Theoretical Study of an Undisclosed Reaction Class: Direct H-Atom Abstraction from Allylic Radicals by Molecular Oxygen
Abstract
1. Introduction
 unsaturated species +HȮ2 has not been found to be important; instead, fuel radicals typically undergo isomerization or β-scission reaction pathways breaking down to smaller species. This can be found in many typical and well-validated kinetic models for oxidation of alkanes [1,2,3], alkenes [4,5,6,7,8], aromatics [9,10,11], alcohols [12,13,14], aldehydes [15], ethers [16,17], and furans [18,19].
unsaturated species +HȮ2 has not been found to be important; instead, fuel radicals typically undergo isomerization or β-scission reaction pathways breaking down to smaller species. This can be found in many typical and well-validated kinetic models for oxidation of alkanes [1,2,3], alkenes [4,5,6,7,8], aromatics [9,10,11], alcohols [12,13,14], aldehydes [15], ethers [16,17], and furans [18,19].2. Computational Methods
- Three sets of quantum chemical methods: Method 1, Method 2, and Method 3.
3. Results and Discussion
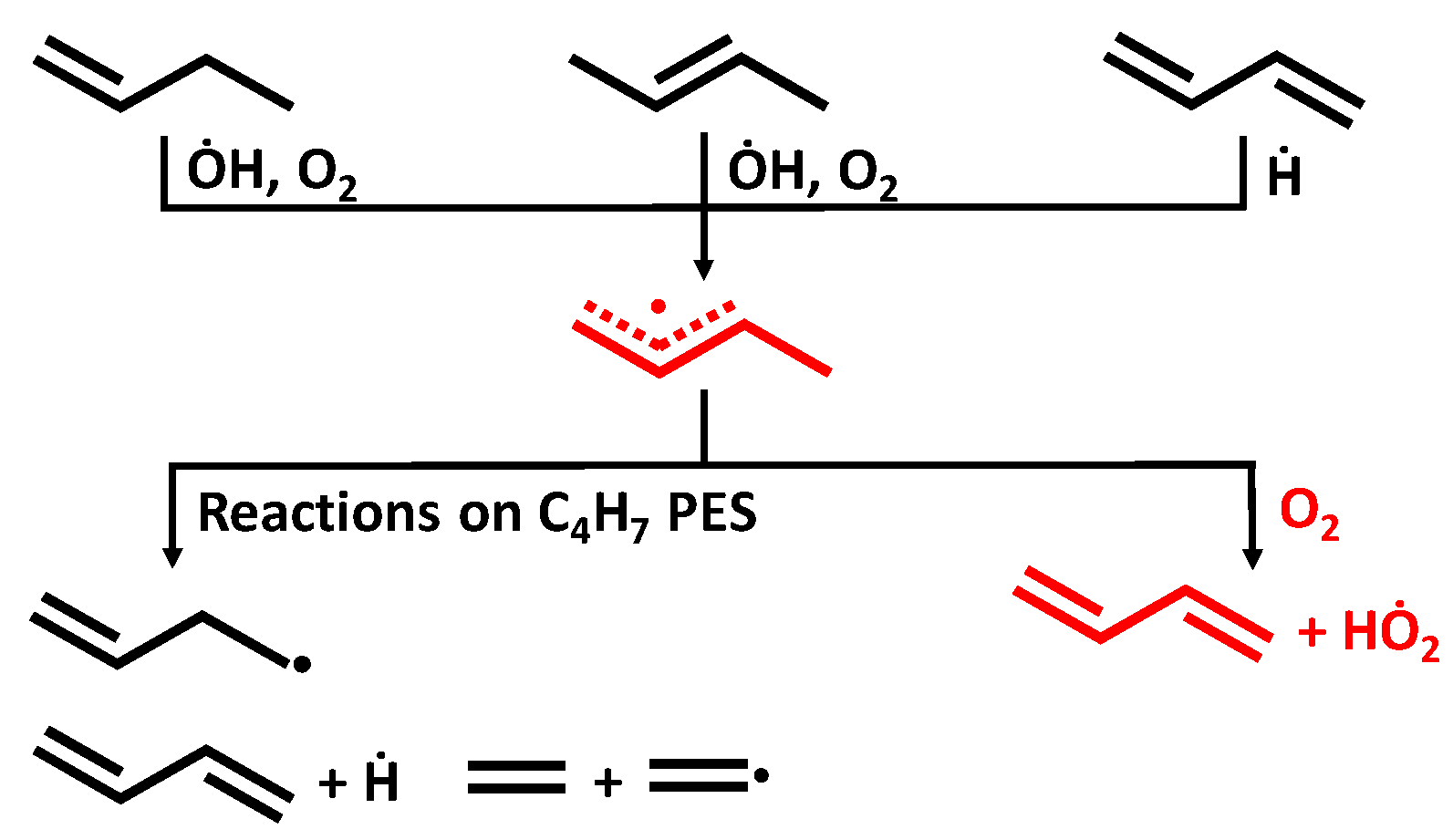
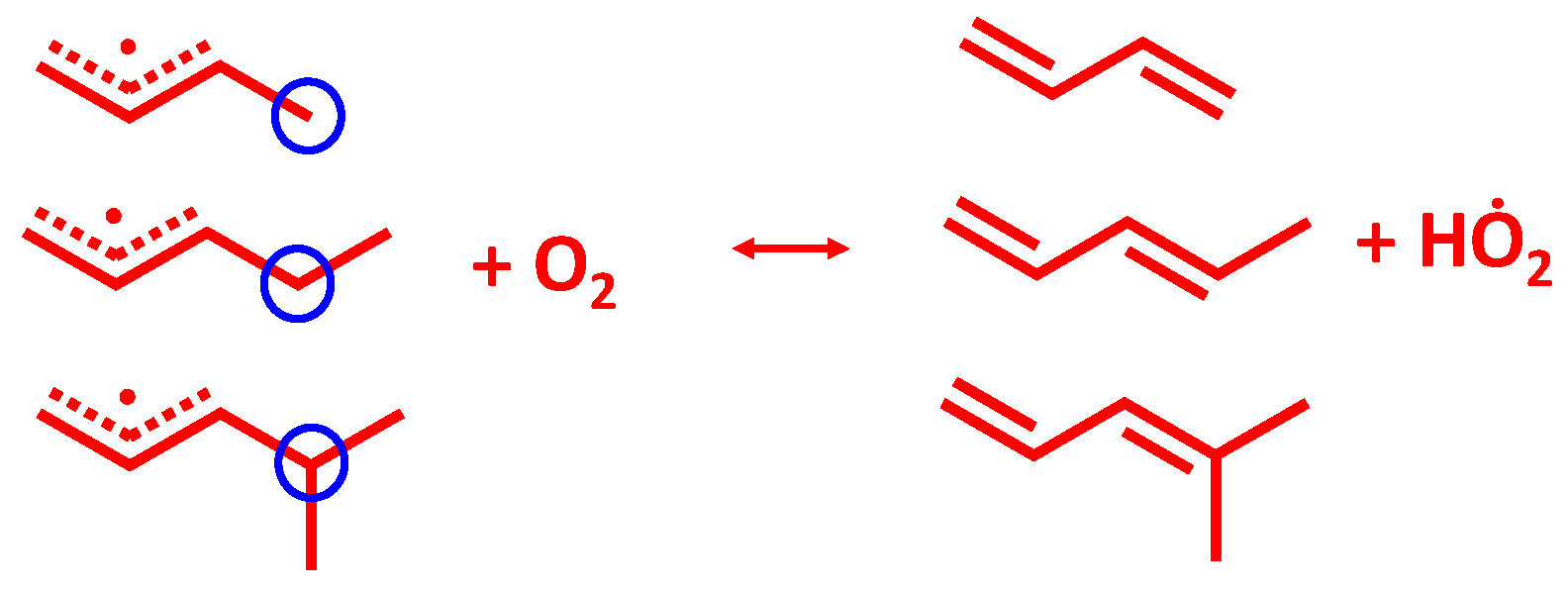
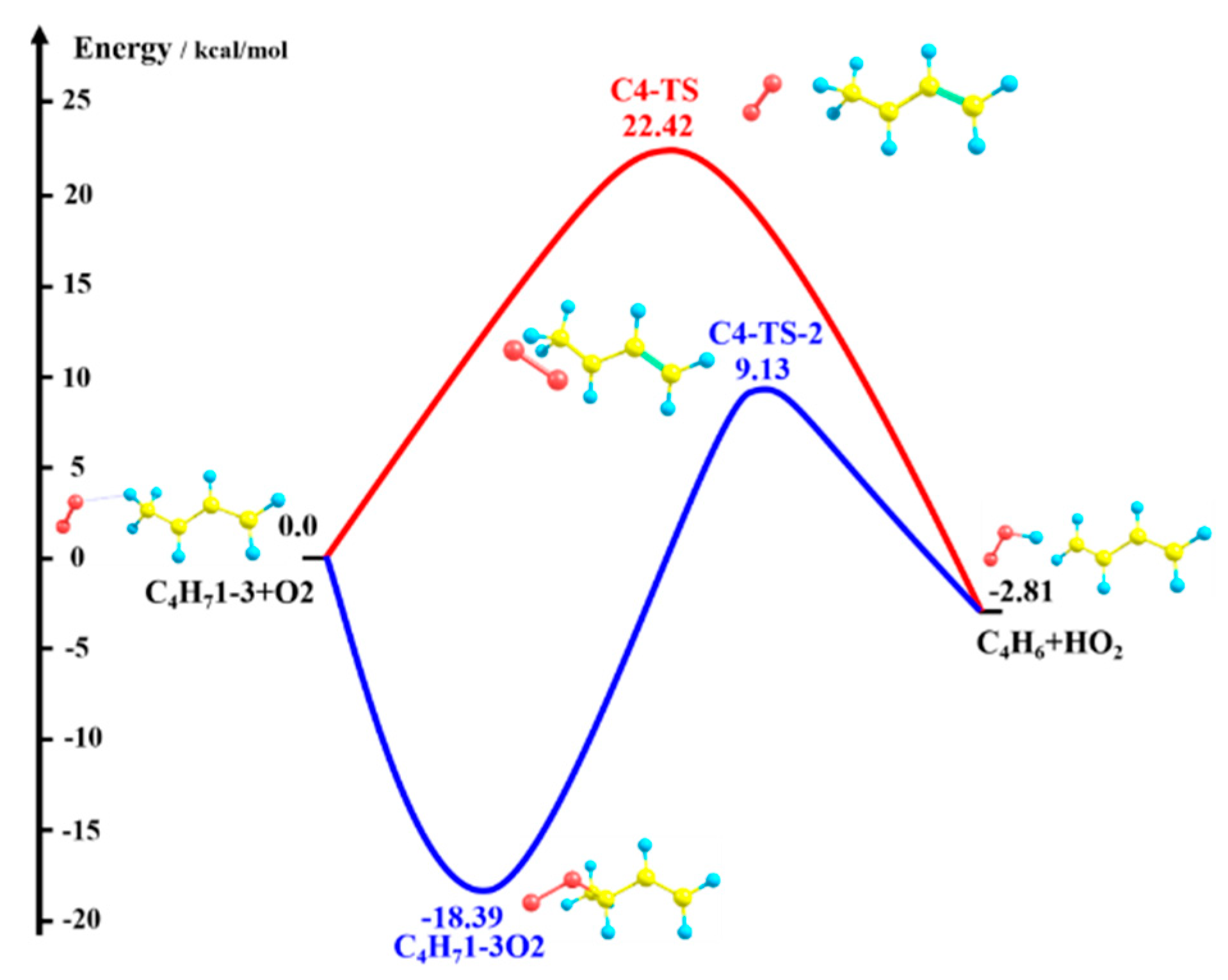
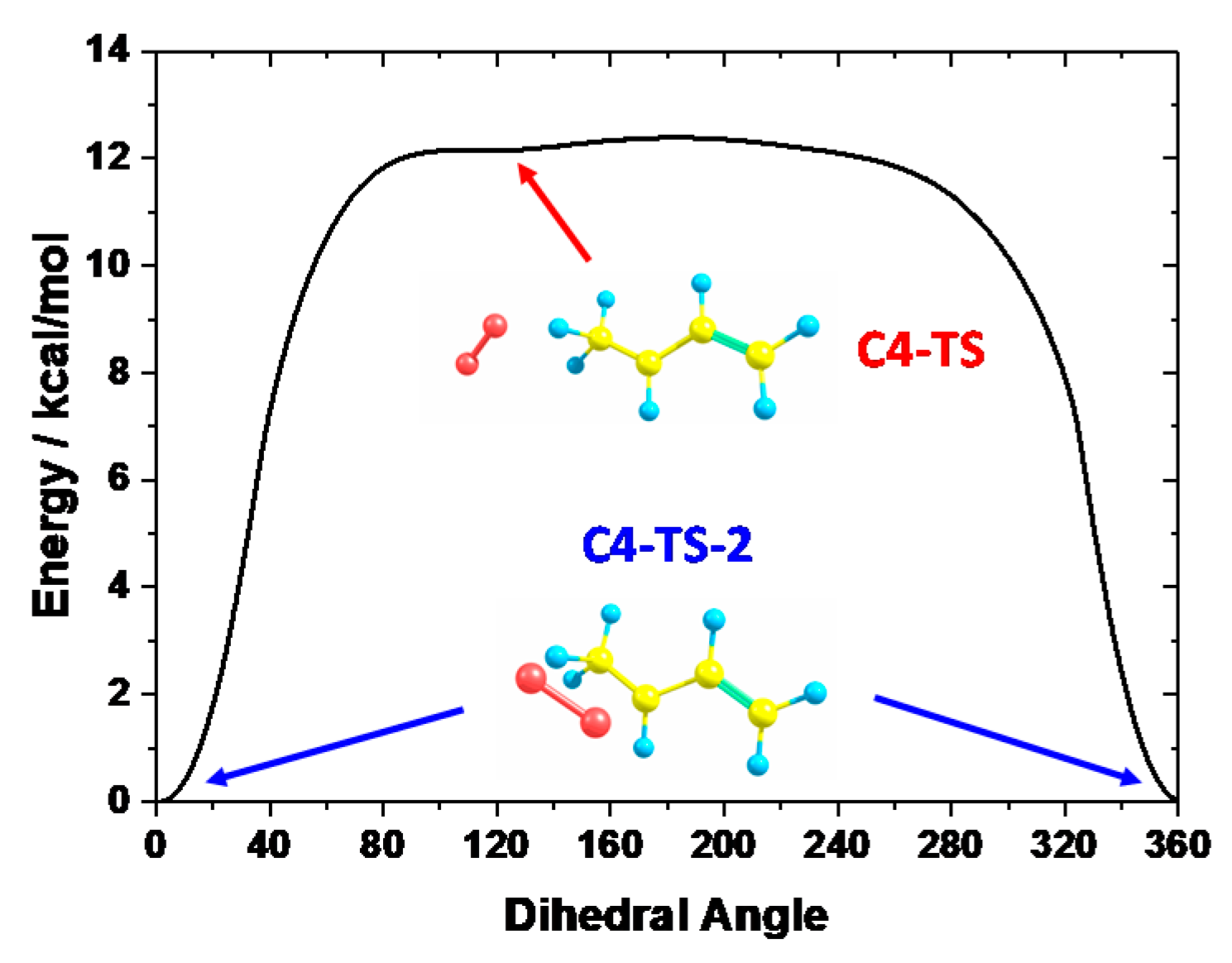
3.1. Potential Energy Surface for C4H71-3 + O2 Reaction
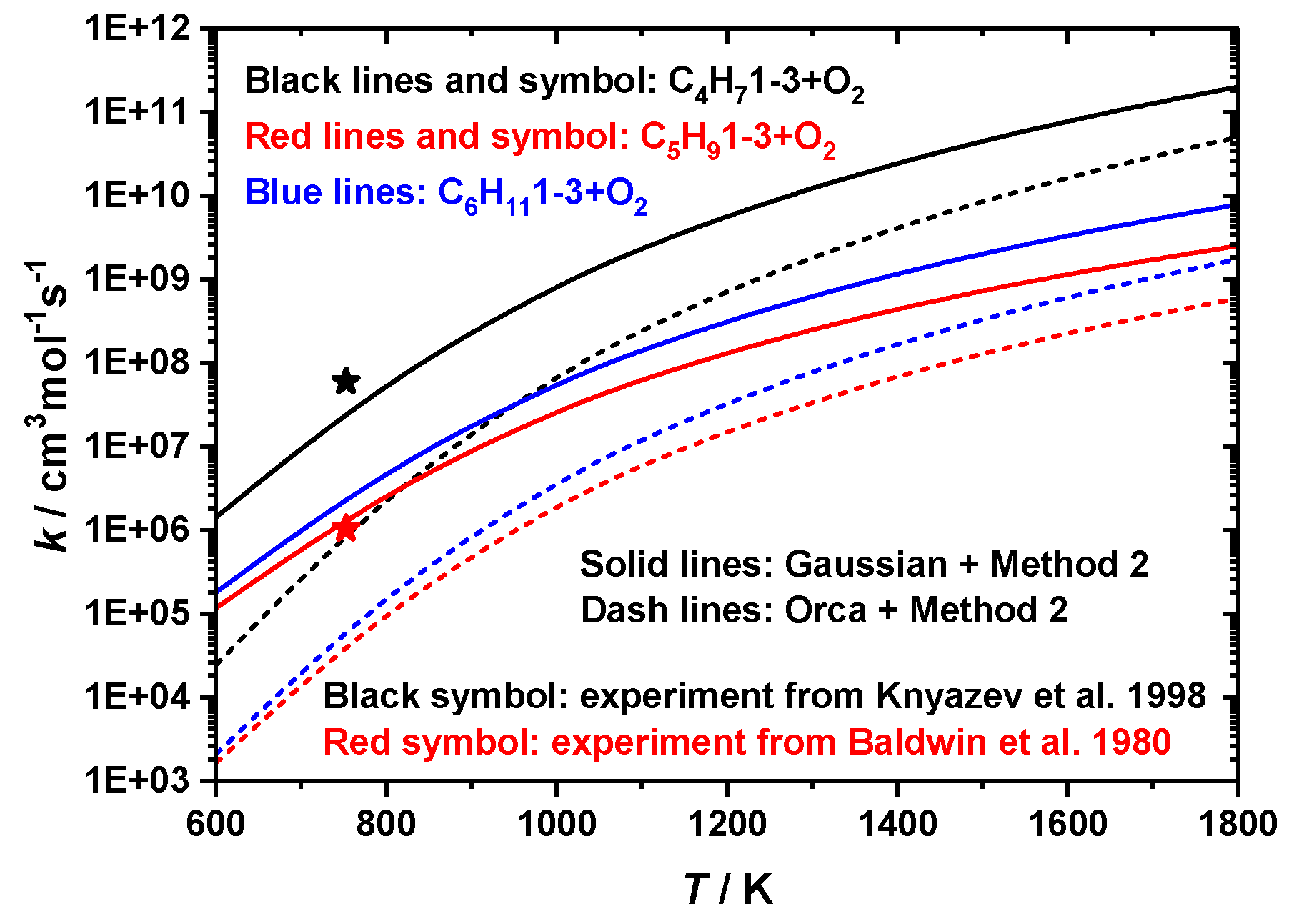
3.2. Comparison of Rate Constants
- Comparing two ab initio solvers (Gaussian and Orca) when using the MultiWell kinetic solver with Method 2
- Comparing two kinetic solvers (Multiwell and PAPR) using ab initio results from Gaussian with Method 2
 C4H6 + HO2 measured by Knyazev et al. [46], and C5 reaction: C5H91-3 + O2
C4H6 + HO2 measured by Knyazev et al. [46], and C5 reaction: C5H91-3 + O2  C5H8 + HO2 measured by Baldwin et al. [47]. H-atom abstraction from the primary site (C4H71-3 + O2) was found to be faster than from secondary (C5H91-3 + O2) and tertiary (C6H111-3 + O2) sites, which seems counter-intuitive. Significant differences were found for the rate constants predicted using two ab initio solvers, being about a factor of 4–86 differences, depending on the temperature, and this is due to the difference in the barrier heights. When compared to experimental measurements, the results obtained from Gaussian solver with Method 2 show about a factor of 2 difference for both C4 and C5 reactions, which is in much better agreement with experimental data relative to the Orca solver.
C5H8 + HO2 measured by Baldwin et al. [47]. H-atom abstraction from the primary site (C4H71-3 + O2) was found to be faster than from secondary (C5H91-3 + O2) and tertiary (C6H111-3 + O2) sites, which seems counter-intuitive. Significant differences were found for the rate constants predicted using two ab initio solvers, being about a factor of 4–86 differences, depending on the temperature, and this is due to the difference in the barrier heights. When compared to experimental measurements, the results obtained from Gaussian solver with Method 2 show about a factor of 2 difference for both C4 and C5 reactions, which is in much better agreement with experimental data relative to the Orca solver.3.3. Thermodynamic Properties
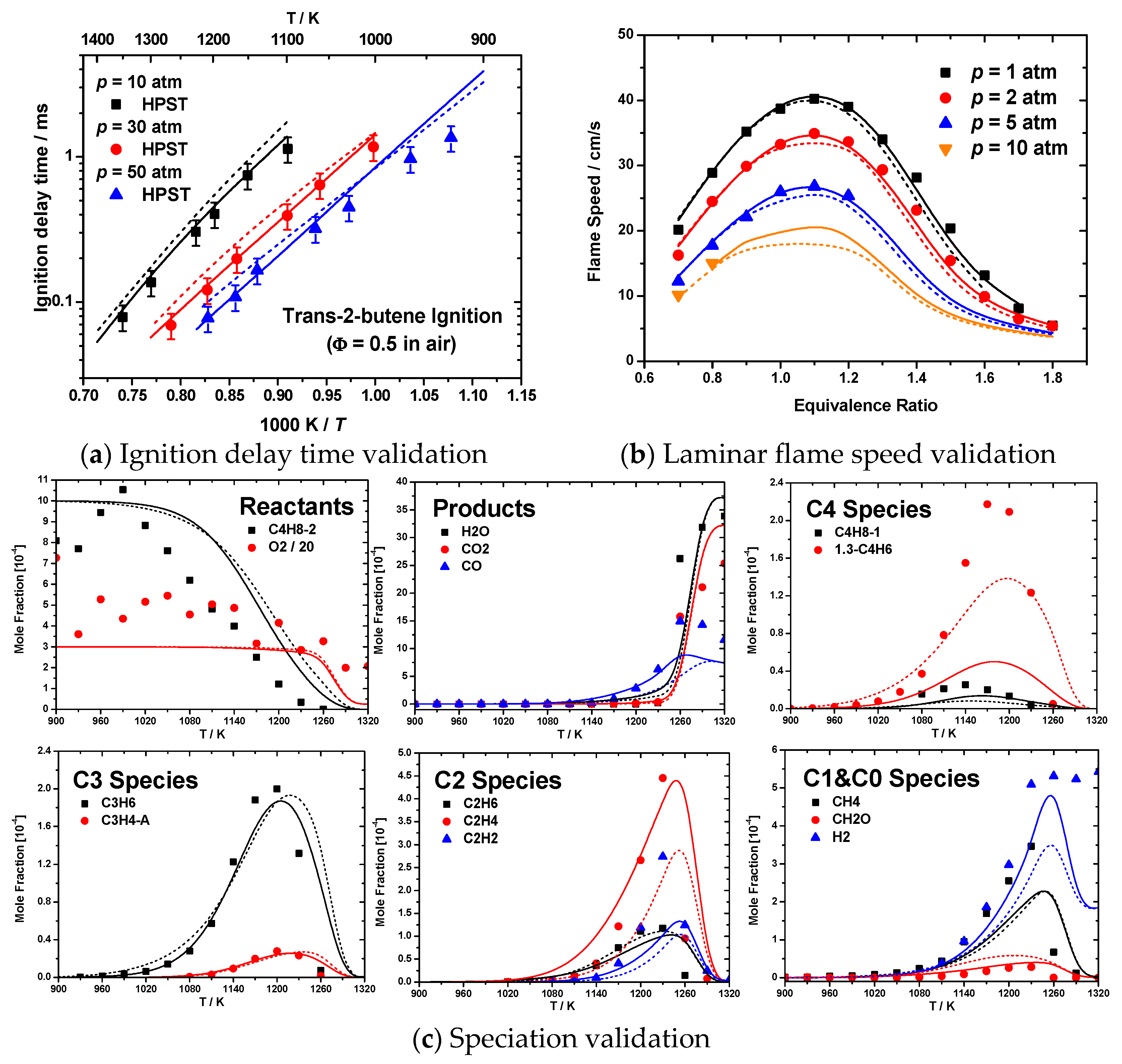
3.4. Application in Kinetic Model Development
 C4H6 + HȮ2 reaction was based on analogy to the theoretical prediction of IĊ3H7 + O2
C4H6 + HȮ2 reaction was based on analogy to the theoretical prediction of IĊ3H7 + O2  C3H6 + HȮ2 reaction from DeSain et al. [53]. A comparison against rates calculated here is given in Figure S2 in Supplementary Material 1. Figure 6 shows the validation results for the following key targets of high-temperature oxidation:
C3H6 + HȮ2 reaction from DeSain et al. [53]. A comparison against rates calculated here is given in Figure S2 in Supplementary Material 1. Figure 6 shows the validation results for the following key targets of high-temperature oxidation:4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, K.; Banyon, C.; Togbé, C.; Dagaut, P.; Bugler, J.; Curran, H.J. An experimental and kinetic modeling study of n-hexane oxidation. Combust. Flame 2015, 162, 4194–4207. [Google Scholar] [CrossRef]
- Zhang, K.; Banyon, C.; Bugler, J.; Curran, H.J.; Rodriguez, A.; Herbinet, O.; Battin-Leclerc, F.; B’Chir, C.; Heufer, K.A. An updated experimental and kinetic modeling study of n-heptane oxidation. Combust. Flame 2016, 172, 116–135. [Google Scholar] [CrossRef]
- Atef, N.; Kukkadapu, G.; Mohamed, S.Y.; Rashidi, M.A.; Banyon, C.; Mehl, M.; Heufer, K.A.; Nasir, E.F.; Alfazazi, A.; Das, A.K.; et al. A comprehensive iso-octane combustion model with improved thermochemistry and chemical kinetics. Combust. Flame 2017, 178, 111–134. [Google Scholar] [CrossRef]
- Kopp, M.M.; Donato, N.S.; Petersen, E.L.; Metcalfe, W.K.; Burke, S.M.; Curran, H.J. Oxidation of Ethylene–Air Mixtures at Elevated Pressures, Part 1: Experimental Results. J. Propuls. Power 2014, 30, 790–798. [Google Scholar] [CrossRef]
- Kopp, M.M.; Petersen, E.L.; Metcalfe, W.K.; Burke, S.M.; Curran, H.J. Oxidation of Ethylene—Air Mixtures at Elevated Pressures, Part 2: Chemical Kinetics. J. Propuls. Power 2014, 30, 799–811. [Google Scholar] [CrossRef]
- Burke, S.M.; Metcalfe, W.; Herbinet, O.; Battin-Leclerc, F.; Haas, F.M.; Santner, J.; Dryer, F.L.; Curran, H.J. An experimental and modeling study of propene oxidation. Part 1: Speciation measurements in jet-stirred and flow reactors. Combust. Flame 2014, 161, 2765–2784. [Google Scholar] [CrossRef]
- Burke, S.M.; Burke, U.; Mc Donagh, R.; Mathieu, O.; Osorio, I.; Keesee, C.; Morones, A.; Petersen, E.L.; Wang, W.; DeVerter, T.A.; et al. An experimental and modeling study of propene oxidation. Part 2: Ignition delay time and flame speed measurements. Combust. Flame 2015, 162, 296–314. [Google Scholar] [CrossRef]
- Zhou, C.-W.; Li, Y.; O’Connor, E.; Somers, K.P.; Thion, S.; Keesee, C.; Mathieu, O.; Petersen, E.L.; DeVerter, T.A.; Oehlschlaeger, M.A.; et al. A comprehensive experimental and modeling study of isobutene oxidation. Combust. Flame 2016, 167, 353–379. [Google Scholar] [CrossRef]
- Zhang, Y.; Somers, K.P.; Mehl, M.; Pitz, W.J.; Cracknell, R.F.; Curran, H.J. Probing the antagonistic effect of toluene as a component in surrogate fuel models at low temperatures and high pressures. A case study of toluene/dimethyl ether mixtures. Proc. Combust. Inst. 2017, 36, 413–421. [Google Scholar] [CrossRef]
- Yuan, W.; Li, Y.; Pengloan, G.; Togbé, C.; Dagaut, P.; Qi, F. A comprehensive experimental and kinetic modeling study of ethylbenzene combustion. Combust. Flame 2016, 166, 255–265. [Google Scholar] [CrossRef]
- Dagaut, P.; Ristori, A.; El Bakali, A.; Cathonnet, M. Experimental and kinetic modeling study of the oxidation of n-propylbenzene. Fuel 2002, 81, 173–184. [Google Scholar] [CrossRef]
- Zhang, Y.; El-Merhubi, H.; Lefort, B.; Le Moyne, L.; Curran, H.J.; Kéromnès, A. Probing the low-temperature chemistry of ethanol via the addition of dimethyl ether. Combust. Flame 2018, 190, 74–86. [Google Scholar] [CrossRef]
- Frassoldati, A.; Cuoci, A.; Faravelli, T.; Niemann, U.; Ranzi, E.; Seiser, R.; Seshadri, K. An experimental and kinetic modeling study of n-propanol and iso-propanol combustion. Combust. Flame 2010, 157, 2–16. [Google Scholar] [CrossRef]
- Sarathy, S.M.; Vranckx, S.; Yasunaga, K.; Mehl, M.; Oßwald, P.; Metcalfe, W.K.; Westbrook, C.K.; Pitz, W.J.; Kohse-Höinghaus, K.; Fernandes, R.X.; et al. A comprehensive chemical kinetic combustion model for the four butanol isomers. Combust. Flame 2012, 159, 2028–2055. [Google Scholar] [CrossRef]
- Pelucchi, M.; Somers, K.P.; Yasunaga, K.; Burke, U.; Frassoldati, A.; Ranzi, E.; Curran, H.J.; Faravelli, T. An experimental and kinetic modeling study of the pyrolysis and oxidation of n-C3C5 aldehydes in shock tubes. Combust. Flame 2015, 162, 265–286. [Google Scholar] [CrossRef]
- Burke, U.; Somers, K.P.; O’Toole, P.; Zinner, C.M.; Marquet, N.; Bourque, G.; Petersen, E.L.; Metcalfe, W.K.; Serinyel, Z.; Curran, H.J. An ignition delay and kinetic modeling study of methane, dimethyl ether, and their mixtures at high pressures. Combust. Flame 2015, 162, 315–330. [Google Scholar] [CrossRef]
- Yasunaga, K.; Gillespie, F.; Simmie, J.M.; Curran, H.J.; Kuraguchi, Y.; Hoshikawa, H.; Yamane, M.; Hidaka, Y. A Multiple Shock Tube and Chemical Kinetic Modeling Study of Diethyl Ether Pyrolysis and Oxidation. J. Phys. Chem. 2010, 114, 9098–9109. [Google Scholar] [CrossRef]
- Somers, K.P.; Simmie, J.M.; Gillespie, F.; Burke, U.; Connolly, J.; Metcalfe, W.K.; Battin-Leclerc, F.; Dirrenberger, P.; Herbinet, O.; Glaude, P.A.; et al. A high temperature and atmospheric pressure experimental and detailed chemical kinetic modelling study of 2-methyl furan oxidation. Proc. Combust. Inst. 2013, 34, 225–232. [Google Scholar] [CrossRef]
- Somers, K.P.; Simmie, J.M.; Gillespie, F.; Conroy, C.; Black, G.; Metcalfe, W.K.; Battin-Leclerc, F.; Dirrenberger, P.; Herbinet, O.; Glaude, P.-A.; et al. A comprehensive experimental and detailed chemical kinetic modelling study of 2,5-dimethylfuran pyrolysis and oxidation. Combust. Flame 2013, 160, 2291–2318. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, C.-W.; Curran, H.J. An extensive experimental and modeling study of 1-butene oxidation. Combust. Flame 2017, 181, 198–213. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, C.-W.; Somers, K.P.; Zhang, K.; Curran, H.J. The oxidation of 2-butene: A high pressure ignition delay, kinetic modeling study and reactivity comparison with isobutene and 1-butene. Proc. Combust. Inst. 2017, 36, 403–411. [Google Scholar] [CrossRef]
- Zhou, C.-W.; Li, Y.; Burke, U.; Banyon, C.; Somers, K.P.; Ding, S.; Khan, S.; Hargis, J.W.; Sikes, T.; Mathieu, O.; et al. An experimental and chemical kinetic modeling study of 1,3-butadiene combustion: Ignition delay time and laminar flame speed measurements. Combust. Flame 2018, 197, 423–438. [Google Scholar] [CrossRef]
- Li, Y.; Klippenstein, S.J.; Zhou, C.-W.; Curran, H.J. Theoretical Kinetics Analysis for Ḣ Atom Addition to 1,3-Butadiene and Related Reactions on the Ċ4H7 Potential Energy Surface. J. Phys. Chem. 2017, 121, 7433–7445. [Google Scholar] [CrossRef]
- Foresman, J.; Ortiz, J.; Cioslowski, J.; Fox, D. Gaussian 09, Revision, D. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Barker, J.R.; Nguyen, T.L.; Stanton, J.F.; Aieta, C.; Ceotto, M.; Gabas, F.; Kumar, T.J.D.; Li, C.G.L.; Lohr, L.L.; Maranzana, A.; et al. MultiWell-2016 Software Suite; Barker, J.R., Ed.; University of Michigan: Ann Arbor, MI, USA, 2016. [Google Scholar]
- Georgievskii, Y.; Miller, J.A.; Burke, M.P.; Klippenstein, S.J. Reformulation and Solution of the Master Equation for Multiple-Well Chemical Reactions. J. Phys. Chem. 2013, 117, 12146–12154. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-W.; Simmie, J.M.; Somers, K.P.; Goldsmith, C.F.; Curran, H.J. Chemical Kinetics of Hydrogen Atom Abstraction from Allylic Sites by 3O2; Implications for Combustion Modeling and Simulation. J. Phys. Chem. 2017, 121, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Bugler, J.; Power, J.; Curran, H.J. A theoretical study of cyclic ether formation reactions. Proc. Combust. Inst. 2017, 36, 161–167. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11−18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Purvis, G.D., III; Bartlett, R.J. A full coupled-cluster singles and doubles model: The inclusion of disconnected triples. J. Chem. Phys. 1982, 76, 1910–1918. [Google Scholar] [CrossRef]
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Curtiss, L.A.; Redfern, P.C.; Raghavachari, K. Gaussian-4 theory. J. Chem. Phys. 2007, 126, 084108. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.J.; Taylor, P.R. A diagnostic for determining the quality of single-reference electron correlation methods. Int. J. Quantum Chem. 1989, 36, 199–207. [Google Scholar] [CrossRef]
- Ochterski, J.W.; Petersson, G.A.; Montgomery, J.A., Jr. A complete basis set model chemistry. V. Extensions to six or more heavy atoms. J. Chem. Phys. 1996, 104, 2598–2619. [Google Scholar] [CrossRef]
- Curtiss, L.A.; Raghavachari, K.; Redfern, P.C.; Rassolov, V.; Pople, J.A. Gaussian-3 (G3) theory for molecules containing first and second-row atoms. J. Chem. Phys. 1998, 109, 7764–7776. [Google Scholar] [CrossRef]
- Eckart, C. The Penetration of a Potential Barrier by Electrons. Phys. Rev. 1930, 35, 1303–1309. [Google Scholar] [CrossRef]
- Eyring, H. The Activated Complex in Chemical Reactions. J. Chem. Phys. 1935, 3, 107–115. [Google Scholar] [CrossRef]
- Gordon, S.; Mcbride, B.J. Computer Program for Calculation of Complex Chemical Equilibrium Compositions, Rocket Performance, Incident and Reflected Shocks, and Chapman-Jouguet Detonations. Nasa Sti 1976, 273, 119–224. [Google Scholar]
- ANSYS, Inc. ANSYS Chemkin-Pro 17.2; ANSYS, Inc.: San Diego, CA, USA, 2016. [Google Scholar]
- Li, Y.; Sarathy, S.M. Probing hydrogen–nitrogen chemistry: A theoretical study of important reactions in NxHy, HCN and HNCO oxidation. Int. J. Hydrog. Energy 2020, 45, 23624–23637. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. PCCP 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Knyazev, V.D.; Slagle, I.R. Thermochemistry and Kinetics of the Reaction of 1-Methylallyl Radicals with Molecular Oxygen. J. Phys. Chem. 1998, 102, 8932–8940. [Google Scholar] [CrossRef]
- Baldwin, R.R.; Bennett, J.P.; Walker, R.W. Addition of pentenes to slowly reacting mixtures of hydrogen and oxygen at 480 °C. J. Chem. Soc. Faraday Trans. Phys. Chem. Condens. Phases 1980, 76, 2396–2412. [Google Scholar] [CrossRef]
- Pedley, J.B. Thermochemical Data of Organic Compounds; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Ruscic, B.; Pinzon, R.E.; Morton, M.L.; von Laszevski, G.; Bittner, S.J.; Nijsure, S.G.; Amin, K.A.; Minkoff, M.; Wagner, A.F. Introduction to Active Thermochemical Tables: Several “Key” Enthalpies of Formation Revisited. J. Phys. Chem. 2004, 108, 9979–9997. [Google Scholar] [CrossRef]
- Branko, R.; Reinhardt, E.P.; Gregor von, L.; Deepti, K.; Alexander, B.; David, L.; David, M.; Albert, F.W. Active Thermochemical Tables: Thermochemistry for the 21st century. J. Phys. Conf. Ser. 2005, 16, 561. [Google Scholar]
- Ruscic, B.; Feller, D.; Peterson, K.A. Active Thermochemical Tables: Dissociation energies of several homonuclear first-row diatomics and related thermochemical values. Theor. Chem. Acc. 2013, 133, 1415. [Google Scholar] [CrossRef]
- Goldsmith, C.F.; Magoon, G.R.; Green, W.H. Database of Small Molecule Thermochemistry for Combustion. J. Phys. Chem. 2012, 116, 9033–9057. [Google Scholar] [CrossRef]
- DeSain, J.D.; Klippenstein, S.J.; Miller, J.A.; Taatjes, C.A. Measurements, Theory, and Modeling of OH Formation in Ethyl + O2 and Propyl + O2 Reactions. J. Phys. Chem. 2003, 107, 4415–4427. [Google Scholar] [CrossRef]
- Zhao, P.; Yuan, W.; Sun, H.; Li, Y.; Kelley, A.P.; Zheng, X.; Law, C.K. Laminar flame speeds, counterflow ignition, and kinetic modeling of the butene isomers. Proc. Combust. Inst. 2015, 35, 309–316. [Google Scholar] [CrossRef]
- Fenard, Y.; Dagaut, P.; Dayma, G.; Halter, F.; Foucher, F. Experimental and kinetic modeling study of trans-2-butene oxidation in a jet-stirred reactor and a combustion bomb. Proc. Combust. Inst. 2015, 35, 317–324. [Google Scholar] [CrossRef]
- Ruiz, R.P.; Bayes, K.D.; Macpherson, M.T.; Pilling, M.J. Direct observation of the equilibrium between allyl radicals, oxygen, and allylperoxy radicals. J. Phys. Chem. 1981, 85, 1622–1624. [Google Scholar] [CrossRef]
- Morgan, C.A.; Pilling, M.J.; Tulloch, J.M.; Ruiz, R.P.; Bayes, K.D. Direct determination of the equilibrium constant and thermodynamic parameters for the reaction. C3H5+ O2⇌ C3H5O2. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1982, 78, 1323–1330. [Google Scholar] [CrossRef]
- Slagle, I.R.; Ratajczak, E.; Heaven, M.C.; Gutman, D.; Wagner, A.F. Kinetics of polyatomic free radicals produced by laser photolysis. 4. Study of the equilibrium isopropyl + oxygen .tautm. isopropylperoxy between 592 and 692 K. J. Am. Chem. Soc. 1985, 107, 1838–1845. [Google Scholar] [CrossRef]
- Baldwin, R.R.; Lodhi, Z.H.; Stothard, N.; Walker, R.W. The oxidation chemistry of allyl radicals and related ‘stable’ radicals. Symp. Int. Combust. 1991, 23, 123–130. [Google Scholar] [CrossRef]
- Lodhi, Z.H.; Walker, R.W. Oxidation of allyl radicals: Kinetic parameters for the reactions of allyl radicals with HO2 and O2 between 400 and 480 °C. J. Chem. Soc. Faraday Trans. 1991, 87, 2361–2365. [Google Scholar] [CrossRef]
- Lodhi, Z.H.; Walker, R.W. Decomposition of 4,4-dimethylpent-1-ene in the presence of oxygen between 400 and 500 °C: Oxidation chemistry of allyl radicals. J. Chem. Soc. Faraday Trans. 1991, 87, 681–689. [Google Scholar] [CrossRef]
- Stothard, N.D.; Walker, R.W. Oxidation chemistry of propene in the autoignition region: Arrhenius parameters for the allyl + O2 reaction pathways and kinetic data for initiation reactions. J. Chem. Soc. Faraday Trans. 1992, 88, 2621–2629. [Google Scholar] [CrossRef]
- Bozzelli, J.W.; Dean, A.M. Hydrocarbon radical reactions with oxygen: Comparison of allyl, formyl, and vinyl to ethyl. J. Phys. Chem. 1993, 97, 4427–4441. [Google Scholar] [CrossRef]
- Chen, C.-J.; Bozzelli, J.W. Thermochemical Property, Pathway and Kinetic Analysis on the Reactions of Allylic Isobutenyl Radical with O2: an Elementary Reaction Mechanism for Isobutene Oxidation. J. Phys. Chem. A 2000, 104, 9715–9732. [Google Scholar] [CrossRef]
- Pratt, D.A.; Mills, J.H.; Porter, N.A. Theoretical Calculations of Carbon−Oxygen Bond Dissociation Enthalpies of Peroxyl Radicals Formed in the Autoxidation of Lipids. J. Am. Chem. Soc. 2003, 125, 5801–5810. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Bozzelli, J.W. Thermochemical and kinetic analysis of the allyl radical with O2 reaction system. Proc. Combust. Inst. 2005, 30, 1015–1022. [Google Scholar] [CrossRef]
- El-Agamey, A.; McGarvey, D.J. First Direct Observation of Reversible Oxygen Addition to a Carotenoid-Derived Carbon-Centered Neutral Radical. Org. Lett. 2005, 7, 3957–3960. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.S.; Shadnia, H.; Chepelev, L.L. Stability of carbon-centered radicals: Effect of functional groups on the energetics of addition of molecular oxygen. J. Comput. Chem. 2009, 30, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Dibble, T.S.; Sha, Y.; Thornton, W.F.; Zhang, F. Cis–Trans Isomerization of Chemically Activated 1-Methylallyl Radical and Fate of the Resulting 2-Buten-1-peroxy Radical. J. Phys. Chem. A 2012, 116, 7603–7614. [Google Scholar] [CrossRef]
| Method 1 | Method 2 | Method 3 | |
|---|---|---|---|
| Ab initio solver | Gaussian and Orca | Gaussian | |
| Geometry, frequency, scan and IRC | M06-2X/6-311++G(d,p) | ||
| SPEs | CCSD(T)/cc-pVTZ | CCSD(T)/cc-pVDZ | G4 |
| CCSD(T)/cc-pVTZ | |||
| CCSD(T)/cc-pVQZ | MP2/cc-pVDZ | ||
| MP2/cc-pVTZ | |||
| MP2/cc-pVQZ | |||
| Zero Kelvin energies | CBS-APNO/G3/G4 | ||
| Method 1 | Method 2 | Method 3 | |
|---|---|---|---|
| Scale factor for ZPEs | 0.9698 | ||
| Scale factor for frequencies | 0.983 | ||
| CBS extrapolation for SPE | ECCSD(T)/cc-pVQZ + (ECCSD(T)/cc-pVQZ − ECCSD(T)/cc-pVTZ) × 44/(54 − 44) | ECCSD(T)/cc-pVTZ + (ECCSD(T)/cc-pVTZ − ECCSD(T)/cc-pVDZ) × 34/(44 − 34) + EMP2/cc-pVQZ + (EMP2/cc-pVQZ − EMP2/cc-pVTZ) × 44/(54 − 44) − EMP2/cc-pVTZ + (EMP2/cc-pVTZ − EMP2/cc-pVDZ) × 34/(44 − 34) | EG4 |
| Method 1 | Method 2 | Method 3 | |||
|---|---|---|---|---|---|
| Reactions | Orca | Gaussian | Orca | Gaussian | Gaussian |
| Forward barrier height | |||||
C4H71-3 + O2  C4H6 + HO2 C4H6 + HO2 | 22.42 | 19.15 | 23.23 | 18.30 | 23.35 |
C5H91-3 + O2  C5H8 + HO2 C5H8 + HO2 | 21.00 | - | 21.80 | 16.65 | 21.61 |
C6H111-3 + O2  C6H10 + HO2 C6H10 + HO2 | 21.12 | - | 21.96 | 16.58 | 21.02 |
| Reverse barrier height | |||||
C4H71-3 + O2  C4H6 + HO2 C4H6 + HO2 | 25.23 | 22.48 | 26.21 | 22.00 | 25.90 |
C5H91-3 + O2  C5H8 + HO2 C5H8 + HO2 | 27.16 | - | 28.09 | 23.63 | 27.06 |
C6H111-3 + O2  C6H10 + HO2 C6H10 + HO2 | 28.39 | - | 29.31 | 24.58 | 26.94 |
| C4H71-3 + O2 Reaction | C5H91-3 + O2 Reaction | C6H111-3 + O2 Reaction | ||||
|---|---|---|---|---|---|---|
| T/K | MultiWell | PAPR | MultiWell | PAPR | MultiWell | PAPR |
| 600 | 1.43E + 06 | 1.28E + 06 | 1.17E + 05 | 5.49E + 05 | 1.81E + 05 | 5.10E + 05 |
| 800 | 6.37E + 07 | 6.12E + 07 | 3.01E + 06 | 2.04E + 07 | 5.58E + 06 | 1.74E + 07 |
| 1000 | 8.61E + 08 | 8.62E + 08 | 2.71E + 07 | 2.41E + 08 | 5.77E + 07 | 1.91E + 08 |
| 1100 | 2.38E + 09 | 2.42E + 09 | 6.35E + 07 | 6.31E + 08 | 1.44E + 08 | 4.88E + 08 |
| 1200 | 5.75E + 09 | 5.92E + 09 | 1.33E + 08 | 1.45E + 09 | 3.17E + 08 | 1.10E + 09 |
| 1300 | 1.24E + 10 | 1.30E + 10 | 2.52E + 08 | 3.02E + 09 | 6.35E + 08 | 2.24E + 09 |
| 1400 | 2.46E + 10 | 2.59E + 10 | 4.44E + 08 | 5.78E + 09 | 1.17E + 09 | 4.21E + 09 |
| 1500 | 4.53E + 10 | 4.81E + 10 | 7.35E + 08 | 1.03E + 10 | 2.04E + 09 | 7.40E + 09 |
| 1600 | 7.82E + 10 | 8.39E + 10 | 1.16E + 09 | 1.73E + 10 | 3.34E + 09 | 1.23E + 10 |
| 1700 | 1.28E + 11 | 1.39E + 11 | 1.74E + 09 | 2.78E + 10 | 5.25E + 09 | 1.96E + 10 |
| 1800 | 2.01E + 11 | 2.20E + 11 | 2.53E + 09 | 4.28E + 10 | 7.91E + 09 | 2.98E + 10 |
| ΔfHӨ | SӨ | Cp | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Molecules | Source | 298 K | 298 K | 300 K | 400 K | 500 K | 600 K | 800 K | 1000 K | 1500 K |
| C4H6 | This study | 26.7 | 66.6 | 18.9 | 24.3 | 28.7 | 32.1 | 37.1 | 40.7 | 46.3 |
| ATcT | 26.5 | - | - | - | - | - | - | - | - | |
| TDOC | 26.3 | - | - | - | - | - | - | - | - | |
| Goldsmith et al. | 26.5 | 65.8 | 18.5 | 24.0 | 28.7 | 32.4 | 37.6 | 41.1 | 46.6 | |
| C5H8 | This study | 18.7 | 76.5 | 24.4 | 30.9 | 36.4 | 41.0 | 47.8 | 52.7 | 60.4 |
| TDOC | 18.2 | - | - | - | - | - | - | - | - | |
| C6H10 | Current study | 11.0 | 84.9 | 29.8 | 37.6 | 44.3 | 49.7 | 58.1 | 64.3 | 74.2 |
| ΔfHӨ | SӨ | Cp | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Radicals | Source | 298 K | 298 K | 300 K | 400 K | 500 K | 600 K | 800 K | 1000 K | 1500 K |
| C4H71-3 | MultiWell | 31.9 | 72.1 | 19.7 | 24.8 | 29.3 | 33.1 | 39.1 | 43.6 | 50.6 |
| PAPR | 31.7 | 72.0 | 19.9 | 24.8 | 29.3 | 33.3 | 39.2 | 43.7 | 50.6 | |
| C5H91-3 | MultiWell | 26.8 | 83.1 | 25.2 | 31.8 | 37.6 | 42.5 | 50.1 | 55.8 | 64.8 |
| PAPR | 27.2 | 83.3 | 25.5 | 32.6 | 38.2 | 43.1 | 50.5 | 56.1 | 64.9 | |
| C6H111-3 | MultiWell | 19.8 | 88.9 | 31.4 | 39.4 | 46.5 | 52.4 | 61.6 | 68.4 | 79.1 |
| PAPR | 19.8 | 89.1 | 31.2 | 39.0 | 46.2 | 52.4 | 61.5 | 68.4 | 79.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wu, J.; Zhao, Q.; Zhang, Y.; Huang, Z. Theoretical Study of an Undisclosed Reaction Class: Direct H-Atom Abstraction from Allylic Radicals by Molecular Oxygen. Energies 2021, 14, 2916. https://doi.org/10.3390/en14102916
Li Y, Wu J, Zhao Q, Zhang Y, Huang Z. Theoretical Study of an Undisclosed Reaction Class: Direct H-Atom Abstraction from Allylic Radicals by Molecular Oxygen. Energies. 2021; 14(10):2916. https://doi.org/10.3390/en14102916
Chicago/Turabian StyleLi, Yang, Jin Wu, Qian Zhao, Yingjia Zhang, and Zuohua Huang. 2021. "Theoretical Study of an Undisclosed Reaction Class: Direct H-Atom Abstraction from Allylic Radicals by Molecular Oxygen" Energies 14, no. 10: 2916. https://doi.org/10.3390/en14102916
APA StyleLi, Y., Wu, J., Zhao, Q., Zhang, Y., & Huang, Z. (2021). Theoretical Study of an Undisclosed Reaction Class: Direct H-Atom Abstraction from Allylic Radicals by Molecular Oxygen. Energies, 14(10), 2916. https://doi.org/10.3390/en14102916







