Vergence Tracking: A Tool to Assess Oculomotor Performance in Stereoscopic Displays
Abstract
Introduction
Methods
Participants
- Monocular visual acuity (evaluated using a decimal scale chart) better than 10/10;
- No history of functional or organic ocular pathology;
- No use of medication that might interfere with oculomotor performance;
- No visual complaint (such as headache, eyestrain, or reddening of the eyes) prior to the experiment.
Apparatus
Stimuli
Procedure
- -
- Did you experience diplopia during the session? Yes / No
- -
- If so, what percentage of time did you experience diplopia on a scale from 10 to 100% (in steps of 10).
- -
- Did you experience any discomfort? Yes / No
- -
- If so, which disorder did you experience (multiple answers can be checked)? Headaches / Reddening of the eyes / Eyes that draw / Fatigue / Blurred vision / Other (explain).
- -
- Please quantify your discomfort (if any) on a scale from 0 to 10, with 10 corresponding to maximum discomfort.
Data processing
Statistical analysis
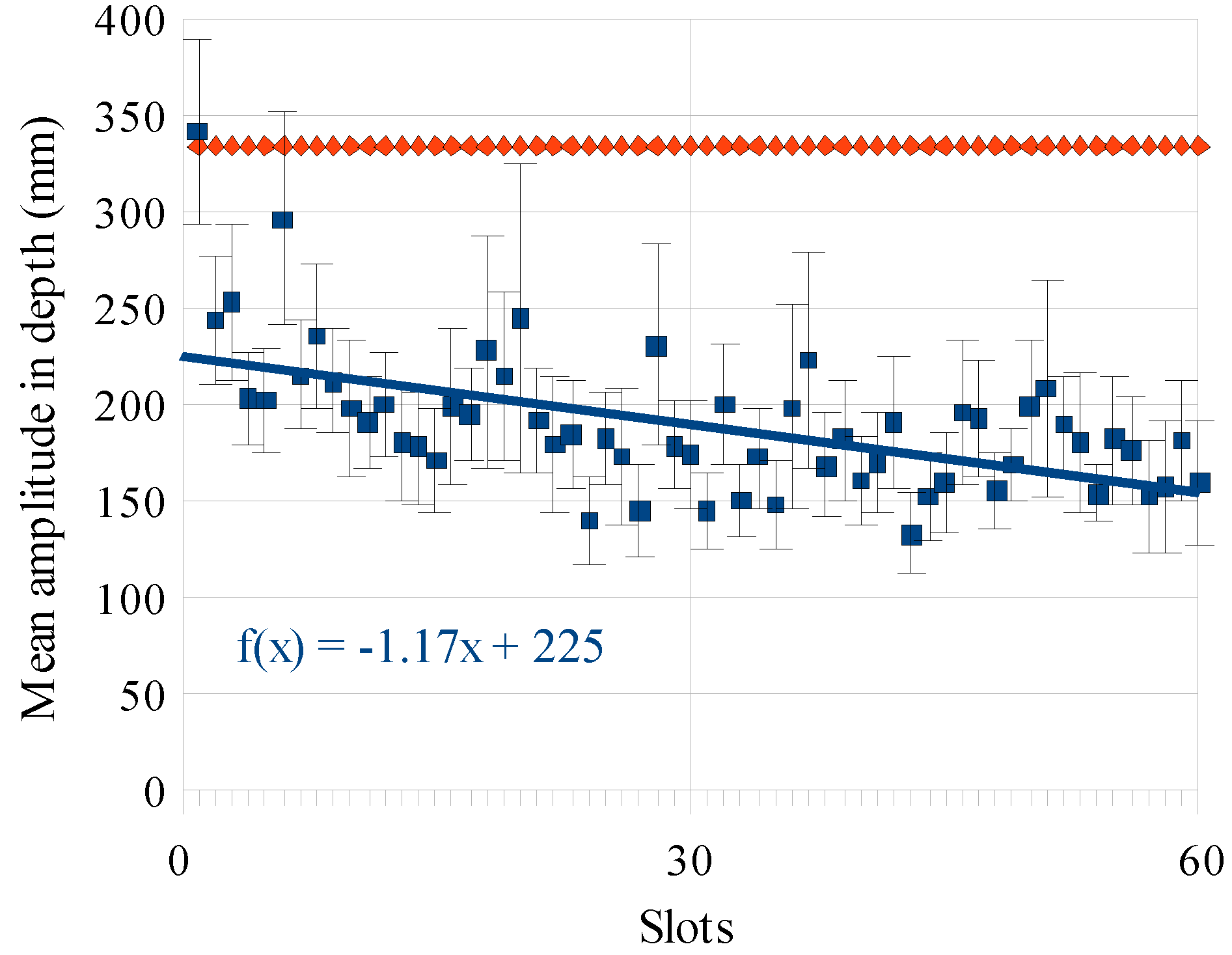
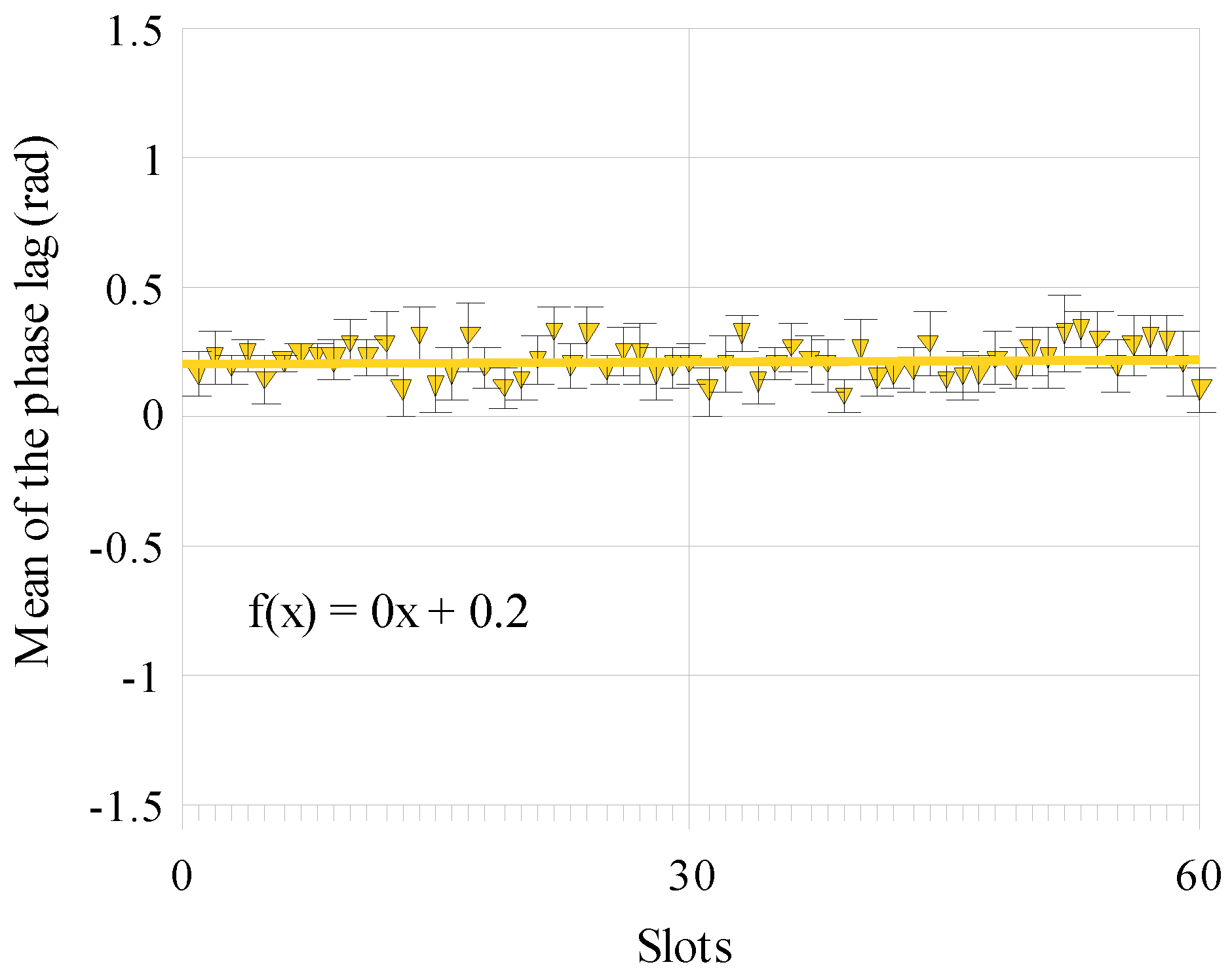
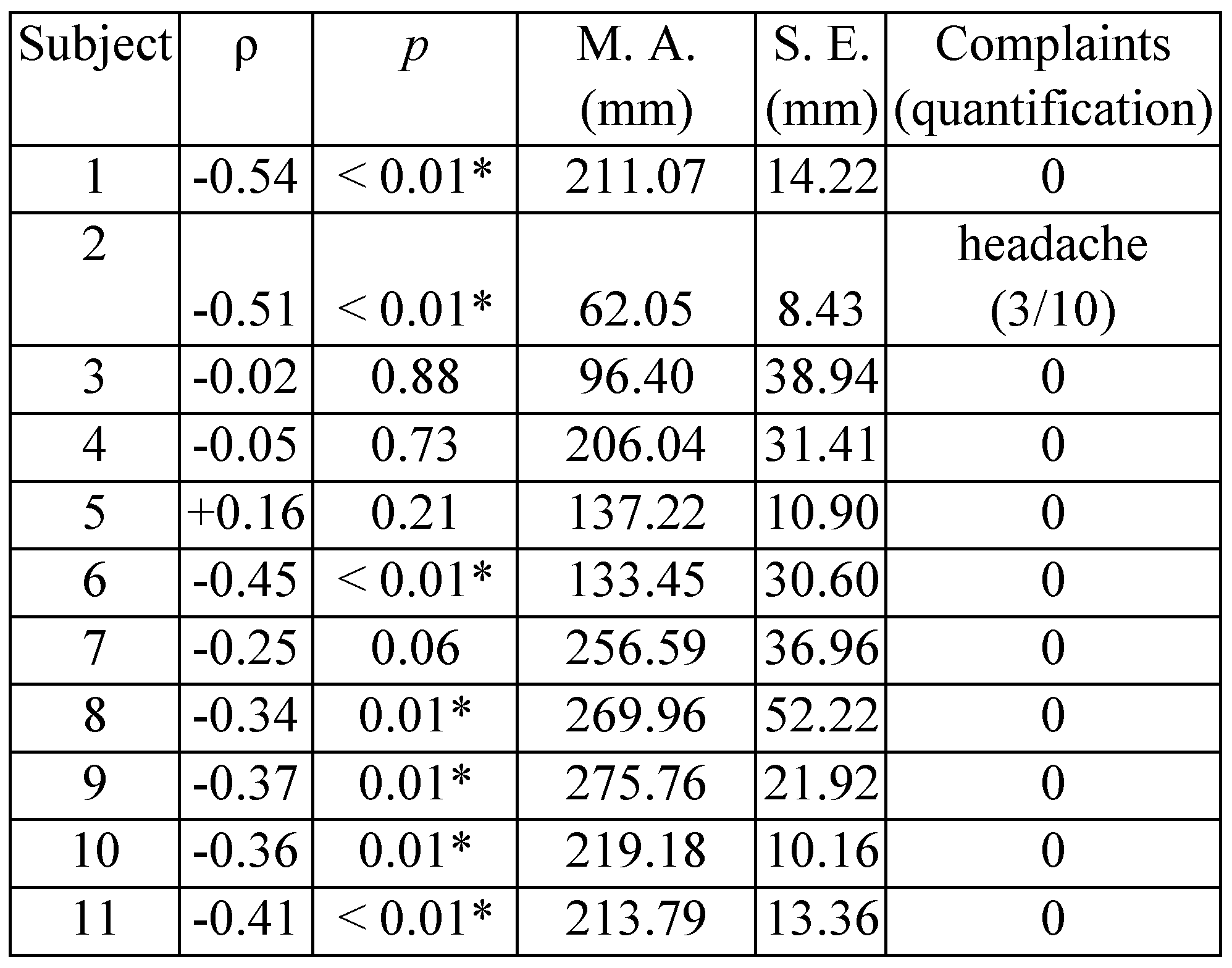
Results
Discussion
Acknowledgments
References
- Akeley, K., S. J. Watt, A. R. Girshick, and M. S. Banks. 2004. A stereo display prototype with multiple focal distances. ACM Transactions on Graphics (TOG) 23, 3: 804–813. [Google Scholar]
- Ciuffreda, K. J. 2006. Accommodation, the pupil, and presbyopia. In Borish's Clinical Refraction, 2nd ed. Edited by W. J. Benjamin. Birmingham, UK: Elsevier: pp. 93–144. [Google Scholar]
- Eadie, A. S., L. S. Gray, P. Carlin, and M. Mon-Williams. 2000. Modelling adaptation effects in vergence and accommodation after exposure to a simulated virtual reality stimulus. Ophthalmic and Physiological Optics 20, 3: 242–251. [Google Scholar]
- Emoto, M., T. Niida, and F. Okano. 2005. Repeated vergence adaptation causes the decline of visual functions in watching stereoscopic television. Journal of Display Technology 1, 2: 328–340. [Google Scholar]
- Emoto, M., Y. Nojiri, and F. Okano. 2004. Changes in fusional vergence limit and its hysteresis after viewing stereoscopic TV. Displays 25, 2–3: 67–76. [Google Scholar]
- Epelboim, J., R. M. Steinman, E. Kowler, Z. Pizlo, C. J. Erkelens, and H. Collewijn. 1997. Gaze-shift dynamics in two kinds of sequential looking tasks. Vision Research 37, 18: 2597–2607. [Google Scholar] [CrossRef] [PubMed]
- Erkelens, C., and H. Collewijn. 1985. Eye movements and stereopsis during dichoptic viewing of moving random-dot stereograms. Vision Research 25, 11: 1689–1700. [Google Scholar]
- Fincham, E. F., and J. Walton. 1957. The reciprocal actions of accommodation and convergence. The Journal of Physiology 137, 3: 488–508. [Google Scholar]
- Fuchs, A. F., and M. D. Binder. 1983. Fatigue resistance of human extraocular muscles. Journal of Neurophysiology 49, 1: 28–34. [Google Scholar] [PubMed]
- Hoffman, D. M., A. R. Girshick, K. Akeley, and M. S. Banks. 2008. Vergence-accommodation conflicts hinder visual performance and cause visual fatigue. Journal of Vision 8, 3: 1–30. [Google Scholar]
- Hofstetter, H. W. 1945. The zone of clear single binocular vision. Part I. American Journal of Optometry 22: 301–333. [Google Scholar] [CrossRef]
- Howard, I. P., X. Fang, R. S. Allison, and J. E. Zacher. 2000. Effects of stimulus size and eccentricity on horizontal and vertical vergence. Experimental Brain Research 130, 2: 124–132. [Google Scholar] [CrossRef] [PubMed]
- Howarth, P. A. 2011. Potential hazards of viewing 3 D stereoscopic television, cinema and computer games: A review. Ophthalmic and Physiological Optics 31, 2: 111–122. [Google Scholar] [CrossRef]
- Jainta, S., J. Hoormann, and W. Jaschinski. 2007. Objective and subjective measures of vergence step responses. Vision Research 47, 26: 3238–3246. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jaschinski, W., S. Jainta, and J. Hoormann. 2008. Comparison of shutter glasses and mirror stereoscope for measuring dynamic and static vergence. Journal of Eye Movement Research 1, 5: 1–7. [Google Scholar] [CrossRef]
- Kim, J., T. Shibata, D. Hoffman, and M. Banks. 2011. Assessing vergence-accommodation conflict as a source of discomfort in stereo displays. Journal of Vision 11, 11: 324–324. [Google Scholar] [CrossRef]
- Krishnan, V., S. Phillips, and L. Stark. 1973. Frequency analysis of accommodation, accommodative vergence and disparity vergence. Vision Research 13, 8: 1545–1554. [Google Scholar] [CrossRef]
- Lambooij, M., W. IJsselsteijn, M. Fortuin, and I. Heynderickx. 2009. Visual discomfort and visual fatigue of stereoscopic displays: A review. Journal of Imaging Science and Technology 53, 3: 1–14. [Google Scholar] [CrossRef]
- Lambooij, M., W. Ijsselsteijn, and I. Heynderickx. 2007. Visual discomfort in stereoscopic displays: A review. Paper presented at the Stereoscopic Displays and Virtual Reality Systems XIV, San Jose, CA. [Google Scholar]
- Le Van Quyen, M., J. Foucher, J. P. Lachaux, E. Rodriguez, A. Lutz, J. Martinerie, and et al. 2001. Comparison of Hilbert transform and wavelet methods for the analysis of neuronal synchrony. Journal of neuroscience methods 111, 2: 83–98. [Google Scholar] [CrossRef]
- Mon-Williams, M., and J. P. Wann. 1998. Binocular virtual reality displays: When problems do and don't occur. Human Factors 40, 1: 42–49. [Google Scholar] [CrossRef]
- Morgan, M. W. 1944a. Accommodation and its relationship to convergence. American Journal of Optometry & Archives of American Academy of Optometry 21: 183–195. [Google Scholar]
- Morgan, M. W. 1944b. Analysis of clinical data. American Journal of Optometry & Archives of American Academy of Optometry 21: 477–491. [Google Scholar]
- Peli, E. 1995. Real vision & virtual reality. Optics and Photonics News 6, 7: 28–34. [Google Scholar]
- Rashbass, C., and G. Westheimer. 1961. Disjunctive eye movements. The Journal of Physiology 159, 2: 339–360. [Google Scholar] [CrossRef] [PubMed]
- Rushton, S. K., and P. M. Riddell. 1999. Developing visual systems and exposure to virtual reality and stereo displays: Some concerns and speculations about the demands on accommodation and vergence. Applied Ergonomics 30, 1: 69–78. [Google Scholar] [CrossRef] [PubMed]
- Saito, S. 1992. Does fatigue exist in a quantitative measurement of eye movements? Ergonomics 35, 5–6: 607–615. [Google Scholar] [CrossRef]
- Semmlow, J., Y. F. Chen, B. Granger-Donetti, and T. L. Alvarez. 2008. Saccadic behavior during the response to pure disprity vergence stimuli I: general properties. Journal of Eye Movement Research 1, 2: 1–11. [Google Scholar]
- Semmlow, J., Y. F. Chen, B. Granger-Donetti, and T. L. Alvarez. 2009. Correction of saccade-induced midline errors in responses to pure disparity vergence stimuli. Journal of Eye Movement Research 2, 5: 1–13. [Google Scholar] [CrossRef]
- Sharples, S., S. Cobb, A. Moody, and J. R. Wilson. 2008. Virtual reality induced symptoms and effects (VRISE): Comparison of head mounted display (HMD), desktop and projection display systems. Displays 29, 2: 58–69. [Google Scholar] [CrossRef]
- Shibata, T., J. Kim, D. M. Hoffman, and M. S. Banks. 2011. The zone of comfort: Predicting visual discomfort with stereo displays. Journal of Vision 11, 8: 1–29. [Google Scholar] [CrossRef]
- Ukai, K., and P. A. Howarth. 2008. Visual fatigue caused by viewing stereoscopic motion images: Background, theories, and observations. Displays 29, 2: 106–116. [Google Scholar] [CrossRef]
- Wann, J. P., and M. Mon-Williams. 2002. Measurement of visual aftereffects following virtual environment exposure. In Handbook of Virtual Environments. Edited by K. M. Stanney. Mahwah, NJ: Lawrence Erlbaum Associates: pp. 731–749. [Google Scholar]
- Wann, J. P., S. Rushton, and M. Mon-Williams. 1995. Natural problems for stereoscopic depth perception in virtual environments. Vision Research 35, 19: 2731–2736. [Google Scholar] [PubMed]
- Yang, S., and J. E. Sheedy. 2011. Effects of vergence and accommodative responses on viewer's comfort in viewing 3D stimuli. Paper presented at the Stereoscopic Displays and Applications XXII, San Francisco, CA. [Google Scholar]
- Yuan, W., and J. L. Semmlow. 2000. The influence of repetitive eye movements on vergence performance. Vision Research 40, 22: 3089–3098. [Google Scholar] [PubMed]
- Zee, D., E. Fitzgibbon, and L. Optican. 1992. Saccade-vergence interactions in humans. Journal of Neurophysiology 68, 5: 1624–1641. [Google Scholar] [CrossRef] [PubMed]


 |
Copyright © 2012. This article is licensed under a Creative Commons Attribution 4.0 International License.
Share and Cite
Neveu, P.; Philippe, M.; Priot, A.-E.; Fuchs, P.; Roumes, C. Vergence Tracking: A Tool to Assess Oculomotor Performance in Stereoscopic Displays. J. Eye Mov. Res. 2012, 5, 1-8. https://doi.org/10.16910/jemr.5.2.1
Neveu P, Philippe M, Priot A-E, Fuchs P, Roumes C. Vergence Tracking: A Tool to Assess Oculomotor Performance in Stereoscopic Displays. Journal of Eye Movement Research. 2012; 5(2):1-8. https://doi.org/10.16910/jemr.5.2.1
Chicago/Turabian StyleNeveu, Pascaline, Matthieu Philippe, Anne-Emmanuelle Priot, Philippe Fuchs, and Corinne Roumes. 2012. "Vergence Tracking: A Tool to Assess Oculomotor Performance in Stereoscopic Displays" Journal of Eye Movement Research 5, no. 2: 1-8. https://doi.org/10.16910/jemr.5.2.1
APA StyleNeveu, P., Philippe, M., Priot, A.-E., Fuchs, P., & Roumes, C. (2012). Vergence Tracking: A Tool to Assess Oculomotor Performance in Stereoscopic Displays. Journal of Eye Movement Research, 5(2), 1-8. https://doi.org/10.16910/jemr.5.2.1



