Evaluation of Biohydrogen Production Depending on the Substrate Used—Examples for the Development of Green Energy
Abstract
1. Introduction
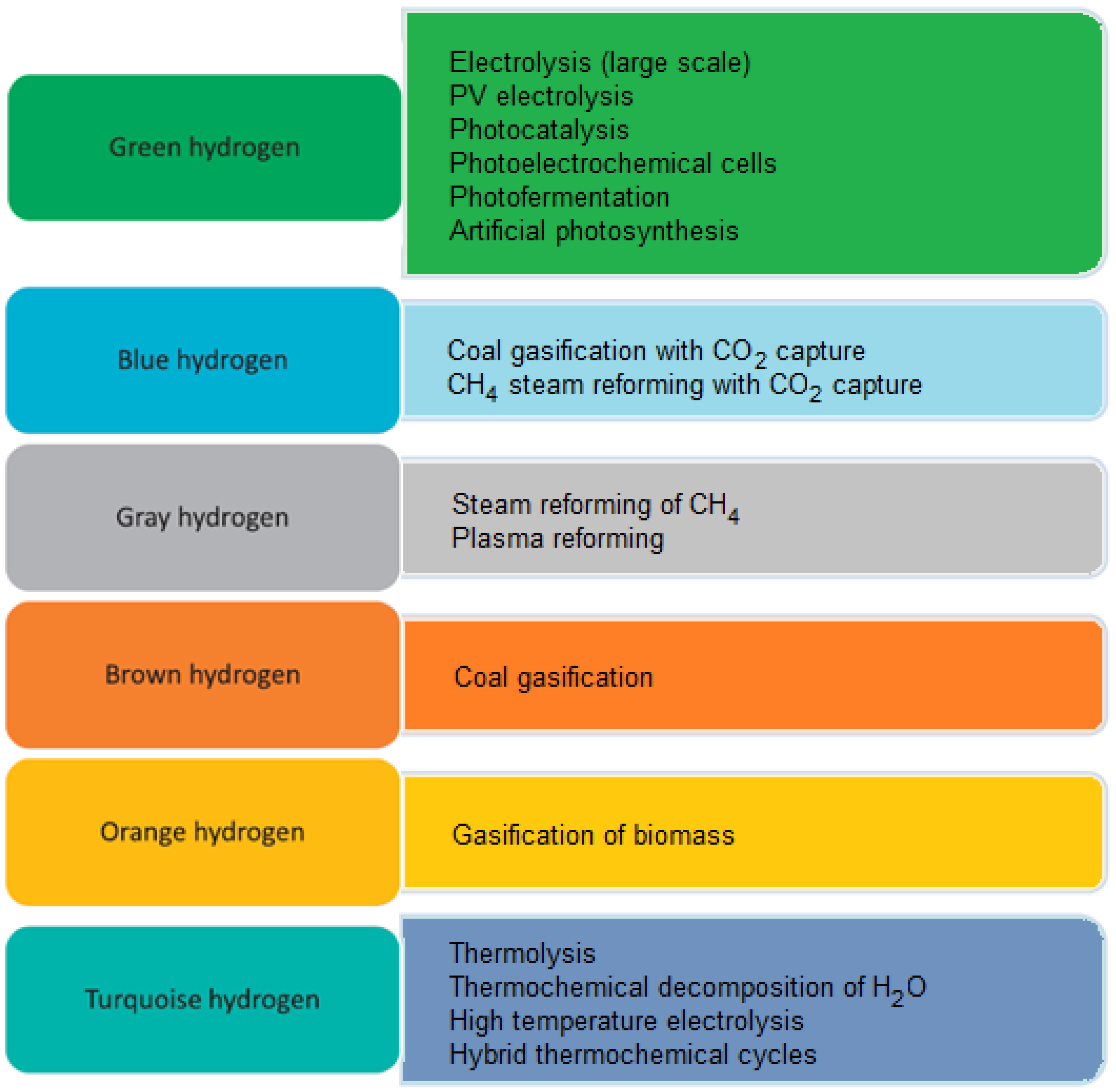
1.1. Aspects of the Production of “Colored” Hydrogen and Biohydrogen
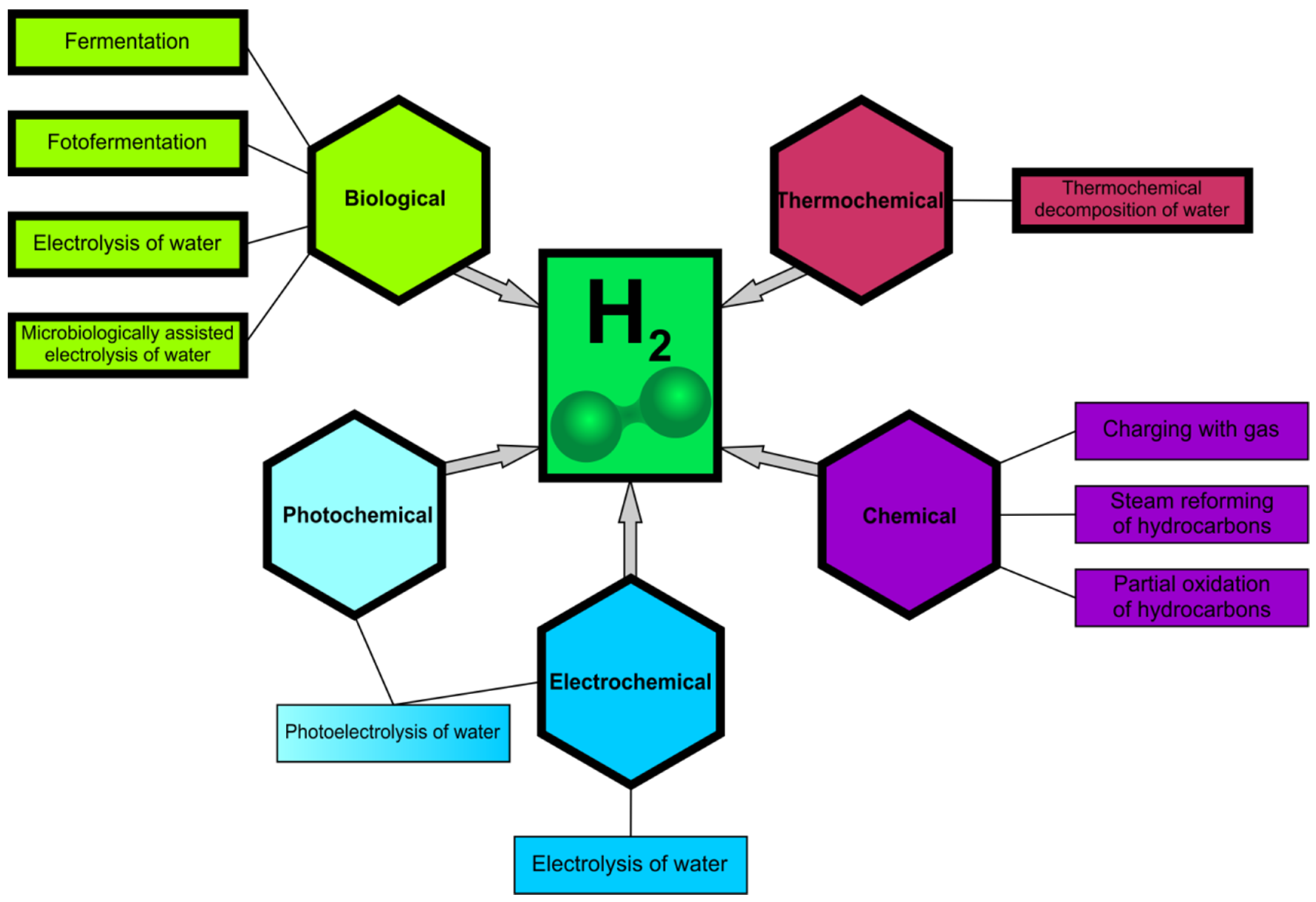
- Large-scale H2O electrolysis powered by electricity from various renewable sources.
- Photoelectrochemical cells (PEC) [19] based on a chain of transformations initiated by sunlight in an aqueous environment. PEC uses photoactive semiconductors that create an electrode and absorb light (energy) to enable H2O catalysis using sunlight and the production of gaseous H2 and oxygen (O2).
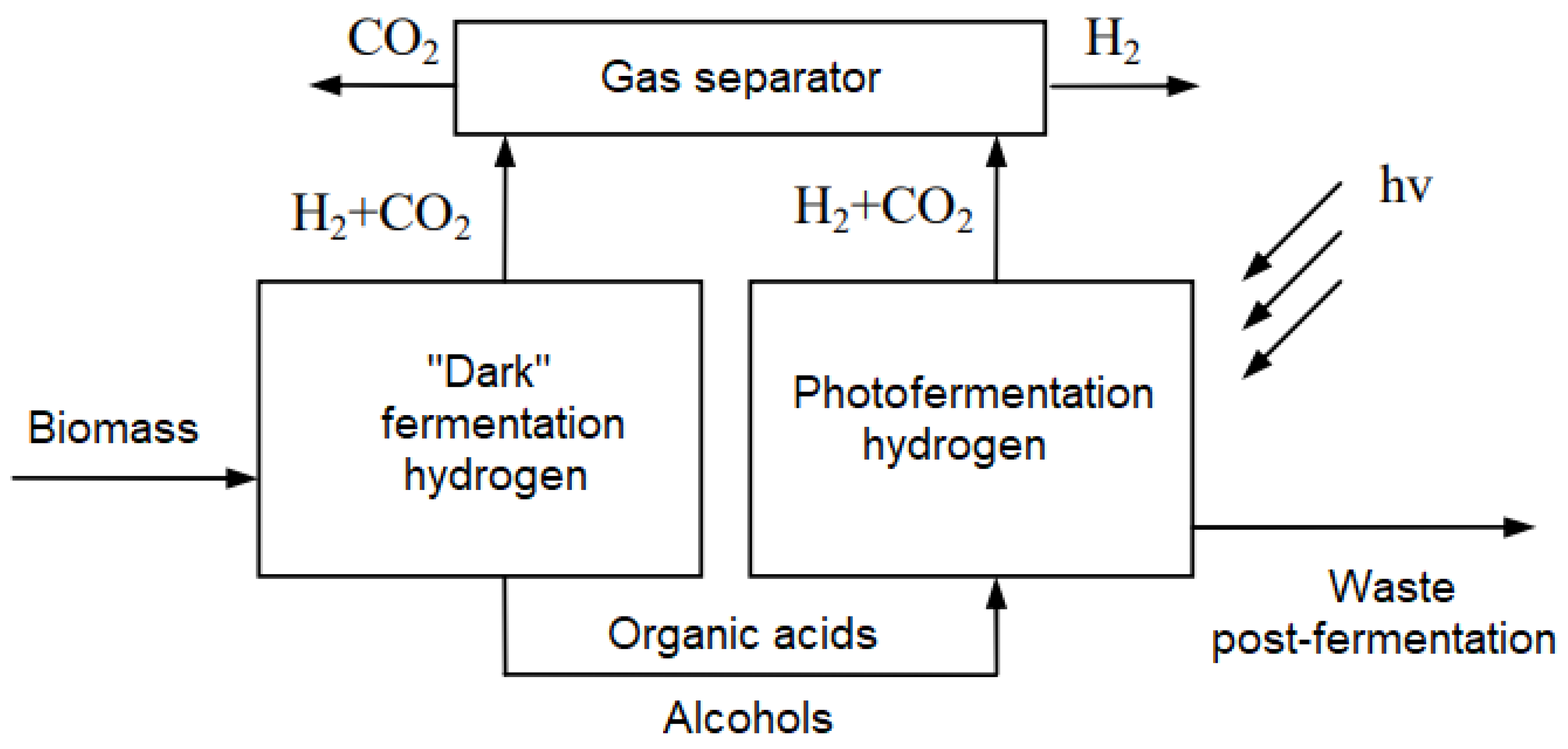
- Photofermentation [20], which uses bacteria for anaerobic fermentation to produce H2 from organic acids under the influence of light (natural or artificial).
- Photocatalysis based on the use of an activated and light-powered catalyst, which creates electron-hole pairs, allowing H2O electrolysis to begin, similar to PEC. This process is subject to numerous limitations (e.g., efficiency or speed), and research into its better understanding is ongoing [17].
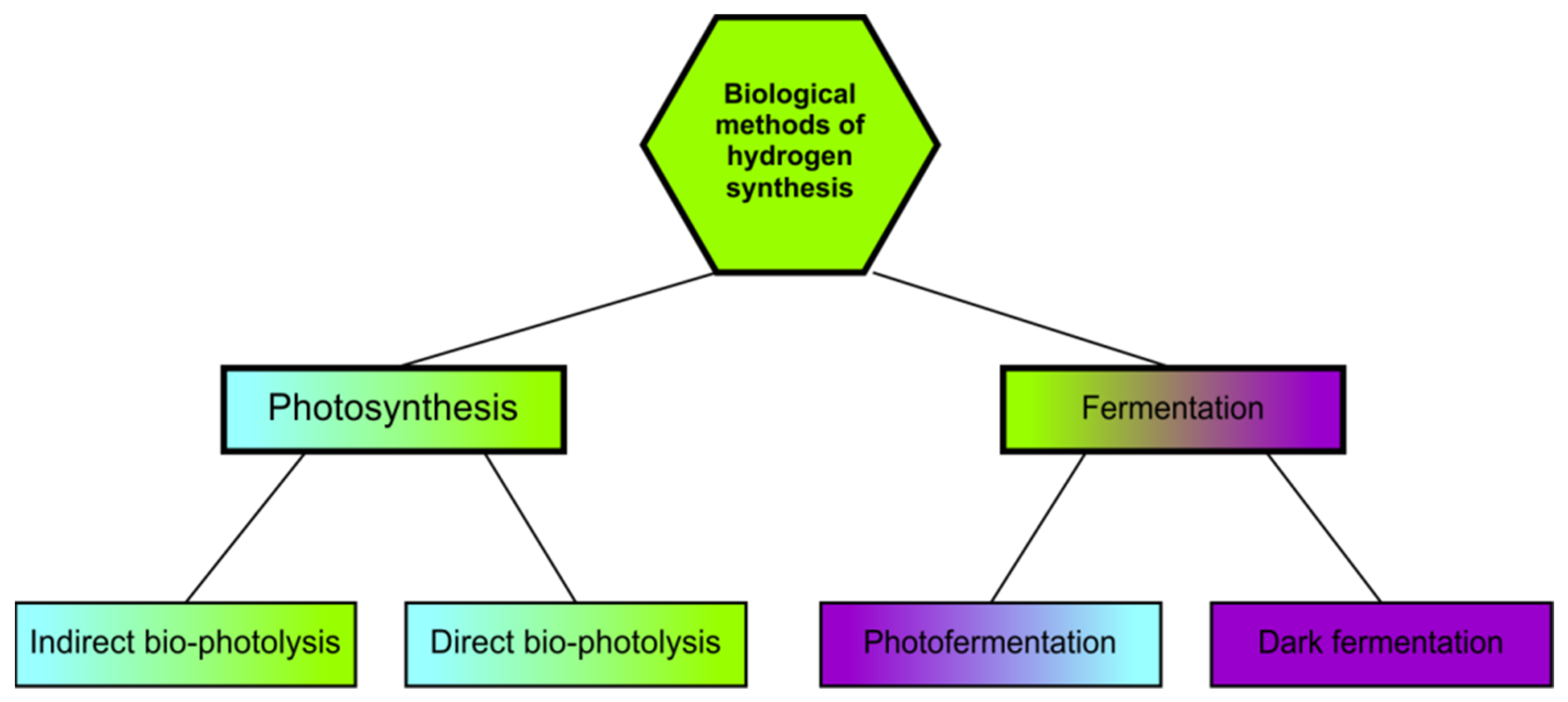
- -
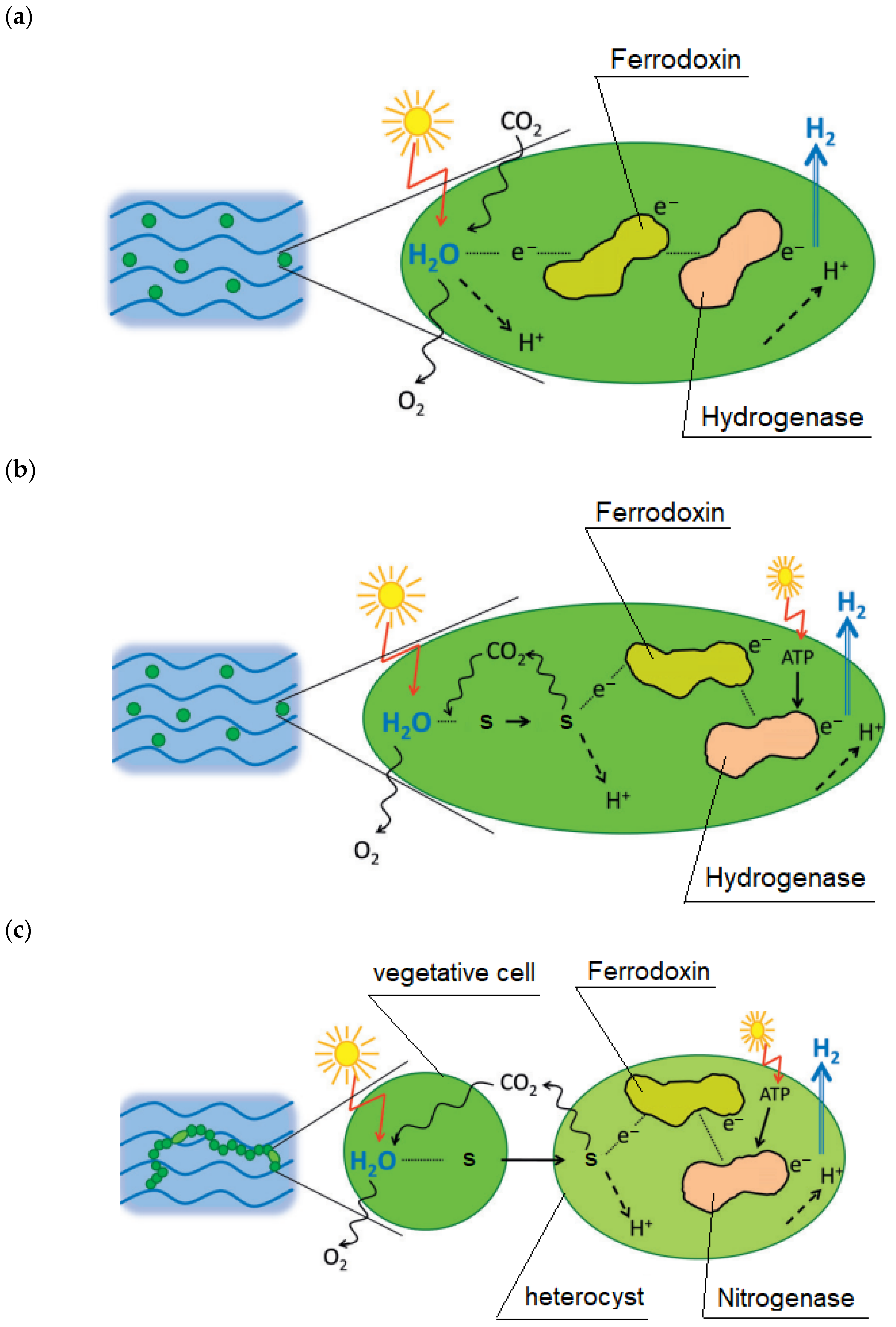
- H2O photolysis;
- -
- Transfer of electrons from photosystems to a protein called ferrhodoxin, which is then a direct donor of electrons transferred to protons;
- -
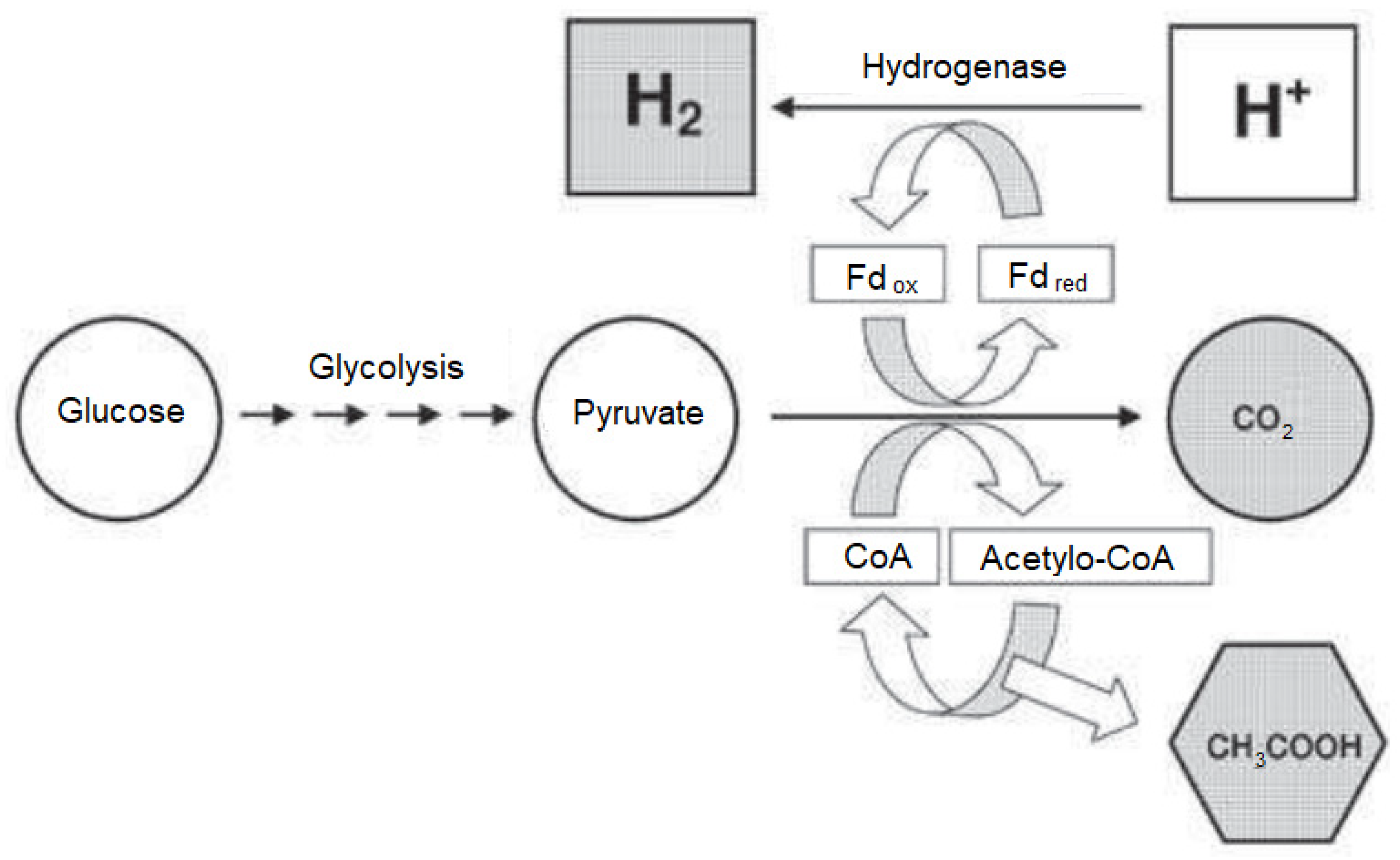
- Biohydrogen synthesis is catalyzed by hydrogenase.
1.2. Biohydrogen Status on Continents (America, Africa, Asia, Australia and Oceania)
1.3. Status of Biohydrogen in Europe
1.4. The Status of Biohydrogen in Poland
- -
- Jastrzębska Spółka Węglowa (JSW)—the separation of H2 from coke oven gas;
- -
- Lotos, Polskie Sieci Elektroenergetyczne—Polish Power Grids (PSE)—the use of electrolyzers with electricity from renewable energy sources (RES);
- -
- Sescom sales—electrolyzers powered by PV;
- -
- Grup Azoty—scaling its own production of “gray” H2 for sale;
- -
- Polenergia—production and use of “green” H2—cogeneration converted to burn H2;
- -
- RB Consulting—the distribution of electrolyzers;
- -
- Pątnów Adamów Konin Power Plant Complex (ZE PAK)—the use of electrolyzers with electricity from biomass;
- -
- Orlen—the use of electrolyzers with electricity from RES;
- -
- Tauron Wytwarzanie—the production of SNG (synthetic natural gas): H2 from electrolysis with electricity from RES and CO2 from emission installations;
- -
- Wałbrzyskie Zakłady Koksownicze “Victoria”—the separation of H2 from coke oven gas;
- -
- Stalprodukt—steam CH4 reforming.
- (1)
- Potentially an excellent energy carrier (Power to Gas—P2G);
- (2)
- A practical energy carrier in the circular economy (CE).
- (1)
- The Energy Policy of Poland until 2040 (specifically, objective 4. Development of energy markets (development of electromobility and alternative fuels));
- (2)
- The Polish Hydrogen Strategy to 2030 with a perspective to 2040 (development of 32 H2 refueling stations and the use of RES for H2 production based on electrolysis (the capacity of electrolyzers will reach 2 GW in 2030));
- (3)
- The Plan for the Development of Electromobility in Poland “Energy for the Future” (improvement of energy security, improvement of air quality and creation of conditions for the development of the electromobility of Poles);
- (4)
- The UN Sustainable Development Goals (Goal 13 Climate Action (reducing CO2 emissions and reducing global warming));
- (5)
- The direction of the Łukasiewicz Research Network: A sustainable economy and energy. The subjects of research projects include the clean and efficient manufacturing, transmission and storage of energy and the effective use of surplus energy from RES, energy from waste and alternative fuels (in particular, H2 technologies).
2. Results and Discussion
- (1)
- The mass percentage of H2, the H2/CO molar ratio and the hydrogen yield were the highest in the steam gasification process. The yield of CH4, LHV and CO was the highest in the CO2 gasification process. The percentage of CO2 was the highest in oxygen gasification and the degree of gas production was the highest in air gasification.
- (2)
- As the modified equivalence ratio (MER) increased, the hydrogen mass percentage, carbon dioxide mass percentage, H2/CO molar ratio, hydrogen yield, and gas production increased. However, the mass percentage yield of CH4, LHV and CO (except CO2 gasification) decreased.
- (3)
- For all gasifiers, the mass percentage of H2 and the mass percentage of CO increased with the increasing free space temperature, but the mass percentage of CO2 and the mass percentage of CH4 decreased.
- (4)
- As the temperature increased, the LHV of the synthesis gas decreased, but the H2 yield, CO yield and gas production rate increased for all gasifying agents. As the free space temperature increased, the H2/CO molar ratio decreased in the case of steam and H2O2 gasification, but increased in the case of O2, air and CO2 gasification [107].
- -
- H2O pollution, excessive soil fertilization and runoff of H2O from fields to the ground and surface;
- -
- Eutrophication, excessive fertilization of inland and marine H2O (algal blooms, reduction of biodiversity and modification of aquatic ecosystems, loss of bottom fauna);
- -
- Microbiological contamination where pathogenic microorganisms contained in slurry pose a serious sanitary threat.
3. Conclusions
- -
- H2O photolysis;
- -
- The transfer of electrons from photosystems to a protein called ferrodoxin, which is then a direct donor of electrons transferred to protons;
- -
- Biohydrogen synthesis which is catalyzed by hydrogenase.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASBR | Anaerobic Sequencing Batch Reactor |
| COD | Chemical Oxygen Demand |
| FW | Food Waste |
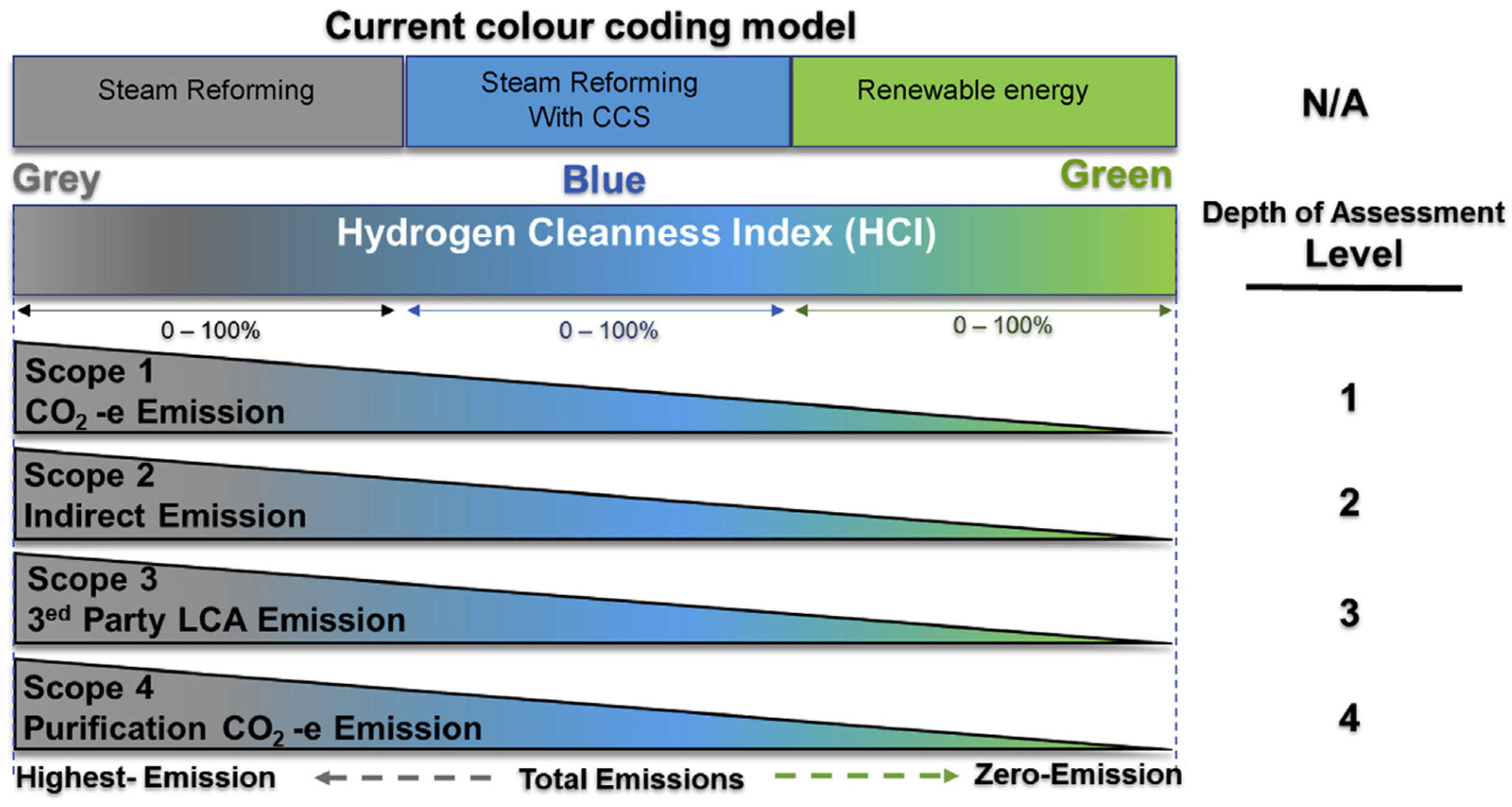
| HCI | Hydrogen Cleanness Index |
| HDR | Hydrogen Direct Reduction |
| HRT | Hydraulic Retention Time |
| LAB | Lactic Acid Bacteria |
| OFMSW | Organic Fraction Composition of Municipal Solid Waste |
| OLR | Organic Load Rates |
| PEC | Photoelectrochemical cells |
| P2G | Power to Gas |
| RES | Renewable Energy Sources |
| SRT | Solids Retention Time |
| TVS | Total Volatile Solid |
| VFA | Volatile Fatty Acids |
| VS | Volatile Solids Loading Index |
References
- Ahmed, S.F.; Liu, G.; Mofijur, M.; Azad, A.K.; Hazrat, M.A.; Chu, Y.M. Physical and Hybrid Modelling Techniques for Earth-Air Heat Exchangers in Reducing Building Energy Consumption: Performance, Applications, Progress, and Challenges. Sol. Energy 2021, 216, 274–294. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Syazmi, Z.A.H.S.; Mofijur, M.; Abas, A.E.P.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent Landscape Review on Biodiesel Production: Technology Updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- Bolatkhan, K.; Kossalbayev, B.D.; Zayadan, B.K.; Tomo, T.; Veziroglu, T.N.; Allakhverdiev, S.I. Hydrogen Production from Phototrophic Microorganisms: Reality and Perspectives. Int. J. Hydrogen Energy 2019, 44, 5799–5811. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Rafa, N.; Mofijur, M.; Badruddin, I.A.; Inayat, A.; Ali, M.S.; Farrok, O.; Khan, T.M.Y. Biohydrogen Production from Biomass Sources: Metabolic Pathways and Economic Analysis. Front. Energy Res. 2021, 9, 753878. [Google Scholar] [CrossRef]
- Levin, D.B.; Pitt, L.; Love, M. Biohydrogen production: Prospects and limitations to practical application. Int. J. Hydrogen Energy 2004, 29, 173–185. [Google Scholar] [CrossRef]
- Show, K.Y.; Lee, D.J.; Tay, J.H.; Lin, C.Y.; Chang, J.S. Biohydrogen production: Current perspectives and the way forward. Int. J. Hydrogen Energy 2012, 37, 15616–15631. [Google Scholar] [CrossRef]
- Chong, M.-L.; Sabaratnam, V.; Shirai, Y.; Hassan, M.A. Biohydrogen production from biomass and industrial wastes by dark fermentation. Int. J. Hydrogen Energy 2009, 34, 3277–3287. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). 10/2022. Available online: https://www.iea.org/data-and-statistics/charts/fossil-fuel-use-by-scenario-2020-2030-and-2050 (accessed on 10 March 2024).
- Energy Transitions Commission (ETC). Making the Hydrogen Economy Possible:Accelerating Clean Hydrogen in an Electrified Economy Version 1.2 April 2021. Available online: https://energy-transitions.org/wp-content/uploads/2021/04/ETC-Global-Hydrogen-Report.pdf (accessed on 10 March 2024).
- Energy Transitions Commision. Version 1.0 April 2023. Available online: https://www.energy-transitions.org/wp-content/uploads/2023/04/2022-053-ETC-Hydrogen-Technical-Annex-Final_.pdf (accessed on 10 March 2024).
- bp Statistical Review of World Energy June 2020. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2020-full-report.pdf (accessed on 12 March 2024).
- Jie, X.; Gonzalez-Cortes, S.; Xiao, T.; Yao, B.; Wang, J.; Slocombe, D.R.; Fang, Y.; Miller, N.; Al-Megren, H.A.; Dilworth, J.R.; et al. The decarbonisation of petroleum and other fossil hydrocarbon fuels for the facile production and safe storage of hydrogen. Energy Environ. Sci. 2019, 12, 238–249. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration (EIA). Electricity Explained; U.S. Energy Information Administration (EIA): Washington, DC, USA, 2019.
- Mandley, S.J.; Daioglou, V.; Junginger, H.M.; van Vuuren, D.P.; Wicke, B. EU bioenergy development to 2050. Renew. Sustain. Energy 2020, 127, 109858. [Google Scholar] [CrossRef]
- Quilcaille, Y.; Gasser, T.; Ciais, P.; Lecocq, F.; Janssens-Maenhout, G.; Mohr, S. Uncertainty in projected climate change arising from uncertain fossil-fuel emission factors. Environ. Res. Lett. 2018, 13, 4. [Google Scholar] [CrossRef]
- Edenhofer, O.R.; Pichs-Madruga, Y.; Sokona, E.; Farahani, S.; Kadner, K.; Seyboth, A.; Adler, I.; Baum, S.; Brunner, P.; Eickemeier, B.; et al. (Eds.) Climate Change 2014: Mitigation of Climate Change; Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Acar, C.; Dincer, I. Selection criteria and ranking for sustainable hydrogen production options. Int. J. Hydrogen Energy 2022, 47, 40118–40137. [Google Scholar] [CrossRef]
- Jia, J.; Seitz, L.C.; Benck, J.D.; Huo, Y.; Chen, Y.; Desmond Ng, J.W.; Bilir, T.; Harris, J.S.; Jaramillo, T.F. Solar water splitting by photovoltaic-electrolysis with a solar-to-hydrogen efficiency over 30%. Nat. Commun. 2016, 7, 13237. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Li, B.; Zhu, H.; Shi, J. 3D interconnected nanoporous Ta3N5 films for photoelectrochemical water splitting: Thickness-controlled synthesis and insights into stability. Sci. China Mater. 2021, 64, 1876–1888. [Google Scholar] [CrossRef]
- Putatunda, C.; Behl, M.; Solanki, P.; Sharma, S.; Bhatia, S.K.; Walia, A.; Bhatia, R.K. Current challenges and future technology in photofermentation-driven biohydrogen production by utilizing algae and bacteria. Int. J. Hydrogen Energy 2023, 48, 21088–21109. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, G.; Zhang, J. A hybrid artificial photosynthesis system with molecular catalysts covalently linked onto TiO2 as electron relay for efficient photocatalytic hydrogen evolution. J. Mater. Sci. Technol. 2020, 50, 147–152. [Google Scholar] [CrossRef]
- Abas, N.; Kalair, E.; Kalai, A.; Ul Hasan, Q.; Khan, N. Nature inspired artificial photosynthesis technologies for hydrogen production: Barriers and challenges. Int. J. Hydrogen Energy 2020, 45, 20787–20799. [Google Scholar] [CrossRef]
- Klaster Technologii Wodorowch. Available online: https://klasterwodorowy.pl/ (accessed on 12 March 2024).
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Kisiel, A. e-Biotechnologia.pl. 2019. Available online: http://www.e-biotechnologia.pl/Artykuly/Biowodor/ (accessed on 12 March 2024).
- International Energy Agency (IEA). Electricity Market Report 2023. Available online: https://www.iea.org/reports/electricity-market-report-2023 (accessed on 12 March 2024).
- Demirbas, A.H. Biofuels for future transportation necessity. Energy Educ. Sci. Technol. Part A Energy Sci. Res. 2010, 26, 13–23. [Google Scholar]
- Manara, P.; Zabaniotou, A. Towards sewage sludge based biofuels via thermochemical conversion—A review. Renew. Sustain. Energy Rev. 2012, 16, 2566–2582. [Google Scholar] [CrossRef]
- Kass, M.D.; Abdullah, Z.; Biddy, M.J.; Drennan, C.; Haq, Z.; Hawkins, T.; Wang, M. Understanding the Opportunities of Biofuels for Marine Shipping (No. ORNL/TM-2018/1080); Oak Ridge National Lab. (ORNL): Oak Ridge, TN, USA, 2018. [Google Scholar]
- Lehman, Clarence and Selin, Noelle Eckley. “biofuel”. Encyclopedia Britannica, 15 Sep. 2021. Available online: https://www.britannica.com/technology/biofuel (accessed on 10 December 2021).
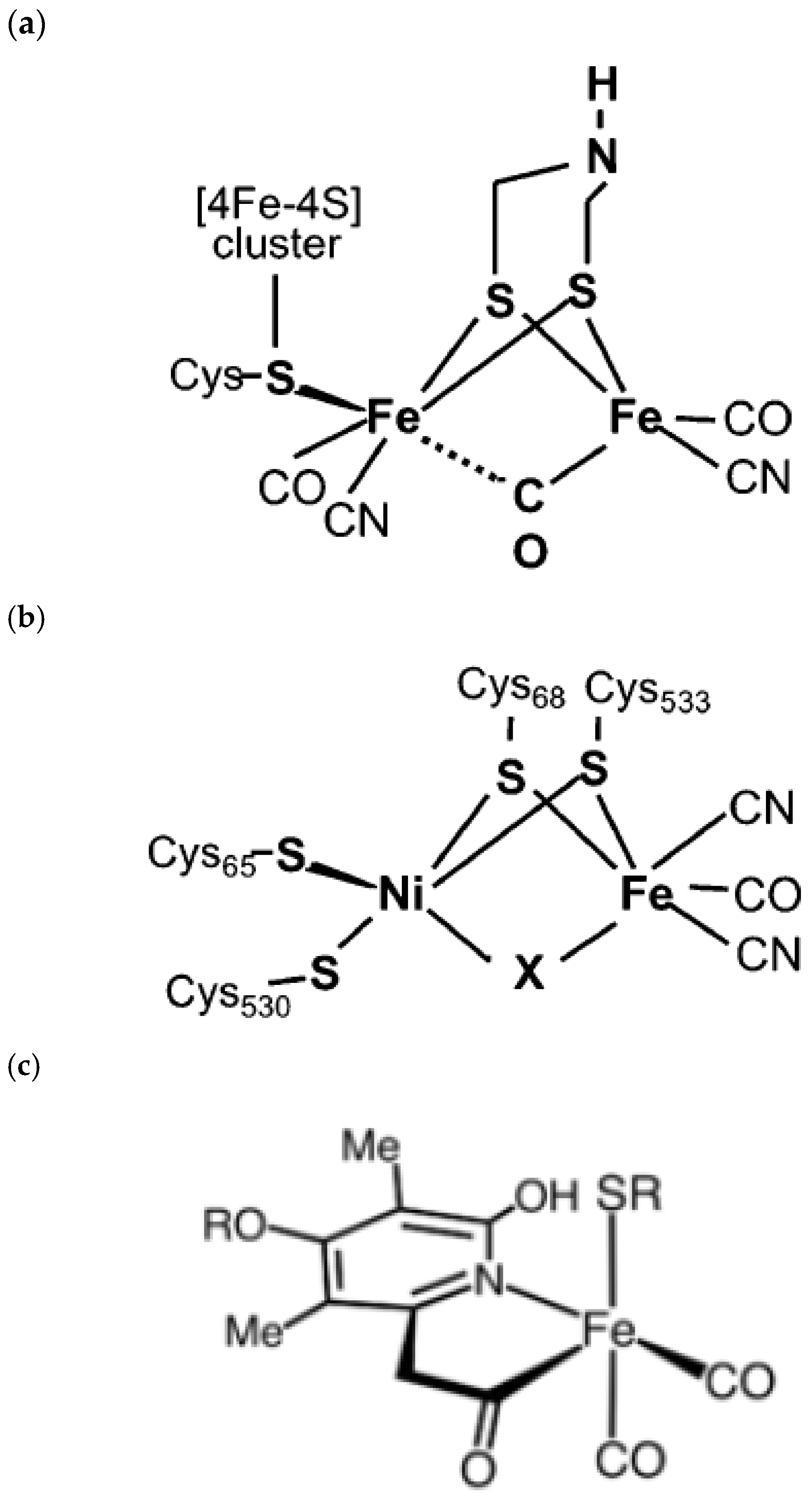
- Lubitz, W.; Ogata, H.; Rüdiger, O.; Reijerse, E. Hydrogenases. Chem. Rev. 2014, 114, 4081–4148. [Google Scholar] [CrossRef]
- Vignais, P.M.; Billoud, B. Occurrence, Classification, and Biological Function of Hydrogenases: An Overview. Chem. Rev. 2007, 107, 4206–4272. [Google Scholar] [CrossRef]
- Land, H.; Ceccaldi, P.; Mészáros, L.S.; Lorenzi, M.; Redman, H.J.; Senger, M.; Stripp, S.T.; Berggren, G. Discovery of novel [FeFe]-hydrogenases for biocatalytic H2-production. Chem. Sci. 2019, 10, 9941–9948. [Google Scholar] [CrossRef] [PubMed]
- Morra, S. Fantastic [FeFe]-Hydrogenases and Where to Find Them. Front. Microb. 2022, 13, 853626. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Koo, J. O2 sensitivity and H2 production activity of hydrogenases—A review. Biotechnol. Bioeng. 2019, 116, 3124–3135. [Google Scholar] [CrossRef]
- Hemschemeier, A.; Melis, A.; Happe, T. Analytical approaches to photobiological hydrogen production in unicellular green algae. Photosynth. Res. 2009, 102, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Melis, A.; Zhang, L.; Forestier, M.; Ghirardi, M.L.; Seibert, M. Sustained Photobiological Hydrogen Gas Production upon Reversible Inactivation of Oxygen Evolution in the Green AlgaChlamydomonas reinhardtii. Plant Physiol. 2000, 122, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Peden, E.A.; Boehm, M.; Mulder, D.W.; Davis, R.; Old, W.M.; King, P.W.; Ghirardi, M.L.; Dubini, A. Identification of Global Ferredoxin Interaction Networks in Chlamydomonas reinhardtii. J. Biol. Chem. 2013, 288, 35192–35209. [Google Scholar] [CrossRef] [PubMed]
- Volgusheva, A.; Styring, S.; Mamedov, F. Increased photosystem II stability promotes H2 production in sulfur-deprived Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2013, 110, 7223–7228. [Google Scholar] [CrossRef] [PubMed]
- Tamagnini, P.; Axelsson, R.; Lindberg, P.; Oxelfelt, F.; Wünschiers, R.; Lindblad, P. Hydrogenases and Hydrogen Metabolism of Cyanobacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 1–20. [Google Scholar] [CrossRef]
- Mona, S.; Kumar, S.S.; Kumar, V.; Parveen, K.; Saini, N.; Deepak, B.; Pugazhendhi, A. Green technology for sustainable biohydrogen production (waste to energy): A review. Sci. Total Environ. 2020, 728, 138481. [Google Scholar] [CrossRef]
- Dziga, D. Biohydrogen—The fuel of the future? Universe 2015, 116, 44–48. [Google Scholar]
- Moritz, M. Biological methods of obtaining hydrogen. Chemist 2012, 66, 827–834. [Google Scholar]
- Das, D.; Veziroglu, T.N. Advances in biological hydrogen production processes. Int. J. Hydrogen Energy 2008, 33, 6046. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Parisa, T.A.; Islam, N.; Kusumo, F.; Inayat, A.; Le, V.G.; Bad, I.A. Progress and Challenges of Contaminate Removal from Wastewater Using Microalgae Biomass. Chemosphere 2021, 286, 131656. [Google Scholar] [CrossRef]
- Hutsol, T.; Glowacki, S.; Tryhuba, A.; Kovalenko, N.; Pustova, Z.; Rozkosz, A.; Sukmaniuk, O. Current Trends of Biohydrogen Production from Biomass—Green Hydrogen; Monograph. Libra-Print: Łomża, Poland, 2021. ISBN 978-83-8237-021-8. Available online: https://dglib.nubip.edu.ua/handle/123456789/8103 (accessed on 12 March 2024). [CrossRef]
- Hawkes, F.R.; Dinsdale, R.; Hawkes, D.L.; Hussy, I. Sustainable fermentative hydrogen production: Challenges for process optimization. Int. J. Hydrogen Energy 2002, 27, 1339–1347. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H.; Sumathy, K. An overview of hydrogen production from biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Krzemińska, I.; Kwietniewska, E. Processes of biological hydrogen production, Buses—Technology, Operation, Transport Systems 10/2021. Available online: https://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-article-BWAW-0016-0034 (accessed on 14 March 2024).
- Ferraren-De Cagalitan, D.D.T.; Abundo, M.L.S. A review of biohydrogen production technology for application towards hydrogen fuel cells. Renew. Sustain. Energy Rev. 2021, 151, 111413. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Biofuels, biodiesel and biohydrogen production using bioprocesses. A review. Environ. Chem. Lett. 2020, 18, 1049–1072. [Google Scholar] [CrossRef]
- Chai, Y.H.; Mohamed, M.; Cheng, Y.W.; Chin, B.L.F.; Yiin, C.L.; Yusup, S.; Lam, M.K. A review on potential of biohydrogen generation through waste decomposition technologies. Biomass Convers. Biorefinery 2023, 13, 8549–8574. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Schneemann, A.; White, J.L.; Kang, S.; Jeong, S.; Wan, L.F.; Cho, E.S.; Stavila, V. Nanostructured metal hydrides for hydrogen storage. Chem. Rev. 2018, 118, 10775–10839. [Google Scholar] [CrossRef]
- Miao, J.; Lang, Z.; Xue, T.; Li, Y.; Li, Y.; Cheng, J.; Tang, Z. Revival of Zeolite-Templated Nanocarbon Materials: Recent Advances in Energy Storage and Conversion. Adv. Sci. 2020, 7, 2001335. [Google Scholar] [CrossRef]
- Wu, R.; Zhang, X.; Liu, Y.; Zhang, L.; Hu, J.; Gao, M.; Pan, H. A unique double-layered carbon nanobowl-confined lithium borohydride for highly reversible hydrogen storage. Small 2020, 16, 2001963. [Google Scholar] [CrossRef]
- Gest, H.; Kamen, M.D. Photoproduction of molecular hydrogen by Rhodospirillum rubrum. Science 1949, 109, 558. [Google Scholar] [CrossRef]
- Eroğlu, E.; Eroğlu, İ.; Gündüz, U.; Türker, L.; Yücel, M. Biological hydrogen production from olive mill wastewater with two-stage processes. Int. J. Hydrogen Energy 2006, 31, 1527. [Google Scholar] [CrossRef]
- Urbaniec, K.; Grabarczyk, R. Directions of Research on Biological Methods of Obtaining Hydrogen as an Energy Carrier. Inżynieria Systemów Bioagrotechnicznych 5(14); Powierża, L., Ed.; Department of Systems Engineering, Warsaw University of Technology in Płock: Plock, Poland, 2005; pp. 209–214. ISBN 83-915395-3-9. [Google Scholar]
- Nanda, S.; Pattnaik, F.; Patra, B.R.; Kang, K.; Dalai, A.K. A Review of Liquid and Gaseous Biofuels from Advanced Microbial Fermentation Processes. Fermentation 2023, 9, 813. [Google Scholar] [CrossRef]
- Yagüe, L.; Linares, J.I.; Arenas, E.; Romero, J.C. Levelized Cost of Biohydrogen from Steam Reforming of Biomethane with Carbon Capture and Storage (Golden Hydrogen)—Application to Spain. Energies 2024, 17, 1134. [Google Scholar] [CrossRef]
- North America Hydrogen Market Analysis: Industry Market Size, Plant Capacity, Production, Operating Efficiency, Demand & Supply, End-User Industries, Sales Channel, Regional Demand, Company Share, Manufacturing Process, 2015–2032. Available online: https://www.chemanalyst.com/industry-report/north-america-hydrogen-market-2950 (accessed on 14 March 2024).
- Becerra-Quiroz, A.-P.; Rodríguez-Morón, S.-A.; Acevedo-Pabón, P.-A.; Rodrigo-Ilarri, J.; Rodrigo-Clavero, M.-E. Evaluation of the Dark Fermentation Process as an Alternative for the Energy Valorization of the Organic Fraction of Municipal Solid Waste (OFMSW) for Bogotá, Colombia. Appl. Sci. 2024, 14, 3437. [Google Scholar] [CrossRef]
- Albuquerque, M.M.; Martinez-Burgos, W.J.; De Bona Sartor, G.; Letti, L.A.J.; De Carvalho, J.C.; Soccol, C.R.; Medeiros, A.B.P. Advances and Perspectives in Biohydrogen Production from Palm Oil Mill Effluent. Fermentation 2024, 10, 141. [Google Scholar] [CrossRef]
- Hydrogen Development in Latin America. CSIS. Available online: https://www.csis.org/analysis/hydrogen-development-latin-america (accessed on 14 March 2024).
- El-kebeer, A.A.; Mahmoud, U.F.; Ismail, S.; Jalal, A.A.E.; Kowal, P.; Al-Hazmi, H.E.; Hassan, G.K. Maximizing Bio-Hydrogen and Energy Yields Obtained in a Self-Fermented Anaerobic Bioreactor by Screening of Different Sewage Sludge Pretreatment Methods. Processes 2024, 12, 118. [Google Scholar] [CrossRef]
- Dar, M.A.; Xie, R.; Zabed, H.M.; Ali, S.; Zhu, D.; Sun, J. Termite Microbial Symbiosis as a Model for Innovative Design of Lignocellulosic Future Biorefinery: Current Paradigms and Future Perspectives. Biomass 2024, 4, 180–201. [Google Scholar] [CrossRef]
- Ren, N.; Li, Y.; Zadsar, M.; Hu, L.; Li, J. Biological Hydrogen Production In China: Past, Present and Future. In Proceedings of the ASME 2005 International Solar Energy Conference. Solar Energy, Orlando, FL, USA, 6–12 August 2005; ASME: New York, NY, USA, 2005; pp. 663–666. [Google Scholar] [CrossRef]
- Meng, X.; Chen, M.; Gu, A.; Wu, X.; Liu, B.; Zhou, J.; Mao, Z. China’s hydrogen development strategy in the context of double carbon targets. Nat. Gas Ind. B 2022, 9, 521–547. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, Z. Overview of hydrogen storage and transportation technology in China. Unconv. Resour. 2023, 3, 291–296. [Google Scholar] [CrossRef]
- Available online: https://www.iea.org/policies/16977-hydrogen-industry-development-plan-2021-2035 (accessed on 12 March 2024).
- Available online: https://www.dcceew.gov.au/energy/hydrogen (accessed on 12 March 2024).
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. A Hydrogen Strategy for a Climate-Neutral Europe COM (2020) 301 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52020DC0301 (accessed on 12 March 2024).
- Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions REPowerEU Plan COM (2022) 230 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52022DC0230 (accessed on 12 March 2024).
- Pwaelec, G.; Muron, M.; Bracht, J.; Bonnet-Cantalloube, B.; Floristean, A.; Brahy, N. Hydrogen Europe Clean Hydrogen Monitor. 2020. Available online: https://hydrogeneurope.eu/wp-content/uploads/2021/11/Clean-Hydrogen-Monitor-2020.pdf (accessed on 12 March 2024).
- Cihlar, J.; Villar Lejarreta, A.; Wang, A.; Melgar, F.; Jens, J.; Rio, P.; Leun, v.d.K. ASSET Study on Hydrogen Generation in Europe: Overview of Costs and Key Benefits; European Commission: Brussels, Belgium; Luxembourg, 2021. [Google Scholar]
- International Energy Agency (IEA). IEA G20 Hydrogen Report: Assumptions. 2019. Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 12 March 2024).
- H21 NoE. H21 North of England. 2018. Available online: https://www.h21.green/wp-content/uploads/2019/01/H21-NoE-PRINT-PDF-FINAL-1.pdf (accessed on 12 March 2024).
- Taibi, E.; Miranda, R.; Vanhoudt, W.; Winkel, T.; Lanoix, J.C.; Barth, F.; International Renewable Energy Agency IRENA. Hydrogen from Renewable Power: Technology Outlook for the Energy Transition. 2018. Available online: https://www.irena.org/publications/2018/Sep/Hydrogen-from-renewable-power (accessed on 12 March 2024).
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkens, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrogen Energy 2017, 34, 30470–30492. [Google Scholar] [CrossRef]
- van Wijk, A.; Chatzimarkakis, J. Hydrogen Europe: Green Hydrogen for a European Green Deal, A 2x40 GW Initiative. 2020. Available online: https://hydrogeneurope.eu/ (accessed on 12 March 2024).
- JRC. JRC EU Times model Hydrogen Module. 2019. Available online: http://data.europa.eu/89h/5839d35a-6b1e-4f47-ab9b-df3f1dafe4e6 (accessed on 12 March 2024).
- Jakobsen, D.; Åtland, V. Concepts for Large Scale Hydrogen Production. NTNU. 2016. Available online: https://ntnuopen.ntnu.no/ntnu-xmlui/handle/11250/240255 (accessed on 12 March 2024).
- Polish Economic Institute. Hydrogen Economy in Poland: Observations Based on the Research Framework of the Technological Innovation System; POLICY PAPER 5/2020; Polish Economic Institute: Warsaw, Poland, 2020. [Google Scholar]
- Lefranc, L.; Linares, J.I.; Santos, A.M.; Arenas, E.; Martín, C.; Moratilla, Y. The role of golden hydrogen in the decarbonising of urban buses. In Proceedings of the ECOS 2023—36th International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems, Las Palmas de Gran Canaria, Spain, 25–30 June 2023; Available online: https://files.griddo.comillas.edu/ecos-2023-cte-rev1.pdf (accessed on 14 March 2024).
- Susmozas, A.; Iribarren, D.; Dufour, J. Assessing the Life-Cycle Performance of Hydrogen Production via Biofuel Reforming in Europe. Resources 2015, 4, 398–411. [Google Scholar] [CrossRef]
- Panagiotopoulos, J.A.; Bakker, R.R.; de Vrije, T.; Urbaniec, K.; Koukios, E.G.; Claassen, P.A.M. Prospects of utilization of sugar beet carbohydrates for biological hydrogen production in the EU. J. Clean. Prod. 2010, 18 (Suppl. S1), S9–S14. [Google Scholar] [CrossRef]
- Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC135018 (accessed on 12 March 2024).
- Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal/repowereu-affordable-secure-and-sustainable-energy-europe_en (accessed on 12 March 2024).
- Available online: https://energy.ec.europa.eu/topics/energy-systems-integration/hydrogen_en#eu-hydrogen-strategy (accessed on 14 March 2024).
- European Commision. Key Actions of the EU Hydrogen Strategy. Available online: https://energy.ec.europa.eu/topics/energy-systems-integration/hydrogen/key-actions-eu-hydrogen-strategy_en (accessed on 12 March 2024).
- Letter of Intent for Establishing a Partnership for the Development of the Hydrogen Economy and Concluding a Sectoral Hydrogen Agreement. Warsaw 2020. Available online: https://www.gov.pl/attachment/ebf105f5-babb-4ae9-9251-47fe7186e73 (accessed on 14 March 2024).
- “New Energy Technologies” PROGRAM. Warsaw, December 2020. Available online: https://www.ncbj.gov.pl/nowe-technologie-zakresie-energii (accessed on 14 March 2024).
- Polish Economic Institute. Development Directions of the Hydrogen Economy in Poland; Working Paper 7/2019; Polish Economic Institute: Warsaw, Poland, 2019. [Google Scholar]
- Mebs, S.; Duan, J.; Wittkamp, F.; Stripp, S.T.; Happe, T.; Apfel, U.-P.; Winkler, M.; Haumann, M. Differential Protonation at the Catalytic Six-Iron Cofactor of [FeFe]-Hydrogenases Revealed by 57Fe Nuclear Resonance X-ray Scattering and Quantum Mechanics/Molecular Mechanics Analyses. Inorg. Chem. 2019, 58, 4000–4013. [Google Scholar] [CrossRef]
- Vignais, P.M.; Colbeau, A. Molecular biology of microbial hydrogenases. Curr Issues Mol Biol 2004, 6, 159–188. [Google Scholar]
- Land, H.; Sekretareva, A.; Huang, P.; Redman, H.J.; Németh, B.; Polidori, N.; Mészáros, L.S.; Senger, M.; Stripp, S.T.; Berggren, G. Characterization of a putative sensory [FeFe]-hydrogenase provides new insight into the role of the active site architecture. Chem. Sci. 2020, 11, 12789–12801. [Google Scholar] [CrossRef]
- Grinter, R.; Kropp, A.; Venugopal, H.; Senger, M.; Badley, J.; Cabotaje, P.R.; Jia, R.; Duan, Z.; Huang, P.; Stripp, S.T.; et al. Structural basis for bacterial energy extraction from atmospheric hydrogen. Nature 2023, 615, 541–547. [Google Scholar] [CrossRef]
- Morra, S.; Valetti, F.; Gilardi, G. FeFe-hydrogenases as biocatalystsin bio-hydrogen production. Rend. Lincei Sci. Fis. E Nat. 2017, 28, 183–194. [Google Scholar] [CrossRef]
- Lu, F.; Smith, P.R.; Mehta, K.; Swartz, J.R. Development of a synthetic pathway to convert glucose to hydrogen using cell free extracts. Int. J. Hydrogen Energy 2015, 40, 9113–9124. [Google Scholar] [CrossRef]
- Hołaj-Krzak, J.T. Long-Range Dynamic Cooperative Interactions between Hydrogen Bonds in Model Lattices of Dicarboxylic Acid Crystals in the Light of Spectral Studies in the Infrared Range; University of Silesia: Katowice, Poland, 2017. [Google Scholar]
- Ntaikou, I.; Antonopoulou, G.; Lyberatos, G. Biohydrogen Production from Biomass and Wastes via Dark Fermentation: A Review. Waste Biomass Valor. 2010, 1, 21–39. [Google Scholar] [CrossRef]
- Chong, M.L.; Raha, A.R.; Shirai, Y.; Hassan, M.A. Biohydrogen production by Clostridium butyricum EB6 from palm oil mill effluent. Int. J. Hydrogen Energy 2009, 34, 764–771. [Google Scholar] [CrossRef]
- Lee, K.S.; Lin, P.J.; Fangchiang, K.; Chang, J.S. Continuous hydrogen production by anaerobic mixed microflora using a hollow-fiber microfiltration membrane bioreactor. Int. J. Hydrogen Energy 2007, 32, 950–957. [Google Scholar] [CrossRef]
- Mishra, S.; Upadhyay, R.K. Review on biomass gasification: Gasifiers, gasifying mediums, and operational parameters. Mater. Sci. Energy Technol. 2021, 4, 329–340. [Google Scholar] [CrossRef]
- Sansaniwal, S.K.; Pal, K.; Rosen, M.A.; Tyagi, S.K. Recent advances in the development of biomass gasification technology: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 72, 363–384. [Google Scholar] [CrossRef]
- Islam, M.W. Effect of different gasifying agents (steam, H2O2, oxygen, CO2, and air) on gasification parameters. Int. J. Hydrogen Energy 2020, 45, 31760–31774. [Google Scholar] [CrossRef]
- Chee, M.K.T.; Ng, B.-J.; Chew, Y.-H.; Chang, W.S.; Chai, S.-P. Photocatalytic Hydrogen Evolution from Artificial Seawater Splitting over Amorphous Carbon Nitride: Optimization and Process Parameters Study via Response Surface Modeling. Materials 2022, 15, 4894. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Yang, X.; Chen, A.; Chen, P.; Yang, L.; Yan, C.; Shi, Y. Enhanced Photocatalysis of Black TiO2/Graphene Composites Synthesized by a Facile Sol–Gel Method Combined with Hydrogenation Process. Materials 2022, 15, 3336. [Google Scholar] [CrossRef]
- Grushevskaya, S.; Belyanskaya, I.; Kozaderov, O. Approaches for Modifying Oxide-Semiconductor Materials to Increase the Efficiency of Photocatalytic Water Splitting. Materials 2022, 15, 4915. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-W.; Chung, J. Bioproduction of Hydrogen from Food Waste by Pilot-Scale Combined Hydrogen/Methane Fermentation. Int. J. Hydrogen Energy 2010, 35, 11746–11755. [Google Scholar] [CrossRef]
- Shuang, L.; Chunying, W.; Lili, Y.; Wenzhe, L.; Zhongjiang, W.; Lina, L. Optimization of Hydrogen Production from Agricultural Wastes Using Mixture Design. Int. J. Agric. Biol. Eng. 2017, 10, 246–254. [Google Scholar] [CrossRef]
- Rangel, C.; Sastoque, J.; Calderón, J.; Gracia, J.; Cabeza, I.; Villamizar, S.; Aceved, P. Pilot-Scale Assessment of Biohydrogen and Volatile Fatty Acids Production via Dark Fermentation of Residual Biomass. Chem. Eng. Trans. 2022, 92, 61–66. [Google Scholar] [CrossRef]
- Hernandez, M.; Gonzalez, A.J.; Suarez, F.; Ochoa, C.; Candela, A.M.; Cabeza, I. Assessment of the Biohydrogen Production Potential of Different Organic Residues in Colombia: Cocoa Waste, Pig Manure and Coffee Mucilage. Chem. Eng. Trans. 2018, 65, 247–252. [Google Scholar] [CrossRef]
- Jung, K.-W.; Kim, D.-H.; Shin, H.-S. Continuous Fermentative Hydrogen Production from Coffee Drink Manufacturing Wastewater by Applying UASB Reactor. Int. J. Hydrogen Energy 2010, 35, 13370–13378. [Google Scholar] [CrossRef]
- Han, S.-K.; Shin, H.-S. Performance of an Innovative Two-Stage Process Converting Food Waste to Hydrogen and Methane. J. Air Waste Manag. Assoc. 2004, 54, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Tenca, A.; Schievano, A.; Perazzolo, F.; Adani, F.; Oberti, R. Biohydrogen from Thermophilic Co-Fermentation of Swine Manure with Fruit and Vegetable Waste: Maximizing Stable Production without PH Control. Bioresour. Technol. 2011, 102, 8582–8588. [Google Scholar] [CrossRef]
- Alibardi, L.; Cossu, R. Composition Variability of the Organic Fraction of Municipal Solid Waste and Effects on Hydrogen and Methane Production Potentials. Waste Manag. 2015, 36, 147–155. [Google Scholar] [CrossRef]
- Alibardi, L.; Cossu, R. Effects of Carbohydrate, Protein and Lipid Content of Organic Waste on Hydrogen Production and Fermentation Products. Waste Manag. 2016, 47, 69–77. [Google Scholar] [CrossRef]
- Dong, L.; Zhenhong, Y.; Yongming, S.; Xiaoying, K.; Yu, Z. Hydrogen Production Characteristics of the Organic Fraction of Municipal Solid Wastes by Anaerobic Mixed Culture Fermentation. Int. J. Hydrogen Energy 2009, 34, 812–820. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, S.-H.; Shin, H.-S. Hydrogen Fermentation of Food Waste without Inoculum Addition. Enzyme Microb. Technol. 2009, 45, 181–187. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jang, S.; Yun, Y.-M.; Lee, M.-K.; Moon, C.; Kang, W.-S.; Kwak, S.-S.; Kim, M.-S. Effect of Acid-Pretreatment on Hydrogen Fermentation of Food Waste: Microbial Community Analysis by next Generation Sequencing. Int. J. Hydrogen Energy 2014, 39, 16302–16309. [Google Scholar] [CrossRef]
- Jang, S.; Kim, D.-H.; Yun, Y.-M.; Lee, M.-K.; Moon, C.; Kang, W.-S.; Kwak, S.-S.; Kim, M.-S. Hydrogen Fermentation of Food Waste by Alkali-Shock Pretreatment: Microbial Community Analysis and Limitation of Continuous Operation. Bioresour. Technol. 2015, 186, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Elbeshbishy, E.; Nakhla, G. Comparative Study of the Effect of Ultrasonication on the Anaerobic Biodegradability of Food Waste in Single and Two-Stage Systems. Bioresour. Technol. 2011, 102, 6449–6457. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.C.; Giannis, A.; Chen, C.-L.; Wang, J.-Y. Evaluation of Hydrogen Producing Cultures Using Pretreated Food Waste. Int. J. Hydrogen Energy 2014, 39, 19337–19342. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Xu, K.-Q.; Kobayashi, T.; Li, Y.-Y.; Inamori, Y. Effect of Organic Loading Rate on Continuous Hydrogen Production from Food Waste in Submerged Anaerobic Membrane Bioreactor. Int. J. Hydrogen Energy. 2014, 39, 16863–16871. [Google Scholar] [CrossRef]
- Kim, S.-H.; Han, S.-K.; Shin, H.-S. Optimization of Continuous Hydrogen Fermentation of Food Waste as a Function of Solids Retention Time Independent of Hydraulic Retention Time. Process Biochem. 2008, 43, 213–218. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, S.-H.; Kim, K.-Y.; Shin, H.-S. Experience of a Pilot-Scale Hydrogen-Producing Anaerobic Sequencing Batch Reactor (ASBR) Treating Food Waste. Int. J. Hydrogen Energy 2010, 35, 1590–1594. [Google Scholar] [CrossRef]
- Cavinato, C.; Giuliano, A.; Bolzonella, D.; Pavan, P.; Cecchi, F. Bio-Hythane Production from Food Waste by Dark Fermentation Coupled with Anaerobic Digestion Process: A Long-Term Pilot Scale Experience. Int. J. Hydrogen Energy 2012, 37, 11549–11555. [Google Scholar] [CrossRef]
- La Licata, B.; Sagnelli, F.; Boulanger, A.; Lanzini, A.; Leone, P.; Zitella, P.; Santarelli, M. Bio-Hydrogen Production from Organic Wastes in a Pilot Plant Reactor and Its Use in a SOFC. Int. J. Hydrogen Energy 2011, 36, 7861–7865. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y. A Bench Scale Study of Fermentative Hydrogen and Methane Production from Food Waste in Integrated Two-Stage Process. Int. J. Hydrogen Energy 2009, 34, 245–254. [Google Scholar] [CrossRef]
- Kim, S.-H.; Cheon, H.-C.; Lee, C.-Y. Enhancement of Hydrogen Production by Recycling of Methanogenic Effluent in Two-Phase Fermentation of Food Waste. Int. J. Hydrogen Energy 2012, 37, 13777–13782. [Google Scholar] [CrossRef]
- Chu, C.-F.; Ebie, Y.; Xu, K.-Q.; Li, Y.-Y.; Inamori, Y. Characterization of Microbial Community in the Two-Stage Process for Hydrogen and Methane Production from Food Waste. Int. J. Hydrogen Energy 2010, 35, 8253–8261. [Google Scholar] [CrossRef]
- Ntaikou, I.; Gavala, H.N.; Kornaros, M.; Lyberatos, G. Hydrogen production from sugars and sweet sorghum biomass using Ruminococcus albus. Int. J. Hydrogen Energy 2008, 33, 1153–1163. [Google Scholar] [CrossRef]
- de Vrije, T.; Bakker, R.R.; Budde, M.A.W.; Lai, M.H.; Mars, A.E.; Claassen, P.A.M. Efficient hydrogen production from the lignocellulosic energy crop Miscanthus by the extreme thermophilic bacteria Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Biotechnol. Biofuels 2009, 2, 12. [Google Scholar] [CrossRef]
- Kyazze, G.; Dinsdale, R.; Hawkes, F.R.; Guwy, A.J.; Premier, G.C.; Donnison, I.S. Direct fermentation of fodder maize, chicory fructans and perennial ryegrass to hydrogen using mixed microflora. Bioresour. Technol. 2008, 99, 8833–8839. [Google Scholar] [CrossRef]
- Chairattanamanokorn, P.; Penthamkeerati, P.; Reungsang, A.; Lo, Y.-C.; Lu, W.-B.; Chang, J.-S. Production of biohydrogen from hydrolyzed bagasse with thermally preheated sludge. Int. J. Hydrogen Energy 2009, 34, 7612–7617. [Google Scholar] [CrossRef]
- Levin, D.B.; Islam, R.; Cicek, N.; Sparling, R. Hydrogen production by Clostridium thermocellum 27405 from cellulosic biomass substrates. Int. J. Hydrogen Energy 2006, 31, 1496–1503. [Google Scholar] [CrossRef]
- Datar, R.; Huang, J.; Maness, P.-C.; Mohagheghi, A.; Czernik, S.; Chornet, E. Hydrogen production from the fermentation of corn stover biomass pretreated with a steam-explosion process. Int. J. Hydrogen Energy 2007, 32, 932–939. [Google Scholar] [CrossRef]
- Ivanova, G.; Rakhely, G.; Kovacs, K.L. Thermophilic biohydrogen production from energy plants by Caldicellulosiruptor saccharolyticus and comparison with related studies. Int. J. Hydrogen Energy 2009, 34, 3659–3670. [Google Scholar] [CrossRef]
- Klimek, K.; Kapłan, M.; Syrotyuk, S.; Konieczny, R.; Anders, D.; Dybek, B.; Karwacka, A.; Wałowski, G. Production of Agricultural Biogas with the Use of a Hydrodynamic Mixing System of a Polydisperse Substrate in a Reactor with an Adhesive Bed. Energies 2021, 14, 3538. [Google Scholar] [CrossRef]
- Wałowski, G. Development of biogas and biorafinery systems in Polish rural communities. J. Water Land Dev. 2021, 49, 156–168. [Google Scholar] [CrossRef]
- Wałowski, G. Assessment of polydisperse substrate flow in a fermentor for computational fluid dynamics modeling. J. Water Land Dev. 2022, 56, 1–7. [Google Scholar] [CrossRef]
- Smurzyńska, A.; Czekała, W.; Kupryaniuk, K.; Cieślik, M.; Kwiatkowska, A. Types and properties of liquid manure and the possibilities of its management. Agric. Eng. Probl. 2016, 4, 117–127. [Google Scholar]
- Clemens, J.; Trinborn, M.; Weiland, P.; Amon, B. Mitigation of greenhouse gas emissions by anaerobic digestion of cattle slurry. Agric. Ecosyst. Environ. 2006, 112, 171–177. [Google Scholar] [CrossRef]
- Rodhe, L.K.K.; Ascue, J.; Willén, A.; Vegerfors Persson, B.; Nordberg, Å. Greenhouse gas emissions from storage and field application of anaerobically digested and non-digested cattle slurry. Agric. Ecosyst. Environ. 2015, 199, 358–368. [Google Scholar] [CrossRef]
- Kwiecińska, A. Ecological Slurry Management Using Membrane Techniques. Work Carried Out as Part of Scientific Work. Financed from the Funds for Science in the Years 2010–2012 as a Research Project No. N N523 559038; Silesian University of Technology, Faculty of Environmental and Energy Engineering, Department of Environmental Chemistry and Membrane Processes: Gliwice, Poland, 2013. [Google Scholar]
- IPPC Directive 1996. Council Directive 96/61/EC Concerning Integrated Pollution Prevention and Control. 24 September 1996. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31996L0061 (accessed on 14 March 2024).
- HELCOM 2020. Dostęp do Strony 22.05.2020 r. Available online: http://www.helcom.fi/ (accessed on 14 March 2024).
- Kutera, J. Slurry Management; Publishing House of the Agricultural University: Wrocław, Poland, 1994; p. 370. [Google Scholar]
- International Energy Agency (MAE/IEA). Global Hydrogen Review 2022. Available online: https://www.iea.org/reports/global-hydrogen-review-2022/executive-summary (accessed on 14 March 2024).
- Vignais, P.M.; Magnin, J.-P.; Willison, J.C. Increasing biohydrogen production by metabolic engineering: An overview. Int. J. Hydrogen Energy 2006, 31, 1478–1483. Available online: https://www.researchgate.net/publication/222402243_Increasing_biohydrogen_production_by_metabolic_engineering (accessed on 14 March 2024). [CrossRef]
- Sickerman, N.S.; Hu, Y.; Ribbe, M.W. Nitrogenases. In Metalloproteins: Methods and Protocols; Hu, Y., Ed.; Springer: New York, NY, USA, 2019; pp. 3–24. [Google Scholar]
- Einsle, O.; Rees, D.C. Structural enzymology of nitrogenase enzymes. Chem. Rev. 2020, 120, 4969–5004. [Google Scholar] [CrossRef]
- Golden, J.W.; Yoon, H.S. Heterocyst development in Anabaena. Curr. Opin. Microbiol. 2003, 6, 557–563. [Google Scholar] [CrossRef]
- Morra, S.; Arizzi, M.; Valetti, F.; Gilardi, G. Oxygen stability in the new [FeFe]-hydrogenase from Clostridium beijerinckii SM10 (CbA5H). Biochemistry 2016, 55, 5897–5900. [Google Scholar] [CrossRef]
- Burgdorf, T.; Lenz, O.; Buhrke, T.; van der Linden, E.; Jones, A.K.; Albracht, S.P.J.; Friedrich, B. [NiFe]-hydrogenases of Ralstonia eutropha H16: Modular enzymes for oxygen-tolerant biological hydrogen oxidation. J. Mol. Microbiol. Biotechnol. 2005, 10, 181–196. [Google Scholar] [CrossRef]
- Lukey, M.J.; Parkin, A.; Roessler, M.M.; Murphy, B.J.; Harmer, J.; Palmer, T.; Sargent, F.; Armstrong, F.A. How Escherichia coli is equipped to oxidize hydrogen under different redox conditions. J. Biol. Chem. 2010, 285, 3928–3938. [Google Scholar] [CrossRef]
- Topin, J.; Diharce, J.; Fiorucci, S.; Antonczak, S.; Golebiowski, J. O2 migration rates in [NiFe] hydrogenases. A joint approach combining free-energy calculations and kinetic modeling. J. Phys. Chem. B 2014, 118, 676–681. [Google Scholar] [CrossRef]
- Wu, X.; Liang, Y.; Li, Q.; Zhou, J.; Long, M. Characterization and cloning of oxygen-tolerant hydrogenase from Klebsiella oxytoca HP1. Res. Microbiol. 2011, 162, 330–336. [Google Scholar] [CrossRef]
- Rudiger, O.; Gutierrez-Sanchez, C.; Olea, D.; Pereira, I.A.C.; Velez, M.; Fernandez, V.M.; De Lacey, A.L. Enzymatic anodes for hydrogen fuel cells based on covalent attachment of Ni-Fe hydrogenases and direct electron transfer to SAM-modified gold electrodes. Electroanalysis 2010, 22, 776–783. [Google Scholar] [CrossRef]
- Liu, J.; Wu, W.J.; Fang, F.; Zorin, N.A.; Chen, M.; Qian, D.J. Immobilization of hydrogenase on carbon nanotube polyelectrolytes as heterogeneous catalysts for electrocatalytic interconversion of protons and hydrogen. J. Nanopart. Res. 2016, 18, 220. [Google Scholar] [CrossRef]
- Rüdiger, O.; Abad, J.M.; Hatchikian, E.C.; Fernandez, V.M.; De Lacey, A.L. Oriented Immobilization of Desulfovibrio gigas Hydrogenase onto Carbon Electrodes by Covalent Bonds for Nonmediated Oxidation of H2. J. Am. Chem. Soc. 2005, 127, 16008–16009. [Google Scholar] [CrossRef]
- Reddy, K.R.; Hassan, M.; Gomes, V.G. Hybrid nanostructures based on titanium dioxide for enhanced photocatalysis. Appl. Catal. A 2015, 489, 1–16. [Google Scholar] [CrossRef]
- Liu, X.; Risbakk, S.; Carvalho, P.A.; Yang, M.Y.; Backe, P.H.; Bjoras, M.; Norby, T.; Chatzitakis, A. Immobilization of FeFe-hydrogenase on black TiO2 nanotubes as biocathodes for the hydrogen evolution reaction. Electrochem. Commun. 2022, 135, 107221. [Google Scholar] [CrossRef]
- Wang, Y.M.; Song, Y.H.; Ma, C.L.; Xia, H.Q.; Wu, R.R.; Zhu, Z.G. Electrochemical characterization of a truncated hydrogenase from Pyrococcus furiosus. Electrochim. Acta 2021, 387, 138502. [Google Scholar] [CrossRef]
- Siebel, J.F.; Adamska-Venkatesh, A.; Weber, K.; Rumpel, S.; Reijerse, E.; Lubitz, W. Hybrid [FeFe]-hydrogenases with modified active sites show remarkable residual enzymatic activity. Biochemistry 2015, 54, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Slater, J.W.; Shafaat, H.S. Nickel-substituted rubredoxin as a minimal enzyme model for hydrogenase. J. Phys. Chem. Lett. 2015, 6, 3731–3736. [Google Scholar] [CrossRef] [PubMed]
- Esmieu, C.; Raleiras, P.; Berggren, G. From protein engineering to artificial enzymes—Biological and biomimetic approaches towards sustainable hydrogen production. Sustain. Energy Fuels 2018, 2, 724–750. [Google Scholar] [CrossRef] [PubMed]

| Technology | Year | Investment Cost | Efficiency | Ref. |
|---|---|---|---|---|
| EUR * Million/MWh | % | |||
| Green—Alkaline electrolyzers (ALK) | 2020 | 0.628–1.955 | 63–70 | [77] |
| 2020 | 0.444–0.947 | 63–68 | [78] | |
| 1.395 | 51 | [79] | ||
| 1.158–2.837 | 49–69 | [80] | ||
| 2030 | 0.496–1.151 | 65–71 | [77] | |
| 0.361–0.740 | 68–69 | [81] | ||
| 0.700 | 65 | [79] | ||
| 0.736–1.531 | 52–73 | [80] | ||
| 2050 | 0.220–0.880 | 70–80 | [77] | |
| 0.289 | 69 | [81] | ||
| Green—Membrane cells with polymer electrolyte (PEM) | 2020 | 1.613–2.828 | 56–60 | [77] |
| 1.997 | 57 | [79] | ||
| 1.474–3.402 | 55–63 | [82] | ||
| 1.266–3.596 | 52–63 | [80] | ||
| 2030 | 0.841–2.095 | 63–68 | [77] | |
| 1.037 | 64 | [79] | ||
| 0.998–2.257 | 59–68 | [82] | ||
| 0.772–2.739 | 52–69 | [80] | ||
| Green—Oxide Electrolyzers (SOEC) | 2020 | 3.041–6.658 | 74–81 | [77] |
| 1.066 | 76 | [82] | ||
| 2.132–3.664 | 80 | [80] | ||
| 2030 | 0.838–3.199 | 77–84 | [77] | |
| 2.132–3.664 | 80 | [82] | ||
| 0.799–3.331 | 80 | [80] | ||
| 2050 | 0.489–1.143 | 77–90 | [77] | |
| 0.388 | 80 | [82] | ||
| Blue—CCS for existing Steam Methane Reforming plant (SMR) | 2020 | 0.701 | - | [83] |
| Blue—New for steam methane reforming (SMR) and CCS | 2020 | 1.650 | - | [83] |
| 2020 | 0.963 | - | [76] | |
| 1.594 | 69 | [77] | ||
| 0.792–1.408 | - | [77] | ||
| 0.963 | - | [76] | ||
| 2030 | 0.909 | - | [76] | |
| 1.290 | 69 | [77] | ||
| 2050 | 0.856 | - | [76] | |
| 1.214 | 69 | [77] | ||
| Blue—CCS for existing autothermal reforming (ATR) | 2020 | 0.688 | - | [83] |
| Blue—New installation autothermal reforming (ATR) and CCS | 2020 | 1.498 | - | [83] |
| 0.952 | - | [78] |
| Technology | Advantages | Disadvantages |
|---|---|---|
| photofermentation |
|
|
| dark fermentation |
|
|
| direct biophotolysis |
|
|
| indirect biophotolysis |
|
|
| microbial electrolysis |
|
|
| Microorganism | Strain | Benefits | Limitations |
|---|---|---|---|
| green algae |
|
|
|
| cyanobacteria |
|
|
|
| photosynthetic bacteria |
|
|
|
| fermentative bacteria |
|
|
|
| Source | Biogas Production Potential | Hydrogen Production Potential | Technology | Ref. |
|---|---|---|---|---|
| Food waste | Biogas 2.446 Nm3/d | H2 1.0 Nm3/d | A two-stage fermentation process for hydrogen/methane production | [111] |
| Mixture of food waste, cattle manure, potato pulp and pig manure | H2 21.0 mL/g VS | Multi-component system, laboratory scale | [112] | |
| Pig manure (pm), coffee mucilage (cfm) and cocoa mucilage | 91.85 mL H2/g VS, | 4.367 mL H2 | The pilot plant was operated under mesophilic conditions | [113] |
| Coffee mucilage Cocoa waste Pig manure | Coffee 2.80 Cocoa waste 4.88 Pig manure 3.30 L/Lsolution | Coffee 2.12 L/Lsolution Cocoa 0.07 L/Lsolution Pig manure 0.48 L/Lsolution | [114] | |
| Coffee drink manufacturing wastewater | 1.29 mol H2/mol hexose added | 0.07 L H2//L/H | Up-flow anaerobic sludge blanket reactor | [115] |
| Food waste | H2 and 0.31 m3/kg· VSadded CH4 and 0.21 m3/kg· VSadded | H2 3.63 m3/m3·day CH4 1.75 m3/m3·day | [116] | |
| Fruit–vegetable waste with swine manure ratio of 35/65 | 126 mL H2 g−1VS-added | 3.27 L H2 L−1 d−1 | Anaerobic fermentation | [117] |
| Organic waste: fruits (F), vegetables (V), meat–fish–cheese (MFC), bread–pasta (BP) and rejected materials | 142 mL CH4/gVS/d | 232 mLH2/gVS/d using only carbohydrates | Anaerobic digestion plant: Batch (35 °C, pH 5.5) | [118] |
| Organic waste: meat–fish–cheese (MFC), fruits (F), vegetable (V), bread–pasta (BP). The fraction MFC was composed of raw chicken breast, tuna chunks in brine and butter; the fraction F was composed of apple–banana mousse; the fraction V was composed of lyophilized minestrone soup; the fraction BP was composed of bread crumbs and raw pasta | 244 mL/gVS | 129 mL H2/gVS | Dark fermentation batch tests were carried out in 1-litre batch reactors under mesophilic conditions (35 ± 1 °C). | [119] |
| Rice Potato Lettuce | 134 H2 mL/g-VS 106 H2 mL/g-VS 50 H2 mL/g-VS | Batch: (37 °C, pH 5.5) | [120] | |
| Food waste; Pre-treatment: 90 °C, 20 min | 148.7 H2 mL/g-VS | Batch (35 °C, pH 7.0) | [121] | |
| Food waste (Cafeteria); pre-treatment acid: 12 h, pH 2 | 158 H2 mL/g-VS | Batch (37 °C, pH 8.0) | [122] | |
| Food waste (cafeteria); pre-treatment alkaline: 6 h, pH 12 | 162 H2 mL/g-VS | Batch 37 °C, pH 6.0 | [123] | |
| Food waste; pre-treatment: ultrasonic, 30 min | 140 H2 mL/g-VS | Batch (30 °C) | [124] | |
| Food waste; pre-treatment: Autoclaving (121 °C, 15 min) | 38.6 H2 mL/g-VS | Batch (35 °C) | [125] | |
| Food waste (Cafeteria) | Biogas production rate 62.5 L/day with OLR (125.4 kg-COD/m3/day) | 111.11 H2 mL/gVS added | Membrane bioreactor MBR, working volume 5 L 55 °C, pH 5.5 | [126] |
| Food waste | 80.9 H2 mL/gVS added | Anaerobic sequencing batch reactors (ASBR), working volume 4.5 L, 35 °C | [127] | |
| Food waste | 0.54 mol H2/mol hextose | Anaerobic sequencing batch reactors (ASBR), working volume 150 L, HRT 36 h, pH 5.3, 35 °C | [128] | |
| Food waste | 66.7 H2 L/kgVS | Stirred tank reactors (CSTR) with a working volume of 0.2 m3. pH 4.7, 55 °C, HRT 3.3 d | [129] | |
| Fruit and vegetable unsold stock | 240 L H2 containing H2 (49%) | Dark anaerobic fermentation in a pilot-scale reactor (V: 35 L) | [130] | |
| Food waste | 0.065 H2 m3/kgVS | Rotating drum: pH 5.2–5.8, 40 °C, SRT 160 h | [131] | |
| Food waste; Heat pretreatment at 70 °C for 60 min | ASBR, working volume 12 L Produced CH4: 0.92 m3/m3 d | H2: 1.76 m3/m3 d | ASBR, working volume 3.6 L. 35 °C | [132] |
| Food waste | CSTR, working volume 40 L, HRT 5 d, 35 °C, pH 7.5 Produced CH4: 464 mL/gVS added | Produced H2: 205 mL/gVS added | CSTR, working volume 10 L, HRT 1.3 d, 55 °C, pH 5.5 | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarosz, Z.; Kapłan, M.; Klimek, K.; Anders, D.; Dybek, B.; Herkowiak, M.; Hołaj-Krzak, J.T.; Syrotyuk, S.; Korobka, S.; Syrotyuk, H.; et al. Evaluation of Biohydrogen Production Depending on the Substrate Used—Examples for the Development of Green Energy. Energies 2024, 17, 2524. https://doi.org/10.3390/en17112524
Jarosz Z, Kapłan M, Klimek K, Anders D, Dybek B, Herkowiak M, Hołaj-Krzak JT, Syrotyuk S, Korobka S, Syrotyuk H, et al. Evaluation of Biohydrogen Production Depending on the Substrate Used—Examples for the Development of Green Energy. Energies. 2024; 17(11):2524. https://doi.org/10.3390/en17112524
Chicago/Turabian StyleJarosz, Zbigniew, Magdalena Kapłan, Kamila Klimek, Dorota Anders, Barbara Dybek, Marcin Herkowiak, Jakub T. Hołaj-Krzak, Serhiy Syrotyuk, Serhiy Korobka, Hanna Syrotyuk, and et al. 2024. "Evaluation of Biohydrogen Production Depending on the Substrate Used—Examples for the Development of Green Energy" Energies 17, no. 11: 2524. https://doi.org/10.3390/en17112524
APA StyleJarosz, Z., Kapłan, M., Klimek, K., Anders, D., Dybek, B., Herkowiak, M., Hołaj-Krzak, J. T., Syrotyuk, S., Korobka, S., Syrotyuk, H., & Wałowski, G. (2024). Evaluation of Biohydrogen Production Depending on the Substrate Used—Examples for the Development of Green Energy. Energies, 17(11), 2524. https://doi.org/10.3390/en17112524