Abstract
Deploying onshore wind energy as a cornerstone of future global energy systems challenges societies and decision-makers worldwide. Expanding wind energy should contribute to a more sustainable electricity generation without harnessing humans and their environment. Opponents often highlight the negative environmental impacts of wind energy to impede its expansion. This study reviews 152 studies to synthesize, summarize, and discuss critically the current knowledge, research gaps, and mitigation strategies on the environmental impacts of onshore wind energy. The investigated effects comprise impacts on the abiotic and biotic environment, with birds and bats in particular, noise and visual impacts. Effects are discussed in the context of social acceptance, other energy technologies, and wind energy expansion in forests. This review illustrates that many effects are highly case-specific and must be more generalizable. Studies are biased regarding the research focus and areas, needing more standardized research methods and long-term measurements. Most studies focus on the direct mortality of birds and bats at wind farms and are concentrated in Europe and North America. Knowledge gaps persist for many impact categories, and the efficacy of mitigation strategies has yet to be proven. More targeted, unbiased research is required that allows for an objective evaluation of the environmental impacts of wind energy and strategies to mitigate them. Impacts, such as those on biodiversity, need to be addressed in the context of other anthropogenic influences and the benefits of wind energy. This forms the basis for a socially acceptable, efficient, and sustainable expansion of wind energy.
1. Introduction
Transitioning global energy systems from fossil fuels to renewable energy (RE) is a globally promoted, effective strategy to combat global climate change and its impacts [1]. Renewable energy sources (RESs) provide affordable, sustainable, and free-obtained energy crucial for decarbonizing the energy sector and achieving carbon neutrality [2].
Onshore and offshore wind energy (WE) are supposed to account for more than one-third (35%) of the total generation mix in 2050 [3]. Climate policies and reductions in technology costs have increased the global attractiveness of wind energy and enhanced the growth of the wind sector [4]. Wind energy provides environmental benefits compared to conventional, fossil-fueled power plants, particularly regarding greenhouse gas emissions, air pollutants, or water consumption [5]. To achieve the target, global onshore wind energy capacity needs to be expanded to more than 5000 GW by 2050 [3] compared to around 840 GW in 2022 [6]. In countries with forests as the dominant land cover type, there are high demands to use forested areas for renewable energy to meet zero-emission goals [7]. Hence, substantial onshore wind energy infrastructure installation in forested areas is needed. Intact forests, fulfilling important functions such as biodiversity, carbon sequestration and storage, water provision, or human health, are increasingly threatened by human actions such as urbanization, agriculture, or infrastructure. Their preservation is an urgent priority to counter the biodiversity crisis and climate change [8].
Broad public acceptance is essential for the further expansion of wind energy. It is affected by the multitude of ecological and societal impacts that arise from WE utilization [4]. A wide variety of studies covers those impacts [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160] and comprise impacts on the abiotic environment (A), impacts on the biotic environments (B), impacts on birds and bats (C), noise impacts (D), visual impacts (E), other impacts (F), social acceptance (G), and multiple impacts (H).
Noise and light pollution are considered sources of disturbance to humans and other species [72]. Visual impact and noise of wind energy are among the most significant environmental hindrances to the development of the wind power industry, influencing the acceptance of wind energy by the public and by authorities [35,37,131]. Visual and aesthetic impacts on the landscape annoy people [35] and increase their opposition to wind power [110]. Wind farms (WFs) installed at exposed sites are visible from a great distance [78].
According to a previous study, land use constantly changes due to nature and human interactions [42]. Wind energy facilities have been reported to be very land-use intensive [114]. Installing wind turbines (WTs) and transmission infrastructure takes up land, affecting landscapes and biodiversity [102]. Wind energy-related land use changes trigger biodiversity decline and emphasize the necessity for meeting climate and biodiversity goals [73]. On the other hand, wind energy potential can be affected by land use changes [47].
The WF operation has measurable impacts on meteorological variables [88]. Assumed as a sink of energy and source of turbulence [14], wind turbines alter microscale and possibly macroscale weather [76] and impact regional climate [35]. Soil carbon cycling is directly affected by micrometeorological changes in air temperature, soil moisture, and radiation induced by wind turbines and indirectly affected by micrometeorological changes in plants and soil microbial activities [102].
The large-scale deployment of wind energy contributing to climate change mitigation can have trade-offs with biodiversity [56,57], which is also called the green–green dilemma [43]. Wind energy development often creates severe concerns about biodiversity [80] and negatively impacts associated sustainability targets [97]. Co-occurrence of RE infrastructure and wildlife may mean that emission targets are met at the expense of conservation objectives [41]. Wind turbine impacts on wildlife comprise direct impacts such as collision mortality and indirect impacts, including habitat alterations and behavioral impacts [37,76,81]. Birds and bats are consistently considered the most affected taxonomic groups with different responses to the before-mentioned impact pathways [81,149]. The impacts of wind energy on flora and fauna and the reduction in wildlife are also reasons for social opposition to wind energy [43].
An increasing number of wind turbines and an expansion into new sites potentially exacerbate environmental problems. Taller, giant wind turbines with larger, faster-moving blades produce more energy but may also increase environmental adverse effects [76]. Understanding the full consequences of energy production is necessary for meeting the energy demand while safeguarding ecological systems and human societies [72] and striving for a sustainable energy transition [150].
This review aims to collocate evidence on the environmental impacts of large-scale onshore wind turbines in the categories mentioned above, summarize adequate and efficient strategies to mitigate impacts, and reveal significant research gaps and requirements. Due to the increasing number of wind turbines in forested areas, this review discusses the consequences of environmental impacts on forest ecosystems. Furthermore, this study contextualizes wind energy impacts with other human activities and energy technologies to conclude the justification of wind energy opponent arguments.
2. Materials and Methods
This review is based on a systematic literature review in the scientific databases Google Scholar (Mountain View, CA, USA) (scholar.google.de, accessed on 14 November 2023), ScienceDirect (Amsterdam, Netherlands) (sciendirect.com, accessed on 14 November 2023), Taylor & Francis Online (London, United Kingdom) (Tandfonline.com, accessed on 14 November 2023), Springer (Berlin, Germany) (springer.com, accessed on 14 November 2023), Wiley Online Library (New York City, NY, USA) (onlinelibrary.wiley.com, accessed on 14 November 2023), and Web of Science (Philadelphia, PA, USA) (www.webofscience.com/wos/woscc/basic-search, accessed on 14 November 2023). Several search queries were built containing and combining the keywords of “onshore” and “wind energy” with “forest”, “ecology”, “biodiversity”, “climate”, “climatic”, “microclimate”, “ecosystem”, “noise”, “visual”, and “environmental impacts”. Studies were selected from the subject areas of “Energy”, “Environmental Science”, “Agricultural and Biological Sciences”, “Social Sciences”, and “Engineering”. The resulting studies were filtered according to the criteria of (1) being a peer-reviewed academic article, (2) being published in the past 14 years (2010 to 2023), and (3) dealing with impacts related to onshore wind energy. Due to the low availability of studies regarding micrometeorological impacts of wind energy, one study was included that was published in 2004. Based on an initial screening, the impact categories A to F were identified as the main impact pathways in the literature. They form the basis for the structure of this review. For selecting appropriate studies, the incidence of these categories was surveyed in the titles, abstracts, article structures, and conclusions of all potential studies. In total, 152 studies were selected and analyzed systematically (Table 1). This review is a global assessment and includes studies from most continents and 31 different countries.

Table 1.
Evaluated studies including publication year, journal, and study area.
Within the main impact categories, several key topics and keywords are addressed by the literature. To evaluate the focus and research gaps of the recent literature, the occurrence of these critical issues was counted and visualized. The count considered the exact wording and included synonyms or paraphrases. The results of this systematic assessment are provided in an overview section and figures for each main category.
3. Results and Discussion
3.1. General Characteristics of the Reviewed Studies
In 2018–2023, 55% of the selected studies were published, and 16% were published before 2015. The 152 studies are published in 81 different journals, indicating a broad diversity of addressed critical issues. The five most represented journals are Biological Conservation (11 studies), Energy Policy (8 studies), Renewable and Sustainable Energy Reviews (8 studies), Science of the Total Environment (7 studies), and Renewable Energy (6 studies). Together, they comprise 26% of the studies included in this review.
Field studies and literature reviews are the most frequent types of studies. However, less than one-third of the studies are based on measured data, while the majority rely on datasets or databases, modeling and simulation approaches, surveys, and media analyses or summarize and synthesize information from other studies. A large spatial heterogeneity is found related to the study areas. Europe (45%) and North America (21%) together account for two-thirds of all studies (Figure 1). Both continents are characterized by high installed onshore wind energy capacities of 210 GW and 163 GW in 2022 [161]. Only 5% of the studies refer to an Asian study area, although Asia has the highest onshore wind energy capacity (393 GW). In contrast to all other regions, the proportion of studies addressing impacts on birds and bats is very low in Asia. Social acceptance-related studies only refer to Europe and North America. Most studies focus on impacts on birds and bats (50%) or the biotic environment (11%), while information on noise impacts (3%) is scarce.
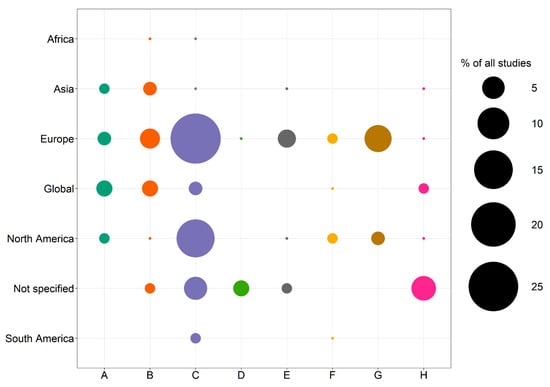
Figure 1.
A spatial distribution of the reviewed studies addressing the categories’ impacts on the abiotic environment (A), impacts on the biotic environment (B), impacts on birds and bats (C), noise impacts (D), visual impacts (E), other impacts (F), social acceptance of onshore wind energy (G), and multiple impacts (H). The size of the circles represents the share of studies addressing the critical topic in the geographical regions.
3.2. Impacts on the Abiotic Environment (A)
3.2.1. Overview
WTs interact with their abiotic environment and are discussed in the context of ecosystem processes, micrometeorology, soil physics, and pollution (Figure 2). Micrometeorological impacts are the most investigated abiotic impacts in all regions except Europe.
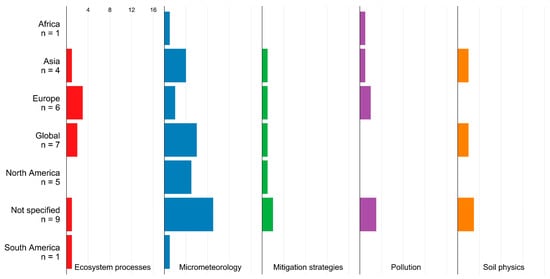
Figure 2.
Abiotic impacts in studies from different regions. N denotes the number of studies.
The term pollution refers to chemical processes, including the dissemination of pollutants or nutrients. Mitigation strategies are rarely discussed in the context of abiotic effects.
3.2.2. Micrometeorology
Wind turbines can lead to micrometeorological alterations of immediate areas (Table S1 in Supplementary Material) [38,146]. They extract kinetic energy and momentum from the atmosphere [32,87,113], reduce wind speed in the downwind region [14,88,113], and cause downwind turbulence (wakes) [34,113] which alter the surface–atmosphere exchange of energy, momentum, mass, and moisture [37,151], and stimulate vertical mixing [13,14,32].
Under a stable atmospheric boundary layer, typically formed at night, WTs might cause an increase in near-surface air temperature (Ta) and land surface temperature (LST) [11,13,14,88,112,132,138] by downward mixing of warmer air in the wakes of WTs [11,14,112,132] or heat flux convergence below the rotor [112].
During the daytime, when the atmospheric boundary layer is unstable and the air is well mixed, this effect is negligible and LST changes are mostly insignificant [14,112,132] or even negative [13,132]. More significant warming effects of LST are found in grassland and cropland compared to forests, probably due to the higher surface roughness, smaller WFs [112], and the intensification of latent heat flux in forests [132]. LST impacts decline with increasing distance from the WF [11] and are detectable up to 10 km [88,112].
Overall, WF impacts on LST are small in magnitude compared to the background inter-annual variability of LST [132] but higher compared to solar photovoltaic systems [32]. The aforementioned temperature-related effects do not occur at all wind farms, exhibit seasonal variations, and depend on WF characteristics, environmental conditions, and local factors such as topography and land use [112].
The enhanced vertical mixing affects the vertical distribution of humidity. Wind turbine-induced vertical mixing may impact cloud formation and local precipitation [102,151]. The drying of near-surface air due to the downward mixing of dry air under stable conditions might only be valid for wet and cool soil conditions [14] and is contradicted by findings of another study [11] in peatland systems. Several studies consistently indicate that WFs promote an increase in evapotranspiration (ET) [14,88], which, together with a higher LST, can reduce soil moisture [153]. Another study [138] found a lower ET in wind farm areas than in control areas and mentioned reduced transpiration as a reason for a higher LST.
Based on the studies mentioned above, large WFs can have measurable impacts on local to regional weather and climate [66,76,151]. While a large-scale deployment of wind farms could change the global distributions of rainfall and clouds [35], it is expected that the impacts of WTs on the global average surface air temperature [66] and global wind speed patterns [83] are insignificant as the extent of the effects are minor compared to land, topography, and background climate impacts [32]. A huge expansion of wind energy capacity would be needed to influence global circulation patterns and climate [69,94].
It is challenging to accurately measure and extract the specific impact from WTs, particularly in forests, where the micrometeorological impacts of WTs are even more complex to assess due to structural diversity, increased turbulence intensity and wind shear, unpredictable airflows, and forest-specific microclimate [42,44,89]. Besides the direct micrometeorological impacts of WF operations, the consequences of land use changes associated with installation must be considered [93]. Positive impacts of micrometeorological changes due to WFs could be that the reduction in wind speed helps to mitigate hazards of sandstorms [37] and that rising local Ta could benefit local vegetation growth and agriculture during cold periods by reducing plant frost, particularly at night [93,102].
3.2.3. Ecosystem Processes
Micrometeorological changes due to WF deployment directly affect the soil carbon cycle and indirectly impact the biotic environment that regulates the soil carbon cycle [102,153]. Implications for biogeochemical ecosystem processes are highly uncertain [13,153].
3.2.4. Soil Physics and Pollution
Removing surface plants and topsoil and replacing them with impermeable surfaces for the transport of wind turbines, grid expansion, and site preparation affect the soil structure, soil compaction, and erosion, and cause changes in hydrologic features [37,72,89,102,113].
Pollution or changes in macronutrient concentrations and cycles in soils or rivers arise during several life cycle stages of wind energy, e.g., as a consequence of WF construction activities such as forest-felling [35,67,88,109,113].
3.2.5. Research Gaps
Although micrometeorological impacts are still the most investigated abiotic impacts in all regions except for Europe, the impact is not yet well understood as studies on the impacts of WTs on land–atmosphere exchanges are relatively new, and effects on the local microclimate are highly variable and difficult to predict [146,151]. Most of the studies (89%) addressing micrometeorological impacts in this review did not measure effects but they did summarize results from other studies, analyze remote sensing data, or present modeling results. Detailed explanations of physical processes and spatial variations in wind farm–atmosphere interactions, particularly hydrometeorology, remain largely unknown and debatable due to observational limitations and model deficiencies [132].
More research is needed to improve the understanding of these processes [151] and reduce the lack of data regarding the potential impacts of wake effects on the environment [113]. The monitoring of WFs needs to be strengthened to reveal the long-term impacts of WFs on local climates and implications for ecosystems [88]. Information about the impacts of different WF development activities on fluvial macronutrient concentration and export in peatlands is scarce [67].
3.2.6. Mitigation Strategies
Some strategies for minimizing impacts on the abiotic environment are presented in Table 2 but their evidence is scarce.

Table 2.
Proposed measures for minimizing abiotic impacts of wind energy.
Large parts of North and Central America, the southern tip of South America, northern Russia, northern China, parts of Africa, southern Australia, and New Zealand are proposed as ideal regions for WFs with low micrometeorological impacts, due to high natural turbulence and frictional dissipation [13].
3.3. Impacts on the Biotic Environment Excluding Bird and Bats (B)
3.3.1. Overview
The biotic impacts of WE include impacts on birds and bats, other wildlife, forests, vegetation, and general impacts on biodiversity (Figure 3). Impacts on birds and bats are the most evidenced critical topics in the context of biotic impacts across almost all study regions. In Europe, wind energy impacts on forest ecosystems are also in the focus of research.
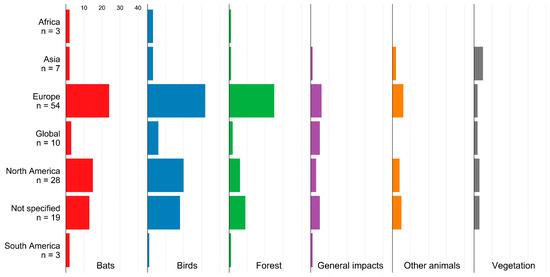
Figure 3.
Biotic impacts in studies from different regions. N denotes the number of studies.
The term general impacts relates to holistic findings on biodiversity. Other animals mainly comprise non-volant mammals and insects. Birds and bats listed in the overview figure are treated in a separate section due to the multitude of studies and findings.
3.3.2. General Biotic Impacts
Although RE impact is broadly positive on biodiversity through climate change mitigation and the reduction in air pollution, the large-scale deployment of RE can have some biodiversity tradeoffs (green–green dilemma) [56,57]. The impacts of WFs on biodiversity and wildlife species comprise habitat loss [56,62,76], habitat degradation [60,76], habitat fragmentation [62,65], vegetation disturbance [62], a potential suppression of ecosystem functions [138], and alterations of species behavior [76]. RE facilities increasingly overlap with conservation areas in more biodiverse regions such as Southeast Asia or Central America, which could substantially intensify impacts on the biotic environment [114,122]. The potential conflicts between energy production and biodiversity conservation seem to be lower for wind energy than for bioenergy as wind energy also allows other uses of the same land [122]. Areas of opportunity for developing wind energy with little harm to biodiversity exist in several world regions [122].
3.3.3. Forests
Due to the more extensive exploitation of land for wind farms and land scarcity, an increasing number of WTs have been recently increasingly planned and constructed in or close to natural habitats such as shrub- and woodlands [16,30,59,126,134] where WT installation has become technically and economically feasible.
Forest hilltops and ridgelines typically offer above-average wind resource availability and are far away from residential areas, which reduces impacts on humans [30] but might interfere with landscape aesthetics as forests represent areas of greater naturalness [131]. Clearings of forests for wind turbine pads and access roads create more openings and edges [33], increase bare grounds [50], decrease natural habitat cover [50], put additional pressure on already stressed forest ecosystems [29,50], and might affect the commercial viability of a WE project [42]. WF constructions in forests can strongly fragment forest ecosystems [89] and require a high level of land transformation [126]. In commercial forests, WTs could be compatible with timber production and represent an additional income for forest landowners [30] but could also negatively affect timber provisioning due to deforestation [93].
3.3.4. Vegetation
The alterations of the microclimate and vegetation through WF construction could affect local vegetation growth [132,138]. Although some WFs do not negatively affect vegetation growth either spatially or temporally and may increase crop yields [88], WFs can affect vegetation structure and vitality negatively [89,102,112] and induce an inhibiting effect if Ta changes reduce soil moisture and enhance water stress resulting in and causing inhibited photosynthesis, primary production, and vegetation growth [37,102,138,153]. WFs can affect protected plant communities where vegetation has been removed, and maintenance activities regularly occur [145].
The estimated inhibiting effects of WFs on vegetation growth cannot be generally applied to all WFs [138], and vegetation impacts can be either positive, negative, or non-detectable [112] depending on local conditions and ecosystems of different study areas [88,89].
3.3.5. Wildlife
Aves (birds) and Chiroptera (bats) are the most evidenced taxa regarding onshore WE impacts. The impacts on other taxa are hardly investigated. It is hypothesized that insects are attracted by WTs [100] because they accumulate in the lee of tall structures and swarm above prominent high points in landscapes [34], rest on the surface of towers [121], or are attracted by light-colored turbines [52,59,120], warm surface temperature induced by blade rotation [120], heat emission [59], or the herbaceous vegetation cover around wind turbine bases [108].
Mammalian predators are not expected to suffer from high mortality rates in wind farms [33]. Different non-volant mammals were found to avoid WF areas and their related infrastructure [32,73,80] or can even live within or near WF areas by adapting their behavior [85]. Decreasing soil moisture can adversely affect feeding activities and the community structures of soil invertebrates [153]. The displacement of one species often has direct consequences for other species because of cascading effects induced by WTs [60]. Wind power installations can also positively impact some land animals and contribute to higher survival rates due to enhanced resource availability and declining predator populations [56].
3.3.6. Research Gaps
Although the state of knowledge on the biotic impacts of wind energy is constantly improving, many gaps and uncertainties remain [79]. Compared to the taxa Aves and Chiroptera, impacts on other wildlife [34] and vegetation [37,88,132] are understudied and even missing in Africa and Latin America. Data and knowledge gaps persist as natural processes or responses of species and populations often take a long time or are difficult to monitor and model [111]. Much of the existing research is species- or location-specific and only applies under particular circumstances [72]. More holistic, integrative, system-focused landscape or ecosystem approaches that consider multiple elements of the structure and functioning of an ecosystem are urgently required to make robust assessments that address direct, indirect, long-term, and cumulative effects [72,155]. The quality, quantity, and transparency of scientific assessments must be improved by establishing clear and rigorous standards, better access to existing data, and a broader focus [72].
3.3.7. Mitigation Strategies
Several mitigation strategies are proposed to minimize biotic environmental impacts (Table 3). Overall, energy development and conservation goals need not be mutually exclusive but will require a perspective change for wind energy development [72]. Future expansion of RE and protected areas is possible with relatively little overlap with appropriate strategies [41].

Table 3.
Proposed measures for minimizing biotic impacts of wind energy.
From a conservational point of view, WFs should be located in fragmented land outside protected areas and land take should be avoided [73]. If this is not possible, conflicts should be mitigated by onsite and best management practices [142].
3.4. Impacts on Birds and Bats (C)
3.4.1. Overview
Birds and bats are identified as the main wildlife groups affected by WTs [30,81] and suffer disproportionately from WTs [62]. The impacts include direct mortality related to species’ activity, habitat alterations and loss, behavioral impacts, and vague and cumulative impacts (Figure 4) [16,17,35,62,80,81,95,97,102,127,154]. Most studies focus on direct mortality in Europe, North America, or in general (no area specified) while neglecting other study areas and providing weaker evidence on habitat alterations and behavioral impacts.
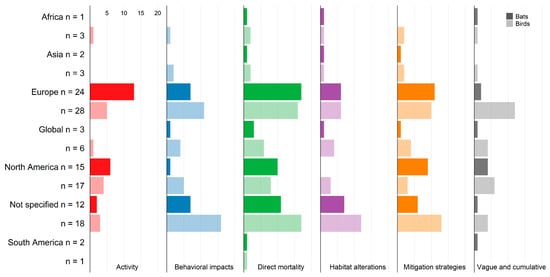
Figure 4.
Impacts on birds and bats in studies from different regions. N denotes the number of studies.
The overview highlights that the impacts on birds and bats are very complex and very present in studies from Europe and North America. The classification into sub-categories is not always entirely clear, due to interacting effects.
3.4.2. Activity and Direct Mortality
Birds and bats suffer from lethal and sublethal injuries in WFs due to collision with WT components and associated infrastructure, electrocution at transmission lines (giant birds), or barotrauma (bats) caused by a sudden pressure drop near the turbine edges [35,59,72,127,140].
WTs affect many bird and bat species [102] at all land-cover types [45,75]. Still, the actual number of fatalities is highly variable (Table S2), indicating that vulnerability and mortality reflect a combination of taxon-, season-, site-, population-, and turbine-specific factors [9,46,79,81,95,152,154]. The fatalities per wind turbine presented in the literature range from 0 to 125 birds per year and from 0 to 287 bats per year.
At the global scale, birds that most likely collide with WTs are of the orders Accipitriformes, Ciconiiformes, Falconiformes, many species within Charadriiformes, Podicipediformes, Strigiformes, Cuculiformes, or Bucerotiformes [126,141]. Small passerines are the most common bird fatalities caused by collision with WTs in Latin America [9], the USA, and Canada, followed by upland game birds and diurnal raptors. In North America, less than one permille of the continent-wide population for each species is estimated to be killed annually by collisions with WTs [45].
In most studies, bat fatalities outnumber birds [79,127,141]. They are estimated to be tens per turbine per year [56]. Molossidae suffer the most fatalities in Latin America [9]. Together with Hipposideridae, Molossidae have the highest death rates globally [141]. Bats in the genus Lasiurus are among the most collision-susceptible bats in the USA [9]. Nearly one-quarter of all bat species in the USA and Canada are affected by WT-related bat mortalities [35]. The species with the highest collision numbers in Europe are the common pipistrelle (Pipistrellus pipistrellus), Nathusius’s pipistrelle (P. nathusii), and the common noctule (Nyctalus noctule), which account together with five other species for 98% of all bat fatalities at WTs in northwestern Europe [59]. The largest bat family, Vespertilionidae, includes the five bat species most vulnerable to collision [141].
Some areas are susceptible to bird and bat fatalities. Hotspots of bird vulnerability are often within critical migratory corridors and along coastlines [58,141] or regions where raptors use thermals to gain altitude to move between locations and forage [151,152]. The most significant number of collisions for bats is observed and predicted in North America at WFs along forested ridgetops in the eastern USA and agricultural regions of southwestern Alberta, Canada, and Europe [141,151]. Cumulative direct mortality estimates of birds and bats from WFs mainly focus on the USA and exhibit high uncertainty and a wide value range (Table S3). Annual fatalities in the USA might amount to 679,000 birds and 888,000 bats.
Collision risk depends on multiple factors such as season, WT and WF properties, species characteristics, behavior, number of birds and bats found in the area, climatic conditions, topography, food availability, and habitat quality in general [35,37,46,96,108,136,151].
Many studies indicate that the seasonal effects of collision mortality are likely due to varying bird and bat behavior, particularly in habitat use and flight activity [56,81,127]. For many migratory passerine and raptor species, spring, fall, and early winter migration, pre-migration, and post-migration periods are characterized by peak mortality at WTs [45,86,127,151,152]. In the temperate Northern Hemisphere, fatalities of migratory bats are concentrated during late summer and autumn migration, with a smaller peak in early spring [28,34,35,59,81,117,127,143].
WT and WF properties are likely to influence collision risk [127,152]. Increasing hub height and greater tower heights of WTs were found to be correlated with higher collision rates for birds and bats in some studies [35,45,51,60,81], although low heights of turbines might attract bird nesting [66]. Different studies found no significant or marginal impact of turbine and rotor dimensions and speed on the collision risk of birds and bats [35,46,81]. Local factors likely lead to substantial variation in mortality rate and obscure any possible effects of WT size in WFs [46]. For birds, in contrast to bats [59,127], collision risk is not limited to operating phases but also occurs at inoperable stages or vacant towers [133,159].
The species’ characteristics and behavior strongly determine whether they collide with WF structures. The activity of birds and bats, particularly at rotor height [59], positively correlates with fatality rates [20,119,120,140]. Differences in collision risk between species result from ecological and morphological factors [58,81]. Flight height, speed, and style of birds passing through the rotor-swept zone are essential parameters for predicting collision risk [74,127,137,144,154]. Bat species particularly vulnerable to collision, though of different genera, have some morphological and ecological similarities [127]. Migratory, tree-roosting, high-flying, edge- or open-space aerial foraging, insectivorous bats seem to be at particular collision risk [9,28,34,52,59,60,79,103,119,127,143,156]. Still, both migratory and resident bat species are prone to collisions with WTs throughout Europe [59]. Collisions of bats with WTs do not appear as chance events. Evidence has been found that bats conduct activities near WTs and use them [20]. There are different hypotheses as to why bats could be attracted to wind turbines, including bats seeking shelter (roosts), social opportunities (conspecifics; mating), or food (insect prey) at wind turbines [34,52,59,70,79,121,127]. Bats could also be attracted by swishing sounds, heat, acoustic, or visual attractors [35,59,79,100,103,133]. Furthermore, bats may misperceive the smooth surfaces of WT towers to be water [52], misperceive WTs as trees [34,35,100], or suffer from echolocation failure and electromagnetic disorientation [133].
Weather conditions influence bird abundance, flight altitude, and flight direction [33,66,81,106,118,126,152]. Bats have the highest collision risk during nights with low wind speed, relatively high air temperature, and no precipitation due to higher activity [32,34,59,62,79,80,81,117,130,156]. Many authors consider meteorological conditions the most critical environmental variables, with immediate and significant influence on bat activity levels [130].
The potential collision risk of migrating raptors and other species is linked to the terrain [74] and is exceptionally high along or on forested ridgelines [35,60,74,81,147]. Topographic features can result in large numbers of birds being funneled through an area of turbines (bottlenecks) [152]. Migrating bats use linear aspects of the landscape for navigation and movement [81,120,127].
Habitat suitability in the vicinity of a WF is a crucial factor driving collision risk [90]. The highest fatalities often occur near habitats attractive to birds [56,68,140,152] and bats [29,77,90,120,130,143]. The larger a facility is, the more critical the specific spatial and environmental context becomes in determining bat mortality [90].
Overall, the susceptibility of volant wildlife species to collide with WTs is highly species-specific and linked to the morphological and behavioral traits of the species. Responses may change in space and with time. Thus, general conclusions on risks for species assemblages are hardly possible, even within one species or one taxonomic group [126].
Direct bird mortality at WTs is lower than other anthropogenic mortality sources (Table S4) [81,86,135,140]. Hundreds of millions die each year in a collision with constructed structures, such as glass windows, buildings, communication towers, transmission lines, and vehicles, or fall victim to cats [135,140]. The cause-specific annual mortality in the US varies from billions (cat predation) to hundreds of millions (building and automobile collisions), tens of millions (power line collisions), and hundreds of thousands (WT collisions) [86]. For every 250 deaths caused by humans, only one bird dies due to WTs [113].
An ongoing rapid expansion of WE, and a projected increase in turbine size, however, could lead to substantially greater mortality and annual fatality numbers [9,86,152]. Wind energy is likely less harmful to wildlife than other energy sources, such as nuclear power and fossil fuels [135,136]. By reducing the reliance on fossil fuels and nuclear power, WTs prevent the injuries of wildlife that would otherwise occur [135], particularly climate change impacts.
Studies on other anthropogenic causes for bat mortality are scarce. Adverse effects from fossil fuels, traffic, or other anthropogenic structures are likely, and the number of bat fatalities from traffic may be notably higher than from wind energy [79,127]. Most anthropogenic causes of faunal mortality are unquantified [140].
3.4.3. Habitat Alterations
The loss, degradation, and fragmentation of habitats threatens bird and bat species [38,95]. Areas with abundant wind resources might coincide with habitats used or visited by sensitive bird [16,62,74] or bat [140] species. High levels of transformations are required to establish new WTs in forests and shrublands [81,126]. Habitat alterations include the construction of turbines and associated infrastructure [59]. The extent to which these habitat modifications affect wildlife species depends on species-specific habitat requirements and tolerances to disturbance, the extent of development, and distance thresholds [50,95,126,154]. Specialist species are more vulnerable than wide-ranging and generalist ones [81]. Habitat alterations in forests due to WTs are linked to a decrease in the density of forest species [48,50]. Contrary results regarding birds were detected within other studies and indicate that recolonization or habitat improvement after initial construction disturbance is possible [48,82,95,102,126].
Bats might lose foraging habitats or roosts through the replacement or fragmentation of natural habitats [28]. Forests are one of the most critical habitats for many bat species [30], and alterations in these ecosystems might induce harmful impacts on bat species least affected by collision mortality [59]. The altered environment may be more favorable for bat species [81] that use flyways and patchworks of attractive foraging patches within which WTs act as an ecological trap [77]. Modifications of the landscape may attract more bats if they create a favorable environment for aerial insects upon which most insectivorous bats feed [151].
Installing new wind farms aggravates other pressures on habitats and intensifies the regional cumulative impact by inducing additional habitat loss and deterioration of habitat quality [18,50,51].
3.4.4. Behavioral Impacts
In long-existing WFs, the direct collision of animals due to turbine blades is often negligible [80], while behavioral impacts dominate. WFs impact birds’ foraging, flight, re-orientation, breeding, feeding, and roosting behavior [32,35,42,140]. The response of birds to wind energy may vary spatially and temporally, depending on biological and behavioral patterns, wind, topography, and WT conditions [12,96,108]. The capacity of birds to perceive and respond to a possible threat depends on species-specific sensory faculties, physiological considerations, behavioral aspects, and environmental conditions [96,108].
Birds might recognize and become habituated to the presence of WTs and behave to avoid them [33,48,62,81,123,139,154,159,160]. Macro-avoidance describes the avoidance of an entire wind farm by adjusting flight paths to avoid entering the area occupied by groups of WTs [81,95,108] and is a trade-off between avoidance-induced habitat loss and reduced collision mortality [106,159]. Meso- and micro-avoidance occur inside the WF space, directly affecting collision risk [81,95].
For bats, WE exhibits one repulsive effect at the scale of a WF and one attractive effect at the scale of the WT itself, as described above. Avoidance effects affect most bat species and could be related to the geographic origin of individual bats [100,115]. Reasons for changes in bat behavior due to WTs are complex and difficult to understand [130].
3.4.5. Cumulative and Population-Level Impacts
The variable results of the studies illustrate that many effects seem to underlie a strong site, species, season, and turbine specificity [127] and underscore the role of both local and regional factors that may contribute to adverse effects on birds and bats at wind energy projects [154]. In most instances, the collision results in lower impacts for birds than habitat loss, disturbance, displacement, and barrier effects [72,81,97,98].
While collisions are a form of direct mortality, the other impact pathways affect species indirectly through reduced fitness due to stress response, increased energy expenditure, or changed foraging behavior. Among the orders with the highest cumulative impact of WE, three (Anseriformes, Charadriiformes, and Podicipediformes) are linked to wetland systems and marine intertidal habitats [16]. At the same time, two (Accipitriformes and Falconiformes) have their habitat predominantly in forests, open areas, and shrubland [97]. Species within orders may vary in vulnerability depending on their behavior [97,101]. For bats, collision is likely the leading cause of impacts, and indirect effects are relatively small [29,81].
The most frequently mentioned bird and bat species in the scientific literature are not necessarily those with the highest vulnerability (Table S5). A high frequency could also arise due to the awareness, frequency, size, perceptibility, or ecological importance of a species. Birds from the order Accipitriformes are frequently mentioned potentially due to their susceptibility to collision risk but also their prominence as indicator species [128]. Vespertilionidae is the bat family with the highest species richness and contains some species with the highest collision risk [141]. In total, more than 380 different bird species and 55 different bat species were mentioned in the investigated literature.
Under specific circumstances, population-level consequences from WFs on birds and bats occur [154]. The impacts vary due to interspecific variation, e.g., flight behavior, natural history, habitat use, conservation status, and population sensitivity to additive mortality [16]. Some species could experience population declines because of turbine collisions [39] or barrier effects [48]. Population-level effects of wind energy development are generally minor for most bird and bat species but may be significant for some raptors [39] and migratory bats [53,54]. Bird and bat species with low fecundity, late ages of maturity, long generation times, low reproductive output, low natural mortality, small population sizes, or high habitat specialization, such as soaring birds or bats, are most sensitive to additive mortality and population decline and more likely to be impacted by WE [16,45,129,140,141]. They have a lower ability to compensate for the cumulative effects of anthropogenic factors, which, in isolation, may not pose a threat at a population level [39,157].
Mortality from wind energy could lead to localized population-level impacts, and the cumulative result of wind energy with other anthropogenic sources of mortality may cause widespread declines in avian and bat populations [72]. The cumulative impacts of WE on birds and bats are acceptable, and population effects are unlikely if siting in areas with concentrations of sensitive species is avoided [140].
Overall, the current knowledge on the effects of WE is controversial and relatively scarce for shrub- and woodland-dwelling wildlife species. The literature provides evidence that WFs might affect birds’ and bats’ mortality, behavior, and habitat suitability. However, responses can be harmful, positive, or indifferent, and some studies do not generate clear patterns [126].
3.4.6. Research Gaps
The studies examined in this review show that the research often needs improvement. Observed or estimated fatality rates are often inaccurate, uncorrected, uncalibrated, and biased [9,21,28,33,75,117,151] and do not account for search area, searcher efficiency, or potential carcass removal from scavengers [35,126,154] which might cause an underestimation of fatalities [21,141], particularly in agricultural landscapes and dense forest ridge tops [29,103,136].
The comparability of mortality studies is also limited due to using different reference units, such as “fatalities per turbine”, which do not consider the variation in actual power generation, WT properties, and fatality rates among turbines [54,136]. As many studies are conducted on a single or a few WF sites [126,136], studies differ in design and duration, and the results exhibit season, site, and taxon specificity [33]; findings from local studies cannot be ubiquitously transferred [156]. In many cases, more monitoring data exist but have never been publicly available [9,12,75,86], and studies must rely on opportunistic data [68]. Long-term biodiversity monitoring around WFs based on standardized, systematic assessments is lacking in many landscapes [48,80].
The methodological weaknesses and incompleteness of studies favor the persistence of substantial knowledge gaps regarding the impacts of wind energy on avian and chiropteran wildlife. Significant knowledge gaps comprise the links between pre-construction activity and post-construction fatalities at WFs for bird and bat species [75,119,127,140], the reasons under what conditions, why, how, and how often birds and bats are killed at WFs [20,33,35,42,62,151,152], the indirect effects of WTs on birds and bats [75,81,97,139], cumulative and population-level impacts [12,28,53,54,56,59,79,86,111,157], and empirical evidence demonstrating the effectiveness of mitigation strategies [12,59,82,111].
To allow for more general conclusions on WF effects on wildlife species and population-level effects, many studies claim for a long-term before–after control–impact (BACI) study design for multiple areas [33,48,75,126,139,154,157]. If BACI design is impossible, similar sites should be studied simultaneously to compare areas with and without turbines [154] to determine background mortality [45,133]. Studies need to be harmonized and standardized [33], should be performed over several years (>10) [33,48,82], conducted systematically at all existing and planned facilities in a region [54,90,124], and should assess the effects on ecological communities or ecosystems as a whole [50]. More and better monitoring and hypothesis-based field studies [152] for species diversity and locations in existing wind farms are needed [46] just as large-scale data synthesis that reaches beyond the scale of a single case study [126,136,139] and quantifies overall mortality and its spatiotemporal and taxonomic variations is also needed [86].
Even though it is vital to avoid and mitigate impacts on wildlife resulting from wind energy, it is more important to assess the impacts of other anthropogenic activities and structures, particularly other energy sources [127]. There is significant uncertainty about the independent and cumulative impacts of mortality from different sources on avian populations, for instance, whether multiple mortality sources act additively or compensatory [86].
3.4.7. Mitigation Strategies
Solutions that allow continued growth of WE development without risking biodiversity loss are available (Table 4) but require rapid implementation [54]. Often, a hierarchical concept consisting of (1) avoidance, (2) minimization and reduction, and (3) compensatory mitigation is recommended [12,56,96].

Table 4.
Proposed measures for minimizing wind energy impacts on birds and bats.
Minimization options may have limited applicability across taxa, and there is high uncertainty regarding their effectiveness [17], which is expected to be site-specific [12] and species-specific about audible, optical, and biomechanical constraints and options [12,96]. Effective means should reduce bird and bat fatality without compromising turbine performance and energy output or affecting human life [52,62].
Overall, the avoidance of high-risk sites for development, curtailment during operation, and compensation is deemed most promising in addressing potential impacts across taxa [12]. Curtailment measures during periods of high bat activity are the most effective ways to avoid bat fatalities at WTs [150]. Adjusting turbine operation and warning or deterring signals is expected to be most functional for birds [96]. On-site mitigation measures may also indirectly affect the overall habitat quality as deterrence measures may trigger birds to move away from the WF areas to other possibly suboptimal habitats [96].
Repowering could be a defensible strategy to increase wind energy production without substantially increasing the collision risk of some bird species [51,58,127]. For equivalent wind power, fewer and larger turbines are thought to be preferred over many small turbines to minimize collision risk per energy output to birds [46,51,98,102,141,154]. The largest WTs might, however, increase the danger for bats [79,141]. Further study is required to clarify this subject [46]. Due to the development of standardized methods for siting WTs and monitoring avian impacts, many new developments have reduced the risk of collisions [151]. Decreasing death rates in recent years can be attributed to larger blades that turn more slowly, advanced thermal monitoring, and radar tracking to site WTs more carefully [136]. When mitigation is still impossible or has no desired effect, funding research employing scientifically defensible monitoring methods may render new insights into the species’ ecology for long-term conservation [96]. International cooperative efforts to reduce the environmental impacts of WFs should be enforced [35,59,149,154].
3.5. Noise Impacts (D)
3.5.1. Overview
The impacts of WT noise are rarely discussed in South American or Asian studies and are even missing in studies from Africa (Figure 5). Most information is provided by studies not related to specific study areas. Taking together information from all study regions, evidence on noise propagation and the impacts of noise on human health is scarce. In contrast, more studies quantify noise intensity and discuss potential disturbance for wildlife.
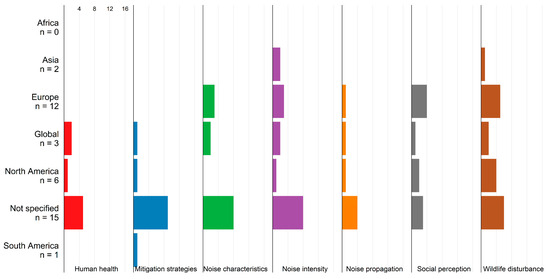
Figure 5.
Noise impacts in studies from different regions. N denotes the number of studies.
The relevance of noise for social perception is further discussed in Section 3.8.
3.5.2. Noise Characteristics and Noise Intensity
Wind turbines produce aerodynamic and mechanical noise [22,35,37,66,84,102,113,151]. The total noise emissions of a WT result from a combination of mechanical and aerodynamic noise [35,151] and are quantified by the sound pressure level [37] which might vary from 49 to 111 dB at the turbine base (Table S6). The noise intensity is higher near the WT base [113] and decreases with distance from WT. The noise effect of several WTs is not additive [35]. The measurement of noise emissions from WTs is complex, mainly due to background noise that may mask the turbine noise [151].
Aerodynamic noise is caused by turbine blades passing through the air and consists of non-periodic signals that vary with turbine size, wind speed, and blade rotation speed [35,37,55]. Larger WTs are expected to produce greater noise [35,37,55].
Noise generated by mechanical and electrical parts [151], such as internal gears or generators [35,37], is affected by wear and tear, lack of maintenance, and poor component design [66]. Besides the operational phase, WT-related noise occurs during transport, construction, maintenance, and decommissioning activities [72]. Effects resulting from construction and decommissioning noise are temporary and occur for other non-renewable energy facilities [135].
3.5.3. Noise Propagation
The noise propagation depends on meteorological conditions, barriers, topography, building characteristics, and ground surface materials [19,22,35]. Background noise can diminish the noise perception from WTs [35]. Stable atmospheric conditions enhance the transmission of characteristic WT noise signals [26].
3.5.4. Social Perception and Human Health
According to the World Health Organization, noise over 55 dB in the daytime or over 40 dB in the nighttime influences residents [84] and wind farm workers [32] and can provoke adverse subjective effects such as annoyance or dissatisfaction or even induce serious health issues such as hearing problems, sleep disorders, headaches, weakening of the immune system, or damage of the vestibular system depending on the noise frequencies [35,66,84,113]. It has not yet been proven that WT noises cause serious health problems per se [32,35].
3.5.5. Impacts on Wildlife
Noise pollution generally contributes to disturbance, habitat degradation, and wildlife displacement and masks auditory life-history traits essential for survival and reproduction [72]. It affects habitat use, territorial behavior, and the breeding success of animals [126]. Consequences are, for instance, reduced bird densities [50,126], avoidance behavior of bats [59] and birds [123], alterations of wildlife migration routes [65], or other behavioral impacts [60,126,139]. Operation noise can be a deterrent [133] or an attractive effect [151]. For wildlife, the loudness of noise is relatively less critical than the consistency of noise [72]. In general, the impacts of WT noise on wildlife are highly species-specific [95], and some species even occur in zones with the highest noise exposure [85].
Forests can act as effective noise barriers depending on the distance that sound travels through the forest, the size and density of trees, and land topography [162].
3.5.6. Research Gaps
There still needs to be more evidence regarding the correlation between WT noise and annoyance or health issues [35], and a common framework for estimating impacts on wildlife in which intensity, frequency, and noise timing are accounted for is lacking [72]. Environmental responses to noise pollution are complex and inefficient to detect empirically [72]. A better understanding of the coupling between noise generation and propagation is required to control noise emissions and meet regulations regarding immission levels at the dwellings [22]. Some new time-frequency analysis methods in signal processing are needed to de-noise effectively according to the unique WT characteristics [84]. Another study [26] recommends (i) conducting long-term measurements that enable the detection of fluctuating weather conditions, (ii) including shutdown times, especially during the night time, to differentiate WT noise and background noise, and (iii) combining measurements at the emission and immission point to identify WT noise components and derive conclusions concerning sound propagation.
3.5.7. Mitigation Strategies
By selecting appropriate mitigation strategies, the noise impacts of WTs can be minimized or even avoided (Table 5).

Table 5.
Proposed measures for minimizing noise impacts of wind energy.
Besides siting, wind turbine technology is crucial for noise mitigation [60]. Recently, advanced machinal design has reduced machinal noise effectively and it is no longer considered to be as important as aerodynamic noise [151]. The modification of the turbine blade design is directly related to economic aspects as it affects the energy yield [22]. Optimizations on blade design can reduce the level of noise by 0.5 to 3.2 dB [66].
3.6. Visual Impacts (E)
3.6.1. Overview
Landscape aesthetics and visual effects are the focus of studies addressing the visual impacts of WTs across all study regions (Figure 6). Most studies are related to Europe or a non-specific study area. Evidence from Africa, Asia, and Latin America on visual impacts is scarce.
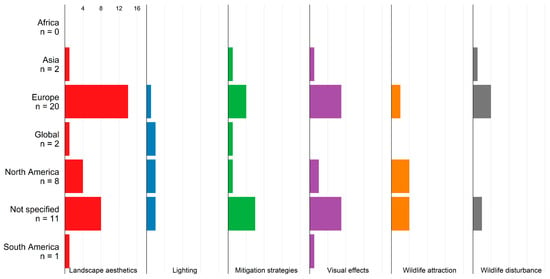
Figure 6.
Visual impacts in studies from different regions. N denotes the number of studies.
Visual effects include WT lighting and shadow effects. Regarding the impacts on wildlife, it has to be differentiated between attractive and disturbing effects.
3.6.2. Landscape Aesthetics
Wind turbines and their infrastructure impact the aesthetics of landscapes [105]. The aesthetic value of a landscape with WTs depends on the number of WTs and the initial attractiveness of the landscape before constructing WTs [23,35]. Higher numbers of WTs generally increase the visual impact, particularly in landscapes with high initial attractiveness and scenic quality, whereas greater distances between the observer and the WT diminish visual impact [23,35,66]. Threshold distances after which the negative visual impact of a WT might disappear are 2 to 12 km [23,43].
Factors related to the landscape surroundings, such as the topography, influence the visual impact of wind farms [131]. The visual impact of WTs increases when the surrounding landscapes suggest a natural character, e.g., with a high proportion of forests compared to a landscape affected by a high degree of anthropogenic elements [131]. However, forests can provide effective visual barriers reducing visual impacts depending on tree size, density, and species [162]. WTs built in high-relief landscapes might be perceived as more contrasting and dominant [19,131].
Moreover, WT properties, such as the height, size, colors, contrast, shape, number of blades, and blade rotating direction, influence their visual impact [24,35,37,42,66]. Rotating blades induce less intense adverse visual effects, and a regular layout of WTs in a WF creates a better sense of visual regularity [35].
The psychophysiological responses to WTs were less harmful than other industrial constructions, such as other energy production facilities [91]. However, the change in landscapes due to WFs may influence a resident’s mental health [32].
3.6.3. Visual Effects and Wind Turbine Lighting
Besides the WT impacts on landscape aesthetics, impacts also comprise other types of visual impairment. Driven by interactions with the sunlight, WTs can cause flashing effects due to reflections of the blades and shadow flickering when turbine blades move through sunshine and the shadows of the blades are cast on static objects [35,37,113]. Utility-scale WTs must be featured with aviation lights to comply with air traffic safety regulations [72,110]. Evidence for substantial or harmful annoyance and stress caused by aviation lights has not been found yet [110].
3.6.4. Impacts on Wildlife
The visual impacts of wind energy facilities on wildlife are often discussed but hardly evidenced. Infrastructure visibility is reduced in habitats with dense vegetation, such as forested areas [126]. WTs may represent a visual disturbance for wildlife [50] that could trigger avoidance behavior [59,123,127]. Otherwise, it is found that insects, birds, or bats might be visually attracted to WTs and their aviation lights, particularly under specific weather conditions, which exposes them to collision risk [52,59,66,133,152]. However, regarding bats, most studies do not support this hypothesis [70,79,127]. Non-renewable energies, such as oil and gas fields, are sources of light pollution with nighttime light propagation from gas flares or vehicle headlights [72].
3.6.5. Research Gaps
The consequences of WT light pollution are weakly evidenced as environmental responses to light pollution are complex and inefficient to detect empirically [72]. Overall, the visual impact of WTs on the landscape is a highly subjective issue [35,131] and difficult to quantify [32,66], hindering a generalization of the study design.
3.6.6. Mitigation Strategies
Several strategies for minimizing the visual impacts of WE are proposed in the literature (Table 6).

Table 6.
Proposed measures for minimizing visual impacts of wind energy.
Repowering is a vital strategy to achieve increments in energy yield with minor additional visual effects [92] as the number of turbines is minimized [24]. Landscape evaluation models allow us to compare changes in landscape quality before and after the project or between different projects [64].
3.7. Other Impacts (F)
3.7.1. Electromagnetic Interference
The electromagnetic field of WTs can, although weak and confined to a small range, create electromagnetic interferences for wireless services [35] by distorting transmissions of exiting signals and generating their own electromagnetic radiation [37]. Air surveillance radar systems, broadcast communication such as radio and television, and navigational systems have been reported to be affected by WTs [32,35,37]. High reflectivity of WT components can reduce the sensitivity of radar systems by increasing background noise, false readings, and shadowing areas of radar coverage [84]. Artificial low-frequency electromagnetic fields from WTs or associated high-voltage power lines may also affect the orientation of magnetic-sensitive species such as birds [96] or could interfere with receptors that bats use to guide flights [151].
The low frequency of investigated studies broaching the issue of WT-caused electromagnetic interferences indicates that scientific evidence on this topic is scarce. The investigated studies do not report, e.g., the distances at which interferences occur, conditions that promote interferences, impacts on wildlife, or the relationship between WT properties and size on electromagnetic interferences. The potential impacts of electromagnetic fields from wind turbines on health have not been documented [37]. Although the electromagnetic radiation and interference of wind turbines are minimal, there are scenarios when electromagnetic interference causes problems [35]. Appropriate mitigation strategies are listed in Table 7.

Table 7.
Proposed measures for minimizing impacts on electromagnetic interference from wind energy.
Impacts on radars have been solved to a great extent in recent times [32].
3.7.2. Land Use Footprint and Changes
All forms of energy production impact the environment through land use [37]. Due to the necessary spacing between WTs, the construction of access roads, electricity substations, and transmission lines, the footprint of wind energy development can be extensive [19]. WTs located in flat areas typically use more land than those in hilly areas, and land requirements are proportional to the dimensions of the blades as WT spacing based on the rotor diameter is applied [37]. Usually, WTs use less than 10% of WF areas [66].
Other land use activities, such as agriculture or grazing, could persist between the WTs without hampering energy production and are only temporarily disturbed during construction [37]. Estimates of the direct disturbance footprint of a WT vary between 0.2 and 1.0 ha per WT or from 0.3 to 2.0 ha per MW [19,30,33,89], while the footprint, including the area not directly disturbed, can rise to 75 ha/MW [15].
Compared to other energy systems, wind energy has a minimal land footprint [62,66]. The average oil and gas footprint is 191% larger [19]. Solar photovoltaics has, on average, a much smaller spatial footprint than wind [41], and per energy unit produced, wind energy requires almost twice the footprint of oil and gas. The trade-off between energy production and environmental footprint depends partly on the energy yields in a particular landscape [72].
More research on the factual footprints of modern turbines is needed to estimate the extent of better-used land and land use changes [37,97]. Even if wind energy development results in relatively small habitat loss in a specific region, the footprint might stretch across the entire zone, fragmenting the habitat [19]. Appropriate strategies include proper siting, monitoring, and reducing the amount of land occupied [19], using already disturbed sites [19,72], or co-locating WTs with other forms of energy development or land use [37,62].
WT installations often result in land use changes that put increasing pressure on ecosystems such as peatlands [67]. Deforestation questions the greenhouse gas-related benefits of wind energy. A wind energy project in Scotland showed that after the clear-felling of a forest, 12 years of WT operation are required to repay the carbon payback [42]. Land use changes might evoke shifts in wind energy potential [47].
3.8. Impact Pathways in the Context of Social Acceptance (G)
The impact categories discussed (A–F) directly or indirectly affect human life and are commonly used as arguments for wind energy opposition. As impacts on wildlife (B, C) have gained importance among the motives for local opposition mainly due to cases of avifauna mortality [40], mitigating those impacts is essential for public acceptance [12]. The public is concerned about the potential effects of wind energy on biodiversity. It acknowledges the existing conflicts between wildlife conservation and climate protection [150], no matter whether or not people feel disturbed by WTs [99]. Residents often perceive collision with rotating WT blades as the most harmful threat to birds and bats. It may, therefore, attract more media attention [140] while less obvious but likely more severe impacts (e.g., habitat alterations) are rarely considered in the media [98]. Stakeholders are often highly concerned about issues related to biodiversity losses and claim for an ecologically sustainable energy transition that accepts revenue losses and project delays to resolve the green–green dilemma [150]. Ignoring these concerns may hinder effective collaborations and agreements among stakeholders. Wildlife impacts are an ongoing challenge in WF approval procedures and legal proceedings [111]. No other project types potentially causing higher bird collision mortality such as power lines, roads, or buildings have been controlled that strictly [79].
Noise pollution is among the primary motives for local opposition to WE in Europe [40], annoying [110], inducing potential health risks [40], and leading to a reduction in property values [55]. Both opponents and supporters mention noise impacts from WTs [63], but opponents might express higher annoyance over identical noise levels [49]. Psychological factors influence the perception and annoyance of noise as it is found to be increased with a negative attitude to the visual impact of WTs on the landscape [91] and reduced if people benefit economically from WTs [32]. Closing scientific and technological gaps on WT noise might reduce adverse reactions toward WFs [22].
Besides noise impacts, the visual impacts of turbines on landscapes influence the public perception of wind energy [66]. They are of high policy relevance [24] as basic arguments of wind power opposition are centered on aesthetics and visual degradation [10,40,111]. Visual impacts affect welfare measures [24] in terms of the economy, such as a loss of property value or limitations for tourism activity [40,55]. In general, economic interests strongly influence the local acceptance of WFs [40].
Although WE has been framed as a solution more often than a cause of a problem in recent years [25], existing environmental opposition to WE is expected to grow. It could limit WE expansion [10] unless local actors are convinced about the broader benefits for society and the environment [25]. Whether scientifically valid or not, perceptions of individuals may become “truth” to them [63].
Without clearly defined siting criteria, land use conflicts will stand in the way of energy transitions and planning initiatives [25,78], particularly in densely populated countries [99]. Social acceptance can be a powerful barrier to the diffusion of WE due to its ability to inhibit and delay the implementation of wind farm projects [40,78] and increase the risk of failures or cost escalation [43]. The lack of social acceptance is often explained as a result of the “Not in My Backyard” (NIMBY) effect, which describes the phenomenon that wind energy projects are accepted but only if it is implemented far from the backyard of residents [40]. Active wind energy critics, however, deliberately avoid the impression of being NIMBY people and use a set of arguments that cover a broad range of common reasonable goals that bear the potential for broad local support [116]. Local opposition occurs mainly if community involvement is lacking in WE permitting and development [40].
Social acceptance can be achieved by informing and involving a broad consortium of stakeholders, adopting a more integrated, collaborative planning process in the beginning, and providing incentives [43,63,107]. Combining a phased development with collaborative planning approaches could provide the required trust and social capital [79]. Wind energy supporters and opponents find fault with the turbines in one form or another, emphasizing the room for improvement [63]. However, WTs with minimal environmental impacts or environmental benefits that are aesthetically pleasing and contribute to local economics will, by and large, be socially accepted [43].
4. Conclusions
Wind energy is considered a central component of the global energy transition but is often criticized and rejected due to its potential environmental impact. In this systematic literature review, the state of knowledge, research gaps, and avoidance strategies of over 150 studies from more than 30 countries on potentially critical effects of wind energy were compiled and discussed in the context of social acceptance. A particular focus was placed on forests, where a significant expansion of wind energy is expected. The review provides an in-depth compilation of existing and lacking knowledge, mitigation strategies, and other drivers of negative effects and discusses critically the field of tension between installing wind turbines close to human or natural environments.
It is found that the overall quantity of studies varies considerably between different regions, and the focus of research varies from region to region. In most parts of the world, the social acceptance of wind energy is not addressed, while in Europe, it is of great relevance.
In principle, current research only allows for a few generalizable statements about the environmental impact of wind energy. Most adverse effects, particularly regarding the biotic environment, are not as clear-cut as they are presented in media, are not considered in a broader context of other anthropogenic influences, and could be avoided with adequate mitigation strategies. The direct mortality of birds and bats at wind turbines is a good example that shows that the most obvious and discussed effects are not necessarily the most severe. Effects mainly result from a complex interplay of multiple factors whose contributions are challenging to distinguish. For all impact pathways, local factors and the direct surroundings of a wind farm drive the occurrence and magnitude of effects. Therefore, the effects in forested areas are assumed to differ from those in agricultural or urban areas.
The reviewed literature provides no evidence that the biodiversity crisis is a direct consequence of mortality at wind farms and indicates that other anthropogenic impacts are likely much more influential. Land use changes and habitat alterations driving biodiversity decline are holistic anthropogenic problems in which wind energy is only an additional factor, if at all. Therefore, the question arises as to why wind energy expansion, in contrast to other human interventions, is so intensely debated and regulated in some parts of the world. It may be based on subjective perceptions that are not objectifiable.
In light of the findings of this study on various impact pathways and the persisting research gaps, arguments of wind power opponents should be discussed critically to differentiate between evidence and pseudo-arguments. More scientific research conducted in regions with an expected increase in onshore wind energy capacity has to provide scientific evidence confirming or falsifying arguments of wind energy opponents. Research must be improved by reinforcing before–after control–impact design for all impact pathways to investigate the temporal progress and spatial extent of effects.
In contrast to studies claiming the precautionary principle, wind power should not be categorically excluded as it is a cornerstone of sustainable energy generation, and adverse effects do not necessarily have to occur. To reinforce wind energy expansion, it has to be highlighted to make society more sustainable and minimize the critical environmental impacts of fossil fuels, such as climate change and air pollution.
Wind energy outperforms all other renewable energy sources in terms of power-generating capacity and environmental impacts [113]. More efficient siting prioritization concepts [163] must be applied instead of delaying and inhibiting planning processes. Installing and using wind energy facilities at sites with abundant wind resources might locally increase environmental impact but contribute to reducing overall impacts on humans, land use, or biodiversity by minimizing the number of required wind farms and mitigating climate change. In case of doubt, individual environmental impacts must be accepted to protect other, more critical assets and reduce cumulative impacts. The required expansion of wind energy is associated with trade-offs between humans and nature or urban and natural environments. However, the overall target of wind energy and other renewables is to protect both by mitigating climate change. Wind energy cannot be sacrificed, but it must be ensured that adverse effects are avoided as far as possible. Installing WTs in forests is unavoidable for many countries and might distract adverse effects from humans but could increase pressure on the natural environment. It should be noted that without wind energy, many of these ecosystems will not persist due to climate change. Therefore, the central future challenge is to ensure that the environmental impact, including climate change, can be minimized by maximizing the energy yield of renewable energy and reducing impacts from other human activities that do not strive for sustainability.
This review forms the basis for systematic, targeted research and wind energy expansion as it critically scrutinizes the significance and avoidance possibilities of different impacts and provides concrete deficits of the current research and proposals for further research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en17133098/s1, Table S1: Observed or estimated changes of meteorological variables and their intensity under specific conditions; Table S2: Summarized ranges of annual avian and chiropteran fatalities per turbine and fatalities per rated power and species affected in studies from different countries; Table S3: Cumulative country- or region-wide annual fatality of birds and bats; Table S4: Mortality numbers of birds (in millions) due to several anthropogenic causes in USA (unless otherwise stated); Table S5: Most frequently mentioned bird and bat species of wind energy-related studies; Table S6: Typical sound pressure level (SPL) values from onshore wind turbines depending on WT number, type, and the distance between facility and measurement.
Author Contributions
Conceptualization, L.S., C.J. and D.S.; methodology, L.S., C.J. and D.S.; software, L.S.; validation, L.S., C.J. and D.S.; formal analysis, L.S.; resources, L.S.; data curation, L.S.; writing—original draft preparation, L.S.; writing—review and editing, L.S., D.S. and C.J.; visualization, L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are available upon request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kim, S.K.; Park, S. Impacts of renewable energy on climate vulnerability: A global perspective for energy transition in a climate adaptation framework. Sci. Total Environ. 2023, 859, 160175. [Google Scholar] [CrossRef]
- Osman, A.I.; Chen, L.; Yang, M.; Msigwa, G.; Farghali, M.; Fawzy, S.; Rooney, D.W.; Yap, P.-S. Cost, environmental impact, and resilience of renewable energy under a changing climate: A review. Environ. Chem. Lett. 2023, 21, 741–764. [Google Scholar] [CrossRef]
- IRENA. Future of Wind: Deployment, Investment, Technology, Grid Integration and Socio-Economic Aspects. 2019. Available online: https://www.irena.org/publications/2019/Oct/Future-of-wind (accessed on 9 February 2024).
- Kandy, D.M.; Mörtberg, U.; Wretling, V.; Kuhlefelt, A.; Byström, G.; Polatidis, H.; Barney, A.; Balfors, B. Spatial multicriteria framework for sustainable wind-farm planning–Accounting for conflicts. Renew. Sustain. Energy Rev. 2024, 189, 113856. [Google Scholar] [CrossRef]
- Sayed, E.T.; Wilberforce, T.; Elsaid, K.; Rabaia, M.K.H.; Abdelkareem, M.A.; Chae, K.J.; Olabi, A.G. A critical review on environmental impacts of renewable energy systems and mitigation strategies: Wind, hydro, biomass and geothermal. Sci. Total Environ. 2021, 766, 144505. [Google Scholar] [CrossRef]
- GWEC. Global Wind Report 2023. Available online: https://gwec.net/globalwindreport2023/ (accessed on 9 February 2024).
- McKay, R.A.; Johns, S.E.; Bischof, R.; Matthews, F.; van der Kooij, J.; Yoh, N.; Eldegard, K. Wind energy development can lead to guild-specific habitat loss in boreal forest bats. Wildl. Biol. 2024, 2024, e01168. [Google Scholar] [CrossRef]
- Watson, J.E.; Evans, T.; Venter, O.; Williams, B.; Tulloch, A.; Stewart, C.; Thompson, I.; Ray, J.C.; Murray, K.; Salazar, A.; et al. The exceptional value of intact forest ecosystems. Nat. Ecol. Evol. 2018, 2, 599–610. [Google Scholar] [CrossRef]
- Agudelo, M.S.; Mabee, T.J.; Palmer, R.; Anderson, R. Post-construction bird and bat fatality monitoring studies at wind energy projects in Latin America: A summary and review. Heliyon 2021, 7, e07251. [Google Scholar] [CrossRef] [PubMed]
- Anshelm, J.; Simon, H. Power production and environmental opinions—Environmentally motivated resistance to wind power in Sweden. Renew. Sustain. Energy Rev. 2016, 57, 1545–1555. [Google Scholar] [CrossRef]
- Armstrong, A.; Burton, R.R.; Lee, S.E.; Mobbs, S.; Ostle, N.; Smith, V.; Waldron, S.; Whitaker, J. Ground-level climate at a peatland wind farm in Scotland is affected by wind turbine operation. Environ. Res. Lett. 2016, 11, 044024. [Google Scholar] [CrossRef]
- Arnett, E.B.; May, R.F. Mitigating Wind Energy Impacts on Wildlife: Approaches for Multiple Taxa. Hum.-Wildl. Interact. 2016, 10, 28–41. [Google Scholar]
- Baidya Roy, S.; Traiteur, J.J. Impacts of wind farms on surface air temperatures. Proc. Natl. Acad. Sci. USA 2010, 107, 17899–17904. [Google Scholar] [CrossRef] [PubMed]
- Baidya Roy, S.; Pacala, S.W.; Walko, R.L. Can large wind farms affect local meteorology? J. Geophys. Res. 2004, 109, D19101. [Google Scholar] [CrossRef]
- Balotari-Chiebao, F.; Brommer, J.E.; Saurola, P.; Ijäsc, A.; Laaksonen, T. Assessing space use by pre-breeding white-tailed eagles in the context of wind-energy development in Finland. Landsc. Urban Plan. 2018, 177, 251–258. [Google Scholar] [CrossRef]
- Balotari-Chiebao, F.; Valkama, J.; Byholm, P. Assessing the vulnerability of breeding bird populations to onshore wind-energy developments in Finland. Ornis. Fenn. 2021, 98, 59–73. [Google Scholar] [CrossRef]
- Balotari-Chiebao, F.; Santangeli, A.; Piirainen, S.; Byholm, P. Wind energy expansion and birds: Identifying priority areas for impact avoidance at a national level. Biol. Conserv. 2023, 277, 109851. [Google Scholar] [CrossRef]
- Bastos, R.; Pinhanços, A.; Santos, M.; Fernandes, R.F.; Vicente, J.R.; Morinha, F.; Honrado, J.P.; Travassos, P.; Barros, P.; Cabral, J.A. Model-assisted monitoring of biodiversity—Evaluating the regional cumulative impact of wind farms on birds: How can spatially explicit dynamic modelling improve impact assessments and monitoring? J. Appl. Ecol. 2016, 53, 1330–1340. [Google Scholar] [CrossRef]
- Baynard, C.W.; Mjachina, K.; Richardson, R.D.; Schupp, R.W.; Lambert, J.D.; Chibilyev, A.A. Energy Development in Colorado’s Pawnee National Grasslands: Mapping and Measuring the Disturbance Footprint of Renewables and Non-Renewables. Environ. Manag. 2017, 59, 995–1016. [Google Scholar] [CrossRef] [PubMed]
- Bennett, V.J.; Hale, A.A.; Williams, D.A. When the excrement hits the fan: Fecal surveys reveal species-specific bat activity at wind turbines. Mammal. Biol. 2017, 87, 125–129. [Google Scholar] [CrossRef]
- Bernard, E.; Paese, A.; Machado, R.B.; de Souza Aguiar, L.M. Blown in the wind: Bats and wind farms in Brazil. Nat. Conserv. 2014, 12, 106–111. [Google Scholar] [CrossRef]
- Bertagnolio, F.; Herr, M.; Madsen, K.D. A roadmap for required technological advancements to further reduce onshore wind turbine noise impact on the environment. Wiley Interdiscip. Rev. Energy Environ. 2023, 12, e469. [Google Scholar] [CrossRef]
- Betakova, V.; Vojar, J.; Sklenicka, P. Wind turbines location: How many and how far? Appl. Energy 2015, 151, 23–31. [Google Scholar] [CrossRef]
- Bishop, I.D. Evidence synthesis in landscape aesthetics: An honourable endeavour yet insufficient applicable knowledge. Socio-Ecol. Pract. Res. 2019, 1, 93–108. [Google Scholar] [CrossRef]
- Bjärstig, T.; Mancheva, I.; Zachrisson, A.; Neumann, W.; Svensson, J. Is large-scale wind power a problem, solution, or victim? A frame analysis of the debate in Swedish media. Energy Res. Soc. Sci. 2022, 83, 102337. [Google Scholar] [CrossRef]
- Blumendeller, E.; Kimmig, I.; Huber, G.; Rettler, P.; Cheng, P.W. Investigations on low frequency noises of onshore wind turbines. Acoustics 2020, 2, 353–365. [Google Scholar] [CrossRef]
- Bose, A.; Dürr, T.; Klenke, R.A.; Henle, K. Assessing the spatial distribution of avian collision risks at wind turbine structures in Brandenburg, Germany. Conserv. Sci. Pract. 2020, 2, e199. [Google Scholar] [CrossRef]
- Browning, E.; Barlow, K.E.; Burns, F.; Hawkins, C.; Boughey, K. Drivers of European bat population change: A review reveals evidence gaps. Mammal. Rev. 2021, 51, 353–368. [Google Scholar] [CrossRef]
- Buchholz, S.; Kelm, V.; Ghanem, S.J. Mono-specific plantations are valuable bat habits: Implication for wind turbine development. Eur. J. Wildl. Res. 2021, 67, 1. [Google Scholar] [CrossRef]
- Bunzel, K.; Bovet, J.; Thrän, D.; Eichhorn, M. Hidden outlaws in the forest? A legal and spatial analysis of onshore wind energy in Germany. Energy Res. Soc. Sci. 2019, 55, 14–25. [Google Scholar] [CrossRef]
- Cerri, J.; Fozzi, I.; De Rosa, D.; Aresu, M.; Apollonio, M.; Berlinguer, F. Griffon Vulture movements are concentrated around roost and supplementary feeding stations: Implications for wind energy development on Mediterranean islands. Glob. Ecol. Conserv. 2023, 47, e02651. [Google Scholar] [CrossRef]
- Chowdhury, N.E.; Shakib, M.A.; Xu, F.; Salehin, S.; Islam, M.R.; Bhuiyan, A.A. Adverse environmental impacts of wind farm installations and alternative research pathways to their mitigation. Clean Eng. Technol. 2022, 7, 100415. [Google Scholar]
- Coppes, J.; Braunisch, V.; Bollmann, K.; Storch, I.; Mollet, P.; Grünschachner-Berger, V.; Taubmann, J.; Suchant, R.; Nopp-Mayr, U. The impact of wind energy facilities on grouse: A systematic review. J. Ornithol. 2020, 161, 1–15. [Google Scholar] [CrossRef]
- Cryan, P.M.; Gorresen, P.M.; Hein, C.D.; Schirmacher, M.R.; Diehl, R.H.; Huso, M.M.; Hayman, D.T.S.; Fricker, P.D.; Bonaccorso, F.J.; Johnson, D.H.; et al. Behavior of bats at wind turbines. Proc. Natl. Acad. Sci. USA 2014, 11, 15126–15131. [Google Scholar] [CrossRef]
- Dai, K.; Bergot, A.; Liang, C.; Xiang, W.N.; Huang, Z. Environmental issues associated with wind energy: A review. Renew. Energy 2015, 75, 911–921. [Google Scholar] [CrossRef]
- Darabi, S.; Monavari, S.M.; Jozi, S.A.; Rahimi, R.; Vafaeinejad, A. Visual impact assessment of renewable energy developments with the application of multi-criteria decision-making method. Environ. Dev. Sustain. 2023, 25, 4437–4451. [Google Scholar] [CrossRef]
- Dhar, A.; Naeth, M.A.; Jennings, P.D.; El-Din, M.G. Perspectives on environmental impacts and a land reclamation strategy for solar and wind energy systems. Sci. Total Environ. 2020, 718, 134602. [Google Scholar] [CrossRef]
- Dhunny, A.Z.; Allam, Z.; Lobine, D.; Lollchund, M.R. Sustainable renewable energy planning and wind farming optimization from a biodiversity perspective. Energy 2019, 185, 1282–1297. [Google Scholar] [CrossRef]
- Diffendorfer, J.E.; Stanton, J.C.; Beston, J.A.; Thogmartin, W.E.; Loss, S.R.; Katzner, T.E.; Johnson, D.H.; Erickson, R.A.; Merrill, M.D.; Corum, M.D. Demographic and potential biological removal models identify raptor species sensitive to current and future wind energy. Ecosphere 2021, 12, e03531. [Google Scholar] [CrossRef]
- Diógenes, J.R.F.; Claro, J.; Rodrigues, J.C.; Loureiro, M.V. Barriers to onshore wind energy implementation: A systematic review. Energy Res. Soc. Sci. 2020, 60, 101337. [Google Scholar] [CrossRef]
- Dunnett, S.; Holland, R.A.; Taylor, G.; Eigenbrod, F. Predicted wind and solar energy expansion has minimal overlap with multiple conservation priorities across global regions. Proc. Natl. Acad. Sci. USA 2022, 119, e2104764119. [Google Scholar] [CrossRef]
- Enevoldsen, P. Onshore wind energy in Northern European forests: Reviewing the risks. Renew. Sustain. Energy Rev. 2016, 60, 1152–1262. [Google Scholar] [CrossRef]
- Enevoldsen, P.; Sovacool, B.K. Examining the social acceptance of wind energy: Practical guidelines for onshore wind project development in France. Renew. Sustain. Energy Rev. 2016, 53, 178–184. [Google Scholar] [CrossRef]
- Enevoldsen, P.; Valentine, S.V. Do onshore and offshore wind farm development patterns differ? Energy Sustain. Dev. 2016, 35, 41–51. [Google Scholar] [CrossRef]
- Erickson, W.P.; Wolfe, M.M.; Bay, K.J.; Johnson, D.H.; Gehring, J.L. A comprehensive analysis of small-passerine fatalities from collision with turbines at wind energy facilities. PLoS ONE 2014, 9, e107491. [Google Scholar] [CrossRef] [PubMed]
- Everaert, J. Collision risk and micro-avoidance rates of birds with wind turbines in Flanders. Bird Study 2014, 61, 220–230. [Google Scholar] [CrossRef]
- Fang, J.; Peringer, A.; Stupariu, M.S.; Pătru-Stupariu, I.; Buttler, A.; Golay, F.; Porté-Agel, F. Shifts in wind energy potential following land-use driven vegetation dynamics in complex terrain. Sci. Total Environ. 2018, 639, 374–384. [Google Scholar] [CrossRef]
- Farfán, M.A.; Duarte, J.; Muñoz, A.R.; Fa, J.E.; Vargas, J.M. Differential recovery of habitat use by birds after wind farm installation: A multi-year comparison. Environ. Impact Assess. Rev. 2017, 64, 8–15. [Google Scholar] [CrossRef]
- Fast, S.; Mabee, W. Place-making and trust-building: The influence of policy on host community responses to wind farms. Energy Policy 2015, 81, 27–37. [Google Scholar] [CrossRef]
- Fernández-Bellon, D.; Wilson, M.W.; O’Halloran, J. Effects of development of wind energy and associated changes in land use on bird densities in upland areas. Conserv. Biol. 2018, 33, 413–422. [Google Scholar] [CrossRef]
- Ferreira, D.; Freixo, C.; Cabral, J.A.; Santos, M. Is wind energy increasing the impact of socio-ecological change on Mediterranean mountain ecosystems? Insights from a modelling study relating wind power boost options with a declining species. J. Environ. Manag. 2019, 238, 283–295. [Google Scholar] [CrossRef]
- Foo, C.F.; Bennett, V.J.; Hale, A.M.; Korstian, J.M.; Schildt, A.J.; Williams, D.A. Increasing evidence that bats actively forage at wind turbines. PeerJ 2017, 5, e3985. [Google Scholar] [CrossRef]
- Frick, W.F.; Baerwald, E.F.; Pollock, J.F.; Barclay, R.M.R.; Szymanski, J.A.; Weller, T.J.; Russel, A.L.; Loeb, S.C.; Medellin, R.A.; McGuire, L.P. Fatalities at wind turbines may threaten population viability of a migratory bat. Biol. Conversat. 2017, 209, 172–177. [Google Scholar] [CrossRef]
- Friedenberg, N.A.; Frick, W.F. Assessing fatality minimization for hoary bats amid continued wind energy development. Biol. Conservat. 2021, 262, 109309. [Google Scholar] [CrossRef]
- García, J.H.; Cherry, T.L.; Kallbekken, S.; Torvanger, A. Willingness to accept local wind energy development: Does the compensation mechanism matter? Energy Policy 2016, 99, 165–173. [Google Scholar] [CrossRef]
- Gasparatos, A.; Doll, C.N.H.; Esteban, M.; Ahmed, A.; Olang, T.A. Renewable energy and biodiversity: Implications for transitioning to a Green Economy. Renew. Sustain. Energy Rev. 2017, 70, 161–184. [Google Scholar] [CrossRef]
- Gasparatos, A.; Ahmed, A.; Voigt, C. Facilitating policy responses for renewable energy and biodiversity. Trends Ecol. Evol. 2021, 36, 377–380. [Google Scholar] [CrossRef]
- Gauld, J.G.; Silva, J.P.; Atkinson, P.W.; Record, P.; Acácio, M.; Arkumarev, V.; Blas, J.; Bouten, W.; Burton, N.; Catry, I.; et al. Hotspots in the grid: Avian sensitivity and vulnerability to collision risk from energy infrastructure interactions in Europe and North Africa. J. Appl. Ecol. 2022, 59, 1496–1512. [Google Scholar] [CrossRef]
- Gaultier, S.P.; Blomberg, A.S.; Ijäs, A.; Vasko, V.; Vesterinen, E.J.; Brommer, J.E.; Lilley, T.M. Bats and wind farms: The role and importance of the Baltic Sea countries in the European context of power transition and biodiversity conservation. Environ. Sci. Technol. 2020, 54, 10385–10398. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.; Wilman, E.N.; Laurance, W.F. How Green is ‘Green’ Energy? Trends Ecol. Evol. 2017, 32, 922–935. [Google Scholar] [CrossRef]
- González, A.; Connell, P. Developing a renewable energy planning decision-support tool: Stakeholder input guiding strategic decisions. Appl. Energy 2022, 312, 118782. [Google Scholar] [CrossRef]
- Gorman, C.E.; Torsney, A.; Gaughran, A.; McKeon, C.M.; Farrell, C.A.; White, C.; Donohue, I.; Stout, J.C.; Buckley, Y.M. Reconciling climate action with the need for biodiversity protection, restoration and rehabilitation. Sci. Total Environ. 2023, 857, 159316. [Google Scholar] [CrossRef]
- Groth, T.M.; Vogt, C. Residents’ perceptions of wind turbines: An analysis of two townships in Michigan. Energy Policy 2014, 65, 251–260. [Google Scholar] [CrossRef]
- Guan, J. Landscape Visual Impact Evaluation for Onshore Wind Farm: A Case Study. ISPRS Int. J. Geo-Inf. 2022, 11, 594. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, X.; Du, S.; Li, C.; Siu, Y.L.; Rong, Y.; Yang, H. The impact of onshore wind power projects on ecological corridors and landscape connectivity in Shanxi, China. J. Clean Prod. 2020, 254, 120075. [Google Scholar] [CrossRef]
- Hamed, T.A.; Alshare, A. Environmental impact of solar and wind energy—A review. J. Sustain Dev. Energy Water Environ. Syst. 2022, 10, 1–23. [Google Scholar] [CrossRef]
- Heal, K.; Phin, A.; Waldron, S.; Flowers, H.; Bruneau, P.; Coupar, A.; Cundill, A. Wind farm development on peatlands increases fluvial macronutrient loading. Ambio 2020, 49, 442–459. [Google Scholar] [CrossRef] [PubMed]
- Heuck, C.; Hermann, C.; Levers, C.; Leitão, P.J.; Krone, O.; Brandl, R.; Albrecht, J. Wind turbines in high quality habitat cause disproportionate increases in collision mortality of the white-tailed eagle. Biol. Conserv. 2019, 236, 44–51. [Google Scholar] [CrossRef]
- Jacobson, M.Z.; Archer, C.L. Saturation wind power potential and its implications for wind energy. Proc. Natl. Acad. Sci. USA 2012, 109, 15679–15684. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.W.; Willis, C.K.R. Activity of tree bats at anthropogenic tall structures: Implications for mortality of bats at wind turbines. Anim. Behav. 2014, 97, 145–152. [Google Scholar] [CrossRef]
- Janhunen, S.; Hujala, M.; Pätäri, S. Owners of second homes, locals and their attitudes towards future rural wind farm. Energy Policy 2014, 73, 450–460. [Google Scholar] [CrossRef]
- Jones, N.F.; Pejchar, L.; Kiesecker, J.M. The Energy Footprint: How oil, natural gas, and wind energy affect land for biodiversity and the flow of ecosystem services. BioScience 2015, 65, 290–301. [Google Scholar] [CrossRef]
- Kati, V.; Kassara, C.; Vrontisi, Z.; Moustakas, A. The biodiversity-wind energy-land use nexus in a global biodiversity hotspot. Sci. Total Environ. 2021, 768, 144471. [Google Scholar] [CrossRef] [PubMed]
- Katzner, T.E.; Brandes, D.; Miller, T.; Lanzone, M.; Maisonneuve, C.; Tremblay, J.A.; Mulvihill, R.; Merovich, G.T., Jr. Topography drives migratory flight altitude of golden eagles: Implications for on-shore wind energy development. J. Appl. Ecol. 2012, 49, 1178–1186. [Google Scholar] [CrossRef]
- Katzner, T.; Bennett, V.; Miller, T.; Duerr, A.; Braham, M.; Hale, A. Wind Energy Development: Methods for Assessing Risks to Birds and Bats Pre-Construction. Hum.-Wildl. Interact. 2016, 10, 42–52. [Google Scholar]
- Katzner, T.E.; Nelson, D.M.; Diffendorfer, J.E.; Duerr, A.E.; Campbell, C.J.; Leslie, D.; Vander Zanden, H.B.; Yee, J.L.; Sur, M.; Huso, M.M.P.; et al. Wind energy: An ecological challenge. Science 2019, 366, 1206–1207. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, L.; Oldfield, I.F.; Park, K. Responses of bats to clear fell harvesting in Sitka Spruce plantations, and implications for wind turbine installation. For. Ecol. Manag. 2017, 395, 1–8. [Google Scholar] [CrossRef]
- Kokologos, D.; Tsitoura, I.; Kouloumpis, V.; Tsoutsos, T. Visual impact assessment method for wind parks: A case study in Crete. Land Use Policy 2014, 39, 110–120. [Google Scholar] [CrossRef]
- Köppel, J.; Dahmen, M.; Helfrich, J.; Schuster, E.; Bulling, L. Cautious but committed: Moving towards adaptive planning and operation strategies for renewable energy’s wildlife implications. Environ. Manag. 2014, 54, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Kumara, H.N.; Babu, S.; Rao, G.B.; Mahato, S.; Bhattacharya, M.; Rao, N.V.R.; Tamiliniyan, D.; Parengal, H.; Deepak, D.; Balakrishnan, A.; et al. Responses of birds and mammals to long-established wind farms in India. Sci. Rep. 2022, 12, 1339. [Google Scholar] [CrossRef]
- Laranjeiro, T.; May, R.; Verones, F. Impacts of onshore wind energy production on birds and bats: Recommendations for future life cycle impact assessment developments. Int. J. Life Cycle Assess. 2018, 23, 2007–2023. [Google Scholar] [CrossRef]
- Lemaître, J.; Lamarre, V. Effects of wind energy production on a threatened species, the Bicknell’s Thrush Catharus bicknelli, with and without mitigation. Bird Conserv. Int. 2020, 30, 194–209. [Google Scholar] [CrossRef]
- Li, G.; Yan, C.; Wu, H. Onshore wind farms do not affect global wind speeds or patterns. Heliyon 2023, 9, e12879. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Y. A review on wind turbine noise mechanism and de-noising techniques. Renew. Energy 2017, 108, 311–320. [Google Scholar] [CrossRef]
- Łopucki, R.; Perzanowski, K. Effects of wind turbines on spatial distribution of the European hamster. Ecol. Indic. 2018, 84, 433–436. [Google Scholar] [CrossRef]
- Loss, S.R.; Will, T.; Marra, P.P. Direct mortality of birds from anthropogenic causes. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 99–120. [Google Scholar] [CrossRef]
- Lundquist, J.K.; DuVivier, K.K.; Kaffine, D.; Tomaszewski, J.M. Costs and consequences of wind turbine wake effects arising from uncoordinated wind energy development. Nat. Energy 2019, 4, 26–34. [Google Scholar] [CrossRef]
- Luo, L.; Zhuang, Y.; Duan, Q.; Dong, L.; Yu, Y.; Liu, Y.; Chen, K.; Gao, X. Local climatic and environmental effects of an onshore wind farm in North China. Agric. Meteorol. 2021, 308, 108607. [Google Scholar] [CrossRef]
- Ma, B.; Yang, J.; Chen, X.; Zhang, L.; Zeng, W. Revealing the ecological impact of low-speed mountain wind power on vegetation and soil erosion in South China: A case study of a typical wind farm in Yunnan. J. Clean. Prod. 2023, 419, 138020. [Google Scholar] [CrossRef]
- MacGregor, K.A.; Lemaître, J. The management utility of large-scale environmental drivers of bat mortality at wind energy facilities: The effects of facility size, elevation and geographic location. Glob. Ecol. Conserv. 2020, 21, e00871. [Google Scholar] [CrossRef]
- Maehr, A.M.; Watts, G.R.; Hanratty, J.; Talmi, D. Emotional response to images of wind turbines: A psychophysiological study of their visual impact on the landscape. Landsc. Urban Plan 2015, 142, 71–79. [Google Scholar] [CrossRef]
- Manchado, C.; Gomez-Jauregui, V.; Lizcano, P.E.; Iglesias, A.; Galvez, A.; Otero, C. Wind farm repowering guided by visual impact criteria. Renew. Energy 2019, 135, 197–207. [Google Scholar] [CrossRef]
- Martínez-Martínez, Y.; Dewulf, J.; Casas-Ledón, Y. GIS-based site suitability analysis and ecosystem services approach for supporting renewable energy development in south-central Chile. Renew. Energy 2022, 182, 363–376. [Google Scholar] [CrossRef]
- Marvel, K.; Kravitz, B.; Caldeira, K. Geophysical limits to global wind power. Nat. Clim. Chang. 2013, 3, 118–121. [Google Scholar] [CrossRef]
- May, R.F. A unifying framework for the underlying mechanisms of avian avoidance of wind turbines. Biol. Conservat. 2015, 190, 179–187. [Google Scholar] [CrossRef]
- May, R.; Reitan, O.; Bevanger, K.; Lorentsen, S.H.; Nygård, T. Mitigating wind-turbine induced avian mortality: Sensory, aerodynamic and cognitive constraints and options. Renew. Sustain. Energy Rev. 2015, 42, 170–181. [Google Scholar] [CrossRef]
- May, R.; Jackson, C.R.; Middel, H.; Stokke, B.G.; Verones, F. Global life-cycle impacts of onshore wind-power plants on bird richness. Environ. Sustain. Indic. 2020, 8, 100080. [Google Scholar] [CrossRef]
- May, R.; Middel, H.; Stokke, B.G.; Jackson, C.; Verones, F. Life-cycle impacts of wind energy development on bird diversity in Norway. Environ. Impact Assess. Rev. 2021, 90, 106635. [Google Scholar] [CrossRef]
- Meyerhoff, J. Do turbines in the vicinity of respondents’ residences influence choices among programmes for future wind power generation? J. Choice Model. 2013, 7, 58–71. [Google Scholar] [CrossRef]
- Millon, L.; Julien, J.F.; Julliard, R.; Kerbiriou, C. Bat activity in intensively farmed landscapes with wind turbines and offset measures. Ecol. Eng. 2015, 75, 250–257. [Google Scholar] [CrossRef]
- Morkūne, R.; Marciukaitis, M.; Jurkin, V.; Gecevicius, G.; Morkunas, J.; Raudonikis, L.; Markevičius, A.; Narščius, A.; Gasiūnaitė, Z.R. Wind energy development and wildlife conservation in Lithuania: A mapping tool for conflict assessment. PLoS ONE 2020, 15, e0227735. [Google Scholar] [CrossRef]
- Msigwa, G.; Ighalo, J.O.; Yap, P.S. Considerations on environmental, economic, and energy impacts of wind energy generation: Projections towards sustainability initiatives. Sci. Total Environ. 2022, 849, 157755. [Google Scholar] [CrossRef]
- Müller, J.; Brandl, R.; Buchner, J.; Pretzsch, H.; Seifert, S.; Strätz, C.; Veith, M.; Fenton, B. From ground to above canopy—Bat activity in mature forests is driven by vegetation density and height. For. Ecol. Manag. 2013, 306, 179–184. [Google Scholar] [CrossRef]
- Ottinger, G.; Hargrave, T.J.; Hopson, E. Procedural justice in wind facility siting: Recommendations for state-led siting processes. Energy Policy 2014, 65, 662–669. [Google Scholar] [CrossRef]
- Palmer, J.F. The contribution of key observation point evaluation to a scientifically rigorous approach to visual impact assessment. Landsc. Urban Plan. 2019, 183, 100–110. [Google Scholar] [CrossRef]
- Pearse, A.T.; Metzger, K.L.; Brandt, D.A.; Shaffer, J.A.; Bidwell, M.T.; Harrell, W. Migrating Whooping Cranes avoid wind-energy infrastructure when selecting stopover habitat. Ecol. Appl. 2021, 31, e02324. [Google Scholar] [CrossRef]
- Pepermans, Y.; Loots, I. Wind farm struggles in Flanders fields: A sociological perspective. Energy Policy 2013, 59, 321–328. [Google Scholar] [CrossRef]
- Pescador, M.; Gómez Ramírez, J.I.; Peris, S.J. Effectiveness of a mitigation measure for the lesser kestrel (Falco naumanni) in wind farms in Spain. J. Environ. Manag. 2019, 231, 919–925. [Google Scholar] [CrossRef]
- Piasecka, I.; Tomporowski, A.; Flizikowski, J.; Kruszelnicka, W.; Kasner, R.; Mrozinski, A. Life Cycle Analysis of Ecological Impacts of an Offshore and a Land-Based Wind Power Plant. Appl. Sci. 2019, 9, 231. [Google Scholar] [CrossRef]
- Pohl, J.; Hübner, G.; Mohs, A. Acceptance and stress effects of aircraft obstruction markings of wind turbines. Energy Policy 2012, 50, 592–600. [Google Scholar] [CrossRef]
- Ponitka, J.; Boettner, S. Challenges of future energy landscapes in Germany—A nature conservation perspective. Energy Sustain. Soc. 2020, 10, 17. [Google Scholar] [CrossRef]
- Qin, Y.; Li, Y.; Xu, R.; Hou, C.; Armstrong, A.; Bach, E.; Wang, Y.; Fu, B. Impacts of 319 wind farms on surface temperature and vegetation in the United States. Environ. Res. Lett. 2022, 17, 024026. [Google Scholar] [CrossRef]
- Rahman, A.; Farrok, O.; Haque, M.M. Environmental impact of renewable energy source based electrical power plants: Solar, wind, hydroelectric, biomass, geothermal, tidal, ocean, and osmotic. Renew. Sustain. Energy Rev. 2022, 161, 112279. [Google Scholar] [CrossRef]
- Rehbein, J.A.; Waston, J.E.M.; Lane, J.L.; Sonter, L.J.; Venter, O.; Atkinson, S.C.; Allan, J.R. Renewable energy development threatens many globally important biodiversity areas. Glob. Chang. Biol. 2020, 26, 3040–3051. [Google Scholar] [CrossRef]
- Reusch, C.; Lozar, M.; Kramer-Schadt, S.; Voigt, C.C. Coastal onshore wind turbines lead to habitat loss for bats in Northern Germany. J. Environ. Manag. 2022, 310, 114715. [Google Scholar] [CrossRef]
- Reusswig, F.; Braun, F.; Heger, I.; Ludewig, T.; Eichenauer, E.; Lass, W. Against the wind: Local opposition to the German Energiewende. Util. Policy 2016, 41, 214–227. [Google Scholar] [CrossRef]
- Rnjak, D.; Janeš, M.; Križan, J.; Antonić, O. Reducing bat mortality at wind farms using site-specific mitigation measures: A case study in the Mediterranean region, Croatia. Mammalia 2023, 87, 259–270. [Google Scholar] [CrossRef]
- Robinson Willmott, J.; Forcey, G.M.; Hooton, L.A. Developing an automated risk management tool to minimize bird and bat mortality at wind facilities. Ambio 2015, 44, 557–571. [Google Scholar] [CrossRef]
- Roemer, C.; Disca, T.; Coulon, A.; Bas, Y. Bat flight height monitored from wind masts predicts mortality risk at wind farms. Biol. Conserv 2017, 215, 116–122. [Google Scholar] [CrossRef]
- Roemer, C.; Bas, Y.; Disca, T.; Coulon, A. Influence of landscape and time of year on bat-wind turbines collision risks. Landsc. Ecol. 2019, 34, 2869–2881. [Google Scholar] [CrossRef]
- Rydell, J.; Bogdanowicz, W.; Boonman, A.; Petterson, S.; Suchecka, E.; Pomorski, J.J. Bats may eat diurnal flies that rest on wind turbines. Mamm. Biol. 2016, 81, 331–339. [Google Scholar] [CrossRef]
- Santangeli, A.; Toivonen, T.; Montesino Pouzols, F.; Pogson, M.; Hastings, A.; Smith, P.; Moilanen, A. Global change synergies and trade-offs between renewable energy and biodiversity. Glob. Chang. Biol. Bioenergy 2016, 8, 941–951. [Google Scholar] [CrossRef]
- Santos, C.D.; Ramesh, H.; Ferraz, R.; Franco, A.; Wikelski, M. Factors influencing wind turbine avoidance behaviour of a migrating soaring bird. Sci. Rep. 2022, 12, 6441. [Google Scholar] [CrossRef] [PubMed]
- Schaub, M. Spatial distribution of wind turbines is crucial for the survival of red kite populations. Biol. Conserv. 2012, 155, 111–118. [Google Scholar] [CrossRef]
- Schmuecker, S.J.; Becker, D.A.; Lanzone, M.J.; Fogg, B.; Romano, S.P.; Katzner, T.E.; Miller, T.A. Use of Upland and Riparian Areas by Wintering Bald Eagles and Implications for Wind Energy. J. Wildl. Manag. 2020, 84, 1578–1589. [Google Scholar] [CrossRef]
- Schöll, E.M.; Nopp-Mayr, U. Impact of wind power plants on mammalian and avian wildlife species in shrub- and woodlands. Biol. Conserv. 2021, 256, 109037. [Google Scholar] [CrossRef]
- Schuster, E.; Bulling, L.; Köppel, J. Consolidating the State of Knowledge: A synoptical review of wind energy’s wildlife effects. Environ. Manag. 2015, 56, 300–331. [Google Scholar] [CrossRef] [PubMed]
- Sebastián-González, E.; Pérez-García, J.M.; Carrete, M.; Donázar, J.A.; Sánchez-Zapata, J.A. Using network analysis to identify indicator species and reduce collision fatalities at wind farms. Biol. Conserv 2018, 224, 209–212. [Google Scholar] [CrossRef]
- Serrano, D.; Margalida, A.; Pérez-García, J.M.; Juste, J.; Traba, J.; Valera, F.; Carrete, M.; Aihartza, J.; Real, J.; Mañosa, S.; et al. Renewables in Spain threaten biodiversity. Science 2020, 370, 1282–1283. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Cabral, J.A.; Hughes, S.J.; Santos, M. A modelling framework to predict bat activity patterns on wind farms: An outline of possible applications on mountain ridges of North Portugal. Sci. Total Environ. 2017, 581, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Sklenicka, P.; Zouhar, J. Predicting the visual impact of onshore wind farms via landscape indices: A method for objectivizing planning and decision processes. Appl. Energy 2018, 209, 445–454. [Google Scholar] [CrossRef]
- Slawsky, L.M.; Zhou, L.; Baidya Roy, S.; Xia, G.; Vuille, M.; Harris, R.A. Observed thermal impacts of wind farms over northern Illinois. Sensors 2015, 15, 14981–15005. [Google Scholar] [CrossRef]
- Smallwood, K.S.; Bell, D.A. Effects of wind turbine curtailment on bird and bat fatalities. J. Wildl. Manag. 2020, 84, 685–694. [Google Scholar] [CrossRef]
- Sorkhabi, S.Y.D.; Romero, D.A.; Yan, G.K.; Gu, M.D.; Moran, J.; Morgenroth, M.; Amon, C.H. The impact of land use constraints in multi-objective energy-noise wind farm layout optimization. Renew. Energy 2016, 85, 359–370. [Google Scholar] [CrossRef]
- Sovacool, B.K. The avian and wildlife costs of fossil fuels and nuclear power. J. Integr. Environ. Sci. 2012, 9, 255–278. [Google Scholar] [CrossRef]
- Sovacool, B.K. The avian benefits of wind energy: A 2009 update. Renew. Energy 2013, 49, 19–24. [Google Scholar] [CrossRef]
- Stantial, M.L.; Cohen, J.B. Estimating flight height and flight speed of breeding Piping Plovers. J. Field Ornithol. 2015, 86, 369–377. [Google Scholar] [CrossRef]
- Tang, B.; Wu, D.; Zhao, X.; Zhou, T.; Zhao, W.; Wei, H. The observed impacts of wind farms on local vegetation growth in northern China. Remote Sens 2017, 9, 332. [Google Scholar] [CrossRef]
- Taubmann, J.; Kämmerle, J.L.; Andrén, H.; Braunisch, V.; Storch, I.; Fiedler, W.; Suchant, R.; Coppes, J. Wind energy facilities affect resource selection of capercaillie Tetrao urogallus. Wildl. Biol. 2021, 2021, 1–13. [Google Scholar] [CrossRef]
- Tesfahunegny, W.; Datiko, D.; Wale, M.; Hailay, G.E.; Hunduma, T. Impact of wind energy development on birds and bats: The case of Adama wind farm, Central Ethiopia. J. Basic Appl. Zoöl. 2020, 81, 41. [Google Scholar] [CrossRef]
- Thaxter, C.B.; Buchanan, G.M.; Carr, J.; Butchart, S.H.M.; Newbold, T.; Green, R.E.; Tobias, J.A.; Foden, W.B.; O`Brien, S.; Pearce-Higgins, J.W. Bird and bat species’ global vulnerability to collision mortality at wind farms revealed through a trait-based assessment. Royal. R. Soc. B. 2017, 284, 20170829. [Google Scholar] [CrossRef]
- Thomas, K.A.; Jarchow, C.J.; Arundel, T.R.; Jamwal, P.; Borens, A.; Drost, C.A. Landscape-scale wildlife species richness metrics to inform wind and solar energy facility siting: An Arizona case study. Energy Policy 2018, 116, 145–152. [Google Scholar] [CrossRef]
- Thompson, M.; Beston, J.A.; Etterson, M.; Diffendorfer, J.A.; Loss, S.R. Factors associated with bat mortality at wind energy facilities in the United States. Biol. Conserv. 2017, 215, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, H.; Rytkönen, S.; Karlin, O.P.; Ollila, T.; Pakanen, V.M.; Tuohimaa, H.; Orell, M. Modelling golden eagle habitat selection and flight activity in their home ranges for safer wind farm planning. Env. Impact Assess. Rev. 2018, 71, 120–131. [Google Scholar] [CrossRef]
- Urziceanu, M.; Anastasiu, P.; Rozylowicz, L.; Sesan, T.E. Local-scale impact of wind energy farms on rare, endemic, and threatened plant species. PeerJ. 2021, 9, e11390. [Google Scholar] [CrossRef] [PubMed]
- Veers, P.; Dykes, K.; Lantz, E.; Barth, S.; Bottasso, C.L.; Carlson, O.; Clifton, A.; Green, J.; Green, P.; Holttinen, H. Grand challenges in the science of wind energy. Science 2019, 366, eaau2027. [Google Scholar] [CrossRef] [PubMed]
- Vignali, S.; Lörcher, F.; Hegglin, D.; Arlettaz, R.; Braunisch, V. A predictive flight-altitude model for avoiding future conflicts between an emblematic raptor and wind energy development in the Swiss Alps. R. Soc. Open Sci. 2022, 9, 211041. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Patraca, R.; Macgregor-Fors, I.; Ortiz-Martínez, T.; Pérez-Sánchez, C.E.; Herrera-Alsina, L.; Muñoz-Robles, C. Bird-community shifts in relation to wind farms: A case study comparing a wind farm, croplands, and secondary forests in Southern Mexico. Condor 2012, 114, 711–719. [Google Scholar] [CrossRef]
- Voigt, C.C.; Popa-Lisseanu, A.G.; Niermann, I.; Kramer-Schadt, S. The catchment area of wind farms for European bats: A plea for international regulations. Biological Conserv. 2012, 153, 80–86. [Google Scholar] [CrossRef]
- Voigt, C.C.; Straka, T.M.; Fritze, M. Producing wind energy at the cost of biodiversity: A stakeholder view on a green-green dilemma. J. Renew. Sustain. Energy 2019, 11, 063303. [Google Scholar] [CrossRef]
- Wang, S.; Wang, S. Impacts of wind energy on environment: A review. Renew. Sustain. Energy Rev. 2015, 49, 437–443. [Google Scholar] [CrossRef]
- Wang, S.; Wang, S.; Smith, P. Ecological impacts of wind farms on birds: Questions, hypotheses, and research needs. Renew. Sustain. Energy Rev. 2015, 44, 599–607. [Google Scholar] [CrossRef]
- Wang, G.; Li, G.; Liu, Z. Wind farms dry surface soil in temporal and spatial variation. Sci. Total Environ. 2023, 857, 159293. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.T.; Kolar, P.S.; Ferrer, M.; Nygård, T.; Johnston, N.; Hunt, W.G.; Smit-Robinson, H.A.; Farmer, C.J.; Huso, M.; Katzner, T.E. Raptor interactions with wind energy: Case studies from around the world. J. Raptor Res. 2018, 52, 1–18. [Google Scholar] [CrossRef]
- Wawrzyczek, J.; Lindsay, R.; Metzger, M.J.; Quétier, F. The ecosystem approach in ecological impact assessment: Lessons learned from windfarm developments on peatlands in Scotland. Environ. Impact Assess. Rev. 2018, 72, 157–165. [Google Scholar] [CrossRef]
- Wellig, S.D.; Nusslé, S.; Miltner, D.; Kohle, O.; Glaizot, O.; Braunisch, V.; Obrist, M.K.; Arlettaz, R. Mitigating the negative impacts of tall wind turbines on bats: Vertical activity profiles and relationships to wind speed. PLoS ONE 2018, 13, e0192493. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.W.; Fernández-Bellon, D.; Irwin, S.; O’Halloran, J. Hen Harrier Circus cyaneus population trends in relation to wind farms. Bird Study 2017, 64, 20–29. [Google Scholar] [CrossRef]
- Wu, X.; Hu, W.; Huang, Q.; Chen, C.; Jacobson, M.Z.; Chen, Z. Optimizing the layout of onshore wind farms to minimize noise. Appl. Energy 2020, 267, 114896. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, H.; Zhong, S.; Song, N.; Liu, N.; Wang, Z.; Li, B.; Wang, T. Risk evaluation of onshore wind farms in relation to wild duck (Anatidae) movements in the Yangtze River Mouth, China. IET Renew. Power Gener. 2021, 16, 470–477. [Google Scholar] [CrossRef]
- Zwart, M.C.; Robson, P.; Rankin, S.; Whittingham, M.J.; McGowan, P.J.K. Using environmental impact assessment and post-construction monitoring data to inform wind energy developments. Ecosphere 2015, 6, 26. [Google Scholar] [CrossRef]
- IRENA. Renewable Capacity Statistics 2023. Available online: https://www.irena.org/Publications/2023/Mar/Renewable-capacity-statistics-2023 (accessed on 20 November 2023).
- Selkimäki, M.; Riippi, J.; Rana, P.; Lamula, L.; Antila, M.; Heinonen, T.; Tokola, T. Forest landscape shield models for assessing audio-visual disturbances of wind turbines. J. Env. Manag. 2024, 352, 120070. [Google Scholar] [CrossRef] [PubMed]
- Sander, L.; Jung, C.; Schindler, D. New concept of renewable energy priority zones for efficient onshore wind and solar expansion. Energy Convers. Manag. 2023, 294, 117575. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).