (iii)
- The generated HO* decomposes into hydrogen (H*) and oxygen (O*) species.
- (iv)
- Protons (H+) are transferred to the cathode through the electrolyte.
- (v)
- Finally, gaseous oxygen materializes through desorption [35].
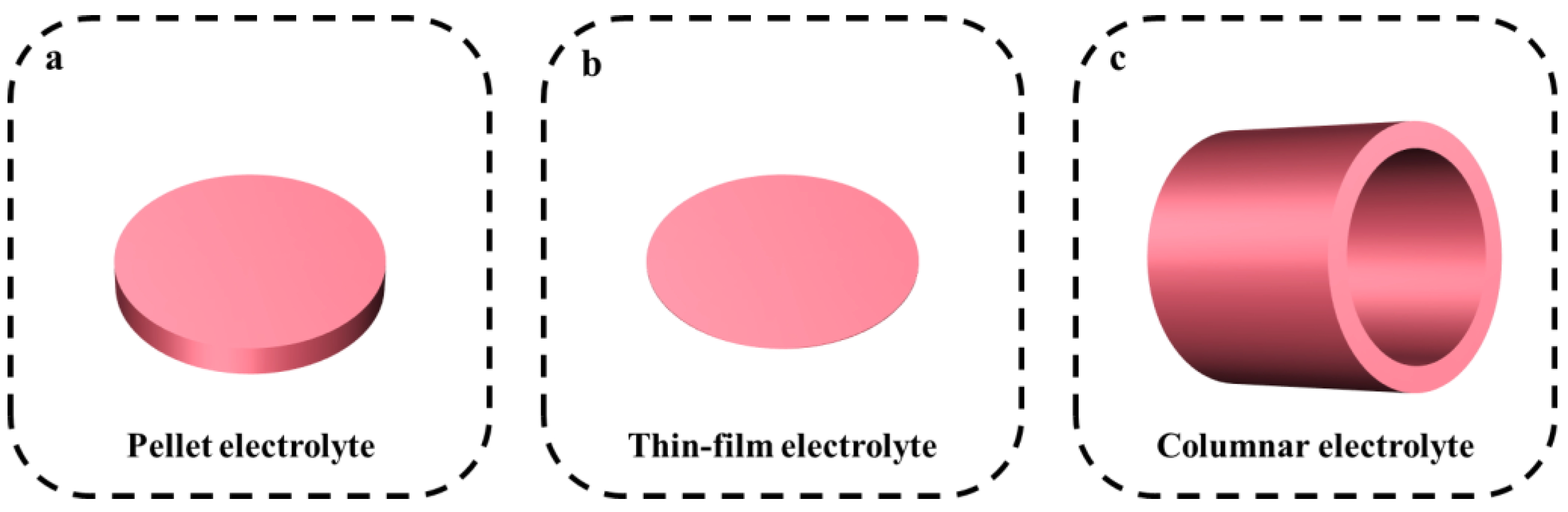
3. PCEC Design Strategies
3.1. Electrolyte Design Strategies
3.2. Electrode Design Strategies
4. Current Progress
5. Conclusions
- A more scalable approach must be investigated to deposit Fe- and Co-based perovskite electrodes to reduce catalyst wastage.
- A more complex catalyst must be developed for the NRR because existing materials are not as advanced as OER catalysts.
- Stability and thermal mismatch issues for the OER must be addressed to decrease wastage and increase cell stability.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teichmann, D.; Stark, K.; Müller, K.; Zöttl, G.; Wasserscheid, P.; Arlt, W. Energy Storage in Residential and Commercial Buildings via Liquid Organic Hydrogen Carriers (LOHC). Energy Environ. Sci. 2012, 5, 9044–9054. [Google Scholar] [CrossRef]
- Kandemir, T.; Schuster, M.E.; Senyshyn, A.; Behrens, M.; Schlögl, R. The Haber–Bosch Process Revisited: On the Real Structure and Stability of “Ammonia Iron” under Working Conditions. Angew. Chem. Int. Ed. 2013, 52, 12723–12726. [Google Scholar] [CrossRef] [PubMed]
- Haber, F.; Rossignol, R. Le Über Die Technische Darstellung von Ammoniak Aus Den Elementen. Zeitschrift für Elektrochemie und Angew. Phys. Chemie 1913, 19, 53–72. [Google Scholar] [CrossRef]
- Aika, K.i.; Hori, H.; Ozaki, A. Activation of Nitrogen by Alkali Metal Promoted Transition Metal I. Ammonia Synthesis over Ruthenium Promoted by Alkali Metal. J. Catal. 1972, 27, 424–431. [Google Scholar] [CrossRef]
- IEA Ammonia Technology Roadmap. Available online: https://www.iea.org/reports/ammonia-technology-roadmap (accessed on 4 October 2023).
- Brown, T. Feeding Life 2030: The Vision of Fertilizers Europe. Available online: https://www.ammoniaenergy.org/articles/feeding-life-2030-the-vision-of-fertilizers-europe/ (accessed on 4 October 2023).
- Shipman, M.A.; Symes, M.D. Recent Progress towards the Electrosynthesis of Ammonia from Sustainable Resources. Catal. Today 2017, 286, 57–68. [Google Scholar] [CrossRef]
- Foster, S.L.; Bakovic, S.I.P.; Duda, R.D.; Maheshwari, S.; Milton, R.D.; Minteer, S.D.; Janik, M.J.; Renner, J.N.; Greenlee, L.F. Catalysts for Nitrogen Reduction to Ammonia. Nat. Catal. 2018, 1, 490–500. [Google Scholar] [CrossRef]
- Jeerh, G.; Zhang, M.; Tao, S. Recent Progress in Ammonia Fuel Cells and Their Potential Applications. J. Mater. Chem. A 2021, 9, 727–752. [Google Scholar] [CrossRef]
- Gunduz, S.; Deka, D.J.; Ozkan, U.S. A Review of the Current Trends in High-Temperature Electrocatalytic Ammonia Production Using Solid Electrolytes. J. Catal. 2020, 387, 207–216. [Google Scholar] [CrossRef]
- Liu, F.; Ding, D.; Duan, C. Protonic Ceramic Electrochemical Cells for Synthesizing Sustainable Chemicals and Fuels. Adv. Sci. 2023, 10, e2206478. [Google Scholar] [CrossRef]
- Giddey, S.; Badwal, S.P.S.; Kulkarni, A. Review of Electrochemical Ammonia Production Technologies and Materials. Int. J. Hydrogen Energy 2013, 38, 14576–14594. [Google Scholar] [CrossRef]
- Garagounis, I.; Kyriakou, V.; Skodra, A.; Vasileiou, E.; Stoukides, M. Electrochemical Synthesis of Ammonia in Solid Electrolyte Cells. Front. Energy Res. 2014, 2, 1. [Google Scholar] [CrossRef]
- Wang, B.; Li, T.; Gong, F.; Othman, M.H.D.; Xiao, R. Ammonia as a Green Energy Carrier: Electrochemical Synthesis and Direct Ammonia Fuel Cell—A Comprehensive Review. Fuel Process. Technol. 2022, 235, 107380. [Google Scholar] [CrossRef]
- Juangsa, F.B.; Irhamna, A.R.; Aziz, M. Production of Ammonia as Potential Hydrogen Carrier: Review on Thermochemical and Electrochemical Processes. Int. J. Hydrogen Energy 2021, 46, 14455–14477. [Google Scholar] [CrossRef]
- Medvedev, D. Trends in Research and Development of Protonic Ceramic Electrolysis Cells. Int. J. Hydrogen Energy 2019, 44, 26711–26740. [Google Scholar] [CrossRef]
- Shen, H.; Choi, C.; Masa, J.; Li, X.; Qiu, J.; Jung, Y.; Sun, Z. Electrochemical Ammonia Synthesis: Mechanistic Understanding and Catalyst Design. Chem 2021, 7, 1708–1754. [Google Scholar] [CrossRef]
- Trivinho-Strixino, F.; Santos, J.S.; Souza Sikora, M. 3—Electrochemical Synthesis of Nanostructured Materials. In Nanostructures; Da Róz, A.L., Ferreira, M., de Lima Leite, F., Oliveira, O.N.B.T.-N., Eds.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 53–103. ISBN 978-0-323-49782-4. [Google Scholar]
- Droushiotis, N.; Grande, F.D.; Dzarfan Othman, M.H.; Kanawka, K.; Doraswami, U.; Metcalfe, I.S.; Li, K.; Kelsall, G. Comparison between Anode-supported and Electrolyte-supported Ni-CGO-LSCF Micro-tubular Solid Oxide Fuel Cells. Fuel Cells 2014, 14, 200–211. [Google Scholar] [CrossRef]
- Iwahara, H. Oxide-Ionic and Protonic Conductors Based on Perovskite-Type Oxides and Their Possible Applications. Solid State Ionics 1992, 52, 99–104. [Google Scholar] [CrossRef]
- Choi, S.; Kucharczyk, C.J.; Liang, Y.; Zhang, X.; Takeuchi, I.; Ji, H.I.; Haile, S.M. Exceptional Power Density and Stability at Intermediate Temperatures in Protonic Ceramic Fuel Cells. Nat. Energy 2018, 3, 202–210. [Google Scholar] [CrossRef]
- Liu, M.; Yang, L.; Wang, S.; Blinn, K.; Liu, M.; Liu, Z.; Cheng, Z. Enhanced Sulfur and Coking Tolerance of a Mixed Ion Conductor for SOFCs: BaZr0.1Ce0.7y0.2−XYbXO3−δ. Science 2009, 326, 126–129. [Google Scholar] [CrossRef]
- Zhu, H.; Kee, R.J. Membrane Polarization in Mixed-Conducting Ceramic Fuel Cells and Electrolyzers. Int. J. Hydrogen Energy 2016, 41, 2931–2943. [Google Scholar] [CrossRef]
- Yun, D.S.; Joo, J.H.; Yu, J.H.; Yoon, H.C.; Kim, J.N.; Yoo, C.Y. Electrochemical Ammonia Synthesis from Steam and Nitrogen Using Proton Conducting Yttrium Doped Barium Zirconate Electrolyte with Silver, Platinum, and Lanthanum Strontium Cobalt Ferrite Electrocatalyst. J. Power Sources 2015, 284, 245–251. [Google Scholar] [CrossRef]
- Sharma, R.K.; Patel, H.; Mushtaq, U.; Kyriakou, V.; Zafeiropoulos, G.; Peeters, F.; Welzel, S.; Van De Sanden, M.C.M.; Tsampas, M.N. Plasma Activated Electrochemical Ammonia Synthesis from Nitrogen and Water. ACS Energy Lett. 2021, 6, 313–319. [Google Scholar] [CrossRef]
- Li, C.I.; Matsuo, H.; Otomo, J. Effective Electrode Design and the Reaction Mechanism for Electrochemical Promotion of Ammonia Synthesis Using Fe-Based Electrode Catalysts. Sustain. Energy Fuels 2021, 5, 188–198. [Google Scholar] [CrossRef]
- Liu, D.; Chen, M.; Du, X.; Ai, H.; Lo, K.H.; Wang, S.; Chen, S.; Xing, G.; Wang, X.; Pan, H. Development of Electrocatalysts for Efficient Nitrogen Reduction Reaction under Ambient Condition. Adv. Funct. Mater. 2021, 31, 2008983. [Google Scholar] [CrossRef]
- Huang, Y.; Babu, D.D.; Peng, Z.; Wang, Y. Atomic Modulation, Structural Design, and Systematic Optimization for Efficient Electrochemical Nitrogen Reduction. Adv. Sci. 2020, 7, 1902390. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, K.C.; Holland, P.L. Recent Developments in the Homogeneous Reduction of Dinitrogen by Molybdenum and Iron. Nat. Chem. 2013, 5, 559–565. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Jia, R.; Yu, Y.; Zhang, B. Unveiling the Activity Origin of a Copper-Based Electrocatalyst for Selective Nitrate Reduction to Ammonia. Angew. Chemie Int. Ed. 2020, 59, 5350–5354. [Google Scholar] [CrossRef]
- Han, J.; Liu, Z.; Ma, Y.; Cui, G.; Xie, F.; Wang, F.; Wu, Y.; Gao, S.; Xu, Y.; Sun, X. Ambient N2 Fixation to NH3 at Ambient Conditions: Using Nb2O5 Nanofiber as a High-Performance Electrocatalyst. Nano Energy 2018, 52, 264–270. [Google Scholar] [CrossRef]
- Mars, P.; van Krevelen, D.W. Oxidations Carried out by Means of Vanadium Oxide Catalysts. Chem. Eng. Sci. 1954, 3, 41–59. [Google Scholar] [CrossRef]
- Abghoui, Y.; Garden, A.L.; Howalt, J.G.; Vegge, T.; Skúlason, E. Electroreduction of N2 to Ammonia at Ambient Conditions on Mononitrides of Zr, Nb, Cr, and V: A DFT Guide for Experiments. ACS Catal. 2016, 6, 635–646. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, W.; Kane, N.; Luo, Z.; Pei, K.; Sasaki, K.; Choi, Y.; Chen, Y.; Ding, D.; Liu, M. An Efficient Bifunctional Air Electrode for Reversible Protonic Ceramic Electrochemical Cells. Adv. Funct. Mater. 2021, 31, 2105386. [Google Scholar] [CrossRef]
- Ding, D.; Zhang, Y.; Wu, W.; Chen, D.; Liu, M.; He, T. A Novel Low-Thermal-Budget Approach for the Co-Production of Ethylene and Hydrogen via the Electrochemical Non-Oxidative Deprotonation of Ethane. Energy Environ. Sci. 2018, 11, 1710–1716. [Google Scholar] [CrossRef]
- Duan, C.; Kee, R.; Zhu, H.; Sullivan, N.; Zhu, L.; Bian, L.; Jennings, D.; O’Hayre, R. Highly Efficient Reversible Protonic Ceramic Electrochemical Cells for Power Generation and Fuel Production. Nat. Energy 2019, 4, 230–240. [Google Scholar] [CrossRef]
- Han, D.; Liu, X.; Bjørheim, T.S.; Uda, T. Yttrium-Doped Barium Zirconate-Cerate Solid Solution as Proton Conducting Electrolyte: Why Higher Cerium Concentration Leads to Better Performance for Fuel Cells and Electrolysis Cells. Adv. Energy Mater. 2021, 11, 2003149. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.L.; Wu, C.; Gao, J.; Xu, H.; Li, Y.; Guo, X.; Li, H.; Zhou, W. A Multilayer Ceramic Electrolyte for All-Solid-State Li Batteries. Angew. Chemie Int. Ed. 2021, 60, 3781–3790. [Google Scholar] [CrossRef] [PubMed]
- Brett, D.J.L.; Aguiar, P.; Clague, R.; Marquis, A.J.; Schöttl, S.; Simpson, R.; Brandon, N.P. Application of Infrared Thermal Imaging to the Study of Pellet Solid Oxide Fuel Cells. J. Power Sources 2007, 166, 112–119. [Google Scholar] [CrossRef]
- Feng, W.; Wu, W.; Jin, C.; Zhou, M.; Bian, W.; Tang, W.; Gomez, J.Y.; Boardman, R.; Ding, D. Exploring the Structural Uniformity and Integrity of Protonic Ceramic Thin Film Electrolyte Using Wet Powder Spraying. J. Power Sources Adv. 2021, 11, 100067. [Google Scholar] [CrossRef]
- Skodra, A.; Stoukides, M. Electrocatalytic Synthesis of Ammonia from Steam and Nitrogen at Atmospheric Pressure. Solid State Ionics 2009, 180, 1332–1336. [Google Scholar] [CrossRef]
- Yilmaz, S.; Kavici, B.; Ramakrishnan, P.; Celen, C.; Horri, B.A. Highly Conductive Cerium- and Neodymium-Doped Barium Zirconate Perovskites for Protonic Ceramic Fuel Cells. Energies 2023, 16, 4318. [Google Scholar] [CrossRef]
- François, M.; Lescure, V.; Heintz, O.; Combemale, L.; Demoisson, F.; Caboche, G. Synthesis of Y-Doped BaZrO3 Proton Conducting Electrolyte Material by a Continuous Hydrothermal Process in Supercritical Conditions: Investigation of the Formation Mechanism and Electrochemical Performance. Ceram. Int. 2023, 49, 25344–25352. [Google Scholar] [CrossRef]
- Konwar, D.; Yoon, H.H. A Methane-Fueled SOFC Based on a Thin BaZr0.1Ce0.7Y0.1Yb0.1O3−δ Electrolyte Film and a LaNi0.6Co0.4O3 Anode Functional Layer. J. Mater. Chem. A 2016, 4, 5102–5106. [Google Scholar] [CrossRef]
- Fan, Z.; Cao, D.; Zhou, M.; Zhu, Z.; Chen, M.; Liu, J. Barium Cerate-Zirconate Electrolyte Powder Prepared by Carbonate Coprecipitation for High Performance Protonic Ceramic Fuel Cells. Ceram. Int. 2023, 49, 8524–8532. [Google Scholar] [CrossRef]
- Wang, S.; Shen, J.; Zhu, Z.; Wang, Z.; Cao, Y.; Guan, X.; Wang, Y.; Wei, Z.; Chen, M. Further Optimization of Barium Cerate Properties via Co-Doping Strategy for Potential Application as Proton-Conducting Solid Oxide Fuel Cell Electrolyte. J. Power Sources 2018, 387, 24–32. [Google Scholar] [CrossRef]
- Malešević, A.; Radojković, A.; Žunić, M.; Dapčević, A.; Perać, S.; Branković, Z.; Branković, G. Evaluation of Stability and Functionality of BaCe1−xInxO3−δ Electrolyte in a Wider Range of Indium Concentration. J. Adv. Ceram. 2022, 11, 443–453. [Google Scholar] [CrossRef]
- Kane, N.; Luo, Z.; Zhou, Y.; Ding, Y.; Weidenbach, A.; Zhang, W.; Liu, M. Durable and High-Performance Thin-Film BHYb-Coated BZCYYb Bilayer Electrolytes for Proton-Conducting Reversible Solid Oxide Cells. ACS Appl. Mater. Interfaces 2023, 15, 32395–32403. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Chang, W.; Jeong, H.J.; Kim, K.H.; Park, H.S.; Shim, J.H. High Performance of Protonic Ceramic Fuel Cells with 1-Μm-Thick Electrolytes Fabricated by Inkjet Printing. Addit. Manuf. 2023, 71, 103590. [Google Scholar] [CrossRef]
- Bae, K.; Lee, S.; Jang, D.Y.; Kim, H.J.; Lee, H.; Shin, D.; Son, J.W.; Shim, J.H. High-Performance Protonic Ceramic Fuel Cells with Thin-Film Yttrium-Doped Barium Cerate-Zirconate Electrolytes on Compositionally Gradient Anodes. ACS Appl. Mater. Interfaces 2016, 8, 9097–9103. [Google Scholar] [CrossRef]
- Kim, D.; Bae, K.T.; Kim, K.J.; Im, H.N.; Jang, S.; Oh, S.; Lee, S.W.; Shin, T.H.; Lee, K.T. High-Performance Protonic Ceramic Electrochemical Cells. ACS Energy Lett. 2022, 7, 2393–2400. [Google Scholar] [CrossRef]
- Lee, K.R.; Tseng, C.J.; Jang, S.C.; Lin, J.C.; Wang, K.W.; Chang, J.K.; Chen, T.C.; Lee, S.W. Fabrication of Anode-Supported Thin BCZY Electrolyte Protonic Fuel Cells Using NiO Sintering Aid. Int. J. Hydrogen Energy 2019, 44, 23784–23792. [Google Scholar] [CrossRef]
- Choi, S.M.; An, H.; Yoon, K.J.; Kim, B.K.; Lee, H.W.; Son, J.W.; Kim, H.; Shin, D.; Ji, H.I.; Lee, J.H. Electrochemical Analysis of High-Performance Protonic Ceramic Fuel Cells Based on a Columnar-Structured Thin Electrolyte. Appl. Energy 2019, 233–234, 29–36. [Google Scholar] [CrossRef]
- Zhong, Z.; Li, Z.; Li, J.; Guo, X.; Hu, Q.; Feng, Y.; Sun, H. A Facile Method to Synthesize BaZr0.1Ce0.7Y0.1Yb0.1O3−δ (BZCYYb) Nanopowders for the Application on Highly Conductive Proton-Conducting Electrolytes. Int. J. Hydrogen Energy 2022, 47, 40054–40066. [Google Scholar] [CrossRef]
- Wang, M.; Wu, W.; Lin, Y.; Tang, W.; Gao, G.; Li, H.; Stewart, F.F.; Wang, L.; Yang, Y.; Ding, D. Improved Solid-State Reaction Method for Scaled-Up Synthesis of Ceramic Proton-Conducting Electrolyte Materials. ACS Appl. Energy Mater. 2023, 6, 8316–8326. [Google Scholar] [CrossRef]
- Sari, S.N.; Nieroda, P.; Pasierb, P. The BaCeO3-Based Composite Protonic Conductors Prepared by Spark Plasma Sintering (SPS) and Free-Sintering Methods. Int. J. Hydrogen Energy 2023, 48, 29748–29758. [Google Scholar] [CrossRef]
- Timurkutluk, C.; Timurkutluk, B.; Kaplan, Y. Experimental Optimization of the Fabrication Parameters for Anode-Supported Micro-Tubular Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2020, 45, 23294–23309. [Google Scholar] [CrossRef]
- Ren, C.; Xu, P.; Zhang, Y.; Liu, T. Understanding the Polymer Binder Effect on the Microstructure and Performance of Micro-Tubular Solid Oxide Fuel Cells with Continuously Graded Pores Fabricated by the Phase Inversion Method. Appl. Surf. Sci. 2023, 612, 155928. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, S.; Li, C.; Rainwater, B.; Liu, Y.; Zhang, L.; Zhang, Y.; Li, C.; Liu, M. Atmospheric Plasma-Sprayed BaZr0.1Ce0.7Y0.1Yb0.1O3−δ (BZCYYb) Electrolyte Membranes for Intermediate-Temperature Solid Oxide Fuel Cells. Ceram. Int. 2016, 42, 19231–19236. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, M.; Bao, D.; Wang, Z.; Jin, F.; Wang, Y.; He, T. Performance of Fuel Electrode-Supported Tubular Protonic Ceramic Cells Prepared through Slip Casting and Dip-Coating Methods. Catalysts 2023, 13, 182. [Google Scholar] [CrossRef]
- Hou, M.; Zhu, F.; Liu, Y.; Chen, Y. A High-Performance Fuel Electrode-Supported Tubular Protonic Ceramic Electrochemical Cell. J. Eur. Ceram. Soc. 2023, 43, 6200–6207. [Google Scholar] [CrossRef]
- Oneill, B.J.; Jackson, D.H.K.; Lee, J.; Canlas, C.; Stair, P.C.; Marshall, C.L.; Elam, J.W.; Kuech, T.F.; Dumesic, J.A.; Huber, G.W. Catalyst Design with Atomic Layer Deposition. ACS Catal. 2015, 5, 1804–1825. [Google Scholar] [CrossRef]
- Kosaka, F.; Nakamura, T.; Otomo, J. Electrochemical Ammonia Synthesis Using Mixed Protonic-Electronic Conducting Cathodes with Exsolved Ru-Nanoparticles in Proton Conducting Electrolysis Cells. J. Electrochem. Soc. 2017, 164, F1323–F1330. [Google Scholar] [CrossRef]
- Kim, J.; Jun, A.; Gwon, O.; Yoo, S.; Liu, M.; Shin, J.; Lim, T.H.; Kim, G. Hybrid-Solid Oxide Electrolysis Cell: A New Strategy for Efficient Hydrogen Production. Nano Energy 2018, 44, 121–126. [Google Scholar] [CrossRef]
- Li, W.; Guan, B.; Ma, L.; Hu, S.; Zhang, N.; Liu, X. High Performing Triple-Conductive Pr2NiO4+δ Anode for Proton-Conducting Steam Solid Oxide Electrolysis Cell. J. Mater. Chem. A 2018, 6, 18057–18066. [Google Scholar] [CrossRef]
- Ding, H.; Wu, W.; Jiang, C.; Ding, Y.; Bian, W.; Hu, B.; Singh, P.; Orme, C.J.; Wang, L.; Zhang, Y.; et al. Self-Sustainable Protonic Ceramic Electrochemical Cells Using a Triple Conducting Electrode for Hydrogen and Power Production. Nat. Commun. 2020, 11, 1907. [Google Scholar] [CrossRef]
- Pei, K.; Zhou, Y.; Xu, K.; Zhang, H.; Ding, Y.; Zhao, B.; Yuan, W.; Sasaki, K.; Choi, Y.M.; Chen, Y.; et al. Surface Restructuring of a Perovskite-Type Air Electrode for Reversible Protonic Ceramic Electrochemical Cells. Nat. Commun. 2022, 13, 2207. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhou, Y.; Hu, T.; Xu, Y.; Hou, M.; Zhu, F.; Liu, D.; Zhang, H.; Xu, K.; Liu, M.; et al. An Efficient High-Entropy Perovskite-Type Air Electrode for Reversible Oxygen Reduction and Water Splitting in Protonic Ceramic Cells. Adv. Mater. 2023, 35, e2209469. [Google Scholar] [CrossRef]
- Choi, S.; Davenport, T.C.; Haile, S.M. Protonic Ceramic Electrochemical Cells for Hydrogen Production and Electricity Generation: Exceptional Reversibility, Stability, and Demonstrated Faradaic Efficiency. Energy Environ. Sci. 2019, 12, 206–215. [Google Scholar] [CrossRef]
- Saqib, M.; Choi, I.G.; Bae, H.; Park, K.; Shin, J.S.; Kim, Y.D.; Lee, J.I.; Jo, M.; Kim, Y.C.; Lee, K.S.; et al. Transition from Perovskite to Misfit-Layered Structure Materials: A Highly Oxygen Deficient and Stable Oxygen Electrode Catalyst. Energy Environ. Sci. 2021, 14, 2472–2484. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, C.; Zhang, X.; Zhu, X.; Wei, P.; Ji, L.; Guo, Y.; Gao, S.; Luo, Y.; Wang, Z.; et al. An Ultrasmall Ru2P Nanoparticles-Reduced Graphene Oxide Hybrid: An Efficient Electrocatalyst for NH3 Synthesis under Ambient Conditions. J. Mater. Chem. A 2020, 8, 77–81. [Google Scholar] [CrossRef]
- Skúlason, E.; Bligaard, T.; Gudmundsdóttir, S.; Studt, F.; Rossmeisl, J.; Abild-Pedersen, F.; Vegge, T.; Jónsson, H.; Nørskov, J.K. A Theoretical Evaluation of Possible Transition Metal Electro-Catalysts for N2 Reduction. Phys. Chem. Chem. Phys. 2012, 14, 1235–1245. [Google Scholar] [CrossRef]
- Tao, H.; Choi, C.; Ding, L.X.; Jiang, Z.; Han, Z.; Jia, M.; Fan, Q.; Gao, Y.; Wang, H.; Robertson, A.W.; et al. Nitrogen Fixation by Ru Single-Atom Electrocatalytic Reduction. Chem 2019, 5, 204–214. [Google Scholar] [CrossRef]
- Back, S.; Jung, Y. On the Mechanism of Electrochemical Ammonia Synthesis on the Ru Catalyst. Phys. Chem. Chem. Phys. 2016, 18, 9161–9166. [Google Scholar] [CrossRef]
- Montoya, J.H.; Tsai, C.; Vojvodic, A.; Nørskov, J.K. The Challenge of Electrochemical Ammonia Synthesis: A New Perspective on the Role of Nitrogen Scaling Relations. ChemSusChem 2015, 8, 2180–2186. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.J.; Wei, L.; Zhao, X.S.; Wei, Y.S.; Li, J.W.; Sheng, T.; Zhu, F.C.; Tian, N.; Zhou, Z.Y.; Sun, S.G. Excavated Cubic Platinum-Iridium Alloy Nanocrystals with High-Index Facets as Highly Efficient Electrocatalysts in N2 Fixation to NH3. Chem. Commun. 2019, 55, 9335–9338. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Sun, W.; Liu, Q.; Liu, X.; Chen, J.; Lv, X.; Li, W.; Liu, Y.p.; Shen, Z. Efficient Electrochemical Nitrogen Fixation over Isolated Pt Sites. Small 2020, 16, e2000015. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.R.; Rohr, B.A.; Schwalbe, J.A.; Cargnello, M.; Chan, K.; Jaramillo, T.F.; Chorkendorff, I.; Nørskov, J.K. Electrochemical Ammonia Synthesis—The Selectivity Challenge. ACS Catal. 2017, 7, 706–709. [Google Scholar] [CrossRef]
- Xia, J.; Huang, K.; Yao, Z.; Zhang, B.; Li, S.; Chen, Z.; Wu, F.; Wu, J.; Huang, Y. Ternary Duplex FeCoNi Alloy Prepared by Cathode Plasma Electrolytic Deposition as a High-Efficient Electrocatalyst for Oxygen Evolution Reaction. J. Alloy. Compd. 2022, 891, 161934. [Google Scholar] [CrossRef]
- Li, S.Y.; Nguyen, T.X.; Su, Y.H.; Lin, C.C.; Huang, Y.J.; Shen, Y.H.; Liu, C.P.; Ruan, J.J.; Chang, K.S.; Ting, J.M. Sputter-Deposited High Entropy Alloy Thin Film Electrocatalyst for Enhanced Oxygen Evolution Reaction Performance. Small 2022, 18, e2106127. [Google Scholar] [CrossRef]
- Vøllestad, E.; Strandbakke, R.; Tarach, M.; Catalán-Martínez, D.; Fontaine, M.L.; Beeaff, D.; Clark, D.R.; Serra, J.M.; Norby, T. Mixed Proton and Electron Conducting Double Perovskite Anodes for Stable and Efficient Tubular Proton Ceramic Electrolysers. Nat. Mater. 2019, 18, 752–759. [Google Scholar] [CrossRef]
- Hu, T.; Zhu, F.; Xia, J.; He, F.; Du, Z.; Zhou, Y.; Liu, Y.; Wang, H.; Chen, Y. In Situ Engineering of a Cobalt-Free Perovskite Air Electrode Enabling Efficient Reversible Oxygen Reduction/Evolution Reactions. Adv. Funct. Mater. 2023, 33, 2305567. [Google Scholar] [CrossRef]
- Wan, Y.; Xing, Y.; Li, Y.; Huan, D.; Xia, C. Thermal Cycling Durability Improved by Doping Fluorine to PrBaCo2O5+Δ as Oxygen Reduction Reaction Electrocatalyst in Intermediate-Temperature Solid Oxide Fuel Cells. J. Power Sources 2018, 402, 363–372. [Google Scholar] [CrossRef]


| Electrolyte | Method | Conductivity (S cm−1) | Thickness (mm) | Reference |
|---|---|---|---|---|
| SrCe0.95Yb0.05O3−δ | sol–gel | Unknown | 1.5 | [41] |
| BaZr0.8−x−yCexNdyY0.1Yb0.1O3−δ | Pechini method | 500 °C: 3.77 × 10−4 | 0.8–1.5 | [42] |
| BaZr0.85Y0.15O3−δ | hydrothermal process | 600 °C: 2.5 × 10−3 | 1.6 | [43] |
| Electrolyte | Method | Conductivity (S cm−1) | Thickness (µm) | Reference |
|---|---|---|---|---|
| BaCe0.7Zr0.1Y0.2 | co-precipitation solid-state reaction dip-coating | 650 °C: 2.8 × 10−2 | ~20 | [45] |
| BaCe0.8Y0.2−xNdxO3−δ | citrate–nitrate combustion | 350 °C: 8.5 × 10−3 | ~20 | [46] |
| BaCe1−xInxO3−δ | auto-combustion reaction | 700 °C: 5 × 10−3 | 20–25 | [47] |
| BaZr0.1Ce0.7Y0.1Yb0.1 | solid-state reaction | 500 °C: 1.2 × 10−2 | 10 | [48] |
| BaHf0.8Yb0.2O3−δ | pulsed laser deposition (PLD) | 500 °C: 2.5 × 10−3 | 110 | [48] |
| BaZr0.1Ce0.7Y0.1Yb0.1 | solid-state reaction | 500 °C: 1.3 × 10−2 | ~10 | [22] |
| BaZr0.4Ce0.4Y0.1Yb0.1 | solid-state reaction | 500 °C: 5.6 × 10−3 | ~15 | [21] |
| BaZr0.2Ce0.6Y0.1Yb0.1O3−δ | Pechini method inkjet printing | 600 °C: 24.39 | 1 | [49] |
| BaCe0.5Zr0.35Y0.15O3−δ | citric nitrate method PLD | Unknown | 2–4 | [50] |
| BaZr1−x−yCexYyO3 | ultrafast microwave-assisted sintering tape casting | Unknown | ~12 | [51] |
| BaZr0.2Ce0.6Y0.2O3 | solid-state reaction spin coating | 800 °C: 1 × 10−2 | ~7 | [52] |
| BaCe0.55Zr0.3Y0.15O3−δ | screen printing | Unknown | ~2.5 | [53] |
| Electrolyte | Method | Conductivity | Reference |
|---|---|---|---|
| BaZr0.4Ce0.4Y0.15Zn0.05O3 | solid-state reaction | Unknown | [60] |
| BaZr0.1Ce0.7Y0.1Yb0.1 | solid-state reaction | Unknown | [61] |
| Cathode | Deposition Method | Thickness (µm) | Reference |
|---|---|---|---|
| La0.6Sr0.4Co0.2Fe0.8O3−δ | Unknown | 44 | [24] |
| Ag | - | 4 | |
| Pt | - | 8 | |
| Fe | doctor blade | 15–25 | [26] |
| 10-Fe-BCY | doctor blade | 15–25 | |
| 0.5W-10Fe-BCY | doctor blade | 15–25 | |
| PrBa0.5Sr0.5Co1.5Fe0.5O5+δ | - | 10–20 | [36] |
| Ru–Ag/MgO | Unknown | - | [41] |
| Ni-BCYR | - | - | [63] |
| NdBa0.5Sr0.5Co1.5Fe0.5O5+δ (NBSCF)-BZCYYb | drop coating | 15 | [64] |
| Pr2NiO4-BZCY | screen printing | 13 | [65] |
| PrCo0.05Ni0.5O3−δ | tape casting | 29 | [66] |
| Ba0.9Co0.7Fe0.2Nb0.1O3−δ | screen printing | 15 | [67] |
| Pr0.2Ba0.2Sr0.2La0.2Ca0.2CoO3−δ | spray coating | 20 | [68] |
| PrBa0.5Sr0.5Co1.5Fe0.5O5+δ | PLD | 20 | [69] |
| Gd0.3Ca2.7Co3.82Cu0.18O9−δ | screen printing | 30 | [70] |
| Cathode * | Electrolyte | NH3 Production Rate [mol cm−2 s−1] × 10−9 | Thickness (µm) | Reference |
|---|---|---|---|---|
| La0.6Sr0.4Co0.2Fe0.8O3−δ | BaZr0.8Y0.2O3−δ | 0.0850 | 44 | [24] |
| Ag | BaZr0.8Y0.2O3−δ | 0.0490 | 4 | |
| Pt | BaZr0.8Y0.2O3−δ | <0.0010 | 8 | |
| Fe | BaCe0.9Y0.1O3−δ | 14.000 | 15–25 | [26] |
| 10-Fe-BCY | BaCe0.9Y0.1O3−δ | 0.4200 | 15–25 | |
| 0.5W-10Fe-BCY | BaCe0.9Y0.1O3−δ | 0.5700 | 15–25 | |
| Ru–Ag/MgO | SrCe0.95Yb0.05O3−δ | 0.0003 | - | [41] |
| Ni-BCYR | BaCe0.9Y0.1O3−δ | 0.0110 | - | [63] |
| Anode | Electrolyte | Current Density @1.3 V and 550 °C [A cm−2] | Thickness (µm) | Reference |
|---|---|---|---|---|
| Pr0.2Ba0.2Sr0.2La0.2Ca0.2CoO3−δ | BaZr0.1Ce0.7Y0.1Yb0.1O3−δ | −0.800 | 20 | [68] |
| PrBa0.5Sr0.5Co1.5Fe0.5O5+δ | BaZr0.4Ce0.4Y0.1Yb0.1O3−δ | −1.059 | 20 | [69] |
| Gd0.3Ca2.7Co3.82Cu0.18O9−δ | BaZr0.1Ce0.7Y0.1Yb0.1O3−δ | −1.241 | 30 | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieri, H.M.; Kim, M.-C.; Badakhsh, A.; Choi, S.H. Electrochemical Synthesis of Ammonia via Nitrogen Reduction and Oxygen Evolution Reactions—A Comprehensive Review on Electrolyte-Supported Cells. Energies 2024, 17, 441. https://doi.org/10.3390/en17020441
Vieri HM, Kim M-C, Badakhsh A, Choi SH. Electrochemical Synthesis of Ammonia via Nitrogen Reduction and Oxygen Evolution Reactions—A Comprehensive Review on Electrolyte-Supported Cells. Energies. 2024; 17(2):441. https://doi.org/10.3390/en17020441
Chicago/Turabian StyleVieri, Hizkia Manuel, Moo-Chang Kim, Arash Badakhsh, and Sun Hee Choi. 2024. "Electrochemical Synthesis of Ammonia via Nitrogen Reduction and Oxygen Evolution Reactions—A Comprehensive Review on Electrolyte-Supported Cells" Energies 17, no. 2: 441. https://doi.org/10.3390/en17020441
APA StyleVieri, H. M., Kim, M.-C., Badakhsh, A., & Choi, S. H. (2024). Electrochemical Synthesis of Ammonia via Nitrogen Reduction and Oxygen Evolution Reactions—A Comprehensive Review on Electrolyte-Supported Cells. Energies, 17(2), 441. https://doi.org/10.3390/en17020441








