1. Introduction
The production of mushrooms in Poland is growing rapidly. During 2006–2018, the production of mushrooms increased from 196 thousand Mg·year
−1 to 325 thousand Mg·year
−1, which currently accounts for 24.15% of the total production in the European Union (EU) [
1,
2]. Poland is the largest grower of mushrooms in Europe and the third in the world. One of the abundant wastes arising during the cultivation of mushrooms is a worn-out substrate—mushroom spent compost (
MSC)—which accounts to ~5 kg of
MSC per 1 kg of produced mushrooms [
3]. This amounts to 5.6 and 1.6 million Mg·year
−1 of
MSC, in the EU and Poland, respectively [
1,
4].
The sustainable management of
MSC is an important issue for the industry. The
MSC is currently considered as an ‘industrial waste’ (020199 code, according to European Waste Classification), which is classified as wastes from agriculture [
5]. Potential waste treatment methods depend on
MSC properties. For example, a white mushroom (
Agaricus bisporus) is grown on a bedding consisting mainly of straw and poultry manure. The manure also contains gypsum, urea, peat, coconut, and soy proteins [
6]. The mushroom production cycle takes about 6 to 8 weeks on this bedding with three harvestings of mushrooms. After this period, the
MSC cannot be regenerated and used again for production. It is a waste which needs to be treated [
7]. The
MSC is a source of biogenic elements, such as C, N, P, K, and S (
Table 1). The low level of heavy metals content allows the
MSC to be considered as an organic fertilizer [
8]. In addition, the
MSC also contains a residual fraction of organic compounds, humic and fulvic acids, fraction decalcification, and bitumen [
7]. An overview of the properties of the
MSC is presented in
Table 1.
Thus far, various methods for
MSC management have been developed and used. Those methods were aimed to obtain compost, bioethanol, biogas, enzyme lactase, xylooligosaccharides, or hydrogen. The most popular use of
MSC is composting due to its organic nature and balanced ratio of C/N 12.3–18:1 (
Table 1). The work of Kalembasa et al. [
7] showed that compost from
MSC could be an excellent fertilizer for improving soil structure. However, research to date shows some concerns as well [
13]. For example, compost from
MSC can be highly variable and does not always meet the legal criteria for a fertilizer [
8]. This is due to the high variability of the compost parameters such as N, P, and K, biogenic elements, C/N ratio, pH, electrolytic conductivity, and the Ca, Na, Mg, Cu, Fe, Zn, Mn, Cr, Cd, Pb, and Ni content. The share and content of hemicellulose, cellulose, and lignin can also vary [
14]. Thus, the variable
MSC composition may cause technological problems of the process and heterogeneity of the obtained fertilizer quality.
Bioethanol production from
MSC can be facilitated via hydrolysis with acids or bases, physical treatment using steam followed by fermentation. The difficulty comes from high lignin content. Steam explosion is used for delignification (the breakdown of lignin structures) into simpler cellulose. The literature shows that the highest potential of conversion of
MSC to bioethanol requires 20 bar steam pressure [
15]. Research on
MSC hydrolysis with the addition of surfactants was published [
16].
The
MSC may also be a source of valuable substances. During the hydrothermal conversion of
MSC rich in nutrients (e.g., proteins), production of xylooligosaccharides may be possible [
17,
18]. Thanks to the high purity of the obtained product, this process has industrial potential [
15]. The
MSC may also be an efficient source of lactase enzyme, which is widely used in paper, clothing, and food production [
4].
Biogas production is also feasible. However, it has been found that this process is not as effective as fermentation of other biomass. Anaerobic fermentation of
MSC generates ~122 dm
3·kg
−1 d.m. of biomethane, while corn silage can produce 320 dm
3·kg
−1 d.m. [
19]. The biogas yield from other available substrates: cattle manure (324 ± 15.5 dm
3·kg
–1 d.m.), corn silage (653 ± 28.8 dm
3·kg
–1 d.m.), waste fruit and vegetables (678 ± 15.8 dm
3·kg
–1 d.m.), sugar beet pulp (634 ± 235 dm
3·kg
–1 d.m.), whey (736 ± 15.5 dm
3·kg
–1 d.m.) [
20], shows that biogas production from
MSC is not competitive enough. Another interesting use of
MSC is the production of hydrogen, which has already been tested at the lab-scale. Two-step hydrolysis with acid and base resulted in 2.52 moles of H
2·g COD
−1 [
21].
Law regulations regarding the management of waste from mushroom production allow using
MSC for energy purposes [
22]. The 2009/28/WE (23 April 2009) [
23] indicates the possibility of considering the
MSC as biomass, and in consequence, the energy produced from
MSC as energy from a renewable source. However, due to the high moisture,
MSC is considered as fuel with low calorific value (
Table 1) [
24]. The
MSC, with approximately 70% moisture, has an
LHV of ~4.6 MJ·kg
−1 d.m. [
3].
MSC’s characteristics (
Table 1) do not indicate high profitability of the incineration process [
25].
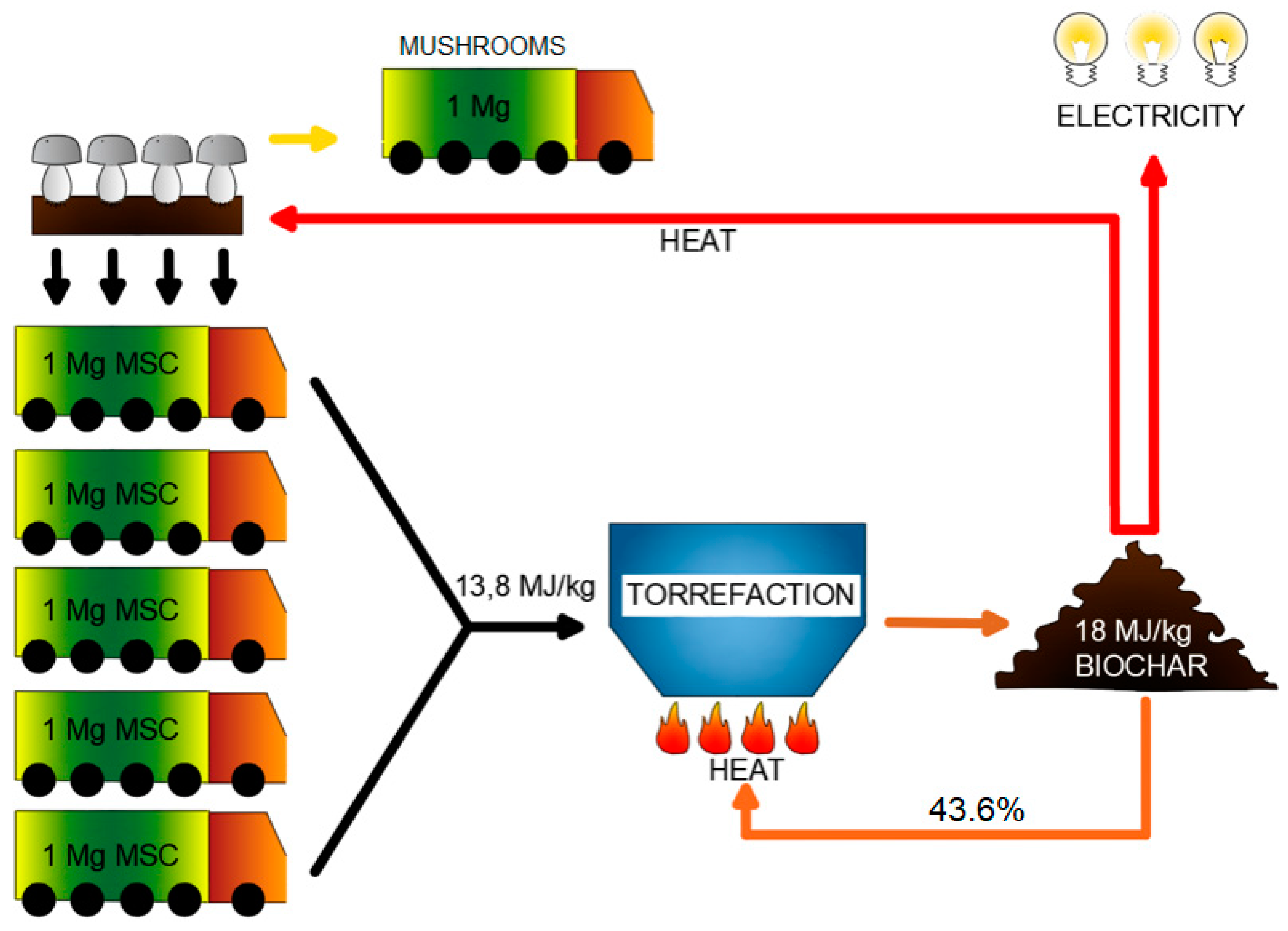
We propose to convert the
MSC into a more efficient solid fuel, according to the ‘Waste to Carbon’ approach [
26] (
Figure 1). One of the ways of
MSC conversion is the process of torrefaction, otherwise known as high-temperature drying, low-temperature pyrolysis, or biomass ‘roasting’. It consists of roasting the organic compounds of plant origin out of a substance [
27]. Torrefaction is characterized by the range of temperatures 200–300 °C [
28], while according to the act on renewable sources energy [
29], the temperature range is 200–320 °C. The residence (process) time of torrefaction depends on the water or volatile content, as well as the type of reactor or substrate type [
30]. The residence time usually does not exceed 60 min. The products of torrefaction are solid biocoal and gas (‘torgas’). Torgas consists of nonflammable substances such as water or CO
2 and flammable substances such as CO, CH
4, or H
2 [
31]. Depending on process parameters and properties of the substrate, a different ratio of flammable to noncombustible parts may be achieved [
28]. The torgas is not an attractive product of torrefaction due to the high content of nonflammable substances [
32,
33].
The main product of the torrefaction, biocoal, according to Polish regulations, should have
LHV not less than 21 MJ·kg
−1 d.m. and the feedstock should be from solid biomass/waste of vegetable, animal, or biodegradable origin to be considered as a ‘renewable source of energy’ [
29].
During the torrefaction process, the loss of mass, as well as the loss of chemical energy from raw material, is observed. However, the degree of mass loss is between 30 and 40%, while the energy content drop does not exceed 10%. Due to this difference, energy densification in solid fuels occurs [
28]. However, the bulk density of the product is increasing, which positively affects the logistics of fuel [
25] transport. Torrefaction increases the C content in the biocoal, which improves its calorific value. The product also becomes more homogenous. This affects the further thermal transformation of biocoal as a fuel. Because the abrasiveness is reduced, the mechanical grinding requires less energy compared to the raw substrate. There is also a possibility of pelletization of biocoal from torrefaction [
34]. Additionally, the ratios of H/C and O/C are reduced, which improves the fuel properties of the biocoal in relation to the used substrate [
35,
36].
Torrefaction of waste is a growing research area. Examples include fractions of industrial and municipal wastes [
27,
37], sewage sludge [
38], brewers’ spent grain [
39], Oxytree biomass [
40] prunings, woodchips, olives waste, and Virginia mallow (considered as a potential energy crop) [
22]. High-quality fuels can be obtained due to the torrefaction process. Therefore, it is necessary to carry out research on new substrates, aimed to optimize the torrefaction process to obtain the highest calorific value. For torrefaction optimization, it is necessary to determine the appropriate temperature and residence time for a given substrate to achieve the most beneficial fuel parameters [
25,
41].
The
MSC is a new substrate which has not yet been tested for its conversion to solid fuel during the torrefaction process. The torrefaction of
MSC and reusing part of the heat from biocoal combustion for torrefaction energy demand and
MSC farm energy demand is at the early stage of technology readiness. Therefore, the aim of this study was the determination of the
MSC torrefaction process kinetics parameters and the preliminary selection of technological parameters under which biocoal with the highest calorific value may be obtained. This article presents pioneering, novel research as a proof-of-concept of a new technology of
MSC reuse, by conversion to high-quality solid fuel, which may be internally reused to achieve heat self-sufficiency (
Figure 1).
3. Results
The
k values significantly (
p < 0.05) increased with the temperature of the torrefaction process. The lowest
k value was recorded at 200 °C (
k = 1.7 × 10
−5 s
−1) and the highest (
k = 4.6 × 10
−5 s
−1) at 300 °C (
Table 3). Similar dependence was confirmed by Dhanavath et al. [
52]. However, the increase in
k in relation to torrefaction temperature was not linear. Significant (
p < 0.05) differences between the
k values observed for 300 °C and the values obtained in temperatures between 200 and 260 °C; however, no significant increase between 200 °C and 260 °C was observed (
Table A1). Torrefaction at 280 °C caused a significant increase of the
k value in comparison to 200 °C. The results indicate that the temperature must be >280 °C to achieve significant acceleration of the torrefaction process.
Similar to [
53,
54], we estimated the activation energy for the whole torrefaction process. On the base of estimated
k values, the activation energy of
MSC torrefaction, reaching about 22.3 kJ·mol
−1, was determined (
Table 3). The obtained
Ea value is low in comparison with typical woody biomass, where, for example, for beech and spruce, these values are 150 and 155 kJ·mol
−1, respectively [
55], for willow from 46 to 152 kJ·mol
−1, [
56] pine 131 kJ·mol
−1, and fir 128 kJ·mol
−1 [
57]. However, these values have been determined by different methods and models. The model used in the present work had a global first-order character, as it was part of the preliminary study. This model allows estimating solid mass yield at a specific temperature and time of the process. Because of the high ash content in
MSC and the narrow range of used temperatures, we recommend additional experiments on the kinetics of the process, using more complex models based on first-order kinetics with distributed activation energy models (DAEMs), pseudokinetics, or multicomponent first-order kinetic models.
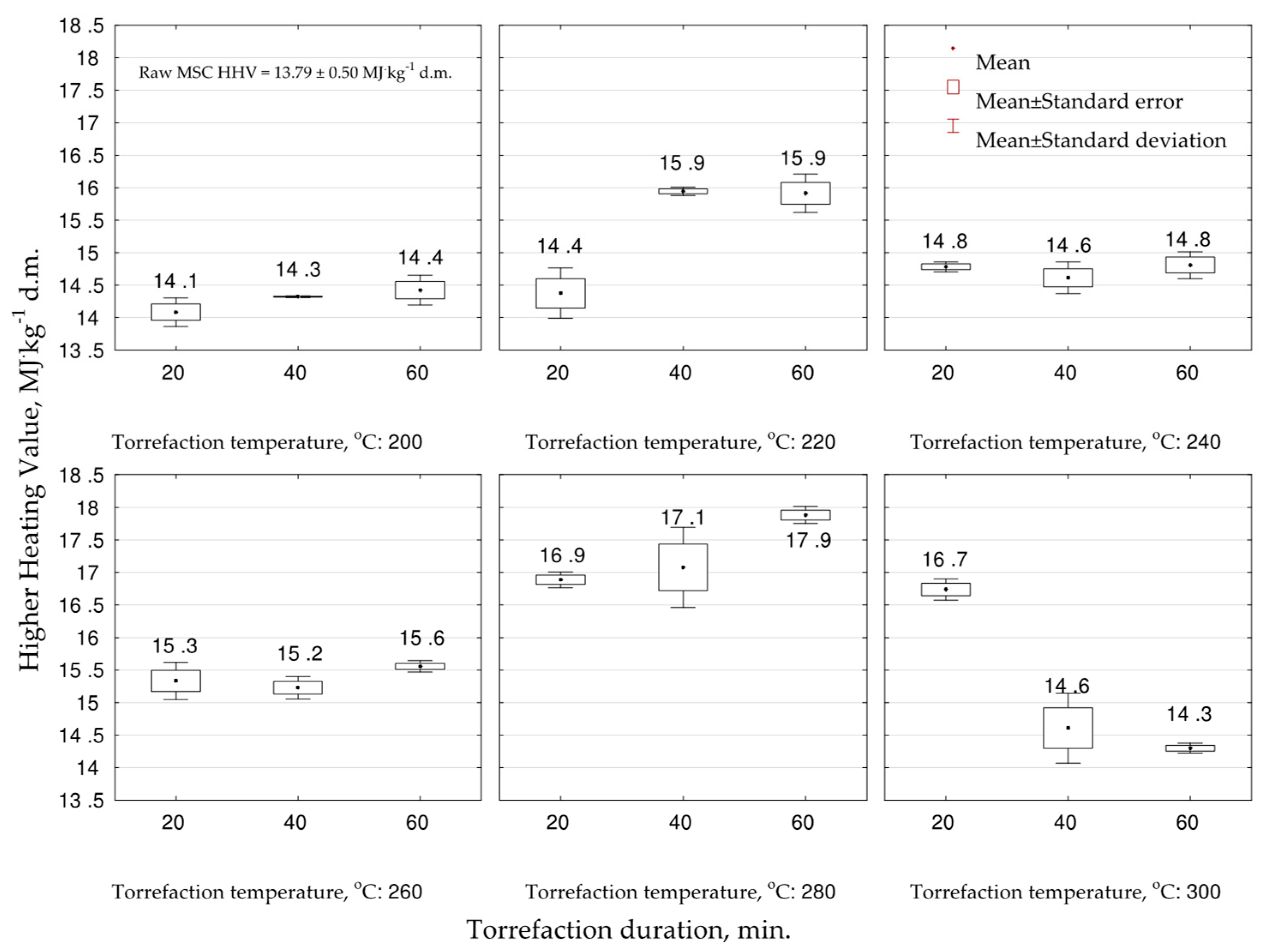
The experiment showed that the
HHV increased due to torrefaction in all studied variants in comparison to raw
MSC biomass (13.7 MJ·kg
−1 d.m.) (
Figure 2). The significantly (
p < 0.05) lowest values were noted for temperature 200 °C (all durations) from 14.1 to 14.4 MJ·kg
−1 d.m. The best (
p < 0.05) fuel properties were noted for biocoals obtained from
MSC under 280 °C 16.9–17.9 MJ·kg
−1 d.m. In the case of 220 °C, a significant (
p < 0.05) influence of torrefaction duration on the increase of
HHV was noted (
Table A2). On the other hand, the increase of torrefaction time decreased (
p < 0.05) the
HHV under the temperature of 300 °C, which could be related to organic matter volatilization and mineralization. Additionally, the increase of the temperature from 200 to 280 °C, for the same duration, increased (
p < 0.05) the
HHV (
Figure 2,
Table A2). The experiment showed that to achieve the highest
HHV of the biocoal,
MSC should be torrefied under the temperature of 280 °C for a duration of 60 min.
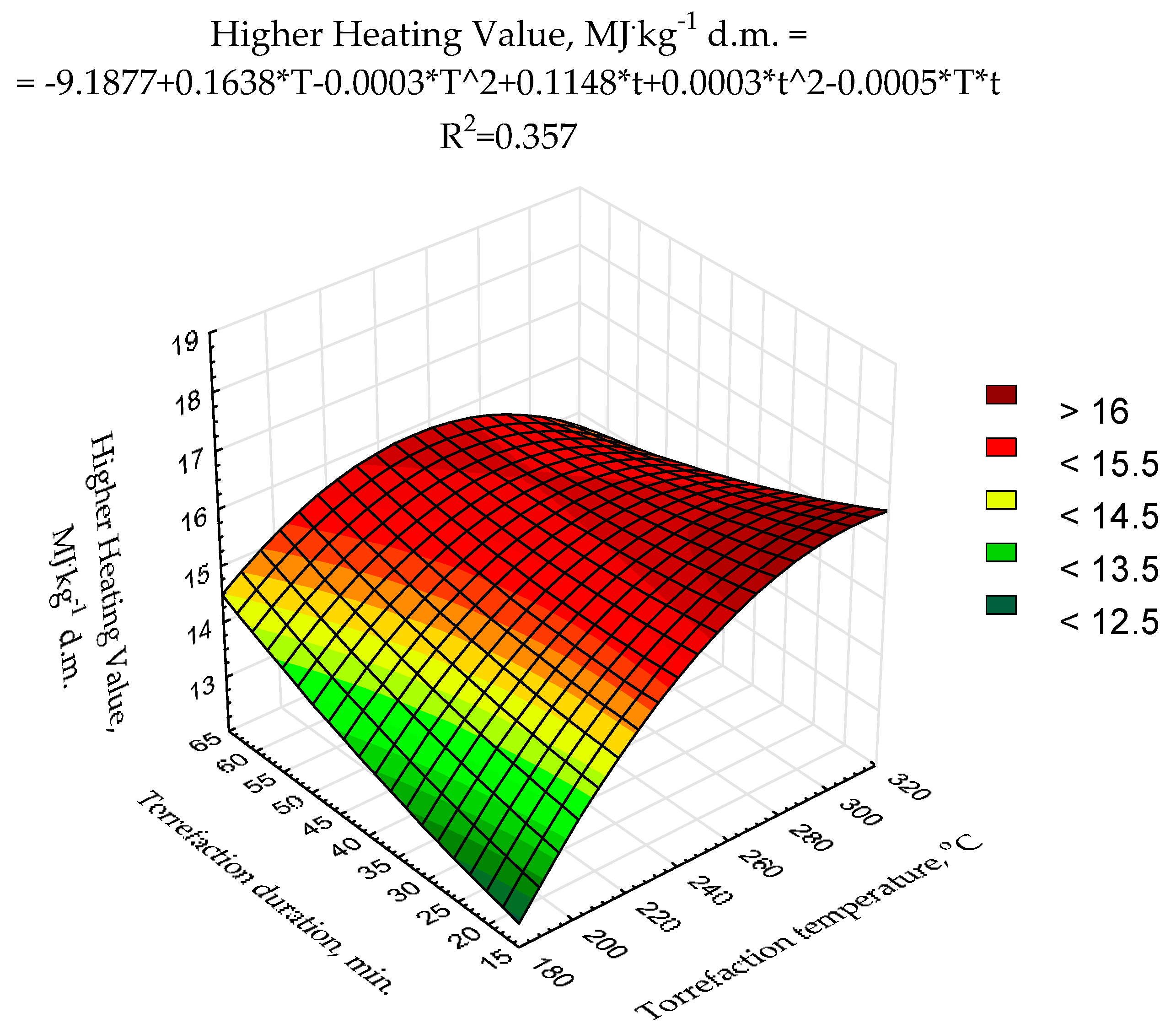
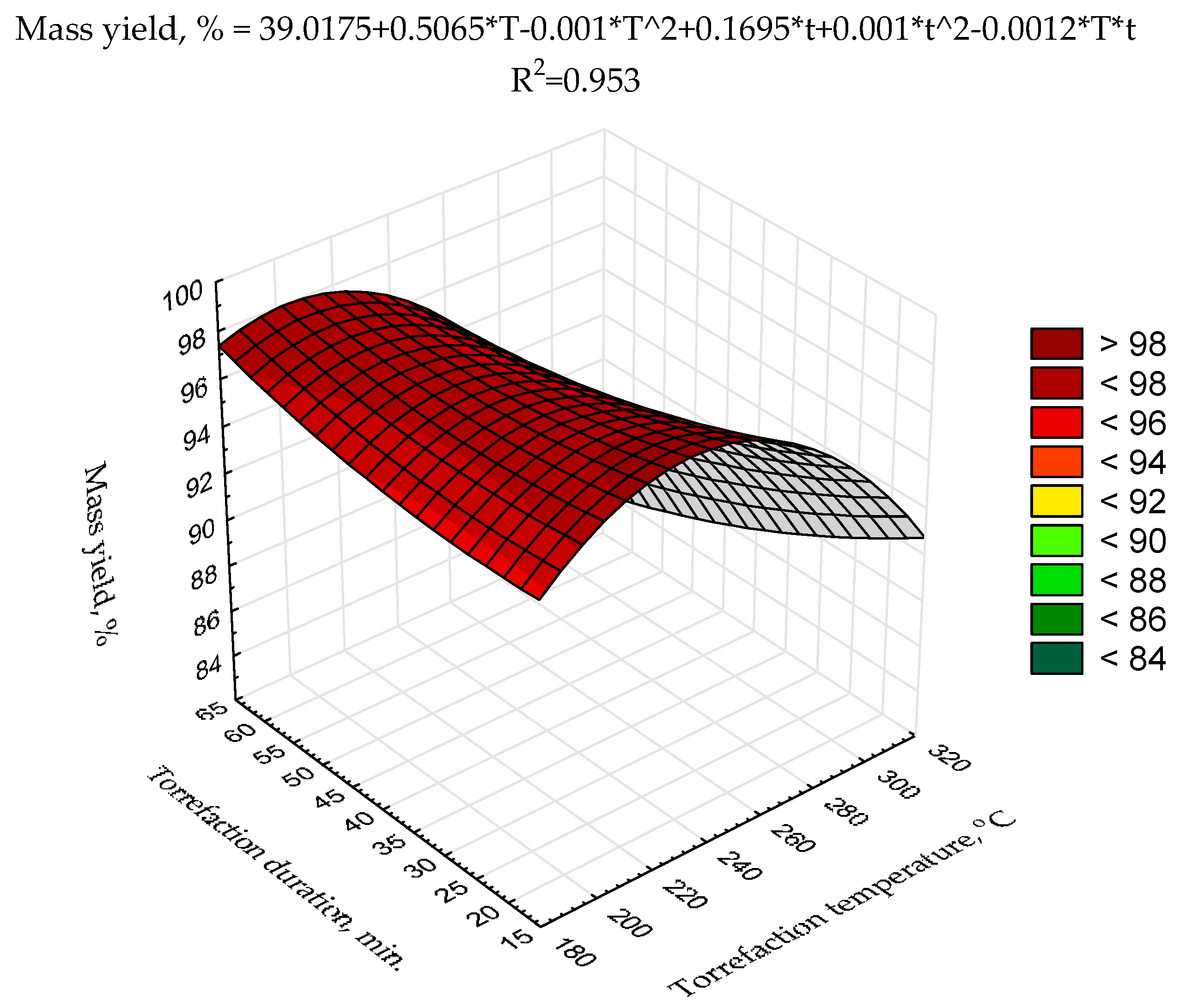
The second-degree polynomial model of torrefaction temperature and duration influence on
HHV was proposed (
Figure 3) using raw data presented previously [
43]. The statistical evaluation showed that the determination coefficient was relatively low (0.357) (
Figure 3) and only two model regression coefficients (a
3, and a
6) were statistically significant (
p < 0.05) (
Table A3). Such a low fitting degree of the model parameters to existing data could be due to a relatively low number of repetitions (n = 3 for each variant) and/or a low degree of the polynomial. To achieve better results suitable for
MSC torrefaction optimization, more sophisticated research with a higher number of repetitions (to reduce the degree of the heterogeneity of the results) with the application of better-fitting models is required.
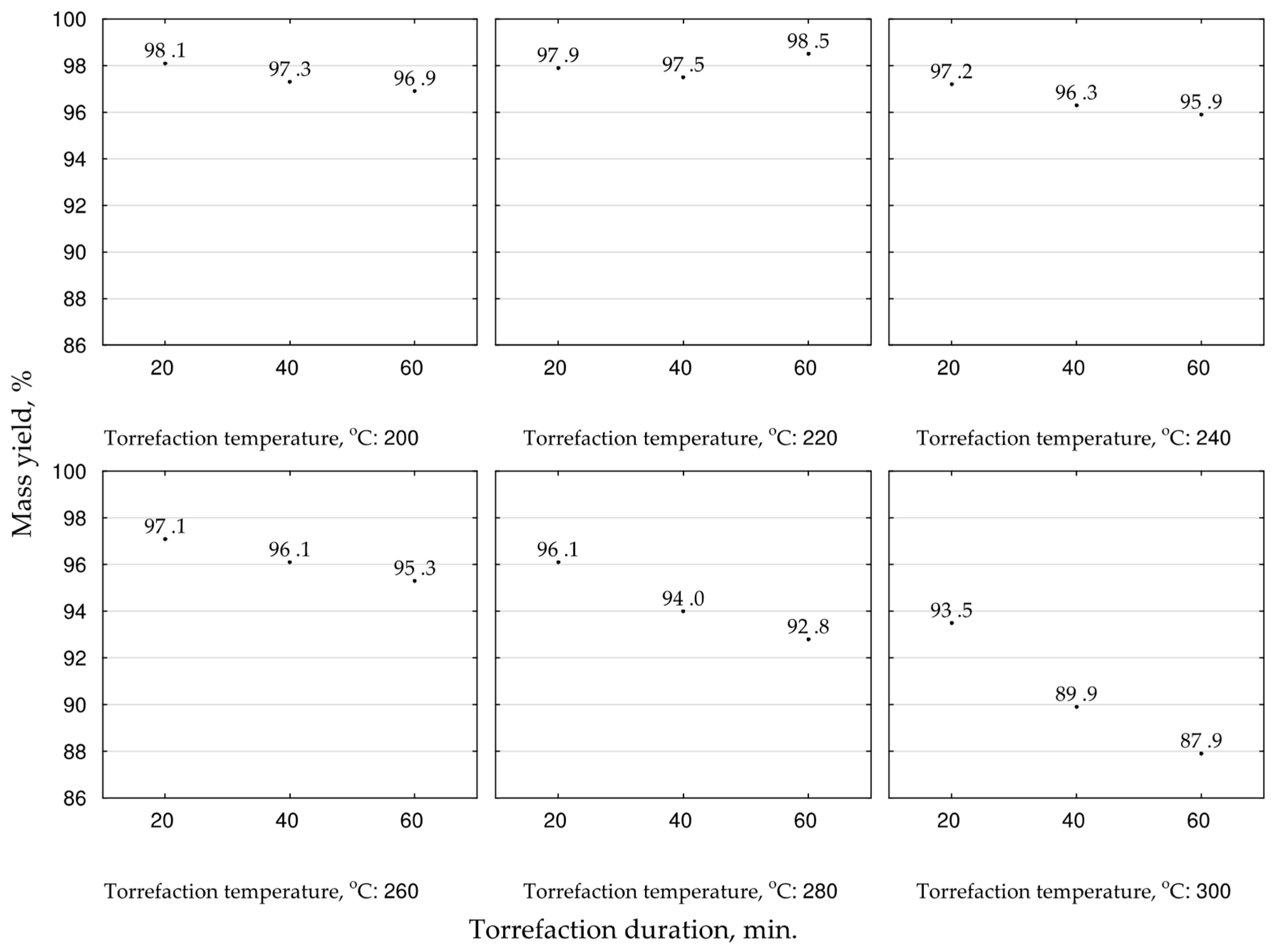
We determined the mass yields and energy yields to analyze the treatment efficiency. These parameters were calculated according to Equations (6)–(8) on the base of the mean values of
HHV and masses from the TGA [
43] for a given torrefaction temperature and duration. The highest mass yield of biocoal was achieved for temperatures 200 and 220 °C and ranged between 96.9 and 98.5%, respectively (
Figure 4). The mass yield under higher temperatures decreased and depended on torrefaction duration. The increase of torrefaction time decreased the mass yield. The lowest achieved value, 87.9%, was in the case of 300 °C, and 60 min duration (
Figure 4). For scaling-up the process, high mass yield is important when biomass or waste is converted to biocoal for agricultural purposes. Therefore, for the optimization of that goal, the second-degree polynomial model of torrefaction temperature and duration influence on biocoals’ mass yield was proposed (
Figure 5). The statistical evaluation showed that the determination coefficient was high (0.953) (
Figure 5), but only the model regression coefficients related to torrefaction temperature (a
2, a
3, and a
6) and the intercept (a
1) were statistically significant (
p < 0.05) (
Table A4).
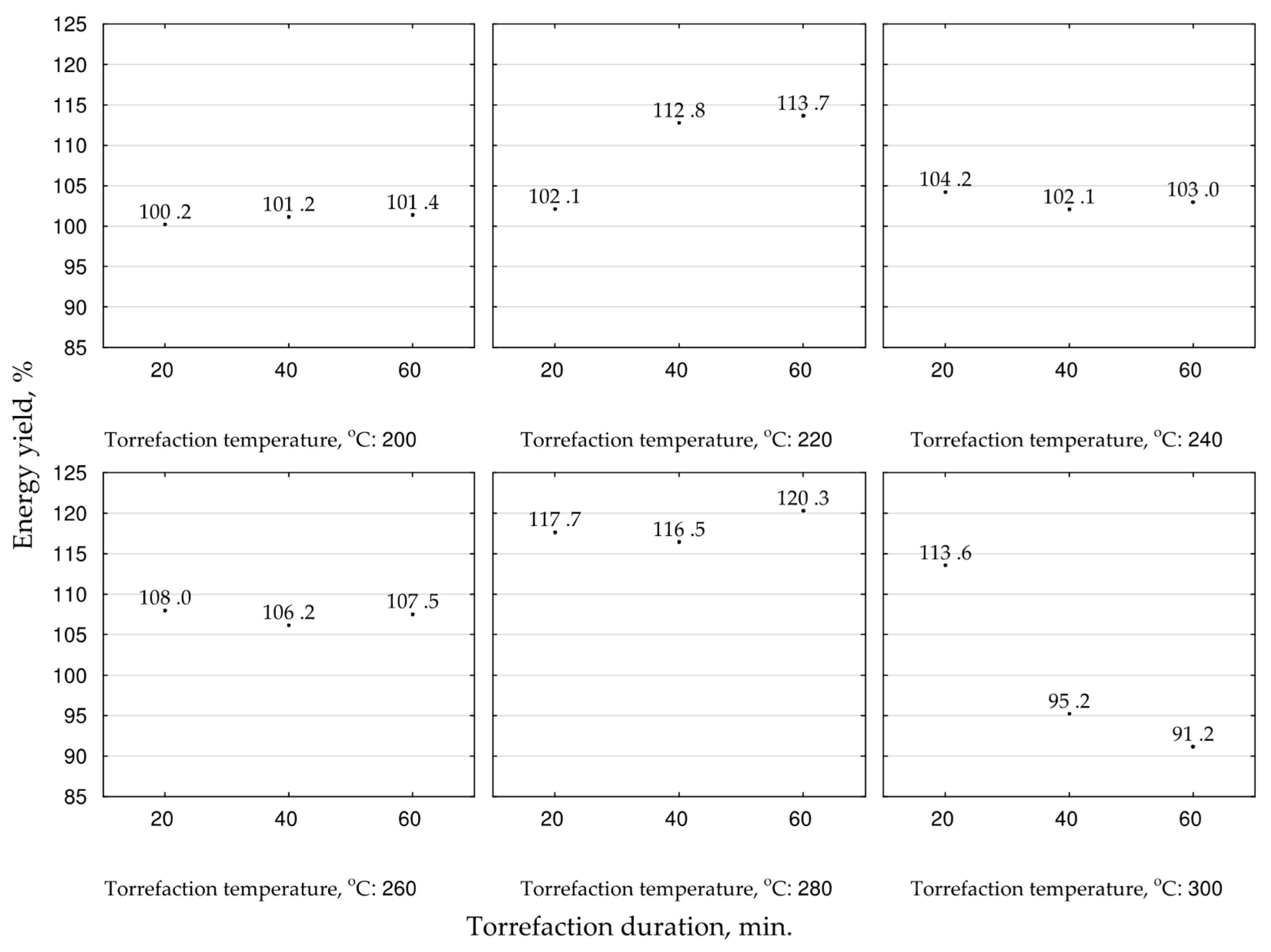
The energy yield in biocoal indicates the efficiency of the chemical energy densification due to thermochemical processes concerning the mass loss of the treated
MSC. The highest energy yield values, exceeding 116.5–120.3%, were noted at 280 °C, and were correlated with process duration. The increase of the temperature to 300 °C decreased the energy yield to 91%. Thus, for fuel production, the temperature of 280 °C and the process duration of 60 min should be applied (
Figure 6). Additionally, for optimization of
MSC conversion to biocoal for energy purposes, the second-degree polynomial model of torrefaction temperature and duration influence on biocoals’ energy yield was proposed (
Figure 7). The statistical evaluation showed that determination coefficient was not high (0.314) (
Figure 7); however, all the regression coefficients were significant (
p < 0.05) (
Table A5). The low degree of model fitting to the obtained data could be related to a similarly low degree of fitting of the model proposed for the
HHV.
HHV values are used for energy yield calculations. Similarly, to achieve better results suitable for
MSC torrefaction optimization, more complex research with a higher number of repetitions (to reduce the degree of the
HHV results’ heterogeneity) with the application of better-fitting models is required.
We have shown an alternative utilization of abundant biowaste (
MSC).
MSC has potential for improved management that is both sustainable and economical [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25]. We have proposed treating
MSC via torrefaction to produce biocoal with improved fuel properties. This research showed that by using torrefaction at 280 °C for 60 min, it is possible to increase the
HHV of raw
MSC from ~13.7 MJ·kg
−1 to ~18 MJ·kg
−1. This calorific value of obtained biocoal is comparable with nonagglomerating highly volatile coals (
HHV from 17.4 to 23.9 MJ·kg
−1) or lignite coal (
HHV below 17.4 MJ·kg
−1) [
58]. Our research also showed that the highest efficiency (i.e., the energy yield) was achieved at the temperature of 280 °C for a duration of 60 min.
Based on these results and using the best variant (
T and
t), a simple model [
49] was proposed (after some modifications) for estimating the possibility of achieving heat self-sufficiency of biocoal production. The model also calculates the theoretical mass yield after biocoal recirculation of the torrefied biomass to maintain the torrefaction process itself (biocoal recirculation—
Figure 1). The calculations and results were gathered in
Table 4.
The estimated value of torrefied
MSC recirculation rate was obtained for 1 Mg of wet
MSC (65.32% moisture content). For
MSC torrefaction (280 °C, 60 min), the mass yield of
MSC biocoal was 92.8%, and
HHV of torrefied
MSC was 17.9 MJ·kg
−1. For these conditions, the estimated biocoal recirculation rate for obtaining the
MSC torrefaction heat self-sufficiency was 43.6%. Therefore the 56.4% of produced biocoal (181.6 kg produced form 1 Mg of wet
MSC) may be used for heating the mushroom farm (
Figure 1) or be sold to external users.
The presented simplified calculation of the biocoal (considered as a fuel) recirculation rate is the first step for the evaluation of the feasibility of utilization of torrefaction technology for MSC treatment. The model was based on simple assumptions. Therefore, more advanced and precise models with a comprehensive analysis of mass and energy balances are warranted as a separate work.