Abstract
Biofuels used as biocomponents for transport fuels should meet quality requirements. Their properties have a significant impact on the proper functioning of the engine supply system and the wear of its components. Changes in the performance of biofuel functionality may already occur during storage. Therefore, the present study aimed to evaluate changes in selected rheological and tribological parameters of higher fatty acid esters depending on the time and method of their storage by considering different types of substrates used for their production. The presence of possible microbiological contamination, which may affect the examined parameters of biofuels, was also analyzed. The dynamic viscosity of the biofuels tested changed depending on the substrate used. The biofuel produced from waste oil had the highest viscosity. Tribological studies show that both the linear wear of samples and the friction moment were higher after the storage period. The acid number of the esters did not exceed the permissible value recommended by the standard. The type of raw material used for the production of biodiesel and the conditions of its storage affected biodeterioration, proved by the growth of microorganisms. The highest number of microorganisms was recorded in biofuels prepared from waste oil.
1. Introduction
With the increase in energy demand, currently met mainly by fossil fuels, carbon dioxide emissions have also significantly increased [1]. Considering energy security and, most importantly, environmental issues, alternative solutions are being sought. One of them is the production of bioenergy, i.e., renewable energy from biomass [2]. The main stage of energy use of biomass is its conversion to heat, electricity or biofuels [3]. Much focus has been given to transport biofuels, the production of which could enable sustainable use of biomass on a large scale [4]; additionally, the use of bioethanol or biodiesel allows us to reduce the use of conventional fuels.
Biodiesel is composed of monoalkyl esters of long-chain fatty acids derived from renewable fatty compounds [5]. The basic raw material for its production is oil, including soybean, rapeseed, sunflower, palm, and jatropha [6,7,8,9,10], and animal fats, especially waste animal fat such as tallow and chicken fat [11,12]. Biodiesel is produced by diluting oils with solvents, microemulsion, pyrolysis or transesterification [13], resulting in fatty acid esters (Figure 1). The esters produced in this process have a high cetane value, low viscosity, and an appropriate calorific value [14].
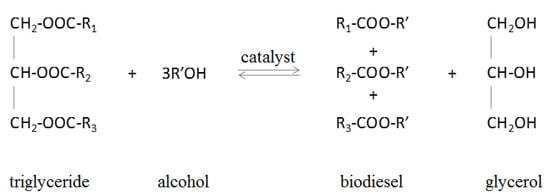
Figure 1.
Scheme of triglyceride transesterification [15].
The type of substrate used to produce biodiesel affects the emissions of CO, CO2, NOx, and particular matter (PM) [16]. Compared to conventional fuels, the combustion of biofuels does not cause emission of sulfur and aromatic compounds [17]; however, increased emission of NOx is observed. An exception is biodiesel obtained from palm oil and oil obtained from microalgae cells [16].
One of the main issues associated with the use of biodiesel is its stability, which can change during its storage, mainly due to changes in physical and chemical parameters [18]. These changes may be due to microbiological contamination. Fuel components can be a valuable source of carbon and energy for microorganisms [19], which lead to their biodegradation. The susceptibility of biodiesel to this type of contamination is associated with its higher hygroscopy than that of conventional fuel [20]. The basic condition for the development of microorganisms is the presence of chemically unbound water in the fuel [21] because they grow intensively mainly at the water/fuel interface [22]. An increase in the water content of biodiesel may be due to inappropriate storage conditions, especially high temperatures [23]. The presence of water leads to the hydrolysis of esters, resulting in the formation of free fatty acids and glycerol. These conditions are also conducive to microbiological corrosion (MIC), which adversely affects both the storage process and the subsequent use of biofuels [24]. Biofilms are formed under conditions favorable for the growth and activity of microorganisms, and the presence of biofilms is also found on the bottom and walls of fuel tanks. Bacteria, yeasts, and fungi mainly grow in fuels [25]. Storage causes aging of the fuel, which changes its performance. Aging processes can also be catalyzed by chemical compounds and materials present in the structural elements of the process infrastructure. Because of ageing, the fuel becomes turbid, delaminated, or shows formation of deposits. In addition to the impact of aging on the basic parameters of biofuel, attention should also be given to the changes in the tribological properties of the fuel, i.e., the lubricating factor for the elements of frictional vapors present in power supply systems.
The present study aims to evaluate changes in selected rheological and tribological properties of higher fatty acid esters, depending on the time and method of their storage, by considering different types of substrates used for their production. The presence of possible microbiological contamination, which may affect the examined parameters of biofuels, is also analyzed.
2. Materials and Methods
2.1. Materials
Esters of higher fatty acids prepared under laboratory conditions in the one-stage transesterification process from oil pressed from rapeseed (R) and sunflower seeds (S), waste oil (W), and bioester (BE) provided by a Polish refinery were used in the experiments. The BE met all the necessary requirements specified in EN 14214 standard.
2.2. Testing and Analysis Procedure
The waste oil was heated and filtered through filter papers to remove solid particles and other residues that could interfere with the transesterification process and contaminate the product. The oil obtained by pressing was left for 24 h to sediment the plant residues and was then filtered. The crude oil was subjected to the process of transesterification by using methanol and KOH as a catalyst. The oil was heated in a reaction flask to a temperature of 60 °C, and the prepared process catalyst (potassium methoxide) was added. A molar ratio of oil to methanol of 1: 6 was used. The entire reaction mixture was placed on a magnetic stirrer and stirred intensively with a magnetic rod (600–650 rpm). After cooling, the mixture was poured into a separating funnel. After phase separation, the lower glycerin phase was separated. The upper layer of esters was purified using water, which eliminates soap formation and residues of glycerol or the catalyst. Additional vacuum cleaning with anhydrous magnesium sulfate was performed. After purification, the obtained esters were placed in 1 L tightly closed glass containers and stored under dark light for five months in the laboratory under outdoor real conditions at constant or variable temperature. Eight test variants were thus created, depending on the type of substrate used for production and storage parameters (Table 1).

Table 1.
Description of the experimental variants.
Physicochemical (dynamic viscosity, density, and pH) and microbiological (bacterial and fungal count) analyses were performed on the day of conducting the experiment and after 150 days. After the storage period, the acid number (AN) and tribological properties of the esters were determined.
Dynamic viscosity, which is a measure of the internal friction of the tested biofuels, was measured at 10 °C, 20 °C, and 40 °C. A Brookfield DV-II + viscometer was used in the study. The temperature of the biofuel sample with a volume of 17 mL in the viscometer measuring cylinder was maintained by a thermostat. For each measuring point, the temperature was maintained for 10 min to stabilize the measurement. The torque of the shaft in the cylinder filled with biofuel samples was measured. Dynamic viscosity was measured as the resistance of the rotating roller of the device. The measurement was repeated three times for the adopted temperatures, and the mean values of dynamic viscosity were then estimated.
The acid number was determined by the titration method using phenolphthalein as an indicator and calculated using the following formula:
where 56.1 is the molecular weight of KOH; V is the volume of KOH (mL); CKOH is the concentration of KOH (mol L−1); and m is the amount of ester (g).
Acid number (AN) = (56.1 V CKOH) m−1,
The number of bacteria and fungi in the esters was determined by an indirect method using membrane filtration. Fuel samples (1 mL) were taken from individual objects and filtered under vacuum through a 0.22 µM filter. Membrane filters were washed with 3 mL of 0.85% NaCl and then placed on the surface of appropriate solidified medium in Petri dishes. Nutrient Agar (NA) medium with the following composition [g L−1]: Peptone 5, beef extract 3, and agar 15, was used to determine the number of bacteria. The number of fungi was determined on potato dextrose agar (PDA) medium with the following composition [g L−1]: Agar 17, potato extract 4, and glucose 20. The dishes were incubated at 25 °C for 48 h (bacteria) or 5 days (fungi) and the number of microorganisms was then determined. The results were expressed as colony forming units (CFU) per 1 mL.
The influence of time and method of storage of the above-mentioned biofuels on lubricating properties was also assessed in terms of the friction moment and wear in the tribological node. The reference values in the tests were the results obtained for diesel oil tested under the same conditions. In tribological tests, because of the possibility of a rapid increase in wear, samples made of aluminum alloy in the form of shafts with 10 mm diameter and 16 mm length were used, while the counter-sample was a ring with 43 mm diameter and a 8 mm width made of ISO 100Cr6 bearing steel with a hardness of 62 HRC and a roughness of Ra 0.19 µm. The work in the friction node starts in a concentrated point contact and as the sample wear progresses, it is aimed at the distributed contact characteristic of the sliding nodes.
The T 05 tribometer schematically shown in Figure 2 was used for the study. The measuring station consists of a drive system connected through a torque meter (7) to the counter-sample (5). The load is applied by a pneumatic system (1), and the value of the load force is controlled by a force sensor (2) placed between the actuator and the sample holder (3). The displacement of the sample in relation to the counter-sample is measured using an inductive displacement sensor (8). The counter-sample is lubricated by immersion in the tank (6) filled to three-fourths of the height with 75 mL of the tested fuel. The device is also equipped with two thermocouples, which enables us to measure the total temperature of the sample and the ambient temperature.
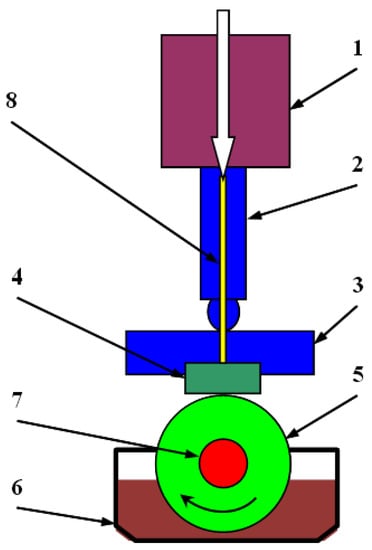
Figure 2.
Diagram of the test stand; 1—pneumatic load transfer system, 2—load transfer system with control system, 3—sample holder, 4—sample, 5—counter-sample, 6—lubricant tank, 7—drive system with torque meter, 8—displacement sensor.
Each preparation of the test stand and the start of the tests were performed in accordance with the guidelines of ASTM G77 (Standard Test Method for Ranking Resistance of Materials to Sliding Wear Using Block-on-Ring Wear Test). The sample was fixed in the tribometer holder, the tank was filled with biofuel, and the test run was started. After each test run, the biofuel was replaced and the friction node was cleaned and cooled to the initial temperature. Each run started with an initial distribution of the biofuel over the frictional steam elements during the acceleration of the counter-sample to a preset linear speed in 15 s and without contact of the frictional steam elements. After obtaining the set linear velocity and applying the tested biofuel to the elements of frictional steam, a proper test run was conducted without stopping the counter-sample. The tests were performed at the set linear velocity of 1 m s−1. In the tests, a constant speed of load force accumulation over time and a constant test duration were assumed for each variant of the tested biofuel. The value of the load was increased gradually from 0 N to 500 N, and the test time was 100 s, which corresponds to 100 m of the friction path. Thus, a 5 N s−1 increase in the loading force was obtained.
2.3. Statistical Analysis
All the determinations were performed in triplicate. The measured values were expressed as mean ± standard deviation. The significance of differences was evaluated using Tukey’s honestly significant difference test at the level of α = 0.05. All statistical analyses were performed using the statistical software package for Windows (Dell Statistica (data analysis software system) version 13.3 (2016); Dell Inc., Tulsa, OK, USA).
3. Results and Discussion
3.1. Microbial Contamination of Biofuels
Compared to conventional diesel, the esters of higher fatty acids contain fewer carbon and hydrogen atoms and approximately 12% more oxygen, which can promote the development of microorganisms and cause a change in the parameters of biofuel [26]. After one month of storage in containers containing esters produced from sunflower oil (S), regardless of the storage conditions, sediments were observed at the bottom of the tank. Similar changes were also observed after 90 days for rapeseed oil (R) and waste oil (W) esters. Because of the presence of microorganisms, sediments and sludges negatively affect the quality of stored fuel and may also damage tanks and equipment in which such fuel is used [22]. No sediments were noted in the BE1 and BE2 commercial bioesters.
Microbes were not noted initially; however, after 150 days the presence of both bacteria and fungi was observed in most biofuel samples taken from individual facilities (Figure 3). Microorganisms can grow in fuel because of the availability of free water, which can accumulate due to temperature changes [27]. This is confirmed by the results obtained in the present study. Biofuels stored under variable environmental conditions showed a higher number of microorganisms. Favorable conditions for the development of microorganisms were noted in esters prepared from waste oil. On the one hand, this finding may suggest that the method used for the initial purification of these esters before the transesterification process could be insufficient, and the esters contained residues that promoted the growth of both bacteria and fungi. On the other hand, the observed changes may also be influenced by changes in the physical and chemical properties of fuels during storage, as noted in several previous studies [28,29,30].
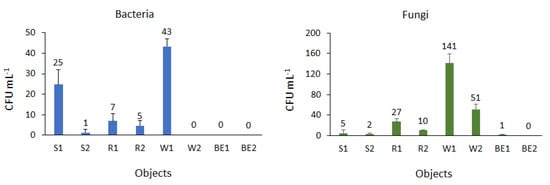
Figure 3.
The number of bacteria and fungi in the tested biofuels.
The rapeseed ester samples showed higher fungal growth than bacterial growth. The beneficial effect of rapeseed methyl esters (RME) on these microorganisms, compared to bacteria, is also confirmed by studies on the microbiological stability of fuels conducted by Schlaicher et al. [31]. Moreover, biodiesel may contain different proportions of fatty acids depending on the type of feedstock used for their production; this explains the differences in the number of microorganisms observed in particular ester samples in the present study. According to Bucker et al. [32], the changes in the properties of fuels during storage may determine the development of specific groups of microorganisms best suited to the conditions.
Microorganisms can grow across a wide range of temperatures, but most of them prefer warmer conditions [33]. According to Klofutar and Golob [22], storing fuel at a temperature below 10 °C can prevent microbial growth. In the present study, the low storage temperature of the esters limited microbial proliferation; however, significant differences in bacterial and fungal counts were noted in the S2 and W2 samples and in the R2 and W2 samples, respectively.
In crude oil and its derivatives, if the number of microorganisms is less than 50 per mL, it indicates that the product is pure; furthermore, the product is considered to be slightly contaminated if the bacterial and fungal counts are approximately 105 per mL and 103–104 per mL, respectively, and significantly contaminated if these values increase to 106–108 per mL and 104–106 per mL, respectively [34]. In the present study, sample W1 had the highest number of bacteria (43 CFU mL−1). These microorganisms were not found in commercial bioesters (BE1 and BE2) and in W2. Therefore, these biofuels tested can be considered to be free of bacterial contamination. The average number of fungi in biofuels ranged from 1 CFU mL−1 in BE1 to ≥ 140 CFU mL−1 in W1. No fungi were found in samples taken from the BE2 site. The detected values were below the level that corresponds to contaminated fuels. The presence of microorganisms in fuel not only affects its quality, but it may also cause damage and corrosion of tanks during storage [35]; moreover, the biomass of microorganisms that develop during storage may block filters in the fuel system of vehicles [21]. Different types of biocides are used to prevent microbial growth; however, the consequences of their use are not yet well understood [36]. In the present study, the detected levels of microbial growth were below those that correspond to contaminated fuels, which may indicate that the growth and development of microorganisms can be limited by storing the esters under appropriate conditions. This could enable us to reduce the amount of antimicrobials introduced into biofuels, especially for biodiesels that contain such additives and have a negative environmental influence [37].
3.2. Acidity of Biofuels
Acid number is a good parameter to monitor fuel quality, as it shows the amount of free fatty acids (FFA) formed by oxidation processes [25]. According to EN 14214 and ASTM D6751, the acid number for biodiesel should be less than 0.5 mg KOH g−1. Regardless of the type of substrate from which the biodiesel was prepared and the storage time and conditions, the acid value of the tested esters was well below the value set by the standards. After 150 days of storage, the acid number ranged from 0.31 mg KOH g−1 for esters prepared from sunflower oil to 0.73 mg KOH g−1 for commercial BE (Figure 4). The acid number indicates the corrosive potential of biodiesel, which is important for determining its durability in relation to fuel tanks and engines [38].
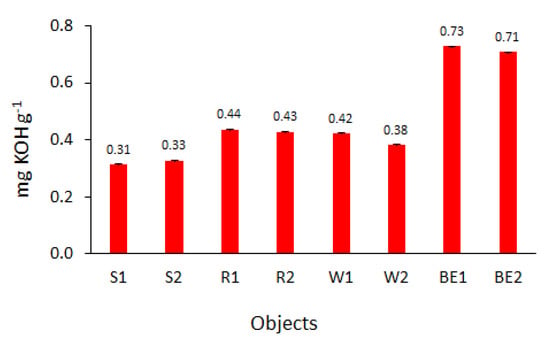
Figure 4.
Acid number of the tested biofuels after 150 days of storage.
3.3. Dynamic Viscosity and Tribological Tests
Analysis of the dynamic viscosity of biofuels shows that biofuel W prepared from waste oil had the highest value of dynamic viscosity (Figure 5), while biofuel S derived from sunflower oil had the lowest dynamic viscosity. The conditions and time of storage of the samples, as described in the test methodology, did not affect the increase in the dynamic viscosity of the tested biofuels and even caused a slight decrease compared to that of fresh biofuels. The biofuels stored in closed tanks were kept at ambient temperature (conditions (1), outside, in the range of 11 °C to 23 °C) or (2) at −4 °C. Comparable observations were obtained in similar studies [28] conducted in Malaysia, where the average ambient temperature is much higher than that in Poland. The authors found that during the storage of biofuels, especially in open tanks and at elevated temperatures (above 25 °C), oxidation processes played a dominant role and increased the density and viscosity of biofuels. Although these processes can be minimized by using chemical stabilizers [39], they often increase the production costs of biofuels, and moreover, chemical stabilizers pose a threat to the environment. The authors of studies on the oxidation stability of low-sulfur diesel fuel with the addition of biodiesel also confirmed the above observations [40].
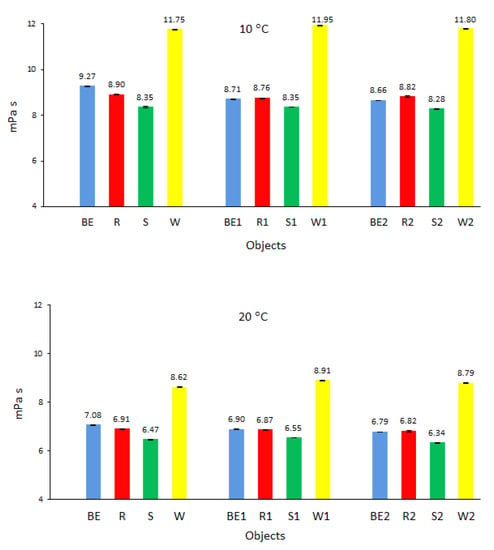
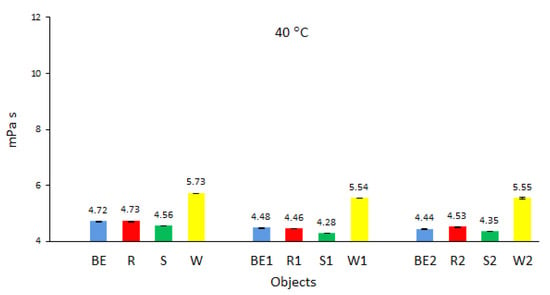
Figure 5.
Dynamic viscosity of the tested biofuels.
The slight decrease in the dynamic viscosity of biofuels after the storage period noted in the present study was also caused by the precipitation of small amounts of sediments in biofuels W, S, and R. These sediments, with a higher dynamic viscosity than that of pure biofuels, were deposited at the bottom of the tanks and were not taken for testing; this contributed to a lower dynamic viscosity than that of new biofuels.
Figure 6 presents the percentage values of the obtained friction moment for the maximum load of the tested biofuels with respect to diesel (D).
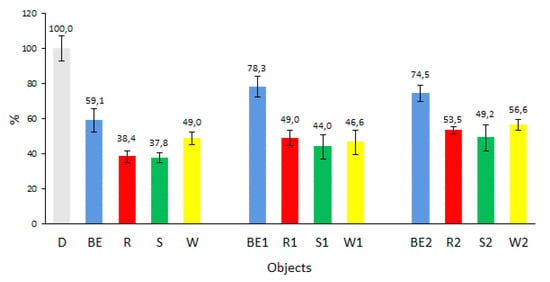
Figure 6.
Friction moment of the tested biofuels with respect to diesel (D).
Analysis of the friction moment values obtained for the tested biofuels at the end of the test showed that the biofuels exhibited a significant decrease in the value of this parameter with respect to diesel. This is also confirmed by the results obtained by other authors [41]. Ucha et al. [42] also confirmed the beneficial effect of glycerin added to diesel to improve its lubricating properties.
The largest change was observed for new biofuels. The results of the friction moment obtained for biofuels R, S, and W ranged from 38% to 49% of that achieved by D. Biofuel BE in the tested friction node had a frictional moment value of 59% of the reference value at the end of the test. Storage of the tested fuels at an ambient temperature influenced the changes in the friction moment in the tested node from 49% for R1 to 44% for biofuel S1, 47% for W1, and 78% for BE1. Storage of the tested fuels in the constant temperature increased the friction moment for the tested biofuels; however, the values were still lower than those obtained for diesel. Biofuel S2 showed an increase to 49% of the value obtained by diesel and an 11% increase with respect to the biofuel tested after the production process. Biofuels R2 and BE2 showed an increase in the frictional moment, i.e., 15% of that of new biofuels.
In summary, biofuel BE had the highest friction moment values regardless of the storage method. Biofuels R, S, and W showed the influence of the storage method on the friction moment. The lowest friction moment was recorded for new biofuels and the highest one for biofuels stored under ambient conditions; this finding confirms the effect of storage time on biofuels.
Concurrent with the results of the friction moment test, the linear wear of samples lubricated by the biofuel under test was also assessed. Figure 7 shows the view of samples lubricated with the tested biofuels after the tests.

Figure 7.
View of the signs of wear of selected samples lubricated with biofuels after tests.
Analysis of the results of linear wear (Figure 8) shows that the tested biofuels reduced the wear of sample material as compared to diesel fuel, regardless of the method and time of storage. The better lubricating properties of biofuels are due to their dipole structure and better adhesion to metal surfaces, which create abrasion-resistant layers. These results have been demonstrated in studies [43,44].
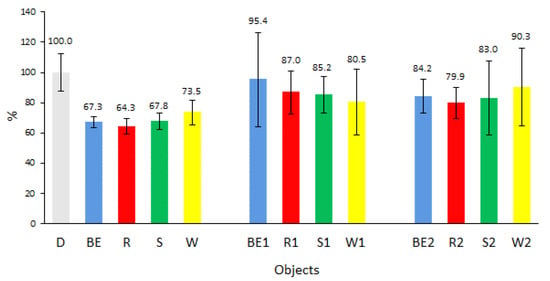
Figure 8.
Linear wear of biofuel-lubricated sample material with respect to diesel (D).
The lowest wear was observed for new biofuels in relation to diesel, similar to that observed for the friction moment. The lowest wear (64%) was noted for biofuel R and the highest one (73%) for W. Biofuels BE and S had very similar linear wear rates of 67% and 68% of the sample material, respectively.
Analysis of the effect of the storage method on the value of linear wear of the sample material showed an increase in wear in both tested versions as compared to new biofuels. A greater differentiation of wear was observed for biofuels stored under stable environmental conditions than for biofuels stored under variable conditions. Storage under ambient temperature conditions caused an increase in wear for all biofuels in the following order: BE1 by 28%, R1 by 23%, S1 by 17%, and W1 by 8%, compared to new biofuels, and it was also higher than the wear recorded for biofuels stored under constant conditions. Storage under constant temperature conditions resulted in the occurrence of physicochemical phenomena in the tested biofuels, which reduced the value of wear in relation to most biofuels stored at ambient temperature, despite the fact that the friction moment test showed an increase. The linear wear of the sample material increased by 17% in BE1, by 16% in R1, by 15% in S1, and by 17% in W1 in relation to new biofuels.
4. Conclusions
The present study demonstrated changes in the selected rheological and tribological parameters of higher fatty acid esters depending on the time and method of their storage. The type of feedstock used for the production of biodiesel and the conditions of its storage affect the growth and development of microorganisms. Biofuel prepared from waste oil had the highest number of microorganisms. The acid number of the laboratory-prepared esters after the storage time did not exceed the permissible value recommended by the standard. The time and method of storage did not cause an increase in the dynamic viscosity of the tested biofuels. The biofuel produced from waste oil had the highest viscosity. Regardless of the storage conditions, after 150 days, the dynamic viscosity of the biofuels showed a slight decrease. The time and method of storage affected the tribological properties expressed in terms of the friction moment and the linear wear index of the sample material of the tested biofuels. These properties deteriorated after the storage period under both conditions, but were still better than those observed for diesel.
Author Contributions
Conceptualization, M.H.-P. and A.K.; methodology, M.H.-P., A.K. and P.S.; formal analysis, M.H.-P., A.K., P.S. and D.S.; investigation, M.H.-P., A.K., P.S. and D.S.; writing—original draft preparation, M.H.P. and A.K.; writing—review and editing, M.H.-P. and A.K.; visualization, M.H.-P. and A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ho, D.P.; Ngo, H.H.; Guo, W. A mini review on renewable sources for biofuel. Bioresour. Technol. 2014, 169, 742–749. [Google Scholar] [CrossRef]
- Rasool, U.; Hemalatha, S. A review on bioenergy and biofuels: Sources and their production. Braz. J. Biol. Sci. 2016, 3, 3–21. [Google Scholar] [CrossRef]
- Chang, S.P.; Roy, P.S.; Kim, S.H. Current developments in thermochemical conversion of biomass to fuels and chemicals. IntechOpen 2018. [Google Scholar] [CrossRef]
- Sharma, S.; Meena, R.; Sharma, A.; Goyal, P.K. Biomass conversion technologies for renewable energy and fuels: A Review note. IOSR J. Mech. Civ. Eng. 2014, 11, 28–35. [Google Scholar] [CrossRef]
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energ. Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Du, W.; Xu, Y.-Y.; Liu, D.-H.; Li, Z.-B. Study on acyl migration in immobilized lipozyme TL-catalyzed transesterification of soybean oil biodiesel production. J. Mol. Catal. B Enzym. 2005, 37, 68–71. [Google Scholar] [CrossRef]
- Pleanjai, S.; Gheewala, S.H.; Garivait, S. Environmental evaluation of biodiesel production from palm oil in a life cycle perspective. Asian J. Energy Environ. 2007, 8, 15–32. [Google Scholar]
- Berchmans, H.J.; Hirata, S. Biodiesel production from crude Jatropha curcas L. seed oil with a high content of free fatty acids. Biores. Technol. 2008, 99, 1716–1721. [Google Scholar] [CrossRef]
- Porte, A.V.; Schneider, R.C.S.; Kaercher, J.A.; Klamt, R.A.; Schmatz, W.L.; Da Silva, W.L.T.; Filho, W.A.S. Sunflower biodiesel production and application in family farms in Brazil. Fuel 2010, 89, 3718–3724. [Google Scholar] [CrossRef]
- Yoo, S.J.; Veriansyah, B.; Kim, J.; Kim, J.D.; Lee, Y.W. Synthesis of biodiesel from rapeseed oil using supercritical methanol with metal oxide catalyst. Biores. Technol. 2010, 101, 8686–8689. [Google Scholar] [CrossRef]
- Alptekin, E.; Canakci, M.; Sanli, H. Biodiesel production from vegetable oil and waste animal fats in a pilot plant. Waste Manag. 2014, 34, 2146–2154. [Google Scholar] [CrossRef]
- Banković-Ilić, I.B.; Stojković, I.J.; Stamenkovic, O.S.; Veljkovic, V.B.; Hung, Y.-T. Waste animal fats as feedstocks for biodiesel production. Renew. Sustain. Energy Rev. 2014, 32, 238–254. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Singh, B.; Upadhyay, S.N. Advancements in development and characterization of biodiesel: A review. Fuel 2008, 87, 2355–2373. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, S. A review on biodiesel production technique. Eng. J. Appl. Scopes 2018, 3, 5–8. [Google Scholar]
- Leung, D.Y.C.; Koo, B.C.P.; Guo, Y. Degradation of biodiesel under different storage conditions. Biores. Technol. 2006, 97, 250–256. [Google Scholar] [CrossRef]
- Kim, D.S.; Hanifzadeh, M.; Kumar, A. Trend of biodiesel feedstock and its impact on biodiesel emission characteristics. Environ. Prog. Sustain. Energy 2018, 37, 7–19. [Google Scholar] [CrossRef]
- Patil, P.D.; Mulla, M.Z. Review on different sources for the production of biodiesel. Int. J. Latest Technol. Eng. Manag. Appl. Sci. 2017, 6, 21–25. [Google Scholar]
- Komariah, L.N.; Aprisah, M.S.; Rosa, Y.S.L. Storage tank materials for biodiesel blends; the analysis of fuel property changes. MATEC Web Conf. 2017, 101, 02012. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Smolik, B.; Kamieniecka, A. Preliminary study of the efficiency of biodiesel biological decomposition with autochthonous soil microflora. Ecol. Chem. Eng. 2011, 18, 1401–1406. [Google Scholar]
- Fregolente, P.B.L.; Fregolente, L.V.; Maciel, M.R.W. Water content in biodiesel, diesel, and biodiesel–diesel blends. J. Chem. Eng. Data. 2012, 57, 1817–1821. [Google Scholar] [CrossRef]
- Siegert, W. Microbial Contamination in Diesel Fuel—Are New Problems Arising from Biodiesel Blends? In Proceedings of the 11th International Conference on Stability, Handling and Use of Liquid Fuels, Prague, Czech Republic, 18–22 October 2009; p. 3. [Google Scholar]
- Klofutar, B.; Golob, J. Microorganisms in diesel and in biodiesel fuels. Acta Chim. Slov. 2007, 54, 744–758. [Google Scholar]
- Zakaria, H.; Khalid, A.; Sies, M.F.; Mustaffa, N.; Manshoor, B. Effect of storage temperature and storage duration on Biodiesel properties and characteristics. Appl. Mech. Mater. 2014, 465–466, 316–321. [Google Scholar] [CrossRef]
- Malarvizhi, S.; Krishnamurthy, S.R. Microbiologically influenced corrosion of carbon steel exposed to biodiesel. Int. J. Corros. 2016. [Google Scholar] [CrossRef]
- Dodos, G.S.; Konstantakos, T.; Longinos, S.; Zannikos, F. Effects of microbiological contamination in the quality of biodiesel fuels. Global Nest J. 2012, 14, 175–182. [Google Scholar]
- Biernat, K. Biofuels in storage and operating conditions. In Storage Stability of Fuels; IntechOpen: London, UK, 2015. [Google Scholar]
- Sørensen, G.; Pedersen, D.V.; Norgaard, A.K.; Sorensen, K.B.; Nygaard, S.D. Microbial growth studies in biodiesel blends. Bioresour. Technol. 2011, 102, 5259–5264. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Tamaldin, N.; Jaat, M.; Ali, M.F.M.; Manshoor, B.; Zaman, I. Impacts of biodiesel storage duration on fuel properties and emissions. Proc. Eng. 2013, 68, 225–230. [Google Scholar] [CrossRef]
- Mazumdar, P.; Borugadda, V.B.; Goud, V.V.; Sahoo, L. Effect of storage parameters on stability of Jatropha-derived biodiesel. Int. J. Energy Environ. Eng. 2013, 4, 13. [Google Scholar] [CrossRef]
- Christensen, E.; McCormick, R.L. Long-term storage stability of biodiesel and biodiesel blends. Fuel Process. Technol. 2014, 128, 339–348. [Google Scholar] [CrossRef]
- Schleicher, T.; Werkmeister, R.; Russ, W.; Meyer-Pittroff, R. Microbiological stability of biodiesel–diesel-mixtures. Bioresour. Technol. 2009, 100, 724–730. [Google Scholar] [CrossRef]
- Bücker, F.; Santestevan, N.A.; Roesch, L.F.; Jacques, R.J.S.; Peralba, M.C.R.; Camargo, F.A.O.; Bento, F.M. Impact of biodiesel on biodeterioration of stored Brazilian diesel fuel. Int. Biodeterior. Biodegrad. 2011, 65, 172–178. [Google Scholar] [CrossRef]
- Hill, E.C.; Hill, G.C. Strategies for Resolving Problems Caused by Microbial Growth in Terminals and Retail Sites Handling Biodiesels. In Proceedings of the 11th International Conference on Stability, Handling and Use of Liquid Fuels, Prague, Czech Republic, 18–22 October 2009; Available online: http://toc.proceedings.com/07515webtoc.pdf (accessed on 14 October 2020).
- Allsopp, D.; Seal, K.J.; Gaylarde, C.C. Introduction to Biodeterioration, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Stamps, B.W.; Bojanowski, C.L.; Drake, C.A.; Nunn, H.S.; Lloyd, P.F.; Floyd, J.G.; Emmerrich, K.A.; Neal, A.R.; Crookes-Goodson, W.J.; Stevenson, B.S. In situ linkage of fungal and bacterial proliferation to microbiologically influenced corrosion in B20 biodiesel storage tanks. Front. Microbiol. 2020, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Luz, G.; Sousa, B.; Guedes, A.; Barreto, C.; Brasil, L. Biocides used as additives to biodiesels and their risks to the environment and public health: A review. Molecules 2018, 23, 2698. [Google Scholar] [CrossRef] [PubMed]
- Hawrot-Paw, M.; Koniuszy, A.; Zając, G.; Szyszlak-Bargłowicz, J. Ecotoxicity of soil contaminated with diesel fuel and biodiesel. Sci. Rep. 2020, 10, 16436. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, F.B.; Sobral, S.P. A new method for determining the acid number of biodiesel based on coulometric titration. Talanta 2012, 97, 199–203. [Google Scholar] [CrossRef]
- Das, L.M.; Bora, D.K.; Pradhan, S.; Naik, M.K.; Naik, S.N. Long-term storage stability of biodiesel produced from Karanja oil. Fuel 2009, 88, 2315–2318. [Google Scholar] [CrossRef]
- Karavalakis, G.; Stournas, S.; Karonis, D. Evaluation of the oxidation stability of diesel/biodiesel blends. Fuel 2010, 89, 2483–2489. [Google Scholar] [CrossRef]
- Fazal, M.A.; Haseeb, A.S.M.A.; Masjuki, H.H. Investigation of friction and wear characteristics of palm biodiesel. Energ. Convers. Manag. 2013, 67, 251–256. [Google Scholar] [CrossRef]
- Uchôa, I.M.A.; Neto, A.A.D.; Da Silva, S.E.; De Lima, L.F.; De Barros, N.E.L. Evaluation of Lubricating Properties of Diesel Based Fuels Micro Emulsified with Glycerin. Mater. Res. 2017, 20, 701–708. [Google Scholar] [CrossRef]
- Sukjit, E.; Tongroon, M.; Chollacoop, N.; Yoshimura, Y.; Poapongsakorn, P.; Lapuerta, M.; Dearn, K.D. Improvement of the tribological behavior of palm biodiesel via partial hydrogenation of unsaturated fatty acid methyl esters. Wear 2019, 426–427 Pt A, 813–818. [Google Scholar] [CrossRef]
- Hamdan, S.H.; Chong, W.W.F.; Ng, J.-H.; Ghazali, M.J.; Wood, R.J.K. Influence of fatty acid methyl ester composition on tribological properties of vegetable oils and duck fat derived biodiesel. Tribol. Int. 2017, 113, 76–82. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).









