Thermal Properties of Shape-Stabilized Phase Change Materials Based on Porous Supports for Thermal Energy Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Mixtures of Salts for PCM
2.3. Preparation of Shape-Stabilized PCMs
2.4. Methods of Characterization
3. Results and Discussion
3.1. Characterization of the Shape Stabilizers
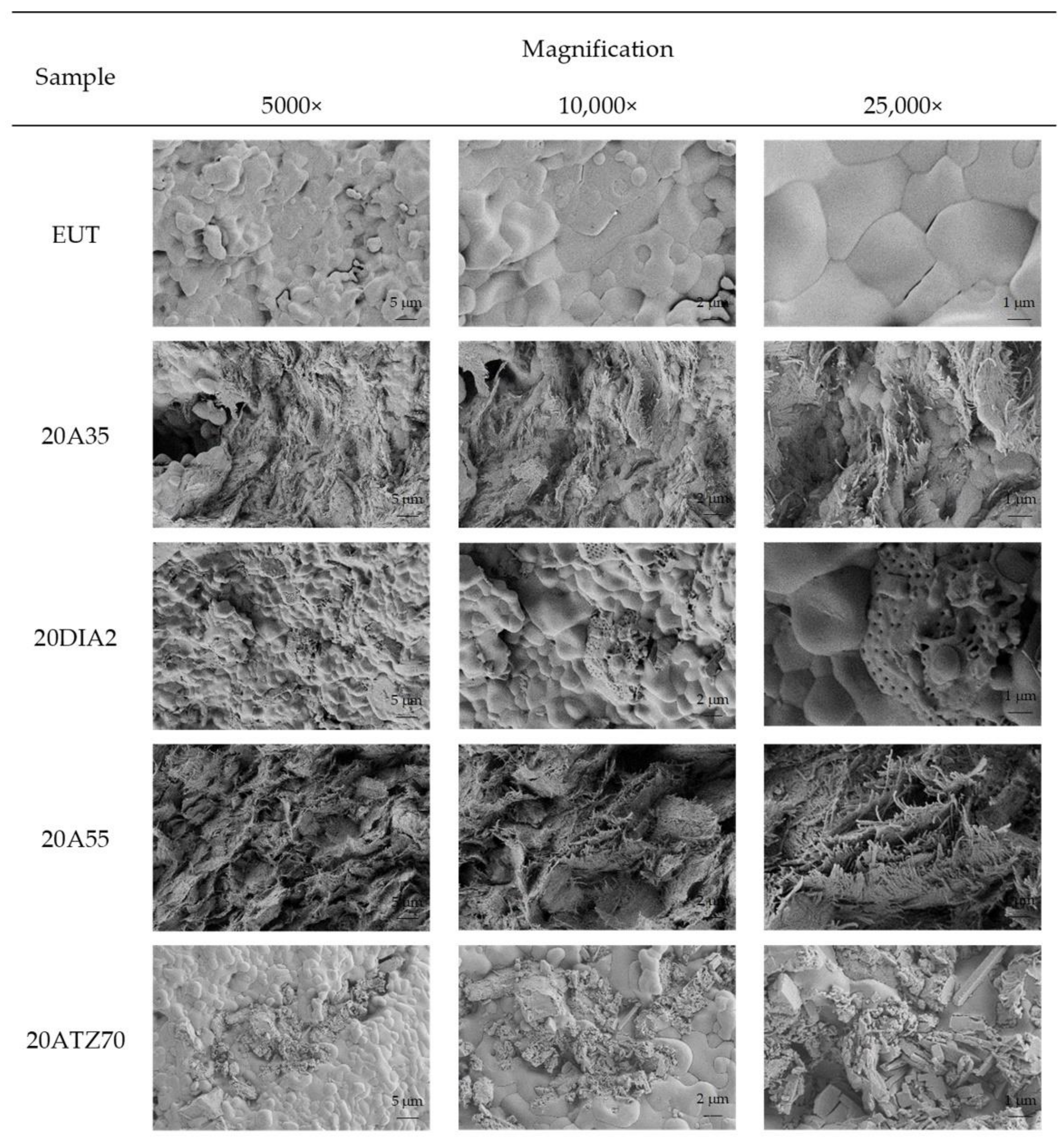
3.1.1. Morphology
3.1.2. Thermal Stability
3.2. Characterization of the Shape-Stabilized Phase Change Materials SSPCM
3.2.1. DSC Results
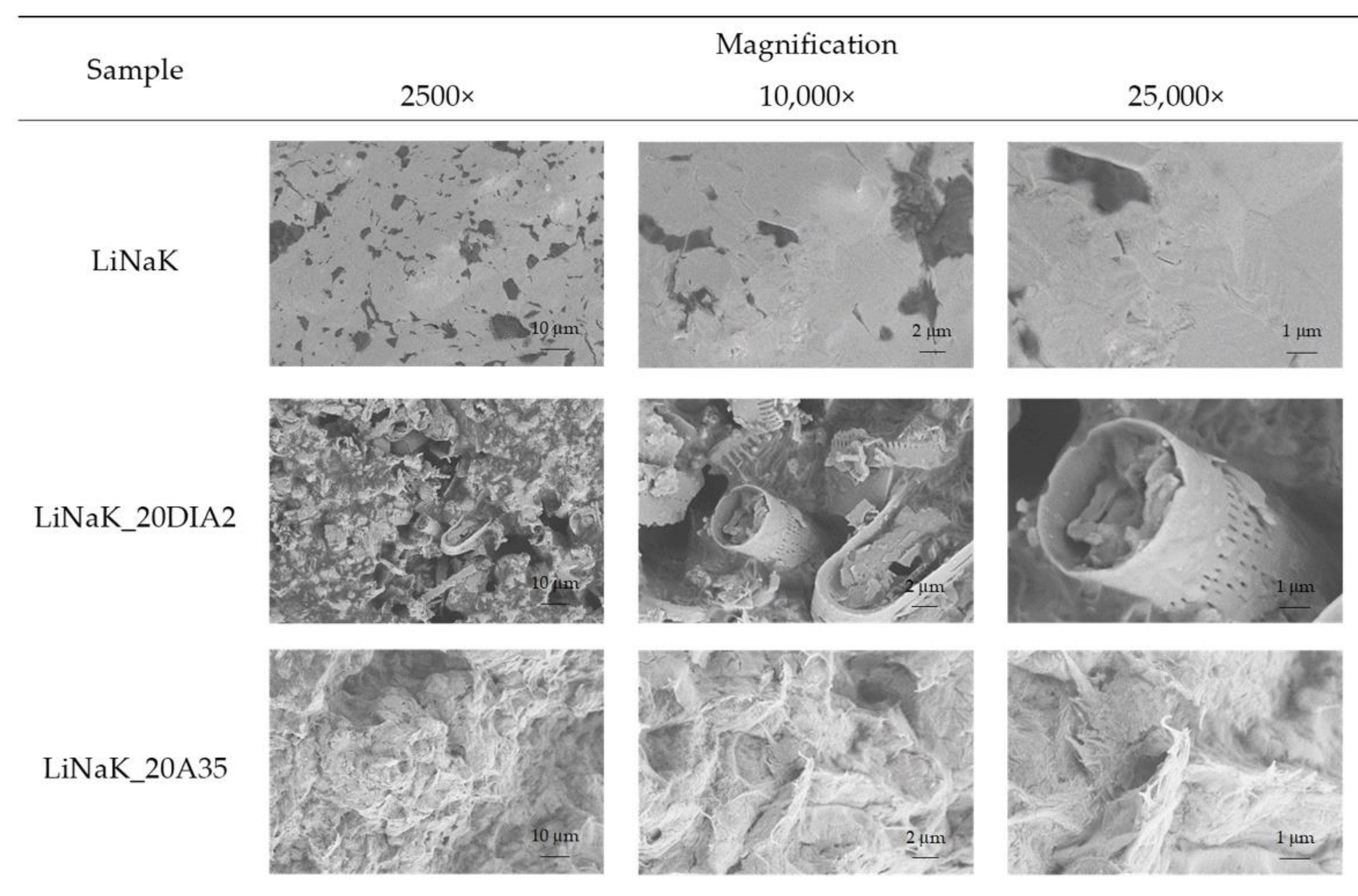
3.2.2. Morphology of SSPCM
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- OECD/IEA—International Energy Agency. World Energy Outlook. 2018. Available online: https://www.iea.org/topics/world-energy-outlook (accessed on 15 September 2021).
- IRENA. Scenarios for the Energy Transition: Global Experiences and Best Practices; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2020; ISBN 9789292602673. [Google Scholar]
- International Renewable Energy Agency. Renewable Power Generation Costs in 2019. 2020. Available online: https://www.irena.org/ (accessed on 18 September 2021).
- U.S. Energy Information Administration. International Energy Outlook 2019; 2019. Available online: https://www.eia.gov/outlooks/ieo/ (accessed on 18 September 2021).
- Sorrell, S. Reducing Energy Demand: A Review of Issues, Challenges and Approaches. Renew. Sustain. Energy Rev. 2015, 47, 74–82. [Google Scholar] [CrossRef]
- Iddrisu, I.; Bhattacharyya, S.C. Sustainable Energy Development Index: A Multi-Dimensional Indicator for Measuring Sustainable Energy Development. Renew. Sustain. Energy Rev. 2015, 50, 513–530. [Google Scholar] [CrossRef]
- Dargusch, M.; Liu, W.D.; Chen, Z.G. Thermoelectric Generators: Alternative Power Supply for Wearable Electrocardiographic Systems. Adv. Sci. 2020, 7, 2001362. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.D.; Wang, D.Z.; Liu, Q.; Zhou, W.; Shao, Z.; Chen, Z.G. High-Performance GeTe-Based Thermoelectrics: From Materials to Devices. Adv. Energy Mater. 2020, 10, 2000367. [Google Scholar] [CrossRef]
- Liu, M.; Tay, N.S.; Bell, S.; Belusko, M.; Jacob, R.; Will, G.; Saman, W.; Bruno, F. Review on Concentrating Solar Power Plants and New Developments in High Temperature Thermal Energy Storage Technologies. Renew. Sustain. Energy Rev. 2016, 53, 1411–1432. [Google Scholar] [CrossRef]
- Gajendiran, M.; Nallusamy, N. Application of Solar Thermal Energy Storage for Industrial Process Heating. Adv. Mater. Res. 2014, 984–985, 725–729. [Google Scholar] [CrossRef]
- Jacob, R.; Belusko, M.; Liu, M.; Saman, W.; Bruno, F. Using Renewables Coupled with Thermal Energy Storage to Reduce Natural Gas Consumption in Higher Temperature Commercial/Industrial Applications. Renew. Energy 2019, 131, 1035–1046. [Google Scholar] [CrossRef]
- Sarbu, I.; Sebarchievici, C. A Comprehensive Review of Thermal Energy Storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef]
- Elbahjaoui, R.; el Qarnia, H. Performance Evaluation of a Solar Thermal Energy Storage System Using Nanoparticle-Enhanced Phase Change Material. Int. J. Hydrogen Energy 2019, 44, 2013–2028. [Google Scholar] [CrossRef]
- Pirasaci, T.; Wickramaratne, C.; Moloney, F.; Goswami, D.Y.; Stefanakos, E. Influence of Design on Performance of a Latent Heat Storage System at High Temperatures. Appl. Energy 2018, 224, 220–229. [Google Scholar] [CrossRef]
- Kenisarin, M.M. High-Temperature Phase Change Materials for Thermal Energy Storage. Renew. Sustain. Energy Rev. 2010, 14, 955–970. [Google Scholar] [CrossRef]
- Cárdenas, B.; León, N. High Temperature Latent Heat Thermal Energy Storage: Phase Change Materials, Design Considerations and Performance Enhancement Techniques. Renew. Sustain. Energy Rev. 2013, 27, 724–737. [Google Scholar] [CrossRef]
- Nomura, T.; Akiyama, T. High-temperature latent heat storage technology to utilize exergy of solar heat and industrial exhaust heat. In Green Energy and Technology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1207–1224. [Google Scholar]
- Alam, T.E.; Dhau, J.S.; Goswami, D.Y.; Stefanakos, E. Macroencapsulation and Characterization of Phase Change Materials for Latent Heat Thermal Energy Storage Systems. Appl. Energy 2015, 154, 92–101. [Google Scholar] [CrossRef]
- Zhang, F.; Zhong, Y.; Yang, X.; Lin, J.; Zhu, Z. Encapsulation of Metal-Based Phase Change Materials Using Ceramic Shells Prepared by Spouted Bed CVD Method. Sol. Energy Mater. Sol. Cells 2017, 170, 137–142. [Google Scholar] [CrossRef]
- Wang, J.-W.; Zhang, C.-Z.; Li, Z.-H.; Zhou, H.-X.; He, J.-X.; Yu, J.-C. Corrosion Behavior of Nickel-Based Superalloys in Thermal Storage Medium of Molten Eutectic NaCl-MgCl2 in Atmosphere. Sol. Energy Mater. Sol. Cells 2017, 164, 146–155. [Google Scholar] [CrossRef]
- Gomez-Vidal, J.C.; Tirawat, R. Corrosion of Alloys in a Chloride Molten Salt (NaCl-LiCl) for Solar Thermal Technologies. Sol. Energy Mater. Sol. Cells 2016, 157, 234–244. [Google Scholar] [CrossRef]
- Milián, Y.E.; Gutiérrez, A.; Grágeda, M.; Ushak, S. A Review on Encapsulation Techniques for Inorganic Phase Change Materials and the Influence on Their Thermophysical Properties. Renew. Sustain. Energy Rev. 2017, 73, 983–999. [Google Scholar] [CrossRef]
- Huang, X.; Chen, X.; Li, A.; Atinafu, D.; Gao, H.; Dong, W.; Wang, G. Shape-Stabilized Phase Change Materials Based on Porous Supports for Thermal Energy Storage Applications. Chem. Eng. J. 2019, 356, 641–661. [Google Scholar] [CrossRef]
- Diao, Y.H.; Liang, L.; Zhao, Y.H.; Wang, Z.Y.; Bai, F.W. Numerical Investigation of the Thermal Performance Enhancement of Latent Heat Thermal Energy Storage Using Longitudinal Rectangular Fins and Flat Micro-Heat Pipe Arrays. Appl. Energy 2019, 233–234, 894–905. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, L.; She, X.; Li, C.; Cang, D.; Liu, X.; Xuan, Y.; Ding, Y. Skeleton Materials for Shape-Stabilization of High Temperature Salts Based Phase Change Materials: A Critical Review. Renew. Sustain. Energy Rev. 2020, 119, 109539. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Min, X.; Deng, Y.; Guan, W.; Ning, L. Diatomite: A Promising Natural Candidate as Carrier Material for Low, Middle and High Temperature Phase Change Material. Energy Convers. Manag. 2015, 98, 34–45. [Google Scholar] [CrossRef]
- Qin, Y.; Leng, G.; Yu, X.; Cao, H.; Qiao, G.; Dai, Y.; Zhang, Y.; Ding, Y. Sodium Sulfate-Diatomite Composite Materials for High Temperature Thermal Energy Storage. Powder Technol. 2015, 282, 37–42. [Google Scholar] [CrossRef]
- Jiang, F.; Ge, Z.; Ling, X.; Cang, D.; Zhang, L.; Ding, Y. Improved Thermophysical Properties of Shape-Stabilized NaNO3 Using a Modified Diatomite-Based Porous Ceramic for Solar Thermal Energy Storage. Renew. Energy 2021, 179, 327–338. [Google Scholar] [CrossRef]
- Miliozzi, A.; Chieruzzi, M.; Torre, L. Experimental Investigation of a Cementitious Heat Storage Medium Incorporating a Solar Salt/Diatomite Composite Phase Change Material. Appl. Energy 2019, 250, 1023–1035. [Google Scholar] [CrossRef]
- Miliozzi, A.; Dominici, F.; Candelori, M.; Veca, E.; Liberatore, R.; Nicolini, D.; Torre, L. Development and Characterization of Concrete/PCM/Diatomite Composites for Thermal Energy Storage in CSP/CST Applications. Energies 2021, 14, 4410. [Google Scholar] [CrossRef]
- Zalba, B.; Marın, J.M.; Cabeza, L.F.; Mehling, H. Review on Thermal Energy Storage with Phase Change: Materials, Heat Transfer Analysis and Applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Yu, Q.; Jiang, Z.; Cong, L.; Lu, T.; Suleiman, B.; Leng, G.; Wu, Z.; Ding, Y.; Li, Y. A Novel Low-Temperature Fabrication Approach of Composite Phase Change Materials for High Temperature Thermal Energy Storage. Appl. Energy 2019, 237, 367–377. [Google Scholar] [CrossRef]
- Zhang, P.; Xiao, X.; Ma, Z.W. A Review of the Composite Phase Change Materials: Fabrication, Characterization, Mathematical Modeling and Application to Performance Enhancement. Appl. Energy 2016, 165, 472–510. [Google Scholar] [CrossRef]
- Huang, X.; Alva, G.; Jia, Y.; Fang, G. Morphological Characterization and Applications of Phase Change Materials in Thermal Energy Storage: A Review. Renew. Sustain. Energy Rev. 2017, 72, 128–145. [Google Scholar] [CrossRef]
- Voronin, D.V.; Ivanov, E.; Gushchin, P.; Fakhrullin, R.; Vinokurov, V. Clay Composites for Thermal Energy Storage: A Review. Molecules 2020, 25, 1504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Feng, D.; Shi, L.; Wang, L.; Jin, Y.; Tian, L.; Li, Z.; Wang, G.; Zhao, L.; Yan, Y. A Review of Phase Change Heat Transfer in Shape-Stabilized Phase Change Materials (Ss-PCMs) Based on Porous Supports for Thermal Energy Storage. Renew. Sustain. Energy Rev. 2021, 135, 110127. [Google Scholar] [CrossRef]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel Strategies and Supporting Materials Applied to Shape-Stabilize Organic Phase Change Materials for Thermal Energy Storage—A Review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Xie, N.; Luo, J.; Li, Z.; Huang, Z.; Gao, X.; Fang, Y.; Zhang, Z. Salt Hydrate/Expanded Vermiculite Composite as a Form-Stable Phase Change Material for Building Energy Storage. Sol. Energy Mater. Sol. Cells 2019, 189, 33–42. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z. Paraffin/Diatomite Composite Phase Change Material Incorporated Cement-Based Composite for Thermal Energy Storage. Appl. Energy 2013, 105, 229–237. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, R.; Tang, C.; Wu, B.; Huang, Z.; Min, X.; Huang, Y.; Liu, Y.; Fang, M.; Wu, X. Thermal Conductivity Enhancement of Polyethylene Glycol/Expanded Perlite with Carbon Layer for Heat Storage Application. Energy Build. 2016, 130, 113–121. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H. Stearic Acid Hybridizing Coal-Series Kaolin Composite Phase Change Material for Thermal Energy Storage. Appl. Clay Sci. 2014, 101, 277–281. [Google Scholar] [CrossRef]
- Lv, P.; Liu, C.; Rao, Z. Experiment Study on the Thermal Properties of Paraffin/Kaolin Thermal Energy Storage Form-Stable Phase Change Materials. Appl. Energy 2016, 182, 475–487. [Google Scholar] [CrossRef]
- Ran, X.; Wang, H.; Zhong, Y.; Zhang, F.; Lin, J.; Zou, H.; Dai, Z.; An, B. Thermal Properties of Eutectic Salts/Ceramics/Expanded Graphite Composite Phase Change Materials for High-Temperature Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2021, 225. [Google Scholar] [CrossRef]
- Sari, A.; Karaipekli, A. Thermal Conductivity and Latent Heat Thermal Energy Storage Characteristics of Paraffin/Expanded Graphite Composite as Phase Change Material. Appl. Therm. Eng. 2007, 27, 1271–1277. [Google Scholar] [CrossRef]
- Li, X.; Sanjayan, J.G.; Wilson, J.L. Fabrication and Stability of Form-Stable Diatomite/Paraffin Phase Change Material Composites. Energy Build. 2014, 76, 284–294. [Google Scholar] [CrossRef]
- Xu, G.; Leng, G.; Yang, C.; Qin, Y.; Wu, Y.; Chen, H.; Cong, L.; Ding, Y. Sodium Nitrate—Diatomite Composite Materials for Thermal Energy Storage. Sol. Energy 2017, 146, 494–502. [Google Scholar] [CrossRef]
- Konuklu, Y.; Ersoy, O. Preparation and Characterization of Sepiolite-Based Phase Change Material Nanocomposites for Thermal Energy Storage. Appl. Therm. Eng. 2016, 107, 575–582. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Li, S.; Zhang, T.; Zhang, D.; Guo, P. Thermophysical Properties of Three-Dimensional Palygorskite Based Composite Phase Change Materials. Appl. Clay Sci. 2020, 184, 105367. [Google Scholar] [CrossRef]
- Choi, J.; Valtchev, V.; Moteki, T.; Ogura, M. Phase Change Material-Containing Mesoporous Zeolite Composite for Adsorption Heat Recovery. Adv. Mater. Interfaces 2021, 8, 2001085. [Google Scholar] [CrossRef]
- de Matos Degues, K.; Cypriano, M.G.; Coelho, K.B.; Luza, A.L.; Montedo, O.R.K.; de Castro, L.C.; Angioletto, E. Assessment of PCM-Impregnated Zeolite as a Matrix for Latent Heat Storage. Mater. Sci. Forum 2018, 912, 87–92. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; Qian, T.; Guan, W.; Wang, X. Preparation and Characterization of KNO3/Diatomite Shape-Stabilized Composite Phase Change Material for High Temperature Thermal Energy Storage. J. Mater. Sci. Technol. 2017, 33, 198–203. [Google Scholar] [CrossRef]
- Jeong, S.G.; Jeon, J.; Lee, J.H.; Kim, S. Optimal Preparation of PCM/Diatomite Composites for Enhancing Thermal Properties. Int. J. Heat Mass Transf. 2013, 62, 711–717. [Google Scholar] [CrossRef]
- Leng, G.; Qiao, G.; Jiang, Z.; Xu, G.; Qin, Y.; Chang, C.; Ding, Y. Micro Encapsulated & Form-Stable Phase Change Materials for High Temperature Thermal Energy Storage. Appl. Energy 2018, 217, 212–220. [Google Scholar] [CrossRef]
- Yin, X.; Yu, X.; Wu, X.; Fu, X.; Wu, H.; Zeng, D. Solubility Prediction and Measurement of the System KNO3-LiNO3-NaNO3-H2O. J. Chem. Eng. Data 2013, 58, 1839–1844. [Google Scholar] [CrossRef]
- Tartaglione, G.; Tabuani, D.; Camino, G. Thermal and Morphological Characterisation of Organically Modified Sepiolite. Microporous Mesoporous Mater. 2008, 107, 161–168. [Google Scholar] [CrossRef]
- Lescano, L.; Castillo, L.; Marfil, S.; Barbosa, S.; Maiza, P. Alternative Methodologies for Sepiolite Defibering. Appl. Clay Sci. 2014, 95, 378–382. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, F.; Tang, M.; Liang, J.; Ren, C. Study on Pore Distribution and Formation Rule of Sepiolite Mineral Nanomaterials. J. Nanomater. 2012, 2012, 382603. [Google Scholar] [CrossRef]
- de’ Gennaro, R.; Cappelletti, P.; Cerri, G.; de’ Gennaro, M.; Dondi, M.; Langella, A. Zeolitic Tuffs as Raw Materials for Lightweight Aggregates. Appl. Clay Sci. 2004, 25, 71–81. [Google Scholar] [CrossRef]
- Rice, S.B. Transmission Electron Microscope Observations of Planar Defects in Ferrierite from Kamloops Lake, British Columbia. Am. Miner. 1995, 80, 930–936. [Google Scholar] [CrossRef]
- Önal, M.; Yilmaz, H.; Sarikaya, Y. Some Physicochemical Properties of the White Sepiolite Known as Pipestone from Eskìşehìr, Turkey. Clays Clay Miner. 2008, 56, 511–519. [Google Scholar] [CrossRef]
- Hojati, S.; Khademi, H. Thermal Behavior of a Natural Sepiolite from Northeastern Iran. J. Sci. Islamic Repub. Iran 2013, 24, 129–134. [Google Scholar]
- Meradi, H.; Atoui, L.; Bahloul, L.; Boubendira, K.; Bouazdia, A.; Ismail, F. Characterization by Thermal Analysis of Natural Kieselguhr and Sand for Industrial Application. In Proceedings of the Energy Procedia, Beirut, Lebanon, 17–20 April 2015; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 74, pp. 1282–1288. [Google Scholar] [CrossRef][Green Version]
- Meradi, H.; L’Hadi, A.; Ghabeche, W.; Bahloul, L. Contribution to Characterization of Natural Diatomite. In Proceedings of the International Conference on Water, Environment, Energy and Society ICWEES’ 2018, Djerba Island, Tunisia, 8–11 May 2018; Available online: https://www.icwees2018.tn (accessed on 22 September 2021).
- Knowlton, G.D.; White, T.R.; Mckague, H.L. Thermal Study of Types of Water Associated with Clinoptilolite. Clays Clay Miner. 1981, 29, 403–411. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Cerritelli, G.F.; Miliozzi, A.; Kenny, J.M. Effect of Nanoparticles on Heat Capacity of Nanofluids Based on Molten Salts as PCM for Thermal Energy Storage. Nanoscale Res. Lett. 2013, 8, 448. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Sakamoto, R.; Kamimoto, M. Heat Capacities and Latent Heats of LiNO3, NaNO3, and KNO3. Int. J. Thermophys. 1988, 9, 1081–1090. [Google Scholar] [CrossRef]
- Trujillo, U.; Rueckert, T.L.; Reddy, R.G.; United States Department of Energy. Novel Molten Salts Thermal Energy Storage for Concentrating Solar Power Generation. 2013. Available online: https://www.osti.gov/servlets/purl/1111584/ (accessed on 18 September 2021).
- Coscia, K.; Nelle, S.; Elliott, T.; Mohapatra, S.; Oztekin, A.; Neti, S. Thermophysical Properties of LiNO3–NaNO3–KNO3 Mixtures for Use in Concentrated Solar Power. J. Sol. Energy Eng. Trans. ASME 2013, 135, 034506. [Google Scholar] [CrossRef]
- Bradshaw, R.W.; Meeker, D.E. High-Temperature Stability of Ternary Nitrate Molten Salts for Solar Thermal Energy Systems. Sol. Energy Mater. 1990, 21, 51–60. [Google Scholar] [CrossRef]
- Wang, T.; Mantha, D.; Reddy, R.G. Thermal Stability of the Eutectic Composition in LiNO3–NaNO3–KNO3 Ternary System Used for Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2012, 100, 162–168. [Google Scholar] [CrossRef]
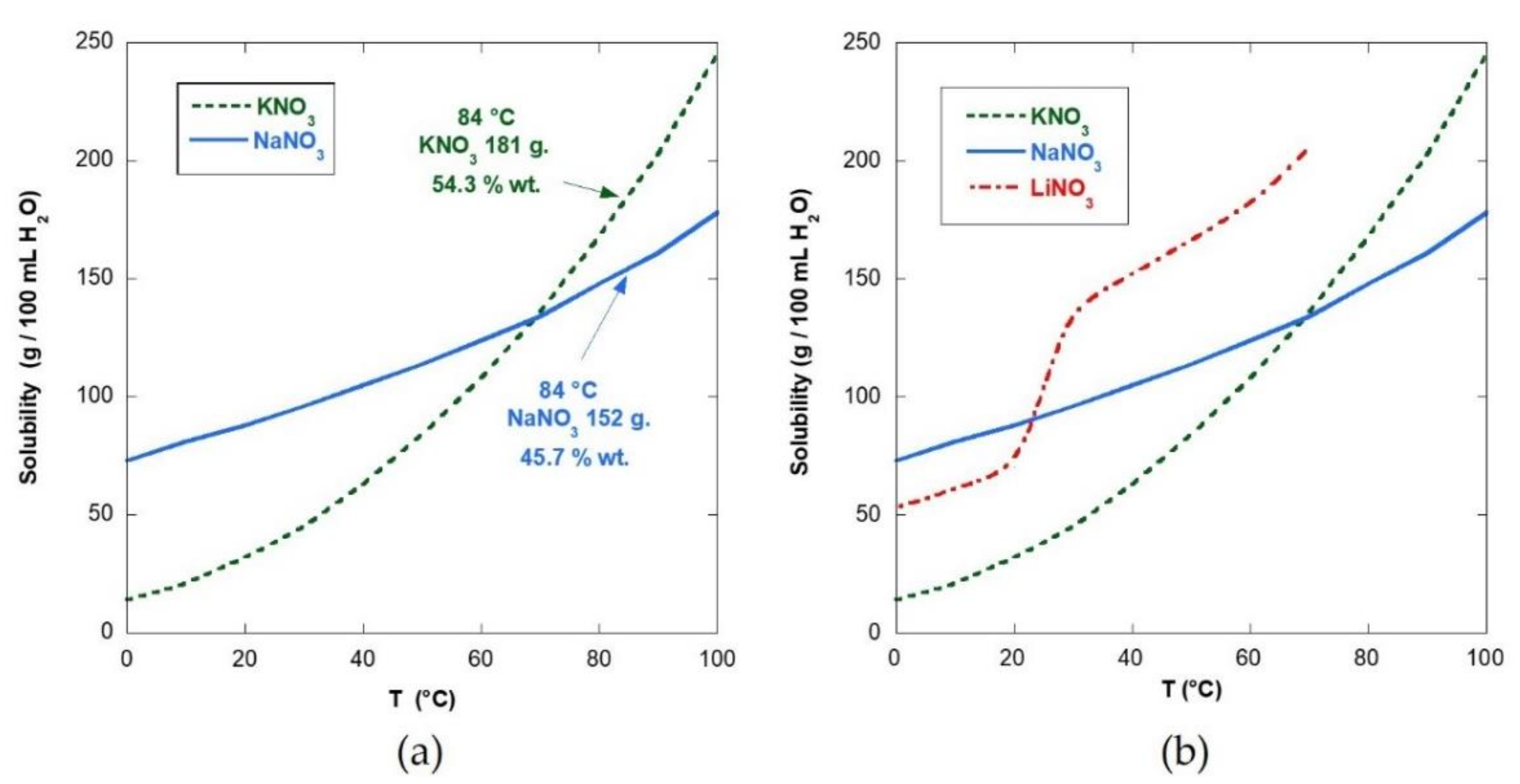

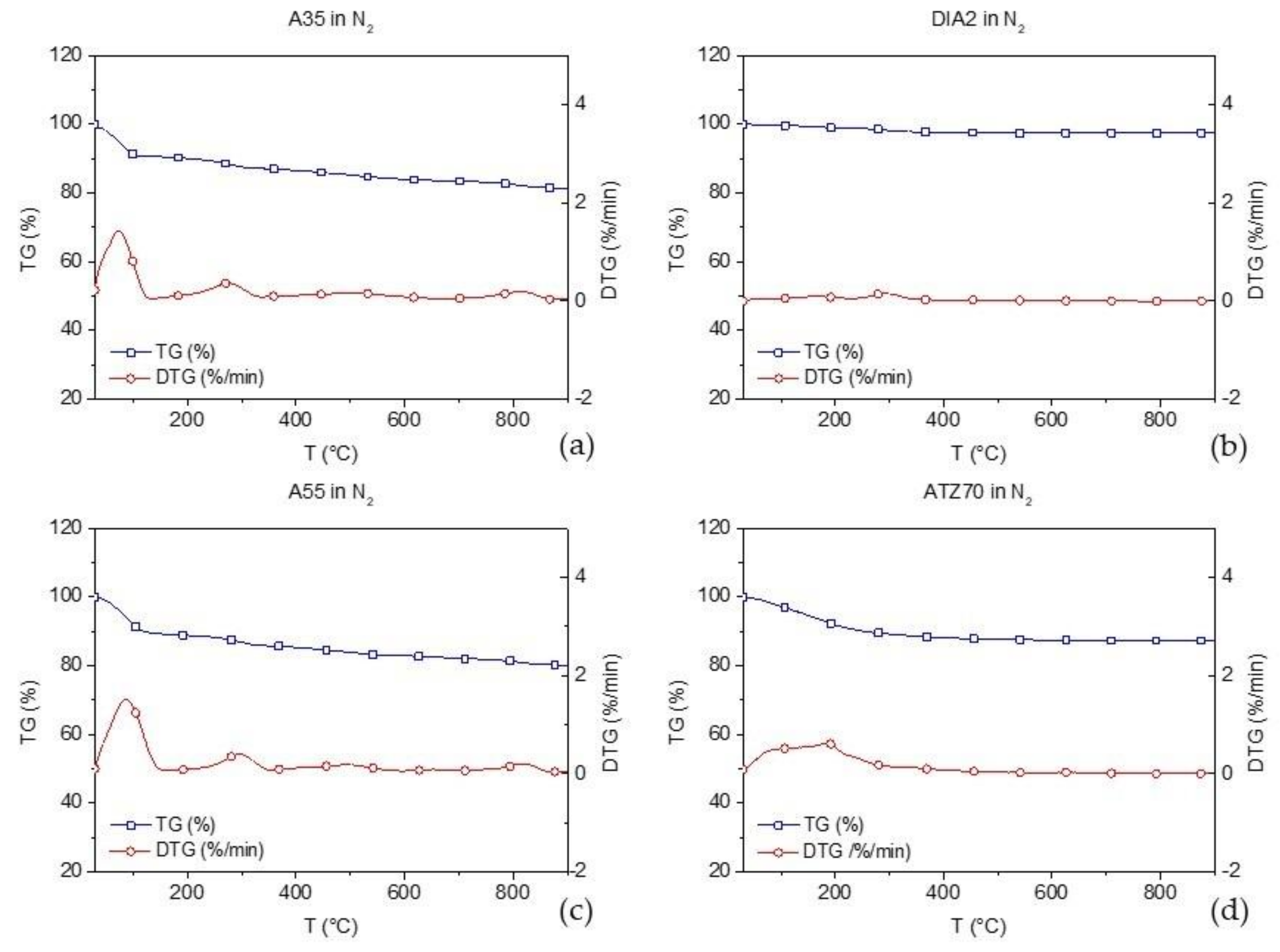
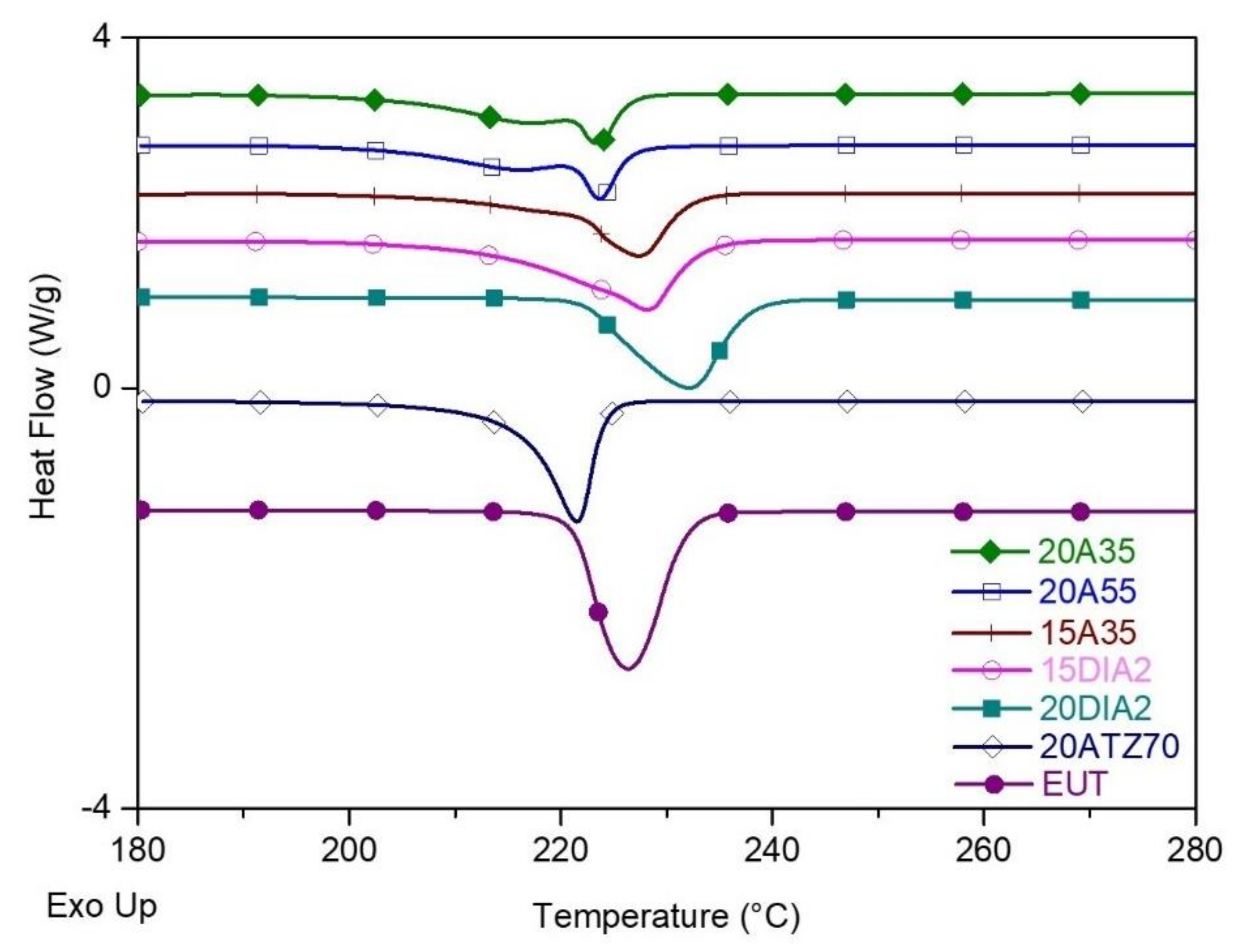
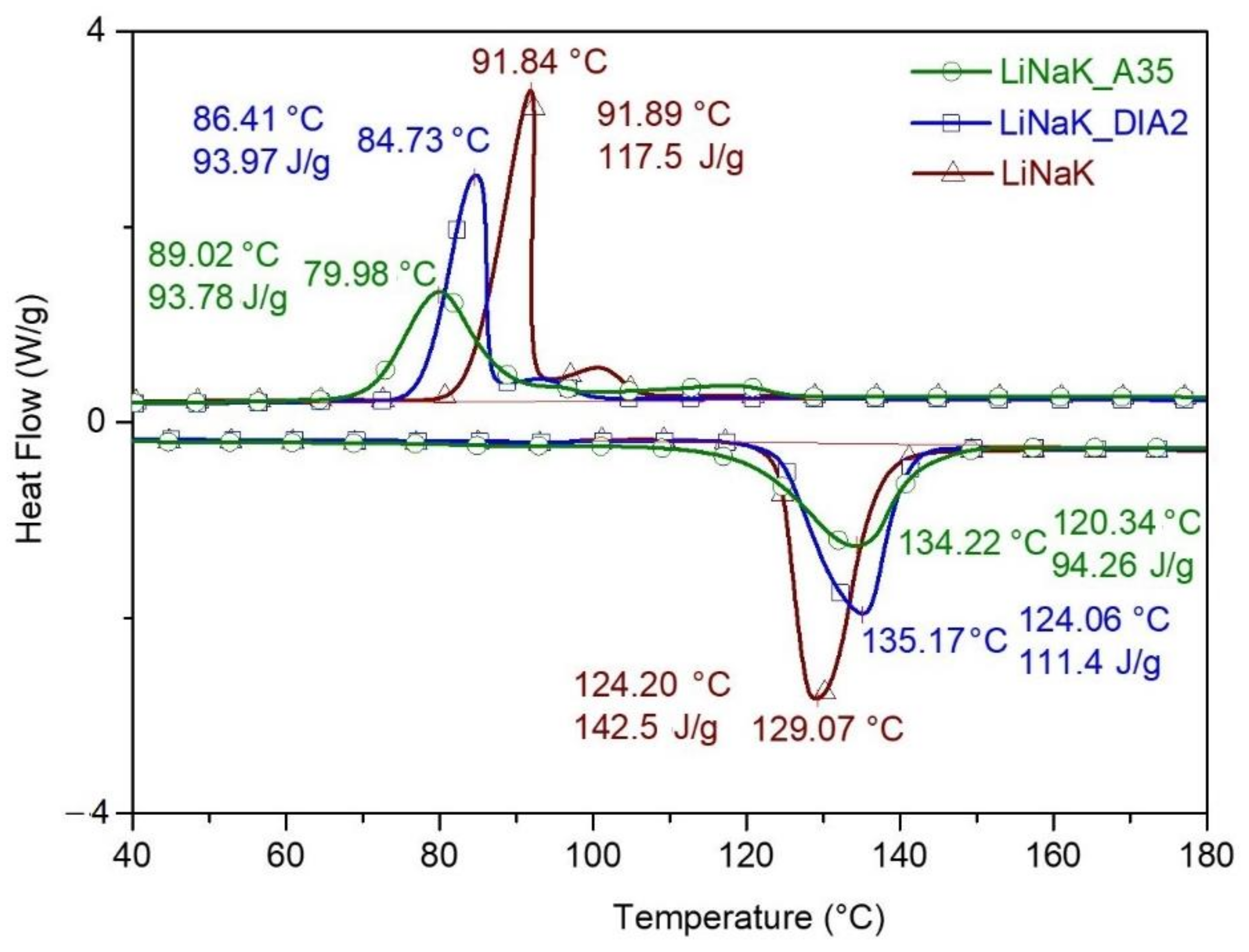
| Code | Name | Composition |
|---|---|---|
| A35 | Cimsil A35 | Hydrous Magnesium Silicate |
| DIA2 | Deref DIA2 | Calcined Diatomite |
| A55 | Cimsil A55 | Mg4Si6O15(OH)2 6H2O |
| ATZ70 | Zeolite ATZ 70 mm | Chabazite/Phillipsite |
| EUT | Eutectic mix KNO3/NaNO3 | KNO3/NaNO3 54.3/45.7% wt. |
| LiNaK | Triple mix (Li-Na-K) NO3 | LiNO3/NaNO3/KNO3 30/20/50% wt. |
| Sample | Shape Stabilizer | SS % wt. | Phase Change Material | PCM % wt. |
|---|---|---|---|---|
| 20A35 | CIMSIL A35 | 20 | EUT | 80 |
| 15A35 | CIMSIL A35 | 15 | EUT | 85 |
| 20DIA2 | DIA2 | 20 | EUT | 80 |
| 15DIA2 | DIA2 | 15 | EUT | 85 |
| 20A55 | CIMSIL A55 | 20 | EUT | 80 |
| 20ATZ70 | ATZ70 | 20 | EUT | 80 |
| LiNaK_20DIA2 | DIA2 | 20 | LiNaK | 80 |
| LiNaK_20A35 | CIMSIL A35 | 20 | LiNaK | 80 |
| Sample | Shape Stabilizer | SS | PCM | Tm | ΔHm | SSc | Tc | ΔHc |
|---|---|---|---|---|---|---|---|---|
| % wt. | % wt. | °C | J/g | % | W/g | J/g °C | ||
| EUT | - | - | 100 | 226 ± 1 | 97.7 ± 2.4 | - | 218 ± 1 | 96.9 ± 2.1 |
| 20A35 | CIMSIL A35 | 20 | 80 | 220 ± 1 | 48.7 ± 4.3 | 62.3 | 218 ± 1 | 49.0 ± 3.8 |
| 15A35 | CIMSIL A35 | 15 | 85 | 224 ± 2 | 57.6 ± 8.2 | 69.4 | 216 ± 3 | 57.7 ± 5.8 |
| 20DIA2 | DIA2 | 20 | 80 | 232 ± 2 | 77.9 ± 2.3 | 99.7 | 211 ± 2 | 77.6 ± 1.9 |
| 15DIA2 | DIA2 | 15 | 85 | 228 ± 3 | 77.7 ± 6.1 | 93.6 | 210 ± 3 | 77.9 ± 6.4 |
| 20A55 | CIMSIL A55 | 20 | 80 | 224 ± 1 | 47.5 ± 4.6 | 60.8 | 219 ± 1 | 51.2 ± 5.3 |
| 20ATZ70 | ATZ70 | 20 | 80 | 221 ± 1 | 68.2 ± 2.2 | 87.3 | 215 ± 2 | 69.4 ± 2.6 |
| Sample | Shape Stabilizer | SS | PCM | Tm | ΔHm | SSc | Tc | ΔHc | SScc |
|---|---|---|---|---|---|---|---|---|---|
| % wt. | % wt. | °C | J/g | % | W/g | J/g °C | % | ||
| LiNaK | - | - | 100 | 129 ± 1 | 142.5 ± 2 | - | 92 ± 1 | 117.5 ± 2 | - |
| LiNaK_20DIA2 | DIA2 | 20 | 80 | 135 ± 1 | 111.4 ± 3 | 97.7 | 85 ± 1 | 94.0 ± 2 | 100 |
| LiNaK_20A35 | CIMSIL A35 | 20 | 80 | 134 ± 2 | 94.3 ± 3 | 82.7 | 80 ± 2 | 93.8 ± 2 | 99.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominici, F.; Miliozzi, A.; Torre, L. Thermal Properties of Shape-Stabilized Phase Change Materials Based on Porous Supports for Thermal Energy Storage. Energies 2021, 14, 7151. https://doi.org/10.3390/en14217151
Dominici F, Miliozzi A, Torre L. Thermal Properties of Shape-Stabilized Phase Change Materials Based on Porous Supports for Thermal Energy Storage. Energies. 2021; 14(21):7151. https://doi.org/10.3390/en14217151
Chicago/Turabian StyleDominici, Franco, Adio Miliozzi, and Luigi Torre. 2021. "Thermal Properties of Shape-Stabilized Phase Change Materials Based on Porous Supports for Thermal Energy Storage" Energies 14, no. 21: 7151. https://doi.org/10.3390/en14217151
APA StyleDominici, F., Miliozzi, A., & Torre, L. (2021). Thermal Properties of Shape-Stabilized Phase Change Materials Based on Porous Supports for Thermal Energy Storage. Energies, 14(21), 7151. https://doi.org/10.3390/en14217151







