Pressurized Chemical Looping for Direct Reduced Iron Production: Economics of Carbon Neutral Process Configurations
Abstract
1. Introduction
1.1. Background
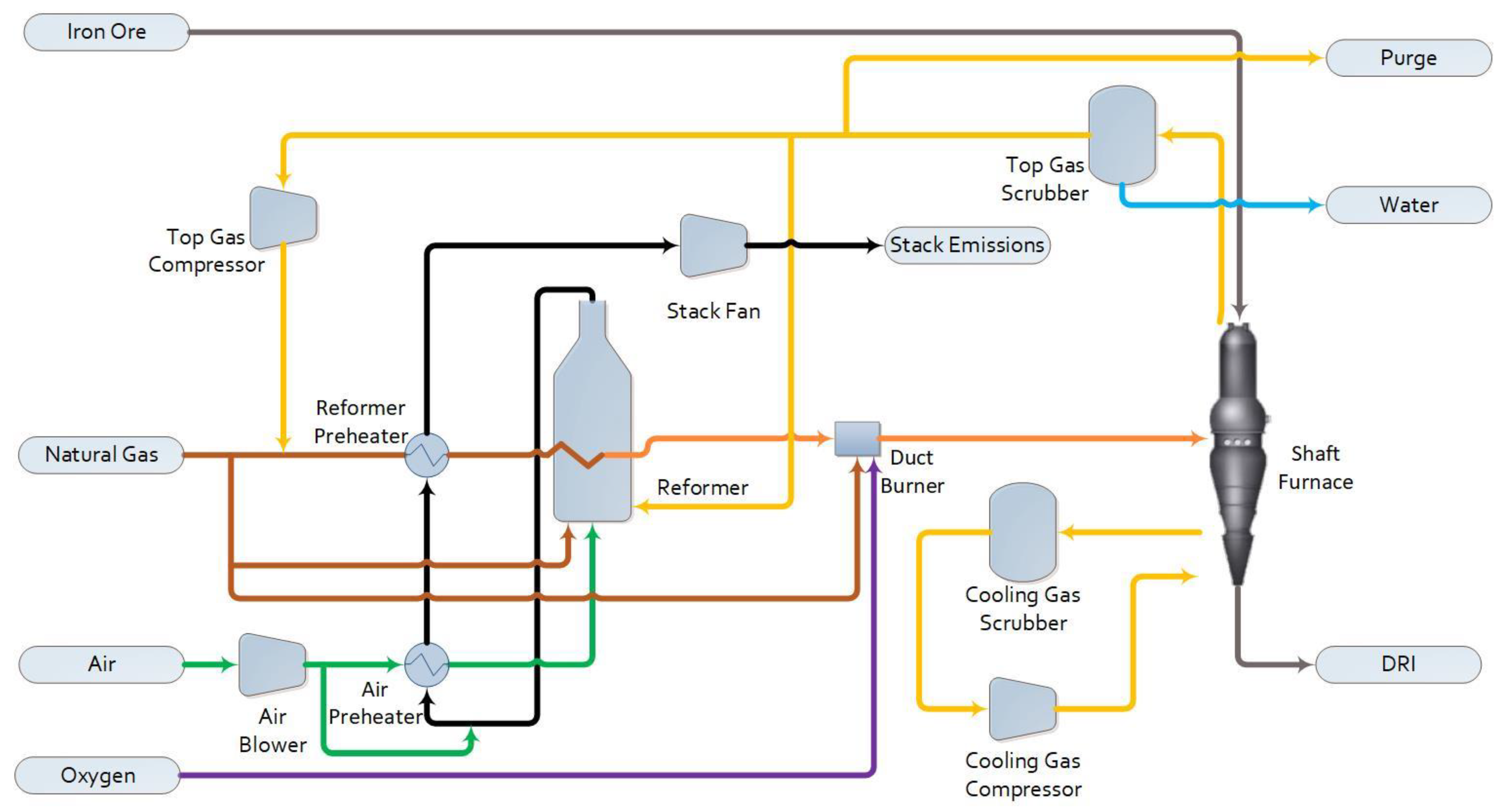
1.2. Process Description
1.2.1. Base Case
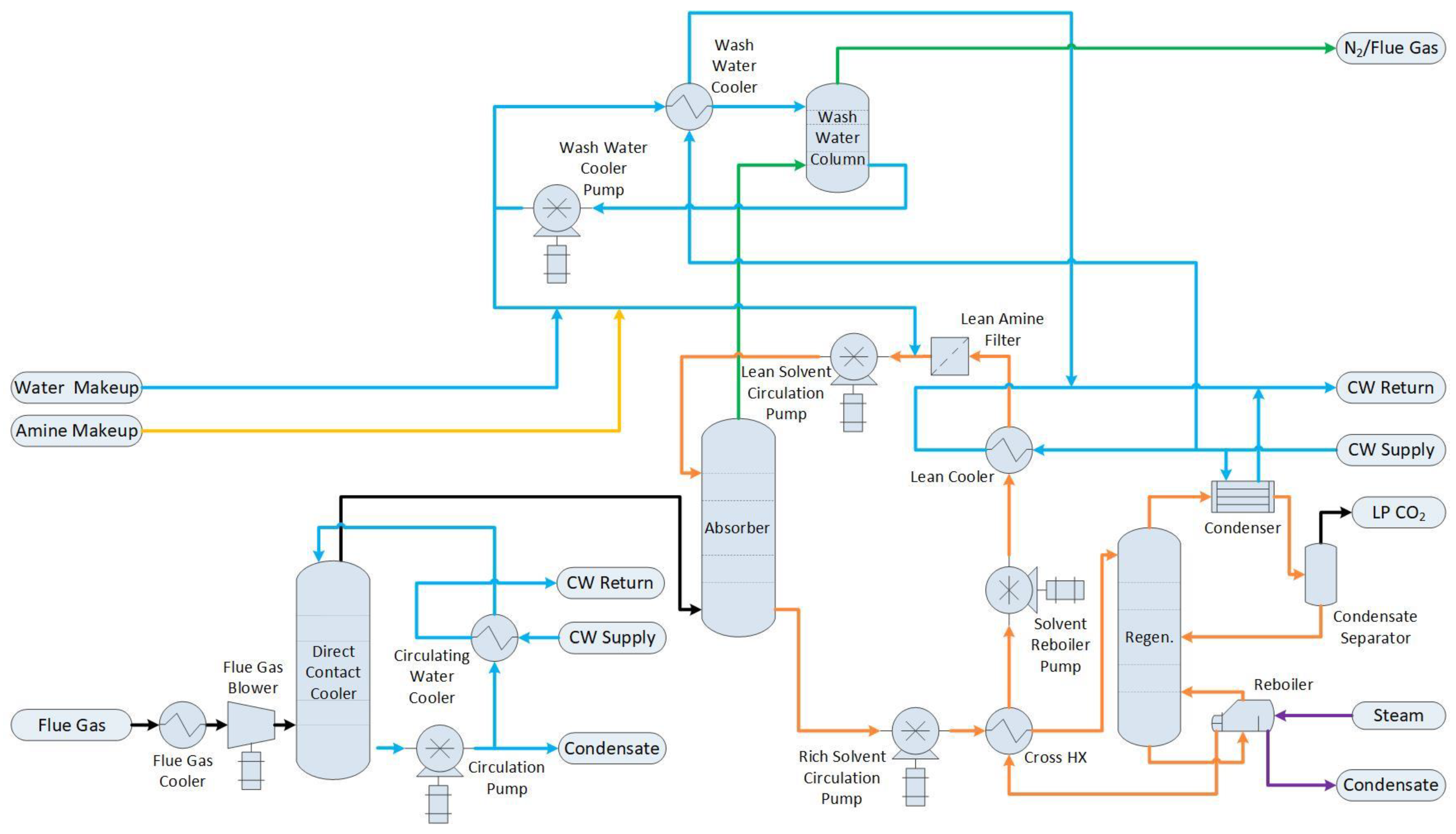
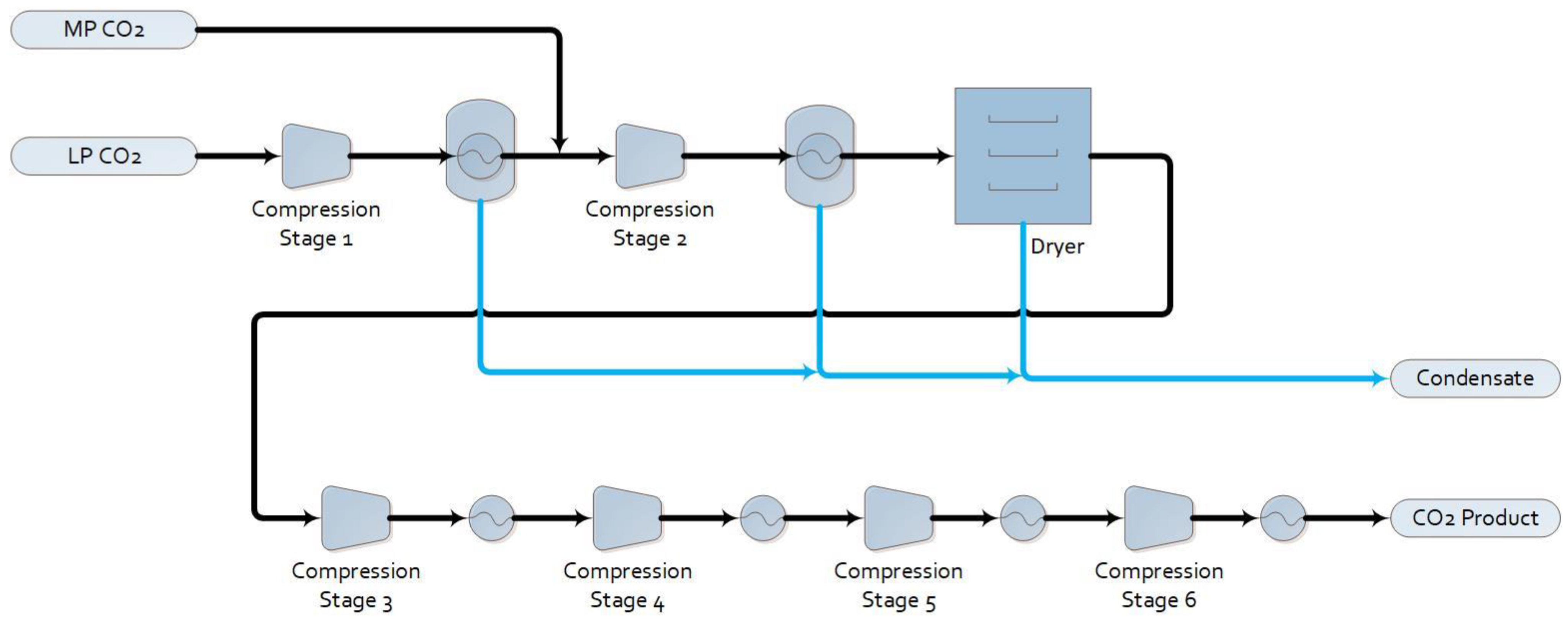
1.2.2. Base Case with Post-Combustion Capture (Base Case + PCC)
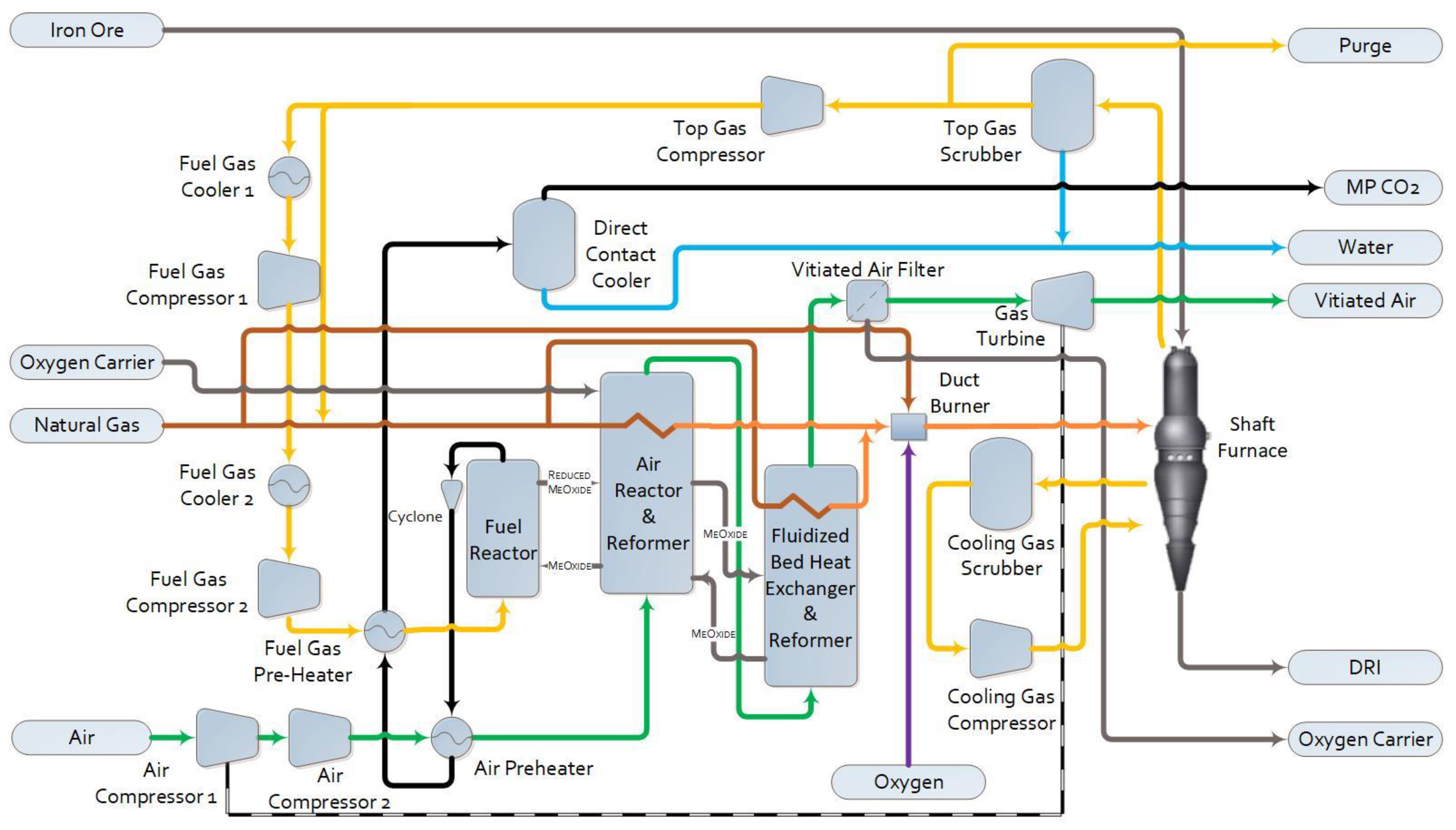
1.2.3. Pressurized Chemical Looping DRI (PCL-DRI)
2. Methods
2.1. Process Simulation
2.2. Capital Cost
2.3. Operating Costs
3. Results
3.1. Capital Cost
3.2. Operating Costs
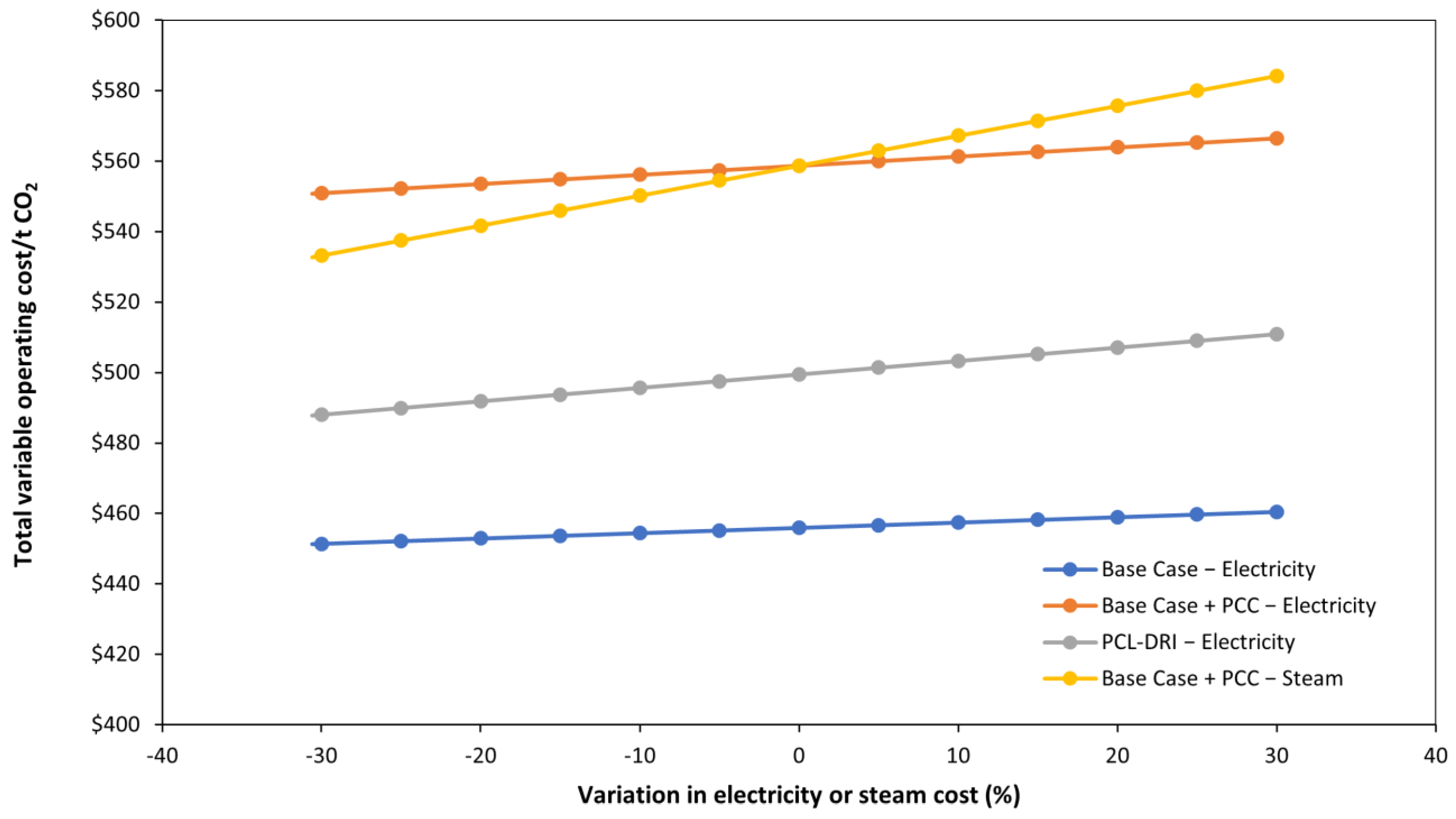
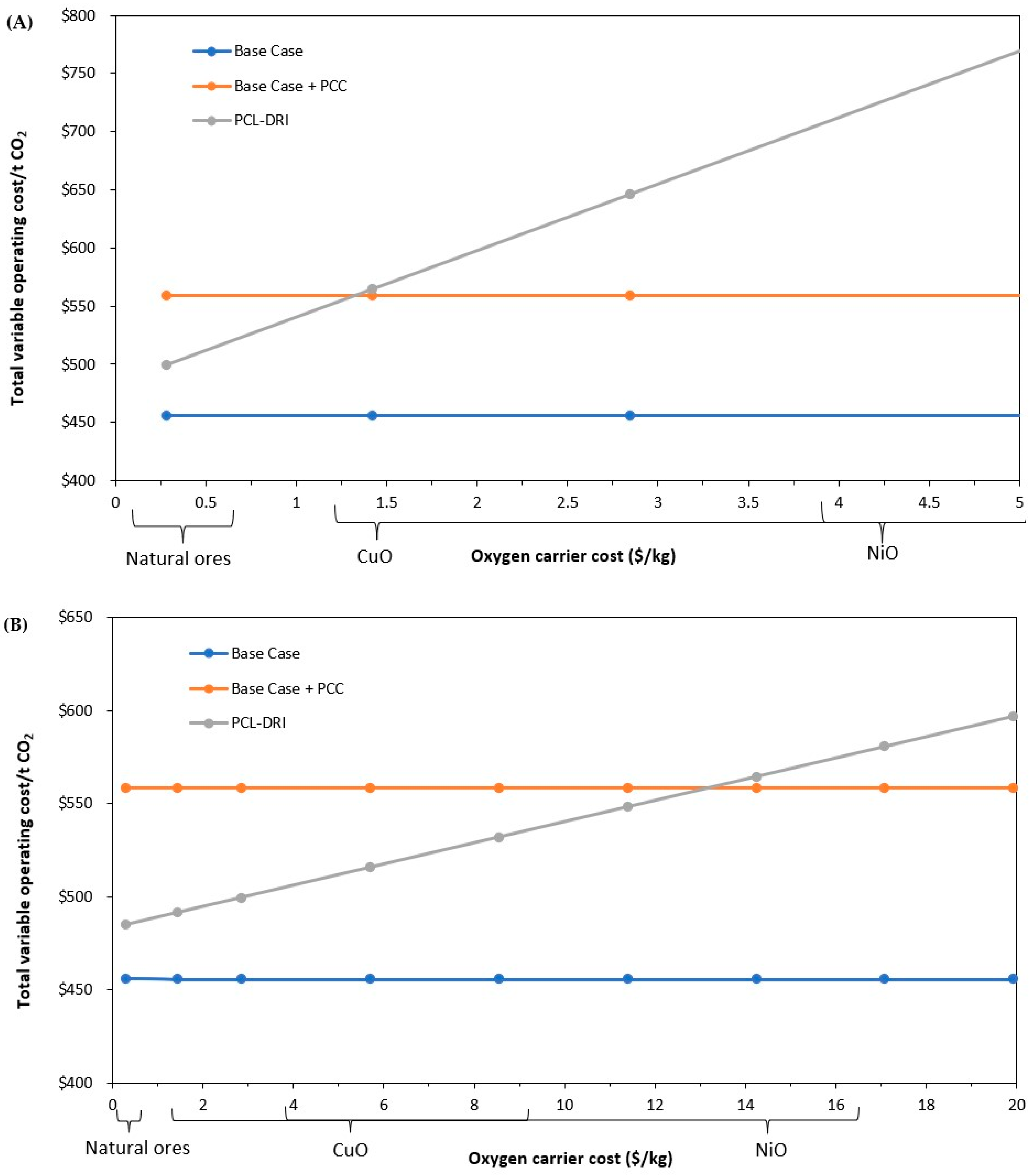
3.3. Parametric Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Energy Agency. Iron and Steel Technology Roadmap—Towards More Sustainable Steelmaking; International Energy Agency: Paris, France, 2020; Available online: https://www.iea.org/reports/iron-and-steel-technology-roadmap (accessed on 15 November 2023).
- Golder Associates Ltd.; Thorn Associates. Canadian Steel Industry Energy & Greenhouse Gas Emissions Intensity, Technology and Carbon Reduction Roadmap; Golder Associates Ltd.: Toronto, ON, Canada, 2021. [Google Scholar]
- Canadian Steel Producers Association. Canada’s Steel Industry: A Sustainable Choice. March 2020. Available online: canadiansteel.ca (accessed on 6 November 2023).
- Chevrier, V. MIDREX H2TM: Ultimate Low-CO2 Ironmaking and Its Place in the New Hydrogen Economy. In AISTech 2018 Proceedings; AIST: Philadelphia, PA, USA, 2018. [Google Scholar]
- Baig, S. Cost Effectiveness Analysis of HYL and Midrex DRI Technologies for the Iron and Steel-Making Industry. Master’s Thesis, Duke University, Durham, NC, USA, 2016. [Google Scholar]
- Hamadeh, H.; Mirgaux, O.; Patisson, F. Detailed Modeling of the Direct Reduction of Iron Ore in a Shaft Furnace. Materials 2018, 11, 1865. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, A.; Assadi, M.; Nikpey Somehsaraei, H. Decarbonization of the Iron and Steel Industry with Direct Reduction of Iron Ore with Green Hydrogen. Energies 2020, 13, 758. [Google Scholar] [CrossRef]
- Wang, R.R.; Zhao, Y.Q.; Babich, A.; Senk, D.; Fan, X.Y. Hydrogen direct reduction (H-DR) in steel industry—An overview of challenges and opportunities. J. Clean. Prod. 2021, 329, 129797. [Google Scholar] [CrossRef]
- Guo, D.; Li, Y.; Cui, B.; Chen, Z.; Luo, S.; Xiao, B.; Zhu, H.; Hu, M. Direct reduction of iron ore/biomass composite pellets using simulated biomass-derived syngas: Experimental analysis and kinetic modelling. Chem. Eng. J. 2017, 327, 822–830. [Google Scholar] [CrossRef]
- Energiron. ENERGIRON HYL: DRI Technology by Tenova and Danieli. Tenova HYL, 2014. Available online: https://www.tenova.com/product/iron-reduction-technologies/ (accessed on 8 May 2020).
- Zhu, L.; He, Y.; Li, L.; Wu, P. Tech-economic assessment of second-generation CCS: Chemical looping combustion. Energy 2018, 144, 915–927. [Google Scholar] [CrossRef]
- Symonds, R.T.; Hughes, R.W.; Lu, D.Y.; Navarri, P.; Ashrafi, O. Systems analysis of pressurized chemical looping combustion for SAGD applications. Int. J. Greenh. Gas Control 2018, 73, 111–123. [Google Scholar] [CrossRef]
- Daneshmand-Jahromi, S.; Sedghkerdar, M.H.; Mahinpey, N. A review of chemical looping combustion technology: Fundamentals, and development of natural, industrial waste, and synthetic oxygen carriers. Fuel 2023, 341, 127626. [Google Scholar] [CrossRef]
- Abuelgasim, S.; Wang, W.; Abdalazeez, A. A brief review for chemical looping combustion as a promising CO2 capture technology: Fundamentals and progress. Sci. Total Environ. 2021, 764, 142892. [Google Scholar] [CrossRef]
- Bond, N.; Symonds, R.; Hughes, R. Pressurized Chemical Looping for Direct Reduced Iron Production: Carbon Neutral Process Configuration and Performance. Energies 2022, 15, 5219. [Google Scholar] [CrossRef]
- Bond, N.; Symonds, R.; Hughes, R. Eliminating CO2 emissions from the Midrex direct reduced iron process: Pressurized chemical looping vs. post-combustion capture. In Proceedings of the 6th International Conference on Chemical Looping, Zaragoza, Spain, 19–22 September 2022. [Google Scholar]
- Primetals Technologies Austria GmbH. 2 MTPY MIDREX® Hot Briquette Iron Plant; Primetals Technologies Ltd.: London, UK, 2019; Available online: https://www.primetals.com/fileadmin/user_upload/content/01_portfolio/1_ironmaking/midrex/2_MTPY_MIDREX_HOT_BRIQUETTED_IRON_PLANT.pdf (accessed on 1 April 2022).
- Midrex Technologies, Inc. The MIDREX® Process; Midrex Technologies, Inc.: Charlotte, NC, USA, 2018; Available online: https://www.midrex.com/wp-content/uploads/MIdrex_Process_Brochure_4-12-18.pdf (accessed on 1 April 2022).
- Hamadeh, H. Modélisation Mathématique Détaillée du Procédé de Réduction Directe du Minerai de fer; Université de Lorraine: Metz, France, 2017; Available online: https://tel.archives-ouvertes.fr/tel-01740462v2 (accessed on 4 April 2020).
- Farhadi, F.; Motemed Hashemi, M.Y.; Bahrami Babaheidari, M. Modelling and simulation of syngas unit in large scale direct reduction plant. Ironmak. Steelmak. 2003, 30, 18–24. [Google Scholar] [CrossRef]
- Béchara, R.; Hamadeh, H.; Mirgaux, O.; Patisson, F. Optimization of the Iron Ore Direct Reduction Process through Multiscale Process Modeling. Materials 2018, 11, 1094. [Google Scholar] [CrossRef]
- Agbonghae, E.O.; Hughes, K.J.; Ingham, D.B.; Ma, L.; Pourkashanian, M. Optimal Process Design of Commercial-Scale Amine-Based CO2 Capture Plants. Ind. Eng. Chem. Res. 2014, 53, 14815–14829. [Google Scholar] [CrossRef]
- Enhance Energy Inc.; Wolf Carbon Solutions; North West Redwater Partnership. Knowledge Sharing Report. Division B: Detailed Report; Calendar Year 2020, Government of Alberta, March 2021. Available online: https://open.alberta.ca/publications/alberta-carbon-trunk-line-project-knowledge-sharing-report-2020 (accessed on 20 November 2023).
- Czakiert, T.; Krzywanski, J.; Zylka, A.; Nowak, W. Chemical Looping Combustion: A Brief Overview. Energies 2022, 15, 1563. [Google Scholar] [CrossRef]
- Moldenhauer, P.; Linderholm, C.; Rydén, M.; Lyngfelt, A. Avoiding CO2 capture effort and cost for negative CO2 emissions using industrial waste in chemical-looping combustion/gasification of biomass. Mitig. Adapt. Strateg. Glob. Chang. 2020, 25, 1–24. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, Y.; Yang, S.; Zhang, Q.; Zhao, J.; Fang, Y.; Hao, X.; Guan, G. Iron-based oxygen carriers in chemical looping conversions: A review. Carbon Resour. Convers. 2019, 2, 23–34. [Google Scholar] [CrossRef]
- Tan, Y.; Ridha, F.N.; Duchesne, M.A.; Lu, D.Y.; Hughes, R.W. Reduction Kinetics of Ilmenite Ore as an Oxygen Carrier for Pressurized Chemical Looping Combustion of Methane. Energy Fuels 2017, 31, 7598–7605. [Google Scholar] [CrossRef]
- Bartocci, P.; Abad, A.; Flores, A.C.; de las Obras Loscertales, M. Ilmenite: A promising oxygen carrier for the scale-up of chemical looping. Fuel 2023, 337, 126644. [Google Scholar] [CrossRef]
- Chitester, D.C.; Kornosky, R.M.; Fan, L.-S.; Danko, J.P. Characteristics of fluidization at high pressure. Chem. Eng. Sci. 1984, 39, 253–261. [Google Scholar] [CrossRef]
- Seider, W.D.; Lewin, D.R.; Seader, J.D.; Widagdo, S.; Gani, R.; Ng, K.M. Product and Process Design Principles: Synthesis, Analysis and Evaluation, 4th ed.; Wiley: Hoboken, NJ, USA, 2017; ISBN 978-0-470-04895-5. [Google Scholar]
- Turton, R.; Bailie, R.; Whiting, W.; Shaeiwitz, J.; Bhattacharyya, D. Analysis, Synthesis, and Design of Chemical Processes, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2012; ISBN 978-0-13-261812-0. [Google Scholar]
- Ulrich, G.D. A Guide to Chemical Engineering Process Design and Economics; John Wiley & Sons, Inc.: New York, NY, USA, 1984. [Google Scholar]
- Peters, M.S.; Timmerhaus, K.D.; West, R.E. Plant Design and Economics for Chemical Engineers, 5th ed.; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]
- Barrington, C. DR-Grade Iron Ore Pellets—A Supply Overview. Available online: https://www.midrex.com/tech-article/dr-grade-iron-ore-pellets-a-supply-overview/ (accessed on 14 November 2023).
- Cabello, A.; Hughes, R.W.; Symonds, R.T.; Champagne, S.; Lu, D.Y.; Mostafavi, E.; Mahinpey, N. Economic analysis of pressurized chemical looping combustion for steam assisted gravity drainage applications. Int. J. Greenh. Gas Control 2019, 90, 102786. [Google Scholar] [CrossRef]
- Tan, Y.; Ridha, F.N.; Lu, D.Y.; Hughes, R.W. Reduction Kinetics of Ilmenite Ore for Pressurized Chemical Looping Combustion of Simulated Natural Gas. Energy Fuels 2017, 31, 14201–14210. [Google Scholar] [CrossRef]
- Hatanaka, T.; Yoda, Y. Attrition of ilmenite ore during consecutive redox cycles in chemical looping combustion. Powder Technol. 2019, 356, 974–979. [Google Scholar] [CrossRef]
- De Vos, Y.; Jacobs, M.; Van Der Voort, P.; Van Driessche, I.; Snijkers, F.; Verberckmoes, A. Development of Stable Oxygen Carrier Materials for Chemical Looping Processes—A Review. Catalysts 2020, 10, 926. [Google Scholar] [CrossRef]
- Liu, F.; Song, C.; Zhu, D.; Li, C.; Ai, L.; Xin, C.; Zeng, X.; Zeng, L.; Huang, N.; Yang, L. Attrition and attrition-resistance of oxygen carrier in chemical looping process—A comprehensive review. Fuel 2023, 333, 126304. [Google Scholar] [CrossRef]
- Adánez-Rubio, I.; Izquierdo, M.T.; Abad, A.; Gayán, P.; de Diego, L.F.; Adánez, J. Spray granulated Cu-Mn oxygen carrier for chemical looping with oxygen uncoupling (CLOU) process. Int. J. Greenh. Gas Control 2017, 65, 76–85. [Google Scholar] [CrossRef]
- Gauthier, T.; Yazdanpanah, M.; Forret, A.; Amblard, B.; Lambert, A.; Bertholin, S. CLC, a promising concept with challenging development issues. Powder Technol. 2017, 316, 3–17. [Google Scholar] [CrossRef]
- Cormos, C.-C. Techno-Economic Evaluations of Copper-Based Chemical Looping Air Separation System for Oxy-Combustion and Gasification Power Plants with Carbon Capture. Energies 2018, 11, 3095. [Google Scholar] [CrossRef]
- Mancuso, L.; Cloete, S.; Chiesa, P.; Amini, S. Economic assessment of packed bed chemical looping combustion and suitable benchmarks. Int. J. Greenh. Gas Control 2017, 64, 223–233. [Google Scholar] [CrossRef]
- Lyngfelt, A.; Leckner, B. A 1000 MW th boiler for chemical-looping combustion of solid fuels—Discussion of design and costs. Appl. Energy 2015, 157, 475–487. [Google Scholar] [CrossRef]
- Newby, R.A.; Keairns, D.L.; Stevens, R.W. Chemical looping combustion oxygen carrier production cost study. Appl. Energy 2023, 345, 121293. [Google Scholar] [CrossRef]
- Lockwood Greene Technologies and Lockheed Martin. Ironmaking Process Alternatives Screening Study; US Department of Energy: Washington, DC, USA, 2000. Available online: https://www.energy.gov/sites/prod/files/2013/11/f4/ironmaking_process.pdf (accessed on 13 May 2020).
- Dansie, J.K.; Sahir, A.H.; Hamilton, M.A.; Lighty, J.S. An investigation of steam production in chemical-looping combustion (CLC) and chemical-looping with oxygen uncoupling (CLOU) for solid fuels. Chem. Eng. Res. Des. 2015, 94, 12–17. [Google Scholar] [CrossRef]
- Osman, M.; Khan, M.N.; Zaabout, A.; Cloete, S.; Amini, S. Review of pressurized chemical looping processes for power generation and chemical production with integrated CO2 capture. Fuel Process. Technol. 2021, 214, 106684. [Google Scholar] [CrossRef]
- Authier, O. Coal Chemical-Looping Combustion for Electricity Generation: Investigation for a 250 MWe Power Plant. Energy Procedia 2013, 37, 588–597. [Google Scholar] [CrossRef]
- Smith, E.; Morris, J.; Kheshgi, H.; Teletzke, G.; Herzog, H.; Paltsev, S. The cost of CO2 transport and storage in global integrated assessment modeling. Int. J. Greenh. Gas Control 2021, 109, 103367. [Google Scholar] [CrossRef]
- Abdalla, A.; Mohamedali, M.; Mahinpey, N. Recent progress in the development of synthetic oxygen carriers for chemical looping combustion applications. Catal. Today 2023, 407, 21–51. [Google Scholar] [CrossRef]
- Siriwardane, R.; Riley, J.; Benincosa, W.; Bayham, S.; Bobek, M.; Straub, D.; Weber, J. Development of CuFeMnAlO4+δ oxygen carrier with high attrition resistance and 50-kWth methane/air chemical looping combustion tests. Appl. Energy 2021, 286, 116507. [Google Scholar] [CrossRef]
- Cabello, A.; Gayán, P.; García-Labiano, F.; de Diego, L.F.; Abad, A.; Adánez, J. On the attrition evaluation of oxygen carriers in Chemical Looping Combustion. Fuel Process. Technol. 2016, 148, 188–197. [Google Scholar] [CrossRef]
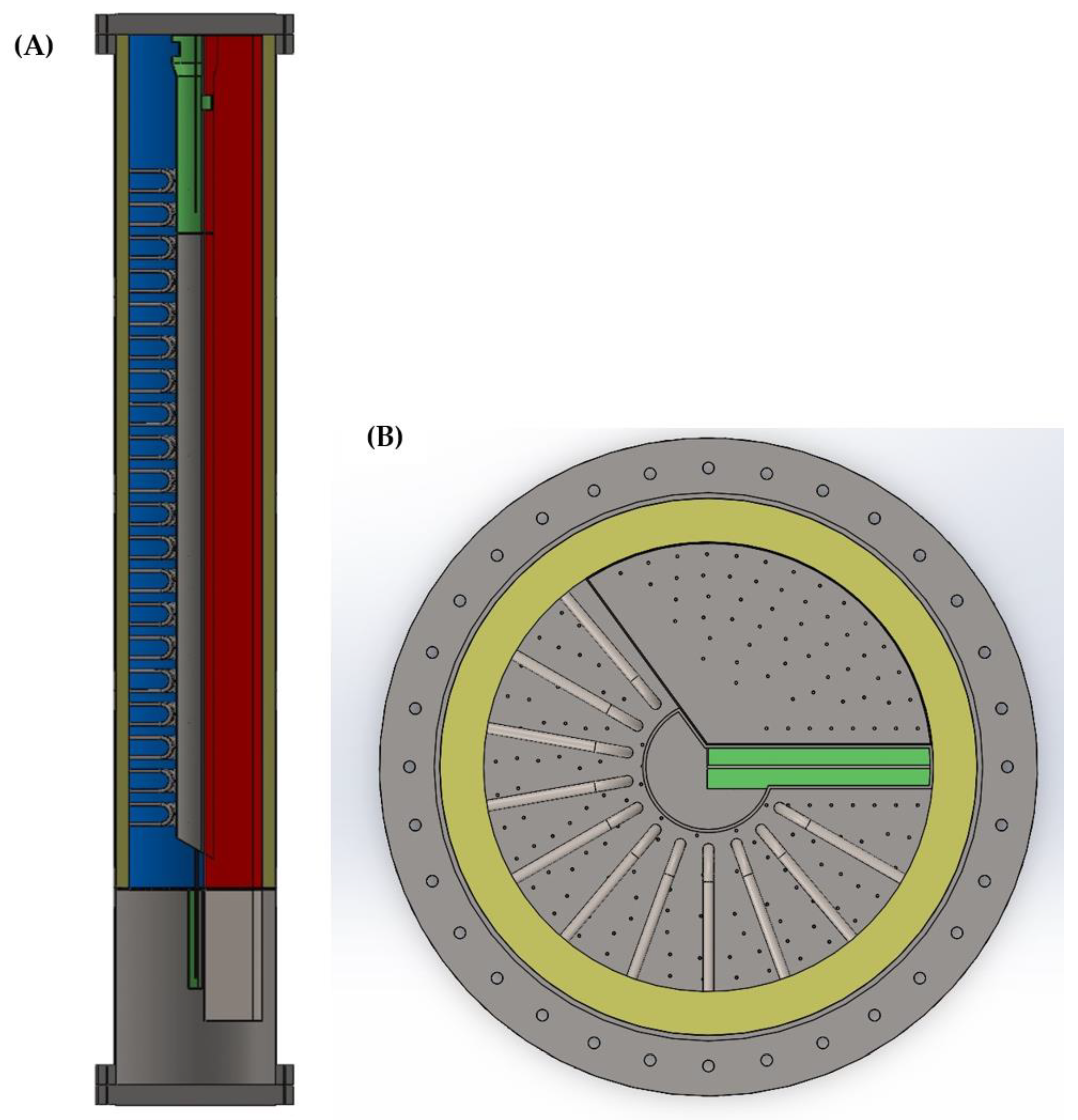
| Oxygen Carrier Properties | Units | Value | |
|---|---|---|---|
| Material | - | Ilmenite ore | |
| Average particle diameter (dsv) | µm | 350 | |
| Particle density | kg/m3 | 4700 | |
| Bed inventory (per PCL reactor) | tonnes | 965 | |
| PCL Reactor Geometry | Units | Value | |
| Pressure vessel height | m | 10 | |
| Pressure vessel outer diameter | m | 7.2 | |
| Refractory thickness | m | 0.20 | |
| No. parallel reactors | - | 4 | |
| Reformer tube area per reactor | m2 | 610 | |
| PCL Reactor Fluidization Parameters | Units | Air reactor | Fuel reactor |
| Bed temperature | °C | 1050 | 1050 |
| Freeboard pressure | kPa(g) | 700 | 700 |
| Cross-sectional area at bottom of bed | m2 | 22.4 | 11.9 |
| umf—bottom of bed (Chitester et al. (1984) [29]) | m/s | 0.08 | 0.12 |
| Gas velocity—bottom of bed | m/s | 0.23 | 0.34 |
| Gas velocity—riser | m/s | 3.9 | 0.34 |
| Parameter | Units | Value |
|---|---|---|
| Orientation | - | Horizontal |
| Pressure vessel length | m | 19 |
| Pressure vessel outer diameter | m | 8.2 |
| Refractory thickness | m | 0.20 |
| No. parallel units | - | 2 |
| Reformer tube area per unit | m2 | 1280 |
| Bed inventory per unit | tonnes | 435 |
| Item | Factor |
|---|---|
| Direct Costs (DC) | |
| Purchased equipment installation | 0.53 × PE |
| Insulation | 0.08 × PE |
| Instrumentation and control | 0.15 × PE |
| Piping | 0.30 × PE |
| Electrical system | 0.20 × PE |
| Buildings | 0.15 × PE |
| Yard improvements | 0.15 × PE |
| Service facilities | 0.15 × PE |
| Land | 0.06 × PE |
| Indirect Costs (IC) | |
| Engineering and supervision | 0.30 × PE |
| Legal expenses | 0.03 × PE |
| Construction expenses | 0.10 × DC |
| Construction overhead | 0.05 × DC |
| Contingency | 0.08 × (DC + IC) |
| Fixed capital investment (FCI) | DC + IC + Contingency |
| Depreciable fixed capital investment | DC + IC + Contingency − Land |
| Startup expense (SE) | 0.09 × FCI |
| Working capital (WC) | 0.17 × FCI |
| Total capital investment (TCI) | FCI + SE + WC |
| Unit | Cost | Source | |
|---|---|---|---|
| Electricity | USD/kWh | 0.0887 | [31] |
| Cooling water | USD/m3 | 0.021 | [31] |
| Steam (medium pressure) | USD/kg | 0.0389 | [31] |
| Natural gas | USD/kg | 0.22 | Industrial expertise |
| Oxygen | USD/kg | 0.07 | Industrial expertise |
| DR pellets | USD/tonne | 68.97 | [34] |
| Oxygen carrier | USD/kg | 0.2847 | [35] |
| Amine makeup | USD/kg | 1.92 | Industrial expertise |
| Non-hazardous waste disposal | USD/kg | 0.036 | [31] |
| Primary waste treatment | USD/m3 | 0.0539 | [31] |
| Cost (USD, 2022) | |||
|---|---|---|---|
| Base Case | Base Case + PCC | PCL-DRI | |
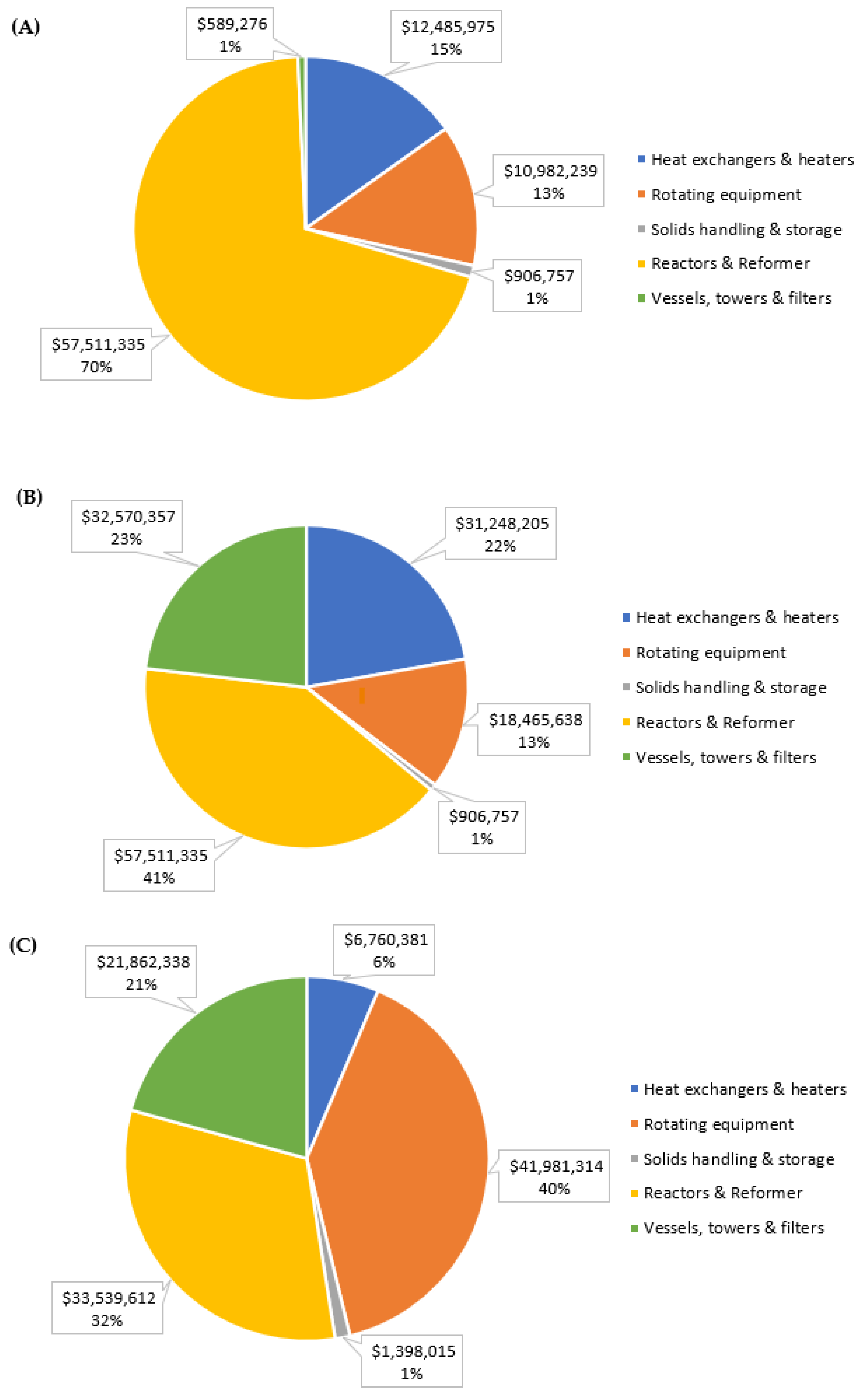
| Total purchased equipment cost | $82,475,582 | $140,702,292 | $105,541,660 |
| Direct costs | $228,457,363 | $389,745,349 | $292,350,397 |
| Indirect costs | $61,485,547 | $104,893,559 | $78,681,307 |
| Contingency | $23,195,433 | $39,571,113 | $29,682,536 |
| Fixed capital investment | $313,138,343 | $534,210,021 | $400,714,241 |
| Depreciable fixed capital investment | $308,189,808 | $525,767,883 | $394,381,741 |
| Startup expenses | $28,182,451 | $48,078,902 | $36,064,282 |
| Working capital | $53,233,518 | $90,815,704 | $68,121,421 |
| Total capital investment | $394,554,312 | $673,104,626 | $504,899,943 |
| Ratio relative to base case | 1.00 | 1.71 | 1.28 |
| Annual Cost (USD, 2022) | ||||||
|---|---|---|---|---|---|---|
| Base Case | Base Case + PCC | PCL-DRI | ||||
| Plant Cost | Cost per Tonne of CO2 | Plant Cost | Cost per Tonne of CO2 | Plant Cost | Cost per Tonne of CO2 | |
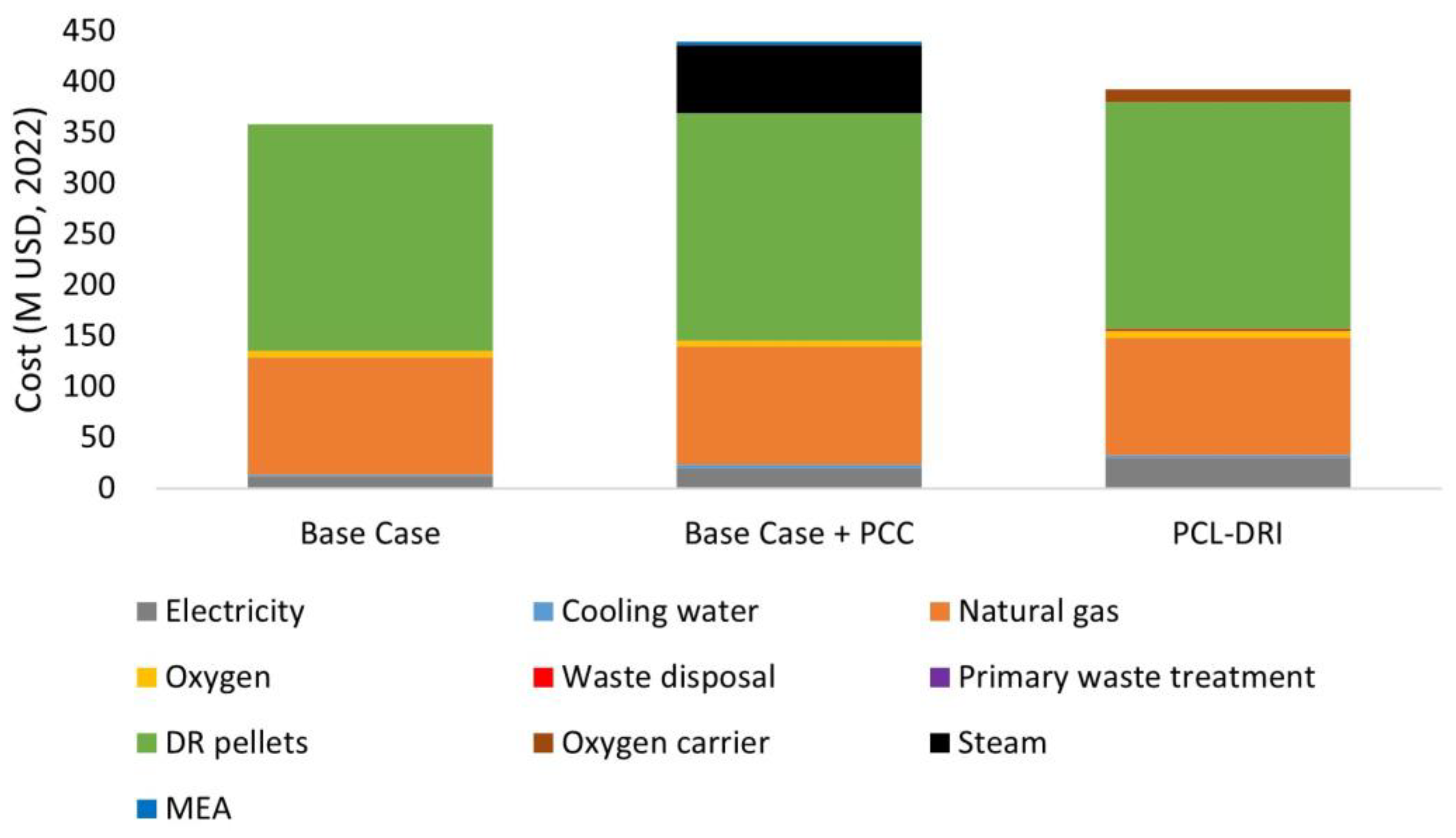
| Electricity | $11,901,990 | $15.13 | $20,424,600 | $25.97 | $29,988,834 | $38.13 |
| Cooling water | $1,180,418 | $1.50 | $3,241,054 | $4.12 | $2,365,634 | $3.01 |
| Steam | $- | $- | $66,889,506 | $85.05 | $- | $- |
| Total utility cost | $13,082,408 | $16.63 | $90,555,160 | $115.14 | $32,354,468 | $41.14 |
| Natural gas | $116,088,270 | $147.61 | $116,088,270 | $147.61 | $116,088,270 | $147.61 |
| Oxygen | $6,119,218 | $7.78 | $6,119,218 | $7.78 | $6,119,218 | $7.78 |
| DR pellets | $223,267,337 | $283.88 | $223,267,337 | $283.88 | $223,267,337 | $283.88 |
| Oxygen carrier | $- | $- | $- | $- | $12,809,796 | $16.29 |
| Amine makeup | $- | $- | $3,361,463 | $4.27 | $- | $- |
| Total raw materials cost | $345,474,825 | $439.27 | $348,863,289 | $443.54 | $358,284,622 | $455.56 |
| Non-hazardous waste disposal | $- | $- | $- | $- | $2,131,605 | $2.71 |
| Primary waste treatment | $17,227 | $0.02 | $47,256 | $0.06 | $45,118 | $0.06 |
| Total waste treatment cost | $17,227 | $0.02 | $47,256 | $0.06 | $2,176,723 | $2.77 |
| Total variable operating costs | $358,574,460 | $456 | $439,438,705 | $559 | $392,815,812 | $499 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bond, N.K.; Symonds, R.T.; Hughes, R.W. Pressurized Chemical Looping for Direct Reduced Iron Production: Economics of Carbon Neutral Process Configurations. Energies 2024, 17, 545. https://doi.org/10.3390/en17030545
Bond NK, Symonds RT, Hughes RW. Pressurized Chemical Looping for Direct Reduced Iron Production: Economics of Carbon Neutral Process Configurations. Energies. 2024; 17(3):545. https://doi.org/10.3390/en17030545
Chicago/Turabian StyleBond, Nicole K., Robert T. Symonds, and Robin W. Hughes. 2024. "Pressurized Chemical Looping for Direct Reduced Iron Production: Economics of Carbon Neutral Process Configurations" Energies 17, no. 3: 545. https://doi.org/10.3390/en17030545
APA StyleBond, N. K., Symonds, R. T., & Hughes, R. W. (2024). Pressurized Chemical Looping for Direct Reduced Iron Production: Economics of Carbon Neutral Process Configurations. Energies, 17(3), 545. https://doi.org/10.3390/en17030545





