Assessing the Impact of Organic Loading Rate on Hydrogen Consumption Rates during In Situ Biomethanation
Abstract
1. Introduction
2. Materials and Methods
2.1. Inoculum and Substrate
2.2. Reactor Operation
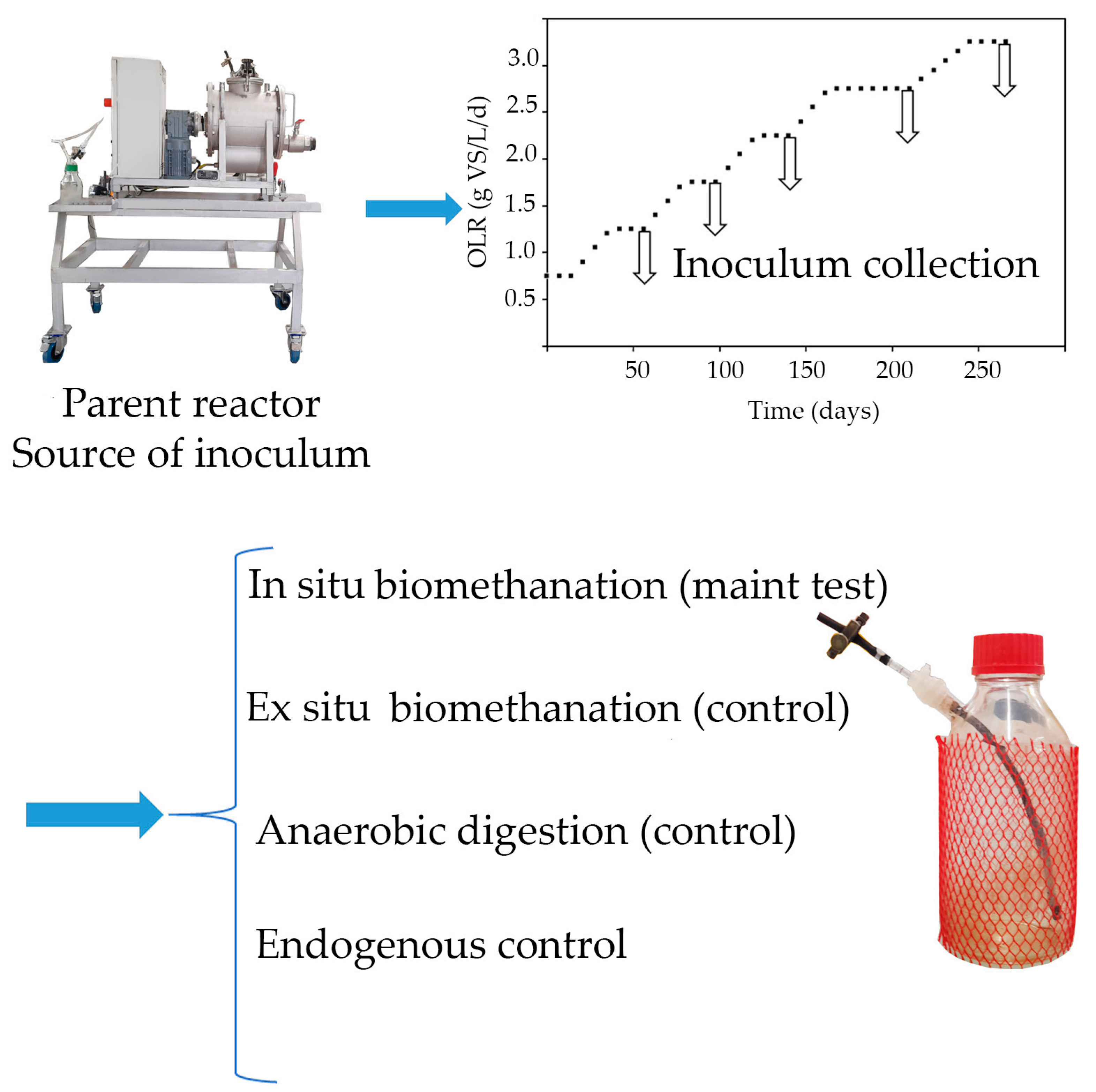
2.2.1. Semi-Continuous AD Reactor (“Parent” Reactor) for Inoculum Generation
2.2.2. Batch Biomethanation Reactors
2.3. Analytical Methods
2.4. Microbial Community Analysis
2.5. Calculations
2.6. Statistical Analysis
3. Results and Discussion
3.1. Performance of the Semi-Continuous “Parent” AD Reactor
3.2. Impact of the OLR on Biomethanation Performance
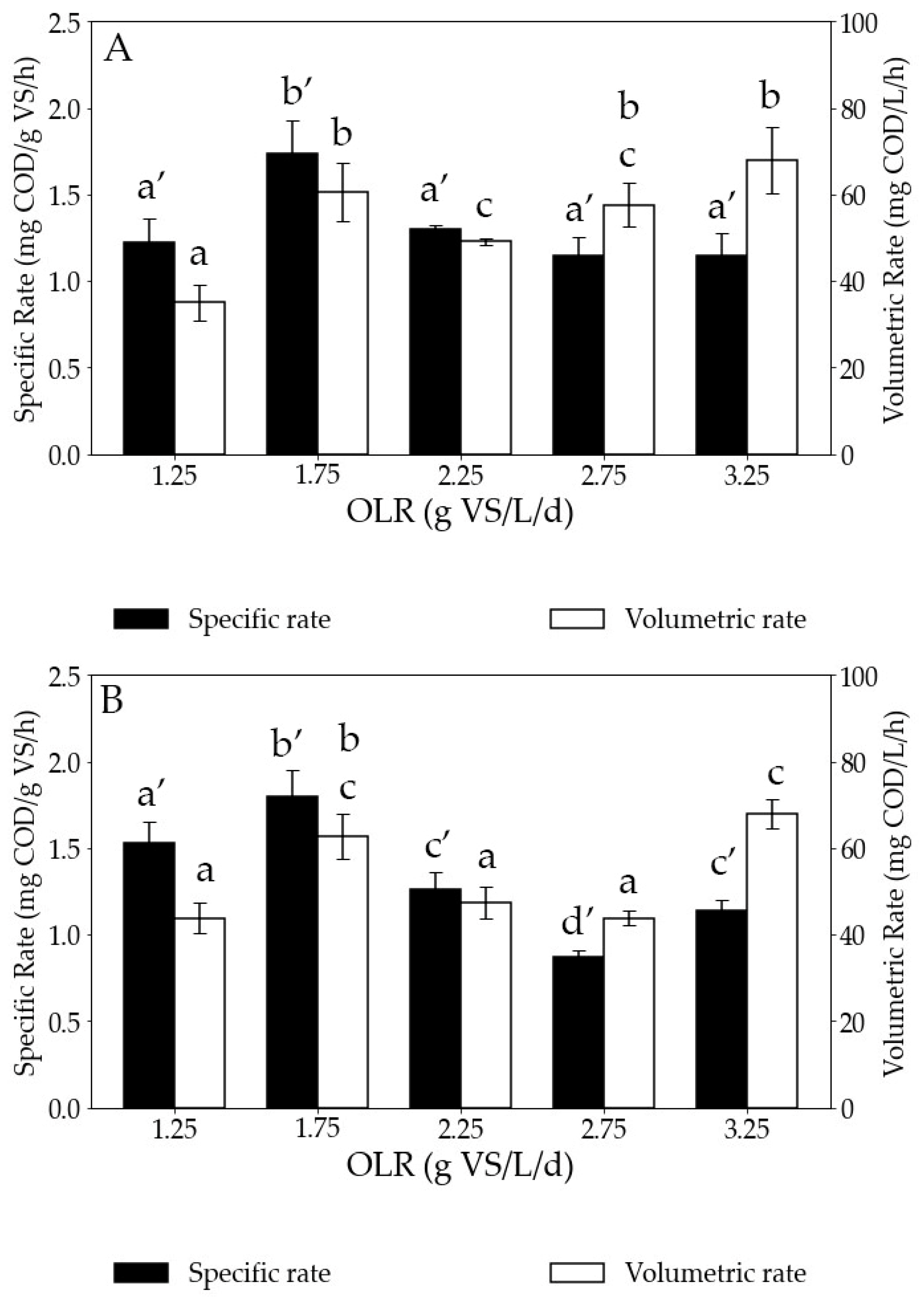
3.2.1. Impact of the OLR on the Hydrogen Consumption Rate
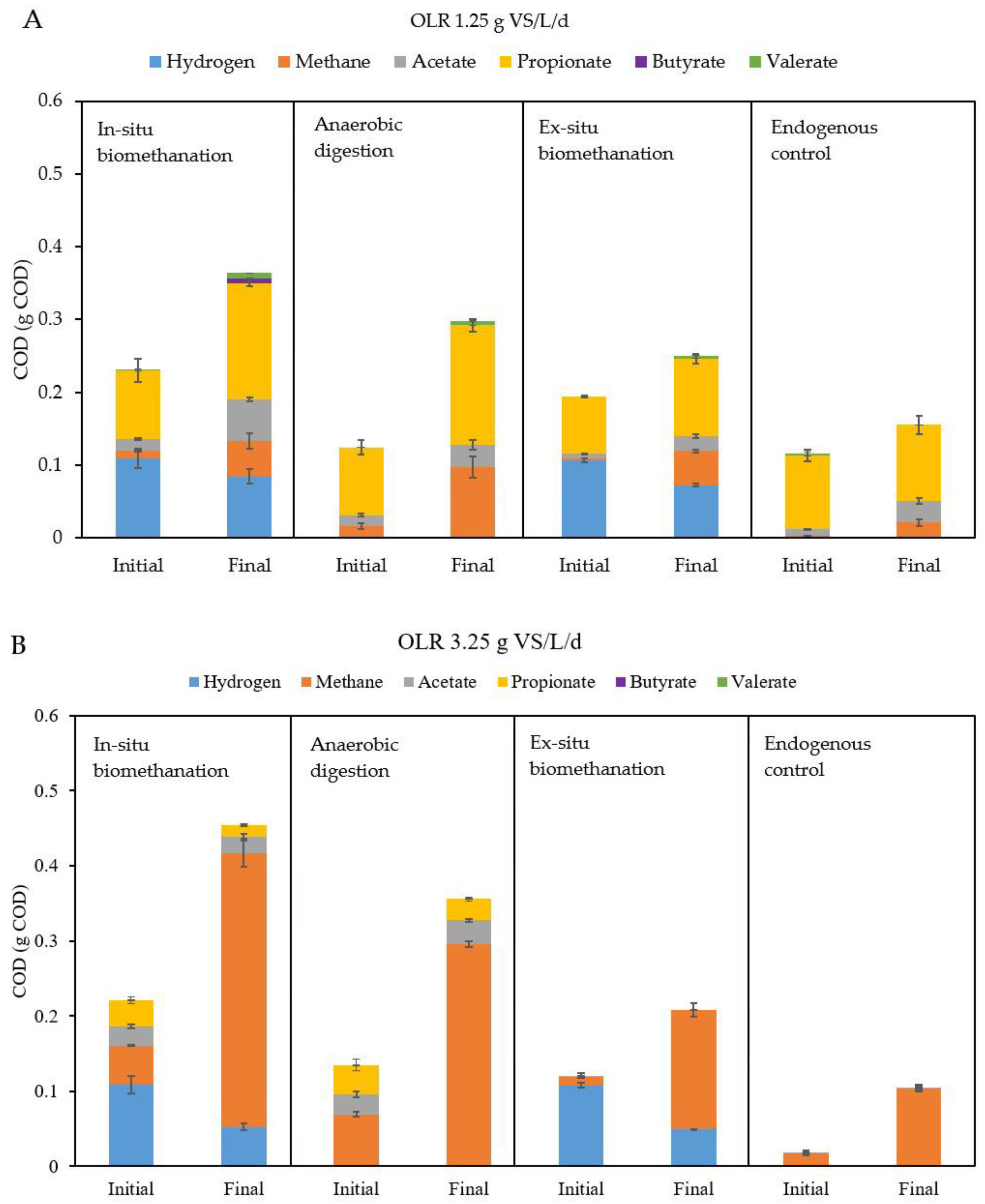
3.2.2. Impact of OLR on Methane and VFA Production
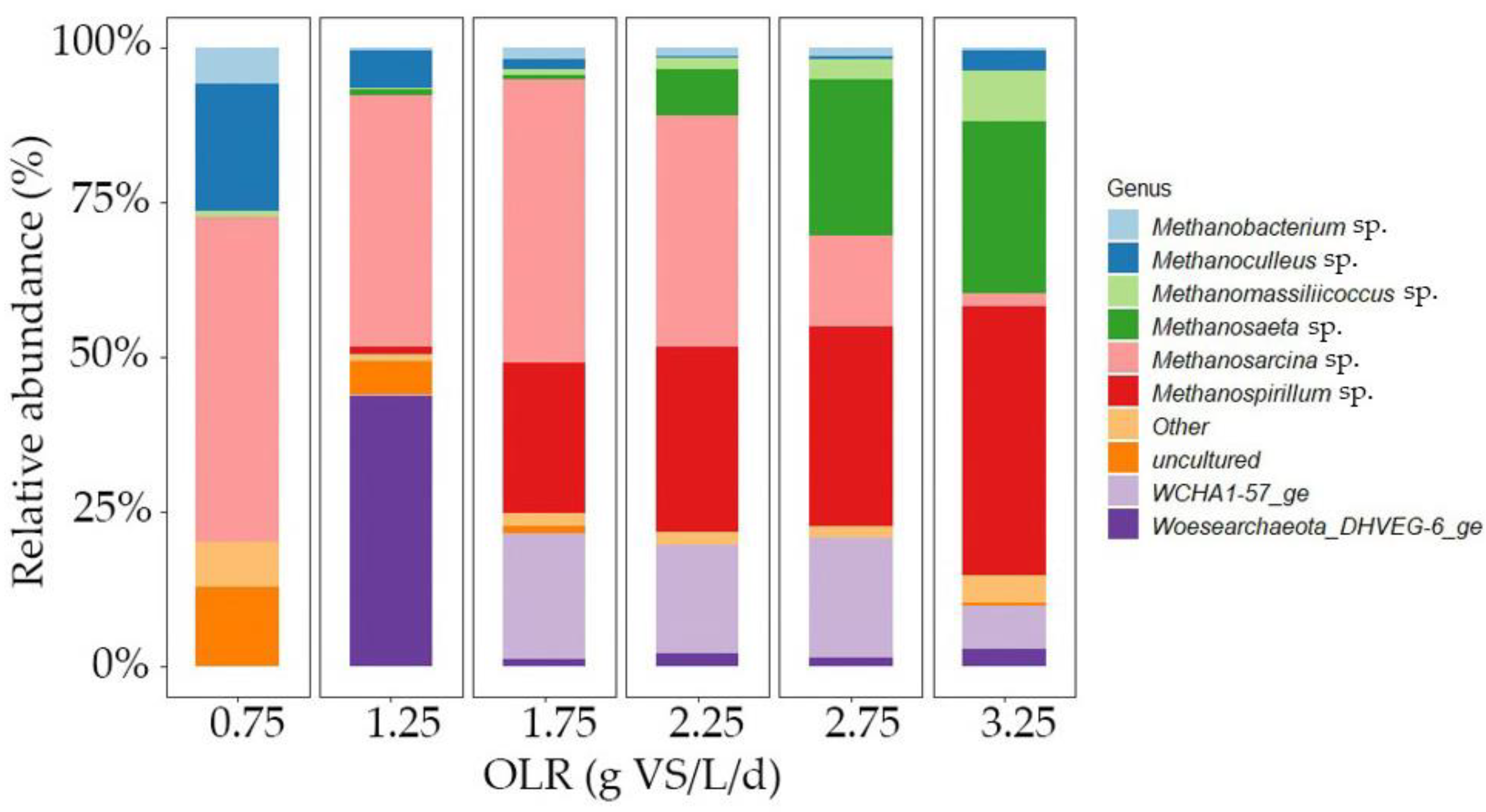
3.2.3. Microbial Community Analysis
3.3. Impact of Short-Period Hydrogen Starvation on Biomethanation Performance
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kacprzak, A.; Włodarczyk, R. Utilization of Organic Waste in a Direct Carbon Fuel Cell for Sustainable Electricity Generation. Energies 2023, 16, 7359. [Google Scholar] [CrossRef]
- Naji, A.; Rechdaoui, S.G.; Jabagi, E.; Lacroix, C.; Azimi, S.; Rocher, V. Pilot-Scale Anaerobic Co-Digestion of Wastewater Sludge with Lignocellulosic Waste: A Study of Performance and Limits. Energies 2023, 16, 6595. [Google Scholar] [CrossRef]
- Tsigkou, K.; Sventzouri, E.; Zafiri, C.; Kornaros, M. Digestate Recirculation Rate Optimization for the Enhancement of Hydrogen Production: The Case of Disposable Nappies and Fruit/Vegetable Waste Valorization in a Mesophilic Two-Stage Anaerobic Digestion System. Renew. Energy 2023, 215, 119010. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Nowicka, A.; Dudek, M. Optimisation of Biogas Production in the Co-Digestion of Pre-Hydrodynamically Cavitated Aerobic Granular Sludge with Waste Fats. Energies 2024, 17, 922. [Google Scholar] [CrossRef]
- Boukouvalas, C.; Kekes, T.; Oikonomopoulou, V.; Krokida, M. Life Cycle Assessment of Energy Production from Solid Waste Valorization and Wastewater Purification: A Case Study of Meat Processing Industry. Energies 2024, 17, 487. [Google Scholar] [CrossRef]
- Sun, Z.-F.; Zhao, L.; Wu, J.-T.; Wang, Z.-H.; Wu, K.-K.; Chen, C.; Xing, D.-F.; Liu, D.-M.; Yang, S.-S.; Wang, A.; et al. Exogenous Hydrogen Supply Improves in-Situ Biogas Upgrading of Sewage Sludge: Performance and Mechanisms. Chem. Eng. J. 2023, 477, 147307. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Dong, R. Recent Progress towards In-Situ Biogas Upgrading Technologies. Sci. Total Environ. 2021, 800, 149667. [Google Scholar] [CrossRef]
- Khan, M.U.; Lee, J.T.E.; Bashir, M.A.; Dissanayake, P.D.; Ok, Y.S.; Tong, Y.W.; Shariati, M.A.; Wu, S.; Ahring, B.K. Current Status of Biogas Upgrading for Direct Biomethane Use: A Review. Renew. Sustain. Energy Rev. 2021, 149, 111343. [Google Scholar] [CrossRef]
- Derakhshesh, S.; Abdollahzadeh Sharghi, E.; Bonakdarpour, B.; Khoshnevisan, B. Integrating Electrocoagulation Process with Up-Flow Anaerobic Sludge Blanket for in-Situ Biomethanation and Performance Improvement. Bioresour. Technol. 2022, 360, 127536. [Google Scholar] [CrossRef]
- Luo, G.; Angelidaki, I. Integrated Biogas Upgrading and Hydrogen Utilization in an Anaerobic Reactor Containing Enriched Hydrogenotrophic Methanogenic Culture. Biotechnol. Bioeng. 2012, 109, 2729–2736. [Google Scholar] [CrossRef]
- Xu, H.; Wang, K.; Zhang, X.; Gong, H.; Xia, Y.; Holmes, D.E. Application of In-Situ H2-Assisted Biogas Upgrading in High-Rate Anaerobic Wastewater Treatment. Bioresour. Technol. 2020, 299, 122598. [Google Scholar] [CrossRef]
- Bellini, R.; Bassani, I.; Vizzarro, A.; Azim, A.A.; Vasile, N.S.; Pirri, C.F.; Verga, F.; Menin, B. Biological Aspects, Advancements and Techno-Economical Evaluation of Biological Methanation for the Recycling and Valorization of CO2. Energies 2022, 15, 4064. [Google Scholar] [CrossRef]
- D’ Silva, T.C.; Isha, A.; Chandra, R.; Vijay, V.K.; Subbarao, P.M.V.; Kumar, R.; Chaudhary, V.P.; Singh, H.; Khan, A.A.; Tyagi, V.K.; et al. Enhancing Methane Production in Anaerobic Digestion through Hydrogen Assisted Pathways – A State-of-the-Art Review. Renew. Sustain. Energy Rev. 2021, 151, 111536. [Google Scholar] [CrossRef]
- Jiang, H.; Wu, F.; Wang, Y.; Feng, L.; Zhou, H.; Li, Y. Characteristics of In-Situ Hydrogen Biomethanation at Mesophilic and Thermophilic Temperatures. Bioresour. Technol. 2021, 337, 125455. [Google Scholar] [CrossRef]
- Agneessens, L.M.; Ottosen, L.D.M.; Andersen, M.; Berg Olesen, C.; Feilberg, A.; Kofoed, M.V.W. Parameters Affecting Acetate Concentrations during In-Situ Biological Hydrogen Methanation. Bioresour. Technol. 2018, 258, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Okoro-Shekwaga, C.K.; Ross, A.; Camargo-Valero, M.A. Enhancing Bioenergy Production from Food Waste by in Situ Biomethanation: Effect of the Hydrogen Injection Point. Food Energy Secur. 2021, 10, e288. [Google Scholar] [CrossRef]
- Poggio, D.; Sastraatmaja, A.; Walker, M.; Michailos, S.; Nimmo, W.; Pourkashanian, M. Experimental Evaluation of Continuous In-Situ Biomethanation of CO2 in Anaerobic Digesters Fed on Sewage Sludge and Food Waste and the Influence of Hydrogen Gas–Liquid Mass Transfer. Processes 2023, 11, 604. [Google Scholar] [CrossRef]
- Kong, X.; Wei, Y.; Xu, S.; Liu, J.; Li, H.; Liu, Y.; Yu, S. Inhibiting Excessive Acidification Using Zero-Valent Iron in Anaerobic Digestion of Food Waste at High Organic Load Rates. Bioresour. Technol. 2016, 211, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Nakakoji, S.; Ng, T.C.A.; Zhu, P.; Tsukada, R.; Tatara, M.; Ng, H.Y. Acclimatizing Waste Activated Sludge in a Thermophilic Anaerobic Fixed-Bed Biofilm Reactor to Maximize Biogas Production for Food Waste Treatment at High Organic Loading Rates. Water Res. 2023, 242, 120299. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Ruiz, D.; Rouez, M.; Crest, M.; Steyer, J.-P.; Bernet, N.; Delgenès, J.-P.; Escudié, R. Accumulation of Propionic Acid during Consecutive Batch Anaerobic Digestion of Commercial Food Waste. Bioresour. Technol. 2017, 245, 724–733. [Google Scholar] [CrossRef]
- Vechi, N.T.; Agneessens, L.M.; Feilberg, A.; Ottosen, L.D.M.; Kofoed, M.V.W. In Situ Biomethanation: Inoculum Origin Influences Acetate Consumption Rate during Hydrogen Addition. Bioresour. Technol. Rep. 2021, 14, 100656. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Trably, E.; Rouez, M.; Crest, M.; Steyer, J.-P.; Delgenès, J.-P.; Escudié, R. Dry Anaerobic Digestion of Food Waste and Cardboard at Different Substrate Loads, Solid Contents and Co-Digestion Proportions. Bioresour. Technol. 2017, 233, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Eaton, A.D.; Franson, M.A.H.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E. Standard Methods for the Examination of Water & Wastewater; American Public Health Association: Washington, DC, USA, 2005; p. 1.v. [Google Scholar]
- Motte, J.-C.; Escudié, R.; Beaufils, N.; Steyer, J.-P.; Bernet, N.; Delgenès, J.-P.; Dumas, C. Morphological Structures of Wheat Straw Strongly Impacts Its Anaerobic Digestion. Ind. Crops Prod. 2014, 52, 695–701. [Google Scholar] [CrossRef]
- Bio2E, INRAE, 2018. Plateforme Biotechnologie et Bioraffinerie Environnementales. [CrossRef]
- Braga Nan, L.; Trably, E.; Santa-Catalina, G.; Bernet, N.; Delgenes, J.-P.; Escudie, R. Microbial Community Redundance in Biomethanation Systems Lead to Faster Recovery of Methane Production Rates after Starvation. Sci. Total Environ. 2022, 804, 150073. [Google Scholar] [CrossRef] [PubMed]
- Venkiteshwaran, K.; Milferstedt, K.; Hamelin, J.; Zitomer, D.H. Anaerobic Digester Bioaugmentation Influences Quasi Steady State Performance and Microbial Community. Water Res. 2016, 104, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Braga Nan, L.; Trably, E.; Santa-Catalina, G.; Bernet, N.; Delgenès, J.-P.; Escudié, R. Biomethanation Processes: New Insights on the Effect of a High H2 Partial Pressure on Microbial Communities. Biotechnol. Biofuels 2020, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, J.; Wu, B.; Liu, J.; Xu, X.; Wu, W.; Zhuang, J.; Li, H.; Huang, T. Enhanced VFAs Production from Microalgal Hydrolytic Acidification with Ultrasonic-Alkali Pretreatment. Algal Res. 2023, 71, 103056. [Google Scholar] [CrossRef]
- Wu, L.-J.; Li, X.-X.; Ye, F.; Yang, F.; Lyu, Y.-K. Determination of Operational Parameters for the First Stage of Continuous Temperature-Phased Anaerobic Digestion of Oily Food Waste: Influent Concentration, Hydraulic Retention Time, pH Control and Temperature. J. Clean. Prod. 2024, 434, 139960. [Google Scholar] [CrossRef]
- Zheng, H.; Li, D.; Stanislaus, M.S.; Zhang, N.; Zhu, Q.; Hu, X.; Yang, Y. Development of a Bio-Zeolite Fixed-Bed Bioreactor for Mitigating Ammonia Inhibition of Anaerobic Digestion with Extremely High Ammonium Concentration Livestock Waste. Chem. Eng. J. 2015, 280, 106–114. [Google Scholar] [CrossRef]
- Marín, D.; Méndez, L.; Suero, I.; Díaz, I.; Blanco, S.; Fdz-Polanco, M.; Muñoz, R. Anaerobic Digestion of Food Waste Coupled with Biogas Upgrading in an Outdoors Algal-Bacterial Photobioreactor at Pilot Scale. Fuel 2022, 324, 124554. [Google Scholar] [CrossRef]
- Choong, Y.Y.; Norli, I.; Abdullah, A.Z.; Yhaya, M.F. Impacts of Trace Element Supplementation on the Performance of Anaerobic Digestion Process: A Critical Review. Bioresour. Technol. 2016, 209, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Bai, T.; Yang, Y.; Li, K.; Tan, H.; Zhuang, S.; Song, F.; Lv, Z.; Wen, H.; Yu, H. Feeding Strategy Determine Flexible Biogas via Shaping Microbial Abundance and Interaction in Anaerobic Digestion. Biomass Bioenergy 2024, 180, 107030. [Google Scholar] [CrossRef]
- Yulisa, A.; Park, S.H.; Chairattanawat, C.; Hwang, S. Effect of Feeding Strategies on the Start-up of Anaerobic Digestion of Fish Waste. Energy 2023, 280, 128199. [Google Scholar] [CrossRef]
- Sasidhar, K.B.; Somasundaram, M.; Ekambaram, P.; Arumugam, S.K.; Nataraj, G.; Murugan, M.A. A Critical Review on the Effects of Pneumatic Mixing in Anaerobic Digestion Process. J. Clean. Prod. 2022, 378, 134513. [Google Scholar] [CrossRef]
- Yu, X.; Yan, L.; Wang, H.; Bi, S.; Zhang, F.; Huang, S.; Wang, Y.; Wang, Y. Anaerobic Co-Digestion of Cabbage Waste and Cattle Manure: Effect of Mixing Ratio and Hydraulic Retention Time. Renew. Energy 2024, 221, 119743. [Google Scholar] [CrossRef]
- Saravanakumar, A.; Sudha, M.R.; Chen, W.-H.; Pradeshwaran, V.; Ashokkumar, V.; Selvarajoo, A. Biomethane Production as a Green Energy Source from Anaerobic Digestion of Municipal Solid Waste: A State-of-the-Art Review. Biocatal. Agric. Biotechnol. 2023, 53, 102866. [Google Scholar] [CrossRef]
- Agyeman, F.O.; Tao, W. Anaerobic Co-Digestion of Food Waste and Dairy Manure: Effects of Food Waste Particle Size and Organic Loading Rate. J. Environ. Manag. 2014, 133, 268–274. [Google Scholar] [CrossRef]
- Paudel, S.; Kang, Y.; Yoo, Y.-S.; Seo, G.T. Effect of Volumetric Organic Loading Rate (OLR) on H2 and CH4 Production by Two-Stage Anaerobic Co-Digestion of Food Waste and Brown Water. Waste Manag. 2017, 61, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Yellezuome, D.; Zhu, X.; Liu, X.; Liu, X.; Liu, R.; Wang, Z.; Li, Y.; Sun, C.; Hemida Abd-Alla, M.; Rasmey, A.-H.M. Integration of Two-Stage Anaerobic Digestion Process with in Situ Biogas Upgrading. Bioresour. Technol. 2023, 369, 128475. [Google Scholar] [CrossRef]
- Gao, M.; Guo, B.; Li, L.; Liu, Y. Role of Syntrophic Acetate Oxidation and Hydrogenotrophic Methanogenesis in Co-Digestion of Blackwater with Food Waste. J. Clean. Prod. 2021, 283, 125393. [Google Scholar] [CrossRef]
- Jensen, M.B.; Kofoed, M.V.W.; Fischer, K.; Voigt, N.V.; Agneessens, L.M.; Batstone, D.J.; Ottosen, L.D.M. Venturi-Type Injection System as a Potential H2 Mass Transfer Technology for Full-Scale in Situ Biomethanation. Appl. Energy 2018, 222, 840–846. [Google Scholar] [CrossRef]
- Kim, S.; Mostafa, A.; Im, S.; Lee, M.-K.; Kang, S.; Na, J.-G.; Kim, D.-H. Production of High-Calorific Biogas from Food Waste by Integrating Two Approaches: Autogenerative High-Pressure and Hydrogen Injection. Water Res. 2021, 194, 116920. [Google Scholar] [CrossRef] [PubMed]
- Figeac, N.; Trably, E.; Bernet, N.; Delgenès, J.-P.; Escudié, R. Temperature and Inoculum Origin Influence the Performance of Ex-Situ Biological Hydrogen Methanation. Molecules 2020, 25, 5665. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Chibwe, K.; Mantilla-Calderon, D.; Ling, F.; He, Z. Meta-Analysis of Biogas Upgrading to Renewable Natural Gas through Biological CO2 Conversion. J. Clean. Prod. 2023, 426, 139128. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Moscoviz, R.; Ruiz, D.; Santa-Catalina, G.; Trably, E.; Rouez, M.; Crest, M.; Steyer, J.-P.; Bernet, N.; Delgenès, J.-P.; et al. Addition of Granular Activated Carbon and Trace Elements to Favor Volatile Fatty Acid Consumption during Anaerobic Digestion of Food Waste. Bioresour. Technol. 2018, 260, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Akbar, S.; Okonkwo, V.; Smith, C.; Khan, S.; Ali Shah, A.; Adnan, F.; Zeeshan Ijaz, U.; Ahmed, S.; Badshah, M. Enrichment of the Hydrogenotrophic Methanogens for, in-Situ Biogas up-Gradation by Recirculation of Gases and Supply of Hydrogen in Methanogenic Reactor. Bioresour. Technol. 2022, 345, 126219. [Google Scholar] [CrossRef] [PubMed]
- Fukuzaki, S.; Nishio, N.; Shobayashi, M.; Nagai, S. Inhibition of the Fermentation of Propionate to Methane by Hydrogen, Acetate, and Propionate. Appl. Environ. Microbiol. 1990, 56, 719–723. [Google Scholar] [CrossRef]
- Cazier, E.A.; Trably, E.; Steyer, J.-P.; Escudie, R. Reversibility of Hydrolysis Inhibition at High Hydrogen Partial Pressure in Dry Anaerobic Digestion Processes Fed with Wheat Straw and Inoculated with Anaerobic Granular Sludge. Waste Manag. 2019, 85, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, X.; Zhang, S.; Liu, Z.; Hu, T.; Wang, X.; Peng, X.; Dai, H.; Wu, J.; Hu, F. Iron-Carbon Micro-Electrolysis Material Enhanced High-Solid Anaerobic Digestion: Performance and Microbial Mechanism. Biochem. Eng. J. 2024, 201, 109132. [Google Scholar] [CrossRef]
- Zhu, C.; Qu, R.; Li, X.; Zuo, X.; Yuan, H. Investigates of Substrate Mingling Ratio and Organic Loading Rate of KOH Pretreated Corn Stover and Pig Manure in Batch and Semi-Continuous System: Anaerobic Digestion Performance and Microbial Characteristics. Chin. J. Chem. Eng. 2023, 62, 114–123. [Google Scholar] [CrossRef]
- Zhang, X.; Jiao, P.; Zhang, M.; Wu, P.; Zhang, Y.; Wang, Y.; Xu, K.; Yu, J.; Ma, L. Impacts of Organic Loading Rate and Hydraulic Retention Time on Organics Degradation, Interspecies Interactions and Functional Traits in Thermophilic Anaerobic Co-Digestion of Food Waste and Sewage Sludge. Bioresour. Technol. 2023, 370, 128578. [Google Scholar] [CrossRef] [PubMed]
- Linyi, C.; Yujie, Q.; Buqing, C.; Chenglong, W.; Shaohong, Z.; Renglu, C.; Shaohua, Y.; Lan, Y.; Zhiju, L. Enhancing Degradation and Biogas Production during Anaerobic Digestion of Food Waste Using Alkali Pretreatment. Environ. Res. 2020, 188, 109743. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, J.; Hennebel, T.; Boon, N.; Verstraete, W. Methanosarcina: The Rediscovered Methanogen for Heavy Duty Biomethanation. Bioresour. Technol. 2012, 112, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Savvas, S.; Donnelly, J.; Patterson, T.; Chong, Z.S.; Esteves, S.R. Methanogenic Capacity and Robustness of Hydrogenotrophic Cultures Based on Closed Nutrient Recycling via Microbial Catabolism: Impact of Temperature and Microbial Attachment. Bioresour. Technol. 2018, 257, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Wahid, R.; Mulat, D.G.; Gaby, J.C.; Horn, S.J. Effects of H2:CO2 Ratio and H2 Supply Fluctuation on Methane Content and Microbial Community Composition during in-Situ Biological Biogas Upgrading. Biotechnol. Biofuels 2019, 12, 104. [Google Scholar] [CrossRef]
- Ashraf, M.T.; Sieborg, M.U.; Yde, L.; Rhee, C.; Shin, S.G.; Triolo, J.M. Biomethanation in a Thermophilic Biotrickling Filter—pH Control and Lessons from Long-Term Operation. Bioresour. Technol. Rep. 2020, 11, 100525. [Google Scholar] [CrossRef]
- Luo, G.; Johansson, S.; Boe, K.; Xie, L.; Zhou, Q.; Angelidaki, I. Simultaneous Hydrogen Utilization and in Situ Biogas Upgrading in an Anaerobic Reactor. Biotechnol. Bioeng. 2012, 109, 1088–1094. [Google Scholar] [CrossRef]
- Logroño, W.; Popp, D.; Nikolausz, M.; Kluge, P.; Harms, H.; Kleinsteuber, S. Microbial Communities in Flexible Biomethanation of Hydrogen Are Functionally Resilient Upon Starvation. Front. Microbiol. 2021, 12, 619632. [Google Scholar] [CrossRef]


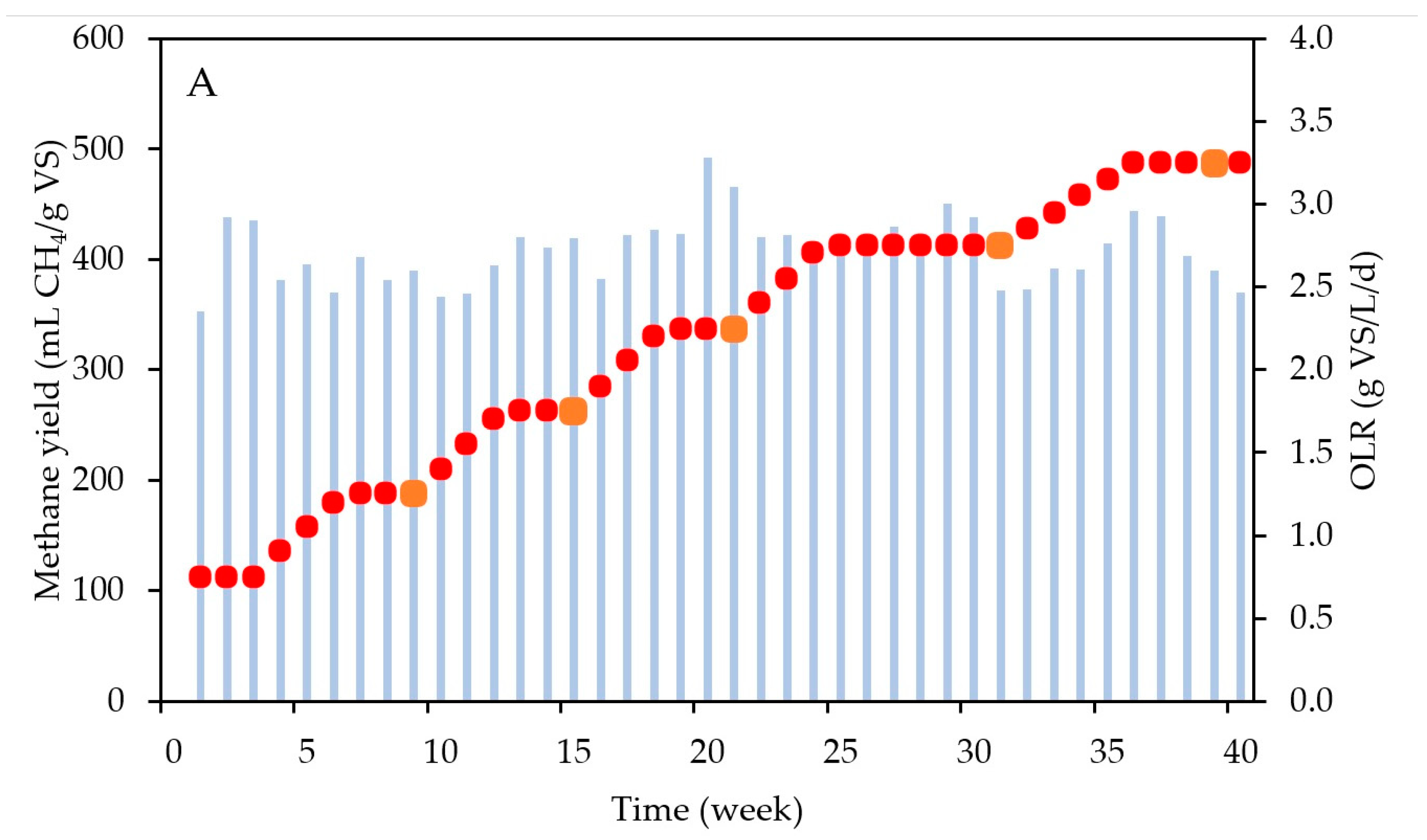
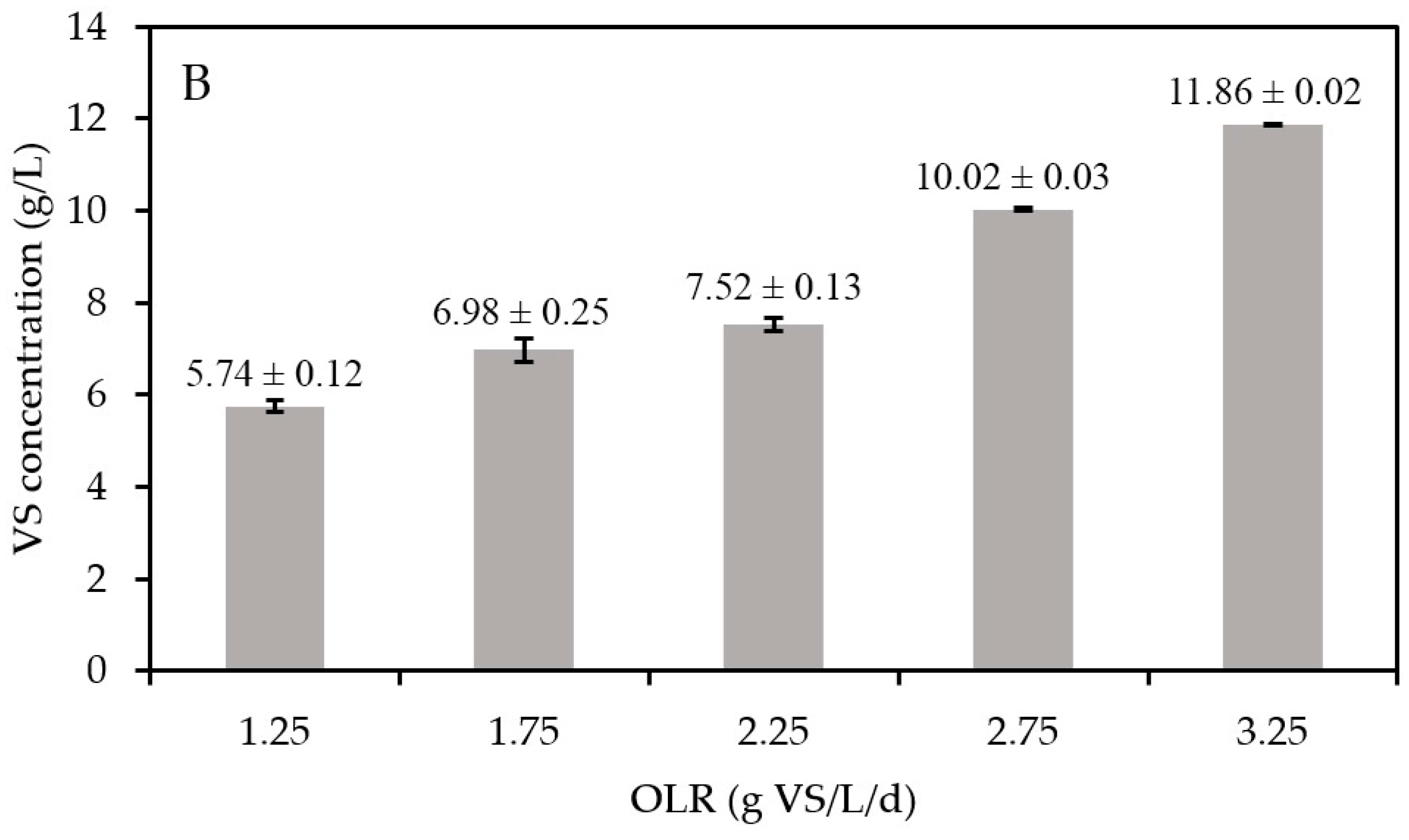
| Substrate Concentration (g VS/L) | OLR (g VS/L/d) |
|---|---|
| 26.25 | 1.25 |
| 36.75 | 1.75 |
| 47.25 | 2.25 |
| 57.75 | 2.75 |
| 68.25 | 3.25 |
| OLR | Shannon | ACE | InvSimpson |
|---|---|---|---|
| 1.25 | 1.32 | 31.71 | 2.76 |
| 1.75 | 1.58 | 33.16 | 3.39 |
| 2.25 | 1.66 | 35.07 | 3.90 |
| 2.75 | 1.76 | 36.04 | 4.47 |
| 3.25 | 1.70 | 37.01 | 3.63 |
| OLR (g VS/L/d) | rH2 (mg COD/L/h) In-Situ 1st Pulse | rH2 (mg COD/L/h) In-Situ 2nd Pulse |
|---|---|---|
| 1.25 | 35 ± 4 | 44 ± 11 |
| 1.75 | 61 ± 7 | 57 ± 6 |
| 2.25 | 49 ± 1 | 73 ± 5 |
| 2.75 | 57 ± 5 | 85 ± 12 |
| 3.25 | 68 ± 8 | 67 ± 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabestani-Rahmatabad, A.; Capson-Tojo, G.; Trably, E.; Delgenès, J.-P.; Escudié, R. Assessing the Impact of Organic Loading Rate on Hydrogen Consumption Rates during In Situ Biomethanation. Energies 2024, 17, 2490. https://doi.org/10.3390/en17112490
Dabestani-Rahmatabad A, Capson-Tojo G, Trably E, Delgenès J-P, Escudié R. Assessing the Impact of Organic Loading Rate on Hydrogen Consumption Rates during In Situ Biomethanation. Energies. 2024; 17(11):2490. https://doi.org/10.3390/en17112490
Chicago/Turabian StyleDabestani-Rahmatabad, Ali, Gabriel Capson-Tojo, Eric Trably, Jean-Philippe Delgenès, and Renaud Escudié. 2024. "Assessing the Impact of Organic Loading Rate on Hydrogen Consumption Rates during In Situ Biomethanation" Energies 17, no. 11: 2490. https://doi.org/10.3390/en17112490
APA StyleDabestani-Rahmatabad, A., Capson-Tojo, G., Trably, E., Delgenès, J.-P., & Escudié, R. (2024). Assessing the Impact of Organic Loading Rate on Hydrogen Consumption Rates during In Situ Biomethanation. Energies, 17(11), 2490. https://doi.org/10.3390/en17112490







