Abstract
Metabolic products such as lipids and proteins produced in cyanobacteria represent an excellent source of biomass and do not compete with agricultural land use unlike soybean and corn. Given their potential use as novel materials for biodiesel production, we aimed to explore the effect of cultivation period and nitrogen concentration on the growth rate and lipid content of Fremyella diplosiphon, a model cyanobacterium. In this study, F. diplosiphon grown in BG11/HEPES medium supplemented with 1.5 g L−1 sodium nitrate (NaNO3) for 7, 10, 15, and 20 days were compared to the untreated control in media amended with 0.25, 0.5, and 1.0 g L−1 NaNO3. Cultures were inoculated in liquid media and grown under continuous fluorescent light in an orbital incubator shaker, and extracted lipids subjected to gravimetric analysis and gas chromatography-mass spectroscopy to determine the best culture conditions for lipid production. Our results demonstrated that a reduction in nitrogen concentration had no significant effect on the growth rate across all cultivation periods; however, the accumulation of total lipid content was significantly influenced by nitrogen concentration. A maximum lipid production (40%) with no reduction in growth was observed in 10-day old cultures in a BG11/HEPES medium supplemented with 1.0 g L−1 NaNO3. Fatty acid methyl ester composition of transesterified lipids demonstrated high amounts of methyl palmitate (50–70%) followed by methyl octadecenoate (17–30%) in the accumulated lipids at all treatments. Trace quantities of methyl dodecanoate, methyl hexadecanoate, methyl octadecanoate, and methyl octadecadienoate (1–8%) were also observed in all tested samples, indicating that nitrogen deprivation in culture media increases lipid production without affecting growth.
1. Introduction
Adopting fossil fuels as the chief energy source has resulted in the surplus emissions of CO2 and other greenhouse gases leading to global climate change. Simultaneously, worldwide nonrenewable energy resource supplies are dwindling, while energy demand is increasing day-by-day [1]. Exhaustion of fossil fuel reserves, increased oil prices, and rising levels of greenhouse gases have driven worldwide interest in renewable energy as an alternative to fossil fuels. Biofuels are renewable green fuels, which have driven interest in methods to maximize production and attracted researchers to meet the growing demand for fuel [2]. Their production offers an opportunity to develop an alternative for fossil fuels while also assisting rural economies [3,4,5,6]. In addition, harmful emissions of carbon monoxide and hydrocarbons can be mitigated, which can decrease greenhouse effects and improve environmental sustainability [7,8]. Most recently, research efforts have been aimed at identifying suitable strains of algae/cyanobacteria which can provide greater energy yields to displace conventional fuels.
According to the 2019 World Gas and Renewables Review, over 2.6 million barrels/day of biofuels were produced worldwide in 2018. The world’s biodiesel supply has exponentially increased from 3.9 billion liters in 2005 to 18.1 billion liters in 2010, and is expected to reach 41.4 billion liters in 2025 [9]. As a result of this growing demand for renewable biodiesel, many researchers have attempted to produce biodiesel from sources such as rapeseed, soybean, peanut, and vegetable oils [10,11]. However, these first-generation fuels are not practical for commercial production since they compete for water and arable lands [12]. The price of biodiesel remains a major hindrance for commercial production, primarily as a result of the high feed cost of vegetable oils [13], thus we are in dire need to identify other new sources. In recent years, microalgae and cyanobacteria have emerged as one of the most promising sources of biodiesel and gained great importance as its high lipid content can serve as raw material for biofuel production [2,14]. These organisms have higher photosynthetic efficiency (10–100 times higher than for plants) and faster growth rate compared to any other energy crop [15,16,17,18]. With a short and non-seasonal life-cycle, these microbes can thrive without the use of agrochemicals. Additionally, cyanobacteria are more efficient and can be cultivated in marginal lands and used wastewater, thus minimizing competition with food crops [1]. Thus, mass cultivation of cyanobacteria as a biofuel feedstock is being evaluated worldwide, especially since these organisms thrive in the presence of basic nutrients, such as water, CO2, mineral salts, and light [19]. Cyanobacteria are also easily subjected to genetic modification allowing the generation of high-value products such as lipids and proteins [20,21,22,23]. In spite of the advantages that these organisms provide, generating significant amounts of bioenergy at a plausible scale to impact the energy economy and the return of investment based on capital input are potential bottlenecks faced. In particular, a key challenge to achieving viability is the cultivation and harvesting steps, which is critical in maximizing yield while minimizing inputs such as light and nutrients. For commercial production of biodiesel from cyanobacteria, factors such as optimized harvesting, oil extraction, and conversion to fuel processes is imperative. Ideal strains need to be selected and optimized for biomass production, and the fatty acid composition analyzed [24]. Unless we reach a high lipid production per gallon per acre from the selected strains, economic viability would be very hard to achieve. Major breakthroughs in this area can be advanced via the induction of lipid biosynthesis by environmental stresses [25,26,27]. Cyanobacterial strains generate varying quantities of carbohydrates, lipids, and proteins; choice species can adjust their metabolism through basic changes to the composition of growth media. A significant increase in lipid content has been reported in several species grown in nitrogen-deficient environments. Factors such as phosphorous, nitrogen, and iron levels in the medium, salt stress, radiation, acidity, heavy metals, temperature, light intensity, and irradiance [28,29,30,31,32] have been reported to impact the lipid content. Under optimal growth, significant quantities of biomass are produced, but with limited lipid abundance, while species with high lipid levels are typically slow-growing. This indicates an inverse relationship between lipid content and nitrate concentration [7]. Several reports suggest that lipids tend to accumulate in nitrogen-deficient conditions [7,10,24].
High-lipid yield is a major prerequisite for commercial biodiesel production. Fremyella diplosiphon is a model organism for studying photosynthetic pathways and exhibits extreme regulation of phycoerythrin and phycocyanin using a process known as complementary chromatic adaptation. Furthermore, its fast generation time and potential to grow in a wide range of light including shaded light, make this organism an ideal candidate for large-scale cultivation while reducing capital input. However, it is crucial to establish stable culture conditions for achieving high lipid yield in scaled-up generation of biofuels from F. diplosiphon, while reducing capital input [33]. Prior efforts to overexpress genes using electroporation-mediated transformation in this strain has resulted in salt tolerant [34] and high-lipid producing strains [35], as well as the identification of fatty acid methyl esters (FAMEs) that prove its efficacy as a biodiesel agent [36]. In this study, we investigated the growth response and lipid yield of F. diplosiphon when subjected to varying levels of sodium nitrate (NaNO3) in the culture medium. We also examined the best culture conditions for maximal lipid production, and analyzed extracted lipids by gravimetric and gas chromatographic methods to determine the impact of nitrogen deprivation on biodiesel quality.
2. Materials and Methods
2.1. Cyanobacterial Strain and Culture Conditions
A short filamentous F. diplosiphon strain (SF33) obtained from Dr. Beronda Montgomery at Michigan State University, capable of growth in both red and green light, was used in this study. Actively growing cells from 3–6 day old cultures were inoculated from plates into sterilized 250 mL flasks containing 150 mL of BG11/HEPES buffer, and grown under continuous shaking at 170 rpm and permanent 30 µmol/m2/s white light at a temperature of 28 °C in an Innova 44R incubator shaker series (Eppendorf). The BG11 medium (hereafter referred to as BG11/HEPES) was composed of stock cultures containing Na2Mg EDTA, ferric ammonium citrate, citric acid, MgSO4.7H2O, K2HPO4.3H2O, Na2CO3, H3BO3, MnCl2.4H2O, ZnSO4.7H2O, CuSO4.5H2O, CoCl2.6H2O, Na2MoO4.2H2O, and 1.5 g of NaNO3.
2.2. Growth of F. diplosiphon in Varying Sodium Nitrate Concentrations
Actively growing cultures were transferred into 500 mL flasks containing 300 mL BGll/HEPES media amended with 0.25, 0.5, 1.0, and 1.5 g of NaNO3 and grown for a period of 7, 10, 15, and 20 days. Cultures grown in 1.5 g/L NaNO3, which is the standard amount in the BG11 media served as the control. Inoculated cultures were grown in culture conditions as mentioned above, and optical densities at 750 nm measured at 48-h intervals throughout the course of the experiment. Three replicated treatments were maintained and the experiment repeated once.
2.3. Lipid Accumulation in F. diplosiphon Grown in Varying Levels of Sodium Nitrate
F. diplosiphon cultures were grown in BGll/HEPES media amended with varying nitrate levels as mentioned in Section 2.1. Cells were centrifuged using a Beckman-Coulter Avani-J25I with a JA 25.50 rotor, lyophilized overnight, and sonicated in 5 mL chloroform:methanol (2:1) for 30 s. Lipids were extracted using a 2:1 chloroform:methanol mixture according to the method of Folch et al. [37]. The mixture was agitated for 15–20 min in an orbital shaker after dispersion at room temperature and the homogenate centrifuged to recover the liquid phase. The solvent was washed with 0.2 volumes (1 for 5 ml) of distilled H2O, vortexed briefly, and centrifuged at 2000 rpm to separate the two phases. The lower phase was transferred to a pre-weighed vial and the interface was rinsed twice using methanol:water (1:1) without mixing the whole preparation. The lower chloroform phase containing lipids was evaporated under vacuum in a rotary evaporator after centrifugation and siphoning. The dried flasks were weighed to establish the total lipid content, which was determined by the conventional gravimetric method [38].
2.4. Simultaneous Transesterification and Lipid Extraction
To investigate the effect of nitrate deprivation on the F. diplosiphon fatty acid profile, cultures were grown under conditions mentioned above and extracted lipids were subjected to one-step direct transesterification in a multimode commercial scientific reaction microwave (CEM Corp, Matthews, NC, USA) as described by Tabatabai et al. [36].
2.5. Gas Chromatography-Mass Spectrometry in F. diplosiphon Grown in Varying Sodium Nitrate Levels
The fatty acid composition of F. diplosiphon transesterified product was determined using Shimadzu GC17A/QP5050A gas chromatography-mass spectrometry (GC-MS) according to the method described by Rosenberg et al. [39] and Tabatabai et al. [36]. Peaks were identified by comparing mass spectra to the lipid Web Archive of FAME mass spectra. Three biological replicates of each sample were analyzed.
2.6. Statistical Analysis
All measurements included triplicate treatments. The mean and standard deviation of the data values were calculated using MS-Excel. The effect of treatments was determined by one-way analysis of variance (ANOVA) and Tukey’s honestly significant difference (HSD) test conducted to determine the statistical significance of the differences between means of various treatments.
3. Results and Discussion
3.1. Effect of Nitrogen Deprivation on F. diplosiphon Growth
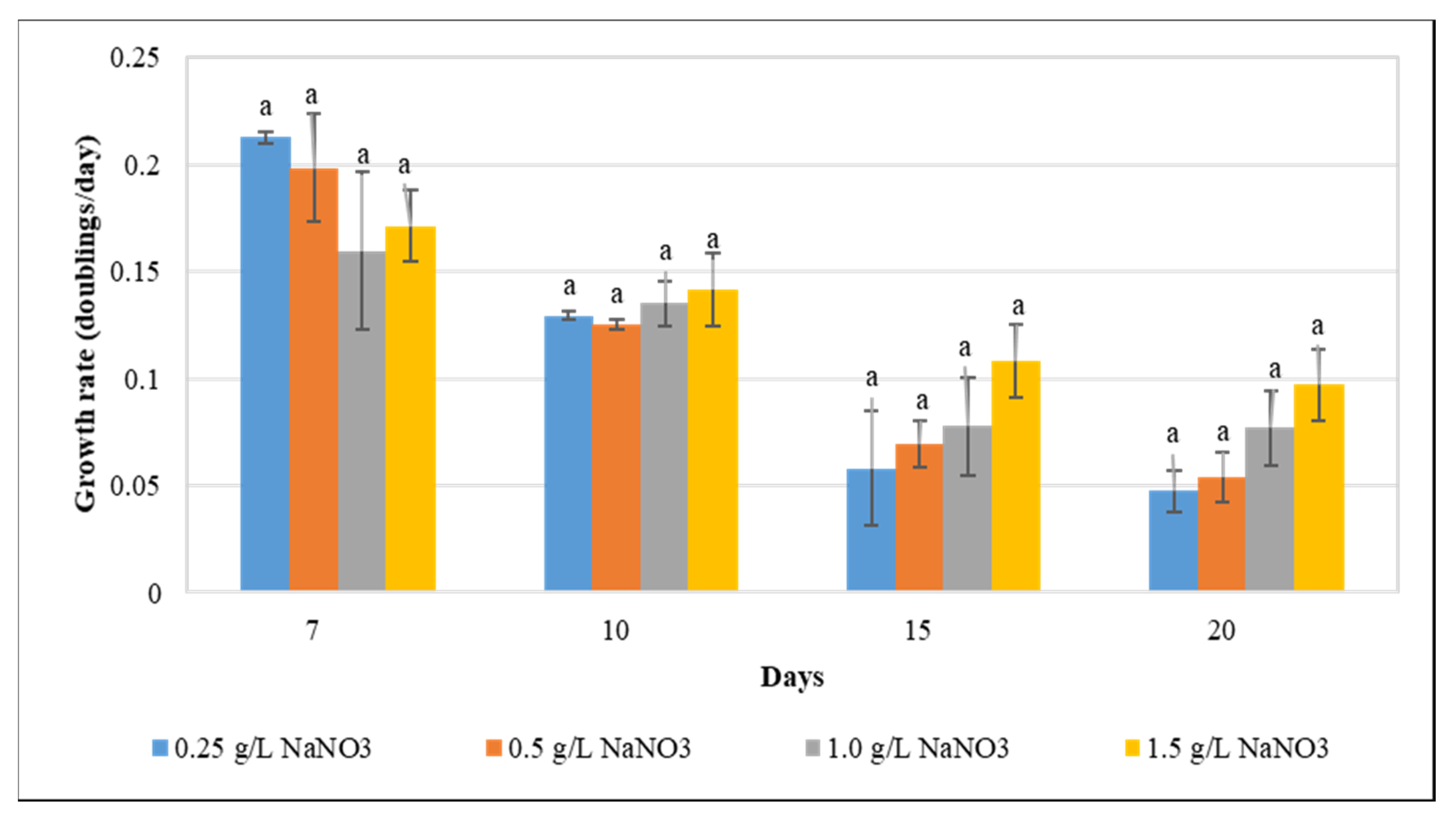
Optimization of culture conditions for maximizing lipid yield is critical to enhance lipid production, and is influenced by various interconnected factors such as growth rate, biomass, and lipid content. The deprivation of essential nutrients including phosphorus, sulfur, nitrogen, and potassium has been reported to result in significant alterations of cellular growth and compositions in algae [40,41]. As an essential macronutrient, nitrogen significantly impacts growth and total lipid content, and is vital for metabolism and development [42]. Exposure to nitrogen starvation has been reported to enhance lipid levels in various microalgal and cyanobacterial species. Reduction in cellular thylakoid levels, acyl hydrolase activation, and phospholipid hydrolysis stimulation have been reported to enhance the intracellular fatty acid abundance [43,44,45,46,47]. Our results revealed that the growth rate was not significantly affected by varying concentrations of NaNO3 (Figure 1), suggesting that partial nitrogen deprivation is not detrimental to F. diplosiphon growth. By contrast, a previous study in Botrycoccus sp., Scenedesmus obliquus, and Chlorella pyrenoidosa revealed that the organism exhibited a significant reduction in growth when subjected to nitrogen starvation [7,28,48,49].
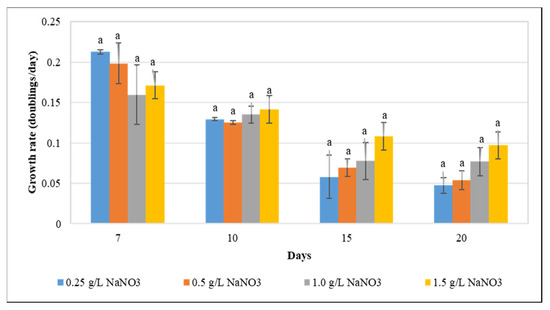
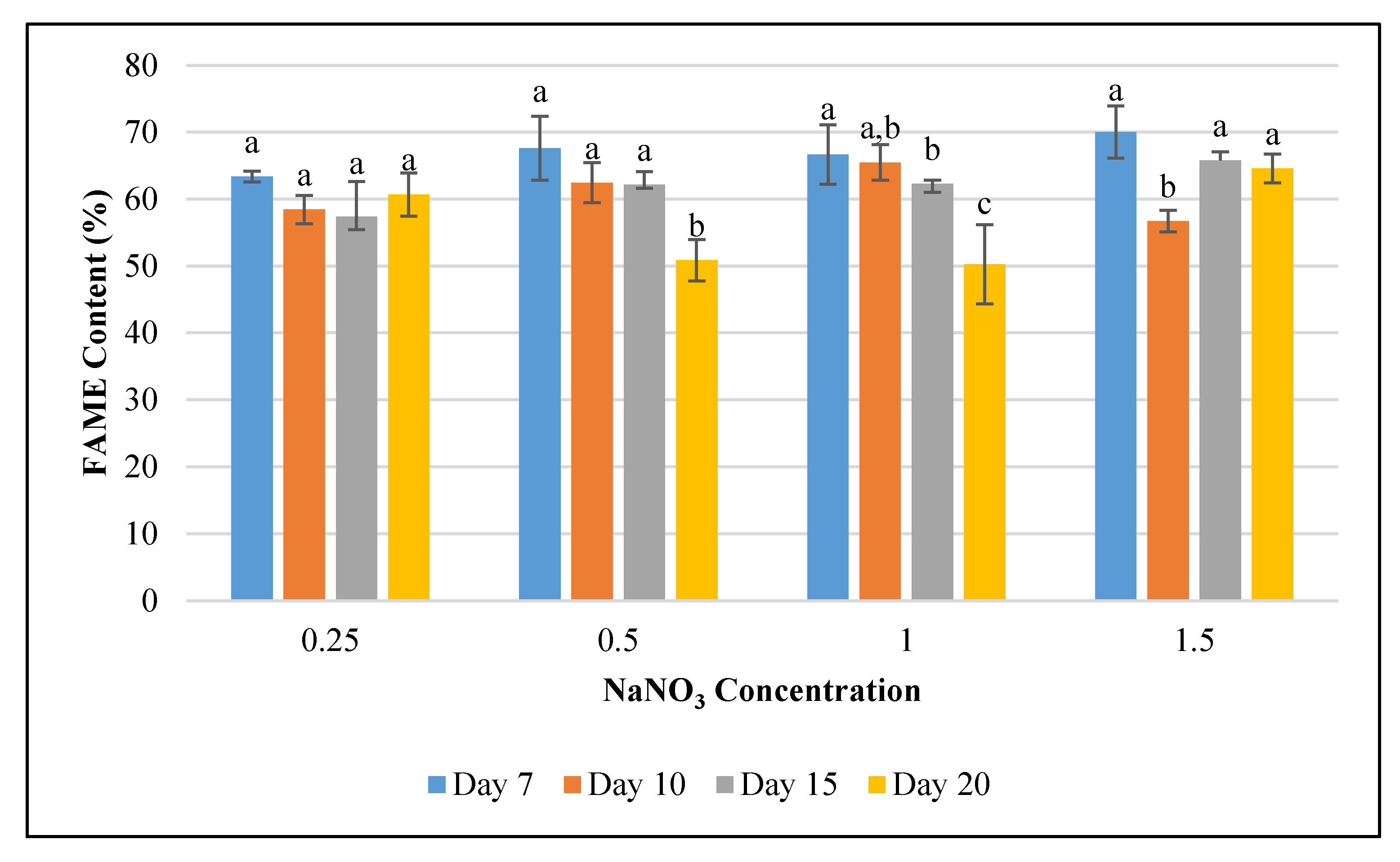
Figure 1.
The impact of varying sodium nitrate (NaNO3) concentrations (0.25, 0.5, 1.0, and 1.5 g L−1) on Fremyella diplosiphon growth rate over a period of 7, 10, 15, and 20 days. The average growth rate (± standard error) for three biological replicates for each treatment is shown. Different letters above bars indicate significance among treatment means (p < 0.05).
The unaffected growth of F. diplosiphon under nitrogen-deprived conditions may also be due to their diazotrophic nature which makes nitrogen available for proper growth by nitrogen fixation. A possible reason for this might be that nitrogen pools could have been consumed to support the cell growth after nitrogen exhaustion [49]. This explanation also finds support from the study of Li et al. [50], who reported that in nitrogen deficient conditions chlorophyll provides the nitrogen required to sustain Chlorella growth. Furthermore, our results find support from the work of Suen et al. [51], who reported the unaltered growth of microalgal species Nannochloropsis in nitrogen deprived conditions. Future studies will be aimed towards investigating the effects of even more drastic reductions in nitrogen supply in F. diplosiphon.
3.2. Effect of Nitrogen Deficiency on F. diplosiphon Total Lipid Content
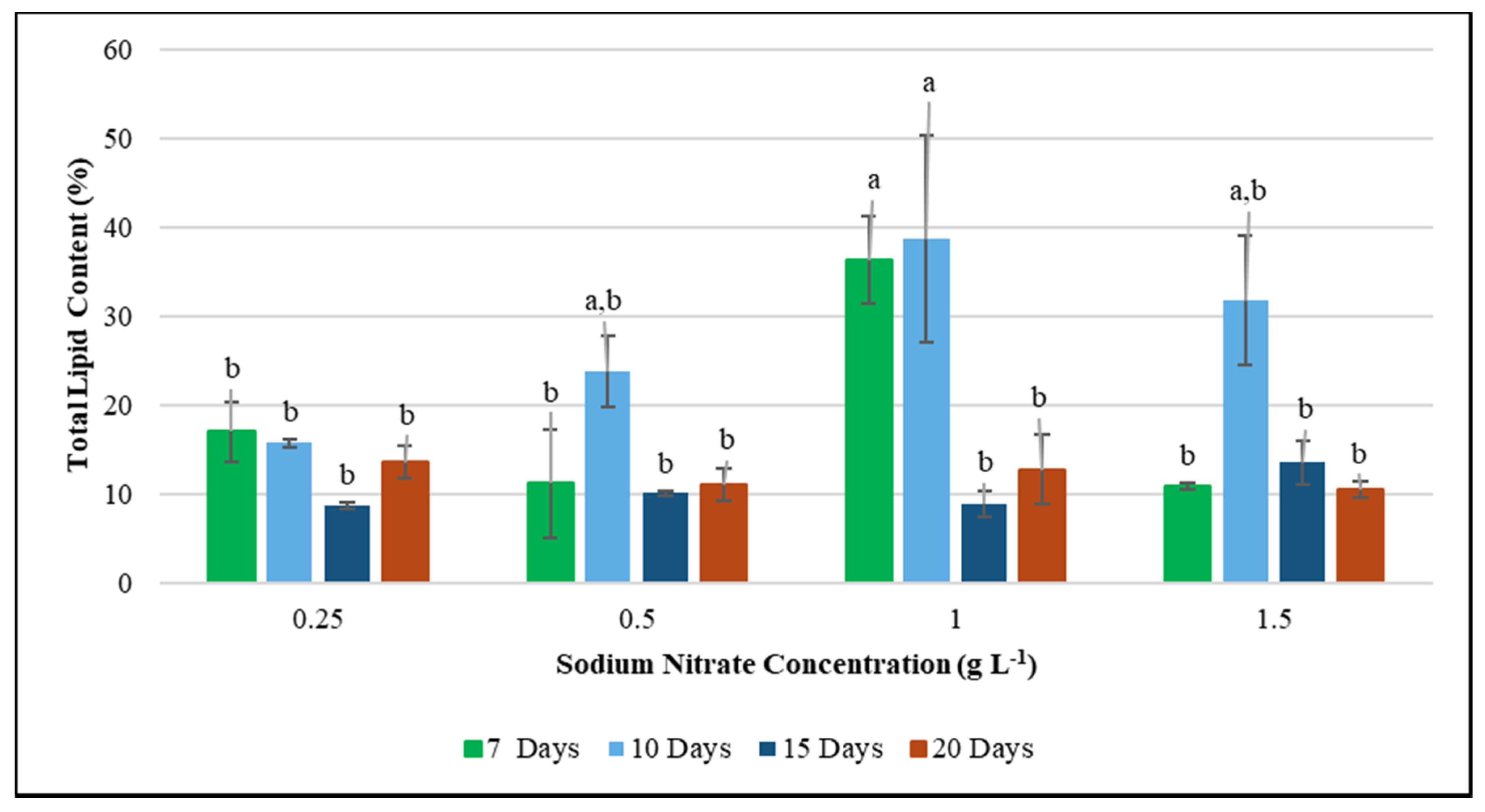
While F. diplosiphon growth was unaffected in varying levels of nitrate concentrations, a reduction in nitrate levels correlated to a significant increase in total lipid content. We observed maximum lipid production in 1.0 g L−1 NaNO3 concentrations on 7 and 10-day-old cultures, indicating that lipid yield in F. diplosiphon can be enhanced under these conditions (Figure 2). These results are in accordance to a report by Stephenson et al. [52], where an increase in lipid content was reported in nitrogen-deprived Chlorella vulgaris cultures. Similar results were also observed in the microalgae Scenedesmus sp., where the lipid content was enhanced six-fold after exposure to nitrogen starvation for 21 days [53]. High-lipid accumulation in a nitrogen-deprived medium was also reported in studies by Stuart et al. [54] and Becker et al. [55]. Accumulation of lipid content under nitrogen deprivation in F. diplosiphon as observed in our study, is comparable to the accumulated lipid content of Synechocystis spp., Oscillatoria spp., Lyngbya semiplena, Limicolaria martensiana, Calothrix spp., and other algae [56]. Further, our results support the findings of Wahlen et al. [57], who demonstrated that microalgae could be better sources for biodiesel production.
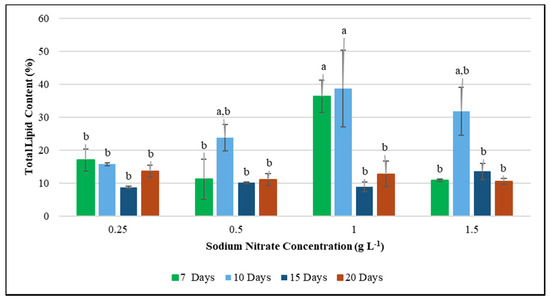
Figure 2.
Effect of total lipid production in Fremyella diplosiphon grown in nitrogen concentrations of 0.25, 0.5, 1.0, and 1.5 g L−1 sodium nitrate over a period of 7, 10, 15, and 20 days. The average % lipid content of total cellular dry weight (±standard error) for three biological replicates of each treatment is shown. Different letters above bars indicate significance among treatment means (p < 0.05).
3.3. Effect of Nitrogen Deficiecny on F. diplosiphon Fatty acid Profile
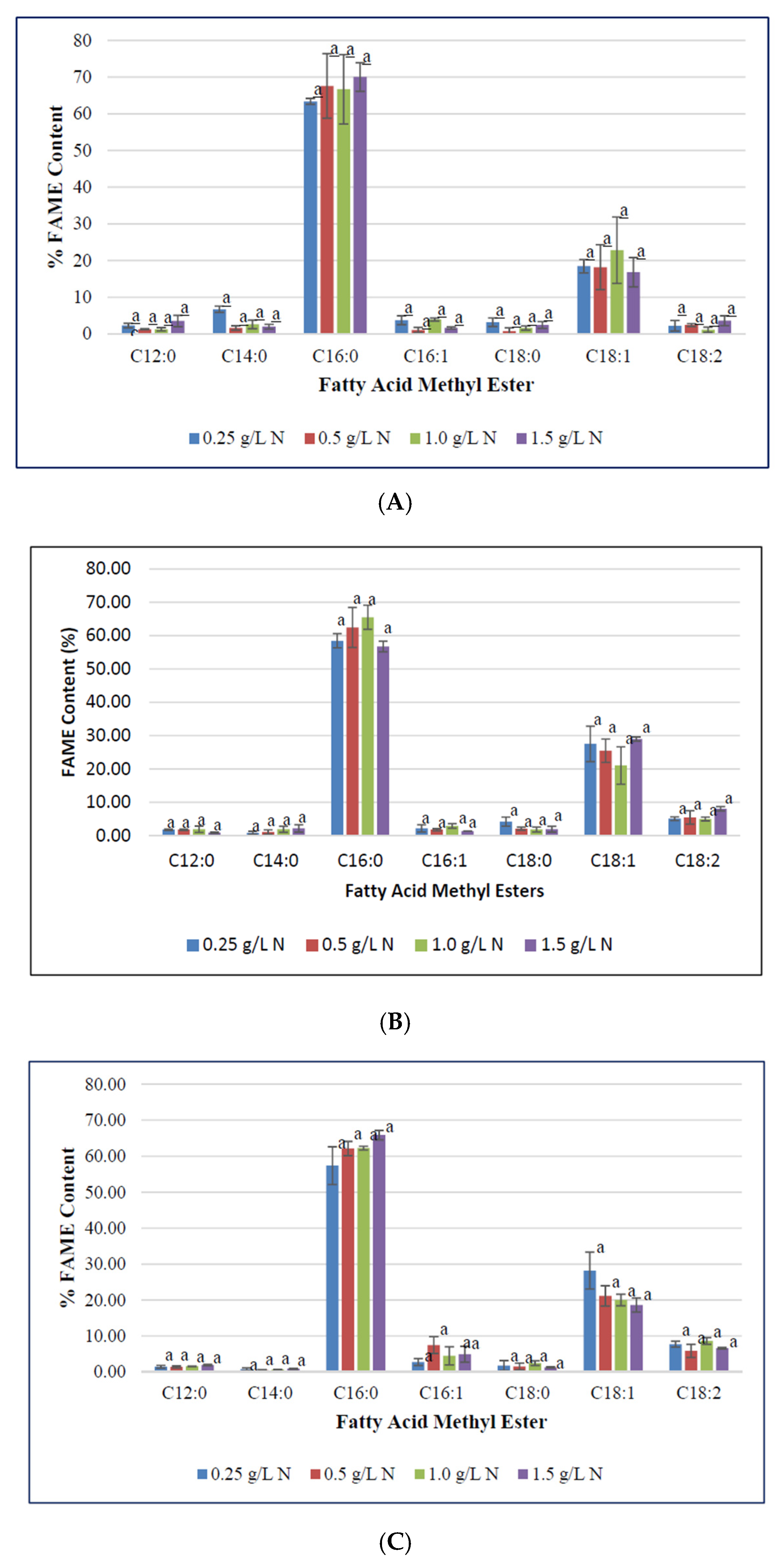
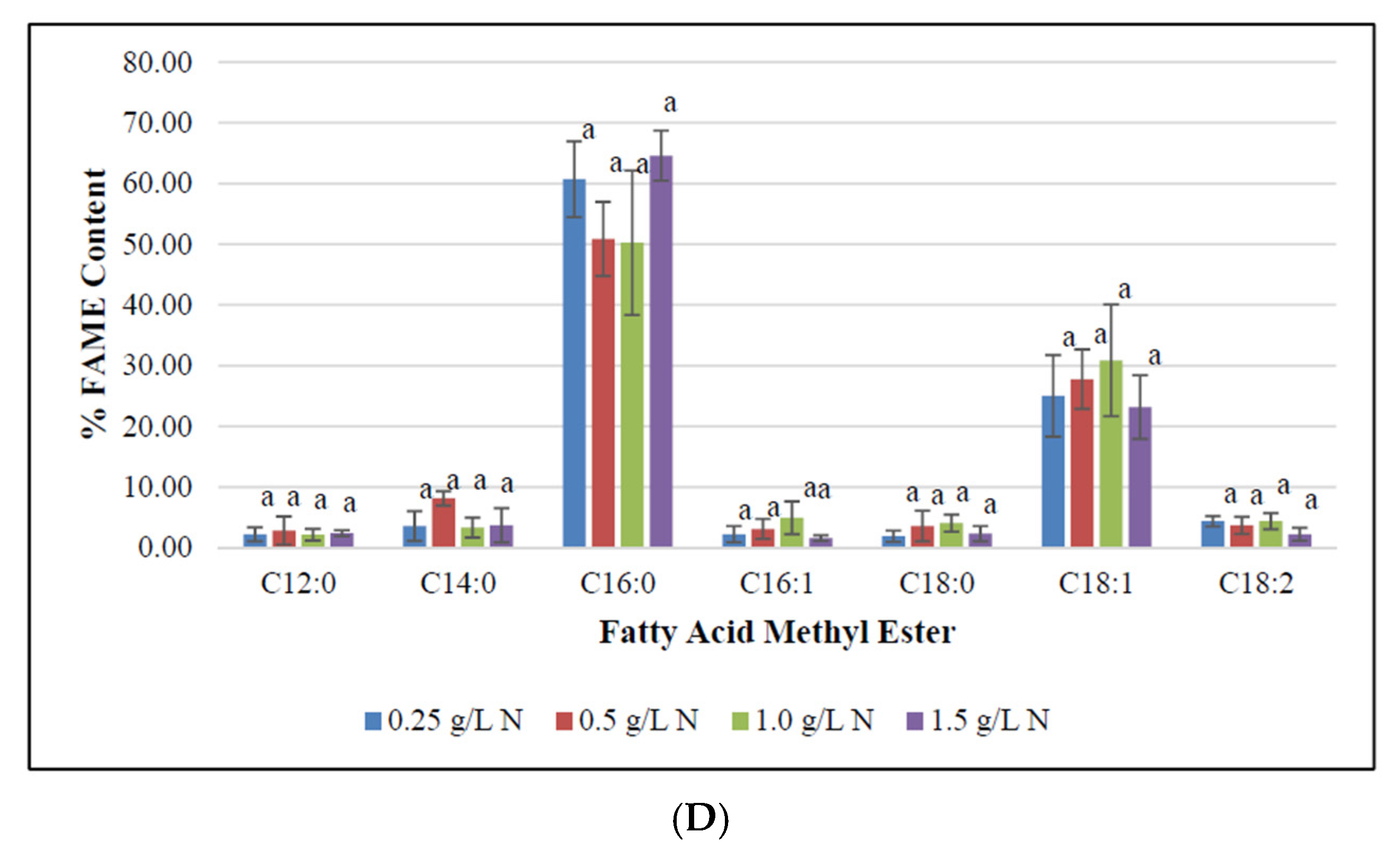
To determine the impact of nitrogen starvation on high-value fatty acid profile in F. diplosiphon, cultures were grown in BG11/HEPES amended with 0.25, 0.5, 1.0, and 1.5 g L−1 NaNO3 and subjected to FAME analysis. The determination of FAME composition using GC-MS showed a high abundance of methyl palmitate (50–70%) followed by methyl octadecenoate (17–30%), trace amounts of methyl dodecanoate, methyl hexadecanoate, methyl octadecanoate, and methyl octadecadienoate (1–8%) in all samples tested (Table 1; Figure 3). Saturated fatty acids such as methyl dodecanoate (12:0), methyl myristate (14:0), methyl palmitate (16:0), and methyl octadecanoate (18:0) attributed for 60–80% of the total FAMEs. Unsaturated fatty acids were present in smaller quantities relative to the saturated fatty acids. Approximately 20–40% of extracted FAMEs were unsaturated, and were mainly comprised of methyl hexadecanoate (16:1), methyl octadecenoate (18:1), and methyl octadecadienoate (18:2). The FAME components, methyl palmitate (C16:0), methyl hexadecanoate (16:1), octadecanoate (18:0), octadecenoate (C18:1), methyl octadecadienoate (C18:2), and methyl hexadecanoate (16:1) were the major components observed in FAME analyses. These results are in accordance to the FAME profile reported in Chlorella minutissima [58]. In addition, prior studies in our laboratory have reported approximately 25% FAME in total transesterified lipids in wild type F. diplosiphon, while up to 70% FAME were present in genetically modified strains [36]. A high ratio of saturated fatty acids in the lipid profile was observed across all treatments, which is a desirable trait for a prospective biofuel agent [59]. We observed methyl palmitate (C16:0) to be the major constituent, which is similar to FAME profiles reported in the microalgae Chlorella pyrenoidosa and C. vulgaris [60,61,62]. Interestingly, the level of methyl palmitate decreased over time in cultures grown in 0.5 and 1.0 g L−1 NaNO3 (Figure 4) indicating that exhaustion of nitrogen supply could have altered the fatty acid composition of F. diplosiphon. These results indicate a direct correlation between the depletion of nitrate in the medium to the fatty acids produced. By contrast, an increase in unsaturated FAME species such as methyl hexadecanoate (16:1), methyl octadecenoate (18:1), and methyl octadecadienoate (18:2) was observed over time, suggesting that lipid profiles of nitrogen-starved cultures contain a higher proportion of unsaturated fatty acids. The presence of saturated fatty acid in lipid profile is valuable for biofuel production but its high melting point could result in fuel gelling in colder climates. On the contrary, the low melting point of unsaturated fatty acids is necessary for biofuel generation, and aids their endurance in cold weather [63]. In the present study, FAME profiling of F. diplosiphon indicated the presence of methyl octadecadienoate (C18:2), which is reported to provide oxidative stability [35]. Further, the presence of both hexadecanoate (16:1) and methyl myristate (14:0) in the lipid profile of F. diplosiphon is beneficial as hexadecanoate (16:1) improves the oxidative stability [63] and methyl myristate (14:0), a shorter chain fatty acid improves NOx emissions [64]. Thus, carbon chain lengths of fatty acid as well as the degree of unsaturation are both key properties for biodiesel quality [65]. Wang et al. [66] demonstrated that enhanced saturated fatty acid abundance in the marine protist, Schizochytrium sp. PKU#Mn4, met the ASTM6751 standards. Tabatabai et al. [36] reported that the high abundance of saturated FAMEs, while beneficial with regards to cetane number and oxidative stability, resulted in high pour and cloud points. This suggests that use of the resultant biofuel from F. diplosiphon in a blend with conventional fuels and additives would the most viable approach. Our findings indicate that nitrogen deprivation at 1.0 g L−1 NaNO3 significantly enhances lipid yield in F. diplosiphon, while achieving a desired saturated:unsaturated fatty acid ratio for biofuel production. Since nitrogen is a major nutrient for chlorophyll production and other proteins, deprivation of this essential macronutrient could inhibit structural and physiological components of photosynthesis [67]. In a report on the changes in lipid composition in Chlorella sp. and Nannochloropsis sp. during nitrogen starvation, the comprehensive fatty acid composition of polar lipids was unaffected, indicating that maintenance of a particular fatty acid profile in each compartment allows its continued function, despite a significant decrease lipid quantity [68].

Table 1.
Fatty acid methyl ester (FAME) composition in Fremyella diplosiphon grown over 7, 10, 15, and 20 days in media containing 0.25, 0.5, 1.0, 1.5 g L−1 sodium nitrate (NaNO3) based on relative abundance of each component (% total extractable FAMEs ± SE).
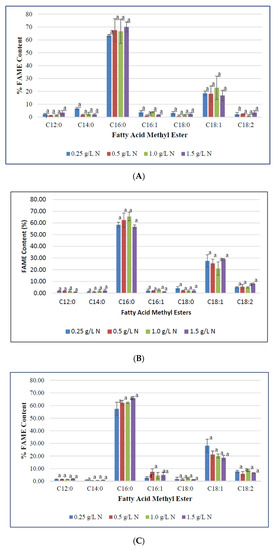
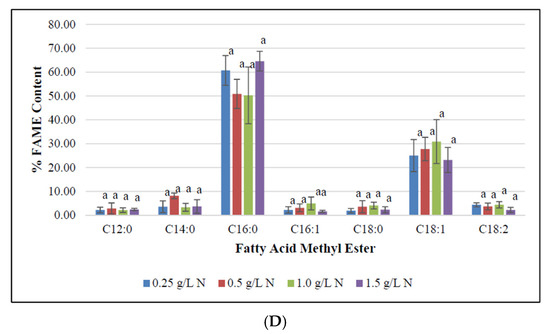
Figure 3.
Fatty methyl ester composition (FAME) of Fremyella diplosiphon grown over (A) 7, (B) 10, (C) 15, and (D) 20 days in media containing 0.25, 0.5, 1.0, 1.5 g L−1 sodium nitrate (NaNO3). Bars represent the average % relative content of specific FAME species (± standard error) for three biological replicates of each nitrate treatment. Different letters above bars indicate significant differences in FAME species abundance between varying nitrate treatments (p < 0.05).
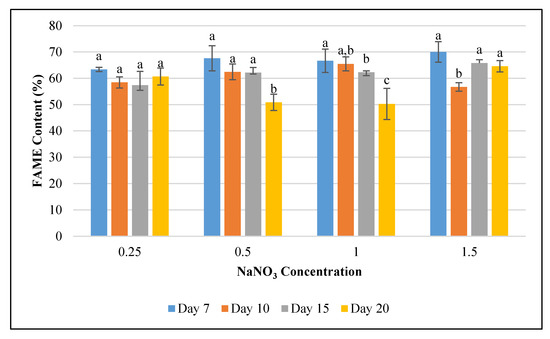
Figure 4.
Methyl palmitate relative abundance in Fremyella diplosiphon grown over 7, 10, 15, and 20 days in media containing 0.25, 0.5, 1.0, 1.5 g L−1 sodium nitrate (NaNO3). Bars represent the average % relative content of specific fatty acid methyl esters (± standard error) for three biological replicates of each nitrate treatment. Different letters above bars indicate significant differences in the methyl palmitate content between varying time periods (p < 0.05).
4. Conclusions
In the present study, we investigated the effect of nitrogen deprivation on growth rate, lipid yield, and FAME composition in F. diplosiphon to identify optimal conditions that enhance lipid accumulation. Maximum lipid productivity was observed under moderately nitrogen-limited conditions (i.e., 1.0 gL−1 sodium nitrate) on the 10th day, without the growth rate being hindered. In addition, we identified and quantified the FAME profile in transesterified F. diplosiphon lipids, which is a prerequisite to evaluate biofuel physical and chemical properties. These findings suggest that manipulating nitrogen input during F. diplosiphon cultivation could enhance lipid production, thus increasing its potential viability as a source of renewable biofuel. Results of this study could lead to a more optimal photobioreactor design for large scale F. diplosiphon cultivation, for the development of natural bio-products across various applications, from fuel to food and cosmetics.
Author Contributions
V.S., B.T., and A.K.S. designed and conceived the study; B.T. and A.A. performed the experiments and analyzed the data; B.T., A.K.S., P.K.S., and V.S. interpreted the data and drafted the manuscript. All authors gave critical comments for the important intellectual content in the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Science Foundation’s Division of Engineering [CBET-1900966] grant awarded to Viji Sitther at Morgan State University. Prashant Kumar Singh is thankful to UGC-Startup Grant, New Delhi, India for the support. The authors are thankful to undergraduate students Christian Jones and LaDonna Wyatt at Morgan State University for technical help.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- John, R.P.; Anisha, G.; Nampoothiri, K.M.; Pandey, A. Micro and macroalgal biomass: A renewable source for bioethanol. Bioresour. Technol. 2011, 102, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Benemann, J. Utilization of carbon dioxide from fossil fuel-burning power plants with biological systems. Energy Convers. Manag. 1993, 34, 999–1004. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Demirbas, A. Use of algae as biofuel sources. Energy Convers. Manag. 2010, 51, 2738–2749. [Google Scholar] [CrossRef]
- Atabani, A.; Silitonga, A.; Badruddin, I.A.; Mahlia, T.; Masjuki, H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Gouveia, L.; Oliveira, A.C. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 2008, 36, 269–274. [Google Scholar] [CrossRef]
- Campbell, P.K.; Beer, T.; Batten, D. Life cycle assessment of biodiesel production from microalgae in ponds. Bioresour. Technol. 2011, 102, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Rouhany, M.; Montgomery, H. Global biodiesel production: The state of the art and impact on climate change: From production to combustion. In Biodiesel; Springer: Cham, Switzerland; 2019; pp. 1–14. [Google Scholar]
- Huang, G.; Chen, F.; Wei, D.; Zhang, X.; Chen, G. Biodiesel production by microalgal biotechnology. Appl. Energy 2010, 87, 38–46. [Google Scholar] [CrossRef]
- Khalid, K.; Khalid, K. Transesterification of palm oil for the production of biodiesel. Am. J. Appl. Sci. 2011, 8, 804–809. [Google Scholar] [CrossRef]
- Takeshita, T. Competitiveness, role, and impact of microalgal biodiesel in the global energy future. Appl. Energy 2011, 88, 3481–3491. [Google Scholar] [CrossRef]
- Lang, X.; Dalai, A.K.; Bakhshi, N.N.; Reaney, M.J.; Hertz, P.B. Preparation and characterization of bio-diesels from various bio-oils. Bioresour. Technol. 2001, 80, 53–62. [Google Scholar] [CrossRef]
- Aikawa, S.; Joseph, A.; Yamada, R.; Izumi, Y.; Yamagishi, T.; Matsuda, F.; Kawai, H.; Chang, J.-S.; Hasunuma, T.; Kondo, A. Direct conversion of Spirulina to ethanol without pretreatment or enzymatic hydrolysis processes. Energy Environ. Sci. 2013, 6, 1844–1849. [Google Scholar] [CrossRef]
- Dismukes, G.C.; Carrieri, D.; Bennette, N.; Ananyev, G.M.; Posewitz, M.C. Aquatic phototrophs: Efficient alternatives to land-based crops for biofuels. Curr. Opin. Biotechnol. 2008, 19, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Angermayr, S.A.; Hellingwerf, K.J.; Lindblad, P.; De Mattos, M.J.T. Energy biotechnology with cyanobacteria. Curr. Opin. Biotechnol. 2009, 20, 257–263. [Google Scholar] [CrossRef]
- Parmar, A.; Singh, N.K.; Pandey, A.; Gnansounou, E.; Madamwar, D. Cyanobacteria and microalgae: A positive prospect for biofuels. Bioresour. Technol. 2011, 102, 10163–10172. [Google Scholar] [CrossRef]
- Jones, C.S.; Mayfield, S.P. Algae biofuels: Versatility for the future of bioenergy. Curr. Opin. Biotechnol. 2012, 23, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Rodolfi, L.; Zittelli, G.C.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef]
- Lu, X. A perspective: Photosynthetic production of fatty acid-based biofuels in genetically engineered cyanobacteria. Biotechnol. Adv. 2010, 28, 742–746. [Google Scholar] [CrossRef]
- Quintana, N.; Van Der Kooy, F.; Van De Rhee, M.D.; Voshol, G.P.; Verpoorte, R. Renewable energy from cyanobacteria: Energy production optimization by metabolic pathway engineering. Appl. Microbiol. Biotechnol. 2011, 91, 471–490. [Google Scholar] [CrossRef]
- Rosgaard, L.; De Porcellinis, A.J.; Jacobsen, J.H.; Frigaard, N.-U.; Sakuragi, Y. Bioengineering of carbon fixation, biofuels, and biochemicals in cyanobacteria and plants. J. Biotechnol. 2012, 162, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Parkhey, P.; Joshi, K.; Mahilkar, A. Design of a microbial fuel cell and its transition to microbial electrolytic cell for hydrogen production by electro-hydro genesis. Indian J. Exp. Biol. 2013, 51, 860–865. [Google Scholar]
- Pruvost, J.; Van Vooren, G.; Le Gouic, B.; Couzinet-Mossion, A.; Legrand, J. Systematic investigation of biomass and lipid productivity by microalgae in photobioreactors for biodiesel application. Bioresour. Technol. 2011, 102, 150–158. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Mulders, K.J.M.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef]
- González, L.E.; Díaz, G.C.; Aranda, D.A.G.; Cruz, Y.R.; Fortes, M.M. Biodiesel production based in microalgae: A biorefinery approach. Nat. Sci. 2015, 7, 358–369. [Google Scholar] [CrossRef]
- Yeesang, C.; Cheirsilp, B. Effect of nitrogen, salt, and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Bioresour. Technol. 2011, 102, 3034–3040. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Yeh, K.-L.; Aisyah, R.; Lee, D.-J.; Chang, J.-S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, A.; Li, J.; He, B. A continuous process for biodiesel production in a fixed bed reactor packed with cation-exchange resin as heterogeneous catalyst. Bioresour. Technol. 2011, 102, 3607–3609. [Google Scholar] [CrossRef] [PubMed]
- Mairet, F.; Bernard, O.; Masci, P.; Lacour, T.; Sciandra, A. Modelling neutral lipid production by the microalga Isochrysis aff. galbana under nitrogen limitation. Bioresour. Technol. 2011, 102, 142–149. [Google Scholar] [CrossRef]
- Fuentes-Grünewald, C.; Garcés, E.; Alacid, E.; Sampedro, N.; Rossi, S.; Camp, J. Improvement of lipid production in the marine strains Alexandrium minutum and Heterosigma akashiwo by utilizing abiotic parameters. J. Ind. Microbiol. Biotechnol. 2011, 39, 207–216. [Google Scholar] [CrossRef]
- Sitther, V.; Tabatabai, B.; Fathabad, S.G.; Gichuki, S.; Chen, H.; Arumanayagam, A.C.S. Cyanobacteria as a biofuel source: Recent advances and applications. Adv. Cyanobacterial Biol. 2020, 269–289. [Google Scholar] [CrossRef]
- Tabatabai, B.; Arumanayagam, A.S.; Enitan, O.; Mani, A.; Natarajan, S.S.; Sitther, V. Overexpression of hlyB and mdh genes confers halotolerance in Fremyella diplosiphon, a freshwater cyanobacterium. Enzym. Microb. Technol. 2017, 103, 12–17. [Google Scholar] [CrossRef]
- Fathabad, S.G.; Arumanayagam, A.S.; Tabatabai, B.; Chen, H.; Lu, J.; Sitther, V. Augmenting Fremyella diplosiphon cellular lipid content and unsaturated fatty acid methyl esters via sterol Desaturase gene overexpression. Appl. Biochem. Biotechnol. 2019, 189, 1127–1140. [Google Scholar] [CrossRef]
- Tabatabai, B.; Chen, H.; Lu, J.; Giwa-Otusajo, J.; McKenna, A.M.; Shrivastava, A.K.; Sitther, V. Fremyella diplosiphon as a biodiesel agent: Identification of fatty acid methyl esters via microwave-assisted direct in situ Transesterification. BioEnergy Res. 2018, 11, 528–537. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Rosenberg, J.N.; Kobayashi, N.; Barnes, A.; Noel, E.A.; Betenbaugh, M.J.; Oyler, G.A. Comparative analyses of three chlorella species in response to light and sugar reveal distinctive lipid accumulation patterns in the microalga C. sorokiniana. PLoS ONE 2014, 9, e92460. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Sigaud-Kutner, T.C.S.; Leitao, M.A.S.; Okamoto, O.K.; Morse, D.; Colepicolo, P. Heavy metal-induced oxidative stress in Algae1. J. Phycol. 2003, 39, 1008–1018. [Google Scholar] [CrossRef]
- Ip, P.-F.; Chen, F. Employment of reactive oxygen species to enhance astaxanthin formation in Chlorella zofingiensis in heterotrophic culture. Process. Biochem. 2005, 40, 3491–3496. [Google Scholar] [CrossRef]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High lipid induction in microalgae for biodiesel production. Energies 2012, 5, 1532–1553. [Google Scholar] [CrossRef]
- Illman, A.; Scragg, A.; Shales, S. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzym. Microb. Technol. 2000, 27, 631–635. [Google Scholar] [CrossRef]
- Singh, S.C.; Sinha, R.P.; Hader, D.P. Role of lipids and fatty acids in stress tolerance in cyanobacteria. Acta Protozool. 2002, 41, 297–308. [Google Scholar]
- Hsieh, C.-H.; Wu, W.-T. Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour. Technol. 2009, 100, 3921–3926. [Google Scholar] [CrossRef]
- Yeh, K.-L.; Chang, J.-S. Nitrogen starvation strategies and photobioreactor design for enhancing lipid content and lipid production of a newly isolated microalga Chlorella vulgaris ESP-31: Implications for biofuels. Biotechnol. J. 2011, 6, 1358–1366. [Google Scholar] [CrossRef]
- Sun, X.; Cao, Y.; Xu, H.; Liu, Y.; Sun, J.; Qiao, D.R.; Cao, Y. Effect of nitrogen-starvation, light intensity and iron on triacylglyceride/carbohydrate production and fatty acid profile of Neochloris oleoabundans HK-129 by a two-stage process. Bioresour. Technol. 2014, 155, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Mallick, N. Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl. Microbiol. Biotechnol. 2009, 84, 281–291. [Google Scholar] [CrossRef]
- Nigam, S.; Rai, M.P.; Sharma, R. Effect of nitrogen on growth and lipid content of Chlorella pyrenoidosa. Am. J. Biochem. Biotechnol. 2011, 7, 124–129. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.; Wu, N.; Lan, C.Q.; Dubois-Calero, N. Biofuels from microalgae. Biotechnol. Prog. 2008, 24, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Suen, Y.; Hubbard, J.S.; Holzer, G.; Tornabene, T.G. Total lipid production of the green alga Nannochloropsis sp. QII under different nitrogen regimes. J. Phycol. 1987, 23, 289–296. [Google Scholar] [CrossRef]
- Stephenson, A.I.; Dennis, J.S.; Howe, C.J.; Scott, S.A.; Smith, A.G. Influence of nitrogen limitation regime on the production by Chlorella vulgaris of lipids for biodiesel feedstocks. Biofuels 2010, 1, 47–58. [Google Scholar] [CrossRef]
- Taher, H.; Al-Zuhair, S.; Al-Marzouqi, A.H.; Haik, Y.; Farid, M. Effective extraction of microalgae lipids from wet biomass for biodiesel production. Biomass Bioenergy 2014, 66, 159–167. [Google Scholar] [CrossRef]
- Scott, S.; Davey, M.P.; Dennis, J.S.; Horst, I.; Howe, C.J.; Lea-Smith, D.J.; Smith, A.G. Biodiesel from algae: Challenges and prospects. Curr. Opin. Biotechnol. 2010, 21, 277–286. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae: Biotechnology and Microbiology; Cambridge University Press: Cambridge, UK, 1994; ISBN 0-521-35020-4. [Google Scholar]
- Nagarkar, S.; Williams, G.A.; Subramanian, G.; Saha, S. Cyanobacteria-dominated biofilms: A high quality food resource for intertidal grazers. Hydrobiologia 2004, 512, 89–95. [Google Scholar] [CrossRef]
- Wahlen, B.D.; Morgan, M.R.; McCurdy, A.T.; Willis, R.M.; Dye, D.J.; Bugbee, B.; Wood, B.D.; Seefeldt, L.C. Biodiesel from microalgae, yeast, and bacteria: Engine performance and exhaust emissions. Energy Fuels 2012, 27, 220–228. [Google Scholar] [CrossRef]
- Tang, H.; Chen, M.; Garcia, M.; Abunasser, N.; Ng, K.S.; Salley, S.O. Culture of microalgae Chlorella minutissima for biodiesel feedstock production. Biotechnol. Bioeng. 2011, 108, 2280–2287. [Google Scholar] [CrossRef]
- Rittmann, B.E. Opportunities for renewable bioenergy using microorganisms. Biotechnol. Bioeng. 2008, 100, 203–212. [Google Scholar] [CrossRef] [PubMed]
- D’Oca, M.G.M.; Viêgas, C.V.; Lemões, J.S.; Miyasaki, E.K.; Morón-Villarreyes, J.A.; Primel, E.G.; Abreu, P.C. Production of FAMEs from several microalgal lipidic extracts and direct transesterification of the Chlorella pyrenoidosa. Biomass Bioenergy 2011, 35, 1533–1538. [Google Scholar] [CrossRef]
- Rai, M.P.; Gautom, T.; Sharma, N. Effect of salinity, pH, light intensity on growth and lipid production of microalgae for bioenergy application. Online J. Biol. Sci. 2015, 15, 260–267. [Google Scholar] [CrossRef]
- Moradi-Kheibari, N.; Ahmadzadeh, H.; Hosseini, M. Use of solvent mixtures for total lipid extraction of Chlorella vulgaris and gas chromatography FAME analysis. Bioprocess Biosyst. Eng. 2017, 40, 1363–1373. [Google Scholar] [CrossRef]
- Bowen, D. Effect of Fatty Acid Structure on Biodiesel. Biodiesel is Good. 2010. Available online: http://biodieselisgood.org/chemistry/effect-of-fatty-acid-structure-on-biodiesel/ (accessed on 3 November 2020).
- Knothe, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Knothe, G. “Designer” biodiesel: Optimizing fatty ester composition to improve fuel properties. Energy Fuels 2008, 22, 1358–1364. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Harrison, S.T. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. Environ. Biol. Fishes 2009, 21, 493–507. [Google Scholar] [CrossRef]
- Wang, Q.; Sen, B.; Liu, X.; He, Y.; Xie, Y.; Wang, G. Enhanced saturated fatty acids accumulation in cultures of newly-isolated strains of Schizochytrium sp. and Thraustochytriidae sp. for large-scale biodiesel production. Sci. Total Environ. 2018, 994–1004. [Google Scholar] [CrossRef]
- Young, E.B.; Beardall, J. Photosynthetic function in Dunaliella tertiolecta (chlorophyta) during a nitrogen starvation and recovery cycle. J. Phycol. 2003, 39, 897–905. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).