Abstract
Low-salinity water-alternating-CO2 (CO2-LSWAG) injection has been widely studied and employed due to its capability to promote enhanced oil recovery (EOR). However, there is no consensus on the dominant mechanisms for oil recovery in carbonates due to the extreme complexity of the oil–brine–rock interactions. This work proposes a comparative investigation of the physicochemical and geochemical effects of continuous CO2 and CO2-LSWAG immiscible injections on oil recovery in a carbonate core. Simulations were carried out using oil PVT properties and relative permeability experimental data from the literature. A comparison of SO42− and Mg2+ as interpolant ions, oil, water and gas production, pressure, and rock and fluid properties along the core and in the effluent was made. The results show a high recovery factor for CO2 (62%) and CO2-LSWAG (85%), even in immiscible conditions. The mineral dissolution and porosity variations were more pronounced for CO2-LSWAG than CO2. The simulation results showed that Mg2+ as an interpolant improves oil recovery more than SO42− because Mg2+ concentration in the aqueous phase after LSW injection leads to relative permeability values, which are more favorable.
1. Introduction
A broad discussion has been observed in recent years regarding greenhouse gas emissions reduction, among which CO2 is one of the main ones. Several studies have evaluated the capture, use and storage of carbon. The CO2 geological storage has been investigated from a few different perspectives. For instance, CO2 injection into depleted oil reservoirs has been considered an alternative for underground gas storage and, simultaneously, to operate as a bioreactor capable of producing natural gas (low-carbon energy) by microbial-mediated CO2 methanation [1]. Another process that has been widely used as an alternative for reducing CO2 release to the atmosphere is the reinjection of this gas to improve the oil recovery factor in reservoirs whose primary methods are no longer technically and economically viable.
CO2 injection is one of the most successful and widely applied enhanced oil recovery (EOR) techniques, achieving a typical incremental oil recovery rate of 15 and 25% when injected after a typical waterflood [2]. CO2 is preferred to other gases, such as hydrocarbons and N2, because it presents the lowest minimum miscibility pressure (MMP). CO2 flooding in miscible conditions leads to oil swelling, oil viscosity reduction, and gas–oil interfacial tension (IFT) reduction due to CO2 dissolution into the oil, increasing the relative permeability of the oil inside the reservoir pores and, consequently, the oil displacement towards production [3,4]. However, it has been reported that even in non-miscible conditions, CO2 flooding still promotes some recovery of residual oil through combining mechanisms related to partial CO2 dissolution in oil and a reduction in capillary and interfacial forces [5,6]. In addition, immiscible CO2 injection allows the use of milder operating conditions, which is better operationally and economically and valued in reservoirs whose pressure is lower than the minimum miscibility pressure of CO2 [7]. Al-Mutairi et al. [8] investigated the injection of immiscible CO2 in carbonates. They experimentally proved that the wettability alteration due to the CO2 solubility in the brine could be an oil recovery mechanism. However, continuous CO2 injection is associated with a high mobility ratio and viscous finger formation, which are undesirable for oil recovery efficiency. For this reason, CO2 injection has been associated with other fluids, such as in low-salinity water-alternating-gas injection or high-salinity water-alternating-gas injection [9].
In turn, water injection with salt concentration and composition variation, as proposed by LSWI, has been studied since the 1990s [10]. The LSWI technique has been tested and investigated at laboratory and field scales, generally showing positive results compared to conventional waterflooding in sandstones and carbonates [11,12,13,14,15,16]. Around 80–90% of the carbonate reservoirs, the primary type of worldwide oil reservoir, are not water-wet due to the positive charge of the carbonate surface that adsorbs the negatively-charged crude oil. Thus, the rock wettability alteration, from oil-wet to water-wet or neutral conditions, enhances oil recovery because the water phase interacts preferentially with the rock surface. In addition, this has been the main reason LSWI leads to oil recovery improvement in carbonate rocks [17,18]. LSWI changes the wettability by combining different mechanisms, such as multicomponent ion exchange, double layer expansion, pH increase, fines migration, and rock dissolution. However, an adequate understanding of the wettability mechanisms in carbonates is still lacking. Some contradictory results have been found due to the high complexity of this type of reservoir, which is an additional motivation to investigate each mechanism’s contributions under several conditions deeply [19,20].
Low-salinity water-alternating-CO2 flooding (CO2-LSWAG) is an EOR technique that has recently attracted scientific and industrial attention. It is very promising because it promotes synergy between the unique advantages of low-salinity water injection (LSWI) and CO2 EOR. The CO2-LSWAG technique has a significant economic and environmental appeal since it does not require the purchase of chemicals, and the CO2 injection into the reservoirs helps mitigate greenhouse gas emissions [21,22]. As stressed by Wilson [23] and Ma and James [16], in addition to improving recovery, the CO2-LSWAG technique helps deal with operational issues, such as the delayed production observed in conventional CO2-WAG (CO2 water-alternating-gas injection). CO2-LSWAG combines the unique advantages of each fluid with the interaction between CO2 and LSW that allows CO2 dissolution into the injected brine. Carbonated brine’s in situ formation leads to improved wettability alteration and brine-oil IFT reduction, decreasing the capillary effects [22].
A few works are investigating the performance of the immiscible CO2-LSWAG technique in carbonate using experimental core flooding tests, as summarized in the review prepared by Ma and James (2022) [16]. Teklu et al. [22] developed an extensive experimental study to compare the miscible CO2-LSWAG case with the typical seawater (SW) injection for carbonate cores. The core flooding results showed that LSW and CO2 alternated injection cycle applied after seawater flooding leads to significant incremental oil recovery. Furthermore, contact angle measurements indicated that the CO2-saturated low-salinity brine led to a higher wettability alteration than SW and LSW brines. In this work, they also evaluated the immiscible CO2-LSWAG, but, like most of the works in the literature, applied it to sandstone cores. Ramanathan et al. [24] demonstrated that rock aging is a necessary procedure before CO2-LSWAG core flooding tests. Their tests showed that immiscible CO2-LSWAG yielded a 36% additional recovery after SW injection in sandstone cores. Neither author focused on examining the mechanisms that lead to wettability alteration in CO2-LSWAG processes.
More recently, Chaturvedi et al. [25] evaluated the impact of water salinity on the performance of CO2-LSWAG core injection, but it is unclear whether the injections were above or below the MMP. They reinforced that the salinity strongly affects the CO2 saturation in water and, consequently, the oil recovery. The authors recommended CO2-LSWAG injection to develop depleted oil reservoirs. However, they considered only sandstone cores, which are out of this study’s scope, and did not perform any mechanistic investigation. Moradpour et al. [9] conducted contact angle, gas solubility in water, emulsion formation, and core flooding experiments to assess the underlying mechanisms of immiscible CO2-LSWAG injection in carbonates, and found an incremental oil recovery of 25.5% after SW injection. They highlighted the ion exchange mechanism as the primary cause of the wettability alteration, i.e., the oil desorption from the rock surface. Solubility and contact angle results indicated that CO2 is more suitable for achieving wettability alteration than N2. Despite their remarkable contributions, Moradpour et al. [9] did not evaluate the effluent ion concentration, which is needed to properly understand the ion exchange mechanism. Other experimental studies have contributed to the CO2-LSWAG evaluation, such as Kumar et al. [26] and Al-Abri et al. [27], who performed core flooding tests applied to sandstones under immiscible conditions. Complementary steady-state laboratory tests, such as the determination of contact angle, CO2 solubility in brines, and oil-brine interfacial tension, were conducted to evaluate the mechanisms behind the effective recovery from the dynamic EOR process. They pointed out interfacial tension reduction due to CO2 solubility in the aqueous phase and the wettability alteration as reasons for additional oil recovery due to LSW injection.
As core flooding experiments are very time-consuming and operationally complex, numerical modeling and simulation have been used to perform comparative, mechanistic, and sensitivity studies on EOR methods. The great challenge for CO2-LSWAG simulation is to incorporate the complexity of the geochemical interactions resulting from the low-salinity water injection and the lack of a consensus on the mechanism of this EOR method [16]. Only a few numerical studies have been conducted on CO2-LSWAG injection. Most of them are applied to sandstones, considering miscible CO2 injection and adopting the wettability alteration as a recovery mechanism for LSWI. Al-Shalabi et al. [28] compared the performance of gas and high- and low-salinity water-alternating-gas injections. They investigated the effect of water salinity on miscible and immiscible WAG injections in a carbonate core using the UTCOMP simulator. They used previous experimental data to validate the simulation results and highlighted that CO2 controls the residual oil saturation. At the same time, the LSW increases oil production through wettability alteration. However, they did not evaluate the interpolant of relative permeability curves. They focused only on evaluating the oil recovery factor and pressure drop. As reinforced by Ma and James [14], the UTCOMP simulator does not include the modeling package of geochemical reactions like ion exchange and mineral dissolution. Using a similar approach, Dang et al. [21] compared conventional waterflooding, LSWI, miscible CO2 injection, and miscible CO2-LSWAG injection in sandstones using the GEMTM compositional simulator with a mechanistic LSW model at the core and field scale. Their results showed the CO2-LSWAG case to be the best scenario. They concluded that the performance of CO2-LSWAG depends on the initial wettability condition, the rock composition, the reservoir heterogeneity, the formation and injection water compositions, the miscibility conditions, and CO2-LSWAG operational parameters, such as flow rate and CO2-LSWAG ratio. Given the limited numerical investigations considering CO2-LSWAG under immiscible conditions in carbonate systems, where most worldwide oil reserves are located, this kind of reservoir requires more in-depth research.
In this context, it is worth highlighting that two major types of mechanisms are involved in oil displacement and recovery during CO2-LSWAG: the geochemical, which is related to rock-fluid interactions, and the physicochemical, which includes all fluid–fluid interactions, such as oil swelling and viscosity reduction due to gas solubilization into the oil [29,30]. In CO2-LSWAG EOR, both the physicochemical and geochemical coincide [22]. Naderi [31] has done a numerical study of CO2-LSWAG to evaluate geochemical and physicochemical modeling. He investigated a sandstone rock with traces of carbonate minerals, such as calcite and dolomite, under miscible conditions, compared with other injection configurations, and considered the use of high-salinity water. However, no comparison of geochemical and physicochemical effects on the performance of immiscible CO2-LSWAG in carbonates is found in the literature. An assessment of how the interpolant ion affects the CO2-LSWAG injection and the difference in the most important reservoir properties comparing different interpolants also lack in the literature. The effects of using different interpolant ions are weakly discussed, even though they are fundamental when performing reservoir simulation because the interpolant is used to obtain the relative permeability curves for the reservoir during the simulation [32].
This work employed the GEMTM simulator to comprehensively investigate continuous CO2 and CO2-LSWAG immiscible injections in a carbonate core. These processes’ physicochemical and geochemical alterations that lead to oil recovery were critically discussed. Oil recovery, water and gas production, reservoir pressure, and rock and fluid properties and composition were carefully assessed in the core and the effluent to understand the physicochemical and geochemical effects. Additionally, for CO2-LSWAG, Mg2+ and SO42− were unprecedentedly compared as interpolant ions in the relative permeability curves.
2. Materials and Methods
This work studied continuous immiscible CO2 injection and low-salinity water-alternating-CO2 injection using the GEM (Generalized Equation-of-State Model Compositional Reservoir Simulator) developed by CMG (Computer Modelling Group) under the same conditions.
Several authors have used this simulator to study enhanced recovery methods. It is essential to highlight the use of this specific tool to evaluate (i) immiscible, miscible, and near-miscible CO2 injection into sandstones as developed by Hatzignatiou and Lu (1994) [33], Kamali et al. (2015) [34] and Kamali et al. (2017) [35] and near miscible CO2 injection simulated by Wang et al. (2021) [5]; (ii) low-salinity water injection into carbonates, by Lee et al. (2020) [36], Moradpour et al. (2020) [37], Adegbite et al. (2018) [38], and Esene et al. (2018) [39]; (iii) miscible and immiscible CO2 water-alternating-gas injection (CO2-WAG) in carbonate by Hu et al. (2020) [40] and Sampaio et al. (2020) [41]; (iv) and also the low-salinity water-alternating-CO2 flooding (CO2-LSWAG), miscible in sandstone by Dang et al. (2016) [21] and Ma and James (2022) [16]. Most of these works validate the simulations of these EOR methods using experimental data from core flooding tests. In general, it is observed that the number of studies focused on the simulation of different immiscible CO2 injection approaches in carbonates, such as continuous injection or CO2-LSWAG, is more limited.
The following sections describe the simulation models, including modeling details, fluid properties, rock and grid simulation characterization, the reactional system and operational requirements specification, and relative permeability data. Additional data required for the simulations are also available in the Supplementary Materials, such as the models to calculate aqueous and hydrocarbons phases properties.
2.1. GEM Modeling of Oil Recovery by Immiscible CO2-LSWAG Injection
The EM simulator presents a numerical formulation to describe multidimensional and multiphase flow in porous media. This tool incorporates the compositional dynamics of the phases through phase behavior and correlations to compute mass transfer. Therefore, it is equation-of-state-based, and fundamentals mass, momentum, and energy balance equations are considered in association with Darcy’s law and the mechanical dispersion/diffusion of components that describe flow in porous media. From this association, multiphase multicomponent flow equations are obtained. The conservation equations that cover all oil, gas, aqueous, and mineral components, as summarized by Dang et al. (2016) [21], are presented as follows:
- For oil and gas components:
- For aqueous components:
- For mineral components:
The reactions listed in Section 2.4 are coupled to these equations and the equations for EOS flash calculations. The chemical equilibrium of intra-aqueous reactions is modeled with chemical equilibrium constants of the reactions, as shown by Dang et al. (2016) [21] and presented below.
where α = 1, …, Raq intra-aqueous reactions; refers to the chemical equilibrium constant for the aqueous reaction α and to the activity product (Equation (5)).
where ak is the activity of component k, calculated with the B-dot model; are the stoichiometry coefficients; and is the number of species in an intra-aqueous equilibrium reaction.
Kinetics of the mineral dissolution and precipitation reactions are also incorporated in the model, as detailed by Dang et al. (2016) [21]:
where β = 1, …, Rmn mineral reactions; refers to the dissolution/precipitation rate per unit bulk volume of the porous medium; to the reactive surface area for mineral β per unit of the porous medium; to the rate constant of mineral reaction; to the activity product for mineral β dissolution reaction; and refers to the chemical equilibrium constant for mineral reaction (available in the literature for many minerals).
Considering the individual contributions of the injected fluids, CO2 and LSW, the simulator can simultaneously capture the recovery mechanisms associated with each one. One of the oil recovery mechanisms related to CO2 injection is the mineral dissolution and release of ions that may participate in other chemical reactions incorporated into the model through the geochemical reactions in Section 2.4. The CO2 distribution between the oil, gas, and water phases is essential to simulate CO2 EOR. Then, the CO2 solubility in the water phase is considered by including Henry’s law. Regarding the contribution of the injection of low-salinity water, the ion exchange reaction (shown in Section 2.4) and the wettability alteration represented by the shifting of the relative permeability curves from an oil-wet condition to a water-wet condition (presented in Section 2.6) are included.
2.2. Fluid Properties
Experimental composition oil data presented by Sequeira [42] were chosen, as indicated in Table 1. As explained in the Supplementary Materials, PVT characterization data and the MMP, also available from Sequeira [32], were used for tuning the EOS to match the oil behavior through the WinProp simulator developed by CMG.

Table 1.
Oil composition [42].
The composition of the connate water (pH = 6) and the injection water is shown in Table 2. The injection water chosen is a typical composition of seawater, diluted 30 times to obtain a low-salinity brine with a concentration of 1000 ppm. This optimal concentration was selected from preliminary studies [43], in which the brine that maximizes the oil recovery factor for the same scenario investigated in this study was evaluated.

Table 2.
Connate water and low-salinity water composition.
2.3. Rock Properties and Simulation Grid
A homogeneous carbonate core with dimensions 1.5″ in diameter and 12″ in length was selected. The simulation grid was built considering the 30 × 1 × 1 blocks with an injection well at coordinates {1,1,1} and production well at coordinates {30,1,1}, approximating the cylindrical shape of the core to cartesian geometry, as illustrated in Figure 1. As a carbonate system, the volumetric composition of the core was 50% calcite and 50% dolomite, which allows for analyzing the different interactions with each type of mineral. The porosity and permeability data are also summarized in Figure 1.
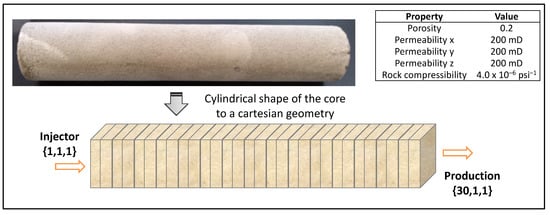
Figure 1.
Schematic diagram of the simulation grid indicating the injector well and production well.
2.4. Reactional System
The simulation model includes relevant chemical reactions related to CO2 injection and CO2-LSWAG, shown in Table 3. Besides the reactions of salt dissociation, the following reactions are worth mentioning: (1) the carbonic acid formation from the CO2 dissociation in water; (2) the rock dissolution of calcite and dolomite due to the presence of an acidic medium from the dissociation of carbonic acid; and (3) the ion exchange reactions.

Table 3.
Reactional system.
To also take into account the kinetic of mineral reactions, in addition to the equilibrium mineral dissolution/precipitation data shown in Table 3, the kinetic parameters of reactions 10 and 11 were included in the simulation model, as presented in Table 4.

Table 4.
Kinetic parameters of mineral reactions.
2.5. Operating Conditions
The initial operating conditions cover a temperature of 238°F (114.4 °C), initial water saturation of 0.0894, and pressure of 1900 psi. The initial core pressure is below the minimum miscibility pressure (6675 psi), determined experimentally by Sequeira [42]. Consequently, the present study was carried out under conditions of immiscible CO2 injection. An overview of the operating conditions used in the simulation is shown in Table 5. Injection well and production well constraints are the same for CO2 continuous flooding, differing only from the non-existence of a water flow.

Table 5.
Operating conditions.
2.6. Relative Permeability Curves
For CO2-LSWAG injection, two simulations were performed, each one using an interpolation criterion. The first simulation considered the sulfate concentration in the aqueous phase to interpolate the relative permeability curves during the process, varying between the high- and low-salinity relative permeability curves provided as input to the simulation. The second scenario used the magnesium concentration in the aqueous phase as the interpolation agent. Mg2+ and SO42− were selected because they have been widely discussed as potential candidates for wettability alteration and oil recovery factor improvement in carbonates when injecting low-salinity water [44]. Although Ca2+ is also mentioned as a possible ion, its effects are more pronounced at lower temperatures than in the analyzed reservoir [44]. The water–oil relative permeability curves for two salinity levels are shown in Figure 2, based on data available from Dang et al. [45]. It is noteworthy that, for the relative permeability curves corresponding to low-salinity water, the interpolant ions concentrations were considered according to Table 2, that is, for magnesium 170 ppm and calcium 142 ppm. Likewise, for the relative permeability curves corresponding to high-salinity water, the concentration of the interpolant ions was specified for connate water in Table 2, that is, for magnesium 4070 ppm and calcium 19,740 ppm. These values are a reference for the curves shown in Figure 2. During the simulation, when the interpolating ion concentration in a grid block assumes an intermediate value to those reported for high and low salinity, an interpolation of the reference curves is performed to determine the value of the relative permeability. The Supplementary Materials provides a detailed discussion on the interpolation of relative water–oil permeability curves, the gas–liquid relative permeability curves, the three-phase relative permeability model, and the hysteresis effect on the gas permeability curves.
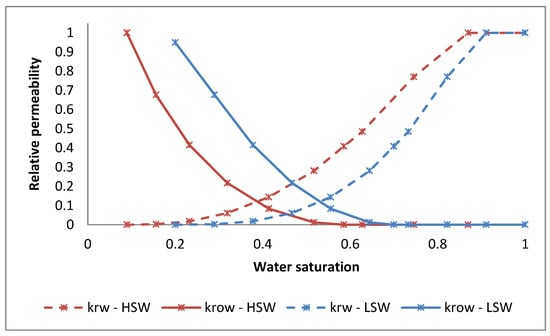
Figure 2.
Water–oil relative permeability curves.
3. Results and Discussion
Before starting the simulation of core-scale recovery methods, adjusting the model’s parameters to calculate the properties of the hydrocarbon phase is necessary, especially for the study conditions involving CO2 injection. The results of these adjustments are in the Supplementary Materials.
3.1. Oil Recovery
The comparison presented in Figure 3 indicates that CO2-LSWAG leads to a higher oil recovery than continuous CO2 injection, regardless of the considered interpolant. However, it is essential to note that even under injection conditions below the minimum miscibility pressure, a high recovery factor of 62% is reached during continuous CO2 injection.
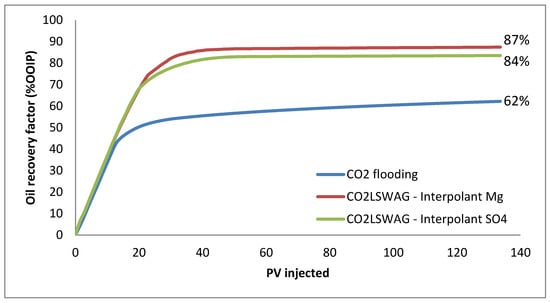
Figure 3.
Oil recovery factor for continuous CO2 injection and CO2-LSWAG considering Mg and SO4 as ion interpolant.
Furthermore, based on Figure 3, the three curves show the same behavior up to approximately the injection of 10 porous volumes. From the 10 PV injected, the core average pressure values, shown in Figure 4, become distinct. As the pressure in the production block is kept constant, this curve reflects the pressure difference between injection and production throughout the displacement. The average pressure corresponding to the continuous CO2 injection shows a slight decrease, while the curves related to CO2-LSWAG present an increase in this average value and an oscillatory trajectory due to the alternate fluids injected. Although the pressure variation was not so significant, the maintenance of a greater pressure gradient is a factor that possibly promotes increased recovery via the CO2-LSWAG method. In addition, in Figure 4, one can observe that at each cycle of CO2 injection, there is an increase in the pressure gradient. Conversely, when the injected slug is of LSW, there is a reduction in this gradient, indicating the loss of CO2 injectivity, i.e., a more significant pressure gradient is required to maintain the same injection flow rate. The water cut and gas–oil ratio graphs, also shown in Figure 5, help to identify the water and gas breakthrough in the simulated scenarios.
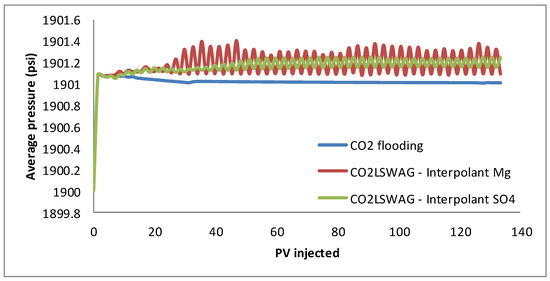
Figure 4.
Average pressure for continuous CO2 injection and CO2-LSWAG considering Mg and SO4 as ion interpolants.
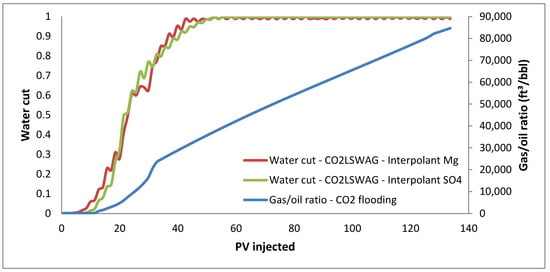
Figure 5.
Water cut for CO2-LSWAG considering Mg and SO4 as interpolant ions and gas–oil ratio for continuous CO2 injection.
Regarding CO2-LSWAG, the water breakthrough occurs at around 30 injected porous volumes, indicated by the increase in the water cut, shown in Figure 5, and by the plateau in the oil recovery curve in Figure 3. Similarly, for continuous CO2 injection, the gas breakthrough is observed at around 20 injected porous volumes, which is associated with the increase in the gas–oil ratio indicated in Figure 5 and the plateau in the recovery curve in Figure 3. All these parameters allow a general overview assessment of oil recovery but do not provide significant details explaining the additional oil production. For instance, a better understanding of porous medium mechanisms that trigger such results is obtained from analyzing the variation in properties in the simulation blocks. Therefore, the following sections present a more detailed analysis. It is important to emphasize that a microscopic analysis on a pore scale has not been done, but instead on what is happening in terms of measurable properties in the smallest simulation control unit (the grid blocks). Usually, the study of the interactions in the medium is done through experimental measurements in the steady state [9,26,27]. On the other hand, in the present work, the variations are analyzed by simulation during the dynamic displacement process.
3.2. Geochemical Mechanisms
Rock–Fluid Interactions and Mineral Dissolution
CO2 injected into the core reacts with water to form carbonic acid, which can react with the rock minerals leading to their dissolution. Variations in the mineral (calcite and dolomite) concentrations and the consequent alterations in permeability and porosity were analysed to assess the impact of mineral dissolution in the porous medium. The Supplementary Materialspresents the approach considered to compute these effects. Three blocks of the grid were selected, located at the coordinates {1,1,1}, {15,1,1}, and {30,1,1}, representing the injection point, the central point of the core, and the production point, respectively. Comparative results for the porosity variation are shown in Figure 6.
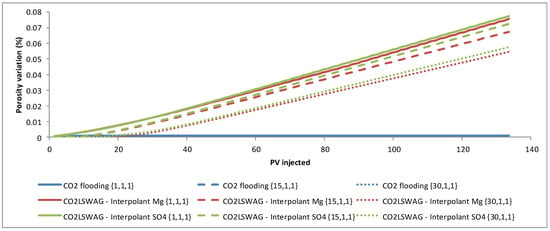
Figure 6.
Porosity variation at the coordinates {1,1,1}, {15,1,1}, and {30,1,1}, representing the injection, the central point of the core, and the production.
The formation damage decreases between the inlet and outlet of the core, with the most significant porosity change occurring at the core inlet, at coordinates {1,1,1}, and the least at coordinates {30,1,1}, as a consequence of the interaction time between CO2, LSW, and the rock. The interaction is gradual as the displacement front of the injected fluids reaches the regions. There is an increasing porosity trend because mineral dissolution predominates in the simulated core flooding tests instead of precipitation. Porosity variations follow the mineral dissolution shown in Figure 7. The results are presented for the {1,1,1} block, which gives a more accentuated mineral variation than the other blocks; following what was observed for porosity, the scenario with more significant dissolution showed a considerable increase in porosity and vice versa. This scenario is due to the gradual CO2 concentration increase in the aqueous phase, which is a consequence of the CO2 contact with the formation water or the formation water + injected water mixture in the cases of continuous CO2 injection and CO2-LSWAG, respectively. Figure 8 highlights the CO2 molality in the aqueous phase from the injection block to the core outlet in the condition of 10 porous volumes injected (before the CO2 breakthrough). In the initial grid blocks, the CO2 concentration is higher for both recovery methods evaluated, favoring the mineral dissolution. Figure 8 shows that the CO2 concentration is higher for the CO2-LSWAG simulation than for the CO2 flooding. One of the main reasons for this difference is the salting-out effect. While CO2-LSWAG is going on, there is a salinity reduction and an increase in CO2 dissolution. On the other hand, during CO2 flooding, the aqueous phase is composed of only the formation water, with high salinity compared to LSW. As a result, less CO2 solubilizes into water.
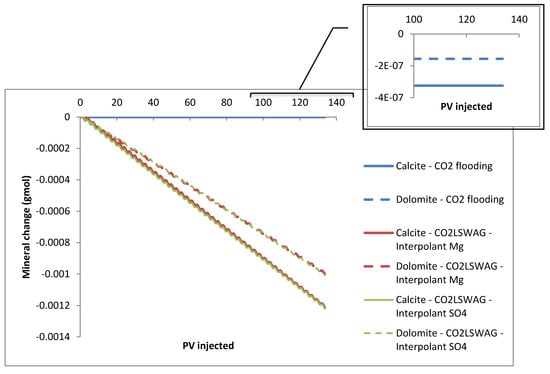
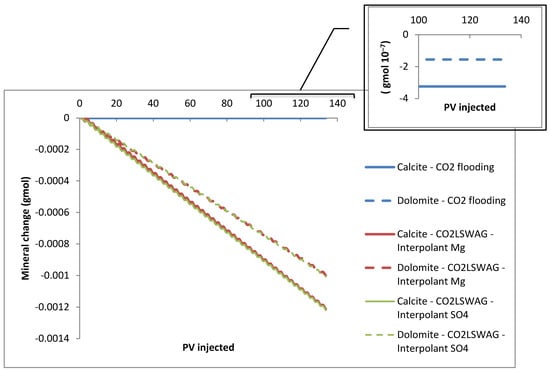
Figure 7.
Mineral dissolution for continuous CO2 injection and CO2-LSWAG considering Mg and SO4 as ion interpolants.
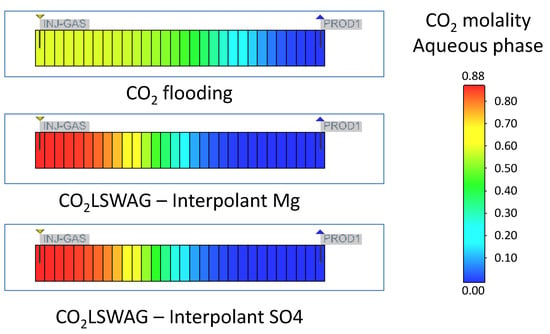
Figure 8.
CO2 molality in the aqueous phase for continuous CO2 injection and CO2-LSWAG considering Mg and SO4 as ion interpolants—10 porous volumes injected.
Although the mineral composition variations are minimal, we can observe some important points:
- For CO2 flooding, there is practically no variation because only gas is injected. Therefore, the water saturation in the medium corresponds to the initial saturation, which is less than 10%, as shown in Figure 9. In addition, since the formation water has high salinity, the already-mentioned salting-out effect reduces the CO2 dissolution in water. A small amount of CO2 solubilizes in water and forms carbonic acid, which is responsible for mineral dissolution.
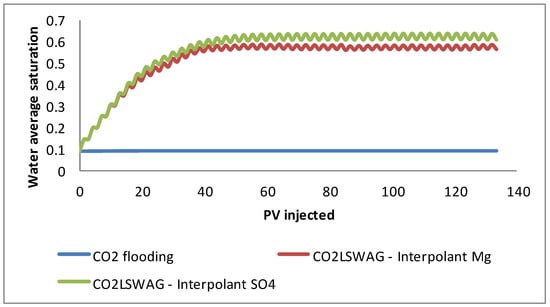 Figure 9. Water average saturation for continuous CO2 injection and CO2-LSWAG considering Mg and SO4 as ion interpolants.
Figure 9. Water average saturation for continuous CO2 injection and CO2-LSWAG considering Mg and SO4 as ion interpolants. - CO2-LSWAG, when sulfate is the interpolant ion, results in more significant mineral variation for dolomite and calcite. The higher variation, in this case, is also associated with water saturation in the core, which is higher than for CO2-LSWAG, where magnesium is the interpolant ion, as indicated in Figure 9.
For the three scenarios evaluated, calcite dissolution is greater than for dolomite because the dissolution rate of CaMg(CO3)2 is slower than CaCO3 dissolution, according to reactions 10 and 11 in Table 3 and Table 4. Xiong et al. (2021) [46] summarize some studies that have discussed these processes and observed the same behavior. Additionally, the authors argue that kinetics control the dissociation and precipitation of dolomite, while thermodynamics control the same reactions for calcite.
No permeability variation was observed in any of the analyzed grid blocks. As previously mentioned, while not negligible, a slight variation in mineral components was identified in Figure 7. Given that this was a core-scale study, insufficient data led to any significant change in the permeability because there is an equilibrium between the mineral dissolution and precipitation reactions indicated by reactions 5, 6, 10, and 11 in Table 3.
As discussed, mineral reactions were more pronounced for CO2-LSWAG than for CO2 flooding, indicating that LSW plays an essential role in geochemical interactions. It occurs because the aqueous phase is fundamental to promoting CO2 dissolution and the formation of carbonic acid. As a result, core properties change. For instance, an increase in porosity increases water saturation and carbonated water formation in situ. CO2 dissolution in the aqueous phase, as represented in Reactions 8 and 9 of Table 3, results in the H+ concentration increase in the medium. In turn, the rise in H+ concentration shifts reactions 10 and 11 in Table 3 towards mineral dissolution (right side). This observation also highlights the role of low-salinity water and its interactional effects with the rock for incremental oil recovery in the evaluated system, because CO2 dissolution in the aqueous phase is necessary to promote mineral dissolution. Moradpour et al. and Teklu et al. [9,22] highlight the rock wettability alteration due to the presence of carbonated water in situ, which makes the rock surface more hydrophilic and, therefore, more water-wet.
The influence of mineral dissolution on the oil recovery factor is better understood if the interactions between the oil components and the rock sites are analyzed. Carboxylic acids (R-COOH) are the main components of oil adsorbed on the rock surface. We observed two active sites for calcite, >CO3− and >Ca+, where the symbol > represents the rock surface. For dolomite, in addition to calcite sites, >Mg+ also appears. The oil polar group (RHCOO−) interacts with the rock sites with positive charges. Oil is apart from the rock and consequently is recovered when it dissolves, as shown in the diagram in Figure 10. In this paper, the mineral reactions considered are related to carbonic acid formation due to the CO2 dissolution in water. However, oil recovery by the rock dissolution mechanism and consequent wettability alteration is also discussed by some authors in low-salinity water flooding studies, such as by Mohammadi et al. [47], Al-Shalabi et al. [48], and Hiorth et al. [49]. Nonetheless, the simulation model developed does not capture the wettability alteration by rock dissolution due to LSW injection in the present study. The effect of LSW on wettability is quantified only through the relative permeability curves. They are set at the simulation beginning but do not consider the CO2 content in water as an interpolation criterion. Regarding rock–fluid interactions, the simulator only includes the influence of formation damage in the recovery estimates, as the porosity variation was less than 1%. Therefore, despite being observed, the rock dissolution mechanism has no significant effect on oil recovery for the simulated core-scale cases.
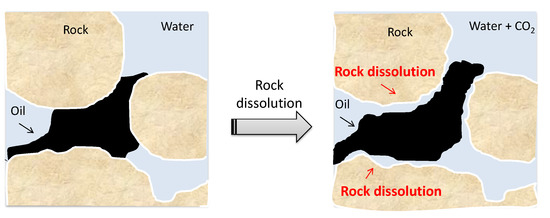
Figure 10.
Schematic diagram of rock dissolution due to carbonated water in situ and oil detachment.
3.3. Physicochemical Interactions
3.3.1. Fluid–Fluid Interaction—Effluent Analysis
To evaluate the fluid–fluid interactions and their influence on the oil recovery estimates for the three simulated scenarios, the results obtained for the last block of the grid, located at the coordinates {30,1,1}, were analyzed. This block corresponds to the core’s production, providing data for a more detailed analysis of the effluent.
Figure 11 shows the pH variations during the core flooding test simulation. Initially, the pH of the effluent is equal to that of the formation water, highlighted in Table 2. Two trends related to pH can be observed in the results. In all cases, the pH decreases due to the presence of CO2 and the formation of carbonic acid. The curve of the continuous CO2 injection evidences this. During CO2-LSWAG, as discussed before, there is a more accentuated dissolution of the rock, both calcite and dolomite. Dissolving these minerals increases the concentration of Ca2+ and Mg2+ ions in the aqueous phase, resulting in a higher pH when compared to continuous CO2 injection. The rise in the HCO3 can infer the increase in these ions concentrations, also shown in Figure 11, from reactions 10 and 11 in Table 3, corresponding to mineral dissolution.
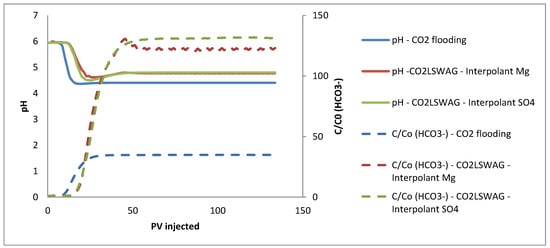
Figure 11.
Results of pH and HCO3− concentration in the effluent for continuous CO2 injection and CO2-LSWAG considering Mg and SO4 as ion interpolants.
Figure 12 shows the concentration variation of the interpolant ions SO42− and Mg2+ for the three simulations. As there is neither additional water supply nor much mineral dissolution, these ions’ concentration during the CO2 flooding can be supposed to be constant and equal to that of the formation water. For CO2-LSWAG cases, there is a reduction in the concentration of the two ions caused by the injection of low-salinity water. As discussed earlier, there is an increase in Mg2+ concentration due to the dissolution of calcite and dolomite. However, the impact of this increase is less than that of dilution caused by injected water. According to the results, the reduction of magnesium concentration before reaching the minimum value is more significant than sulfate in both scenarios.
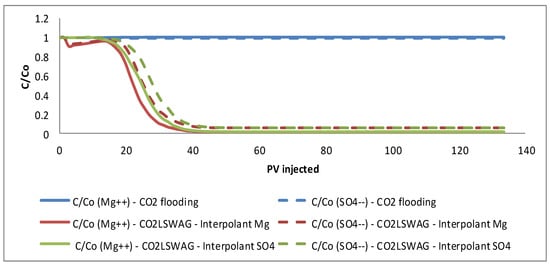
Figure 12.
Results of pH and Mg2+ and SO42− concentration in the effluent for continuous CO2 injection and CO2-LSWAG considering Mg and SO4 as ion interpolants.
For this reason, the trend for the magnesium concentration to be closer to its concentration in the low-salinity water is adopted as a criterion in the relative permeability curves. A higher oil relative permeability favors oil recovery if the interpolant ion concentration is closer to that established for LSW. Therefore, this variation is the primary reason for the oil recovery factor estimated to be higher when magnesium is the interpolant ion instead of sulfate in CO2-LSWAG.
Figure 13 shows the effluent salinity curves in line with the results discussed. There is a dilution of the aqueous phase for CO2-LSWAG due to brine injection with a lower concentration of salts than the formation water initially present in the core. Data on CO2 solubility in the aqueous phase are also shown. Due to the salting-out effect, the solubility is lower for the CO2 flooding scenario because the brine has a higher concentration of salts. Although the CO2 solubility in water reduces the amount of gas that can solubilize in oil, it also leads to mineral dissolution and wettability alteration, which are advantageous, as already mentioned.
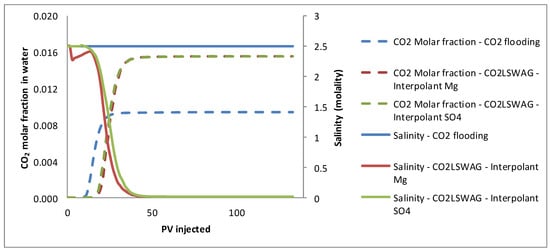
Figure 13.
Results of CO2 molar fraction and salinity of the effluent for continuous CO2 injection and CO2-LSWAG considering Mg and SO4 as ion interpolants.
3.3.2. Fluid–Fluid Interaction—Compositional and Interfacial Properties
In the simulations, CO2 was injected below the minimum miscibility pressure in continuous flooding and CO2-LSWAG. Even for an immiscible injection, compositional effects related to CO2 solubility in the oil can be observed in Figure 14. Experimentally, Bui et al. [50] demonstrated oil swelling at different pressures, including below the MMP. Spivak et al. [51] have already pointed out the contribution of compositional interactions of immiscible CO2 over time during injection in oil reservoirs. As the CO2 molar fraction in oil increases, we can observe the reduction in viscosity and oil swelling caused by a density increase. Both variations improve the displacement properties of this phase. The oil swelling is related to the oil volume increase and, consequently, the oil saturation in the core increase, leading to higher oil relative permeabilities [52]. The peaks identified in the curves shown in Figure 14a with an opposite behavior (increase in viscosity and reduction in density) are related to the compositional effects of mass transfer between the oil and gas phases (oil light components extraction), as can be seen for the methane molar fraction variation presented in Figure 14b.
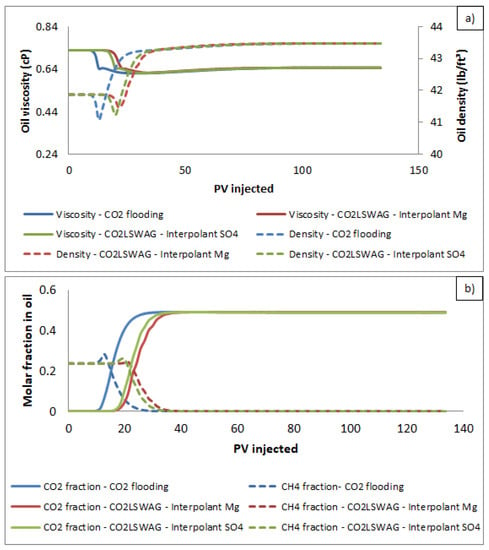
Figure 14.
Results of (a) viscosity and density and (b) CO2 molar fraction and CH4 molar fraction of oil effluent for continuous CO2 injection and CO2-LSWAG considering Mg and SO4 as ion interpolants.
As CO2 is injected under immiscible conditions, gas dissolution in the oil phase is not beneficial. However, the compositional interaction between CO2 and the aqueous phase plays a vital role in oil recovery. As Moradpour et al. [24] pointed out, the mobility of displacing CO2 is enhanced, the viscous fingers are attenuated, and the sweep efficiency is improved.
The effect of interfacial tension reduction is one of the primary mechanisms mentioned in the literature due to CO2 injection. Figure 15a presents the gas/oil interfacial tension results for the grid block located at the intermediate point {15,1,1} and the core production block located at the coordinates {30,1,1}. Initially, the interfacial tension is null because there is no free gas phase in the core. Figure 15b shows the gas saturation. After CO2 injection, the saturation is no longer null, and the interfacial tension is quantified. As there is no alternation of fluids injection, the appearance of this phase occurs before the continuous injection of CO2 for the two grid blocks analyzed. Thus, CO2 injection controls the physicochemical interactions since the variations in oil, gas, and water properties present similar behaviors for all injections. The only difference observed is related to the time the changes occur, faster or slower depending on whether the injection is continuous or LSWAG. In this context, the injection of LSW only delays the contact of CO2 with the phases and, consequently, the physicochemical interactions. The results also indicate the presence of CO2 causing the observed physicochemical alteration in the system. The interfacial tension values are close for the three simulated cases, tending to the same value in the final simulation time. Comparing the CO2-LSWAG injection results reveals no difference in the gas/oil interfacial tension. That occurs due to the polar oil components, which interact in the interface with gas, even when the gas saturation is higher for the scenario in which magnesium is the interpolant ion of the relative permeability curves.
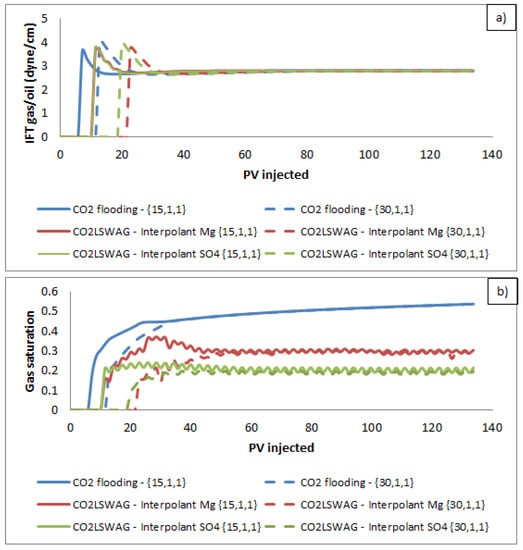
Figure 15.
(a) Interfacial tension gas/oil and (b) gas saturation at grid blocks {15,1,1} and {30,1,1} for continuous CO2 injection and CO2-LSWAG considering Mg and SO4 as ion interpolants.
On the other hand, the brine’s salinity affects the oil/water interfacial tension. In this system, the polar oil components and the salts in the water interact at the interface. However, salinity assumes the same values for the two simulated CO2-LSWAG scenarios. It is essential to clarify that when CO2 solubilizes in the aqueous phase, there is a reduction in the gas–aqueous phase interfacial tension. In addition, due to CO2 diffusion through the brine, CO2 migrates to the oil–brine interface, resulting in the interfacial tension reduction of the oil–brine system and, consequently, CO2 diffusion in the oil [9,22]. All the interfacial effects favor oil recovery and displacement. Moreover, the indirect interaction between CO2 and oil occurs in the WAG process, in which the slugs alternation can prevent the gas from directly contacting the oil.
4. Conclusions
The present work investigated immiscible CO2-LSWAG and continuous CO2 flooding as EOR methods in carbonate systems. Core flooding simulations evaluated the physicochemical and geochemical interactions of CO2-LSWAG on carbonates using experimental oil data from the literature. Oil recovery, water and gas production, reservoir pressure, and rock and fluid properties and composition were carefully assessed in the core and the effluent to understand the contributions behind these obtained results. The main conclusions, based on the results obtained, are summarized in the following:
- Even under injection conditions below the minimum miscibility pressure, continuous CO2 injection achieves a high oil recovery factor of 62%.
- In addition to the unique advantages of each injected fluid, the detailed investigation of physicochemical and geochemical variations highlights that the interaction of CO2 and LSW also contributes to additional oil recovery. The reduction of the aqueous phase salinity accentuates this interaction due to a decrease in the salting-out effect. Despite not using experimental recovery data from core flooding tests, the simulations in this paper allow relating the properties to each other and the consequences of these variations in the oil displacement, making a separate assessment of each interaction rock–fluid or fluid–fluid.
- Results show that mineral reactions were more pronounced for CO2-LSWAG than for CO2 flooding, indicating the critical role of LSW in the geochemical process, even though the simulation model did not include the mineral dissolution mechanism directly related to the LSW injection. Their effects on core properties, such as porosity increase, are controlled by CO2 and LSW interaction associated with in situ formations of carbonate water. The difference between the recovery estimates is due to CO2 dissociation and carbonic acid formation, and this is more noticeable when injecting LSW and the water saturation increases. Furthermore, formation damage was more pronounced for calcite than for dolomite sites in the core, according to the dissolution reaction rate of these minerals.
- CO2 injection controls the physicochemical interactions, even under immiscible conditions. The presence of CO2 highly affects the physicochemical properties. Compositional and interfacial effects related to oil recovery improvement can still be observed at pressures below the MMP, such as CO2 solubility in oil, oil viscosity reduction, oil swelling, gas/oil interfacial tension reduction, and extraction of light hydrocarbons. Oil, gas, and water properties changes present similar behavior for CO2 flooding or CO2-LSWAG, differing just because variations occur faster or slower depending on whether the injection is continuous or water-alternating-gas, as the water slugs delay the contact of the injected CO2 with the phases.
- CO2-LSWAG simulations using Mg2+ as an interpolant improved oil recovery more than SO42−. In this case, magnesium ion concentration in the aqueous phase is closer to the LSW condition after dilution by low-salinity water injection and variation due to mineral dissolution. It leads to relative permeability values, which are more favorable for oil recovery when compared to sulfate concentration.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en16010440/s1, Figure S1: Swelling factor and Saturation pressure fitting as a function of CO2 molar fraction; Figure S2: Gas-liquid relative permeability curve. References [53,54,55,56,57] are cited in the supplementary materials.
Author Contributions
L.d.S.B.: conceptualization, methodology, investigation, writing—original draft preparation, writing—review & editing; I.E.d.S.L.: conceptualization, methodology, investigation, writing—original draft preparation, writing—review & editing; G.M.N.C.: conceptualization, methodology, investigation, writing—original draft preparation, writing—review & editing, supervision, and project administration; S.A.B.V.d.M.: conceptualization, writing—review & editing, supervision, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Petrogal Brasil S.A., grant number 20623-5.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the Agência Nacional de Petróleo, Gás Natural e Biocombustíveis (ANP) and the Petrogal Brasil S.A., related to the grant from the R&D rule, as well as the CMG’s Engineer Juan Mateo.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xiong, Y.; Hou, Z.; Xie, H.; Zhao, J.; Tan, X.; Luo, J. Microbial-Mediated CO2 Methanation and Renewable Natural Gas Storage in Depleted Petroleum Reservoirs: A Review of Biogeochemical Mechanism and Perspective. Gondwana Res. 2022, in press. [Google Scholar] [CrossRef]
- Ghedan, S. Global Laboratory Experience of CO2-EOR Flooding. In Proceedings of the SPE/EAGE Reservoir Characterization and Simulation Conference, Abu Dhabi, UAE, 19–21 October 2009; SPE. [Google Scholar]
- Perera, M.; Gamage, R.; Rathnaweera, T.; Ranathunga, A.; Koay, A.; Choi, X. A Review of CO2-Enhanced Oil Recovery with a Simulated Sensitivity Analysis. Energies 2016, 9, 481. [Google Scholar] [CrossRef]
- Bikkina, P.; Wan, J.; Kim, Y.; Kneafsey, T.J.; Tokunaga, T.K. Influence of Wettability and Permeability Heterogeneity on Miscible CO2 Flooding Efficiency. Fuel 2016, 166, 219–226. [Google Scholar] [CrossRef]
- Wang, G.; Pickup, G.E.; Sorbie, K.S.; Mackay, E.J.; Skauge, A. Multi-Physics Approach to Modelling near-Miscible CO2-WAG Process. J. Pet. Sci. Eng. 2021, 198, 108165. [Google Scholar] [CrossRef]
- Skauge, A.; Serbie, K. Status of Fluid Flow Mechanisms for Miscible and Immiscible WAG. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 31 March–2 April 2014; pp. 891–905. [Google Scholar]
- Qin, Z.; Arshadi, M.; Piri, M. Near-Miscible Supercritical CO2 Injection in Oil-Wet Carbonate: A Pore-Scale Experimental Investigation of Wettability State and Three-Phase Flow Behavior. Adv. Water Resour. 2021, 158, 104057. [Google Scholar] [CrossRef]
- Al-Mutairi, S.M.; Abu-Khamsin, S.A.; Okasha, T.M.; Hossain, M.E. An Experimental Investigation of Wettability Alteration during CO2 Immiscible Flooding. J. Pet. Sci. Eng. 2014, 120, 73–77. [Google Scholar] [CrossRef]
- Moradpour, N.; Pourafshary, P.; Zivar, D. Experimental Analysis of Hybrid Low Salinity Water Alternating Gas Injection and the Underlying Mechanisms in Carbonates. J. Pet. Sci. Eng. 2021, 202, 108562. [Google Scholar] [CrossRef]
- Jadhunandan, P.P.; Morrow, N.R. Spontaneous Imbibition of Water by Crude Oil/Brine/Rock Systems. Situ 1991, 15, 319–345. [Google Scholar]
- Yousef, A.A.; Al-Saleh, S.; Al-Jawfi, M. New Recovery Method for Carbonate Reservoirs through Tuning the Injection Water Salinity: Smart WaterFlooding. In Proceedings of the SPE EUROPEC/EAGE Annual Conference and Exhibition, Vienna, Austria, 23–26 May 2011; SPE. [Google Scholar]
- Zaheri, S.H.; Khalili, H.; Sharifi, M. Experimental Investigation of Water Composition and Salinity Effect on the Oil Recovery in Carbonate Reservoirs. Oil Gas Sci. Technol.–Rev. d’IFP Energies Nouv. 2020, 75, 21. [Google Scholar] [CrossRef]
- Hosseini, E.; Chen, Z.; Sarmadivaleh, M.; Mohammadnazar, D. Applying Low-Salinity Water to Alter Wettability in Carbonate Oil Reservoirs: An Experimental Study. J. Pet. Explor. Prod. Technol. 2021, 11, 451–475. [Google Scholar] [CrossRef]
- Façanha, J.M.F.; Farzaneh, S.A.; Sohrabi, M. Qualitative Assessment of Improved Oil Recovery and Wettability Alteration by Low Salinity Water Injection for Heterogeneous Carbonates. J. Pet. Sci. Eng. 2022, 213, 110312. [Google Scholar] [CrossRef]
- Farhadi, H.; Fatemi, M.; Ayatollahi, S. Experimental Investigation on the Dominating Fluid-Fluid and Rock-Fluid Interactions during Low Salinity Water Flooding in Water-Wet and Oil-Wet Calcites. J. Pet. Sci. Eng. 2021, 204, 108697. [Google Scholar] [CrossRef]
- Ma, S.; James, L.A. Literature Review of Hybrid CO2 Low Salinity Water-Alternating-Gas Injection and Investigation on Hysteresis Effect. Energies 2022, 15, 7891. [Google Scholar] [CrossRef]
- Sarvestani, A.D.; Ayatollahi, S.; Bahari Moghaddam, M. Smart Water Flooding Performance in Carbonate Reservoirs: An Experimental Approach for Tertiary Oil Recovery. J. Pet. Explor. Prod. Technol. 2019, 9, 2643–2657. [Google Scholar] [CrossRef]
- Rahimi, A.; Honarvar, B.; Safari, M. The Role of Salinity and Aging Time on Carbonate Reservoir in Low Salinity Seawater and Smart Seawater Flooding. J. Pet. Sci. Eng. 2020, 187, 106739. [Google Scholar] [CrossRef]
- Namaee-Ghasemi, A.; Behbahani, H.S.; Kord, S.; Sharifi, A. Geochemical Simulation of Wettability Alteration and Effluent Ionic Analysis during Smart Water Flooding in Carbonate Rocks: Insights into the Mechanisms and Their Contributions. J. Mol. Liq. 2021, 326, 114854. [Google Scholar] [CrossRef]
- Nguyen, N.; Dang, C.; Gorucu, S.E.; Nghiem, L.; Chen, Z. The Role of Fines Transport in Low Salinity Waterflooding and Hybrid Recovery Processes. Fuel 2020, 263, 116542. [Google Scholar] [CrossRef]
- Dang, C.; Nghiem, L.; Nguyen, N.; Chen, Z.; Nguyen, Q. Evaluation of CO2 Low Salinity Water-Alternating-Gas for Enhanced Oil Recovery. J. Nat. Gas Sci. Eng. 2016, 35, 237–258. [Google Scholar] [CrossRef]
- Teklu, T.W.; Alameri, W.; Graves, R.M.; Kazemi, H.; AlSumaiti, A.M. Low-Salinity Water-Alternating- CO2 EOR. J. Pet. Sci. Eng. 2016, 142, 101–118. [Google Scholar] [CrossRef]
- Wilson, A. CO2 Low-Salinity Water Alternating Gas: A Promising New Approach for EOR. JPT J. Pet. Technol. 2015, 67, 84–86. [Google Scholar] [CrossRef]
- Ramanathan, R.; Shehata, A.M.; Nasr-El-Din, H.A. Effect of Rock Aging on Oil Recovery during Water-Alternating- CO2 Injection Process: An Interfacial Tension, Contact Angle, Coreflood, and CT Scan Study. In .Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, Oklahoma, USA, 11–13 April 2016; 2016. [Google Scholar]
- Chaturvedi, K.R.; Ravilla, D.; Kaleem, W.; Jadhawar, P.; Sharma, T. Impact of Low Salinity Water Injection on CO2 Storage and Oil Recovery for Improved CO2 Utilization. Chem. Eng. Sci. 2021, 229, 116127. [Google Scholar] [CrossRef]
- Kumar, H.T.; Shehata, A.M.; Nasr-El-Din, H.A. Effectiveness of Low-Salinity and CO2 Flooding Hybrid Approaches in Low-Permeability Sandstone Reservoirs. In Proceedings of the SPE Trinidad and Tobago Section Energy Resources Conference, Port of Spain, Trinidad and Tobago, 13–15 June 2016. [Google Scholar]
- Al-Abri, H.; Pourafshary, P.; Mosavat, N.; Al Hadhrami, H. A Study of the Performance of the LSWA CO2 EOR Technique on Improvement of Oil Recovery in Sandstones. Petroleum 2019, 5, 58–66. [Google Scholar] [CrossRef]
- Al-Shalabi, E.W.; Sepehrnoori, K.; Pope, G. Numerical Modeling of Combined Low Salinity Water and Carbon Dioxide in Carbonate Cores. J. Pet. Sci. Eng. 2016, 137, 157–171. [Google Scholar] [CrossRef]
- Nobakht, M.; Moghadam, S.; Gu, Y. Effects of Viscous and Capillary Forces on CO2 Enhanced Oil Recovery under Reservior Conditions. Energy Fuels 2007, 21, 3469–3476. [Google Scholar] [CrossRef]
- Katende, A.; Sagala, F. A Critical Review of Low Salinity Water Flooding: Mechanism, Laboratory and Field Application. J. Mol. Liq. 2019, 278, 627–649. [Google Scholar] [CrossRef]
- Naderi, S.; Simjoo, M. Numerical Study of Low Salinity Water Alternating CO2 Injection for Enhancing Oil Recovery in a Sandstone Reservoir: Coupled Geochemical and Fluid Flow Modeling. J. Pet. Sci. Eng. 2019, 173, 279–286. [Google Scholar] [CrossRef]
- Dang, C.T.Q.; Nghiem, L.X.; Chen, Z.; Nguyen, Q.P. Modeling Low Salinity Waterflooding: Ion Exchange, Geochemistry and Wettability Alteration. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 30 September–2 October 2013; Volume 6, pp. 4302–4323. [Google Scholar]
- Lu, D.G.H.Y.; Hatzignatiou, D.G. Process in heavy oil reservoirs Feasibility Study of CO2 Immiscible Displacement Process in Heavy Oil Reservoirs. In Proceedings of the Annual Technical Meeting, Calgary, Alberta, 11–14 June 1994. [Google Scholar]
- Kamali, F.; Hussain, F.; Cinar, Y. A Laboratory and Numerical-Simulation Study of Co-Optimizing CO2 Storage and CO2 Enhanced Oil Recovery. SPE J. 2015, 20, 1227–1237. [Google Scholar] [CrossRef]
- Kamali, F.; Hussain, F. Field-Scale Simulation of CO2 Enhanced Oil Recovery and Storage through SWAG Injection Using Laboratory Estimated Relative Permeabilities. J. Pet. Sci. Eng. 2017, 156, 396–407. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.; Wang, J.; Sung, W. Relationship between Oil Production and CO2 Storage during Low-Salinity Carbonate Water Injection in Acid Carbonate Reservoirs. J. Ind. Eng. Chem. 2020, 88, 215–223. [Google Scholar] [CrossRef]
- Moradpour, N.; Karimova, M.; Pourafshary, P.; Zivar, D. Effects of Slug Size, Soaking, and Injection Schemes on the Performance of Controlled Ions Water Flooding in Carbonates. ACS Omega 2020, 5, 18155–18167. [Google Scholar] [CrossRef]
- Adegbite, J.O.; Al-Shalabi, E.W.; Ghosh, B. Geochemical Modeling of Engineered Water Injection Effect on Oil Recovery from Carbonate Cores. J. Pet. Sci. Eng. 2018, 170, 696–711. [Google Scholar] [CrossRef]
- Esene, C.; Onalo, D.; Zendehboudi, S.; James, L.; Aborig, A.; Butt, S. Modeling Investigation of Low Salinity Water Injection in Sandstones and Carbonates: Effect of Na+ and SO42−. Fuel 2018, 232, 362–373. [Google Scholar] [CrossRef]
- Hu, G.; Li, P.; Yi, L.; Zhao, Z.; Tian, X.; Liang, X. Simulation of Immiscible Water-Alternating- CO2 Flooding in the Liuhua Oilfield Offshore Guangdong, China. Energies 2020, 13, 2130. [Google Scholar] [CrossRef]
- Sampaio, M.A.; de Mello, S.F.; Schiozer, D.J. Impact of Physical Phenomena and Cyclical Reinjection in Miscible CO2-WAG Recovery in Carbonate Reservoirs. J. Pet. Explor. Prod. Technol. 2020, 10, 3865–3881. [Google Scholar] [CrossRef]
- Sequeira, D.S. Compositional Effects on Gas-Oil Interfacial Tension and Miscibility at Reservoir Conditions. Master’s Thesis, Louisiana State University, Baton Rouge, LA, USA, 2006. [Google Scholar]
- Bastos, L.S.; Lins, I.E.S.; Costa, G.M.N.; de Melo, S.A.B.V. Assessing Water Salinity, Ion Composition, and Interpolant Ion Effects on Oil Recovery by Numerical Simulation of Smart Water Core Flood in Carbonate. In Proceedings of the Petrophase 2022, Bucaramanga, Colombia, 12–16 June 2022. [Google Scholar]
- Sagbana, I.P.; Sarkodie, K.; Nkrumah, W.A. A Critical Review of Carbonate Reservoir Wettability Modi Fi Cation during Low Salinity Water Fl Ooding. Petroleum 2022, in press. [CrossRef]
- Dang, C.T.Q.; Nghiem, L.X.; Chen, Z.; Nguyen, N.T.B.; Nguyen, Q.P. CO2 Low Salinity Water Alternating Gas: A New Promising Approach for Enhanced Oil Recovery. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, Oklahoma, USA, 12–16 April 2014; Volume 1, pp. 578–596. [Google Scholar]
- Xiong, Y.; Hou, Z.; Tan, X.; Luo, J.; Yue, Y.; Wu, K. Constraining Fluid-Rock Interactions during Eogenetic Karst and Their Impacts on Carbonate Reservoirs: Insights from Reactive Transport Modeling. Appl. Geochem. 2021, 131, 105050. [Google Scholar] [CrossRef]
- Mohammadi, S.; Kord, S.; Mohammadzadeh, O.; Moghadasi, J. An Experimental Study into Rock Dissolution Mechanism during Diluted Seawater Injection in Carbonate Rocks. Upstream Oil Gas Technol. 2021, 6, 100031. [Google Scholar] [CrossRef]
- Al-Shalabi, E.W.; Sepehrnoori, K.; Pope, G. Geochemical Interpretation of Low-Salinity-Water Injection in Carbonate Oil Reservoirs. SPE J. 2015, 20, 1212–1226. [Google Scholar] [CrossRef]
- Hiorth, A.; Cathles, L.M.; Madland, M.V. The Impact of Pore Water Chemistry on Carbonate Surface Charge and Oil Wettability. Transp. Porous Media 2010, 85, 1–21. [Google Scholar] [CrossRef]
- Bui, L.; Tsau, J.-S.; Willhite, G.P. Laboratory Investigations of CO2 Near Miscible Application in Arbuckle Reservoir. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, Oklahoma, USA, 24–28 April 2010. [Google Scholar] [CrossRef]
- Spivak, A.; Garrison, W.H.; Ngugen, J.P. Review of an Immiscible CO2 Project, Tar Zone, Fault Block V, Wilmington Field, California. SPE Reserv. Eng. Soc. Pet. Eng. 1990, 5, 155–162. [Google Scholar] [CrossRef]
- Drexler, S.G. Study of the Fluid-Fluid and Rock-Fluid Interactions: Impact of Dissolved CO2 on Interfacial Tension an Wettability for the Brazilian Pre-Salt Scenario. Ph.D. Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2018. [Google Scholar]
- Peng, D.Y.; Robinson, D.B. A New Two-Constant Equation of State. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Jossi, J.A.; Stiel, L.I.; Thodos, G. The Viscosity of Pure Substances in the Dense Gaseous and Liquid Phases. AIChE J. 1962, 8, 59–63. [Google Scholar] [CrossRef]
- Yoonm, P.; Thodos, G. Viscosity of Nonpolar Gaseous Mixtures at Normal Pressures. AIChE J. 1970, 16, 300–304. [Google Scholar] [CrossRef]
- Kestin, J.; Khalifa, H.E.; Sookiazian, H.; Wakeham, W.A. Experimental Investigation of the Effect of Pressure on the Viscosity of Water in the Temperature Range 10-15 Degree C. Ber. Bunsenges./Physical Chem. Chem. Phys. 1978, 82, 180–188. [Google Scholar] [CrossRef]
- Awolayo, A.N.; Sarma, H.K.; Nghiem, L.X. A Comprehensive Geochemical-Based Approach at Modeling and Interpreting Brine Dilution in Carbonate Reservoirs. In Proceedings of the SPE Reservoir Simulation Conference, Montgomery, TX, USA, 20–22 February 2017; pp. 648–674. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).