Kinetic Modeling Study on the Combustion Characterization of Synthetic C3 and C4 Alcohols for Lean Premixed Prevaporized Combustion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Numerical Method
3. Results and Discussions
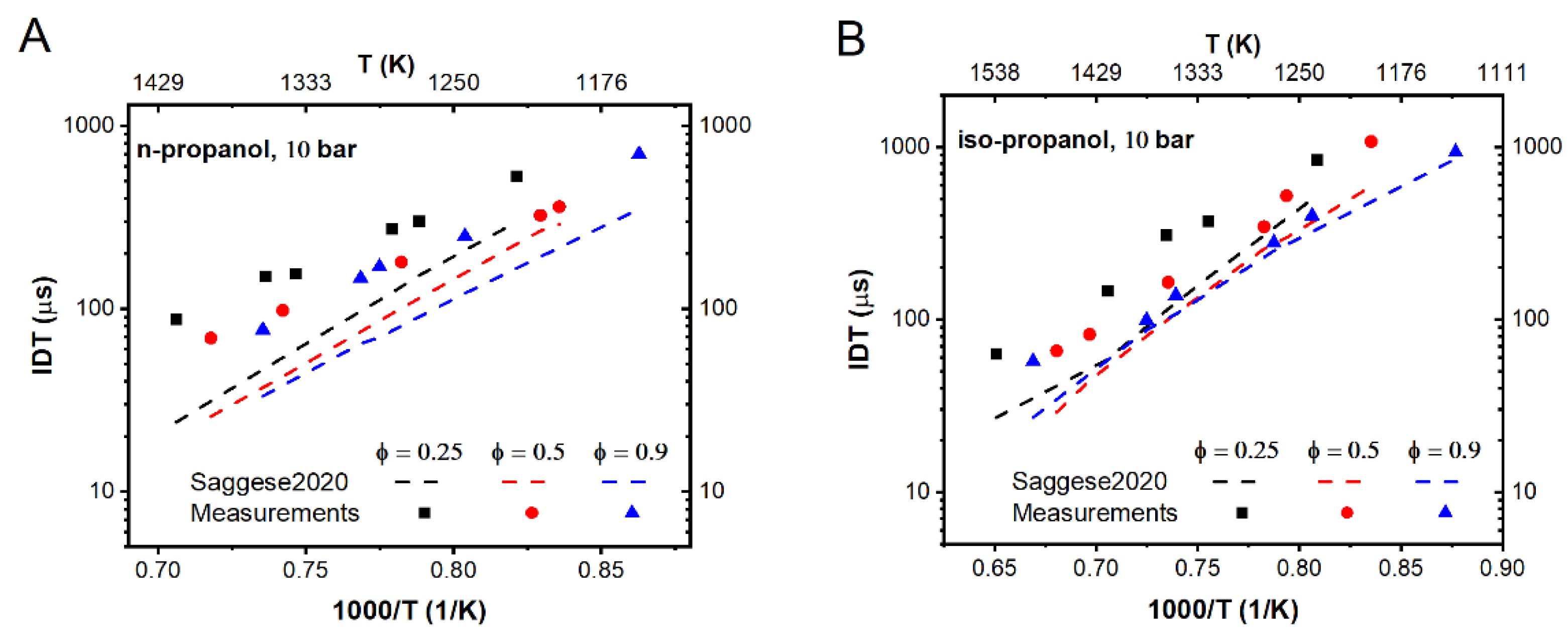
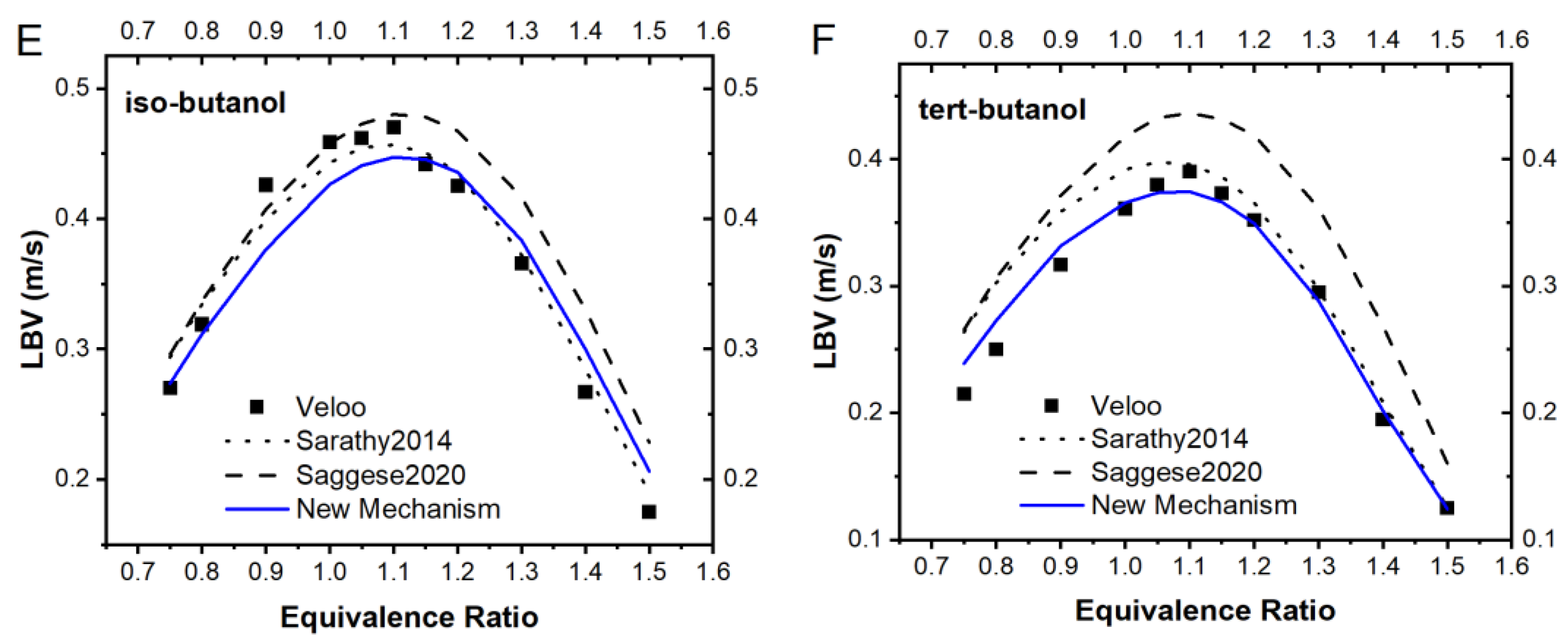
3.1. Literature Mechanisms Validation
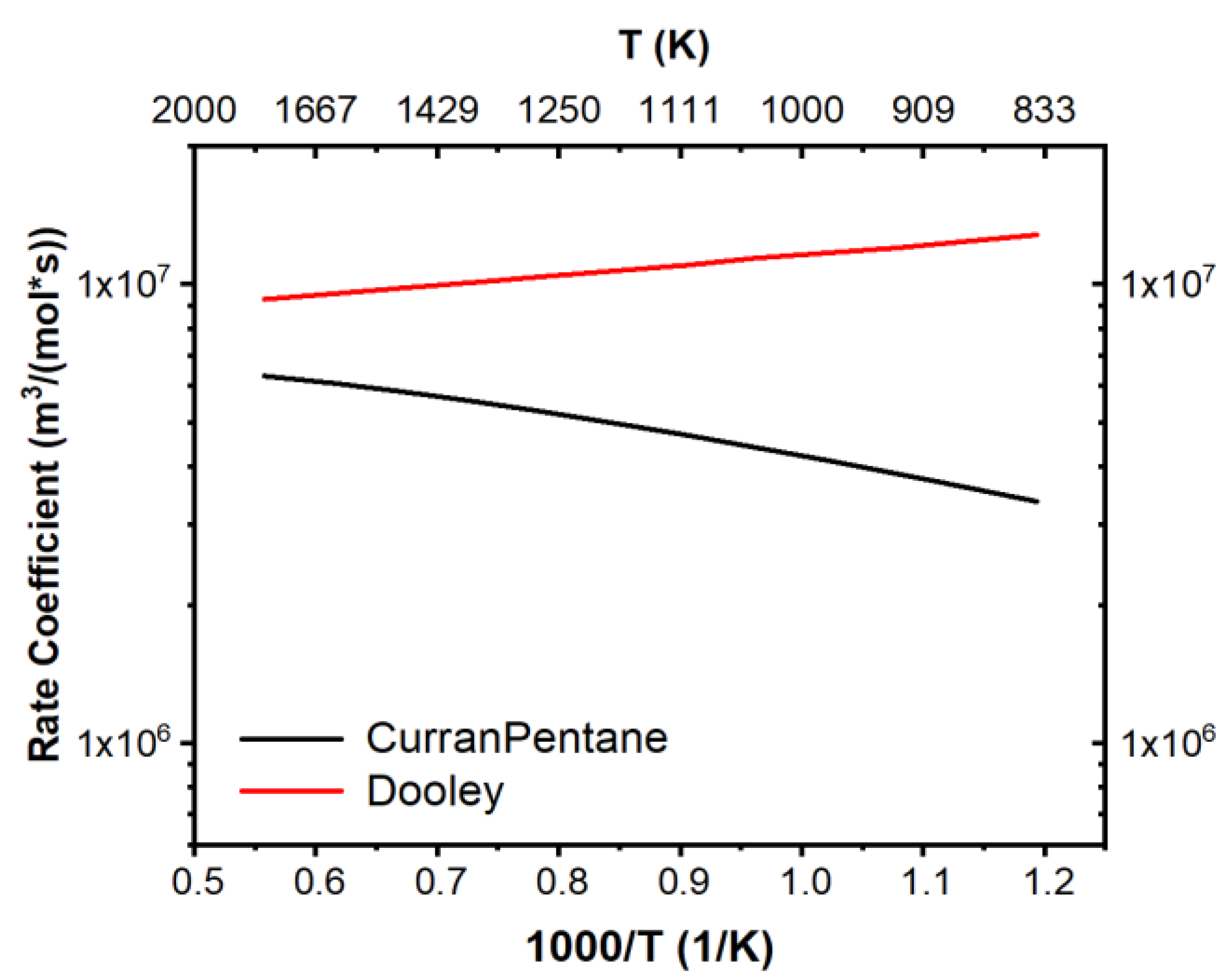
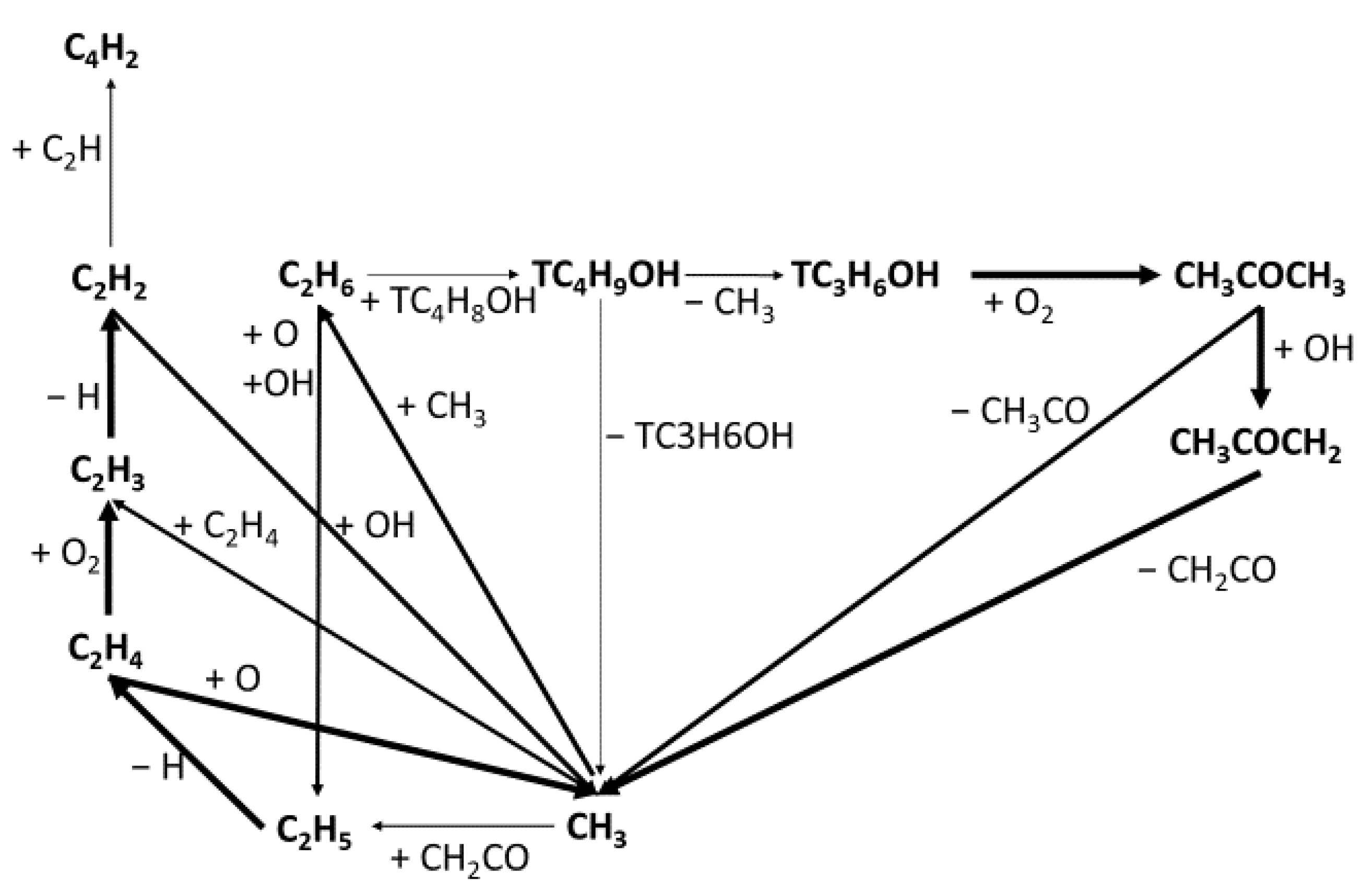
3.2. Mechanism Development
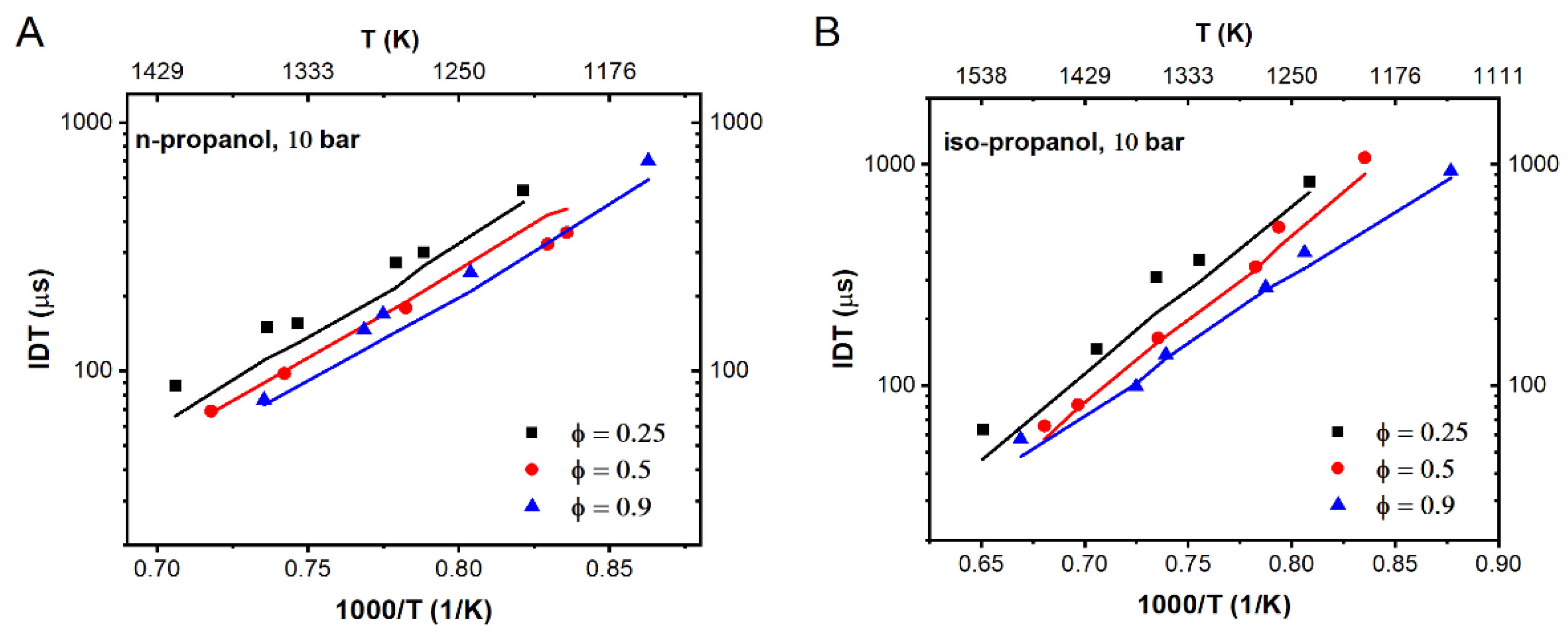
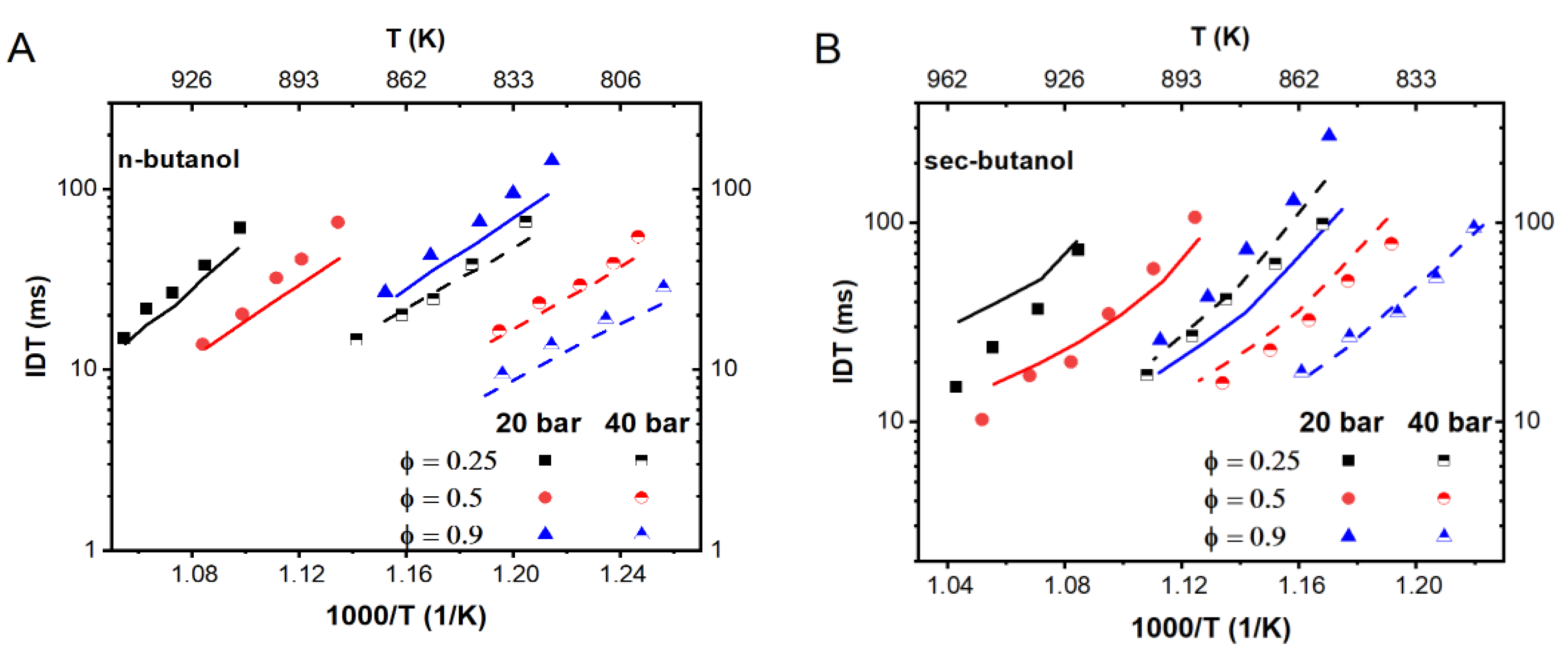
3.3. The New Mechanism Validation
3.4. Modeling LPP for Aviation
3.4.1. Ignition Delay Time and Laminar Burning Velocity Prediction of the Model
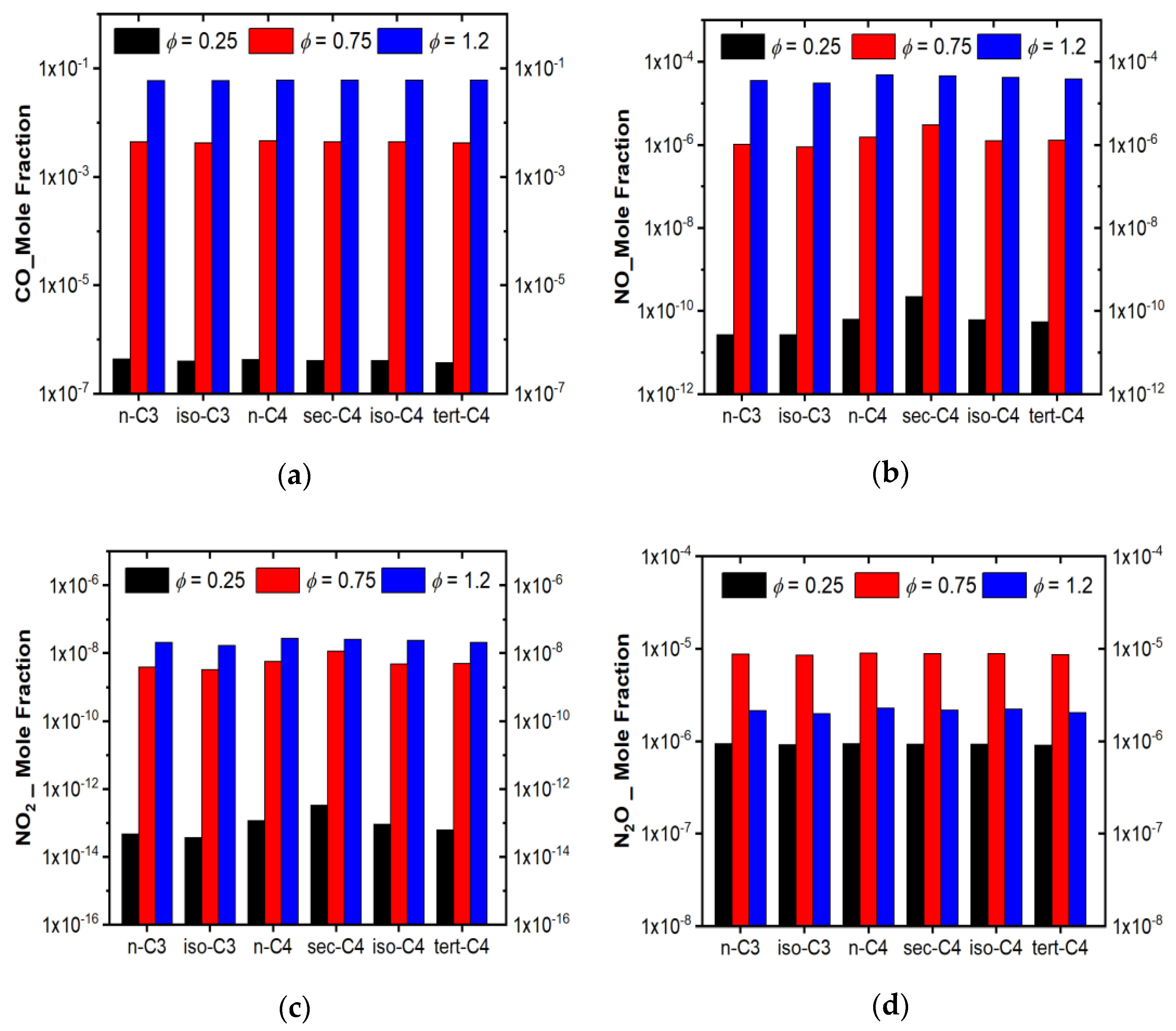
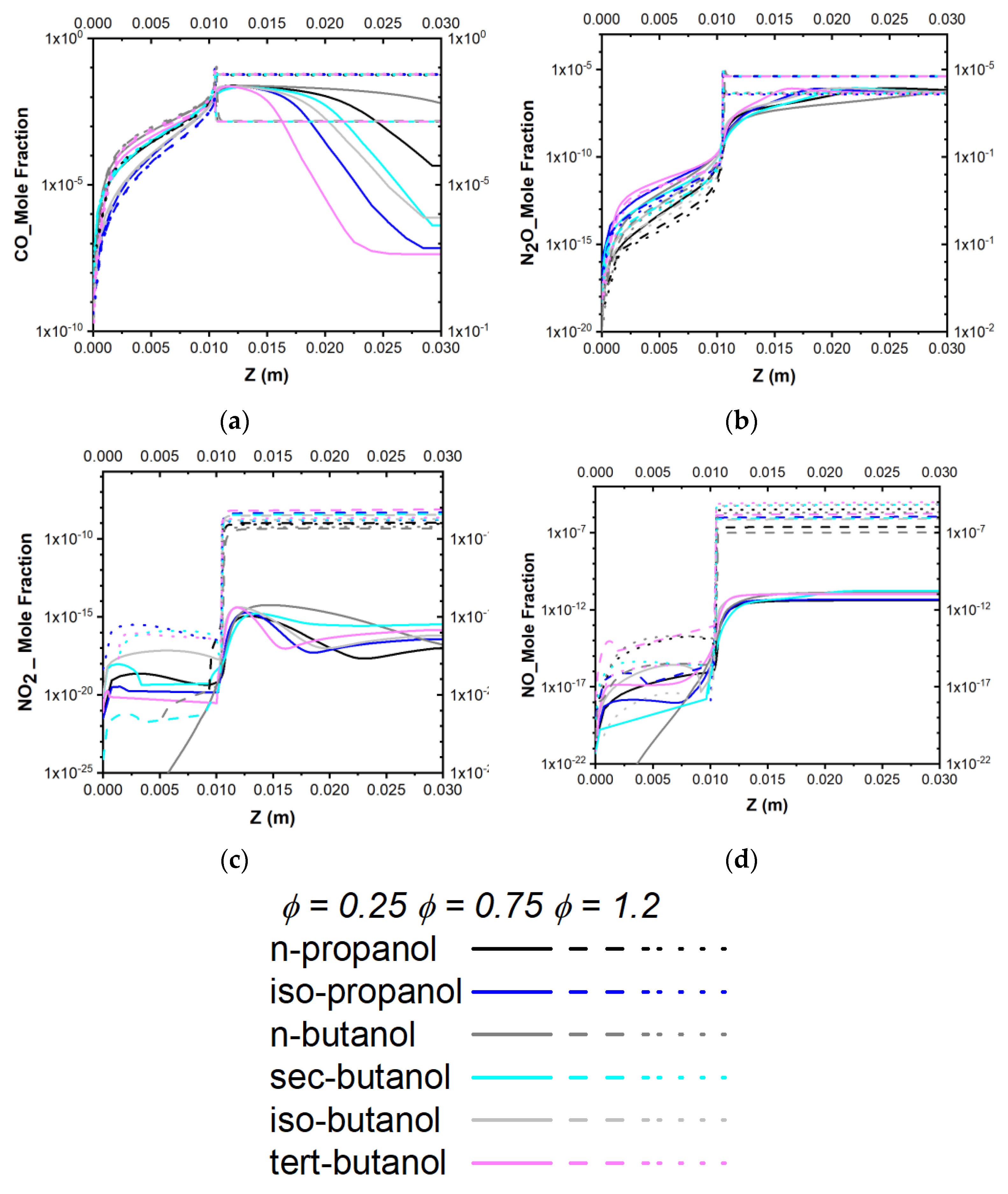
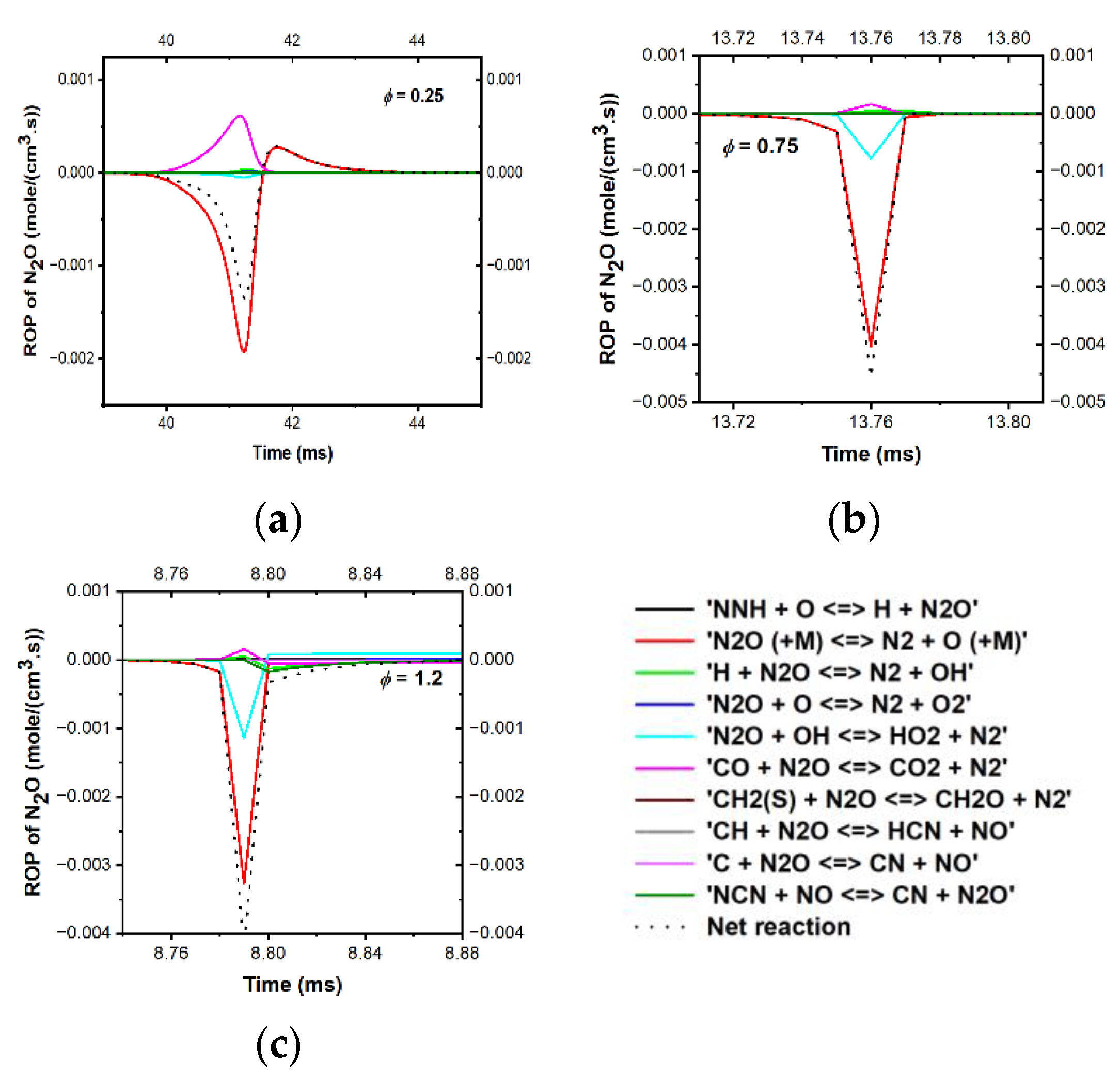
3.4.2. Emission Prediction of the Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sustainable Aviation Fuels. Available online: https://www.easa.europa.eu/eaer/topics/sustainable-aviation-fuels (accessed on 10 July 2021).
- Lefebvre, A.H.; Ballal, D.R. Gas Turbine Combustion: Alternative Fuels and Emissions; CRC Press: Boca Raton, FL, USA, 2010; ISBN 1420086057. [Google Scholar]
- European Green Deal: Commission Proposes Transformation of EU Economy and Society to Meet Climate Ambitions. Available online: https://ec.europa.eu/commission/presscorner/detail/en/IP_21_3541 (accessed on 10 July 2021).
- Skov, I.R.; Schneider, N.; Schweiger, G.; Schöggl, J.-P.; Posch, A. Power-to-X in Denmark: An Analysis of Strengths, Weaknesses, Opportunities and Threats. Energies 2021, 14, 913. [Google Scholar] [CrossRef]
- Albo, J.; Alvarez-Guerra, M.; Castaño, P.; Irabien, A. Towards the Electrochemical Conversion of Carbon Dioxide into Methanol. Green Chem. 2015, 17, 2304–2324. [Google Scholar] [CrossRef]
- Rego de Vasconcelos, B.; Lavoie, J.-M. Recent Advances in Power-to-X Technology for the Production of Fuels and Chemicals. Front. Chem. 2019, 7, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauen, A.; Bitossi, N.; German, L.; Harris, A.; Leow, K. Sustainable Aviation Fuels: Status, Challenges and Prospects of Drop-in Liquid Fuels, Hydrogen and Electrification in Aviation. Johns. Matthey Technol. Rev. 2020, 64, 263–278. [Google Scholar]
- Lee, D.S.; Fahey, D.W.; Forster, P.M.; Newton, P.J.; Wit, R.C.N.; Lim, L.L.; Owen, B.; Sausen, R. Aviation and Global Climate Change in the 21st Century. Atmos. Environ. 2009, 43, 3520–3537. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.S.; Fahey, D.W.; Skowron, A.; Allen, M.R.; Burkhardt, U.; Chen, Q.; Doherty, S.J.; Freeman, S.; Forster, P.M.; Fuglestvedt, J. The Contribution of Global Aviation to Anthropogenic Climate Forcing for 2000 to 2018. Atmos. Environ. 2021, 244, 117834. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, A.; Sauter, W.; Oettinger, M.; Kluge, T.; Schröder, U.; Seume, J.R.; Friedrichs, J.; Dinkelacker, F. A Study on Electrofuels in Aviation. Energies 2018, 11, 392. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, A.H.; Freeman, W.; Cowell, L. Spontaneous Ignition Delay Characteristics of Hydrocarbon Fuel-Air Mixtures; Purdue University, School of Mechanical Engineering: West Lafayette, IN, USA, 1986. [Google Scholar]
- Marek, C.J.; Papathakos, L.; Verbulecz, P. Preliminary Studies of Autoignition and Flashback in a Premixing-Prevaporizing Flame Tube Using Jet-a Fuel at Lean Equivalence Ratios. Contract 1977, 505, 3. [Google Scholar]
- Harvey, B.G.; Meylemans, H.A. The Role of Butanol in the Development of Sustainable Fuel Technologies. J. Chem. Technol. Biotechnol. 2011, 86, 2–9. [Google Scholar] [CrossRef]
- Nair, P.; Meenakshi, H.N. Review on the Synthesis, Performance and Trends of Butanol: A Cleaner Fuel Additive for Gasoline. Int. J. Ambient Energy 2021, 1–17. [Google Scholar] [CrossRef]
- Mourad, M.; Mahmoud, K.R.M. Performance Investigation of Passenger Vehicle Fueled by Propanol/Gasoline Blend According to a City Driving Cycle. Energy 2018, 149, 741–749. [Google Scholar] [CrossRef]
- Kaisan, M.U.; Yusuf, L.O.; Ibrahim, I.U.; Abubakar, S.; Narayan, S. Effects of Propanol and Camphor Blended with Gasoline Fuel on the Performance and Emissions of a Spark Ignition Engine. ACS Omega 2020, 5, 26454–26462. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.T.; Berkowitz, A.M.; Oehlschlaeger, M.A.; Biet, J.; Warth, V.; Glaude, P.-A.; Battin-Leclerc, F. An Experimental and Kinetic Modeling Study of the Oxidation of the Four Isomers of Butanol. J. Phys. Chem. A 2008, 112, 10843–10855. [Google Scholar] [CrossRef] [Green Version]
- Montero, E.A.; Aguilar, F.; Muñoz-Rujas, N.; Alaoui, F.E.M. Thermodynamic Properties of Propanol and Butanol as Oxygenate Additives to Biofuels. Front. Bioenergy Biofuels 2017, 363–389. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Dang, L.; Deng, Y.; Li, J.; Zhao, J. Experimental Study of Flow Dynamics and Fuel Spray Characteristics in Lean Premixed Prevaporized Combustor. Fuel 2015, 144, 197–204. [Google Scholar] [CrossRef]
- Maier, G.; Wittig, S. Fuel Preparation and Emission Characteristics of a Pressure Loaded LPP Combustor. In Proceedings of the 30th Fluid Dynamics Conference, Norfolk, VA, USA, 28 June–1 July 1999; p. 3774. [Google Scholar]
- Imamura, A.; Yoshida, M.; Kawano, M.; Aruga, N.; Nagata, Y.; Kawagishi, M. Research and Development of a LPP Combustor with Swirling Flow for Low NO (X). In Proceedings of the 37th Joint Propulsion Conference and Exhibit, Salt Lake City, UT, USA, 8–11 July 2001; p. 3311. [Google Scholar]
- Gokulakrishnan, P.; Ramotowski, M.J.; Gaines, G.; Fuller, C.; Joklik, R.; Eskin, L.D.; Klassen, M.S.; Roby, R.J. A Novel Low Nox Lean, Premixed, and Prevaporized Combustion System for Liquid Fuels. J. Eng. Gas Turbines Power 2008, 130, 051501. [Google Scholar] [CrossRef]
- Goldmann, A.; Dinkelacker, F. Experimental Investigation and Modeling of Boundary Layer Flashback for Non-Swirling Premixed Hydrogen/Ammonia/Air Flames. Combust. Flame 2021, 226, 362–379. [Google Scholar] [CrossRef]
- Johnson, M.V.; Goldsborough, S.S.; Serinyel, Z.; O’Toole, P.; Larkin, E.; O’Malley, G.; Curran, H.J. A Shock Tube Study of N-and Iso-Propanol Ignition. Energy Fuels 2009, 23, 5886–5898. [Google Scholar] [CrossRef]
- Bourque, G.; Healy, D.; Curran, H.; Zinner, C.; Kalitan, D.; de Vries, J.; Aul, C.; Petersen, E. Ignition and Flame Speed Kinetics of Two Natural Gas Blends with High Levels of Heavier Hydrocarbons. J. Eng. Gas Turbines Power 2010, 132, 021504. [Google Scholar] [CrossRef]
- Man, X.; Tang, C.; Zhang, J.; Zhang, Y.; Pan, L.; Huang, Z.; Law, C.K. An Experimental and Kinetic Modeling Study of N-Propanol and i-Propanol Ignition at High Temperatures. Combust. Flame 2014, 161, 644–656. [Google Scholar] [CrossRef]
- Sarathy, S.M.; Vranckx, S.; Yasunaga, K.; Mehl, M.; Oßwald, P.; Metcalfe, W.K.; Westbrook, C.K.; Pitz, W.J.; Kohse-Höinghaus, K.; Fernandes, R.X. A Comprehensive Chemical Kinetic Combustion Model for the Four Butanol Isomers. Combust. Flame 2012, 159, 2028–2055. [Google Scholar] [CrossRef]
- Frassoldati, A.; Cuoci, A.; Faravelli, T.; Niemann, U.; Ranzi, E.; Seiser, R.; Seshadri, K. An Experimental and Kinetic Modeling Study of N-Propanol and Iso-Propanol Combustion. Combust. Flame 2010, 157, 2–16. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, S.; Cheng, Y.; Huang, Z.; Tang, C.; Zhang, J. A Comparative Study of N-Propanol, Propanal, Acetone, and Propane Combustion in Laminar Flames. Proc. Combust. Inst. 2015, 35, 795–801. [Google Scholar] [CrossRef]
- Weber, B.W.; Sung, C.-J. Comparative Autoignition Trends in Butanol Isomers at Elevated Pressure. Energy Fuels 2013, 27, 1688–1698. [Google Scholar] [CrossRef] [Green Version]
- Karwat, D.M.A.; Wagnon, S.W.; Teini, P.D.; Wooldridge, M.S. On the Chemical Kinetics of N-Butanol: Ignition and Speciation Studies. J. Phys. Chem. A 2011, 115, 4909–4921. [Google Scholar] [CrossRef] [PubMed]
- Heufer, K.A.; Fernandes, R.X.; Olivier, H.; Beeckmann, J.; Röhl, O.; Peters, N. Shock Tube Investigations of Ignition Delays of N-Butanol at Elevated Pressures between 770 and 1250 K. Proc. Combust. Inst. 2011, 33, 359–366. [Google Scholar] [CrossRef]
- Stranic, I.; Chase, D.P.; Harmon, J.T.; Yang, S.; Davidson, D.F.; Hanson, R.K. Shock Tube Measurements of Ignition Delay Times for the Butanol Isomers. Combust. Flame 2012, 159, 516–527. [Google Scholar] [CrossRef]
- Veloo, P.S.; Egolfopoulos, F.N. Flame Propagation of Butanol Isomers/Air Mixtures. Proc. Combust. Inst. 2011, 33, 987–993. [Google Scholar] [CrossRef]
- Veloo, P.S.; Egolfopoulos, F.N. Studies of N-Propanol, Iso-Propanol, and Propane Flames. Combust. Flame 2011, 158, 501–510. [Google Scholar] [CrossRef]
- Saggese, C.; Thomas, C.M.; Wagnon, S.W.; Kukkadapu, G.; Cheng, S.; Kang, D.; Goldsborough, S.S.; Pitz, W.J. An Improved Detailed Chemical Kinetic Model for C3-C4 Linear and Iso-Alcohols and Their Blends with Gasoline at Engine-Relevant Conditions. Proc. Combust. Inst. 2021, 38, 415–423. [Google Scholar] [CrossRef]
- McGillen, M.R.; Baasandorj, M.; Burkholder, J.B. Gas-Phase Rate Coefficients for the OH+ n-, i-, s-, and t-Butanol Reactions Measured between 220 and 380 K: Non-Arrhenius Behavior and Site-Specific Reactivity. J. Phys. Chem. A 2013, 117, 4636–4656. [Google Scholar] [CrossRef] [PubMed]
- Mittal, G.; Burke, S.M.; Davies, V.A.; Parajuli, B.; Metcalfe, W.K.; Curran, H.J. Autoignition of Ethanol in a Rapid Compression Machine. Combust. Flame 2014, 161, 1164–1171. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q. Investigation on the Ignition Properties of Propanol and Butanol Isomers under Fuel Lean Conditions. Master’s Thesis, Technische Universität Braunschweig, Braunschweig, Germany, 2021. [Google Scholar]
- Laguna, A.G. Investigation on the Ignition Delay Time of Butanol Fuels for Aviation Application. Bachelor’s Thesis, Technische Universität Braunschweig, Braunschweig, Germany, 2020. [Google Scholar]
- Wang, Q.; Laguna, A.G.; He, X.; Shu, B.; Li, L. Investigation on the Ignition Properties of 1-Propanol and 1-Butanol under Fuel-Lean Conditions; SAE Technical Paper; SAE: Warrendale, PA, USA, 2021. [Google Scholar]
- Nishida, M. Shock tubes. In Handbook of Shock Waves; Elsevier: Amsterdam, The Netherlands, 2001; pp. 553–585. [Google Scholar]
- Goodwin, D.G.; Moffat, H.K.; Speth, R.L. Cantera: An Object-Oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes. Comput. Sci. 2009. [Google Scholar] [CrossRef]
- Nadiri, S.; Agarwal, S.; He, X.; Kühne, U.; Fernandes, R.; Shu, B. Development of the Chemical Kinetic Mechanism and Modeling Study on the Ignition Delay of Liquefied Natural Gas (LNG) at Intermediate to High Temperatures and High Pressures. Fuel 2021, 302, 121137. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, M.; Tang, C.; Liu, Y.; Zhang, P.; Huang, Z. Promoting “Adiabatic Core” Approximation in a Rapid Compression Machine by an Optimized Creviced Piston Design. Fuel 2019, 251, 328–340. [Google Scholar] [CrossRef]
- Van Geem, K.M.; Pyl, S.P.; Marin, G.B.; Harper, M.R.; Green, W.H. Accurate High-Temperature Reaction Networks for Alternative Fuels: Butanol Isomers. Ind. Eng. Chem. Res. 2010, 49, 10399–10420. [Google Scholar] [CrossRef]
- Sarathy, S.M.; Oßwald, P.; Hansen, N.; Kohse-Höinghaus, K. Alcohol Combustion Chemistry. Prog. Energy Combust. Sci. 2014, 44, 40–102. [Google Scholar] [CrossRef]
- Cheng, S.; Kang, D.; Goldsborough, S.S.; Saggese, C.; Wagnon, S.W.; Pitz, W.J. Experimental and Modeling Study of C2–C4 Alcohol Autoignition at Intermediate Temperature Conditions. Proc. Combust. Inst. 2021, 38, 709–717. [Google Scholar] [CrossRef]
- Bugler, J.; Marks, B.; Mathieu, O.; Archuleta, R.; Camou, A.; Grégoire, C.; Heufer, K.A.; Petersen, E.L.; Curran, H.J. An Ignition Delay Time and Chemical Kinetic Modeling Study of the Pentane Isomers. Combust. Flame 2016, 163, 138–156. [Google Scholar] [CrossRef] [Green Version]
- The Master Chemical Mechanism. Available online: http://mcm.york.ac.uk (accessed on 10 July 2021).
- Senosiain, J.P.; Klippenstein, S.J.; Miller, J.A. Reaction of Ethylene with Hydroxyl Radicals: A Theoretical Study. J. Phys. Chem. A 2006, 110, 6960–6970. [Google Scholar] [CrossRef] [PubMed]
- Dooley, S.; Burke, M.P.; Chaos, M.; Stein, Y.; Dryer, F.L.; Zhukov, V.P.; Finch, O.; Simmie, J.M.; Curran, H.J. Methyl Formate Oxidation: Speciation Data, Laminar Burning Velocities, Ignition Delay Times, and a Validated Chemical Kinetic Model. Int. J. Chem. Kinet. 2010, 42, 527–549. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling Nitrogen Chemistry in Combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef] [Green Version]
- Braeunling, W.J.G. Turbojet Engines. Fundamentals, Aero Thermodynamics, Ideal and Real Cycle Processes, Thermal Turbo Engines, Components, Emissions and Systems. In Flugzeugtriebwerke. Grundlagen, Aero-Thermodynamik, Ideale und Reale Kreisprozesse, Thermische Turbomaschinen, Komponenten, Emissionen und Systeme; Springer: Berlin/Heidelberg, Germany, 2009; pp. 859–972. [Google Scholar] [CrossRef]
- Honnet, S.; Seshadri, K.; Niemann, U.; Peters, N. A Surrogate Fuel for Kerosene. Proc. Combust. Inst. 2009, 32, 485–492. [Google Scholar] [CrossRef]
- Omidvarborna, H.; Kumar, A.; Kim, D.-S. Recent Studies on Soot Modeling for Diesel Combustion. Renew. Sustain. Energy Rev. 2015, 48, 635–647. [Google Scholar] [CrossRef]
- Drakon, A.; Eremin, A.; Mikheyeva, E.; Shu, B.; Fikri, M.; Schulz, C. Soot Formation in Shock-Wave-Induced Pyrolysis of Acetylene and Benzene with H2, O2, and CH4 Addition. Combust. Flame 2018, 198, 158–168. [Google Scholar] [CrossRef]
















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadiri, S.; Zimmermann, P.; Sane, L.; Fernandes, R.; Dinkelacker, F.; Shu, B. Kinetic Modeling Study on the Combustion Characterization of Synthetic C3 and C4 Alcohols for Lean Premixed Prevaporized Combustion. Energies 2021, 14, 5473. https://doi.org/10.3390/en14175473
Nadiri S, Zimmermann P, Sane L, Fernandes R, Dinkelacker F, Shu B. Kinetic Modeling Study on the Combustion Characterization of Synthetic C3 and C4 Alcohols for Lean Premixed Prevaporized Combustion. Energies. 2021; 14(17):5473. https://doi.org/10.3390/en14175473
Chicago/Turabian StyleNadiri, Solmaz, Paul Zimmermann, Laxmi Sane, Ravi Fernandes, Friedrich Dinkelacker, and Bo Shu. 2021. "Kinetic Modeling Study on the Combustion Characterization of Synthetic C3 and C4 Alcohols for Lean Premixed Prevaporized Combustion" Energies 14, no. 17: 5473. https://doi.org/10.3390/en14175473
APA StyleNadiri, S., Zimmermann, P., Sane, L., Fernandes, R., Dinkelacker, F., & Shu, B. (2021). Kinetic Modeling Study on the Combustion Characterization of Synthetic C3 and C4 Alcohols for Lean Premixed Prevaporized Combustion. Energies, 14(17), 5473. https://doi.org/10.3390/en14175473





